Abstract
Potassium channels are the most heterogeneous and widely distributed group of ion channels and play important functions in all cells, in both normal and pathological mechanisms, including learning and memory processes. Being fundamental for many diverse physiological processes, K+-channels are recognized as potential therapeutic targets in the treatment of several Central Nervous System (CNS) diseases, such as multiple sclerosis, Parkinson's and Alzheimer's diseases, schizophrenia, HIV-1-associated dementia, and epilepsy. Blockers of these channels are therefore potential candidates for the symptomatic treatment of these neuropathies, through their neurological effects. Venomous animals have evolved a wide set of toxins for prey capture and defense. These compounds, mainly peptides, act on various pharmacological targets, making them an innumerable source of ligands for answering experimental paradigms, as well as for therapeutic application. This paper provides an overview of CNS K+-channels involved in memory acquisition and storage and aims at evaluating the use of highly selective K+-channel blockers derived from arthropod venoms as potential therapeutic agents for CNS diseases involving learning and memory mechanisms.
1. Introduction
Many efforts have been made to understand the physiological mechanisms responsible for learning and memory. Due to their complexity, different approaches have been used to unlock them and various actors of these phenomena have been often revealed [1, 2]. In the last two decades, a new agent has gained the attention of the scientific community studying the processes of learning and memory: the potassium channels [3].
Potassium channels (KCNs) exhibit a great diversity (for review see [4, 5]). In mammals, nine and ten genes that encode channels for Na+ and Ca2+ have been described, respectively. Nonetheless, for KCN they are 78 genes, at least [5]. In addition to this large number of genes, alternative splicing, RNA editing, posttranslational modifications, and channel formation of heteromeric assembly by the association of different principal subunits also contribute to the diversity of KCN [4]. These channels can be grouped into four families: voltage-gated channels (Kv), calcium-activated channels (KCa), inward-rectifiers channels (Kir) and two tandem-pore channels (K2P). Furthermore, these families have many subfamilies each containing several members: Kv have 12 subfamilies (K v1-K v12), KCa, 5 subfamilies (KCa1-KCa5), the Kir, 7 subfamilies (Kir1-Kir7), and K2P, 15 subfamilies (K2P1-K2P7, K2P9,K2P10, K2P12, K2P13 and K2P15-K2P18) [6–9].
The four families of KCNs are structurally related. Kv, Kir, and KCa are transmembrane proteins formed by four α-subunits, whereas in K2P there are only two. α-Subunits of all Kv, KCa2, and KCa3 have six transmembrane segments (6TM), while in KCa1, KCa4, and KCa5 are 7TM. All Kir have α-subunits with 2TM and those of K2P contain 4TM. When α-subunits join to form a channel, they may be identical and thus generating homodimeric or homotetrameric (homomultimeric) channels or may be different resulting in heterodimeric or heterotetrameric (heteromultimeric) channels. Heteromultimeric channels are formed by the association of different α-subunits of the same subfamily. α-Subunits assembly of K2P forms two pores and the in other KCNs there is one pore (for reviews, see [4, 5, 10–13]). The X-ray structure of mammalian Kv1.2 channel with the β 2 subunit (Figure 1) was reported by Long et al. [14]. In accordance with what is mentioned above, Kv channels are formed by four α-subunits that generate one pore (Figures 1(a) and 1(c)). Each Kv1.2 channel subunit contains six transmembrane segments, termed S1 to S6 (Figure 1(b)). The S5-S6 regions (Figures 1(a)–1(c), green) from the four subunits shape a single pore domain. The S1-S4 helices from each subunit form four surrounding voltage-sensing domains (Figures 1(b) and 1(c)).
Figure 1.
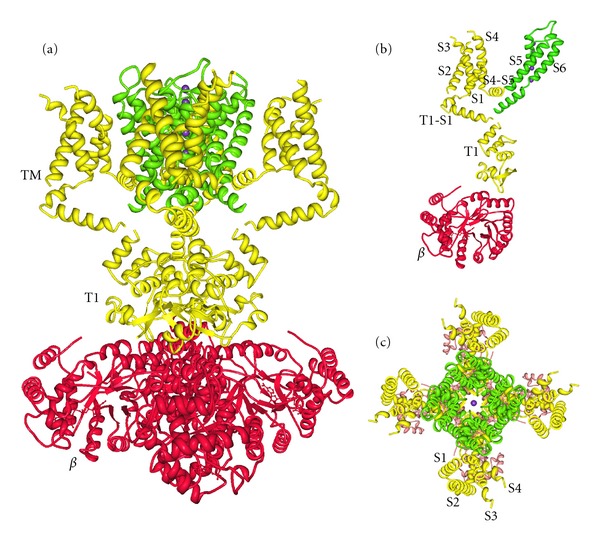
Views of the Kv1.2-β 2 subunit complex. (a) Side view of the Kv1.2-β 2 structure with the extracellular solution above and the intracellular solution below. Four subunits of the channel are colored in yellow (T1 domain and voltage sensor) and green (pore). β subunit tetramer is colored in red. TM indicates the integral membrane component of the complex. (b) Stereoview of a single subunit of the channel and β subunit viewed from the side. Labels correspond to six transmembrane helices (S1 to S6). (c) View of the Kv1.2-β 2 structure from the extracellular side of the membrane. S1–S4 helices from each subunit form four surrounding voltage-sensing domains, S5-S6 regions (green) from the four subunits shape a single pore domain. Purple spheres are potassium ions. Images were generated using Protein Workshop Viewer 3.9 [15] and Protein Data Bank accession ID 2A79 [14].
KCNs have a significant influence on the neuron's activity, functioning of neuronal circuits, and brain plasticity. These channels regulate action potential firing patterns and control neurotransmitter release by constraining local membrane excitability and limiting Ca2+ influx (for review, see [16]). Furthermore, KCNs participate in the induction of synaptic plasticity by shape excitatory postsynaptic potentials (EPSPs) and enhance synaptic integration through their N-methyl-D-aspartate receptor (NMDAR), the cellular analogue for learning and memory (for review, see [3]). Therefore, KCNs may have an important role in cognition.
This possible involvement of KCN in cognitive processes is reinforced by its strong presence in Central Nervous System (CNS). These channels have a wide distribution in the brain of mammals. Several members of the Kv families [17–20], KCa [21–25], Kir [26–29], and K2P [30, 31], are found in the telencephalon, diencephalon, brainstem, and cerebellum of mammals.
The use of KCN blockers has aided to elucidate the function of these channels in the CNS. The tetraethylammonium (TEA) and 4-aminopyridine (4-AP) are traditional (classical) pharmacological blockers for KCN [32]. However, they are not specific, neither act on all types of KCN. TEA blocks different subtypes of Kv channels (except Kv4.2, Kv4.3, Kv5.1, Kv7.1 and all of the subfamilies Kv6, Kv8-Kv12), KCa (except subfamily K Ca2 and KCa3), and subtypes Kir3.4 and Kir7.1, but has no effect on K2P channels (for review see [6–9]). The 4-AP also operates on different subtypes of Kv channels (except Kv3.4, Kv5.1, and all Kv6-Kv12), Kir3.4, and Kir7.1, but not on KCa channels, neither on the K2P [6–9]. Therefore, the potential of these blockers to investigate the role of KCN on cognitive processes is limited.
Their diversity, distribution, and function suggest that the potassium channels could be involved in different cognitive processes, leading to a complex scenario that embarrass the understanding of the role of each K+-channel subtype in these events. This challenge is even more complicated by the lack of drugs that act specifically on each type of KCN. One way to solve this problem has been the use of toxins isolated from invertebrates' venoms as K+-channels blockers.
Many animal toxins, such as those isolated from spider, scorpion, and bee venoms, are specific blockers of potassium channels. While TEA operates in millimolar concentrations, scorpion toxins bind to and block Kv channels with pico- or nanomolar affinities [33]. Apamin, a toxin isolated from the bee venom, blocks KCa2.2 and KCa2.3 channels with affinity of the order of picomolar [34]. Therefore, animal venom toxins may be more useful as pharmacological tools than the classical blockers (TEA and 4-AP) as they act on K+-channels with high potency and selectivity [35].
The aim of this paper is to review the knowledge accumulated on the importance of KCN to the processes of learning and memory. In addition, we present the contribution and potential use of toxins isolated from spiders, scorpions, and bees as pharmacological tools in this investigation. Finally, the participation of KCN in clinical conditions holding cognitive deficits and the possible use of toxins from animals as therapeutic agents are also considered.
2. Potassium Channels in Learning and Memory
The hippocampus has a great importance in learning and memory processes. This limbic structure holds a key in the consolidation of explicit memory. It receives information of events and transfers them to the neocortex where they are stored for a period (even weeks) and then gradually returned to specific regions of the cerebral cortex contributing to the formation of long-term memory [36].
The hippocampus expresses many types of KCN. As shown in Table 1, 39 different types of KCN belonging to the four families (Kv, KCa, K2P, and Kir) have been identified in this neural structure. Of these, the more expressed channels in the hippocampus appear to be the Kv7.2, Kv7.3, KCa1.1, Kir3.2, and Kir3.3, followed by Kv1.1, Kv1.6, Kv3.1, Kv4.2, Kv10.1, KCa2.1, KCa2.2, and Kir3.1 (Table 1). What would be the role of this KCN diversity in the hippocampus?
Table 1.
Distribution of different types of K+ channels in hippocampus.
| Channels | Hippocampus | Employed technique | Ref. | ||
|---|---|---|---|---|---|
| CA1 | CA3 | DG | |||
| Kv1.1 | ++ | +++ | +++ | ISH, IMH, IMC, and CIMP in hippocampus or brain of rat, mouse, or gerbil. | [37–39] |
| Kv1.2 | + | + | ++ | ||
| Kv1.3 | − | − | − | IMH in gerbil hippocampus. | [39] |
| Kv1.4 | ++ | ++ | ++ | ISH, IMH, and IMC in hippocampus or brain of rat, mouse or gerbil. | [20, 38, 39] |
| Kv1.5 | + | + | − | IMH or single-cell RT-PCR in gerbil or rat hippocampus. | [39, 40] |
| Kv1.6 | ++ | +++ | +++ | IMH in gerbil hippocampus. | [39] |
| Kv2.1 | ++ | ++ | Single-cell RT-PCR in rat hippocampus. | [17] | |
| Kv3.1 | ++ | +++ | +++ | Northern blot analysis and ISH in rat brain. | |
| Kv3.2 | +++ | ++ | − | [17] | |
| Kv3.3 | + | + | ++ | ||
| Kv3.4 | − | − | ++ | ||
| Kv4.1 | + | + | ++ | ISH in rat brain. | [18] |
| Kv4.2 | +++ | ++ | +++ | ISH or IMH in rat or mouse brain. | [18, 41, 42] |
| Kv4.3 | + | ++ | +++ | ISH in rat brain. | [18, 42] |
| Kv7.2 | +++ | +++ | +++ | ISH and IMH in rat brain | |
| Kv7.3 | +++ | +++ | +++ | ||
| Kv10.1 | ++ | +++ | ++ | ISH, real time PCR, or IMH in rat brain. | [19, 43] |
| Kv10.2 | − | − | − | ISH and IMH in rat brain. | |
| Kv11.1 | ++ | − | − | ||
| Kv11.2 | − | − | − | [19] | |
| Kv11.3 | +++ | − | − | ||
| Kv12.1 | + | − | + | ||
| Kv12.2 | ++ | − | ++ | ||
| KCa1.1 | +++ | +++ | +++ | ISH, WB analysis, IMH, IMF, IMC, or RLB in mouse or rat brain. | [21, 22, 44] |
| KCa2.1 | ++ | +++ | ++ | ISH, IB analysis, IMH, or RLB in rat brain. | [23, 24] |
| KCa2.2 | +++ | +++ | + | ISH, IB analysis, IMH, or RLB in rat brain. | [23–25] |
| KCa2.3 | + | ++ | + | ||
| K2P1.1 | − | ++ | ++ | ISH in rat and mouse brain. | |
| K2P2.1 | ++ | + | +++ | ISH, WB analysis, IMH, IMF, or IMC in rat or mouse brain. | [31] |
| K2P3.1 | ++ | ++ | ++ | ISH in rat and mouse brain. | |
| K2P4.1 | ++ | +++ | + | [31] | |
| K2P9.1 | ++ | ++ | +++ | ||
| K2P10.1 | − | ++ | + | ||
| Kir2.1 | + | + | +++ | ISH or IMH in mouse or rat brain. | [26–28] |
| Kir2.2 | ++ | + | ++ | ISH in mouse or rat brain. | [26, 27] |
| Kir2.3 | ++ | + | +++ | ||
| Kir3.1 | +++ | ++ | +++ | ISH or IMH in rat brain. | [27, 28] |
| Kir3.2 | +++ | +++ | +++ | ISH or IMH in rat brain. | [27, 45] |
| Kir3.3 | +++ | +++ | +++ | ISH in rat brain | [27] |
| Kir3.4 | + | ++ | + | WB analysis, IMH or ISH in rat or mouse brain. | [27, 45–47] |
| Kir6.2 | ++ | ++ | ++ | ISH, IMH, or IMF in rat or mouse brain. | [29, 48, 49] |
The symbols indicate signal intensity as follows: − (not detected); + (weak); ++ (moderate); +++ (high); CA1, CA3, and DG (Dentate gyrus) are regions of hippocampal formation; Ref.: reference; RT-PCR means reverse transcription polymerase chain reaction; ISH is used for in situ hybridization; IMH for immunohistochemistry; IMC for immunocytochemistry; CIMP for coimmunoprecipitation; IMF for immunofluorescence; WB for western blot; IB for immune blot; RLB for radioligand binding.
Many experimental studies (Table 2) show that KCN may have a significant contribution in learning and memory processes. In these studies, the activity or expression of K+ channels in the brain of rats and mice was altered by different strategies. The impact of this manipulation on the learning and memory was accessed by behavioral tests.
Table 2.
K+ channels manipulations and their effects on experimental behavioral models for learning and memory.
| Channels | Technique | Effect on channel | Behavioral test | Result | Ref. |
|---|---|---|---|---|---|
| Kv, KCa, and Kir | icv of minoxidil, pinacidil, TEA, glibenclamine, gliquidone, and cromakalim. | TEA blocks Kv and KCa. Gliquidone and glibenclamide block Kir. Minoxidil, pinacidil and cromakalim open Kir | PAT in mice | Minoxidil, pinacidil and cromakalim: (−) TEA, glibenclamine, gliquidone: (+) |
[50] |
|
| |||||
| Kv1.1 | icv of antisense oligodeoxyribonucleotide to Kv1.1 mRNA. | Inhibition of channel expression | PAT in mice | (−) | [51] |
| MWM in rats | (−) | ||||
|
| |||||
| Kv12.2 | Kv12.2 (BEC1) knockout mice | Inhibition of channel expression | MWM | (+) | |
| Y-maze test | (+) | ||||
| WFT | (+) | ||||
| Kv12.2 OVER mice | Overexpression of channel in the forebrain | MWM | (−) | [52] | |
| Y-maze test | (−) | ||||
| WFT | (−) | ||||
|
| |||||
| KCa2 | Systemic Infusion of EBIO or CyPPA. | EBIO activates SK channels. CyPPA activates KCa2.2 (SK2)/ KCa2.3 (SK3) subunits over KCa2.1 (SK1), and is more potent than EBIO. | ORT in mice | EBIO: (−) CyPPA: (−) |
|
| Contextual FCP in mice | EBIO: (0) CyPPA: (NT) |
[53] | |||
| Tone FCP in mice | EBIO: (0) CyPPA: (NT) |
||||
|
| |||||
| KCa1.1 | ih (CA1) of paxilline | Paxilline blocks the channel. | Trace eyeblink in rats | (−) | [54] |
|
| |||||
| KCa2.2 | SK2-OVER mice | Overexpression of KCa2.2 (SK2) protein and KCa2.2 mRNA | MWM | (−) | |
| Contextual FCP | (−) | [55] | |||
| Tone FCP | (−) | ||||
|
| |||||
| KCa2.2 | SK2-OVER mice | Overexpression of KCa2.2 (SK2) protein and KCa2.2 mRNA | Contextual FCP | (−) | [56] |
|
| |||||
| KCa2.3 | Doxycycline-induced conditional SK3 channel deficient (T/T) mice | Inhibition of channel expression | PAT | (0) | |
| MWM | (0) | ||||
| ORT | (0) | ||||
| Y-maze test | (−) | [57] | |||
| Five-trial inhibitory avoidance test | (−) | ||||
|
| |||||
| K2P10.1 | Infusion of siRNA in the EC. | Knock down K2p10.1 channels in the EC. | MWM in rats | (+) | [58] |
|
| |||||
| Kir3.4 | Kir3.4 (GIRK4) knockout mice | Inhibition of channel expression | PAT | (0) | [51] |
| MWM | (−) | ||||
|
| |||||
| Kir6.2 | ih (CA3) of diazoxide, or tolbutamide, or both. | Diazoxide opens the channel. Tolbutamide blocks the channel. | Contextual FCP in mice | Diazoxide: (−) Tolbutamide: (0) Both: (0) | [48] |
| Tone FCP in mice | Diazoxide, tolbutamide or both: (0) | ||||
| Kir6.2 knockout mice | Inhibition of channel expression | Contextual FCP | (−) | ||
| Tone FCP | (−) | ||||
| MWM | (0/−) | ||||
(−): impairment; (+): improved; (0): neutral; (0/−): slight impairment; (NT): not tested; Ref.: reference; icv: intracerebroventricular injection; ih: intra-hippocampal injection; EC: entorhinal cortex; OVER: overexpressing; EBIO: 1-ethyl-2-benzimidazolinone; CyPPA: Cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine; siRNA: small interfering RNA; PAT is used for passive-avoidance test; MWM for Morris water maze test; WFT for Water-finding task; ORT for object recognition task; FCP for fear-conditioning paradigm.
Ghelardini et al. [50] worked with mice subjected to passive avoidance test (Table 2). They found that intracerebroventricular (i.c.v.) administration of minoxidil, pinacidil, and cromakalim (KCN openers) produced amnesia. However, TEA, gliquidone and glibenclamide (KCN blockers) prevented this effect. These researchers also used toxins (apamin and charybdotoxin) whose results will be addressed later in the present paper.
Vick et al. [53] studied mice submitted to object recognition task, contextual fear-conditioning paradigm, and tone fear-conditioning paradigm (Table 2). Systemic 1-ethyl-2-benzimidazolinone (EBIO) and cyclohexyl-[2-(3,5-dimethylpyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine (CyPPA), KCa2 channel activators, impaired the encoding, but not retrieval, of object memory in a spontaneous object recognition task. In addition, EBIO did not affect contextual or cued fear memory. They also tested apamin and this treatment will be discussed in Section 4.1.
Matthews and Disterhoft [54] observed rats submitted to trace eyeblink conditioning (Table 2). Intrahippocampal (CA1) injection of paxilline, a KCa1.1 channel blocker, resulted in slowed learning of the task.
Hammond et al. [55] produced transgenic mice that overexpress KCa2.2 subunits by 10-fold and tested them by Morris water maze, contextual fear-conditioning paradigm, and tone fear-conditioning paradigm on mice (Table 2). They found that this condition impaired learning in all tasks. The same results for these animals in contextual fear-conditioning paradigm were obtained for Stackman et al. [56].
Jacobsen et al. [57] tested doxycycline-induced conditional KCa2.3-deficient mice in five distinct learning and memory paradigms: passive-avoidance test, Morris water maze test, object recognition task, Y-maze test, and five-trial inhibitory avoidance test. Impairment was only observed in the last two tasks (with no effect on the others).
Deng et al. [58] worked with rats subjected to Morris water maze test (Table 2). They infused small interfering RNA (siRNA) in the entorhinal cortex (EC) to knock down K2p10.1 channels. Baclofen, a specific γ-aminobutyric acid (GABAB) receptor agonist, was also applied into EC. The treatment of rats with siRNA abolished baclofen-induced inhibition of spatial learning. When administered alone, siRNA tended to improve the learning ability of rats.
Wickaman et al. [46] employed mice lacking a functional Kir3.4 gene and submitted them to passive-avoidance test and Morris water maze test (Table 2). These mice performed similarly to wild-type controls in the first task, however, exhibited impaired performance in latter.
Betourne et al. [48] worked with wild-type and Kir6.2 knockout mice submitted to Morris water maze test, contextual fear-conditioning paradigm, and tone fear-conditioning paradigm (Table 2). In wild-type mice, intra-hippocampal (CA3) injection of diazoxide (Kir6.2 opener) impaired contextual memory. This effect was reversed by co-injecting tolbutamide (Kir6.2 blocker). The Kir6.2 knockout mice presented impairment of contextual and tone memories and slightly impaired performance in Morris water maze (special memory).
It has been described that Kv1.1 channels contribute to the processes of learning and memory. Meiri et al. [51] were able to inhibit the expression of Kv1.1 in the hippocampus, blocking the translation of the mRNA of these channels. They found that this procedure worsened the passive avoidance in mice and spatial memory in rats (Table 2). Kourrich et al. [59], working with odor-discrimination tasks in rats, showed that levels of mRNA expression of Kv1.1 channels in the hippocampus were positively correlated with associative learning.
On the other hand, Kv2.1 channels appear to interfere negatively with learning and memory. Zhong et al. [40] suggested that memory deficits induced by scopolamine in rats may result from exacerbation of potassium currents in hippocampal pyramidal neurons as a consequence of increased mRNA expression of Kv2.1 channels.
It has been shown that Kv12.2 (BEC1) channels have preferential distribution in the forebrain, including the hippocampal and cortical regions [19, 60, 61]. The use of Kv12.2 knockout and overexpression (OVER) mice made possible to verify that this KCN is negatively involved in cognitive function, since the Kv12.2 knockout mice performed behavioral tasks related to working memory, reference memory, and attention better than their wild-type animals. In the OVER mice, on the other hand, the performance of those tasks was impaired [52] (Table 2).
Taken together, these studies strongly suggest that the brain KCNs and their modulation play an important role in the regulation of memory processes. Some hippocampal Kv (Kv1, Kv2 and Kv12), KCa (KCa1 e KCa2), and Kir (Kir3 e Kir6) channels seem to be particularly relevant. On this basis, the KCN blockers could be useful in investigation and the treatment of cognitive deficits.
3. Arthropod K+ Channels Toxins
Arthropod venoms constitute a rich source of peptidyl KCN inhibitors (KTxs). There are two classes of inhibitory peptides based on their mechanism of action: (1) KCN blockers which bind to the outer vestibule and then blocking the ion conductance by the pore occlusion; (2) KCN gating modifiers which shift the channel opening to more positive potentials. Scorpion KCN blockers (scorpions KTxs), exemplified by charybdotoxin (ChTX) [62], and the peptide tertiapin, isolated from bee venom [63], act as pore blockers, while spider KTxs, such as SGTx1 [64], act as a gating modifier. In turn, apamin, another toxin from bee venom, possibly acts as a pore blocker, although residues of the extracellular S3/S4 loop of the KCa2 (SK) channels also affect the apamin binding [65]. According to Lamy et al. [66], based on differences in binding affinity and potency of the blockage, apamin does not behave as a classical pore blocker, and probably the blocking effect occurs by an allosteric mechanism.
These KTxs show different arrangement of their three-dimensional (3D) structures. The folding types earlier found are αα, αββ, and β αββ [67–69]. Despite the conformation differences, most of these peptides have common residues which promote the binding with the potassium-channel vestibule, such as a lysine residue distant from an aromatic residue for 6.6 ± 1.0 Å [70].
Arthropod toxins have been used as pharmacological tools to better understand the role of ion channels, as most of them act in a high specific and potent way. Some of these toxins constitute unique blockers of certain ion channels, such as ergtoxin-1 (Centruroides noxius) [71], and BeKm-1 (Mesobuthus eupeus) [72] for Kv11.1 (HERG), psalmotoxin (Psalmopoeus cambridgei) for ASIC1a channel [73], tertiapin-Q (Apis mellifera) for Kir3.1 (GIRK) [63], and Lq2 (Leiurus quinquestriatus hebraeus) for Kir1.1 (ROMK1) [74].
The scorpion KTxs are formed by 20–95 amino acid residues stabilized by two, three, or four disulfide bonds, making this structure relatively stable. The scorpion KTxs were originally classified into three families named α, β, and γ [75], all of them have the highly conserved secondary structural arrangement α/β stabilized by cysteines (CSα/β). More recently, scorpion KTxs presenting a different structural arrangement, with only two α-helices stabilized by two disulfide bonds, CSα/α, were described, and these peptides were named κ-KTxs [76–78]. Among the almost 190 scorpion KTxs described until now, the α-KTx family, the largest one, contains more than 130 peptides thus far, classified in 20 subfamilies, based on their amino acid homology [75, 79].
Toxins from spider venom can play an important and complementary role in investigation of KCN cognitive function. Unlike most animal toxins obtained from snakes, bees, scorpions and sea anemones venom, which block mainly Kv1 and Kv3 channels, peptide toxins from spiders target Kv2 and Kv4 channels, which are expressed in the CNS and cardiovascular system of mammals (for review see [80]). Moreover, the venom spiders belonging to the Theraphosidae family represent a plentiful source of peptides that modify the gating of Kv channels [81]. Hanatoxin and seemingly others tarantula toxins shift channel opening to more depolarized voltages [81, 82] by stabilizing the resting conformation of the voltage sensor [83]. It has been suggested that these peptides interact with the voltage-sensor paddle within the lipid membrane [84–86].
The bee venom is composed of several classes of peptides, as well as enzymes and biogenic amines. Among the peptides, it can be emphasized the presence of the melittin [87], apamin [88], tertiapin [89], and mast cell degranulating peptide [90]. Certainly, in relation to neurotoxins, the apamin has greatly excelled, since this peptide has been considered a good pharmacological tool and it has provided important information in respect of the functioning of K+ -channels [91].
4. Arthropod KTxs in Learning and Memory
As already mentioned above, KCN classical blockers are not specific and cannot act at all KCNs. So these blockers have a major limitation as tools for studying the role of KCN in the CNS. An alternative to this obstacle is the use of KTxs from arthropods venom. In this section we report studies using these toxins to access the mechanisms of learning and memory in animals submitted to behavioral tests. Table 3 presents a summary of these works. Analyzing this table, we can see that the apamin is the most used KTx in tasks of learning and memory, as detailed below.
Table 3.
Assembled data on the effect of the bee and scorpion venom KTxs on the performance of animals in behavioral tests of learning and memory.
| Species | Toxin | KCN target | Behavioral test | Result | Ref. | |
|---|---|---|---|---|---|---|
| Scorpion | Androctonus mauretanicus mauretanicus | Kaliotoxin | Kv1.1 and Kv1.3 blocker | Olfactory discrimination task in rats | Improvement | [92] |
| Buthus tasmulus | Iberiotoxin (IbTx) | KCa1.1 blocker | Passive avoidance test in chicks | Impaired retention | [93] | |
| Leiurus quinquestriatus hebraeus | Charybdotoxin | Kv1.3 and KCa1.1 blocker | Passive avoidance test in mice | Improvement | [50] | |
| Lei-Dab7 | KCa2.2 blocker | Radial arm maze in rat | No effect | [25] | ||
|
| ||||||
| Bee | Apis mellifera | Apamin | KCa2.2 and KCa2.3 blocker | Bar-pressing response in appetitively motivated mice | Improvement | [94] |
| Object recognition task in rats | Improvement | [95] | ||||
| Habituation task in rats | Improvement | [96] | ||||
| Passive avoidance test in rats or mice | No effect | [96, 97] | ||||
| Passive avoidance test in mice | Improvement | [50, 98] | ||||
| Morris water maze in mice | Improvement | [97, 98] | ||||
| Morris water maze in rats | No effect | [98] | ||||
| Y-maze test in mice | No effect | [97] | ||||
| Radial arm maze test in mice or rats | Improvement | [25, 99] | ||||
| Visual discrimination in rats | No effect | [98] | ||||
| Olfactory discrimination task in rats | Improvement | [100] | ||||
| Olfactory associative task in rats | Improvement | [101] | ||||
| Tone fear-conditioning paradigm in mice | No effect | [53] | ||||
| T-maze test in rats | Improvement | [102] | ||||
| Passive avoidance task in chicks | Impaired retention | [103] | ||||
Ref.: reference.
4.1. Apamin
Apamin is a small peptide that corresponds to less than 2% of bee venom dry weight. Its amino acid sequence was described independently by two groups, revealing completely its primary structure [104]. Like many other peptide neurotoxins, apamin has high cysteine content and a high basicity, but apamin is different from most peptide toxins in its unusual ability to cross the blood brain barrier and act on the Central Nervous System [105]. This peptide is a polypeptide of 18 amino acids having a molecular weight of 2039 Da, with two disulfide bridges connecting position 1 with 11, and position 3 with 15 [106]. According to Vincent et al. [107], the most important part of the apamin sequence for neurotoxic activity appears to be the C- terminal region containing the two arginine residues, given that chemical modification of Argl3 and Arg14 eliminates toxicity (DL50 in mice). Assays with autoradiography of binding sites for apamin revealed that it binds preferentially to the hippocampus, to the habenular nucleus, and to the nucleus medialis septi [108].
Apamin is an antagonist of all three subtypes of small conductance Ca2+-activated K+-channel, KCa2 channels. However, this KTx showed subtype-specific affinity demonstrated by values of half maximal inhibition (IC50) and, dissociation constant (Kd) for KCa2.1 (SK1), KCa2.2 (SK2), and KCa2.3 (SK3) (IC50 = 704pM, 27pM, 4 nM and Kd 390pM, 4pM, 11pM, resp.) [34]. As a consequence of pharmacological blockage, apamin has been considered as a useful tool to investigate the physiological mechanisms involved in higher brain functions, especially cognitive processes or in the control of mood [98, 109].
This bee KTx facilitates spatial and nonspatial learning and improves memory performance in laboratory rodents, accelerating the acquisition of bar-pressing response in appetitively motivated mice [94]. In that report, when apamin was injected (dose 0.2 mg/kg, i.p.) 30 min before the acquisition session, it accelerated lever-press learning and the performance in a retention session 24 h later in BALB/mice. Results showed that immediate administration improved the retention of the lever-press task one day later [94]. Additionally, administration of the apamin in the acquisition of the lever-press task produced a stronger increase in early gene expression, c-fos and c-jus, in the CA1, CA3, and dentate gyrus as compared to trained saline-injected mice [110]. The similar pattern of immediate genes has been observed in the initial activation of neurons during the memory process [99].
Moreover, Deschaux et al. [95] showed that injection of apamin (0.4 mg/kg i.p.) before the training improved learning in an object recognition task in rats. Emphasizing, they found that rats injected with apamin before the first exploration session spent more time in exploring the new object than the familiar object at the second trial, when it took place 24 h after the first trial. Injection of apamin just after the first trial or before the second trial did not modify the difference in exploration time between the new and the familiar object. These results suggest that apamin could improve learning, but not consolidation or restitution of the information, in an object recognition task [95].
Also in 1997, Deschaux and Bizot [96] reported that, in the habituation task, apamin (0.4 mg/kg, i.p) decreased activity (distance travelled and rearing) on the restitution sessions only when it was injected before acquisition sessions, but not when injection took place just after the acquisition session or before the restitution session. In addition, the authors showed that in the passive avoidance test, apamin did not alter performance whenever the time of administration. According to Deschaux and Bizot [96], blockage of the apamin sensitive KCa channels improved the acquisition in nonstressful task, but not in a stressful situation in rats.
In the water maze spatial navigation, apamin (0.2 and 0.06 mg/kg, i.p) administered 30 min before daily training improved the acquisition and reversal learning of septal-lesioned mice, but it did not improve learning and memory in a spontaneous alternation task in a Y-maze and in a passive avoidance task, and did not affect learning and memory in any of three tasks when intact mice were used as subjects [97]. Interestingly, apamin dose dependently (0.2–0.06 mg/Kg, i.p) reversed the lesion-induced defect in the radial arm maze and in the water maze [99]. These results corroborated that blockage of KCa channels can alleviate the spatial reference memory and working memory defect induced by a damaged septohippocampal axis [99].
Van Der Staay et al. [98] make a comprehensive study using series of cognition tests with mice and rats from different strains. They used the standard version and a modified version of the Morris water escape task, the passive and active avoidance tasks, and the operant tasks in the Skinner box as cognitive tests, and the rat forced swimming test and the open-field test after cocaine administration as noncognitive tests. Results showed that apamin appeared to improve the cognitive performance of mice in two versions of the Morris water escape task and the passive avoidance task. However, inconsistence evidence was identified in rats. Moreover, apamin affected the general behavior of rats in the visual discrimination task, in the forced swimming test, and in the open field after cocaine administration [98].
In 2001, it was tested the effect of an i.c.v apamin (0.3 ng) injection on an olfactory associative task. Apamin did not modify the learning of the procedure side of the task or the learning of the odor-reward association. To specifically test reference memory, the rats were trained on a new odor-association problem using the same procedure (acquisition session), and they were tested for memory retention 24 h later. Apamin injected before or after the acquisition session improved retention of the valence of a new odor pair. Thus, the results indicate that the blockage of apamin-sensitive KCa2 channels facilitates reference memory [100].
In order to investigate the effect of potassium channel subtypes on rats learning and memory, Mpari et al. [101] compare the effects of two blockers, apamin (0.3 ng) and lei-Dab7 (3 ng), a modified scorpion KTx that selectively blocks KCa2.2 [111]. The results indicated that the blockage of KCa2.2 and KCa2.3 channels by apamin facilitates consolidation on new odor associations, using olfactory associative task in rats. However, lei-Dab7 remains without effect suggesting an involvement of KCa2.3 channels for the integration of synaptic signaling and plasticity modulation involved in learning and memory processes [101].
In the same way, in a spatial radial-arm maze task with rats, it was shown that lei-Dab7 did not modify attention or memory. However, apamin (specific to KCa2.2 and KCa2.3 channels) improved reference memory and accelerated strategy changes from egocentric to allocentric. These results reinforce that KCa2.3 blockage improves memory in rats [25].
Apamin is also able to facilitate the encoding of contextual fear memory during the limited 1 conditioned stimulus-unconditioned stimulus pairing protocol. It was shown that mice treated with apamin exhibited significantly greater freezing during the context test than did the saline-treated control [53].
More recently, the role of KCa in memory formation was explored in chicks trained on a single-trial discrimination avoidance task. Blockage of KCa2 channels using apamin (1 nM, 0.02 ng/hem, i.c.) impaired long-term memory retention when administered between 10 min before, and 30 min after training [103].
Blockage of KCa2 channels in the prefrontal cortex (PFC) by apamin also improves working memory performance [102] (Table 3). In prefrontal, visual, and somatosensory cortical pyramidal neurons, KCa2 channels mediate a hyperpolarization following calcium-induced calcium release, triggered by activation of muscarinic [112, 113] or glutamate receptors [114]. The PFC is central for working memory, the aptitude to internally symbolize information without an external input, being fundamental for establishing conceptual thinking, and for language, for example. As KCa2 channels play an important role in diminishing the excitability during synaptic transmission in the medial prefrontal cortex (mPFC), their blockage could potentiate synaptic transmission optimizing activity within the mPFC network [115, 116].
All these studies suggest that KCa2 channels are involved in memory processes but only when the task does not implicate a spatial strategy or a stressful situation. Since apamin is effective in some of these examples, but fails to have an effect in others, it appears that apamin-sensitive channels affect only certain circuitries involved in memory processing. Van Der Staay et al. [98] discuss the use of apamin as a tool to study the role of potassium channels in learning and memory. They do not consider apamin a good tool, despite its high selectivity, because the peptide has a very narrow therapeutic window, since there is an apparent overlap between the doses that enhance cognition with those causing side effects. Even so, studies using apamin are of great importance for the understanding of these channels and their influence on processes of learning and memory.
4.2. Charybdotoxin
Charybdotoxin (α-KTx 1.1, ChTX-Lq1, or ChTx-a), isolated from Leiurus quinquestriatus hebraeus (Yellow scorpion) [117], is a potent selective inhibitor of high (large or big) conductance Ca2+-activated potassium channels (KCa1.1, BK, or maxi-K), as well as a Kv1.3 channel [62]. In an autoradiographic study of rat brain it was demonstrated high levels of [125I]-charbydotoxin in white matter regions such as the lateral olfactory tract and fasciculus retroflexus, as well as in gray matter-containing regions such as the zona incerta, medial geniculate, and superior colliculus [118].
Using a [14C]-2-deoxyglucose autoradiographic technique, it was shown that i.c.v. administration of charybdotoxin produced effect on glucose utilization in 21 brain regions predominantly limited to the hippocampus, limbic and motor structures, indicating that glucose utilization was altered within three pathways implicated within learning and memory processes, the septohippocampal pathway, Schaffer collaterals within the hippocampus, and the Papez circuit. These results suggested the possibility that handling of particular subtypes of Kv1 channels by specific scorpion toxins in the hippocampus and related structures could alter cognitive processes without provoking large-scale changes in neural activity throughout the brain [119].
Ghelardini et al. [50] showed that the i.c.v. administration of charybdotoxin, 20 min before the training session in the mouse passive avoidance test, prevented the amnesia induced by potassium-channel openers (minoxidil and pinacidil). The amnesia in mice was produced by opening the KATP potassium channels, and it was reversed by blocking KATP potassium channels. Since the prevention of the amnesia induced by KATP potassium channel openers in the mouse passive-avoidance test is also obtained by blocking voltage-gated and calcium-activated channels, it is plausible to consider that more than one type of KCN appears to be involved in cognitive processes. It is worth mentioning that KCN blockers used by Ghelardini et al. [50] did not improve cognitive abilities when given alone, contrasting with the findings showing that apamin enhances memory in an object recognition task [95]. In fact, apamin, as it blocks KCa channels, affects learning rather than memory, an effect that is noticeable only in an object recognition task and that was observed after an interval of 24 h, when the control animals were not able to remember the exploration of the objects presented in the first session anymore [50].
4.3. Kaliotoxin
Kaliotoxin is a specific inhibitor of Kv1.1 and Kv1.3 isolated from the scorpion Androctonus mauretanicus mauretanicus [120]. The involvement of Kv1.1 and Kv1.3 in learning and memory processes was studied by using kaliotoxin in rats submitted to olfactory associative learning. Kaliotoxin (10 ng) improved learning but not information consolidation in the odor-reward training and increased the long-term retrieval of an odor-reward association tested by a reversal test 1 month after the odor-reward training. The reference memory was also tested by successive odor-pair training. When kaliotoxin was injected before the acquisition or retention session it improved performance. Nevertheless, when kaliotoxin was injected immediately after acquisition no effect was observed, suggesting that the blockage of Kv1.1 or Kv1.3 channels by kaliotoxin facilitates cognitive processes as learning, in particular in a reference representation [92].
4.4. Iberiotoxin (IbTx)
IbTx, a toxin isolated from venom of the scorpion Mesobuthus tamulus, is a selective inhibitor of KCa1.1 channels [121, 122]. These channels have been identified in brain regions that are related to cognition such as the hippocampus [123], and they have also been implicated in memory processing.
In rabbits, it has been shown a strong relationship between classical conditioning and membrane excitability in Purkinje cells which persisted for at least 1 month [124]. In slices of cerebellar lobule, the administration of IbTX mimics the increases in membrane excitability related to conditioning, suggesting that classical conditioning is dependent upon the inhibition of KCa1.1 channels [124].
In chicks submitted to passive avoidance task, Edwards and Rickard [93] showed a transient retention loss (40–70 min after training) associated with the central administration of 50 nM IbTX immediately after training.
4.5. Spider KTxs
Despite Kv2 channels possibly contributing negatively to the mechanisms of learning and memory and spider venom being a rich source of gating modifiers of Kv2 channels, no evaluation of the use of the spider KTxs as probes in cognitive processes was carried out so far. Only one study [125] tested the effect of a spider toxin in mice submitted to behavioral tests of memory, but this toxin was not a KTx.
5. Potassium Channels and Clinical Conditions Holding Cognitive Impairment
The studies presented above suggest that, at least in part, expression modification and conductance modulation of potassium channels are related to learning and memory processes. In some neurological dysfunctions, the relationship between KCN and cognitive aspects is also evident.
5.1. Limbic Encephalitis
Limbic encephalitis is characterized by disorientation, agitation, anxiety, depression, irritability, personality change, acute confusional state, hallucinations, complex partial and secondary generalized seizures, and, mainly, by impairment of short-term memory. Limbic encephalitis may have paraneoplastic or autoimmune origin (for reviews see [126, 127]).
Some cases of limbic encephalitis reinforce that KCNs are involved in learning and memory processes. Patients with this clinical condition and high plasma concentration (as reflected in the cerebrospinal fluid) of antibodies against voltage-dependent potassium channels (anti-Kv) had severe memory impairment. This cognitive function has a neuropsychological improvement after reducing serum levels of anti-Kv by plasma exchange, immunosuppressive treatment or spontaneous fall [126, 128–130].
5.2. Human-Immunodeficiency-Virus-Type-1 (HIV-1-) Associated Dementia (HAD)
HAD is a severe and debilitating form of HIV-1-associated neurocognitive disorders (HANDs). Over 40 million people worldwide are infected by HIV and 20–30% of them displayed symptoms of HAD. This disorder is characterized by cognitive deficits, motor disturbances, and behavioral abnormalities (for reviews see [131, 132]).
In individuals with HAD, the cognitive deficits could be result of Kv channels dysfunction [133, 134]. The brain cortex in individuals with HAD revealed overexpression of genes coding for KCNs that prolong afterhyperpolarization [135].
This participation of KCN in memory in the clinical condition caused by HIV-1 is supported by experimental results. Injection of HIV-1-infected human monocyte-derived macrophages into brain of immunodeficient mice, a model of human HIV-1-encephalitis (HIVE), impaired both long-term potentiation in hippocampus and spatial learning [136, 137]. Interestingly, these two damages are reversed when the HIVE mice received systemic administration of 4-AP, a KCN blocker aforecited [138].
5.3. Schizophrenia
Schizophrenia is a neuropsychiatric disorder that affects approximately 0.8% of the world population. It is characterized by the presence of several symptoms that can be grouped into two categories: positive and negative symptoms as a function of normal behavior. Positive symptoms (in addition to normal behavior) include hallucinations, delusions, and disorganized thoughts. Negative signs (absent in normal behavior) consist of anhedonia and social withdrawal [139, 140].
Schizophrenia is associated with cognitive deficits [141]. Weickert et al. [142] found that 51% of 117 patients with schizophrenia and decline in intelligence quotient (IQ) also exhibited deficits of executive function, memory, and attention. Deficits of executive function and memory also were found in 71% of 73 individuals with schizophrenia that showed intellectually compromised or deteriorated [143].
This memory deficit observed in patients with schizophrenia may be also consequence of dysfunction of potassium channels. Early life exposure of rodents to maternal separation or social isolation is an animal model for schizophrenia [144]. Quan et al. [145] observed deficits in learning and memory in postweaning isolation-reared rats similar to individuals with schizophrenia. When compared to the housed rats, the isolated ones performed worse in probe trials and memory retention tests in Morris water maze. Interestingly, these researchers found that the amplitudes of hippocampal voltage-dependent transient potassium A-type (I (A)) currents were enhanced, and the steady inactivation curve of I (A) currents was shifted towards positive potential by CSF of isolated rats. These K+ currents regulate action potential backpropagation and the induction of specific forms of synaptic plasticity, which is thought to underlie learning and memory [146, 147]. Therefore, Quan et al. [145] suggest that the mechanism by which early stressful experience leads to long-lasting consequences for spatial memory involves hippocampal potassium ion channel currents.
5.4. Alzheimer's Disease
Disorders that impair mental abilities are among the most feared result of aging. Among the kind of dementia, Alzheimer's disease (AD) represents the substantial majority of cases. AD is a progressive neurodegenerative disease characterized by loss of function and death of neurons in various areas of the brain, leading to loss of mental functions such as memory and learning. The clinical diagnosis of AD is usually done when the memory loss speeds up, and other behavioral and cognitive symptoms appear, mainly because the failure in the capacity to remember ordinary facts of everyday life is easily dismissed as normal aging. The episodic memory is related to the hippocampus and the interconnections circuits between this area and cortex goes through changes during aging that are highly susceptible to neurodegeneration in AD. Unfortunately, by the time of AD diagnosis, prominent neuronal loss has already occurred in the entorhinal cortex (EC), the brain's interface between the hippocampal formation and neocortex. It is worthy to mention that neurodegeneration in EC does not occur in healthy brains undergoing aging, and as structural and functional measures of the EC, dentate gyrus and CA3 region circuit display a progressive change on the course to AD. This circuit could represent a suitable target to therapies aimed to modify the disease progression [148].
In addition to the decreased number of acetylcholine (Ach) receptors in the basal forebrain cholinergic neurons (BCFN), Kv3.1 and Kv2.1 have been implicated to AD. The use of immunohistochemical techniques showed that both KCNs are expressed in the BCFN [149]. BFCN voltage-gated KCNs regulate Ach release and may participate in BFCN neurodegeneration in the course of AD.
The accumulation of amyloid beta plaques is an AD characteristic. It seems that these plaques form calcium-conducting ion channels that cause rapid neurodegeneration due to calcium overload [150]. Toxins acting as Ca2+ channel blockers, such as ω-agatoxin, attenuate the increases in Ca2+ concentration in isolated hippocampal nerve endings in the rat [149].
I (A) currents and the KCN behind these currents seem to have a role in AD. KCNs responsible for I (A) currents have been involved in the onset of long-term potentiation in mammalian neurons, which is thought to underlie learning and memory [146, 147]. A change in the steady-state properties of the I (A) current was showed in the amyloid-treated Drosophila cholinergic neurons which was sufficient to increase the threshold for the initiation of repetitive firing [151]. In this study, specific KTxs of the Kv4.2 channel (phrixotoxin-2) and Kv1 channel (α-dendrotoxin) were used to determine which channels triggered these currents in the Drosophila model. It had been shown that treatment of cholinergic neurons with amyloid peptide altered the kinetics of the current and caused a decrease in neuronal viability [152].
Brain microglia and their KCNs are also important to AD. These cells are activated to produce a “respiratory burst,” which produces reactive oxygen species (ROS) that cause the death of target cells in pathological states [153]. However, ROS generated by microglia contribute to the death of neurons in neurodegenerative conditions such as Alzheimer's and Parkinson's diseases, HIV and prion infection, and multiple sclerosis (reviewed by [154]). Kv1.3 and KCa2 and KCa4 are required for the respiratory burst and ROS formation in cultured microglia, and the inhibition of Kv1.3 by agitoxin-2 prevented neuronal killing by the microglia [155]. Moreover, charybdotoxin and α-dendrotoxin, all Kv1.3 blockers, reduced neuron killing by microglia as shown by transwell cell-culture system [156].
5.5. Multiple Sclerosis
Multiple sclerosis (abbreviated MS, known as disseminated sclerosis or encephalomyelitis disseminata) is an inflammatory disease. In MS, the fatty myelin sheaths around the axons of the brain and spinal cord are destroyed, leading to demyelination and scarring as well as a broad spectrum of signs and symptoms such as muscular weakness, loss of coordination and speech, visual disturbances, and cognitive disability. MS is a chronic degenerative disease and the most common neurological cause of disability in young adults in industrialized societies [157]. The major disabling aspect of MS is the cognitive impairment, which is characterized primarily by memory loss, attention deficits, slowed information processing, and failure in executive function [158].
Some MS symptoms may be closely dependent upon changes in KCN [159]. Kv1.1 and Kv1.2 are specifically found in paranodal regions of axons [160]. KCN blockers such as aminopyridines (APs) when applied in vitro to demyelinated axons are able to restore the conduction and also to potentiate synaptic transmission [161–164]. Because of that, much attention has been given to Kv blockers as a prospective agent for MS and other neuropathies treatments [165, 166].
Moreover, MS is a chronic inflammatory autoimmune disease and the central role of T lymphocytes, and their Kv1.3 and KCa1.1 channels, in its pathogenesis has been largely evidenced [167–169]. Kaliotoxin, which blocks the lymphocyte Kv1.3 and the neuronal Kv1.1 channels, ameliorates the symptoms of adoptive experimental autoimmune encephalomyelitis (EAE) in rats [170], a widely used model for the human MS disease [167]. It is worthy to mention that 4-AP has been used to manage some of the symptoms of MS [171, 172]. 4-AP was approved by the US Food and Drug Administration (FDA) on January 22, 2010 for the treatment of MS [173].
5.6. Parkinson's Disease
Parkinson's disease (PD) is a slowly progressive degenerative disorder of the CNS and is characterized by slowness or deficiency of movement (bradykinesia), rigidity, postural instability, and tremor primarily while at rest. The motor symptoms of PD result from the death of dopamine-generating cells in the substantia nigra, a region of the midbrain. In the early stage of the disease, the most obvious symptoms are those that are movement-related, such as shaking, rigidity, slowness of movement, and difficulty with walking and gait. With time, cognitive and behavioral problems may arise, commonly followed by dementia in the advanced phase of the disease. PD is more common in the elderly, with most cases occurring after the age of 50 (for review see [174, 175]).
Potassium channels have been implicated in the pathogenesis of PD. KATP channels comprised of Kir6.2 and Sur1 subunit are abundantly expressed in dopaminergic neurons of substantia nigra [176]. KATP directly couple the cellular metabolic state to membrane excitability. It has been shown that activity of the mitochondrial respiratory chain complex I (CXI, also known as NADH: ubiquinone reductase) is reduced in PD patients, strongly suggesting that metabolic stress is an important trigger factor for the PD neurodegeneration. Dopaminergic midbrain neurons express different types of KATP channels mediating their differential response to the inhibition of mitochondrial complex I [177]. The use of CXI inhibitor leads to the activation of SUR1/Kir6.2-containing KATP channels, which results in a membrane hyperpolarization of the dopaminergic neuron that is associated with a complete loss of spontaneous activity [178]. Studies of dopaminergic midbrain neurons in the weaver mouse, a genetic mouse model of dopaminergic degeneration similar to that in PD [179], sustain the proposal of KATP channel activation as a neuroprotective strategy. Up to now, no KATP channel activators have been described from arthropod venom.
5.7. Epilepsy
The term epilepsy refers to a group of neurological disorders, clinically diverse and with multiple etiologies, characterized by paroxysmal brain discharges referred to as spontaneous recurrent seizures [180, 181]. Epilepsy is the second most common neurological disorder (after stroke), affecting 1-2% of the world's population [182]. Failure to treat epilepsy causes neurobiological, psychological, and social consequences for the patient [183], particularly cognitive impairment [184, 185]. Age of onset of epilepsy, type and duration of seizures, as well as the antiepileptic drug, therapy are strongly related to cognitive dysfunction present in the syndrome [186–188].
Clearly, ion channels are critical for regulating excitability and, contribute significantly to epilepsy pathophysiology. In a recent article, N'Gouemo [189] related that the loss-of-function large conductance KCa channels mutations contribute to neuronal hyperexcitability that can lead to temporal lobe epilepsy, tonic-clonic seizures and alcohol withdrawal seizures, and blockage of these channels can trigger seizures and status epilepticus.
Despite its availability, conventional and “new generation” antiepileptic drugs (AEDs) are commonly associated with side effects, which can vary in frequency and severity [190]. In addition, AEDs are ineffective in controlling seizures in one-third of patients (drug-resistant), reaching 70% in patients with temporal lobe epilepsy, which is the more frequent epilepsy in adults and it is characterized by degeneration of limbic structures (sclerotic hippocampus), directly involved in different memory processes and in their modulation [191–194]. It is noteworthy that cognitive deficits represent a serious neuropsychological problem in people suffering from temporal lobe epilepsy.
Several studies suggest that KCN may be an important new class of targets for anticonvulsant therapies [195, 196], especially for refractory epilepsy. New drugs that act on potassium channels have been provided and approved by the FDA and the European Union, for example, ezogabine (retigabine). In vitro studies indicate that ezogabine acts primarily by opening neuronal Kv7.2–7.5 channels [197].
6. Arthropod Toxins: Therapeutic and Study Tools
Given that the dysfunction of K+ channels has significant contribution in cognitive deficits of several neurological clinical conditions, the drugs that modulate their activity became important allies in the study and treatment of these pathologies. Due to the wide presence and diversity of potassium channels in the brain, these therapeutic agents need to be specific and potent. In this way, the toxins from arthropods turn out to be excellent candidates.
6.1. Potential of KTxs as Pharmacological Tools
KTxs that act on KCN responsible for I (A) currents are of particular interest. These channels regulate firing frequency, spike initiation and waveform in excitable cells and may contribute to specialized functions, such as learning, memory, and behavior [146, 147]. According to the cell type and to the molecular heterogeneity, I (A) currents exhibit a wide diversity of physiological properties. KCNs of subtype Kv4 participate in the generation of I (A) currents in the cerebellum granular cells [198], neostriatal cholinergic interneurons [199], and hippocampal interneurons [147]. Kv2 channels seem to be equally important for the existence of I (A) currents in neocortical pyramidal neurons [200, 201]. Therefore KTxs that influence the I (A) currents, or more specifically in Kv2 and/or Kv4 channels activity, become potential pharmacological tools for studying the participation of KCN in learning memory and its dysfunctions.
Many scorpion KTxs influence the I (A) currents. Noxiustoxin (NTX), from the venom of the Mexican scorpion Centruroides noxius Hoffmann [202], and discrepin, toxin from the Venezuelan scorpion Tityus discrepans [203], belong to α-KTx15 subfamily. Toxins of this subfamily are reported to affect the I (A) currents [198]. Interesting, discrepin blocks irreversible K+-channels of rat cerebellum neurons [203]. BmTX3 (systematic name α-KTx 15.2) is toxin isolated from venom of Manchurian scorpion Mesobuthus martensi is Karsch. This peptide blocked (Kd = 54 nM) completely the I (A) current of striatum neurons in culture, whereas the sustained K+ current was unaffected. The labeled synthetic toxin (125I-sBmTX3) was found in the striatum, hippocampus, superior colliculus, and cerebellum in the adult rat brain [204]. BmTx3B (Martentoxin, systematic name alpha-KTx 16.2) is another toxin isolated from Mesobuthus martensi Karsch. It was tested on two types of voltage-dependent potassium currents recorded from dissociated hippocampal neurons of neonatal rat in whole-cell voltage-clamp mode. BmTx3B selectively inhibited the delayed rectifier potassium current (I (K)), without affecting the I (A) current [205]. Therefore these scorpions KTxs are candidates to become tools for the study of KCN in physiological and pathological mechanisms of learning and memory.
As mentioned above, the spider venom is a source of KTxs that act on Kv2 and Kv4 channels. The first spider toxins described as KCN blockers were the hanatoxins 1 and 2 (HaTx 1 and 2) of the theraphosidae spider Grammostola spatulata [206]. Despite the marked differences in their primary sequence, both blocked the Kv2.1 channel with Kd of 42 nM. HaTxs block Kv1 and Kv3 channels, while the shal-related channel (type Kv4) is sensitive to the toxin. The heteropodatoxins (HpTx 1–3), isolated from the venom of Heteropoda venatoria (Sparassidae), blocked the conductance of Kv4.2 but not of Kv1.4, in a voltage-dependent manner. Other toxins that act on KCN were isolated from the venom of the Theraphosidae Phrixotrichus auratus. The phrixotoxins (Patx 1 and 2) specifically block Kv4.3 and Kv4.2 currents at nanomolar concentrations altering the gating properties of these channels by interacting with the voltage sensor. The subfamilies of shaker (Kv1), shab (Kv2), and shaw (Kv3) were not inhibited by PaTxs [207]. The stromatoxin1, ScTx1, isolated from tarantula Stromatopelma calceata, was the first high-affinity inhibitor described for the Kv2.2 channel. ScTx1 also inhibited Kv2.1 channels, Kv4.2 and Kv2.1/Kv9.3 heterodimer [208]. HmTx 1 and 2, peptides purified from the tarantula spider Heteroscodra maculata, inhibited potassium currents associated with subtypes Kv2 [206]. Guangitoxin-1E (GxTX-1E) is a potent gating modifier peptide of Kv2 channels isolated from the venom of the tarantula Plesiophrictus guangxiensis [209]. Other spider toxins targeting the K+-channel voltage sensor include heteroscodratoxins (HmTx1,2), which target Kv2 and Kv4 channels [206]; TLTx1–3, which preferentially inhibit Kv4 channels [210]; PhTx3-1, which inhibits the outward rectifier A-type K+-channel [211]. SGTx1 (Kappa-theraphotoxin-Scg1a) is a peptide toxin isolated from the venom of the aggressive African Theraphosidae Scodra griseipes that has been shown to inhibit outward K+ currents in rat cerebellar granule neurons [64]. Functionally, SGTx1 reversibly inhibits potassium currents in oocytes expressing Kv2.1 channels and acts by shifting the activation of the channel to more depolarized voltages [212]. Finally, phrixotoxin-1, a peptide purified from the venom of the tarantula Phrixotrichus auratus, is a specific and potent blocker of Kv4.3 [207]. So there are many spider KTxs that are potential tools for the study of KCN in learning and memory and its dysfunction.
Toxins that act on large-conductance Ca2+-activated K+-channels (KCa1.1 or BK) and small-conductance Ca2+-activated K+ channel (KCa2.1 or SK1, KCa2.2 or SK2, and KCa2.3 or SK3) are important too. Neuronal firing is also regulated by KCa1.1 and KCa2 which constitute an exclusive family of ion channels which combine intracellular chemical changes and electric signaling [213, 214]. KCa1.1 are homologous to Kv channel α-subunits, but possess additional hydrophobic segments forming an extracellular N-terminal and a long intracellular C-terminal that holds one of the Ca2+-binding sites [215]. It has been reported that KCa channels are involved in regulation of neocortex pyramidal cell excitability [216, 217]. Therefore KTxs that influence the KCa channels become also potential pharmacological tools.
KCa channels are blocked by many scorpion toxins. Martentoxin, purified from Mesobuthus martensi Karsch venom, is able to block KCa1.1 currents in rat hippocampal neurons [218]. This KCN is also inhibited by slotoxin from Centruroides noxius [219] and noxiustoxin from Centruroides noxius [220]. Slotoxin is described not only as a potent and selective blocker, but it also can differentially inhibit KCa1.1 channels, depending on the presence of β-subunits [219] and on the α-splice variant [221]. KCa2 channels are also blocked by different scorpion toxins. For example, there are scyllatoxin (leiurotoxin I) isolated from Leiurus quinquestriatus hebraeus [222] and tamapin from Mesobuthus tumulus [223]. This last toxin blocks KCa2 channels in pyramidal neurons of the hippocampus as well as in cell lines expressing distinct KCa2 channel subunits, displaying a remarkable selectivity for KCa2.2 (IC50 = 24pM) versus KCa2.1 (≅1750-fold) and KCa2.3 (≅70-fold) channels [223]. These data reinforce the potential of scorpion KTxs as pharmacological tools.
As has been evident, multiple KCNs participate in the regulation of cellular events behind the phenomena of learning and memory and their dysfunctions. Therefore, it is necessary a varied arsenal of pharmacological tools to investigate the role of each potassium channel in these cognitive processes. It also became clear that scorpions and spiders KTxs can provide this diversity of tools.
7. Conclusion
The KCNs are components of the mechanisms responsible for learning and memory. Its diversity and wide distribution in the brain make the action of KCN during the formation of learning and memory retrieval to be complex and variable. To investigate the role of each subtype of KCN in these phenomena, we need also a wide variety of pharmacological tools, specific and potent for KCN. The toxins from the venoms of arthropods can be such tools. These precise potassium channel toxins may not only have an important contribution to uncover the processes underlying learning and memory, but can also become therapeutic agents for many diseases and disorders of the CNS.
Acknowledgments
This paper is financially supported by FAPDF (193.000.418/2008), CNPq (303003/2009-0 to EFS, 479873/2008-9 to MRM). C. D. C. Gati receives scholarship from CNPq.
References
- 1.Nadel L, Hardt O. Update on memory systems and processes. Neuropsychopharmacology. 2011;36(1):251–273. doi: 10.1038/npp.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgado-Bernal I. Learning and memory consolidation: linking molecular and behavioral data. Neuroscience. 2011;176:12–19. doi: 10.1016/j.neuroscience.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Hoffman DA. Potassium channels: newly found players in synaptic plasticity. Neuroscientist. 2008;14(3):276–286. doi: 10.1177/1073858408315041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coetzee WA, Amarillo Y, Chiu J, et al. Molecular diversity of K+ channels. Annals of the New York Academy of Sciences. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 5.Judge SIV, Smith PJ, Stewart PE, Bever CT. Potassium channel blockers and openers as CNS neurologic therapeutic agents. Recent Patents on CNS Drug Discovery. 2007;2(3):200–228. doi: 10.2174/157488907782411765. [DOI] [PubMed] [Google Scholar]
- 6.Gutman GA, Chandy KG, Grissmer S, et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacological Reviews. 2005;57(4):473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 7.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacological Reviews. 2005;57(4):463–472. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 8.Kubo Y, Adelman JP, Clapham DE, et al. International union of pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacological Reviews. 2005;57(4):509–526. doi: 10.1124/pr.57.4.11. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein SAN, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International union of pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacological Reviews. 2005;57(4):527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- 10.Miller C. An overview of the potassium channel family. Genome biology. 2000;1(4) doi: 10.1186/gb-2000-1-4-reviews0004. Article ID REVIEWS0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2002;419(6902):35–42. doi: 10.1038/nature00978. [DOI] [PubMed] [Google Scholar]
- 12.Magleby KL. Gating mechanism of BK (Slo1) channels: so near, yet so far. Journal of General Physiology. 2003;121(2):81–96. doi: 10.1085/jgp.20028721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacological Reviews. 2005;57(4):387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- 14.Long SB, Campbell EB, MacKinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309(5736):897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 15.Moreland JL, Gramada A, Buzko OV, Zhang Q, Bourne PE. The Molecular Biology Toolkit (MBT): a modular platform fro developing molecular visualization applications. BMC Bioinformatics. 2005;6, article no. 21 doi: 10.1186/1471-2105-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu C, Barry J. Function and mechanism of axonal targeting of voltage-sensitive potassium channels. Progress in Neurobiology. 2011;94(2):115–132. doi: 10.1016/j.pneurobio.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiser M, Vega-Saenz De Miera E, Kentros C, et al. Differential expression of Shaw-related K+ channels in the rat central nervous system. Journal of Neuroscience. 1994;14(3):949–972. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serôdio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. Journal of Neurophysiology. 1998;79(2):1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- 19.Saganich MJ, Machado E, Rudy B. Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. Journal of Neuroscience. 2001;21(13):4609–4624. doi: 10.1523/JNEUROSCI.21-13-04609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luján R, De La Vega CDC, Del Toro ED, Ballesta JJ, Criado M, Juiz JM. Immunohistochemical localization of the voltage-gated potassium channel subunit Kv1.4 in the central nervous system of the adult rat. Journal of Chemical Neuroanatomy. 2003;26(3):209–224. doi: 10.1016/j.jchemneu.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Knaus HG, Schwarzer C, Koch ROA, et al. Distribution of high-conductance Ca2+-activated K+ channels in rat brain: targeting to axons and nerve terminals. Journal of Neuroscience. 1996;16(3):955–963. doi: 10.1523/JNEUROSCI.16-03-00955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sausbier U, Sausbier M, Sailer CA, et al. Ca2+-activated K+ channels of the BK-type in the mouse brain. Histochemistry and Cell Biology. 2006;125(6):725–741. doi: 10.1007/s00418-005-0124-7. [DOI] [PubMed] [Google Scholar]
- 23.Stocker M, Pedarzani P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Molecular and Cellular Neurosciences. 2000;15(5):476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- 24.Sailer CA, Hu H, Kaufmann WA, et al. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. Journal of Neuroscience. 2002;22(22):9698–9707. doi: 10.1523/JNEUROSCI.22-22-09698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mpari B, Sreng L, Regaya I, Mourre C. Small-conductance Ca2+-activated K+ channels: Heterogeneous affinity in rat brain structures and cognitive modulation by specific blockers. European Journal of Pharmacology. 2008;589(1–3):140–148. doi: 10.1016/j.ejphar.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Horio Y, Morishige KI, Takahashi N, Kurachi Y. Differential distribution of classical inwardly rectifying potassium channel mRNAs in the brain: comparison of IRK2 with IRK1 and IRK3. FEBS Letters. 1996;379(3):239–243. doi: 10.1016/0014-5793(95)01519-1. [DOI] [PubMed] [Google Scholar]
- 27.Karschin C, Dißmann E, Stühmer W, Karschin A. IRK(1-3) and GIRK(1-4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. Journal of Neuroscience. 1996;16(11):3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyashita T, Kubo Y. Localization and developmental changes of the expression of two inward rectifying K+-channel proteins in the rat brain. Brain Research. 1997;750(1-2):251–263. doi: 10.1016/s0006-8993(96)01365-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, Tanaka O, Suzuki M, et al. Localization of pore-forming subunit of the ATP-sensitive K+-channel, Kir6.2, in rat brain neurons and glial cells. Molecular Brain Research. 2002;101(1-2):23–32. doi: 10.1016/s0169-328x(02)00137-7. [DOI] [PubMed] [Google Scholar]
- 30.Hervieu GJ, Cluderay JE, Gray CW, et al. Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience. 2001;103(4):899–919. doi: 10.1016/s0306-4522(01)00030-6. [DOI] [PubMed] [Google Scholar]
- 31.Talley EM, Solórzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. Journal of Neuroscience. 2001;21(19):7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathie A, Wooltorton JRA, Watkins CS. Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. General Pharmacology. 1998;30(1):13–24. doi: 10.1016/s0306-3623(97)00034-7. [DOI] [PubMed] [Google Scholar]
- 33.Lipkind GM, Fozzard HA. A model of scorpion toxin binding to voltage-gated K+ channels. Journal of Membrane Biology. 1997;158(3):187–196. doi: 10.1007/s002329900256. [DOI] [PubMed] [Google Scholar]
- 34.Grunnet M, Jensen BS, Olesen SP, Klaerke DA. Apamin interacts with all subtypes of cloned small-conductance Ca2+-activated K+ channels. Pflugers Archiv European Journal of Physiology. 2001;441(4):544–550. doi: 10.1007/s004240000447. [DOI] [PubMed] [Google Scholar]
- 35.Harvey AL, Bradley KN, Cochran SA, et al. What can toxins tell us for drug discovery? Toxicon. 1998;36(11):1635–1640. doi: 10.1016/s0041-0101(98)00156-1. [DOI] [PubMed] [Google Scholar]
- 36.Kandel ER, Kupfermann I, Iversen S. Learning and memory. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. New York, NY, USA: McGraw-Hill; 2000. [Google Scholar]
- 37.Monaghan MM, Trimmer JS, Rhodes KJ. Experimental localization of Kv1 family voltage-gated K+ channel α and β subunits in rat hippocampal formation. Journal of Neuroscience. 2001;21(16):5973–5983. doi: 10.1523/JNEUROSCI.21-16-05973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Kunkel DO, Schwartzkroin PA, Tempel BL. Localization of Kv1.1 and Kv1.2, two K+ channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. Journal of Neuroscience. 1994;14(8):4588–4599. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park KH, Chung YH, Shin CM, et al. Immunohistochemical study on the distribution of the voltage-gated potassium channels in the gerbil hippocampus. Neuroscience Letters. 2001;298(1):29–32. doi: 10.1016/s0304-3940(00)01710-9. [DOI] [PubMed] [Google Scholar]
- 40.Zhong CB, Pan YP, Tong XY, Xu XH, Wang XL. Delayed rectifier potassium currents and Kv2.1 mRNA increase in hippocampal neurons of scopolamine-induced memory-deficient rats. Neuroscience Letters. 2005;373(2):99–104. doi: 10.1016/j.neulet.2004.09.069. [DOI] [PubMed] [Google Scholar]
- 41.Varga AW, Anderson AE, Adams JP, Vogel H, Sweatt JD. Input-specific immunolocalization of differentially phosphorylated Kv4.2 in the mouse brain. Learning and Memory. 2000;7(5):321–332. doi: 10.1101/lm.35300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsaur ML, Chou CC, Shih YH, Wang HL. Cloning, expression and CNS distribution of Kv4.3, an A-type K+ channel α subunit. FEBS Letters. 1997;400(2):215–220. doi: 10.1016/s0014-5793(96)01388-9. [DOI] [PubMed] [Google Scholar]
- 43.Martin S, Lino de Oliveira C, Mello de Queiroz F, Pardo LA, Stühmer W, Del Bel E. Eag1 potassium channel immunohistochemistry in the CNS of adult rat and selected regions of human brain. Neuroscience. 2008;155(3):833–844. doi: 10.1016/j.neuroscience.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Sailer CA, Kaufmann WA, Kogler M, et al. Immunolocalization of BK channels in hippocampal pyramidal neurons. European Journal of Neuroscience. 2006;24(2):442–454. doi: 10.1111/j.1460-9568.2006.04936.x. [DOI] [PubMed] [Google Scholar]
- 45.Murer G, Adelbrecht C, Lauritzen I, et al. An immunocytochemical study on the distribution of two G-protein-gated inward rectifier potassium channels (Girk2 and Girk4) in the adult rat brain. Neuroscience. 1997;80(2):345–357. doi: 10.1016/s0306-4522(97)00001-8. [DOI] [PubMed] [Google Scholar]
- 46.Wickman K, Karschin C, Karschin A, Picciotto MR, Clapham DE. Brain localization and behavioral impact of the G-protein-gated K+ channel subunit GIRK4. Journal of Neuroscience. 2000;20(15):5608–5615. doi: 10.1523/JNEUROSCI.20-15-05608.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iizuka M, Tsunenari I, Momota Y, Akiba I, Kono T. Localization of a G-protein-coupled inwardly rectifying K+ channel, CIR, in the rat brain. Neuroscience. 1997;77(1):1–13. doi: 10.1016/s0306-4522(96)00460-5. [DOI] [PubMed] [Google Scholar]
- 48.Betourne A, Bertholet AM, Labroue E, et al. Involvement of hippocampal CA3 KATP channels in contextual memory. Neuropharmacology. 2009;56(3):615–625. doi: 10.1016/j.neuropharm.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Karschin C, Ecke C, Ashcroft FM, Karschin A. Overlapping distribution of KATP channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Letters. 1997;401(1):59–64. doi: 10.1016/s0014-5793(96)01438-x. [DOI] [PubMed] [Google Scholar]
- 50.Ghelardini C, Galeotti N, Bartolini A. Influence of potassium channel modulators on cognitive processes in mice. British Journal of Pharmacology. 1998;123(6):1079–1084. doi: 10.1038/sj.bjp.0701709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meiri N, Ghelardini C, Tesco G, et al. Reversible antisense inhibition of Shaker-like Kv1.1 potassium channel expression impairs associative memory in mouse and rat. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4430–4434. doi: 10.1073/pnas.94.9.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyake A, Takahashi S, Nakamura Y, et al. Disruption of the ether-à-go-go K+ channel gene BEC1/KCNH3 enhances cognitive function. Journal of Neuroscience. 2009;29(46):14637–14645. doi: 10.1523/JNEUROSCI.0901-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vick KA, Guidi M, Stackman RW. In vivo pharmacological manipulation of small conductance Ca2+-activated K+ channels influences motor behavior, object memory and fear conditioning. Neuropharmacology. 2010;58(3):650–659. doi: 10.1016/j.neuropharm.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matthews EA, Disterhoft JF. Blocking the BK channel impedes acquisition of trace eyeblink conditioning. Learning and Memory. 2009;16(2):106–109. doi: 10.1101/lm.1289809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hammond RS, Bond CT, Strassmaier T, et al. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. Journal of Neuroscience. 2006;26(6):1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stackman RW, Jr., Bond CT, Adelman JP. Contextual memory deficits observed in mice overexpressing small conductance Ca2+-activated K+ type 2 (KCa2.2, SK2) channels are caused by an encoding deficit. Learning and Memory. 2008;15(4):208–213. doi: 10.1101/lm.906808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobsen JPR, Redrobe JP, Hansen HH, et al. Selective cognitive deficits and reduced hippocampal brain-derived neurotrophic factor mRNA expression in small-conductance calcium-activated K+ channel deficient mice. Neuroscience. 2009;163(1):73–81. doi: 10.1016/j.neuroscience.2009.05.062. [DOI] [PubMed] [Google Scholar]
- 58.Deng PY, Xiao Z, Yang C, et al. GABAB receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK-2 K+ channels. Neuron. 2009;63(2):230–243. doi: 10.1016/j.neuron.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kourrich S, Manrique C, Salin P, Mourre C. Transient hippocampal down-regulation of Kv1.1 subunit mRNA during associative learning in rats. Learning and Memory. 2005;12(5):511–519. doi: 10.1101/lm.86305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Engeland B, Neu A, Ludwig J, Roeper J, Pongs O. Cloning and functional expression of rat ether-a-go-go-like K+ channel genes. Journal of Physiology. 1998;513(3):647–654. doi: 10.1111/j.1469-7793.1998.647ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyake A, Mochizuki S, Yokoi H, Kohda M, Furuichi K. New ether-a-go-go K+ channel family members localized in human telencephalon. Journal of Biological Chemistry. 1999;274(35):25018–25025. doi: 10.1074/jbc.274.35.25018. [DOI] [PubMed] [Google Scholar]
- 62.Gimenez-Gallego G, Navia MA, Reuben JP, Katz GM, Kaczorowski GJ, Garcia ML. Purification, sequence, and model structure of charybdotoxin, a potent selective inhibitor of calcium-activated potassium channels. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(10):3329–3333. doi: 10.1073/pnas.85.10.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin W, Lu Z. A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry. 1998;37(38):13291–13299. doi: 10.1021/bi981178p. [DOI] [PubMed] [Google Scholar]
- 64.Marvin L, De E, Cosette P, Gagnon J, Molle G, Lange C. Isolation, amino acid sequence and functional assays of SGTx1. The first toxin purified from the venom of the spider Scodra griseipes. European Journal of Biochemistry. 1999;265(2):572–579. doi: 10.1046/j.1432-1327.1999.00726.x. [DOI] [PubMed] [Google Scholar]
- 65.Nolting A, Ferraro T, D’Hoedt D, Stocker M. An amino acid outside the pore region influences apamin sensitivity in small conductance Ca2+-activated K+ channels. Journal of Biological Chemistry. 2007;282(6):3478–3486. doi: 10.1074/jbc.M607213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lamy C, Goodchild SJ, Weatherall KL, et al. Allosteric block of KCa2 channels by apamin. Journal of Biological Chemistry. 2010;285(35):27067–27077. doi: 10.1074/jbc.M110.110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jouirou B, Mouhat S, Andreotti N, De Waard M, Sabatier JM. Toxin determinants required for interaction with voltage-gated K+ channels. Toxicon. 2004;43(8):909–914. doi: 10.1016/j.toxicon.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 68.Mouhat S, Jouirou B, Mosbah A, De Waard M, Sabatier JM. Diversity of folds in animal toxins acting on ion channels. Biochemical Journal. 2004;378(3):717–726. doi: 10.1042/BJ20031860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mouhat S, Andreotti N, Jouriou B, Sabatier JM. Animal toxins acting on voltage-gated potassium channels. Current Pharmaceutical Design. 2008;14(24):2503–2518. doi: 10.2174/138161208785777441. [DOI] [PubMed] [Google Scholar]
- 70.Dauplais M, Lecoq A, Song J, et al. On the convergent evolution of animal toxins. Conservation of a diad of functional residues in potassium channel-blocking toxins with unrelated structures. Journal of Biological Chemistry. 1997;272(7):4302–4309. doi: 10.1074/jbc.272.7.4302. [DOI] [PubMed] [Google Scholar]
- 71.Gurrola GB, Rosati B, Rocchetti M, et al. A toxin to nervous, cardiac, and endocrine ERG K+ channels isolated from Centruroides noxius scorpion venom. FASEB Journal. 1999;13(8):953–962. [PubMed] [Google Scholar]
- 72.Korolkova YV, Kozlov SA, Lipkin AV, et al. An ERG Channel Inhibitor from the Scorpion Buthus eupeus. Journal of Biological Chemistry. 2001;276(13):9868–9876. doi: 10.1074/jbc.M005973200. [DOI] [PubMed] [Google Scholar]
- 73.Escoubas P, Bernard C, Lambeau G, Lazdunski M, Darbon H. Recombinant production and solution structure of PcTx1, the specific peptide inhibitor of ASIC1a proton-gated cation channels. Protein Science. 2003;12(7):1332–1343. doi: 10.1110/ps.0307003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu Z, MacKinnon R. Purification, characterization, and synthesis of an inward-rectifier K+ channel inhibitor from scorpion venom. Biochemistry. 1997;36(23):6936–6940. doi: 10.1021/bi9702849. [DOI] [PubMed] [Google Scholar]
- 75.Tytgat J, Chandy KG, Garcia ML, et al. A unified nomenclature for short-chain peptides isolated from scorpion venoms: α-KTx molecular subfamilies. Trends in Pharmacological Sciences. 1999;20(11):444–447. doi: 10.1016/s0165-6147(99)01398-x. [DOI] [PubMed] [Google Scholar]
- 76.Srinivasan KN, Sivaraja V, Huys I, et al. kappa-Hefutoxin1, a novel toxin from the scorpion Heterometrus fulvipes with unique structure and function. Importance of the functional diad in potassium channel selectivity. Journal of Biological Chemistry. 2002;277(33):30040–30047. doi: 10.1074/jbc.M111258200. [DOI] [PubMed] [Google Scholar]
- 77.Chagot B, Pimentel C, Dai L, et al. An unusual fold for potassium channel blockers: NMR structure of three toxins from the scorpion Opisthacanthus madagascariensis . Biochemical Journal. 2005;388(1):263–271. doi: 10.1042/BJ20041705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Camargos TS, Restano-Cassulini R, Possani LD, et al. The new kappa-KTx 2.5 from the scorpion Opisthacanthus cayaporum . Peptides. 2011;32(7):1509–1517. doi: 10.1016/j.peptides.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 79.Rodríguez De La Vega RC, Possani LD. Current views on scorpion toxins specific for K+-channels. Toxicon. 2004;43(8):865–875. doi: 10.1016/j.toxicon.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 80.Estrada G, Villegas E, Corzo G. Spider venoms: a rich source of acylpolyamines and peptides as new leads for CNS drugs. Natural Product Reports. 2007;24(1):145–161. doi: 10.1039/b603083c. [DOI] [PubMed] [Google Scholar]
- 81.Swartz KJ. Tarantula toxins interacting with voltage sensors in potassium channels. Toxicon. 2007;49(2):213–230. doi: 10.1016/j.toxicon.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swartz KJ, MacKinnon R. Hanatoxin modifies the gating of a voltage-dependent K+ channel through multiple binding sites. Neuron. 1997;18(4):665–673. doi: 10.1016/s0896-6273(00)80306-2. [DOI] [PubMed] [Google Scholar]
- 83.Lee HC, Wang JM, Swartz KJ. Interaction between extracellular hanatoxin and the resting conformation of the voltage-sensor paddle in Kv channels. Neuron. 2003;40(3):527–536. doi: 10.1016/s0896-6273(03)00636-6. [DOI] [PubMed] [Google Scholar]
- 84.Lee SY, MacKinnon R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature. 2004;430(6996):232–235. doi: 10.1038/nature02632. [DOI] [PubMed] [Google Scholar]
- 85.Phillips LR, Milescu M, Li-Smerin Y, Mindell JA, Kim JI, Swartz KJ. Voltage-sensor activation with a tarantula toxin as cargo. Nature. 2005;436(7052):857–860. doi: 10.1038/nature03873. [DOI] [PubMed] [Google Scholar]
- 86.Milescu M, Vobecky J, Roh SH, et al. Tarantula toxins interact with voltage sensors within lipid membranes. Journal of General Physiology. 2007;130(5):497–511. doi: 10.1085/jgp.200709869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raghuraman H, Chattopadhyay A. Melittin: a membrane-active peptide with diverse functions. Bioscience Reports. 2007;27(4-5):189–223. doi: 10.1007/s10540-006-9030-z. [DOI] [PubMed] [Google Scholar]
- 88.Habermann E. Apamin. Pharmacology and Therapeutics. 1984;25(2):255–270. doi: 10.1016/0163-7258(84)90046-9. [DOI] [PubMed] [Google Scholar]
- 89.Xu X, Nelson JW. Solution structure of tertiapin determined using nuclear magnetic resonance and distance geometry. Proteins: Structure, Function and Genetics. 1993;17(2):124–137. doi: 10.1002/prot.340170203. [DOI] [PubMed] [Google Scholar]
- 90.Dotimas EM, Hamid KR, Hider RC, Ragnarsson U. Isolation and structure analysis of bee venom mast cell degranulating peptide. Biochimica et Biophysica Acta. 1987;911(3):285–293. doi: 10.1016/0167-4838(87)90069-0. [DOI] [PubMed] [Google Scholar]
- 91.Weatherall KL, Goodchild SJ, Jane DE, Marrion NV. Small conductance calcium-activated potassium channels: from structure to function. Progress in Neurobiology. 2010;91(3):242–255. doi: 10.1016/j.pneurobio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 92.Kourrich S, Mourre C, Soumireu-Mourat B. Kaliotoxin, a Kv1.1 and Kv1.3 channel blocker, improves associative learning in rats. Behavioural Brain Research. 2001;120(1):35–46. doi: 10.1016/s0166-4328(00)00356-9. [DOI] [PubMed] [Google Scholar]
- 93.Edwards TM, Rickard NS. Pharmaco-behavioural evidence indicating a complex role for ryanodine receptor calcium release channels in memory processing for a passive avoidance task. Neurobiology of Learning and Memory. 2006;86(1):1–8. doi: 10.1016/j.nlm.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 94.Messier C, Mourre C, Bontempi B, Sif J, Lazdunski M, Destrade C. Effect of apamin, a toxin that inhibits Ca2+-dependent K+ channels, on learning and memory processes. Brain Research. 1991;551(1-2):322–326. doi: 10.1016/0006-8993(91)90950-z. [DOI] [PubMed] [Google Scholar]
- 95.Deschaux O, Bizot JC, Goyffon M. Apamin improves learning in an object recognition task in rats. Neuroscience Letters. 1997;222(3):159–162. doi: 10.1016/s0304-3940(97)13367-5. [DOI] [PubMed] [Google Scholar]
- 96.Deschaux O, Bizot JC. Effect of apamin, a selective blocker of Ca2+-activated K+-channel, on habituation and passive avoidance responses in rats. Neuroscience Letters. 1997;227(1):57–60. doi: 10.1016/s0304-3940(97)00301-7. [DOI] [PubMed] [Google Scholar]
- 97.Ikonen S, Schmidt B, Riekkinen P. Apamin improves spatial navigation in medial septal-lesioned mice. European Journal of Pharmacology. 1998;347(1):13–21. doi: 10.1016/s0014-2999(98)00075-2. [DOI] [PubMed] [Google Scholar]
- 98.Van Der Staay FJ, Fanelli RJ, Blokland A, Schmidt BH. Behavioral effects of apamin, a selective inhibitor of the SKCa-channel, in mice and rats. Neuroscience and Biobehavioral Reviews. 1999;23(8):1087–1110. doi: 10.1016/s0149-7634(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 99.Ikonen S, Riekkinen P. Effects of apamin on memory processing of hippocampal-lesioned mice. European Journal of Pharmacology. 1999;382(3):151–156. doi: 10.1016/s0014-2999(99)00616-0. [DOI] [PubMed] [Google Scholar]
- 100.Fournier C, Kourrich S, Soumireu-Mourat B, Mourre C. Apamin improves reference memory but not procedural memory in rats by blocking small conductance Ca2+-activated K+ channels in an olfactory discrimination task. Behavioural Brain Research. 2001;121(1-2):81–93. doi: 10.1016/s0166-4328(00)00387-9. [DOI] [PubMed] [Google Scholar]
- 101.Mpari B, Regaya I, Escoffier G, Mourre C. Differential effects of two blockers of small conductance Ca2+-activated K+ channels, apamin and lei-Dab7, on learning and memory in rats. Journal of integrative neuroscience. 2005;4(3):381–396. doi: 10.1142/s0219635205000884. [DOI] [PubMed] [Google Scholar]
- 102.Brennan AR, Dolinsky B, Vu MAT, Stanley M, Yeckel MF, Arnsten AFT. Blockade of IP3-mediated SK channel signaling in the rat medial prefrontal cortex improves spatial working memory. Learning and Memory. 2008;15(3):93–96. doi: 10.1101/lm.767408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baker KD, Edwards TM, Rickard NS. Blocking SK channels impairs long-term memory formation in young chicks. Behavioural Brain Research. 2011;216(1):458–462. doi: 10.1016/j.bbr.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 104.Haux P, Sawerthal H, Habermann E. Sequence analysis of bee venom neurotoxin (apamine) from its tryptic and chymotryptic cleavage products. Hoppe-Seyler’s Zeitschrift fur Physiologische Chemie. 1967;348(6):737–738. [PubMed] [Google Scholar]
- 105.Habermann E. Bee and wasp venoms. Science. 1972;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 106.Callewaert GL, Shipolini R, Vernon CA. The disulphide bridges of apamin. FEBS Letters. 1968;1(2):111–113. doi: 10.1016/0014-5793(68)80033-x. [DOI] [PubMed] [Google Scholar]
- 107.Vincent JP, Schweitz H, Lazdunski M. Structure-function relationships and site of action of apamin, a neurotoxic polypeptide of bee venom with an action on the central nervous system. Biochemistry. 1975;14(11):2521–2525. doi: 10.1021/bi00682a035. [DOI] [PubMed] [Google Scholar]
- 108.Habermann E, Cheng Raude D. Central neurotoxicity of apamin, crotamin, phospholipase A and α amanitin. Toxicon. 1975;13(6):465–473. doi: 10.1016/0041-0101(75)90176-2. [DOI] [PubMed] [Google Scholar]
- 109.Garcia ML, Galvez A, Garcia-Calvo M, King VF, Vazquez J, Kaczorowski GJ. Use of toxins to study potassium channels. Journal of Bioenergetics and Biomembranes. 1991;23(4):615–646. doi: 10.1007/BF00785814. [DOI] [PubMed] [Google Scholar]
- 110.Heurteaux C, Messier C, Destrade C, Lazdunski M. Memory processing and apamin induce immediately early gene expression in mouse brain. Molecular Brain Research. 1993;18(1-2):17–22. doi: 10.1016/0169-328x(93)90169-p. [DOI] [PubMed] [Google Scholar]
- 111.Shakkottai VG, Regaya I, Wulff H, et al. Design and characterization of a highly selective peptide inhibitor of the small conductance calcium-activated K+ channel, SKCa2. Journal of Biological Chemistry. 2001;276(46):43145–43151. doi: 10.1074/jbc.M106981200. [DOI] [PubMed] [Google Scholar]
- 112.Yamada SI, Takechi H, Kanchiku I, Kita T, Kato N. Small-conductance Ca2+-dependent K+ channels are the target of spike-induced Ca2+ release in a feedback regulation of pyramidal cell excitability. Journal of Neurophysiology. 2004;91(5):2322–2329. doi: 10.1152/jn.01049.2003. [DOI] [PubMed] [Google Scholar]
- 113.Gulledge AT, Park SB, Kawaguchi Y, Stuart GJ. Heterogeneity of phasic cholinergic signaling in neocortical neurons. Journal of Neurophysiology. 2007;97(3):2215–2229. doi: 10.1152/jn.00493.2006. [DOI] [PubMed] [Google Scholar]
- 114.Hagenston AM, Fitzpatrick JS, Yeckel MF. MGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cerebral Cortex. 2008;18(2):407–423. doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Faber ESL, Sah P. Functions of SK channels in central neurons. Clinical and Experimental Pharmacology and Physiology. 2007;34(10):1077–1083. doi: 10.1111/j.1440-1681.2007.04725.x. [DOI] [PubMed] [Google Scholar]
- 116.Faber ESL. Functional interplay between NMDA receptors, SK channels and voltage-gated Ca2+ channels regulates synaptic excitability in the medial prefrontal cortex. Journal of Physiology. 2010;588(8):1281–1292. doi: 10.1113/jphysiol.2009.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miller C, Moczydlowski E, Latorre R, Phillips M. Charybdotoxin a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- 118.Gehlert DR, Gackenheimer SL. Comparison of the distribution of binding sites for the potassium channel ligands [125I]apamin, [125I]charybdotoxin and [125I]iodoglyburide in the rat brain. Neuroscience. 1993;52(1):191–205. doi: 10.1016/0306-4522(93)90192-i. [DOI] [PubMed] [Google Scholar]
- 119.Cochran SM, Harvey AL, Pratt JA. Regionally selective alterations in local cerebral glucose utilization evoked by charybdotoxin, a blocker of central voltage-activated K+-channels. European Journal of Neuroscience. 2001;14(9):1455–1463. doi: 10.1046/j.0953-816x.2001.01770.x. [DOI] [PubMed] [Google Scholar]
- 120.Crest M, Jacquet G, Gola M, et al. Kaliotoxin, a novel peptidyl inhibitor of neuronal BK-type Ca2+-activated K+ channels characterized from Androctonus mauretanicus mauretanicus venom. Journal of Biological Chemistry. 1992;267(3):1640–1647. [PubMed] [Google Scholar]
- 121.Galvez A, Gimenez-Gallego G, Reuben JP, et al. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus . Journal of Biological Chemistry. 1990;265(19):11083–11090. [PubMed] [Google Scholar]
- 122.Giangiacomo KM, Garcia ML, McManus OB. Mechanism of iberiotoxin block of the large-conductance calcium-activated potassium channel from bovine aortic smooth muscle. Biochemistry. 1992;31(29):6719–6727. doi: 10.1021/bi00144a011. [DOI] [PubMed] [Google Scholar]
- 123.Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. Journal of Physiology. 1999;521(1):135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schreurs BG, Gusev PA, Tomsic D, Alkon DL, Shi T. Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. Journal of Neuroscience. 1998;18(14):5498–5507. doi: 10.1523/JNEUROSCI.18-14-05498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Himi T, Saito H, Nakajima T. Spider toxin (JSTX-3) inhibits the memory retrieval of passive avoidance tests. Journal of Neural Transmission. General Section. 1990;80(1):79–89. doi: 10.1007/BF01245024. [DOI] [PubMed] [Google Scholar]
- 126.Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127(3):701–712. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 127.Anderson NE, Barber PA. Limbic encephalitis—a review. Journal of Clinical Neuroscience. 2008;15(9):961–971. doi: 10.1016/j.jocn.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 128.Buckley C, Oger J, Clover L, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Annals of Neurology. 2001;50(1):73–78. doi: 10.1002/ana.1097. [DOI] [PubMed] [Google Scholar]
- 129.Harrower T, Foltynie T, Kartsounis L, De Silva RN, Hodges JR. A case of voltage-gated potassium channel antibody-related limbic encephalitis. Nature Clinical Practice Neurology. 2006;2(6):339–343. doi: 10.1038/ncpneuro0194. [DOI] [PubMed] [Google Scholar]
- 130.Kartsounis LD, de Silva R. Unusual amnesia in a patient with VGKC-Ab limbic encephalitis: a case study. Cortex. 2011;47(4):451–459. doi: 10.1016/j.cortex.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 131.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death and Differentiation. 2005;12(1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 132.Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. Journal of Neuroimmune Pharmacology. 2010;5(3):294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Keblesh JP, Reiner BC, Liu J, Xiong H. Pathogenesis of human immunodeficiency virus type-1 (HIV-1)-associated dementia: role of voltage-gated potassium channels. Retrovirology. 2008;2:1–10. [PMC free article] [PubMed] [Google Scholar]
- 134.Keblesh J, Hu D, Xiong H. Voltage-gated potassium channels in human immunodeficiency virus type-1 (HIV-1)-associated neurocognitive disorders. Journal of Neuroimmune Pharmacology. 2009;4(1):60–70. doi: 10.1007/s11481-008-9106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gelman BB, Soukup VM, Schuenke KW, et al. Acquired neuronal channelopathies in HIV-associated dementia. Journal of Neuroimmunology. 2004;157(1-2):111–119. doi: 10.1016/j.jneuroim.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 136.Zink WE, Anderson E, Boyle J, et al. Impaired spatial cognition and synaptic potentiation in a murine model of human immunodeficiency virus type 1 encephalitis. Journal of Neuroscience. 2002;22(6):2096–2105. doi: 10.1523/JNEUROSCI.22-06-02096.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anderson ER, Boyle J, Zink WE, Persidsky Y, Gendelman HE, Xiong H. Hippocampal synaptic dysfunction in a murine model of human immunodeficiency virus type 1 encephalitis. Neuroscience. 2003;118(2):359–369. doi: 10.1016/s0306-4522(02)00925-9. [DOI] [PubMed] [Google Scholar]
- 138.Keblesh JP, Dou H, Gendelman HE, Xiong H. 4-aminopyridine improves spatial memory in a murine model of HIV-1 encephalitis. Journal of Neuroimmune Pharmacology. 2009;4(3):317–327. doi: 10.1007/s11481-009-9161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stilo SA, Murray RM. The epidemiology of schizophrenia: replacing dogma with knowledge. Dialogues in Clinical Neuroscience. 2010;12(3):305–315. doi: 10.31887/DCNS.2010.12.3/sstilo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 141.Heinrichs RW. The primacy of cognition in schizophrenia. American Psychologist. 2005;60(3):229–242. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]
- 142.Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Archives of General Psychiatry. 2000;57(9):907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- 143.Potter AI, Nestor PG. IQ subtypes in schizophrenia: distinct symptom and neuropsychological profiles. Journal of Nervous and Mental Disease. 2010;198(8):580–585. doi: 10.1097/NMD.0b013e3181ea4e43. [DOI] [PubMed] [Google Scholar]
- 144.Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23(3):223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 145.Quan MN, Tian YT, Xu KH, Zhang T, Yang Z. Post weaning social isolation influences spatial cognition, prefrontal cortical synaptic plasticity and hippocampal potassium ion channels in Wistar rats. Neuroscience. 2010;169(1):214–222. doi: 10.1016/j.neuroscience.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 146.Chen X, Yuan LL, Zhao C, et al. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. Journal of Neuroscience. 2006;26(47):12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bourdeau ML, Morin F, Laurent CE, Azzi M, Lacaille JC. Kv4.3-mediated A-type K+ currents underlie rhythmic activity in hippocampal interneurons. Journal of Neuroscience. 2007;27(8):1942–1953. doi: 10.1523/JNEUROSCI.3208-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gallagher M, Koh MT. Episodic memory on the path to Alzheimer's disease. Current Opinion in Neurobiology. 2011;21(6):929–934. doi: 10.1016/j.conb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dougherty JJ, Wu J, Nichols RA. β-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex. Journal of Neuroscience. 2003;23(17):6740–6747. doi: 10.1523/JNEUROSCI.23-17-06740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Betancourt L, Colom LV. Potassium (K+) channel expression in basal forebrain cholinergic neurons. Journal of Neuroscience Research. 2000;61(6):646–651. doi: 10.1002/1097-4547(20000915)61:6<646::AID-JNR8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 151.Kidd JF, Sattelle DB. The effects of amyloid peptides on A-type K+ currents of Drosophila larval cholinergic neurons: modeled actions on firing properties. Invertebrate Neuroscience. 2006;6(4):207–213. doi: 10.1007/s10158-006-0034-y. [DOI] [PubMed] [Google Scholar]
- 152.Kidd JF, Brown LA, Sattelle DB. Effects of amyloid peptides on A-type K+ currents of Drosophila larval cholinergic neurons. Journal of Neurobiology. 2006;66(5):476–487. doi: 10.1002/neu.20227. [DOI] [PubMed] [Google Scholar]
- 153.Demaurex N, Petheö GL. Electron and proton transport by NADPH oxidases. Philosophical Transactions of the Royal Society B. 2005;360(1464):2315–2325. doi: 10.1098/rstb.2005.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature Reviews Neuroscience. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 155.Khanna R, Roy L, Zhu X, Schlichter LC. K+ channels and the microglial respiratory burst. American Journal of Physiology. 2001;280(4):C796–C806. doi: 10.1152/ajpcell.2001.280.4.C796. [DOI] [PubMed] [Google Scholar]
- 156.Fordyce CB, Jagasia R, Zhu X, Schlichter LC. Microglia Kv1.3 channels contribute to their ability to kill neurons. Journal of Neuroscience. 2005;25(31):7139–7149. doi: 10.1523/JNEUROSCI.1251-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Compston A, Coles A. Multiple sclerosis. The Lancet. 2008;372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 158.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. The Lancet Neurology. 2008;7(12):1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 159.Gutmann L, Gutmann L. Axonal channelopathies: an evolving concept in the pathogenesis of peripheral nerve disorders. Neurology. 1996;47(1):18–21. doi: 10.1212/wnl.47.1.18. [DOI] [PubMed] [Google Scholar]
- 160.Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365(6441):75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- 161.Jankowska E, Lundberg A, Rudomin P, Sykova E. Effects of 4-aminopyridine on transmission in excitatory and inhibitory synapses in the spinal cord. Brain Research. 1977;136(2):387–392. doi: 10.1016/0006-8993(77)90816-2. [DOI] [PubMed] [Google Scholar]
- 162.Sherratt RM, Bostock H, Sears TA. Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibres. Nature. 1980;283(5747):570–572. doi: 10.1038/283570a0. [DOI] [PubMed] [Google Scholar]
- 163.Targ EF, Kocsis JD. 4-Aminopyridine leads to restoration of conduction in demyelinated rat sciatic nerve. Brain Research. 1985;328(2):358–361. doi: 10.1016/0006-8993(85)91049-2. [DOI] [PubMed] [Google Scholar]
- 164.Smith KJ, Felts PA, John GR. Effects of 4-aminopyridine on demyelinated axons, synapses and muscle tension. Brain. 2000;123(1):171–184. doi: 10.1093/brain/123.1.171. [DOI] [PubMed] [Google Scholar]
- 165.Bever CT., Jr. The current status of studies of aminopyridines in patients with multiple sclerosis. Annals of Neurology. 1994;36:S118–S121. doi: 10.1002/ana.410360728. [DOI] [PubMed] [Google Scholar]
- 166.Polman CH, Bertelsmann FW, Van Loenen AC, Koetsier JC. 4-Aminopyridine in the treatment of patients with multiple sclerosis: long-term efficacy and safety. Archives of Neurology. 1994;51(3):292–296. doi: 10.1001/archneur.1994.00540150090022. [DOI] [PubMed] [Google Scholar]
- 167.Holoshitz J, Naparstek Y, Ben-Nun A. T lymphocyte lines induce autoimmune encephalomyelitis, delayed hypersensitivity and bystander encephalitis. European Journal of Immunology. 1984;14(8):729–734. doi: 10.1002/eji.1830140811. [DOI] [PubMed] [Google Scholar]
- 168.Beeton C, Wulff H, Barbaria J, et al. Selective blockade of T lymphocyte K+ channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(24):13942–13947. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fanger CM, Rauer H, Neben AL, et al. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. Journal of Biological Chemistry. 2001;276(15):12249–12256. doi: 10.1074/jbc.M011342200. [DOI] [PubMed] [Google Scholar]
- 170.Beeton C, Barbaria J, Giraud P, et al. Selective blocking of voltage-gated K+ channels improves experimental autoimmune encephalomyelitis and inhibits T cell activation. Journal of Immunology. 2001;166(2):936–944. doi: 10.4049/jimmunol.166.2.936. [DOI] [PubMed] [Google Scholar]
- 171.Solari A, Uitdehaag B, Giuliani G, Pucci E, Taus C. Aminopyridines for symptomatic treatment in multiple sclerosis. Cochrane Database of Systematic Reviews. 2001;(4) doi: 10.1002/14651858.CD001330. Article ID CD001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Korenke AR, Rivey MP, Allington DR. Sustained-release fampridine for symptomatic treatment of multiple sclerosis. Annals of Pharmacotherapy. 2008;42(10):1458–1465. doi: 10.1345/aph.1L028. [DOI] [PubMed] [Google Scholar]
- 173.Medication guide for Ampyra. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM199168.pdf.
- 174.Gibb WRG. Neuropathology of Parkinson’s disease and related syndromes. Neurologic Clinics. 1992;10(2):361–376. [PubMed] [Google Scholar]
- 175.Jankovic J. Parkinson’s disease: clinical features and diagnosis. Journal of Neurology, Neurosurgery and Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 176.Liss B, Haeckel O, Wildmann J, Miki T, Seino S, Roeper J. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nature Neuroscience. 2005;8(12):1742–1751. doi: 10.1038/nn1570. [DOI] [PubMed] [Google Scholar]
- 177.Liss B, Roeper J. ATP-sensitive potassium channels in dopaminergic neurons: transducers of mitochondrial dysfunction. News in Physiological Sciences. 2001;16(5):214–217. doi: 10.1152/physiologyonline.2001.16.5.214. [DOI] [PubMed] [Google Scholar]
- 178.Schapira AHV, Gu M, Taanman JW, et al. Mitochondria in the etiology and pathogenesis of Parkinson’s disease. Annals of Neurology. 1998;44(3):S89–S98. doi: 10.1002/ana.410440714. [DOI] [PubMed] [Google Scholar]
- 179.Patil N, Cox DR, Bhat D, Faham M, Myers RM, Peterson AS. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nature Genetics. 1995;11(2):126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- 180.Tunnicliff G. Basis of the antiseizure action of phenytoin. General Pharmacology. 1996;27(7):1091–1097. doi: 10.1016/s0306-3623(96)00062-6. [DOI] [PubMed] [Google Scholar]
- 181.Löscher W. New visions in the pharmacology of anticonvulsion. European Journal of Pharmacology. 1998;342(1):1–13. doi: 10.1016/s0014-2999(97)01514-8. [DOI] [PubMed] [Google Scholar]
- 182.Blum DE. New drugs for persons with epilepsy. Advances in neurology. 1998;76:57–87. [PubMed] [Google Scholar]
- 183.Guerrini R. Epilepsy in children. Lancet. 2006;367(9509):499–524. doi: 10.1016/S0140-6736(06)68182-8. [DOI] [PubMed] [Google Scholar]
- 184.Aldenkamp A, Arends J. The relative influence of epileptic EEG discharges, short nonconvulsive seizures, and type of epilepsy on cognitive function. Epilepsia. 2004;45(1):54–63. doi: 10.1111/j.0013-9580.2004.33403.x. [DOI] [PubMed] [Google Scholar]
- 185.Dodrill CB. Neuropsychological effects of seizures. Epilepsy and Behavior. 2004;5(1):S21–S24. doi: 10.1016/j.yebeh.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 186.Beghi E, De Maria G, Gobbi G, Veneselli E. Diagnosis and treatment of the first epileptic seizure: guidelines of the Italian League against Epilepsy. Epilepsia. 2006;47(5):2–8. doi: 10.1111/j.1528-1167.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 187.Meador KJ. Cognitive and memory effects of the new antiepileptic drugs. Epilepsy Research. 2006;68(1):63–67. doi: 10.1016/j.eplepsyres.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 188.Hermann B. Cognition in epilepsy and its transient impairment. Epilepsy and Behavior. 2011;22(3):p. 419. doi: 10.1016/j.yebeh.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 189.N'Gouemo P. Targeting BK (big potassium) channels in epilepsy. Expert Opinion on Therapeutic Targets. 2011;15(11):1283–1295. doi: 10.1517/14728222.2011.620607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Meldrum BS. Identification and preclinical testing of novel antiepileptic compounds. Epilepsia. 1997;38(9):S7–S15. doi: 10.1111/j.1528-1157.1997.tb05204.x. [DOI] [PubMed] [Google Scholar]
- 191.Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clinical neurosurgery. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- 192.Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Archives of Neurology. 1997;54(4):369–376. doi: 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- 193.Wieser H-G. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45(6):695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- 194.Saling MM. Verbal memory in mesial temporal lobe epilepsy: beyond material specificity. Brain. 2009;132(3):570–582. doi: 10.1093/brain/awp012. [DOI] [PubMed] [Google Scholar]
- 195.Cooper EC. Potassium channels: how genetic studies of epileptic syndromes open paths to new therapeutic targets and drugs. Epilepsia. 2001;42(5):49–54. doi: 10.1046/j.1528-1157.2001.0420s5049.x. [DOI] [PubMed] [Google Scholar]
- 196.Meldrum BS, Rogawski MA. Molecular targets for antiepileptic drug development. Neurotherapeutics. 2007;4(1):18–61. doi: 10.1016/j.nurt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stafstrom CE, Grippon S, Kirkpatrick P. Ezogabine (retigabine) Nature Reviews Drug Discovery. 2011;10(10):729–730. doi: 10.1038/nrd3561. [DOI] [PubMed] [Google Scholar]
- 198.Vacher H, Prestipino G, Crest M, Martin-Eauclaire MF. Definition of the alpha-KTx15 subfamily. Toxicon. 2004;43(8):887–894. doi: 10.1016/j.toxicon.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 199.Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the a type and attributable to coexpression of Kv4.2 and Kv4.1 subunits. Journal of Neuroscience. 1998;18(9):3124–3137. doi: 10.1523/JNEUROSCI.18-09-03124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Guan D, Tkatch T, Surmeier DJ, Armstrong WE, Foehring RC. Kv2 subunits underlie slowly inactivating potassium current in rat neocortical pyramidal neurons. Journal of Physiology. 2007;581(3):941–960. doi: 10.1113/jphysiol.2007.128454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Guan D, Horton LR, Armstrong WE, Foehring RC. Postnatal development of A-type and Kv1- and Kv2-mediated potassium channel currents in neocortical pyramidal neurons. Journal of Neurophysiology. 2011;105(6):2976–2988. doi: 10.1152/jn.00758.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sitges M, Possani LD, Bayon A. Noxiustoxin, a short-chain toxin from the Mexican scorpion Centruroides noxius, induces transmitter release by blocking K+ permeability. Journal of Neuroscience. 1986;6(6):1570–1574. doi: 10.1523/JNEUROSCI.06-06-01570.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.D’Suze G, Batista CVF, Frau A, et al. Discrepin, a new peptide of the sub-family α-KTx15, isolated from the scorpion Tityus discrepans irreversibly blocks K+-channels (I A currents) of cerebellum granular cells. Archives of Biochemistry and Biophysics. 2004;430(2):256–263. doi: 10.1016/j.abb.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 204.Vacher H, Romi-Lebrun R, Mourre C, et al. A new class of scorpion toxin binding sites related to an A-type K+ channel: pharmacological characterization and localization in rat brain. FEBS Letters. 2001;501(1–3):31–36. doi: 10.1016/s0014-5793(01)02620-5. [DOI] [PubMed] [Google Scholar]
- 205.Li MH, Wang YF, Chen XQ, Zhang NX, Wu HM, Hu GY. BmTx3B, a novel scorpion toxin from Buthus martensi Karsch, inhibits delayed rectifier potassium current in rat hippocampal neurons. Acta Pharmacologica Sinica. 2003;24(10):1016–1062. [PubMed] [Google Scholar]
- 206.Swartz KJ, MacKinnon R. An inhibitor of the Kv2.1 potassium channel isolated from the venom of a Chilean tarantula. Neuron. 1995;15(4):941–949. doi: 10.1016/0896-6273(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 207.Diochot S, Drici MD, Moinier D, Fink M, Lazdunski M. Effects of phrixotoxins on the Kv4 family of potassium channels and implications for the role of Itoj in cardiac electrogenesis. British Journal of Pharmacology. 1999;126(1):251–263. doi: 10.1038/sj.bjp.0702283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Escoubas P, Diochot S, Célérier ML, Nakajima T, Lazdunski M. Novel tarantula toxins for subtypes of voltage-dependent potassium channels in the Kv2 and Kv4 subfamilies. Molecular Pharmacology. 2002;62(1):48–57. doi: 10.1124/mol.62.1.48. [DOI] [PubMed] [Google Scholar]
- 209.Schmalhofer WA, Ratliff KS, Weinglass AB, Kaczorowski GJ, Garcia ML, Herrington J. A KV2.1 gating modifier binding assay suitable for highthroughput screening. Channels. 2009;3(6):437–447. doi: 10.4161/chan.3.6.10201. [DOI] [PubMed] [Google Scholar]
- 210.Ebbinghaus J, Legros C, Nolting A, et al. Modulation of Kv4.2 channels by a peptide isolated from the venom of the giant bird-eating tarantula Theraphosa leblondi . Toxicon. 2004;43(8):923–932. doi: 10.1016/j.toxicon.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 211.Kushmerick C, Kalapothakis E, Beirão PSL, et al. Phoneutria nigriventer toxin Tx3-1 blocks A-type K+ currents controlling Ca2+ oscillation frequency in GH3 cells. Journal of Neurochemistry. 1999;72(4):1472–1481. doi: 10.1046/j.1471-4159.1999.721472.x. [DOI] [PubMed] [Google Scholar]
- 212.Lee CW, Kim S, Roh SH, et al. Solution structure and functional characterization of SGTx1, a modifier of Kv2.1 channel gating. Biochemistry. 2004;43(4):890–897. doi: 10.1021/bi0353373. [DOI] [PubMed] [Google Scholar]
- 213.McManus OB. Calcium-activated potassium channels: regulation by calcium. Journal of Bioenergetics and Biomembranes. 1991;23(4):537–560. doi: 10.1007/BF00785810. [DOI] [PubMed] [Google Scholar]
- 214.Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Current Opinion in Neurobiology. 1998;8(3):321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- 215.Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophysical Journal. 1997;73(3):1355–1363. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yamamoto K, Hashimoto K, Isomura Y, Shimohama S, Kato N. An IP3-assisted form of Ca2+-induced Ca2+ release in neocortical neurons. NeuroReport. 2000;11(3):535–539. doi: 10.1097/00001756-200002280-00022. [DOI] [PubMed] [Google Scholar]
- 217.Yamamoto K, Hashimoto K, Nakano M, Shimohama S, Kato N. A distinct form of calcium release down-regulates membrane excitability in neocortical pyramidal cells. Neuroscience. 2002;109(4):665–676. doi: 10.1016/s0306-4522(01)00486-9. [DOI] [PubMed] [Google Scholar]
- 218.Shi J, He HQ, Zhao R, et al. Inhibition of martentoxin on neuronal BK channel subtype (α+β4): implications for a novel interaction model. Biophysical Journal. 2008;94(9):3706–3713. doi: 10.1529/biophysj.107.122150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Garcia-Valdes J, Zamudio FZ, Toro L, Possan LD. Slotoxin, αKTx1.11, a new scorpion peptide blocker of MaxiK channels that differentiates between α and α+β (β1 or β4) complexes. FEBS Letters. 2001;505(3):369–373. doi: 10.1016/s0014-5793(01)02791-0. [DOI] [PubMed] [Google Scholar]
- 220.Vaca L, Gurrola GB, Possani LD, Kunze DL. Blockade of a KCa channel with synthetic peptides from noxiustoxin: a K+ channel blocker. Journal of Membrane Biology. 1993;134(2):123–129. doi: 10.1007/BF00232748. [DOI] [PubMed] [Google Scholar]
- 221.Nhrke K, Quinn CC, Begenisich T. Molecular identification of Ca2+-activated K+ channels in parotid acinar cells. American Journal of Physiology. 2003;284(2 53-2):C535–C546. doi: 10.1152/ajpcell.00044.2002. [DOI] [PubMed] [Google Scholar]
- 222.Chicchi GG, Gimenez-Callego G, Ber E, Garcia ML, Winquist R, Cascieri MA. Purification and characterization of a unique, potent inhibitor of apamin binding from Leiurus quinquestriatus hebraeus venom. Journal of Biological Chemistry. 1988;263(21):10192–10197. [PubMed] [Google Scholar]
- 223.Pedarzani P, D’Hoedt D, Doorty KB, et al. Tamapin, a venom peptide from the Indian red scorpion (Mesobuthus tamulus) that targets small conductance Ca2+-activated K+ channels and afterhyperpolarization currents in central neurons. Journal of Biological Chemistry. 2002;277(48):46101–46109. doi: 10.1074/jbc.M206465200. [DOI] [PubMed] [Google Scholar]


