Abstract
Background
Previous reports suggest that peripheral airways are associated with asthma control. Patient history, although subjective is used largely to assess asthma control in children because spirometry is many times normal. Impulse oscillometry (IOS) is an objective non-invasive measurement of lung function, which has the potential to examine independently both small and large airway obstruction.
Objective
To determine the utility of IOS in assessing asthma control in children.
Methods
Asthmatic and healthy children (6–17 yrs) were enrolled in the study. Spirometry and IOS (resistance at 5 and 20 Hz, R5 and R20, respectively, reactance at 5 Hz, X5, resonant frequency, Fres, and area under the reactance curve between 5 Hz and Fres, AX) were collected in triplicate before and after a bronchodilator was administered. The physicians were blinded to the IOS measurements and assessed asthma control using ATS guidelines.
Results
Small airway IOS measurements, including R5-20, X5, Fres and AX, of children with uncontrolled asthma (n=44) were significantly different from those of controlled asthmatic (n=57) and healthy (n=14) children, especially prior to the administration of a bronchodilator. However, there was no difference in large airway IOS (R20). No differences were found between controlled asthmatic and healthy children in any of the endpoints. ROC analysis showed cut-points for baseline R5-20 (1.5 cmH2O·L−1·s) and AX (9.5 cmH2O·L−1) that effectively discriminated controlled versus uncontrolled asthma (AUC=0.86 and 0.84), and correctly classified more than 80% of the population.
Conclusion
Uncontrolled asthma is associated with small airways dysfunction, and IOS may be a reliable non-invasive method to assess asthma control in children.
Keywords: reactance, resistance, control, pediatric, lung function
INTRODUCTION
Asthma is a lung disease characterized by airway obstruction and is one of the most common chronic disorders in children. Early diagnosis and control of asthma in children is very important because appropriate treatments may impact the course of the disease. Current guidelines emphasize that treatment decisions should be based on achieving and maintaining asthma control (1). However, assessing asthma control in children is particularly challenging for many reasons including a discrepancy in perceived symptoms between the child and parents (2, 3), and the poor correlation between symptoms and traditional objective tests such as spirometry (4, 5). Therefore, the development of new, reliable, and non-invasive methods to assess of asthma control in children remains a priority and is essential for the effective treatment of asthma.
Increasing evidence indicates that peripheral airway function is associated with asthma control (6–10). Conventional spirometry is regarded as the gold standard assessment of airflow obstruction; however, it has a limited capacity to distinguish distal and proximal airways. For example, the most frequently used measurement (the forced expiratory volume in one second, FEV1) mainly reflects the large airways (11, 12), and the mid-forced expiratory flow (FEF25–75), believed to be a marker of small airways (13, 14), suffers from poor reproducibility (15). Finally, traditional spirometry requires the subject to perform forced expiratory maneuvers (i.e. effort-dependent), which is difficult for young children and also hampers reproducibility.
There are different techniques to detect small airway obstruction, such as heliox flow volume loops (16). However they generally require forced exhalation maneuvers which can be difficult for young children to perform. More recently, a much simpler technique, impulse oscillometry (IOS) has been increasingly used as a noninvasive method to assess airway resistance and reactance in children (17, 18). IOS requires minimal patient cooperation, is effort-independent, and separately quantifies the degree of obstruction in central and peripheral airways (19). IOS has been shown to be useful in the diagnosis of asthma (20, 21) and small airway impairment in children (7) however, studies on the utility of IOS to assess asthma control are limited, and there are no published cut-points for IOS measurements to determine asthma control in children. Therefore, the aim of the study was to investigate the utility of IOS in a pediatric population to detect uncontrolled asthma, and determine the cut-points that discriminate controlled versus uncontrolled asthma.
METHODS
Study participants
Children aged 6 to 17 years who were being actively treated for asthma on the Children’s Hospital of Orange County Breathmobile™ were enrolled in the study. The Breathmobile™ is a mobile asthma clinic that travels to schools, community clinics, and child development centers in low-income neighborhoods throughout Orange County, California and provides comprehensive asthma care to children who have asthma, or are at risk for asthma. Children were included in the study if they were 6–17 years of age and had a clinical diagnosis of asthma by a physician. Patients were excluded from the study if they were diagnosed with any other pulmonary or cardiac disease, had any history of smoking within 12 months of their enrollment, or if they were not able to perform a standard spirometry maneuver. Healthy children without history of asthma, allergies, or other lung diseases were also enrolled in the study as control subjects. The study was approved by the Institutional Review Boards of the University of California, Irvine and the Children’s Hospital of Orange County. Written informed consent and assent were obtained from all participants and their parents or guardians.
Protocol
All study procedures were performed on the Breathmobile™ vans (22). Participants received a nursing assessment to identify their health status, and skin prick testing of eight common allergens to assess atopic status. Categorization of atopic was based on a single positive wheel (3 millimeters greater than negative control). Each subject was required to report a complete symptom history during the past 6–8 weeks, which includes daytime symptoms, nighttime symptoms, exercise symptoms and exacerbations, etc.. Baseline IOS and standard spirometry maneuvers were performed in accordance with ATS/ERS standards (23). IOS was performed prior to spirometry to avoid influence of forced exhalation maneuvers on airway function (24). Albuterol (2 puffs; 180 mcg) was then administered from a metered dose inhaler with a spacer to assess bronchodilator responsiveness. Ten minutes after bronchodilator administration, spirometry and IOS measurements were repeated. Physicians were blinded to the IOS data. They evaluated the participants’ asthma severity, control, and treatment plan using criteria defined in the NAEPP/NHLBI guidelines (25), which included traditional spirometry. For age 5–11, controlled asthma is defined as ≤1/month nighttime symptoms, ≤2 days/wk daytime symptoms or SABA use, ≥80% FEV1 and FEV1/FVC and no interference with normal activities. For ages 12 and older, criteria for control are similar except ≤2/month nighttime symptoms.
Spirometry
Standard spirometry was performed in the sitting position using the Vmax Encore 20c spirometer (CareFusion Respiratory, Yorba Linda, CA). The best spirometric measures of at least 3 reproducible attempts were recorded for analysis. In accordance with ATS guidelines (23), reference values from the Third National Health and Nutrition Examination Study (NHANES III) were used to interpret spirometry results for participants aged 8–17 years (26). For participants younger than 8 years, Morris/Polgar reference values were used (27).
Impulse Oscillometry (IOS)
The Vmax Encore 20c is fully integrated with an IOS system. IOS requires the subject to breath normally (tidal breathing) into a mouthpiece, while a loudspeaker generates an impulse shaped pressure signal into the respiratory system. The IOS system was calibrated each day prior to the measurements using a 3-liter syringe. IOS measurements were performed in the sitting position with participants wearing nose clips. Participants tidally breathed into the IOS mouthpiece for 30 seconds with the cheeks supported by the hands of trained technicians. The technicians evaluated the efforts and made sure each observation consisted of at least 3 reproducible maneuvers which did not have artifacts caused by coughing, swallowing, vocalization or breath holding.
LabManager Version 4.67.0.1 (CareFusion Germany GmbH, Hoechberg, Germany) was used to calculate the pressure-flow relationship and calculate the resistance and reactance of the respiratory system as a function of oscillation frequency. The representative tracing and definitions of the IOS indices including R5, R20, X5, Fres and AX are presented schematically (Fig. 1). Acceptable coherence values (r2>0.6 at 5Hz and r2>0.9 at 10Hz and higher frequencies) were used as recommended (28) to exclude non-linear data. Results were acceptable if the coefficient of variation of at least 2 sets of data was < 10%. Mean values of R5, R20, X5, Fres and AX calculated from the measurements were used for further analysis.
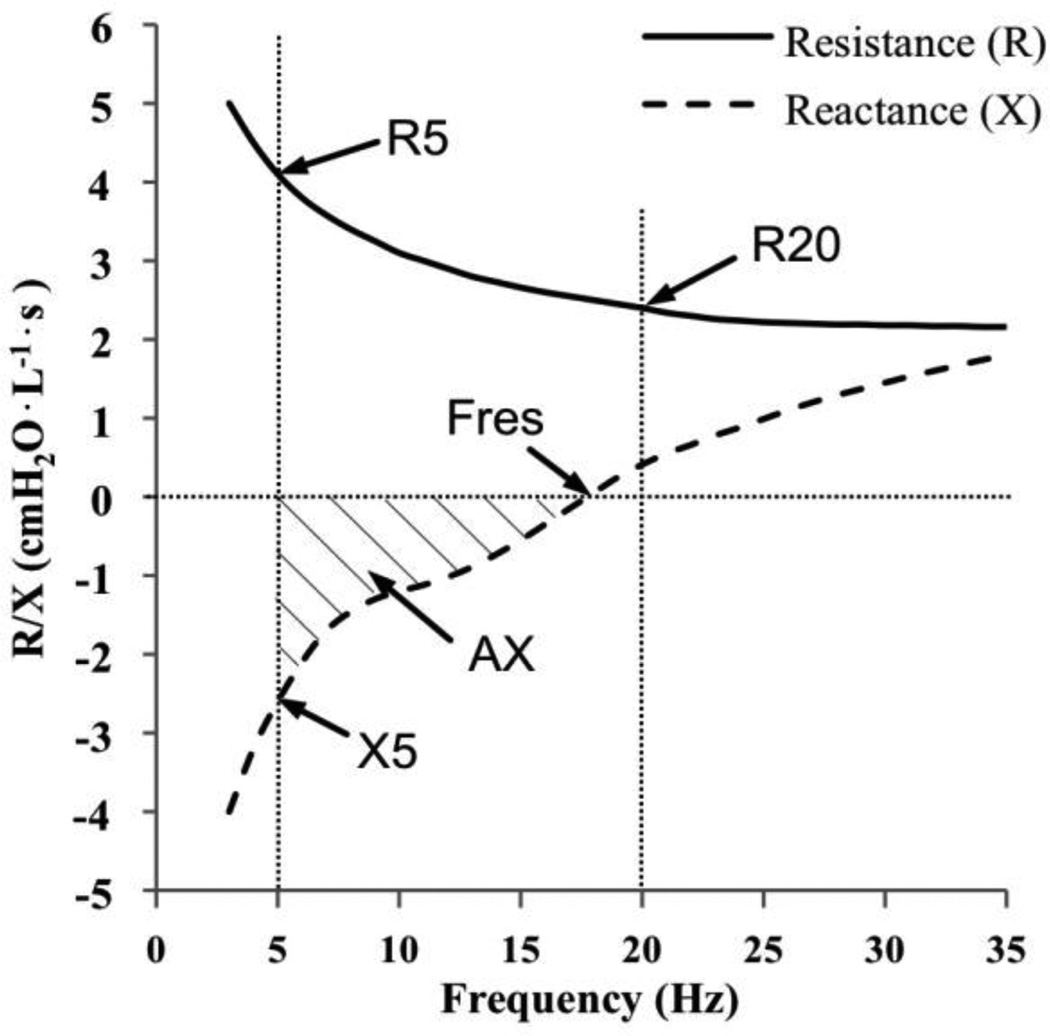
FIG 1.
Schematic illustration of IOS indices over oscillation frequency, including R5, R20, Fres, X5 and AX.
The resistance (R) is the in-phase component of the lung impedance. Because low oscillation frequencies (<15Hz) can be transmitted more distally in the lungs compared to higher frequencies (19), R5 reflects obstruction in both small and large airways, R20 reflects large airways only, and R5-20 is an index of the small airways only (29). The resistance will become more frequency-dependent if peripheral resistance increases (30). Reactance (X) is the out-of-phase component related to the capacitative and the inertive properties of the airways. At low frequencies, capacitative pressure loss is large compared to inertive pressure loss, while at higher frequencies the inertive properties dominate. The intermediate frequency at which the total reactance is 0 is known as the resonant frequency (Fres), when the magnitudes of the capacitative and inertive pressure loss are the same. AX is the total reactance (area under the curve) at all frequencies between 5Hz and Fres (Fig. 1). Thus, X5, Fres and AX all reflect changes in the degree of obstruction in the peripheral airways (19).
Sample size and statistical analysis
Gaylor et al (31) reported a 20–30% decrease in the frequency-dependence of resistance and Saadeh et al (32) found a 40%–50% decrease in AX after inhaled corticosteroid treatment. Thus, we estimated a difference in distal airway IOS of 35% between controlled and uncontrolled asthma pre bronchodilator. Based on this difference, a sample size of 44 subjects in each asthmatic group is needed to provide 90% statistical power to detect a 35% difference at a significance level of 0.05 using one-way analysis of variance.
Because of the non-normal distributions of the measurements and relatively small sample size, the parameters were summarized by medians with ranges, unless indicated otherwise. The non-parametric Mann-Whitney U Test was used to detect the difference of the outcomes between groups. The Paired Wilcoxon Signed Rank Test was applied to test the difference before and after bronchodilator within groups. The receiver operating characteristic (ROC) method was conducted to evaluate the utility of different oscillometric variables in distinguishing children with uncontrolled asthma from controlled asthma. ROC areas with estimated standard errors were calculated for each of the IOS and spirometry variables. In addition, optimized IOS cut-points were calculated, and sensitivity and specificity, positive predictive and negative predictive values, and the correctly classified ratio were estimated at each of the cut-points. General linear regression and analysis of variance (ANOVA) were later applied to describe the relationships between small airway IOS versus asthma control and demographic parameters. The criterion for this analysis was physicians’ assessed asthma control status which included standard spirometry. The statistical analyses were made using R package (2.11.0). Statistical significance was established at P-value < .05.
RESULTS
Study sample
A total of 14 healthy controls and 107 asthmatic subjects were consented for the study. 101 (94%) of the asthmatics were able to perform acceptable IOS maneuvers; 6 patients were excluded from the study because their IOS measurements had coherence lower than the recommended values. Based on physicians’ assessment, 57 (56%) of the 101 asthmatic subjects had controlled asthma and 44 (44%) had uncontrolled asthma. The demographics of the three asthma groups are presented (Table I). The majority of our study population identified themselves as Hispanic (71% of healthy controls and 82% of asthmatics). Of the asthmatics, both controlled and uncontrolled, 77% had positive skin test results, and were categorized as atopic. 92% of the asthmatic patients were diagnosed with mild to moderate asthma. Unpaired Mann-Whitney U Tests showed no statistical difference in age, gender, height, or weight across groups. There was no statistical difference between controlled and uncontrolled asthma in the step level of management. However, the body mass index for uncontrolled asthma was higher compared to controlled and healthy subjects (P-value < 0.05).
TABLE I.
Demographics for different asthma status
| Asthma status | P value* | |||||
|---|---|---|---|---|---|---|
| Healthy (n=14) |
Controlled (n=57) |
Uncontrolled (n=44) |
H vs. C | H vs. U | C vs. U | |
| Age, years | 13 | 12 | 11 | .6945 | .6807 | .4050 |
| Male/Female, % | 36/64 | 51/49 | 59/41 | .3163 | .1327 | .4157 |
| Height, cm | 156 | 154 | 151 | .6962 | .2373 | .2525 |
| Weight, kg | 50 | 51 | 54 | .9137 | .5487 | .2837 |
| Body mass index | 20.9 | 20.8 | 23.8 | .7560 | .0299 | .0086 |
| Atopic, % | 0 | 77 | 77 | <.0001 | <.0001 | .8831 |
| Medication step, % Non-compliant/1/2/3/4 |
27/12/35/21/5 | 27/18/34/16/5 | .5295 | |||
Demographic measurements are presented as median.
Mann-Whitney U Test was applied to detect the group difference between healthy (H) vs. controlled asthma (C), healthy vs. uncontrolled asthma (U) and controlled asthma vs. uncontrolled asthma.
Standard Spirometry
Standard spirometry was compared between healthy, controlled asthma and uncontrolled asthma (Table II). Spirometry was very similar for healthy and controlled asthma. The FEF25–75, FEV1 (% predicted), FEF25–75 (% predicted), and the ratio of FEV1/FVC were higher in healthy and controlled asthma compared to uncontrolled asthma. Bronchodilator response (BDR) of FEV1 (% change from baseline) in healthy and controlled asthma was statistically lower than uncontrolled asthma. Although significant differences were detected, the sensitivities of spirometry outcomes for assessing uncontrolled asthma were low, especially for FEV1 and BDR. In the uncontrolled asthma group, there were 42 (95%), 16 (36%), 17 (39%) and 28 (64%) subjects who had FEV1%predicted, FEF25–75%predicted, FEV1/FVC and BDR, respectively, within the normal range based on the guidelines (25, 33).
TABLE II.
Standard spirometry for different asthma status
| Asthma status | P value* | |||||
|---|---|---|---|---|---|---|
| Healthy (n=14) |
Controlled (n=57) |
Uncontrolled (n=44) |
H vs. C | H vs. U | C vs. U | |
| FVC, L | 3.3 | 3.1 | 3.1 | .9819 | .7359 | .6662 |
| FEV1, L | 3.0 | 2.7 | 2.4 | .6130 | .0982 | .0587 |
| FEF25–75, L · s−1 | 3.1 | 3.0 | 2.3 | .2914 | .0008 | <.0001 |
| FEV% predicted | 102 | 106 | 107 | .2140 | .2477 | .7365 |
| FEV1%predicted† | 104 (100) | 100 (95) | 94 (95) | .4391 | .0195 | .0196 |
| FEF25–75%predicted† | 100 (100) | 92 (96) | 74 (36) | .1218 | .0001 | <.0001 |
| FEV1/FVC, %† | 89 (93) | 87 (79) | 79 (39) | .2102 | <.0001 | <.0001 |
| BDR, %† | 1.6 (100) | 3.2 (95) | 6.4 (64) | .3145 | .0046 | .0009 |
Spirometry measurements are presented as median.
Percentage of patients with the spirometry parameter in normal range are presented in parentheses. FEV1%predicted lower than 80% of predicted, FEF25–75%predicted lower than 65% predicted, FEV1/FVC lower than 80%, or BDR higher than 10% are considered as abnormal.
Mann-Whitney U Test was applied to detect the group difference between healthy (H) vs. controlled asthma (C), healthy vs. uncontrolled asthma (U) and controlled asthma vs. uncontrolled asthma.
IOS
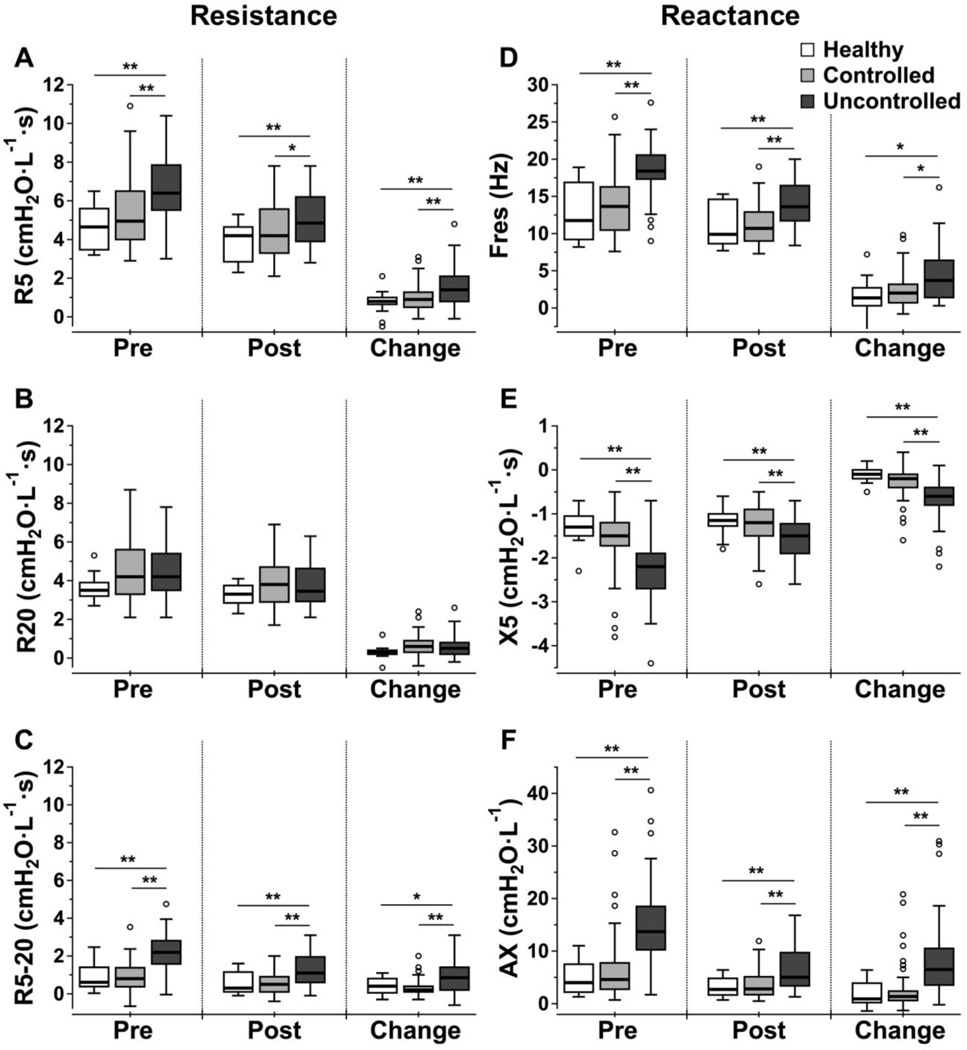
The comparison of IOS measurements between the three groups pre- and post-bronchodilator administration, and the bronchodilator response are presented using box plots (Fig. 2). Healthy subjects and controlled asthmatics had no statistical differences in IOS measurements. For uncontrolled asthma, R20 was also not different from healthy or controlled asthma. However, R5, R5-20, Fres, X5, and AX were all statistically different in uncontrolled asthma compared to healthy and controlled asthma. For each of the five indices, the most significant differences were detected pre-bronchodilator administration. Paired Wilcoxon Signed Rank Tests showed that all IOS outcomes were significantly improved after bronchodilator in all three groups.
FIG 2.
Box plots of IOS measurements (A. R5, B. R20, C. R5-20, D. Fres, E. X5 and F. AX) for different asthma groups before, after bronchodilator and the bronchodilator response. The boxes represent 25th –75th percentile with median, and the top and bottom tails represent the highest/lowest scores without outliers. An outlier is defined as any value that lies more than 1.5 times the interquartile range from either end of the box. Significance level of group difference using unpaired Mann-Whitney U test: * P-value < .05; ** P-value < .01.
Distinguishing Uncontrolled and Controlled Asthma
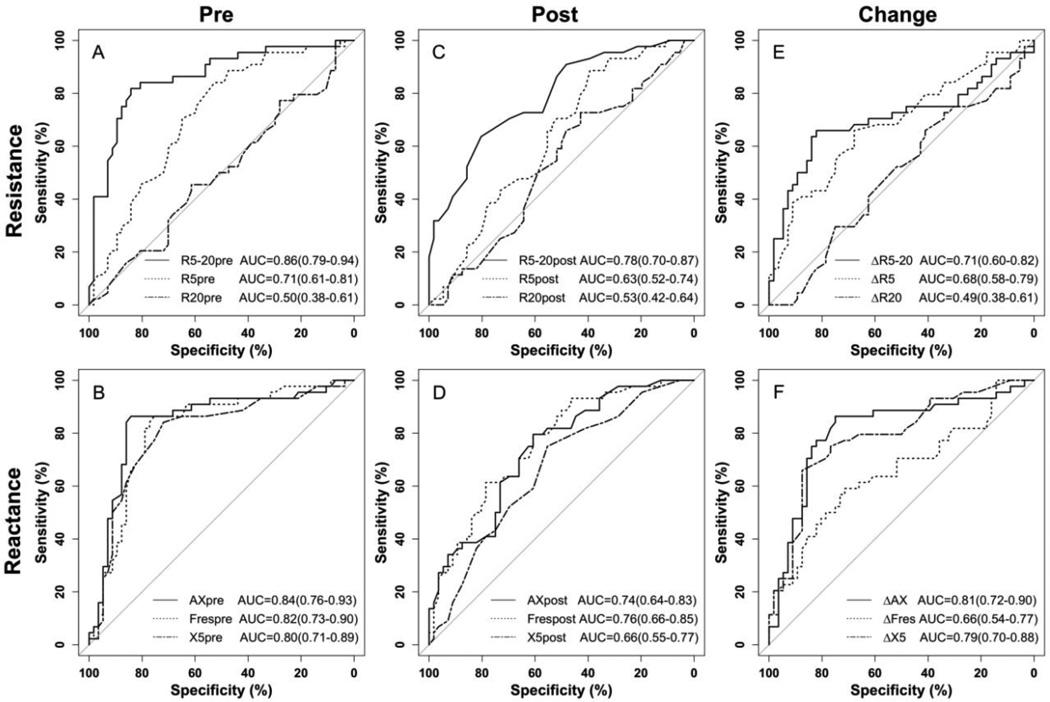
The discriminative properties of the oscillometric variables to distinguish uncontrolled from controlled asthma patients are shown using ROC (Fig. 3). Pre-bronchodilator, the estimated area under the curve (AUC) for R5-20, R5 and R20 were 0.86, 0.71 and 0.5, respectively. The AUC for AX, Fres and X5 pre-bronchodilator were all above 0.8, with AX being slightly better than the other two. Post-bronchodilator, the AUC for R5-20, AX, and Fres decreased below 0.8, R5 and X5 decreased below 0.7 and R20 remained near 0.5. The trends for the bronchodilator response (change from baseline) for the three resistances were similar to those of the post-bronchodilator values. For the bronchodilator response of the reactance indices, the AUC for ΔAX (0.81), where Δ refers to the change from baseline, and ΔX5 (0.79) were similar to the AUC for pre-bronchodilator while the AUC for ΔFres decreased to 0.66.
FIG 3.
ROC curves of IOS measurements in predicting physicians’ assessed uncontrolled asthma, including resistance (A) and reactance (B) before bronchodilator, resistance (C) and reactance (D) after bronchodilator and bronchodilator response of resistance (E) and reactance (F). R5-20, X5, Fres, AX before bronchodilator and bronchodilator response of AX all predict asthma control status (area under the curve > 0.8). AUCs are presented as mean (95% confidence interval)
The receiving operating curves were used to determine the performance of the optimized IOS cut-points in screening uncontrolled from controlled asthma for pre-bronchodilator and the bronchodilator response indices (Table III). The cut-points were selected by maximizing the sum of sensitivity and specificity. Pre-bronchodilator, the best indices were R5-20 and AX, which correctly classified 83.2% and 85.1% of the patients at a cut-point of 1.5 cmH20·L−1.s and 9.5 cmH20·L−1, respectively. These cut-points also had positive and negative predictive values > 0.80.
TABLE III.
Performance of IOS cut-points in screening uncontrolled vs. controlled asthma
| Cut- points† |
Sensitivity | Specificity | PPV(%) | NPV(%) | Correctly classified (%) |
AUC | |
|---|---|---|---|---|---|---|---|
| Before bronchodilator | |||||||
| R5 | 5.2 | 0.84 | 0.53 | 57.8 | 81.1 | 66.3 | 0.71 |
| R5-20 | 1.5 | 0.82 | 0.84 | 80.0 | 85.7 | 83.2 | 0.86 |
| Fres | 16.0 | 0.86 | 0.68 | 67.9 | 86.7 | 76.2 | 0.82 |
| X5 | −1.8 | 0.84 | 0.72 | 69.8 | 85.4 | 77.2 | 0.80 |
| AX | 9.5 | 0.86 | 0.84 | 80.9 | 88.9 | 85.1 | 0.84 |
| Bronchodilator response | |||||||
| ΔR5 | 1.0 | 0.68 | 0.59 | 56.6 | 70.2 | 63.0 | 0.68 |
| ΔR5-20 | 0.6 | 0.66 | 0.82 | 74.4 | 75.4 | 75.0 | 0.71 |
| ΔFres | 3.0 | 0.59 | 0.66 | 57.8 | 67.2 | 63.0 | 0.66 |
| ΔX5 | −0.5 | 0.71 | 0.79 | 72.1 | 77.2 | 75.0 | 0.79 |
| ΔAX | 2.7 | 0.86 | 0.75 | 73.1 | 87.5 | 75.0 | 0.81 |
Cut-points of R5, R5-20, and X5 are cmH2O·L−1·s, cut-point of Fres is Hz and cut-point of AX is cmH2O·L−1. The cut-points were selected by maximizing the total of sensitivity and specificity. Correctly classified ratios higher than 80% and AUCs above 0.80 are in bold.
PPV is positive predictive value; NPV is negative predictive value; AUC is area under the curve.
The best index for the bronchodilator response was ΔAX, which correctly classified 75% of the patients at a cut-point of 2.7, with a positive predictive value and negative predictive value of 73.1% and 87.5%, respectively. Therefore, the bronchodilator response of AX was not as useful as AX pre bronchodilator in screening for uncontrolled asthma. The cut-points for the change in other IOS parameters before and after bronchodilator had AUCs lower than 0.8 and were not good for discriminating asthma control.
DISCUSSION
Our study compared IOS indices of small and large airway resistance and reactance in children with controlled and uncontrolled asthma and established cut-points to identify uncontrolled asthma. Pre-bronchodilator (or baseline) values for small airway resistance (R5-R20) and reactance (AX) performed best, resulting in values for the sensitivity, specificity, positive predictive value, and negative predictive value which all exceeded 0.80. To our knowledge, this is the first study to investigate the utility of IOS parameters to determine asthma control status in a pediatric population. Our results suggest that indices from IOS are useful in determining control status in asthmatic children and add additional information to standard spirometry.
Resistance versus reactance
Previous investigators have shown that peripheral or small airway function evaluated by IOS correlates with healthy status and asthma symptoms in children and adults (9, 34, 35), which is consistent with our results in children. We compared the utility of four peripheral airway variables (R5-20, Fres, X5 and AX) from IOS, which characterize both airways resistance and reactance, in distinguishing asthma control. The results suggested that elevated indices representing both resistance (R5-20) and reactance (AX) were the best indicators of uncontrolled asthma. This suggests that both a decrease in small airway caliber and an increase in airway wall tone contribute to asthmatic symptoms in children. The resistance to flow through a tube is inversely related to the radius of the tube to the fourth power (36); thus, a larger pressure is required to force air through a tube of smaller diameter. In contrast, AX reflects the reactance of the peripheral airways at low frequencies, and thus reflects the ability of the peripheral lung to store capacitative energy. As the peripheral lung becomes less compliant (stiffer), it cannot store as much capacitative energy, and requires a larger pressure to inflate. Thus, an increase in small airway wall tone will decrease (larger negative value) the reactance and increase AX.
R5-20 and AX at baseline are strongly correlated (R2 =0.837), which is consistent with previous reports (19, 30). Airway resistance and reactance are likely coupled, as, at equivalent airway pressures, a stiffer small airway will have a smaller caliber, which would increase the resistance to flow. In either case, the increase in resistance and reactance of the small airways results in a larger pressure during inspiration to inflate the lungs. A larger pressure requires more exertion by the respiratory muscles, and is thus the probable mechanism underlying the relationship between the IOS parameters and asthma control. Therefore, as indices determining asthma control, R5-20 and AX do not provide independent information.
The enhanced discriminatory power of AX relative to the other parameters that reflect reactance in the small airways (Fres and X5) is likely due to the fact that AX is an index that captures the integrated response over the entire range of low frequencies (Fig. 1) (18, 37, 38). As a result, AX is less variable than the reactance at a specific frequency as is the case for both Fres and X5. This is supported by previous work that demonstrates a large variance for X5 in children (24, 34).
Healthy versus controlled asthma
Our study demonstrates that the controlled asthma group and healthy controls have no differences in any of the IOS measurements (Fig. 2). In contrast, studies have shown that the IOS parameters at baseline were statistically different between children with and without asthma (20, 39, 40). However, these latter studies did not consider asthma control. A potential limitation of our study is a relatively small number of normal subjects, which could fail to detect more subtle differences between healthy children and controlled asthmatics.
Bronchodilator response
Previous reports have shown that the IOS-assessed bronchodilator response was useful in discriminating healthy versus asthmatic children (20, 21, 34). This is consistent with our results; however, our results suggest that baseline values of IOS are even more effective at detecting uncontrolled asthma. This is different compared to the traditional bronchodilator response (percent change in FEV1) that has been shown to be a more sensitive indicator of asthma control compared to baseline spirometry (41). This difference may be related to the techniques, the population, status of control, and the fact that IOS can distinguish small and large airways as well as airways resistance and reactance.
Finally, we chose to use the change in the absolute value of the IOS parameters to define the bronchodilator response instead of the percent change, which is commonly used for FEV1. This choice is based on the fact that IOS indices (e.g., AX) increase as asthma symptoms increase, thus creating a larger baseline value, and decrease following administration of a bronchodilator. In contrast, indices from traditional spirometry (e.g., FEV1) decrease with increasing asthma symptoms creating a smaller baseline. Thus, the percent change for IOS will tend to be smaller than traditional spirometry, and the effect of the bronchodilator blunted.
Spirometry versus IOS
Numerous studies have investigated the correlation between traditional spirometry and IOS. For example, R5 correlates with FEV1 at (42, 43) at baseline and during mannitol or methacholine challenge (44, 45). Although FEV1 is the most widely used test for airflow obstruction, it is generally considered to be an index of large airway caliber. In our study, no differences in FEV1 were detected between controlled and uncontrolled asthma, and we found a large proportion (95%) of asthmatic children whose FEV1%predicted was in the normal range (> 80%predicted) despite a physician diagnosis of uncontrolled asthma. One possible explanation is that asthma control status primarily reflects small or peripheral airway obstruction. Alternatively, FEF25–75 is considered to be a more specific marker for obstruction in the distal airways. Our results suggest that FEF25–75%predicted was more sensitive in detecting uncontrolled asthma compared to FEV1, as a lower percentage (36%) of children with uncontrolled asthma were above the normal cutoff (65%predicted) (33). These observations are consistent with our findings in IOS in which only those indices that reflect the small airways could predict asthma control. However, neither FEV1 nor FEF25–75 was as effective as small airway IOS indices in detecting poorly controlled asthma.
Finally, although not rigorously correct since the physician used spirometry as part of the criteria to determine control, we performed additional ROC analysis to gauge the performance of spirometry in detecting uncontrolled asthma. The AUCs for FEF25–75, FEF25–75% predicted, FEV1/FVC and BDR (0.74, 0.79, 0.81 and 0.69, respectively) were all lower than small airway IOS indices or resistance and reactance, despite the fact that spirometry was part of the criteria used by the physician to assess control.
Cut-point values of IOS to discriminate asthma control
Our study was able to determine cut-point values of R5-20 and AX for discriminating asthma control using the absolute value of each index. However, the cut-point values might be affected by other variables such as age, gender, height, weight, BMI and race. Previous studies have shown that IOS measurements correlate with age, gender and height (46–49). In our study, analysis of variance showed that R5-20 or AX had no correlation with gender, weight, or BMI, but did correlate with age and height (P-value < 0.01). Thus, caution should be exercised in using absolute values for cut-points in children who differ in age or height. Furthermore, our population of children was primarily of Hispanic ethnicity, which has been shown to impact baseline values of traditional spirometry (26). There are limited IOS references for baseline values in healthy children for our study age group, and thus additional data is necessary before cut-points expressed as a percent-predicted of normal can be utilized.
Conclusion
The standard asthma history, which incorporates impairment and risk factors as defined by NAEPP guidelines, remains a subjective tool in assessing control. Standard spirometric criteria provide important objective information, but values are usually normal in children with mild to moderate asthma. In addition, spirometry may not accurately reflect small airway dysfunction, which is an important determinant of asthma control. As suggested by our study, IOS, which measures small airway obstruction, can provide additional objective information useful for assessing asthma control in children as an adjunct to the traditional history and spirometry.
Clinical Implication/Key message.
Small airway indices of impulse oscillometry (IOS) identify children with uncontrolled asthma, and thus may be useful in the clinical assessment of asthma control.
ACKNOWLEDGEMENTS
This work was funded by a grant from the National Institutes of Health (R01 HL070645). The authors would also like to thank Michael D. Goldman, MD (in memoriam), Geffen School of Medicine, UCLA, and David Sinks, Director, Technical Marketing of Carefusion, for their expertise in the IOS instrumentation as well as their input and discussions of the clinical application of IOS. The authors would also like to thank the staff of the Children’s Hospital of Orange County Breathmobile, including Jennifer Nguyen, BA, Olga Guijon, MD, and Linh Pham, MD, for their collaborative efforts during data collection and analysis.
Abbreviations used
- BDR
Bronchodilator response of FEV1
- IOS
Impulse oscillometry
- R5
Resistance of the respiratory system at 5Hz
- R20
Resistance of the respiratory system at 20Hz
- R5-20
The difference of R5 and R20
- Fres
Resonant frequency of reactance
- X5
Reactance of the respiratory system at 5Hz
- AX
Reactance Area
- ROC
Receiver operating characteristic
- AUC
Area under the curve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008 Jan;31(1):143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 2.Carroll WD, Wildhaber J, Brand PL. Parent misperception of control in childhood/adolescent asthma: The room to breathe survey. Eur Respir J. 2011 Jun 23; doi: 10.1183/09031936.00048911. [DOI] [PubMed] [Google Scholar]
- 3.Davis KJ, Disantostefano R, Peden DB. Is Johnny wheezing? Parent-child agreement in the Childhood Asthma in America survey. Pediatr Allergy Immunol. 2011 Feb;22 (1 Pt 1):31–35. doi: 10.1111/j.1399-3038.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- 4.Spahn JD, Cherniack R, Paull K, Gelfand EW. Is forced expiratory volume in one second the best measure of severity in childhood asthma? Am J Respir Crit Care Med. 2004 Apr 1;169(7):784–786. doi: 10.1164/rccm.200309-1234OE. [DOI] [PubMed] [Google Scholar]
- 5.Teeter JG, Bleecker ER. Relationship between airway obstruction and respiratory symptoms in adult asthmatics. Chest. 1998 Feb;113(2):272–277. doi: 10.1378/chest.113.2.272. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima N, Mochizuki H, Muramatsu R, Hagiwara S, Mizuno T, Arakawa H. Relationship between exhaled nitric oxide and small airway lung function in normal and asthmatic children. Allergol Int. 2011 Mar;60(1):53–59. doi: 10.2332/allergolint.10-OA-0215. [DOI] [PubMed] [Google Scholar]
- 7.Meraz EG, Nazeran H, Ramos CD, Nava P, Diong B, Goldman MD, et al. Analysis of impulse oscillometric measures of lung function and respiratory system model parameters in small airway-impaired and healthy children over a 2-year period. Biomed Eng Online. 2011;10:21. doi: 10.1186/1475-925X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin RJ. Therapeutic significance of distal airway inflammation in asthma. J Allergy Clin Immunol. 2002 Feb;109(2 Suppl):S447–S460. doi: 10.1067/mai.2002.121409. [DOI] [PubMed] [Google Scholar]
- 9.Takeda T, Oga T, Niimi A, Matsumoto H, Ito I, Yamaguchi M, et al. Relationship between small airway function and health status, dyspnea and disease control in asthma. Respiration. 2010;80(2):120–126. doi: 10.1159/000242113. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J, Douma WR, ten Hacken NH, Vonk JM, Oudkerk M, Postma DS. Ciclesonide improves measures of small airway involvement in asthma. Eur Respir J. 2008 Jun;31(6):1213–1220. doi: 10.1183/09031936.00082407. [DOI] [PubMed] [Google Scholar]
- 11.Annesi I, Oryszczyn MP, Neukirch F, Orvoen-Frija E, Korobaeff M, Kauffmann F. Relationship of upper airways disorders to FEV1 and bronchial hyperresponsiveness in an epidemiological study. Eur Respir J. 1992 Oct;5(9):1104–1110. [PubMed] [Google Scholar]
- 12.Sackner MA. Physiologic features of upper airway obstruction. Chest. 1972 Oct;62(4):414–417. doi: 10.1378/chest.62.4.414. [DOI] [PubMed] [Google Scholar]
- 13.Marseglia GL, Cirillo I, Vizzaccaro A, Klersy C, Tosca MA, La Rosa M, et al. Role of forced expiratory flow at 25%–75% as an early marker of small airways impairment in subjects with allergic rhinitis. Allergy Asthma Proc. 2007 Jan-Feb;28(1):74–78. doi: 10.2500/aap.2007.28.2920. [DOI] [PubMed] [Google Scholar]
- 14.Cirillo I, Klersy C, Marseglia GL, Vizzaccaro A, Pallestrini E, Tosca M, et al. Role of FEF25%–75% as a predictor of bronchial hyperreactivity in allergic patients. Ann Allergy Asthma Immunol. 2006 May;96(5):692–700. doi: 10.1016/S1081-1206(10)61067-8. [DOI] [PubMed] [Google Scholar]
- 15.Studnicka M, Frischer T, Neumann M. Determinants of reproducibility of lung function tests in children aged 7 to 10 years. Pediatr Pulmonol. 1998 Apr;25(4):238–243. doi: 10.1002/(sici)1099-0496(199804)25:4<238::aid-ppul4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Gelb AF, Klein E. The volume of isoflow and increase in maximal flow at 50 percent of forced vital capacity during helium-oxygen breathing as tests of small airway dysfunction. Chest. 1977 Mar;71(3):396–399. doi: 10.1378/chest.71.3.396. [DOI] [PubMed] [Google Scholar]
- 17.Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol. 2003 Aug;112(2):317–322. doi: 10.1067/mai.2003.1627. [DOI] [PubMed] [Google Scholar]
- 18.Larsen GL, Morgan W, Heldt GP, Mauger DT, Boehmer SJ, Chinchilli VM, et al. Impulse oscillometry versus spirometry in a long-term study of controller therapy for pediatric asthma. J Allergy Clin Immunol. 2009 Apr;123(4) doi: 10.1016/j.jaci.2008.10.036. 861-7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman MD, Saadeh C, Ross D. Clinical applications of forced oscillation to assess peripheral airway function. Respir Physiol Neurobiol. 2005 Aug 25;148(1–2):179–194. doi: 10.1016/j.resp.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Song TW, Kim KW, Kim ES, Park JW, Sohn MH, Kim KE. Utility of impulse oscillometry in young children with asthma. Pediatr Allergy Immunol. 2008 Dec;19(8):763–768. doi: 10.1111/j.1399-3038.2008.00734.x. [DOI] [PubMed] [Google Scholar]
- 21.Jee HM, Kwak JH, Jung da W, Han MY. Useful parameters of bronchial hyperresponsiveness measured with an impulse oscillation technique in preschool children. J Asthma. 2010 Apr;47(3):227–232. doi: 10.3109/02770901003624259. [DOI] [PubMed] [Google Scholar]
- 22.Liao O, Morphew T, Amaro S, Galant SP. The Breathmobile: a novel comprehensive school-based mobile asthma care clinic for urban underprivileged children. J Sch Health. 2006 Aug;76(6):313–319. doi: 10.1111/j.1746-1561.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005 Aug;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 24.Goldman MD, Carter R, Klein R, Fritz G, Carter B, Pachucki P. Within- and between- day variability of respiratory impedance, using impulse oscillometry in adolescent asthmatics. Pediatr Pulmonol. 2002 Oct;34(4):312–319. doi: 10.1002/ppul.10168. [DOI] [PubMed] [Google Scholar]
- 25.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007 Nov;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 26.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999 Jan;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 27.Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis. 1971 Jan;103(1):57–67. doi: 10.1164/arrd.1971.103.1.57. [DOI] [PubMed] [Google Scholar]
- 28.Smith RR HJ, Goldman MD. Forced oscillation technique and impulse oscillometry. European Respiratory Monograph. 2003:1–34. [Google Scholar]
- 29.Grimby G, Takishima T, Graham W, Macklem P, Mead J. Frequency dependence of flow resistance in patients with obstructive lung disease. J Clin Invest. 1968 Jun;47(6):1455–1465. doi: 10.1172/JCI105837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oostveen E, MacLeod D, Lorino H, Farre R, Hantos Z, Desager K, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003 Dec;22(6):1026–1041. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 31.Gaylor P, Saadeh CK, Goldman M, Malacara JM, McGee M, Reyes B. Forced oscillation using impulse oscillometry (IOS) provides objective responses to inhaled corticosteroids (ICS) in asthmatic patients when FEV1 fails to improve. Journal of Allergy and Clinical Immunology. 2003 Feb;111:S135. (2 Abstract Supplement) [Google Scholar]
- 32.Saadeh CK, Goldman M, Gaylor P, Malacara JM, McGee M, Gaylor M, et al. Forced oscillation using Impulse Oscillometry (IOS) detects false negative spirometry in symptomatic patients with reactive airways. Journal of Allergy and Clinical Immunology. 2003 Feb;111:S136. (2 Abstract Supplement) [Google Scholar]
- 33.Wang X, Dockery DW, Wypij D, Gold DR, Speizer FE, Ware JH, et al. Pulmonary function growth velocity in children 6 to 18 years of age. Am Rev Respir Dis. 1993 Dec;148(6 Pt 1):1502–1508. doi: 10.1164/ajrccm/148.6_Pt_1.1502. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen KG, Bisgaard H. Discriminative capacity of bronchodilator response measured with three different lung function techniques in asthmatic and healthy children aged 2 to 5 years. Am J Respir Crit Care Med. 2001 Aug 15;164(4):554–559. doi: 10.1164/ajrccm.164.4.2006119. [DOI] [PubMed] [Google Scholar]
- 35.Hozawa S, Terada M, Hozawa M. Comparison of budesonide/formoterol Turbuhaler with fluticasone/salmeterol Diskus for treatment effects on small airway impairment and airway inflammation in patients with asthma. Pulm Pharmacol Ther. 2011 May;23 doi: 10.1016/j.pupt.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Pfitzner J. Poiseuille and His Law. Anaesthesia. 1976;31(2):273–275. doi: 10.1111/j.1365-2044.1976.tb11804.x. [DOI] [PubMed] [Google Scholar]
- 37.van Noord JA, Clement J, van de Woestijne KP, Demedts M. Total respiratory resistance and reactance as a measurement of response to bronchial challenge with histamine. Am Rev Respir Dis. 1989 Apr;139(4):921–926. doi: 10.1164/ajrccm/139.4.921. [DOI] [PubMed] [Google Scholar]
- 38.Meraz EG, Nazeran H, Ramos CD, Nava P, Diong B, Goldman MD, et al. Analysis of impulse oscillometric measures of lung function and respiratory system model parameters in small airway-impaired and healthy children over a 2-year period. Biomed Eng Online. 2011;10:43. doi: 10.1186/1475-925X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paredi P, Goldman M, Alamen A, Ausin P, Usmani OS, Pride NB, et al. Comparison of inspiratory and expiratory resistance and reactance in patients with asthma and chronic obstructive pulmonary disease. Thorax. 2010 Mar;65(3):263–267. doi: 10.1136/thx.2009.120790. [DOI] [PubMed] [Google Scholar]
- 40.Malmberg LP, Pelkonen AS, Haahtela T, Turpeinen M. Exhaled nitric oxide rather than lung function distinguishes preschool children with probable asthma. Thorax. 2003 Jun;58(6):494–499. doi: 10.1136/thorax.58.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galant SP, Morphew T, Newcomb RL, Hioe K, Guijon O, Liao O. The relationship of the bronchodilator response phenotype to poor asthma control in children with normal spirometry. J Pediatr. 2011 Jun;158(6) doi: 10.1016/j.jpeds.2010.11.029. 953-9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song TW, Kim KW, Kim ES, Kim KE, Sohn MH. Correlation between spirometry and impulse oscillometry in children with asthma. Acta Paediatr. 2008 Jan;97(1):51–54. doi: 10.1111/j.1651-2227.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 43.Olaguibel JM, Alvarez-Puebla MJ, Anda M, Gomez B, Garcia BE, Tabar AI, et al. Comparative analysis of the bronchodilator response measured by impulse oscillometry (IOS), spirometry and body plethysmography in asthmatic children. J Investig Allergol Clin Immunol. 2005;15(2):102–106. [PubMed] [Google Scholar]
- 44.Horsman TA, Duke RK, Davenport PW. Airway response to mannitol challenge in asthmatic children using impulse oscillometry. J Asthma. 2009 Aug;46(6):600–603. doi: 10.1080/02770900903006265. [DOI] [PubMed] [Google Scholar]
- 45.Vink GR, Arets HG, van der Laag J, van der Ent CK. Impulse oscillometry: a measure for airway obstruction. Pediatr Pulmonol. 2003 Mar;35(3):214–219. doi: 10.1002/ppul.10235. [DOI] [PubMed] [Google Scholar]
- 46.Nowowiejska B, Tomalak W, Radlinski J, Siergiejko G, Latawiec W, Kaczmarski M. Transient reference values for impulse oscillometry for children aged 3–18 years. Pediatr Pulmonol. 2008 Dec;43(12):1193–1197. doi: 10.1002/ppul.20926. [DOI] [PubMed] [Google Scholar]
- 47.Frei J, Jutla J, Kramer G, Hatzakis GE, Ducharme FM, Davis GM. Impulse oscillometry: reference values in children 100 to 150 cm in height and 3 to 10 years of age. Chest. 2005 Sep;128(3):1266–1273. doi: 10.1378/chest.128.3.1266. [DOI] [PubMed] [Google Scholar]
- 48.Dencker M, Malmberg LP, Valind S, Thorsson O, Karlsson MK, Pelkonen A, et al. Reference values for respiratory system impedance by using impulse oscillometry in children aged 2–11 years. Clin Physiol Funct Imaging. 2006 Jul;26(4):247–250. doi: 10.1111/j.1475-097X.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 49.Park JH, Yoon JW, Shin YH, Jee HM, Wee YS, Chang SJ, et al. Reference values for respiratory system impedance using impulse oscillometry in healthy preschool children. Korean J Pediatr. 2011 Feb;54(2):64–68. doi: 10.3345/kjp.2011.54.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]