Abstract
Non-fibrillar collagen XV is a chondroitin sulphate modified glycoprotein that is associated with the basement membrane zone in many tissues. Its precise functions remain to be fully elucidated though it clearly plays a critical role in the structural integrity of the extracellular matrix. Loss of collagen XV from the basement membrane zone precedes invasion of a number of tumor types and we previously showed that collagen XV functions as a dose-dependent suppressor of tumorigenicity in cervical carcinoma cells. The carboxyl terminus of another non-fibrillar collagen (XVIII) is cleaved to produce endostatin, which has anti-angiogenic effects and thus may act as a tumor suppressor in vivo. Since collagen XV has structural similarity with collagen XVIII, its C-terminal restin domain could confer tumor suppressive functions on the molecule, though our previous data did not support this. We now show that expression of collagen XV enhances the adhesion of cervical carcinoma cells to collagen I in vitro as does the N-terminus and collagenous regions of collagen XV, but not the restin domain. Destruction of a cysteine residue in the collagenous region that is critical for intermolecular interactions of collagen XV abolished the enhanced adhesion to collagen I. Finally, we demonstrate that unlike full length collagen XV, expression of the restin domain alone does not suppress tumorigenicity of cervical carcinoma cells in vivo; hence, this process is dependent on functions and interactions of other parts of the protein.
Keywords: Collagen XV, restin domain, adhesion, tumor suppression
1. Introduction
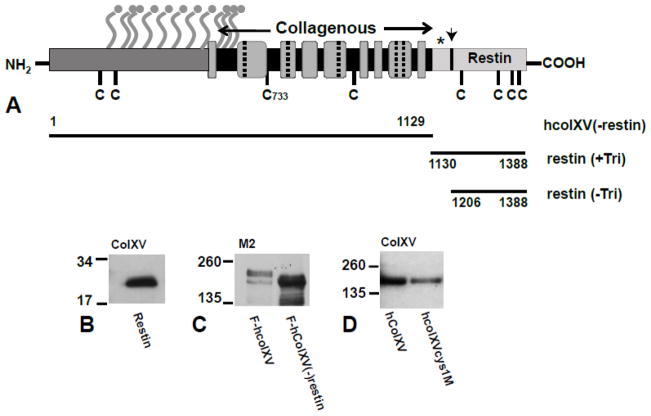
Our interest in collagen XV as a potential tumor suppressor arose from previous data (reviewed in (Harris, 2003)) that mapped a locus for suppression of malignancy to a region between bands A4 and C3 on mouse chromosome 4 (Jonasson et al., 1977). The activity of this locus was dependent on gene dosage (Evans et al., 1982) and correlated with the expression of a dense collagenous matrix (Harris, 1985). A search for potential tumor suppressor and/or collagen genes within the A4 - C3 region identified the gene encoding procollagen XVa1 as the most likely candidate. The 1 chain of human type XV collagen (COL15A1) encodes a 1388 amino acid (aa) protein, with a 25 aa putative signal peptide, a 530 aa N-terminal non-collagenous domain, a 577 residue discontinuous collagenous sequence and a 256 aa C-terminal non-collagenous domain (Hagg et al., 1998). Within the collagenous part of the molecule nine collagenous domains (containing the characteristic Gly-X-Y motif where X and Y may be any other amino acid but an excess of proline and hydroxyproline residues are found) are interrupted by 8 non-collagenous sequences (Fig. 1A). The N-terminal non-collagenous domain of type XV and type XVIII collagen, to which it is highly similar, show sequence homology to thrombospondin. Collagen XV was originally described in sequence derived from a placental cDNA clone (Myers et al., 1992) though it is widely distributed in many human tissues. It is strongly associated with vascular, neuronal, mesenchymal and some epithelial basement membranes implicating a function in adhering the basement membrane to adjacent connective tissue stroma (Myers et al., 1996). More recently a very close association between collagen XV and the fibrillar collagen network was shown subjacent to the basement membrane, a location from which it is ideally placed to contribute to signal transduction pathways (Amenta et al., 2005). Type XV collagen exists in two core protein forms of 250 and 225 kDa, which differ in their carboxyl termini; however, the core proteins are modified by O-linked chondroitin/dermatan sulfate side chains and so may exhibit a much larger apparent molecular weight of about 400kDa or greater (Amenta et al., 2005), (Li et al., 2000). Collagen XV contains a trimerization domain located between the collagenous region and the C-terminal restin domain (Wirz et al., 2011). The trimers are linked by interchain disulphide bonds involving only the two cysteine residues (at amino acids 733 and 965) in the collagenous domain and not the other 8 cysteines located elsewhere in the molecule (Li et al., 2000) (Fig. 1A).
Figure 1. Diagram to show the structure of the collagen XV protein (A) and expression hcolXV domains and mutants (B–D).
A, (From (Amenta et al., 2005)). Amino terminal non-collagenous domain (dark gray); carboxyl domain (restin) (pale gray, with arrow marking location of cleavage sites); collagenous domain (gray boxes) with interruption marked as dashed lines or black bars depending on their length. Ball and stick symbols show consensus sites for GAG attachment; C denotes cysteines; * denotes trimerization domain; horizontal black bars denote the extent of the N-terminal and C-terminal domain constructs. B–D. Western blots of lysates from COS-7 cells transiently transfected with B) restin alone; C) F-hcolXV and F-hcolXV(−)restin; D) hcolXV and hcolXVCys1M). B) and D) probed with anti-colXV antibody and C) with M2 (anti-FLAG). MWs are shown in kDa.
Not only does the location of collagen XV subjacent to the basement membrane support a role in the suppression of the growth of malignant tumors, invasion of the basement membrane by human ductal breast carcinoma cells and colon carcinomas was preceded by the disappearance of this molecule (Amenta et al., 2003), (Amenta et al., 2000). This phenomenon is also true for skin carcinomas and melanomas (Fukushige et al., 2005) and we have preliminary evidence for loss of collagen XV from pancreatic cancers (data not shown). We demonstrated that expression of recombinant collagen XV in a human cervical carcinoma cell line that does not normally express the protein, suppresses tumorigenesis in a dose-dependent manner (Harris et al., 2007). More recently, recombinant collagen XV was also shown to inhibit the adhesion and migration of fibrosarcoma cells in vitro (Hurskainen et al., 2010).
One potential mechanism for the tumor suppressor functions of collagen XV is the reported anti-angiogenic properties of the endostatin domains of collagen XVIII, XV (restin) (Ramchandran et al., 1999), (John et al., 2005) and C-terminal fragments of collagen IV 3 (tumstatin, arresten and canstatin) (reviewed in (Cooke and Kalluri, 2008). The active peptides are derived from the non-triple helical carboxyl-terminal NC1 domains of these collagens, which are cleaved by proteases and released as trimers, though are not active until converted to monomeric forms. Though endostatin derived from collagen XVIII has been shown to inhibit angiogenesis (the sprouting of new blood vessels) and endothelial cell migration and to reduce tumor growth in animal models (O’Reilly et al., 1997) these data are controversial (Harris, 2005) (Brideau et al., 2007). Also the sequence, structure and function of collagen XV- and collagen XVIII-derived endostatins are divergent (Sasaki et al., 2000), (Gaetzner et al., 2005). We previously proposed (Harris et al., 2007) that the tumor suppressor properties of collagen XV reported here involve mechanisms that are different from those mediated by endostatin-like activities for a number of reasons: 1. Collagen XV alters the growth properties of cervical carcinoma cells in three-dimensional culture in vitro where angiogenesis is not relevant. 2. We observe the effects in vivo when an incipient tumor is well below the dimensions at which angiogenesis would be relevant. 3. At high levels of collagen XV expression suppression of malignancy is complete, in contrast to the effects of high doses of endostatin on solid tumors. In the experiments described here we first demonstrate that expression of collagen XV increases adhesion of cervical cancer cells to collagen I substrates, which may recapitulate early events in tumor growth, and that this is conferred by the N-terminal and/or collagenous domains of the protein but not by the restin domain. Moreover, the increased adhesion is dependent on the ability of collagen XV to form wild-type intermolecular interactions. Next, we show that the expression of the restin domain of collagen XV alone, does not inhibit tumor growth in vivo. Thus collagen XV-mediated tumor suppression is not caused by potential anti-angiogenic properties of the restin domain.
2. Results
2.1 Generation of D98 AP2 clones expressing the restin domain of collagen XV; collagen XV without the restin domain; and collagen XV with mutations in a critical cysteine residue
The restin construct was transiently expressed in COS-7 cells to demonstrate that it generated a protein of the expected size (~32 kDa) (Figure 1B). Three additional constructs were evaluated in transient transfection: a FLAG epitope-tagged version of human collagen XV (hcolXV), (F-hcolXV); a construct that encompassed the N terminal and collagenous domains of collagen XV without the restin domain (F-hcolXV(−)restin) (Fig. 1C); and an hcolXV construct with the cysteine residue at 733 mutated to alanine (hcolXVcys1M) (Fig. 1D), which would be predicted to destroy the normal intermolecular interactions of the protein (Fig 1A). As expected, the full-length hcolXV generated a ~225 kDa protein in COS-7 transient transfections (Fig. 1D). Next, stable clones were generated in D98 AP2 cervical carcinoma cells for each construct. Figure 2C shows 3 clones (Rest2, Rest11 and Rest13) that express high levels of the restin domain, moreover, this protein is secreted from the cells as demonstrated by western blot of cell culture medium conditioned by a representative clone Rest 5 (Suppl. Fig. 2C). Three clones that express the F-hcolXV(-restin) construct ((−)Rest1, (−)Rest14, (−)Rest15) are shown in Figure 2D and two clones that express hcolXVcys1M (cys1M3C1, cys1M13C1) are shown in Figure 2F.
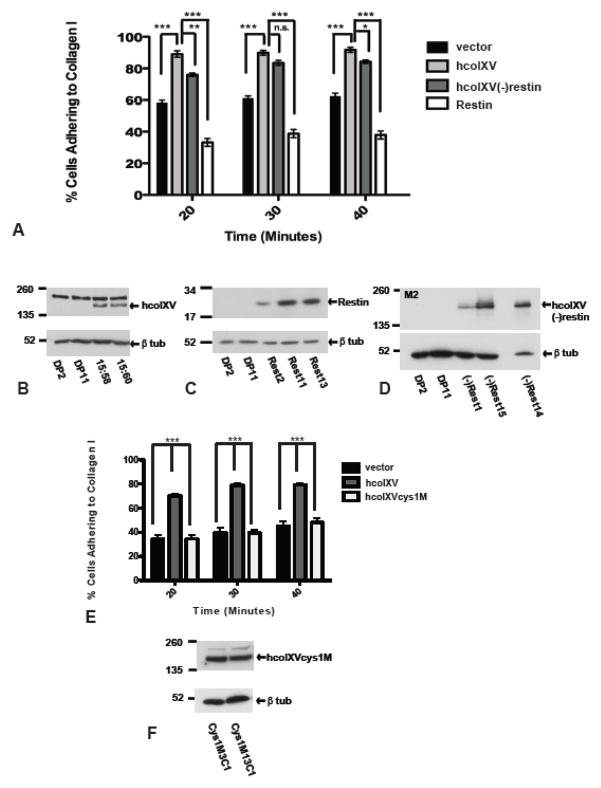
Figure 2. Collagen XV enhances adhesion of D98 AP2 cells to collagen I substrate and mutation of one cysteine residue in the collagenous domain of colXV abolishes this effect.
A. Pooled data from: 2 vector controls (DP2; DP11), 2 hcolXV-expressing clones (15:58; 15:60); 3 hcolXV(−)restin clones ((−)Rest1, (−)Rest14, (−)Rest15), 3 restin clones (Rest2, Rest11, Rest13). HcolXV expressing clones show significantly greater adhesion than vector only controls. The N-terminus/collagenous domains of collagen XV increase adhesion to collagen I, the restin domain does not. (***p<0.001, **p<0.01, *p<0.05, n.s. not significant). B – D. Western blots to show expression levels of hcolXV (B), restin (C) and hcolXV(−)restin (D) in cells used in adhesion assays shown in panel A. B, C probed with anti-colXV Ab, D, with M2. E. Pooled data from: 2 vector controls (DP2; DP11), 2 hcolXV-expressing (15:58; 15:60), 2 Clones expressing hcolXV with the central cysteine mutated to alanine, (Cys1M3C1; Cys1M13C1). The latter adhere like vector controls. (***p<0.001). F. Western blot probed with anti-colXV Ab to show expression levels of hcolXVcys1MC1 and hcolXVcys1M13C1 mutants in cells used in adhesion assays shown in panel E, lower panel probed with anti-tubulin for quantification. Statistical calculations by two way Anova and unpaired t- test with Mann-Whitney test.
2.2 Collagen XV expression in D98 AP2 cells increases adherence to collagen I, while the restin domain alone does not
Adhesion assays were performed to evaluate the interaction of D98 AP2 cells expressing collagen XV with extracellular matrix component substrates including collagen I, collagen IV, fibronectin and laminin. Though expression of collagen XV caused no difference in adhesion to collagen IV, fibronectin and laminin (not shown), it significantly altered adhesion to collagen I. Fig. 2A shows the results of adhesion assays in which the data were pooled from three independent experiments using two vector control clones (DP2 and DP11), two hcolXV clones (15:58 and 15:60) and three individual clones carrying each hcolXV-derived construct, as described in the previous section. Vector-transfected D98 AP2 clones adhere rapidly to collagen I-coated plastic within 20 minutes of plating, though the percentage of cells that adhere does not increase significantly above 60% by 30 or 40 minutes after plating. In contrast clones expressing hcolXV show more than 90% adherence to collagen I by 20 minutes after plating (Figure 2A). The difference between vector controls and hcolXV-expressing clones was highly significant (p<0.001). In contrast, stable expression of the restin domain alone in D98 AP2 cells did not increase their adhesion to collagen I (Fig. 2A). Moreover, the same result was obtained irrespective of whether the restin domain construct included or lacked the N-terminal trimerization domain (Suppl. Fig. 1). Cells expressing hcolXV without the restin domain showed adhesion to collagen I that was very similar to cells which express full-length hcolXV (Fig. 2A). There was a significant increase from the adhesion of vector controls (p<0.001) and though at some time points there were small differences between the adhesion of hcolXV and hcolXV(−)restin clones, this was of low significance (p<0.05) at 40 min and not significant at 30 minutes.
2.3 The collagen XV- induced increase in adherence to collagen I is dependent on its native structure
The two cysteine residues in the collagenous domain of collagen XV were shown (Li et al., 2000) to be critical for intermolecular interactions of the hcolXV molecule. We mutated one of these cysteine residues (C733) to alanine and demonstrated that a stable protein was generated from the construct (hcolXVcys1M Fig. 1D). D98 AP2 clones stably transfected with the hcolXVcys1M construct (Fig. 2F) showed no increase in adhesion to collagen I substrates in comparison to vector control clones (Fig. 2E). Thus the increased adhesion of collagen XV to collagen I requires the ability of hcolXV to form intermolecular interactions.
2.4 Restin does not suppress tumor growth in vivo
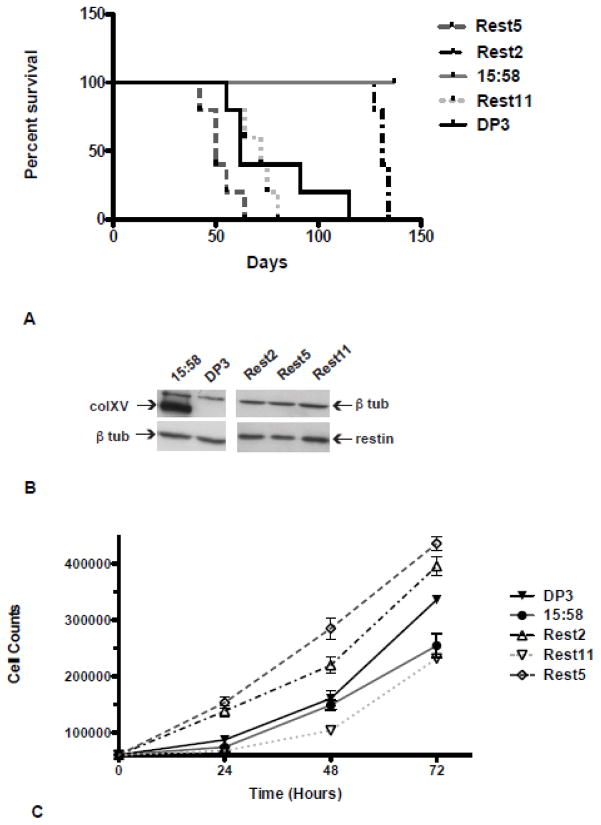
We previously demonstrated the tumor suppressive properties of collagen XV in vivo by subcutaneous injection of D98 AP2 cells into nude mice. While vector controls rapidly generated tumors, cells expressing collagen XV showed a dose-dependent inhibition of tumor growth, with high levels of expression completely abolishing tumor formation (Harris et al., 2007). Here, we evaluated the growth of D98 AP2 cells expressing the restin domain alone in the same in vivo assay. Following subcutaneous injection of 1 × 106 cells, tumors were allowed to develop and animals sacrificed once tumor diameter reached 1.5 cm as we have never observed regression of tumors of this size. Groups of 5 animals were used and the experiment was repeated twice. Figure 3 shows a Kaplan-Meier survival plot of three independent restin domain clones (Rest2, Rest5, Rest11) in comparison to a vector control (DP3) and a collagen XV high expressing clone (15:58), the latter are representative of clones used in previous experiments (Harris et al., 2007). The restin domain clones show no inhibition of tumor growth in comparison to the vector control and in contrast to the hcolXV high expressing clone 15:58 (log-rank test, P<0.0001). Moreover, one of the restin clones (Rest5), generated tumors more rapidly and grew to the 1.5 cm diameter faster than vector control. This observation was not due to differences in growth rate of the clones (Figure 3C), but might reflect the reduced adherence to collagen I observed in the restin clones (Fig. 2A). Appearance of tumors in one restin line (Rest2) occurred later than in the vector control; however, all tumors reached 1.5 cm diameter by 134 days in comparison to 115 days for the DP3 vector clone. As observed previously, high levels of hcolXV in the 15:58 clone completely inhibited tumor growth in nude mice.
Figure 3. Expression of the restin domain of type XV collagen does not suppress tumorigenicity of D98 AP2 cells in nude mice.
A. Kaplan-Meier plots of survival of mice injected with vector-only control clone (DP3), a clone expressing high levels of collagen XV (15:58), restin-only clones (Rest5, Rest2, Rest11), (log-rank tests: Rest5:DP3, P= 0.0584; Rest2:DP3, P=0.0018; Rest11:DP3, P=0.503; 15:58:DP3, P=0.0018; Rest5, Rest2, Rest11: 15:58, P<0.001). B. Western blot to show the expression levels of hcolXV and the restin domain in the clones at the time of injection, in comparison to tubulin. C. In vitro growth rates over 72 hours of clones used in A, DP3, 15:58, Rest2, Rest5, and Rest11.
3. Discussion
We previously showed that human collagen XV altered the growth properties of cervical cancer cells in vitro and suppressed growth of these cells as tumors in vivo, in a dose-dependent manner. It was possible that these observations were due to functions associated with sequence similarity between the carboxyl terminal restin domain of collagen XV and the endostatin domain of collagen XVIII. Endostatin is a potent inhibitor of angiogenesis and so can partially inhibit tumor growth in mice (O’Reilly et al., 1997). However, since we observed an effect of hcolXV on cell growth in vitro and also that the suppression of malignancy by high doses of hcolXV was complete, rather than partial, we proposed that the tumor suppressive properties of collagen XV were independent of the restin domain. Here we demonstrate that unlike endostatin, the restin domain of collagen XV alone is unable to suppress tumor formation in vivo. Moreover, we show using an in vitro cell adhesion assay, which may recapitulate early events in tumor growth, that the combined N-terminus and collagenous domains of collagen XV have similar properties to the full-length molecule, whereas the restin domain does not.
Cervical cancer cells expressing high levels of hcolXV showed significantly increased adhesion to collagen I substrates, though not to collagen IV, laminin or fibronectin, when compared to vector controls. These observations are in contrast to those of Hurskainen et al., (Hurskainen et al., 2010) who showed adhesion of hcolXV to fibronectin and laminin but not collagen I. However, they used recombinant hcolXV expressed in insect cells, which is likely to show different glycosylation from our hcolXV expressed in human cells. Restin domain-expressing clones showed reduced adhesion to collagen I as compared to vector controls, perhaps reflecting their increased tumorigenic capacity in vivo. In contrast, cells expressing the N-terminus and collagenous domain of hcolXV showed almost equivalent levels of adhesion to collagen I as those expressing the full-length protein.
These data encourage further evaluation of the molecular mechanism whereby collagen XV acts as a dose dependent suppressor of tumorigenicity. Our observations that the tumor suppressive properties collagen XV lie in its N-terminus and/or collagenous domains and not in the restin domain and moreover, that these domains interact directly with collagen I, generate novel routes for further investigation.
4. Experimental Procedures
4.1 Collagen XV expression constructs
The full-length human collagen XV cDNA (hcolXV, NM_001855) was kindly donated by Dr Taina Pihlajaniemi and transferred to pcDNA3.1(−)Neo as described previously (Harris et al., 2007). A schematic of the collagen XV protein is shown in Figure 1A. All PCR primers and oligonucleotides used for mutagenesis or cloning are shown in Suppl. Table 1. Two restin domain constructs were generated: 1) includes the collagen XV signal sequence (amino acids 1-27), followed by a large part of NC10 (1130–1388); 2) contains aa 1-27 and 1206–1388, thus just encompassing the restin domain C-terminal region. The signal sequence was cloned from 81 bp oligonucleotides and the C-terminal domains generated by PCR from the hcolXV template using Pfu polymerase. All constructs were cloned into pcDNA3.1(−)Neo. For the construct lacking the restin domain (hcolXV(−)restin, aa 1-1129) site directed mutagenesis was performed on the hcolXV construct, using the Quikchange Site-Directed Mutagenesis Kit (Stratagene/Agilent), for primers see Suppl. Table 1. Briefly, these primers introduce a STOP codon at aa 1130 and move the sequence out of frame, thus inserting multiple additional 3′ STOP codons and preventing inclusion of C-terminal/Restin sequences. To insert the FLAG epitope into hcolXV, site-directed mutagenesis was used to destroy a C-terminal Sac II site (at 4107 bp) without altering the encoded amino acid. Next, purified 2 x FLAG oligonucleotides were annealed and ligated into a N-terminal Sac II site (163 bp, located 12 bases 3′ of the signal sequence). To disrupt the critical cysteine residues (at 733) in the interchain disulphide bonds of hcolXV this amino acid was mutated to alanine (C733A) generating the hcolXVcys1M construct.
4.2 Cell culture, transient transfection, generation of stable cell clones and cell doubling time
Transient transfections were performed in COS-7 cells using CaPO4. D98 AP2 clone B cells (Harris and Bramwell, 1987) were routinely cultured in DMEM and 10% FBS. Stable clones of D98 AP2 carrying hcolXV and pcDNA3.1 vector controls were generated previously (Harris et al., 2007); the various mutants of hcolXV were generated by Lipofectin (Invitrogen) transfection, followed by selection in 650 g/ml neomycin. In vitro growth rates of clones were determined by counting cells at 24, 48 and 72 hours after plating 6 × 104 cells into 24-well plates in triplicate.
4.3 Western blots and antibodies
Cell lysis and western blots were carried out as described previously (Harris et al., 2007) and membranes probed with an antibody specific for the carboxyl terminus of human type XV collagen (Santa Cruz sc-16515) or the FLAG epitope (M2, Sigma F3165). For analysis of secreted forms of hcolXV and mutant constructs, media was conditioned by stable clones for 72h, concentrated to 1/5th the volume using an Amicon Ultra-4 column with Ultracel 10 or Ultracel-100 membranes (Millipore) followed by western blot analysis.
4.4 Subcutaneous injections of tumour cells in athymic mice
One million cells of each indicated cell line were injected subcutaneously into congenitally athymic (nude) mice to analyze tumor growth. Mice were sacrificed once tumors reached a diameter of 1.5 cm.
4.5 Cell adhesion assay
Cell adhesion assays were performed as described previously (Leir et al., 2003).
Supplementary Material
Highlights.
Collagen XV increases adhesion of cells to collagen I.
N-terminal/collagenous domains of collagen XV mediate collagen I adhesion.
The c-terminal Restin domain does not increase adhesion to collagen I.
Mutation of a cysteine residue in the collagenous domain disrupts adhesion.
Restin does not suppress tumorigenicity of cervical cancer cells in vivo.
Acknowledgments
This work was supported by NIH grants CA129258 to AH and P50CA0727 and U01CA111294 to MAH. We thank Dr Taina Pihlajaniemi, for the human colXV cDNA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amenta PS, Briggs K, Xu K, Gamboa E, Jukkola AF, Li D, Myers JC. Type XV collagen in human colonic adenocarcinomas has a different distribution than other basement membrane zone proteins. Hum Pathol. 2000;31:359–366. doi: 10.1016/s0046-8177(00)80251-8. [DOI] [PubMed] [Google Scholar]
- Amenta PS, Hadad S, Lee MT, Barnard N, Li D, Myers JC. Loss of types XV and XIX collagen precedes basement membrane invasion in ductal carcinoma of the female breast. J Pathol. 2003;199:298–308. doi: 10.1002/path.1303. [DOI] [PubMed] [Google Scholar]
- Amenta PS, Scivoletti NA, Newman MD, Sciancalepore JP, Li D, Myers JC. Proteoglycan-collagen XV in human tissues is seen linking banded collagen fibers subjacent to the basement membrane. J Histochem Cytochem. 2005;53:165–176. doi: 10.1369/jhc.4A6376.2005. [DOI] [PubMed] [Google Scholar]
- Brideau G, Makinen MJ, Elamaa H, Tu H, Nilsson G, Alitalo K, Pihlajaniemi T, Heljasvaara R. Endostatin overexpression inhibits lymphangiogenesis and lymph node metastasis in mice. Cancer Res. 2007;67:11528–11535. doi: 10.1158/0008-5472.CAN-07-1458. [DOI] [PubMed] [Google Scholar]
- Cooke VG, Kalluri R. Chapter 1. Molecular mechanism of type IV collagen-derived endogenous inhibitors of angiogenesis. Methods Enzymol. 2008;444:1–19. doi: 10.1016/S0076-6879(08)02801-2. [DOI] [PubMed] [Google Scholar]
- Evans EP, Burtenshaw MD, Brown BB, Hennion R, Harris H. The analysis of malignancy by cell fusion. IX. Re-examination and clarification of the cytogenetic problem. J Cell Sci. 1982;56:113–130. doi: 10.1242/jcs.56.1.113. [DOI] [PubMed] [Google Scholar]
- Fukushige T, Kanekura T, Ohuchi E, Shinya T, Kanzaki T. Immunohistochemical studies comparing the localization of type XV collagen in normal human skin and skin tumors with that of type IV collagen. J Dermatol. 2005;32:74–83. [PubMed] [Google Scholar]
- Gaetzner S, Deckers MM, Stahl S, Lowik C, Olsen BR, Felbor U. Endostatin’s heparan sulfate-binding site is essential for inhibition of angiogenesis and enhances in situ binding to capillary-like structures in bone explants. Matrix Biol. 2005;23:557–561. doi: 10.1016/j.matbio.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Hagg PM, Muona A, Lietard J, Kivirikko S, Pihlajaniemi T. Complete exon-intron organization of the human gene for the alpha1 chain of type XV collagen (COL15A1) and comparison with the homologous COL18A1 gene. J Biol Chem. 1998;273:17824–17831. doi: 10.1074/jbc.273.28.17824. [DOI] [PubMed] [Google Scholar]
- Harris A, Harris H, Hollingsworth MA. Complete suppression of tumor formation by high levels of basement membrane collagen. Mol Cancer Res. 2007;5:1241–1245. doi: 10.1158/1541-7786.MCR-07-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. Suppression of malignancy in hybrid cells: the mechanism. J Cell Sci. 1985;79:83–94. doi: 10.1242/jcs.79.1.83. [DOI] [PubMed] [Google Scholar]
- Harris H. Is collagen XV a tumor suppressor? DNA Cell Biol. 2003;22:225–226. doi: 10.1089/104454903321908601. [DOI] [PubMed] [Google Scholar]
- Harris H. A long view of fashions in cancer research. Bioessays. 2005;27:833–838. doi: 10.1002/bies.20263. [DOI] [PubMed] [Google Scholar]
- Harris H, Bramwell ME. The suppression of malignancy by terminal differentiation: evidence from hybrids between tumour cells and keratinocytes. J Cell Sci. 1987;87(Pt 3):383–388. doi: 10.1242/jcs.87.3.383. [DOI] [PubMed] [Google Scholar]
- Hurskainen M, Ruggiero F, Hagg P, Pihlajaniemi T, Huhtala P. Recombinant human collagen XV regulates cell adhesion and migration. The Journal of biological chemistry. 2010;285:5258–5265. doi: 10.1074/jbc.M109.033787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John H, Radtke K, Standker L, Forssmann WG. Identification and characterization of novel endogenous proteolytic forms of the human angiogenesis inhibitors restin and endostatin. Biochim Biophys Acta. 2005;1747:161–170. doi: 10.1016/j.bbapap.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Jonasson J, Povey S, Harris H. The analysis of malignancy by cell fusion. VII. Cytogenetic analysis of hybrids between malignant and diploid cells and of tumours derived from them. J Cell Sci. 1977;24:217–254. doi: 10.1242/jcs.24.1.217. [DOI] [PubMed] [Google Scholar]
- Leir SH, Holgate ST, Lackie PM. Inflammatory cytokines can enhance CD44-mediated airway epithelial cell adhesion independently of CD44 expression. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1305–1311. doi: 10.1152/ajplung.00255.2002. [DOI] [PubMed] [Google Scholar]
- Li D, Clark CC, Myers JC. Basement membrane zone type XV collagen is a disulfide-bonded chondroitin sulfate proteoglycan in human tissues and cultured cells. J Biol Chem. 2000;275:22339–22347. doi: 10.1074/jbc.M000519200. [DOI] [PubMed] [Google Scholar]
- Myers JC, Dion AS, Abraham V, Amenta PS. Type XV collagen exhibits a widespread distribution in human tissues but a distinct localization in basement membrane zones. Cell Tissue Res. 1996;286:493–505. doi: 10.1007/s004410050719. [DOI] [PubMed] [Google Scholar]
- Myers JC, Kivirikko S, Gordon MK, Dion AS, Pihlajaniemi T. Identification of a previously unknown human collagen chain, alpha 1(XV), characterized by extensive interruptions in the triple-helical region. Proc Natl Acad Sci U S A. 1992;89:10144–10148. doi: 10.1073/pnas.89.21.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Ramchandran R, Dhanabal M, Volk R, Waterman MJ, Segal M, Lu H, Knebelmann B, Sukhatme VP. Antiangiogenic activity of restin, NC10 domain of human collagen XV: comparison to endostatin. Biochem Biophys Res Commun. 1999;255:735–739. doi: 10.1006/bbrc.1999.0248. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Larsson H, Tisi D, Claesson-Welsh L, Hohenester E, Timpl R. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity. J Mol Biol. 2000;301:1179–1190. doi: 10.1006/jmbi.2000.3996. [DOI] [PubMed] [Google Scholar]
- Wirz JA, Boudko SP, Lerch TF, Chapman MS, Bachinger HP. Crystal structure of the human collagen XV trimerization domain: a potent trimerizing unit common to multiplexin collagens. Matrix biology : journal of the International Society for Matrix Biology. 2011;30:9–15. doi: 10.1016/j.matbio.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.