Abstract
This review explores how we become aware of the (integrated) flavor of food. In recent years progress has been made understanding the neural correlates of consciousness. Experimental and computational data has been largely based on the visual system. Contemporary neurobiological frameworks of consciousness are reviewed, concluding that neural reverberation among forward- and back-projecting neural ensembles across brain areas is a common theme.
In an attempt to extrapolate these concepts to the oral-sensory and olfactory systems involved with multimodal flavor perception, the integration of the sensory information of which into a flavor gestalt has been reviewed elsewhere (Verhagen and Engelen 2006), I reconceptualize the flavor-sensory system by integrating it into a larger neural system termed the Homeostatic Interoceptive System (HIS). This system consists of an oral (taste, oral touch, etc.) and non-oral part (non oral-thermosensation, pain, etc) which are anatomically and functionaly highly similar.
Consistent with this new concept and with a large volume of experimental data, I propose that awareness of intraoral food is related to the concomitant reverberant self-sustained activation of a coalition of neuronal subsets in agranular insula and orbitorfrontal cortex (affect, hedonics) and agranular insula and perirhinal cortex (food identity), as well as the amygdala (affect and identity) in humans. I further discuss the functional anatomy in relation essential nodes. These formulations are by necessity to some extent speculative.
Keywords: taste, gustation, smell, olfaction, somatosensory, chemesthesis, flavor, recurrent networks, perirhinal cortex, insular cortex, orbitofrontal cortex, amygdala, food, multisensory integration, affect, attention, perception, consciousness
1. Introduction and Basic anatomy
The neural basis of consciousness have become a topic of great interest during the last decade. Several intriguing ideas have been proposed, yet all of them based on the visual neurosciences. It is the aim of this review to layout parallel concepts, but for the field of flavor neuroscience where such ideas have not yet emerged.
After a general overview of the anatomy of neural sensory systems (section 1), the reader is introduced to the current consensus on neural correlates of awareness as based largely on the visual sciences (section 2a, 2b1). Next, the gustatory system is described as a homeostatic interoceptive system, which allows parallels to be drawn with prior work on awareness that is not considered to be flavor-related (section 2b2). Based on the concepts borrowed from the literature on visual consciousness, the potential roles of flavor-related neural structures in flavor awareness are outlined next (section 2b3), where it is also argued that object and affect awareness are served by separate yet overlapping cortical areas. Following the visual literature, the concepts of essential nodes and recurrent activation are applied to the flavor neurocircuits (section 2b4-5) and attention is discussed last.
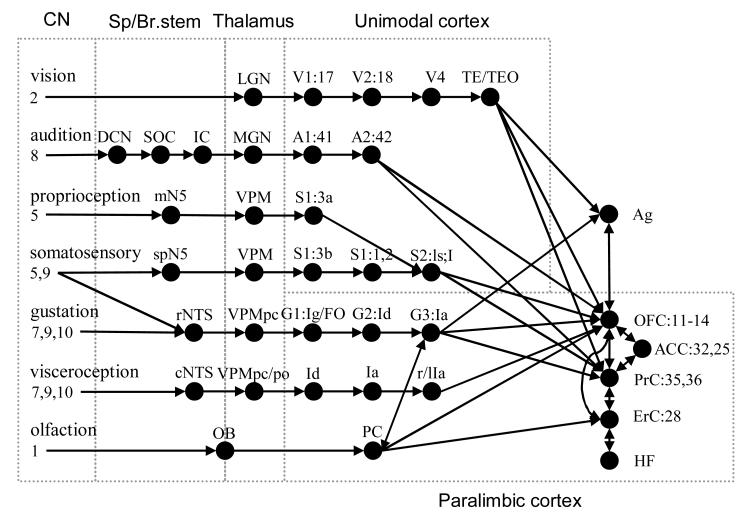
A schematic diagram of the ascending sensory systems contributing to flavor perception is provided in Fig. 1. Most sensory systems of humans are involved with the perception of food. The visual system contributes to food perception mainly in the distal recognition and selection of food (Pangborn 1967; Cardello 1996). Indicated in Fig. 1 are the striate and extrastriate areas of the ventral “what” system (Van Essen and De Yoe 1995; Rolls and Deco 2002). Moving from striate primary visual cortex (V1) to inferotemporal TEO and TE visual receptive field sizes increase and optimal stimuli become increasingly complex. Visual IT projects to amygdala (Ag), orbitofrontal cortex (OFC) and nearby perirhinal cortex (PrC) (Van Essen and De Yoe 1995; Rolls and Deco 2002) . The dorsal “where” system has been omitted for simplicity.
Fig. 1.
Schematic diagram of ascending neural pathways contributing to food perception. Several structures are omitted for simplicity. Brodmann's numerical labels indicated where known. Abbreviations (in order of appearance): LGN: lateral geniculate nucleus; V1, V2 and V4: visual areas 1, 2 and 4; TE/TEO: temporal(-occipital) visual areas; DCN: dorsal cochlear nucleus; SOC: superior olivary complex (non-obligatory relay); IC: inferior colliculus; MGN: medial geniculate nucleus; A1 and A2: auditory areas 1 and 2; mN5: motor nucleus of CN5; VPM(pc): (parvocellular part of the) ventroposteromedial nucleus of the thalamus; S1 and S2: somatosensory areas 1 and 2; spN5: spinal nucleus of CN5; NTS: nucleus of the solitary tract (r: rostral, c: caudal); G1, G2 and G3: gustatory areas 1, 2 and 3; Ig/FO: granular Insula/Frontal Operculum; Id: dysgranular Insula; Ia: agranular Insula; r/lIa: rostral/lateral Ia; OB: olfactory bulb; PC: piriform cortex; Ag: amygdala; OFC: orbitofrontal cortex; ACC: anterior cingulate cortex; PrC: perirhinal cortex; ErC: entorhinal cortex; HF: hippocampal formation. Based on (Norgren 1984; Barbas 1993; Baylis, Rolls et al. 1994; Heimer 1994; Cavada, Company et al. 2000; Mesulam 2000; Sewards and Sewards 2001; Craig 2002).
The auditory system plays a role in the sound of chewing or biting food. Sound is transduced in the spiral cochlear nucleus, projecting to several brainstem nuclei via CN 8, which projects to A1 and A2. A2 projects to OFC and adjacent PrC (Murray and Bussey 1999; Murray and Richmond 2001).
The proprioceptive system aids in the identification of texture, shape and size of foods via active oral exploration of the food, including mastication and tongue movements (Cardello 1996). Proprioceptive information, by way of the motor part of the trigeminal nucleus (mN5) and the ventroposteromedial nucleus of the thalamus (VPM) is represented in Brodmann's area 3a (part of S1), via activation of muscle spindles, Golgi tendon organs, periodontal receptors and deep cutaneous receptors, and projects to S2 (the lateral sulcus and insula (I)) (p. 210 in (Heimer 1994)).
The cranial nerves 5 (trigeminal) and 9 (glossopharyngeal) provide somatosensory input from the oral and nasal cavity to the spinal trigeminal nucleus (spN5), which projects, via the VPM, to the primary and secondary somatosensory cortex (S1 and S2) (Heimer 1994). CN5 and 9 also project to the rostral NTS (rNTS), allowing for the phenomenon of mixed gustatory-somatosensory sensitivity of neurons at the level of the rNTS (Norgren 1983).
The oral input to the gustatory system arrives by way of cranial nerve 7 (facial), 9 (glossopharyngeal) and 10 (vagus). These nerves converge in the rostral NTS, which in primates directly projects to the parvicellular part of the ventroposteromedial nucleus of the thalamus (VPMpc). The VPMpc in turn projects to the anterior insular and opercular areas (AI/FO) which project to the orbitofrontal cortex (OFC) (Norgren 1984; Baylis, Rolls et al. 1994).
Olfactory receptor neurons specifically converge on the mitral cells in the glomeruli of the olfactory bulb (OB) via cranial nerve 1 (olfactory nerve). OB projects to the pyriform cortex, which projects to agranular Insula (Ia), OFC and entorhinal cortex (ErC)(Zald and Pardo 2000; Haberly 2001).
The cortical areas involved with the processing of vision, audition, proprioception and somatosensation belong to the class of unimodal isocortex, involved with the milieu exterieur. Those involved with gustation and visceroception are classed as paralimbic heteromodal cortex. They are multimodal in nature and closely related to the milieu interieur (Mesulam 2000).
The nature of the neural codes in these systems may be spatial and/or temporal, and involve labeled-lines, sparse coding or across-fiber patterns, depending on the sensory system, and on whether the substrate is cortical or not (Hudspeth and Logothetis 2000). Primary cortical areas of the visual, auditory and somatosensory cortex show a topographical (retino- tono- and dermatopic), modular, organization which may imply local neural processing. These modules are context-dependent dynamic entities having non-classical receptive field properties which can be modified continuously by experience through synaptic plasticity (Hudspeth and Logothetis 2000). The visual and auditory system have a parallel hierarchical organization, in which processing occurs simultaneously at various hierarchical stages mediated by back-projections at every stage in the hierarchy (Hudspeth and Logothetis 2000).
Due to space constraints I shall only refer to reviews pertaining to the gustatory and olfactory system in isolation. Extensive reviews are available on gustatory transduction (Lindemann 1996; Gilbertson and Margolskee 2003); psychophysics (Schiffman 2000); neural coding in primates (Scott and Plata-Salaman 1999; Rolls and Scott 2003; Smith and Scott 2003); neural coding in non-primates (Scott and Plata-Salaman 1991; Scott 1992; Smith and St John 1999; Contreras and Lundy 2000; Di Lorenzo 2000; Erickson 2000; Scott and Giza 2000; Scott and Verhagen 2000; Smith, St. John et al. 2000); neural imaging (Small, Zald et al. 1999) and (Verhagen and Engelen 2006).
Reviews are available on olfactory transduction (Buck 1996; Firestein 2001; Moon and Ronnett 2003); psychophysics (Doty and Laing 2003); neural coding in primates (Buck 1996; Haberly 2001; Cleland and Linster 2003; Wilson and Sullivan 2003); neural coding in non-primates (Laurent 1996); neural imaging (Zald and Pardo 2000; Savic 2002; Sobel, Johnson et al. 2003).
2. Conscious experience
A. CONCEPTUAL BACKGROUND
Several global stages of awareness have been described, ranging from coma to alert (Zeman 2001). Here we are concerned only with the specific consciousness (awareness) of experience, called qualia, also know as the hard problem of consciousness. Neuroscientific experimental research in this field has only begun during the last few years. Current investigations are limited to identifying the “neural correlates of consciousness”, a more agreeable starting point than investigating causal relationships.
Crick and Koch (2003) have been influential in this area and below I will outline their framework and compare it to work of others (see also (Rees, Kreiman et al. 2002)). They propose that a conscious percept is related to a transduced stimulus being propagated through the vast neural networks of sensory systems to finally activate the (pre)frontal cortex. From there back projections feed back onto previously activated structures of the sensory systems, ultimately giving rise to a reverberating neural net standing wave among these structures.
The notion of such reverberation has been suggested by several authors, though not all suggest that multimodal cortex is part of it. Dehaene et al. (Dehaene, Sergent et al. 2003) suggest that neural structures of the “global workspace” (any combination of multimodal areas of the cerebral cortex, like the prefrontal cortex) send diffuse backprojections to prior sensory structures. Edelman (2003) has used the term “reentrant processing/signaling” for such reverberating network activation. Grossberg (1995) employs the term “adaptive resonance”. Similarly, Lamme and Roelfsema (2000) argue that recurrent interactions are necessary for awareness (for a graphical depiction of this process, see Fig. 1 there). Llinas and Ribary (2001) speak of “thalamocortical resonance” and Engel and Singer (2001) of “temporal binding”.
Controversy remains on which of the various measured neurophysiological activities (e.g. synchrony, correlated firing, δ-power, γ-power) relate most to conscious experience (see e.g. (Engel and Singer 2001)). Intriguingly, Dehaene and colleagues, employing a neurocomputational model, demonstrated that these measures may well be different aspects of the same underlying phenomenon (Dehaene, Sergent et al. 2003). They showed that a masking task would prevent the reverberating trail of activity due to diffuse backprojections from the global workspace. They further reported that in the network this reverb was likely to be either present or not, which was reflected by the finding that human subjects were either conscious or not of the masked stimulus in a similar task.
Interestingly, using a backward masking visual task (test stimulus before masking stimulus) Rolls (2004) showed a reduction of what may constitute a reverb trail of activity of neurons in monkey inferotemporal cortex (IT, an area at the end of the dorsal visual object stream and analogous to human fusiform gyrus, see Fig. 1) as the masking stimulus was presented at increasingly shorter periods after the onset of the test stimulus. Using the same task in human subjects, when this period became similarly short (∼20 ms) forced choice responses on test-stimulus identity were well above chance (∼75%), yet rated clarity of the stimuli was not, suggesting lack (or very incomplete) consciousness of stimuli despite reasonable (implicit) task performance. Though Rolls attributed this reverb trail to recurrent collateral projections within IT (Rolls 2004), it is equally plausible this was due to back projections from cortical areas further downstream, and consistent with the hypothesis of Crick and Koch (2003). Incidentally, assuming rate coding, Rolls showed that there was a clear relationship between the amount of information available from IT and the apparent clarity/consciousness of the test stimuli, in that it was suggested that the information threshold is higher for conscious experience than for implicit task performance (Rolls 2004). In contrast to many other studies (Engel and Singer 2001), Rolls reported lack of synchrony in the visual system (IT) of awake macaques (Tovee and Rolls 1992).
Despite lack of direct evidence, Crick and Koch further suggest that this reverberation results in the selective and competitive activation of “essential nodes” (Zeki 2001), a set of which is termed a ”coalition”, perhaps in the form of sustained activation of a set of cortical modules (Crick and Koch 2003). It is proposed that we are conscious when coalitions engage in sustained activation.
In summary, the converging evidence of neurophysiological, computational and behavioral studies reviewed very briefly here suggest that back projections (from association cortices) are correlated with, if not necessary for, most (visual) conscious experience.
B. THE NEURAL CORRELATES OF FLAVOR EXPERIENCE
1. BASIC NOTIONS
As the visual system is the most researched among the sensory systems, and has been well characterized in terms of structure-function relationships, most of the aforementioned concepts have been derived from vision science. No similar data is available for the orolfactory senses, though, as outlined below, there is no reason why the neural correlates of consciousness could not also be investigated there.
It should be kept in mind that the olfactory and taste system (and more globally the OFC, Insula, temporal pole, cingulate cortex, rhinal-cortex and parahippocampal region) can be considered to be part of the paralimbic (mesocortical) areas, nested between limbic (pyriform cortex, amygdala, hippocampus) and high-order multimodal association cortex (see fig 1-6 and ch 1.11 in Mesulam (2000)). This area is considered to be involved with memory and learning, channeling emotions and affiliative behaviors, linking visceral state, immune response and endocrine balance (homeostatically) to mental state and with the perception of taste, smell and pain (Mesulam 2000). These areas are much more closely related to the internal milieu, mediated via the hypothalamus, than the unimodal visual, auditory and motor association cortices which are nested between high-order multimodal association cortex and their idiotypic primary sensory cortices which are related to deriving information from and manipulating the external milieu (Mesulam 2000).
One task then is to extrapolate the framework to the orolfactory systems, under the explicit assumption that the derivations based on the visual system will apply. The forward sweep (the initial processing stage, which is insufficient, though necessary, for conscious experience) can readily be seen as consisting of the sequential activation of the neuraxis within each of the “classical” sensory systems (for example for gustatory stimuli: NTS → VPMpc → AI/FO→OFC; for olfactory stimuli: OB → Pyr→AI/FO→OFC), analogous to that of the ventral visual system (RGCs→LgN→V1 → V2 → V4 → IT) (Fig. 1).
At the next stage these downstream areas activate (multimodal) association cortex from which they will in turn receive (diffuse) backprojections allowing for sustained activation to develop in the network (note that within this period also horizontal and early backprojections may have become active). Which cortical areas might provide such backprojections to the orolfactory system? This is analogous to the question of which areas provide the feedback putatively needed for the aforementioned reverb trail in IT.
One answer may be that it is the general “global workspace” of highly interconnected multimodal association cortices (e.g. prefrontal, parietal, temporal and cingulate; (Dehaene, Sergent et al. 2003); see also Fig. 1.11a in (Mesulam 2000), and Fig. 2 in (Mesulam 1998)) that provides this feedback toward any sensory system. Thus, activation of AI/FO would activate excitatory neurons with long-distance axons which would project back onto AI/FO as well as to other high-level areas resulting in global brain scale states of activity (Dehaene, Sergent et al. 2003). Though this ultimately may be true (as these higher association areas are highly interconnected), it will nevertheless be important to explore in which order such multimodal association cortices will become involved. In sections 2-5 these concepts are applied to the gustatory and olfactory system.
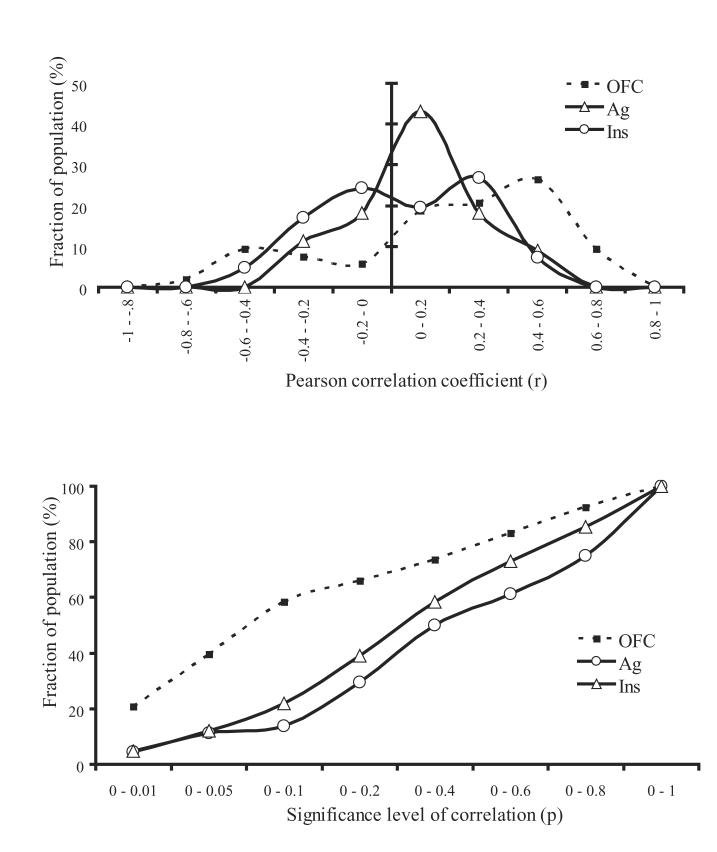
Fig. 2.
Top: The distribution of correlations between activity of single-units evoked by oral stimuli and behavioral acceptance ratings of these stimuli by macaque monkeys for three neural substrates. The distribution of OFC neurons, in contrast to that of neurons from AI/FO and Ag, appears bimodal, suggesting a stronger relation between hedonic evaluation of oral stimuli and the neural activity they evoke in OFC than in AI/FO and Ag (see text for details). Bottom: The distribution of the significance levels of these correlations was different between OFC on one hand and AI/FO and Ag on the other hand. Whereas only a small fraction of AI/FO and Ag neurons showed a significant (p<0.05) correlation between acceptability ratings and neural responses (12.1 and 11.4%, respectively), correlations of nearly half (39.6%) of the OFC neurons were significant.
2. THE HOMEOSTATIC INTEROCEPTIVE SYSTEM
Roughly consistent with this framework, Damasio (1994) has proposed that awareness of the body-self is mediated via recursive meta-representations of homeostatic feelings, as delineated in his somatic-marker hypothesis. It is currently thought that the insula-OFC network represents such interoceptive bodily feelings as pain, temperature hunger, thirst and is necessary for subjective feelings (Craig 2002; Craig 2004). In the following it is argued that taste is associated with interoception, linking it with Damasio's hypothesis and recent imaging studies.
The existence of a cortical system involved with homeostatic interoception has been proposed (Critchley, Mathias et al. 2001; Craig 2002; Craig 2003; Critchley, Wiens et al. 2004). On one hand this was based on anatomical findings of lamina-1 dorsal horn thalamocortical projections, thought to mediate pain, metaboreception, itch and temperature (Craig 2002). On the other hand, human imaging studies consistently showed activation of especially the anterior insula in feelings of the body, including thirst, pain, skin temperature and sexual arousal (Critchley, Wiens et al. 2004). They argued that these sensations are not so much related to somatosensation (and hence mediated via S1, S2), but form a separate system involving homeostasis and interoception (e.g. nociceptive and thermal input related to maintaining proper body function). The identified human neuraxis (Fig. 1, “visceroception”) for fine parasympathetic afferents involves NTS→VPMpc→Id→Ia→lIa, and for fine sympathetic afferents Lamina 1→VMpo→Id→Ia→rIa→OFC (lIa: lateral Ia; rIa: rostral Ia) (Craig 2002). Lamina 1 also projects to NTS, and both lamina 1 and NTS project to VPMpc, in part via PBN. VMpo (posterior part of the ventromedial thalamic nucleus) is rostrally contiguous with the VPMpc (the parvicellular part of the ventroposteromedial nucleus, also known as the basal part of the ventromedial nucleus, VMb)(Craig 2002). This neural system is very similar to that of the gustatory system, especially as recently proposed by Sewards and Sewards (2001), who propose NTS→VPMpc→gI→dI→aI (Fig. 1). The gustatory Ia is located just anterior to the Ia involving the homeostatic interoceptive system (Craig 2002). Thus, the gustatory and homeostatic interoceptive systems run largely parallel in close spatial association to each other from the NTS onward.
This leads us to propose that the homeostatic interoceptive system consists of two major divisions: an oral part, and non-oral part. The oral division of the homeostatic interoceptive system (oHIS) is proposed to represent the same modalities as the non-oral division (noHIS), with as a main difference (besides that of location) that the oHIS further contains an expanded representation of chemosensory sensitivity related to papillary oral input. Note that the idea of the gustatory system as being part of a larger multimodal (gustatory, cutaneous, nociceptive and visceral) system was also proposed by Katz et al. (Katz, Nicolelis et al. 2000) in rodents and also by Scott and colleagues (Scott and Mark 1986), but has not been previously associated with HIS. Arguments, in addition to the strong neuroanatomical similarities, supporting this proposal are as follows.
It is known that the primate gustatory system is not unimodal: at every level of its neuraxis (from cranial nerves to Insula and OFC) that has been tested it has been found that taste sensitive neurons also show sensitivity to touch and temperature (chorda tympani: (Aato, Ogawa et al. 1975); NTS: none tested; VPMpc: (Pritchard, Hamilton et al. 1989); AI/FO: (Scott and Plata-Salaman 1999; Verhagen, Kadohisa et al. 2004); OFC: (Kadohisa, Rolls et al. 2005); Ag: (Kadohisa, Rolls et al. 2005)). This also holds for rodents (chorda tympani: (Ogawa, Sato et al. 1968; Matsuo, Inoue et al. 1995; Shimatani, Grabauskiene et al. 2002); NTS: (Matsuo, Shimizu et al. 1984; Ogawa, Hayama et al. 1988) ; VPMpc: (Verhagen, Giza et al. 2003); GC: (Yamamoto, Yuyama et al. 1981; Kosar, Grill et al. 1986; Yamamoto, Matsuo et al. 1989); (for overview see Table 2 in (Verhagen, Giza et al. 2003)). The reasons for this heteromodality make considerable sense in the context of HIS.
The same has been shown for oral chemesthetic (Green 1996) sensitivity (cranial nerves: (Rentmeister-Bryant and Green 1997); AI/FO: (Verhagen, Kadohisa et al. 2004); OFC: (Kadohisa, Rolls et al. 2005); Ag: (Kadohisa, Rolls et al. 2005)), as in rodents (e.g. cranial n.: (Lundy and Contreras 1993)). These modalities form the core of Craig's HIS modalities. Chemesthetic stimuli have similar sensory effects on the skin as in the mouth. I thus suggest the terms oral common-chemical sense (oCCS, part of oHIS) and non-oral CCS (noCCS, part of noHIS), which provide the advantage of conceptual simplicity.
Virtually all imaging studies related to these modalities on the skin show activation of at least several of the areas of I, OFC, ACC and Ag (Francis, Rolls et al. 1999; Craig, Chen et al. 2000; Craig 2003). This is very similar to imaging studies in taste (O'Doherty, Rolls et al. 1999; Small, Zald et al. 1999; O'Doherty, Rolls et al. 2001; De Araujo, Kringelbach et al. 2003; de Araujo, Rolls et al. 2003; Small, Gregory et al. 2003) (for a metaanalsyis see (Verhagen and Engelen 2006)).
Further, as in the taste system (Norgren 1988), Craig notes that for sub-primates the PBN is an obligatory relay (Craig 2002). Thus, evolutionarily these systems (OHIS and noHIS) developed in parallel, and primates rely more on cortical HIS than sub-primates ((Craig 2003); see (Satinoff 1983) for a related conceptual issue).
Both systems are strongly involved with brainstem homeostatic nuclei (NTS, PBN). In noHIS, the sympathetic Lamina1 division provides the basis for the somato-autonomic reflex arcs at spinal, medullary and mesencephalic levels (see Fig 2 in (Craig 2002)). These exist in the gustatory system as well, primarily mediated via NTS→DMV→efferent vagus nerve to stomach, intestine, liver and pancreas, in the form of parasympathetic cephalic phase reflexes ((Teff 1996); DMV is dorsal motor nucleus of the vagus). In mammals, sensory exposure to food and pure chemicals without ingestions leads to an early miniature version of post-prandrial release of various digestive and metabolic components like saliva, gastric acid, pancreatic enzymes and insulin. This response is considered to be preparatory and to adaptively affect both metabolism and behavior (Teff 1996). Thus, in humans, a 5-minute oral exposure to a sandwich, without swallowing, results in a 33% lower postprandrial glucose levels (i.v. injected glucose) (Teff 1996). Further, just sight and smell of a food can lead to increased glucose clearance. The human salivary and gastric response is correlated with the perceived pleasantness of foods and foods with high hedonic value will be metabolized more rapidly (Teff 1996). Interestingly, in humans cephalic phase reflexes to simple stimuli (e.g. a glucose solution) are much less pronounced than in rodents, whereas responses become more robust as the number of involved modalities increase (e.g. smell, taste, vision (Feldman and Richardson 1986)) or when presented with whole foods (Teff 1996). Thus flavor appears to play a special role in evoking cephalic phase responses in humans, and the primate taste system is as directly involved with homeostatic regulation as the noHIS.
Last, the sense of taste itself is clearly related to homeostatic interoception. It provides the sensory link between milieu exterieur and interieur by allowing informed decisions as to what food is appropriate for the body to ingest and absorb (Scott and Verhagen 2000). These anatomical, functional, electrophysiological and conceptual considerations lead us to suggest the oHIS and noHIS concept.
It should be noted that the anatomical relationship between noHIS and the olfactory system is less evident (Fig. 1, at least sub-cortically), and hence there is no such direct relevance to the olfactory system in Damasio's somatic marker hypothesis or the recent neuroimaging data on interoceptive bodily feelings.
3. THE PERIRHINAL CORTEX, AFFECT AND OBJECT AWARENESS
The notion of the taste system as part of HIS suggests that awareness of taste may be mediated by means as envisioned by Damasio, but does not provide insight into how the neural structures of this system (or those associated with olfaction) are involved with bringing about consciousness. Below the concept of object versus affect awareness is introduced, after which the neural structures and their interactions are discussed in context.
I propose that the Insula-Perirhinal system functions as an orolfactory object recognition system, whereas the Insula-OFC system as an orolfactory affective system. This may suggest that conscious experience of these two aspects of food perception may involve overlapping but also discrete cortical areas. The notion of two separate systems for “object” (quality and intensity) identification versus affective evaluation has previously been suggested by Small et al. (Small, Gregory et al. 2003), Scott and Rolls (in gustation: AI/FO and OFC, resp.; (Scott, Yan et al. 1995)) and Anderson et al. (in olfaction: OFC and amygdala, resp.; (Anderson, Christoff et al. 2003), yet the rhinal cortex played no role there).
With respect to gustatory awareness, such division between sensory and hedonic aspects has previously been suggested by Aurell (in (Sewards 2004)). Analogous to this scheme, Sewards (2004) suggested different neural structures associated with sensory and hedonic awareness than proposed here. He suggests that such functional divisions, based largely on rodents, can be made at any level within the taste system, including the insular cortex, while largely ignoring the overwhelming evidence of involvement of OFC in affective processes (as in reward devaluation or association) and lack of effect of reward devaluation in lower neural areas (Yaxley, Rolls et al. 1985; Rolls, Scott et al. 1988; Scott, Yan et al. 1995). It has long before been proposed that rodents and primates differ substantially in this respect, in that indeed in rodent taste quality and affect are encoded in parallel, whereas they are encoded serially in primates (quality in AI/FO, affect in OFC; (Scott and Plata-Salaman 1999)). I hence take Sewards proposal to only refer to sub-primates.
Prescott (1999) has provided a similar object-based approach to food perception. He argues that the survival value of the orolfactory system lies in its ability to correctly identify foods by jointly identifying its nutrients (gustatory: e.g. salt, carbohydrates, proteins), the food-source or food-state (retronasal olfactory: e.g. volatiles perceived as floral, peanut butter, rotten; texture: thick, lumpy, hard, slimy), and toxins (e.g. taste: alkaloids; chemesthesis: capsaicin). In relation to retronasal aromas as being perceptually associated with the oral cavity/taste, Prescott mentions Gibson's ecological view of perception, which entails that the physiological origins of sensations are less important than that the sensations can be used for object identification. Thus, the identification of an intraoral food relates to the integrated perception of all concurrently stimulated modalities available.
This object-affect dichotomy is strengthened by a novel analysis of the distribution of the correlations between the mean single-unit responses to a large (n=16-25) array of oral stimuli of neurons in AI/FO (n=41), clOFC (n=53) and Ag (n=44) in awake rhesus monkeys and the individual acceptability ratings (method described and neurally validated in (Rolls, Sienkiewicz et al. 1989)) of the monkeys these neurons were recorded from of the same stimuli (Fig. 2, top; new analyses based on data from (Rolls, Verhagen et al. 2003; Kadohisa, Rolls et al. 2004; Verhagen, Kadohisa et al. 2004; Kadohisa, Rolls et al. 2005); analyzed with SPSS V12, SPSS Inc.). The Pearson correlation coefficient between the neural and behavioral responses across the stimulus array were computed for each neuron. The assumption is that if neural encoding involves affective processing, such is reflected in a more negative or positive correlation between neural responses and behavioral measures of hedonic evaluation. As expected, a larger fraction of the clOFC population had more positive and negative correlations than seen in AI/FO and Ag (Fig. 2, top). The distribution for the clOFC appears bimodal, with peaks at correlations between −0.6 and −0.4 and between 0.4 and 0.6, the latter indicating higher neural responses being associated with higher acceptability ratings. The distribution of AI/FO and Ag neurons peaked between −0.2 and 0.4. Indeed, the proportion of variance explained (r2) was higher in clOFC (0.16±0.13, mean±sd) than in AI/FO (0.07±0.08) and Ag (0.06±0.08) as evaluated with a 1-sided t-test (p<10−5and p<10−4, respectively), but did not differ between AI/FO and Ag (p=0.201). In line with this, nearly half (39.6%) the population of clOFC neurons had significant (p<0.05) correlations between their individual responses and the acceptability ratings, whereas this was only 12.1% in AI/FO and 11.4% in Ag. For many clOFC neurons (20.8%) this correlation was highly significant (p<0.01) and the cumulative histogram of their p-values was consistently higher for clOFC than for AI/FO and Ag, as indicated in Fig. 2 (bottom). For example, 58.5% of clOFC neurons had correlations with associated p-values smaller than 0.1, while this was only 13.6% for Ag and 22.0% for AI/FO. Thus, consistent with our proposal, activity of clOFC neurons to oral stimuli is often related to the hedonic evaluation of these stimuli by rhesus macaques, whereas this relation holds to a much lower extent for neurons in AI/FO and Ag. It should be noted that even though such strong associations were present in clOFC, the hedonic ratings explained on average 16±13% of the variance in OFC (maximally 42%), suggesting that hedonic evaluation is not the sole neural correlate of clOFC activity.
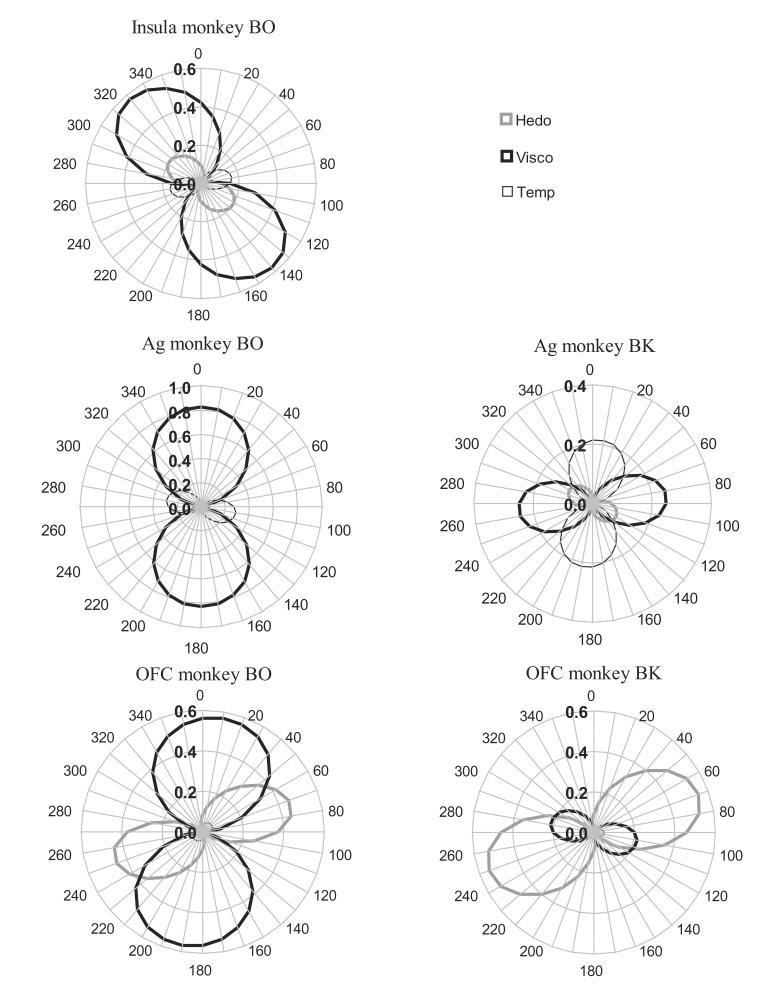
This notion is strengthened by a population-analysis based on the same data (see Table 1 and Fig. 3). The representation of the stimuli in each area (AI/FO, amygdala and OFC) for each monkey (BO and BK) was quantified using multi-dimensional scaling in 2-dimensions (all explaining >90% of the stimulus correlations' variance, except for OFC in BK (77%)). The relation between this abstraction and the monkey's behavioral acceptability of the stimuli, stimulus viscosity and stimulus temperature, were evaluated by rotating the MDS solutions and computing the Pearson correlation coefficient between the neww stimulus location and acceptibility rating in 5° steps in Excel. It should be noted that this is a valid approach as an MDS solution is rotation-invariant. The associated proportion of variance explained (r2) is indicated in Fig. 3 as a function of angle of MDS rotation. Table 1 indicates the maximum r2 obtained by rotation for each of the factors, brain areas and subjects, as well as the associated angle, and F- and p-value of their regression analysis. Consistent with the above analysis based on these same individual neurons, this population analysis suggests that the relation between population-encoding and hedonic value of stimuli is strongest in the OFC for both monkeys (proportion of variance explained is ∼50%), intermediate for I (r2=0.201) and, again, weakest in Ag (<10%). This analysis further suggests that the representation of hedonic value and viscosity (where correlations are significant) are most orthagonalized at the level of the OFC: the difference between the angles at which their correlations are maximal are 16° in AI/FO (140°-124°), and 62° (monkey BO: 73°-11°) and 34° (monkey BK: 104°-70°) in OFC (Table 1).
Table 1.
The maximum proportion of variance (r2) of MDS stimulus location for three brain areas (Ins=insular cortex/frontal operculum, Ag=amygdala and OFC= orbitofrontal cortex) in two monkeys (BO and BK) explained by the three factors of behavioral oral stimulus acceptablity (“Hedonic”), stimulus viscosity and stimulus temperature (see Fig. 3 for details). Indicated are the angle (“max angle”) of the rotation of the MDS at which the r2 was maximal (“max r2”, using the solver function in Excel 2003, Microsoft Corp.), and the associated F- (“F”) and p-value (“p”) of the regression. The r2 of each MDS solution is also presented (“MDS r2”). Significance levels are boldfaced if significant (α=0.05). MDSs were based on >20 neurons and >20 stimuli, except for Ag of BK (number of neurons=5). The acceptability rating explained as much as ∼50% of the variance only in the OFC in both monkeys, and no more than 21% in the AI/FO (20.1%) or amaygdala (9.1%). It further appears that representations of viscosity and hedonic value (where both are significantly correlated with rotated MDS stimulus locations) are most orthagonalized in the OFC (angle-difference is 16° (140°-124°) in Ins of BO, 62° in OFC of BO and 34° in OFC of BK). See text for validation of the analysis.
| BO | BK | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hedonic | Viscosity | Temperat. | MDS r2 |
Hedonic | Viscosity | Temperat. | MDS r2 |
||
| Ins | max angle (°) | 124 | 140 | 79 | 0.927 | ||||
| max r2 | 0.201 | 0.574 | 0.164 | ||||||
| F | [1,23]=5.8 | [1,23]=31.0 | [1,23]=4.5 | ||||||
| p | 0.025 | 1.2*10 − 05 | 0.045 | ||||||
| Ag | max angle (°) | 92 | 179 | 102 | 0.910 | 119 | 86 | 8 | 0.977 |
| max r2 | 0.049 | 0.834 | 0.293 | 0.091 | 0.251 | 0.217 | |||
| F | [1,23]=1.2 | [1,23]=115.9 | [1,23]=9.5 | [1,21]=2.1 | [1,21]=7.0 | [1,21]=5.8 | |||
| p | 0.290 | 1.9*10 − 10 | 0.005 | 0.163 | 0.015 | 0.025 | |||
| OFC | max angle (°) | 73 | 11 | 51 | 0.930 | 70 | 104 | 92 | 0.770 |
| max r2 | 0.456 | 0.571 | 0.057 | 0.550 | 0.218 | 0.051 | |||
| F | [1,21]=17.6 | [1,21]=27.9 | [1,21]=1.3 | [1,20]=24.5 | [1,20]=5.6 | [1,20]=1.1 | |||
| p | 4.0*10 − 04 | 3.1*10 − 05 | 0.274 | 7.8*10 − 05 | 0.028 | 0.310 | |||
Fig. 3.
To complement the individual neuronal-level analysis of Fig. 2, this figure provides a novel population-level analysis between the locations of stimuli on a 2-dimensional scale (MDS, a means to visualize neural population representation of stimuli; Systat v. 10, SPSS Inc.) and behavioral acceptability ratings (“Hedo”), and additionally between these locations and stimulus viscosity (“Visco”) and stimulus temperature (“Temp”). The five MDSs were based on stimulus response similarity as quantified by the Pearson correlation coefficient of the neural responses the stimuli evoked in each population. The figure shows for each area and monkey the r2 (the proportion of the variance explained) of the correlation (r) between stimulus location and these three factors as a function of the rotation of the MDS (no behavioral acceptiblity data was available for insula in monkey BK). Note that rotation of the MDS, as well as scaling, inversion and translation, yield equivalent MDS solutions. In all three neural areas of monkey BO viscosity correlated highly with MDS stimulus position (see Table 1 for details). Importantly, only at the level of the OFC did the behavioral acceptablity of stimuli (measured and analyzed separately for each monkey) correlate highly with the neural representation of the stimuli.
To validate this novel method of analysis, I randomly assigned the acceptance ratings and viscosity levels to the stimuli and performed the same maximalization-of-r2-by-rotation analysis 20 times for each monkey, area and factor. Note that for 24 stimuli the number of permutations of randomly assigned values is 6.2*1023, and it is hence exceedingly unlikely that the values are assigned to the stimuli they actually belong to. This analysis confirmed that the reported maximum r2 values are highly unlikely to occur by chance: the mean (±sd) of the maximum r2 between randomly reshuffled hedonic ratings and rotated MDS of insular neurons in the insula in BO was 0.074±0.056, whereas the maximum r2 for the actual ratings (0.201, see Table 1) was 2.3 sds higher (p<0.012). For the OFC the randomized control analysis revealed a mean r2 of 0.099±0.105, where the actual data (r2=0.456) was 3.4 sds (p<0.001) above control. In the Ag of OB the very high r2 for viscosity (0.834) was 9.8 sds (p<<0.001) above control (0.090±0.076). It hence is clear that the analysis is unlikely to suggest strong relationships by chance.
In conclusion, these analyses substantiate the notion that cortical and subcortical stuctures in primates are to different degrees involved with representing affective information.
Sewards and Sewards (2001) suggest that the perirhinal cortex is a final common pathway for the visual, auditory and somatosensory systems, and that this may hold for the gustatory system as well. They present five arguments why the perirhinal cortex is the “terminus” of the taste system. First, the end of the dorsal visual system (TE), the somatosensory posterior insular cortex, the auditory superior temporal gyrus, and the gustatory posterior agranular insular cortex are all adjacent to the perirhinal cortex. Second, the insular secondary and tertiary taste cortices as proposed above project to perirhinal cortex. Third, it is the highest order area for the visual, somatosensory and auditory systems. Fourth, lesions to this area result in taste recognition deficits. Indeed, the perirhinal cortex is generally believed to be important for object recognition in all other sensory modalities, as well as cross-modal memory of objects and events (see below). Fifth, parahippocampal gyrus (near the perirhinal cortex, in IT) has been shown to be activated in human imaging studies (Sewards and Sewards 2001).
Our cluster analysis-based meta-analysis of odor and taste neuroimaging (Verhagen and Engelen 2006) also suggests activation of PrC. The PrC (BA 35 and 36) is located around x= ±20 mm (range ±17 to ±25), y= −20 mm (−10 to −40), and z= −12 mm (−8 to −32) according to the map by Talairach and Tournoux (1988). These coordinates overlap with right cluster 10 (18.7, −5.4, −13.9; x-, y- and z-coordinates, respectively) and left cluster 5 (−21.4, −10.3, −7.1; Table 2B in (Verhagen and Engelen 2006)). Talairach Daemon found BA 35 11 times (5 taste and 6 odor; from 8 studies) and BA 36 7 times (5 taste and 2 odor, 4 activations being the same as for BA 35; from 7 studies) when searching within an 11mm cube of the peak coordinates of the 296 individual activations.
Limiting the search to a 5mm cube resulted in four identifications of BA 35 (taste study 2: 19, −4, −23; odor study 7: −22, −18.4, −14.9; odor study 11: 18, −12, −21 and −21, −15, −15; x-, y- and z-coordinates, respectively; see Table 1A in (Verhagen and Engelen 2006)) but never BA 36. According to the map by Talairach and Tournoux (1988), each of these four activations was located in BA 28 (ErC), although the authors refer to it as amygdala and hippocampus, which is functionally and spatially closely associated with PrC and hippocampus (Verhagen and Engelen 2006). Thus, within the typical range of variation of neuroimaging studies (see (Verhagen and Engelen 2006)) PrC is not an unlikely candidate to be activated by odor and/or taste stimuli. It will be of considerable interest to further investigate the involvement of PrC and ErC in orolfactory neural processes.
Interestingly, the tertiary gustatory cortex (Ia) is known to have reciprocal connections with the perirhinal cortex (ref 13,37,95 in (Sewards and Sewards 2001)). Thus, the perirhinal cortex may provide the feedback to Ia which I suggest to be necessary for conscious experience/recognition of food as objects in a multimodal fashion, and is part of the temporal cortex that has been suggested to be one of the four multimodal cortices necessary for conscious experience via feedback (Dehaene, Sergent et al. 2003).
Evidence has been presented in several reviews that the PrC, located at the ventromedial surface of the temporal lobe, is important in object recognition (Murray and Bussey 1999; Murray and Richmond 2001). The PrC represents an object's many (multimodal) attributes, while recognizing that it remains an entity. It has reciprocal connections with a vast number of cortical areas: insular cortex, cingulate cortex, OFC, amygdala, STS (auditory), TE and TEO (vision), as well as enthorhinal cortex (which receives strong pyriform input, as well as olfactory bulb input), hippocampal formation, parahippocampal complex (see ref 13-15 in (Murray and Richmond 2001)), providing the necessary connections for such multimodal input (and output). Indeed, the PrC associates different views and non-visual attributes (like touch or smell) mediating object identification. For example, the PrC has been found to be necessary for crossmodal association memory, in that lesioned monkeys cannot choose a visible object first sampled by touch (Goulet and Murray 2001). Monkeys with rhinal cortical lesions are unable to select visible objects that were selectively and arbitrarily assigned to either a peanut or a sultana which three monkeys orally sampled in full darkness (Parker and Gaffan 1998). Even after 500 trials the mean error rate was 232 (not above chance level), without a tendency to improve. Although the authors argue that the deficit was due to a loss of flavor-vision association memory, they could not rule out the possibility of “flavor blindness”, as they didn't test flavor discrimination abilities (which is what our model would predict). Deficits in a food preference task were not apparent in one of the monkeys (and in none of those with specific perirhinal lesions in the study of Gaffan (1994)), despite inability to perform the association task. This too is consistent with our model, in that food preference is a function of relative hedonic value of foods, encoded in the affective system (the neural correlates have been found in the OFC (Schultz, Tremblay et al. 2000)), and hence predicted to be dissociable from the identification (Ia-PrC) system. Indeed, the PrC has no role in object-reward associations, in that it is not necessary for reward devaluations (Thornton, Malkova et al. 1998), whereas the OFC and amygdala are crucially involved in such associations (ref 41, 42 in (Murray and Richmond 2001)). The PrC has further been shown to be important for discriminating visual features, and hence perception, in humans and monkeys (ref 29, 33, 34 in (Murray and Richmond 2001)). These findings lend further support for the neural basis of orolfaction model proposed here, in that the PrC is critically involved with multimodal (food) object representations, in a Gestalt-like fashion (Murray and Bussey 1999), and that is not related to (food) object reward associations (which I propose is mediated by the aI-OFC-Ag-ACC network, see also (Kringelbach 2004)). This hypothesis could be investigated with cell-body-specific lesions of the PrC on orolfactory food discrimination. It should be noted however that direct, specific evidence for a role for PrC in flavor processing remains absent.
In parallel, the anterior cingulate cortex, another multimodal cortex tentatively involved with such feedback, has dense reciprocal connections with the OFC (notably the medial OFC). I speculate that these areas may be necessary for the conscious perception of the affect-awareness of food. These ideas are represented in Fig. 4.
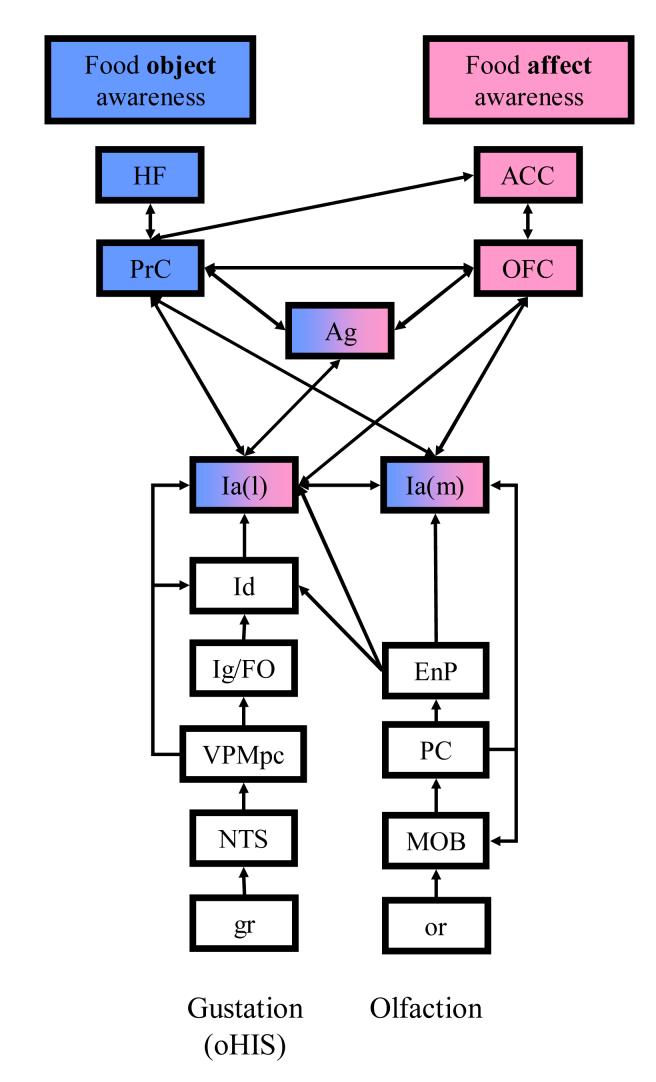
Fig. 4.
Proposed model of neural structures involved with the conscious experience of food. I propose that two different, but overlapping, large-scale networks are involved for 1) food object recognition awareness (Ia, PrC and Ag) and 2) food reward value awareness (Ia, OFC, ACC and Ag). Only the olfactory and gustatory (oHIS: oral division of the homeostatic interoceptive system) systems are shown for simplicity. Abbreviations: gr: gustatory receptors; NTS: nucleus of the solitary tract; VPMpc: parvocellular part of the ventroposteromedial nucleus of the thalamus; Ig/FO: granular Insula/Frontal Operculum; Id: dysgranular Insula; Ia: agranular Insula; PrC: perirhinal cortex; HF: hippocampal formation; Ag: amygdala; or: olfactory receptors; MOB: main olfactory bulb; PC: piriform cortex; EnP: endopyriform nucleus; OFC: orbitofrontal cortex; ACC: anterior cingulate cortex. Entorhinal cortex is incorporated in HF, and its direct connections with OB are not shown for clarity. Partially based on (Barbas 1993; Murray and Bussey 1999; Rolls 1999; Cavada, Company et al. 2000; Murray and Richmond 2001; Sewards and Sewards 2001).
The amygdala and OFC, which have extensive reciprocal connections, may also be critically involved with affect awareness. Indeed, all areas speculated here to be involved with conscious experience via reverberation are considered by Mesulam (2000) to be “neural epicenters” in his model of large-scale neural networks. Such epicenters (or “transmodal areas”) which are higher multimodal association areas, are reciprocally connected to each other, receive reciprocal connections from many more lower order cortical areas and ultimately from the thalamus. Combinations of epicenters may be dynamically formed in relation to the cognitive task at hand (for example the limbic large scale network for memory and emotion, consisting of the hippocampo-enthorhinal complex and amygdala as two interconnected epicenters, receiving input from paralimbic areas, the hypothalamus and limbic thalamus). Mesulam (2000) suggests that the epicenters are also crucially involved with consciousness, which, given that they may all be reciprocally interconnected, is similar to Dehaene's global work space model mentioned earlier.
However, the right anterior insula (rAI), part of noHIS, has recently been implicated in representing the visceral self (Craig 2002; Craig 2004; Critchley, Wiens et al. 2004) and as part of the I-OFC-ACC system, which is the core of the somatic marker theory (Damasio 1994), in explicit subjective awareness and in emotion. For example, the rAI becomes activated during various emotions as anger, happiness (Critchley, Wiens et al. 2004), and disgust (Wicker, Keysers et al. 2003). I argue that the same may hold for the gustatory system, in that the oral part of the aI (also with a bias towards the right hemisphere, see (Verhagen and Engelen 2006)) is similarly involved with affective awareness of food, which is based on the above considerations of the gustatory system being effectively the oral part of HIS. Human imaging studies provide evidence for this as well. For example, Small et al. reported effects of eating chocolate to satiety on the insula and OFC in a human imaging study (Small, Zatorre et al. 2001). Thus it seems plausible that AI is also involved with orolfactory affect in humans, though to a lesser extent than OFC. Furthermore, intraprimate species differences should not be excluded, and have been found with respect to prevalence of spindle cells which exclusively occur in these areas (see ref 22 in (Craig 2004)). This hypothesis is a modification of the exclusive involvement of the OFC among cortical areas in orolfactory affect as proposed by Rolls (Rolls 1999) and has been incorporated in Fig. 4.
In conclusion, I present evidence that the neural circuitry involved with object versus affect awareness of flavor is both overlapping and distinct.
4. ESSENTIAL NODES
With respect to the subsequent stage in the framework of consciousness, we may ask which neural substrates in the orolfactory systems could act as the tentative essential nodes? In the visual, auditory and somatosensory system these may consist of cortical modules (each with coherent featural sensitivity; (Mountcastle 1997)) showing sustained activity, each within a cortical area with clearly defined properties needed for explicit awareness of that property (e.g. V4 for color vision, TE and TEO for complex configural object representation).
With respect to the proposed neural structures involved with food object awareness, such modularity and larger scale cortical functional divisions do not appear to exist at higher cortical levels for the orolfactory systems (Ia-PrC for orolfactory object awareness and Ia/OFC for orolfactory affect awareness). Scott and Plata-Salaman (1999) have found no clear evidence for modularity in the macaque AI/FO, in terms of similarity in gustatory tuning of neurons located along recording tracts orthogonal to the cortical surface. In contrast, a recent optical imaging study of rat gustatory cortex has indicated an anterior-posterior map reflecting responsiveness to sucrose or NaCl (Yoshimura, Sugai et al. 2004). Further, a recent human neuroimaging study of the insular/opercular cortex has suggested gustatory chemotopy (Schoenfeld, Neuer et al. 2004). Differential, yet overlapping, haemodynamic activations to five prototypical tastants of same intensity of this area were stable between imaging sessions within subjects, but varied between subjects (Schoenfeld, Neuer et al. 2004). This may indicate that the preferential reverberation between PrC and a subregion within Ia may consistently be related to awareness of a salty food in one subject, but to another taste in another subject. Alternatively, in absence of clear functional maps for gustation and oral somatosensation in Ia, these nodes may consist of ensembles of neurons with similar tuning profiles that do not show clear spatial (e.g. modular) organization.
The only modularity found thus far in the orolfactory systems is that of the olfactory bulb with its glomerular structure ((Bozza, McGann et al. 2004), three synapses upstream from the Ia). Thus, for olfaction essential nodes may consist of (groups of) glomeruli, and indeed reciprocal connections exist between PFC and OB in rat and it has been found that orbitofrontal regions can activate and inhibit the OB in rat (Cinellar, Ferreyra-Moyano et al. 1987). Backprojections from the anterior piriform cortex (aPC) to the OB have also been identified in rodents (Haberly 2001). Based on this evidence, Haberly states that “the exceedingly large number of backprojecting pyramidal cell axons from aPC (much larger than in the LOT) suggests that, despite its inhibitory action on mitral tufted cells, this system is intimately involved in information processing” (Haberly 2001). In contrast to the OB, no discrete spatial odorant activations have been found in the PC in rats using c-fos technique (Illig and Haberly 2003), nor using 2-deoxyglucose and optical imaging in rat PC (Cattarelli, Astic et al. 1988). A genetic tracer study, employing single olfactory receptor clones, reported well-defined patches of labeled neurons in the aPC (Zou, Horowitz et al. 2001). This spatial organization is thus lost post-synaptically, due to overlapping input to the aPC (for example odorants can activate multiple olfactory receptors) and due to associational fibers (Illig and Haberly 2003). It may well be that the combined OB-aPC-pPC-OFC/Ag/PrC/ErC (see (Haberly 2001)) large-scale network is involved. Hence, it may be that the essential nodes/neural ensembles as related to vision may be of a different structure than those of the orolfactory systems.
With respect to the proposed food affect awareness, neurons with vastly different tuning profiles within a modality have been encountered in the caudolateral OFC (Walker's area 12), as well as with different sensitivity to different modalities, in individual electrode tracks made orthogonal to the cortical laminae (e.g. (Rolls, Verhagen et al. 2003)). Further, inspection of maps of reconstructed positions of responding neurons has not revealed any organizing principle related to modality sensitivity in either AI/FO, OFC or amygdala ((Rolls, Verhagen et al. 2003; Verhagen, Rolls et al. 2003; Verhagen, Kadohisa et al. 2004; Kadohisa, Rolls et al. 2005)). Indeed, given our current hypothesis that OFC would be mainly involved with the affect awareness, we would expect an organization related to reward value, rather than stimulus features. In a recent review (which included a meta-analysis of 87 imaging studies) it was indeed found that the functional organization may be along a medial-lateral axis, reflecting reward versus punishment (Kringelbach and Rolls 2004). The second identified functional axis (anterior-posterior) was suggested to be related to the level of abstraction of the stimulus or task. Indeed such a gradient may be sufficient to encode both the magnitude and value of the reward. More work on the functional organization of higher cortex of the orolfactory systems seems warranted. Thus I propose that awareness of intraoral food is related to the concomitant reverberant self-sustained activation of a coalition (in Crick and Koch's terminology) of neuronal subsets in Ia-OFC (affect, hedonics) and Ia-PrC (food identity), as well as the amygdala (affect and identity) in humans.
5. RECURRENT ACTIVATION
A more fundamental question is whether recurrent activation occurs at all in AI/FO. Lamme and Roelfsema (2000) have presented several arguments suggesting recurrent activation in the visual system: dynamic tuning and non-classical receptive fields. At this point there is no direct evidence for recurrent activation in orolfactory neural structures derived from higher order reciprocal cortical connections. Experiments employing techniques such as temporal lesions (cooling, TMS) of higher order cortex (PrC, ACC and OFC) may be able to reduce the amplitude of the phasic trail of neural activity in Ia, and to reduce the ability/speed to recognize food and modulate its pleasantness.
The necessity of feedback mediated reverberation has been questioned by Zeki. He (2001) proposes the term “microconsciousness”, pertaining to activity of neural areas related to processing of particular features (e.g. V4 color coding, MT motion coding). This is presented in a context where the brain is not so much thought to merely represent reality out there, but to be actively involved in its reconstruction, in order to acquire knowledge. It is emphasized that different aspects of visual entities may not become “microconscious” at the same time as others, and thus that a “neural glue” is required to enable a subject to become aware of all features of stimulus at the same time. He suggests that the CNS may require methods to align different microconsciousnesses (feature representations) in time, but that this merging process would be “post-conscious” (consciousness at a later stage; interestingly, Mesulam (2000) mentions the functionality of the projections of neural epicenters to the striatum to be “synchronizers”; could this be Zeki's “neural glue”?). A problem in this hypothesis is that objects/events (with respect to color of an object and its movement for example) are “bound” together in our conscious experience, even though under specific laboratory conditions they may become separable, and that hence the “microconscious”/“post-consciousness” interpretation has limited ecological use (i.e. finding that processes are dissociable does not imply they are typically dissociated).
6. ATTENTION
For completeness I provide a very brief discussion on covert attention, which may be considered a process that selects which high level information may enter the limited capacity processes of consciousness (the “bottleneck”). Attentional blindness and change blindness paradigms have shown we only become aware of a fraction of the information that lies within our visual fields (Rensink 2002). Attention has been shown to enhance neural responses in lower visual and auditory areas without changing neural tuning (Chun and Marois 2002), which is called attentional priming (Gazzaniga, Ivry et al. 2002). Attentional priming occurs in the visual areas which are related to the location and features that attention is directed to (Corbetta, Miezin et al. 1991). For example, attending to either a house or a face, in images where both stimuli were superimposed on each other, activated either the parahippocampal place area or the fusiform face area (fusiform gyrus, thought to be the human analogue of macaque IT), respectively (O'Craven, Downing et al. 1999).
Attentional priming has similarly been reported for the olfactory system in a study investigating event-related potentials (ERP). Attending to orthonasally presented odor, compared to ignoring it, lead to a decrease in latency of early components and to an increase in amplitude of late components (Krauel, Pause et al. 1998). Behaviorally, Ashkenazi and Marks (2004) reported that attending to retronasally presented vanillin did not improve its detectability, though attention did improve detectability of sucrose (Ashkenazi and Marks 2004) and citric acid (Marks and Wheeler 1998). Attention did not enhance detectability of sucrose or vanillin when they were presented in the same oral aqueous solution, which was suggested to be due to their quality fusion into a unique new flavor (Ashkenazi and Marks 2004). Future studies should explore the possibility of behavioral and neural differences when attending to orthonasal versus retronasal odorants, as well as to the possibility of neural priming in gustation. It will furthermore be of interest to assess to what extent differences in odor versus taste processing as modulated by attention reflects their differential involvement with thalamo-cortical gating mediated by the thalamic reticular nucleus (Crick 1984), as the olfactory system anatomically lacks such obligatory thalamic mediation (Fig. 1).
Unattended information may be highly processed without awareness thereof, up to the semantic level, as shown in extinction experiments with patients showing unilateral neglect (Gazzaniga, Ivry et al. 2002) and experiments on change blindness and attentional blink, arguing for a “late selection” process (Chun and Marois 2002). Gazzaniga and colleagues (1999) have shown, in a well-controlled fMRI experiment, that frontal and parietal cortical areas mediate the internal “spotlight” of visual spatial attention, including the frontal eye fields, PPC and cingulate gyrus, as have others (Pessoa, Kastner et al. 2003). This network of areas overlaps with the one revealed in neuroimaging studies on attention to visual object features (Pessoa, Kastner et al. 2003). It has been proposed that this frontal-parietal network is required for explicit awareness of visual events that are neurally processed up to the category- and item-specific level (Chun and Marois 2002), by providing top-down biasing signals modulating activity in the visual cortex (Pessoa, Kastner et al. 2003). It remains to be tested whether this also holds for orolfactory food perception.
Emotionally salient stimuli can act as exogenous cues for attention (Gazzaniga, Ivry et al. 2002). For example, aversive words are detected more readily and produce smaller attentional blink errors (Chun and Marois 2002). It has been found that the amygdala plays a major role in the emotional modulation of attention (Anderson and Phelps 2001). Amygdala activation can be elicited by emotionally salient stimuli without awareness (Whalen, Rauch et al. 1998) and requires attention (Pessoa, McKenna et al. 2002). As orolfactory stimuli can be primary reinforcers (Rolls 1999) this suggests that the amygdala may be critical for the exogenous attentional cuing to these stimuli. There, the amygdala may show interaction with the right temporoparietal junction and inferior and middle temporal gyri, which have been suggested to be involved in exogenous attentional functions (Corbetta and Shulman 2002).
3. Conclusions
In summary, there is converging evidence (mainly from the visual neurosciences) that conscious experience is correlated with activity in higher association cortices like frontal, parietal, temporal and cingulate cortex. Neurodynamically, such activity sets up a self-sustained reentrant/recurrent net standing wave between higher (heteromodal) and lower (unimodal) cortical areas (essential nodes).
I suggest that the modality of taste may be considered one among several modalities that are represented in a unified system encoding input of the oral cavity, the oral homeostatic interoceptive sensory system (oHIS), which exists in parallel with Craig's non-oral homeostatic interoceptive system. Conceiving of the taste system as part of a homeostatic system explains several observations not integrated before and may be useful in the investigation of the neural correlates of consciousness. This may have implications for understanding pathological conditions such as body image and eating disorders as well.
Based on this synthesis and anatomical, electrophysiological and imaging studies, I propose that two overlapping but distinct networks are involved in food object (recognition) awareness and food affective awareness: the agranular insula-perirhinal cortex network and the agranular insula-orbitofrontal cortex-anterior cingulate network, respectively. I suggest that the essential nodes involve different neural sets than those of vision, e.g. via differential activation of gradients across the Ia and OFC. The overall conscious experience of food identity and affect would involve the synchronous reciprocal activation among these areas.
The function of various brain areas (especially the perirhinal cortex, anterior cingulate, insula and OFC) in flavor perception needs further exploration, for example by studying the perception of flavors in patients or monkeys with well-defined lesions in these areas and other standard techniques.
Unfortunately the neural correlates of attention to and consciousness of food flavor or its components have thus far been unexplored. I suggest that masking tasks and perhaps bistable percepts, as have been employed in vision, may be valuable means for such research.
Acknowledgements
JVV wishes to thank Drs. Thomas R. Scott, Barbara K. Giza, Stuart A. McCaughey, Edmund T.Rolls and Mikiko Kadohisa for their collaboration with the multimodal electrophysiology. The comments and discussion by Dr. Barry Green are gratefully acknowledged. JVV is supported by NIH grant R03 DC008197-01 to JVV.
Abbreviations in text
- ACC
anterior cingulate cortex
- Ag
amygdala
- AI/FO
anterior insula/frontal operculum
- CN
cranial nerve
- cNTS
caudal nucleus of the solitary tract
- cP
centiPoise
- CT
chorda tympani
- DMV
dorsal vagus motor nucleus
- ErC
entorhinal cortex (BA 28)
- FO
frontal operculum
- HIS
homeostatic interoceptive system
- I
insula
- Ia
agranular insula
- Id
dysgranular insula
- Ig
granular insula
- IT
inferotemporal cortex
- LGN
lateral geniculate nucleus
- LOT
lateral olactory tract
- mN5
motor trigeminal nucleus
- MOB
main olfactory bulb
- NaCl
sodium chloride
- noHIS
non-oral part of the homeostatic interoceptive system
- NTS
nucleus of the solitary tract
- OB
olfactory bulb
- OFC
orbitofrontal cortex
- oHIS
oral part of the homeostatic interoceptive system
- ors
olfactory receptors
- PBN
parabrachial nucleus
- PC
piriform cortex (also called pyriform, prepyriform, primary olfactory or olfactory paleocortex)
- pPC
posterior piriform cortex
- PPC
posterior parietal cortex
- PrC
perirhinal cortex (BA 35, 36)
- rNTS
rostral nucleus of the solitary tract
- spN5
spinal trigeminal nucleus
- STS
suprior temporal suclus
- TEO
temporal-occipital area
- VPM
ventroposteromedial nucleus of the thalamus
- VPMpc
parvicelular part of the ventroposteromedial nucleus of the thalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aato M, Ogawa H, et al. Response properties of macaque chorda tympani fibers. J Gen Physiol. 1975;66(6):781–810. doi: 10.1085/jgp.66.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Marks LE. Effect of endogenous attention on detection of weak gustatory and olfactory flavors. Percept & Psychophys. 2004;66(4):596–608. doi: 10.3758/bf03194904. [DOI] [PubMed] [Google Scholar]
- Barbas H. Organization of cortical afferent input to orbitofrontal areas in the rhesus monkey. Neuroscience. 1993;56(4):841–64. doi: 10.1016/0306-4522(93)90132-y. [DOI] [PubMed] [Google Scholar]
- Baylis LL, Rolls ET, et al. Afferent connections of the orbitofrontal cortex taste area of the primate. Neuroscience. 1994;64:801–812. doi: 10.1016/0306-4522(94)00449-f. [DOI] [PubMed] [Google Scholar]
- Bozza T, McGann JP, et al. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42:9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- Buck L. Information coding in the vertebrate olfactory system. Ann Rev Neurosc. 1996;19:517–544. doi: 10.1146/annurev.ne.19.030196.002505. [DOI] [PubMed] [Google Scholar]
- Cardello AV. Chapter 1. The role of the human senses in food acceptance. In: Meiselman HL, MacFie HJH, editors. Food choice, acceptance and consumption. Blackie Academic; London: 1996. [Google Scholar]
- Cattarelli M, Astic L, et al. Metabolic mapping of 2DG uptake in the rat piriform cortex using computerized image processing. Brain Res. 1988;442:180–184. doi: 10.1016/0006-8993(88)91449-7. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, et al. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10(3):220–42. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Chun MM, Marois R. The dark side of visual attention. Curr Opin Neurobiol. 2002;12:184–189. doi: 10.1016/s0959-4388(02)00309-4. [DOI] [PubMed] [Google Scholar]
- Cinellar AR, Ferreyra-Moyano H, et al. Reciprocal functional connections of the olfactory bulbs and other olfactory related areas with the prefrontal cortex. Brain Res Bull. 1987;19(6):651–662. doi: 10.1016/0361-9230(87)90051-7. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Linster C. Central olfactory pathways. In: Doty RL, editor. Handbook of olfaction and gustation. Marcel Dekker; New York: 2003. [Google Scholar]
- Contreras RJ, Lundy RF., Jr. Gustatory neuron types in the periphery: a functional perspective. Phys & Behav. 2000;69:41–52. doi: 10.1016/s0031-9384(00)00187-6. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, et al. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci. 1991;11(8):2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosc. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosc. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003;26(6):303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: why are some more aware than others? Trends Cogn Sci. 2004;8(6):239–241. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, et al. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3(2):184–90. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Nat Acad Sc. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F, Koch C. A framework for consciousness. Nat Rev Neurosc. 2003;6(2):119–126. doi: 10.1038/nn0203-119. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, et al. Neuroanatomical basis for first- and second-order representations of bodily states. Nat Neurosci. 2001;4(2):207–212. doi: 10.1038/84048. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, et al. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' Error. Putnam; New York: 1994. [Google Scholar]
- De Araujo IET, Kringelbach ML, et al. The representation of umami taste in the human brain. Journal of Neurophysiology. 2003;90:313–319. doi: 10.1152/jn.00669.2002. [DOI] [PubMed] [Google Scholar]
- de Araujo IET, Rolls ET, et al. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosc. 2003;18:2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Sergent C, et al. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc Nat Acad Sc. 2003;100(14):8520–8525. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo PM. The neural code for taste in the brain stem: response profiles. Phys & Behav. 2000;69:87–96. doi: 10.1016/s0031-9384(00)00191-8. [DOI] [PubMed] [Google Scholar]
- Doty RL, Laing DG. Psychophysical measurement of human olfactory function, including odorant mixture assesment. In: Doty RL, editor. Handbook of olfaction and gustation. Marcel Dekker; New York: 2003. [Google Scholar]
- Edelman GM. Naturalizing consciousness: A theoretical framework. Proc Nat Acad Sc. 2003;100(9):5520–5524. doi: 10.1073/pnas.0931349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory consciousness. Trends Cogn Sci. 2001;5(1):16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Erickson RP. The evolution of neural coding ideas in the chemical senses. Phys & Behav. 2000;69:3–13. doi: 10.1016/s0031-9384(00)00193-1. [DOI] [PubMed] [Google Scholar]
- Feldman M, Richardson CT. Role of thought, sight, smell, and taste of food in the cephalic phase of gastric acid secretion in humans. Gastroenterology. 1986;90:428–433. doi: 10.1016/0016-5085(86)90943-1. [DOI] [PubMed] [Google Scholar]
- Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- Francis S, Rolls ET, et al. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport. 1999;10(3):453–9. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: evidence for multiple memory systems in the primate temporal lobe. Exp Brain Res. 1994;99(3):411–22. doi: 10.1007/BF00228977. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Ivry RB, et al. Cognitive neuroscience. W.W. Norton & Company; 2002. Selective attention and orienting; pp. 244–299. [Google Scholar]
- Gilbertson TA, Margolskee RF. Molecular physiology of gustatory transduction. In: Doty RL, editor. Handbook of olfaction and gustation. Marcel Dekker; New York: 2003. [Google Scholar]
- Gitelman DR, Nobre AC, et al. A large-scale distributed network for covert spatial attention. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Goulet S, Murray EA. Neural substrates of crossmodal association memory in monkeys: the amygdala versus the anterior rhinal cortex. Behav Neurosc. 2001;115(2):271–284. [PubMed] [Google Scholar]
- Green BG. Chemesthesis: Pungency as a component of flavor. Trends Food Sc Technol. 1996;7:415–420. [Google Scholar]
- Grossberg S. The attentive brain. Am Sci. 1995;83:438–449. [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insight from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Heimer L. The human brain and spinal cord. Springer-Verlag; New York: 1994. [Google Scholar]
- Hudspeth AJ, Logothetis NK. Sensory systems. Curr Opinion Neurobiol. 2000;10:631–641. doi: 10.1016/s0959-4388(00)00133-1. [DOI] [PubMed] [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol. 2003;457:361–367. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Rolls ET, et al. Orbitofrontal cortex: neuronal representation of oral temperature and capsaicin in addition to taste and texture. Neurosc. 2004;127(1):207–221. doi: 10.1016/j.neuroscience.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Rolls ET, et al. The primate amygdala: neuronal representations of the viscosity, fat texture, grittiness, and taste of foods. Neurosc. 2005;132:33–48. doi: 10.1016/j.neuroscience.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Katz DB, Nicolelis MA, et al. Nutrient tasting and signaling mechanisms in the gut. IV. There is more to taste than meets the tongue. Am J Physiol Gastrointest Liver Physiol. 2000;278(1):G6–9. doi: 10.1152/ajpgi.2000.278.1.G6. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, et al. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1986;379(2):329–41. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- Krauel K, Pause BM, et al. Attentional modulation of central odor processing. Chem Senses. 1998;23(4):423–432. doi: 10.1093/chemse/23.4.423. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. Food for thought: hedonic experience beyond homeostasis in the human brain. Neurosc. 2004;126:807–819. doi: 10.1016/j.neuroscience.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lamme VAF, Roelfsema PR. The distinct modes of vision offered by feedforard and recurrent processing. Trends Neurosci. 2000;23(11) doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- Laurent G. Dynamical representation of odors by oscilating and evolving neural assemblies. Trends Neurosci. 1996;19(11):489–496. doi: 10.1016/S0166-2236(96)10054-0. [DOI] [PubMed] [Google Scholar]
- Lindemann B. Taste reception. Physiol Rev. 1996;76(3):719–765. doi: 10.1152/physrev.1996.76.3.719. [DOI] [PubMed] [Google Scholar]
- Llinas R, Ribary U. Consciousness and the brain. The thalamocortical dialogue in health and disease. Ann N Y Acad Sci. 2001;929:166–175. [PubMed] [Google Scholar]
- Lundy RF, Jr., Contreras RJ. Taste prestimulation increases the chorda tympani nerve response to menthol. Physiol & Behav. 1993;54(1):65–70. doi: 10.1016/0031-9384(93)90044-g. [DOI] [PubMed] [Google Scholar]
- Marks LE, Wheeler ME. Attention and the detectibility of weak taste stimuli. Chem Senses. 1998;23(1):19–29. doi: 10.1093/chemse/23.1.19. [DOI] [PubMed] [Google Scholar]
- Matsuo R, Inoue T, et al. Neural activity of chorda tympani mechanosensitive fibers during licking behavior in rats. Brain Res. 1995;689(2):289–98. doi: 10.1016/0006-8993(95)00582-b. [DOI] [PubMed] [Google Scholar]
- Matsuo R, Shimizu N, et al. Lateral hypothalamic modulation of oral sensory afferent activity in nucleus tractus solitarius neurons of rats. J Neurosci. 1984;4(5):1201–7. doi: 10.1523/JNEUROSCI.04-05-01201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M. From sensation to perception. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Principles of behavioral and cognitive neurology. Oxford University Press; Oxford: 2000. [Google Scholar]
- Moon C, Ronnett GV. Molecular neurobiology of olfactory transduction. In: Doty RL, editor. handbook of olfaction and gustation. Marcel Dekker; New York: 2003. [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120(4):701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Perceptual-mnemonic function of the perirhinal cortex. Trends in Cognitive Sciences. 1999;3(4):142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Murray EA, Richmond BJ. Role of perirhinal cortex in object perception, memory, and associations. Curr Opin Neurobiol. 2001;11:188–193. doi: 10.1016/s0959-4388(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Norgren R. The gustatory system in mammals. Am J Otolaryngol. 1983;4(4):234–7. doi: 10.1016/s0196-0709(83)80064-7. [DOI] [PubMed] [Google Scholar]
- Norgren R. Central neural mechanisms of taste. In: Darian-Smith I, editor. Handbook of physiology-The nervous system. Vol. 3. Am Physiol Soc.; Bethesda: 1984. pp. 1087–1128. [Google Scholar]
- Norgren R. The central gustatory system in humans. In: Paxinos G, editor. The Human Nervous System. Academic; New York: 1988. [Google Scholar]
- O'Craven KM, Downing PE, et al. fMRI evidence for objects as the units of attentional selection. Nature. 1999;401:584–587. doi: 10.1038/44134. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, et al. The representation of pleasant and aversive taste in the human brain. Journal of Neurophysiology. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, et al. Areas of the orbitofrontal cortex and amygdala activated by pleasant and unpleasant taste. Society for Neuroscience Abstracts. 1999;25 [Google Scholar]
- Ogawa H, Hayama T, et al. Thermal sensitivity of neurons in the rostral part of the rat solitary nucleus. Br Res. 1988;454(1-2):321–331. doi: 10.1016/0006-8993(88)90833-5. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Sato M, et al. Multiple sensitivity of chorda typani fibres of the rat and hamster to gustatory and thermal stimuli. J Physiol (Lond) 1968;199(1):223–40. doi: 10.1113/jphysiol.1968.sp008650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangborn RM. Some aspects of chemo-reception in human nutrition. In: Kare MR, Maller O, editors. The chemical senses and nutrition. Johns Hopkins University Press; Baltimore, MD: 1967. pp. 45–60. [Google Scholar]
- Parker EA, Gaffan D. Lesions of the primate rhinal cortex cause deficits in flavour-visual associative memory. Behavioural Brain Research. 1998;93:99–105. doi: 10.1016/s0166-4328(97)00148-4. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, et al. Neuroimagng studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23(10):3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, et al. Neural processing of emotional faces requires attention. Proc Nat Acad Sc. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J. Flavour as a psychological construct: implications for perceiving and measuring the sensory qualities of foods. Food Qual Pref. 1999;10:349–356. [Google Scholar]
- Pritchard TC, Hamilton RB, et al. Neural coding of gustatory information in the thalamus of Macaca mulatta. J Neurophysiol. 1989;61(1):1–14. doi: 10.1152/jn.1989.61.1.1. [DOI] [PubMed] [Google Scholar]
- Rees G, Kreiman G, et al. Neural correlates of consciousness in humans. Nat Rev Neurosc. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- Rensink RA. Change detection. Annu Rev Psychol. 2002;53:245–277. doi: 10.1146/annurev.psych.53.100901.135125. [DOI] [PubMed] [Google Scholar]
- Rentmeister-Bryant H, Green BG. Perceived irritation during ingestion of capsaicin or piperine: comparison of trigeminal and non-trigeminal areas. Chem Senses. 1997;22(3):257–266. doi: 10.1093/chemse/22.3.257. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The Brain and Emotion. Oxford University Press; Oxford: 1999. [Google Scholar]
- Rolls ET. Consciousness absent and present: a neurophysiological exploration. Progress in Brain Research. 2004;144:95–106. doi: 10.1016/S0079-6123(03)14406-8. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Baylis LL. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J Neurosci. 1994;14(9):5437–52. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Deco G. Computational Neuroscience of Vision. Oxford University Press; Oxford: 2002. [Google Scholar]
- Rolls ET, Scott TR. Central taste anatomy and neurophysiology. In: Doty RL, editor. Handbook of Olfaction and Gustation. Vol. 32. Dekker. chap; New York: 2003. pp. 679–705. [Google Scholar]
- Rolls ET, Scott TR, et al. The responsiveness of neurones in the frontal opercular gustatory cortex of the macaque monkey is independent of hunger. J Physiol (Lond) 1988;397:1–12. doi: 10.1113/jphysiol.1988.sp016984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Sienkiewicz ZJ, et al. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. Eur J Neurosc. 1989;1:53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Verhagen JV, et al. Representations of the texture of food in the primate orbitofrontal cortex: neurons responding to viscosity, girttiness and capsaicin. J. Neurophysiol. 2003;90:3711–3724. doi: 10.1152/jn.00515.2003. [DOI] [PubMed] [Google Scholar]
- Satinoff E. A reevaluation of the concept of the homeostatic organization of temperature regulation. In: Satinoff E, Teitelbaum P, editors. Handbook of behavioral neurobiology. Vol. 6. Plenum Press; New York: 1983. pp. 442–472. Motivation: [Google Scholar]
- Savic I. Imaging of brain activation by odorants in humans. Curr Opin Neurobiol. 2002;12:455–461. doi: 10.1016/s0959-4388(02)00346-x. [DOI] [PubMed] [Google Scholar]
- Schiffman SS. Taste quality and neural coding: implications from psychophysics and neurophysiology. Physiol Behav. 2000;69:147–159. doi: 10.1016/s0031-9384(00)00198-0. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Neuer G, et al. Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience. 2004;127:347–353. doi: 10.1016/j.neuroscience.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, et al. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10(3):272–84. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Scott TR. Taste: the neural basis of body wisdom. In: Simopoulos A, editor. Nutritional triggers for health and disease. Vol. 67. Karger; Basel: 1992. pp. 1–39. [DOI] [PubMed] [Google Scholar]
- Scott TR, Giza BK. Issues of gustatory neural coding: where they stand today. Physiol & Behav. 2000;69:65–76. doi: 10.1016/s0031-9384(00)00189-x. [DOI] [PubMed] [Google Scholar]
- Scott TR, Mark GP. Feeding and taste. Progress Neurobiol. 1986;27:293–317. doi: 10.1016/0301-0082(86)90004-3. [DOI] [PubMed] [Google Scholar]
- Scott TR, Plata-Salaman CR. Coding of taste quality. In: Getchell TV, editor. Smell and taste in health and disease. Raven Press; New York: 1991. [Google Scholar]
- Scott TR, Plata-Salaman CR. Taste in the monkey cortex. Phys & Behav. 1999;67(4):489–511. doi: 10.1016/s0031-9384(99)00115-8. [DOI] [PubMed] [Google Scholar]
- Scott TR, Verhagen JV. Taste as a factor in the management of nutrition. Nutrition. 2000;16:874–885. doi: 10.1016/s0899-9007(00)00423-8. [DOI] [PubMed] [Google Scholar]
- Scott TR, Yan J, et al. Brain mechanisms of satiety and taste in macaques. Neurobiology. 1995;3(3-4):281–92. [PubMed] [Google Scholar]
- Sewards TV. Dual separate pathways for sensory and hedonic aspects of taste. Br Res Bull. 2004;62:271–283. doi: 10.1016/j.brainresbull.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Cortical association areas in the gustatory system. Neurosc & Biobehav Rev. 2001;25:395–407. doi: 10.1016/s0149-7634(01)00021-5. [DOI] [PubMed] [Google Scholar]
- Shimatani Y, Grabauskiene S, et al. Long-term recording from the chorda tympani nerve in rats. Phys & Behav. 2002;76(1):143–9. doi: 10.1016/s0031-9384(02)00684-4. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, et al. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;14:701–11. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Small DM, Zald DH, et al. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport. 1999;10(1):7–14. doi: 10.1097/00001756-199901180-00002. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, et al. Changes in brain activity related to eating chocolate from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Smith DV, Scott TR. Gustatory neural coding. In: Doty RL, editor. Handbook of olfaction and gustation. Marcel Dekker; New York: 2003. [Google Scholar]
- Smith DV, John SJ. Neural coding of gustatory information. Curr Opin Neurobiol. 1999;9(4):427–35. doi: 10.1016/S0959-4388(99)80064-6. [DOI] [PubMed] [Google Scholar]
- Smith DV, John SJ, et al. Neural cell types and taste quality coding. Physiol&Behav. 2000;69:77–85. doi: 10.1016/s0031-9384(00)00190-6. [DOI] [PubMed] [Google Scholar]
- Sobel N, Johnson BN, et al. Functional neuroimaging of human olfaction. In: Doty RL, editor. Handbook of olfaction and gustation. Marcel Dekker; New York: 2003. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers, Inc; New York: 1988. [Google Scholar]
- Teff KL. Physiological effects of flavour perception. Trends Food Sc Technol. 1996;7:448–452. [Google Scholar]
- Thornton JA, Malkova L, et al. Rhinal cortex ablations fail to disrupt reinforcer devaluation effects in rhesus monkeys (Macaca mulatta) Behav Neurosc. 1998;112(4):1020–1025. doi: 10.1037//0735-7044.112.4.1020. [DOI] [PubMed] [Google Scholar]
- Tovee MJ, Rolls ET. Oscillatory activity is not evident in the primate temporal visual cortex with static stimuli. Neuroreport. 1992;3(4):369–372. doi: 10.1097/00001756-199204000-00020. [DOI] [PubMed] [Google Scholar]
- Turner BH, Mishkin M, et al. Organization of the amygdalopetal modality-specific cortical association areas in the monkey. Journal of Comparative Neurology. 1980;191:515–543. doi: 10.1002/cne.901910402. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, De Yoe EA. Concurrent processing in the primate visual cortex. In: Gazzaniga M, editor. The Cognitive Neurosciences. MIT Press; Cambridge, Mass.: 1995. pp. 383–400. [Google Scholar]
- Verhagen JV, Engelen L. The neurocognitive bases of human multimodal food perception: Sensory integration. Neurosc Biobehav Rev. 2006;30(5):613–650. doi: 10.1016/j.neubiorev.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Giza BK, et al. Responses to taste stimulation in the ventroposteromedial nucleus of the thalamus in rats. J Neurophys. 2003;89:265–275. doi: 10.1152/jn.00870.2001. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Kadohisa M, et al. The primate insular/opercular taste cortex: neuronal representations of the viscosity, fat texture, grittiness, temperature and taste of foods. J Neurophys. 2004;92:1685–1699. doi: 10.1152/jn.00321.2004. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Rolls ET, et al. Neurons in the primate orbitofrontal cortex respond to fat texture independently of viscosity. Journal of Neurophysiology. 2003;90:1514–1525. doi: 10.1152/jn.00320.2003. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, et al. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Keysers C, et al. Both of us disgusted in my insula: The common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Sensory physiology of central olfactory pathways. In: Doty RL, editor. Handbook of olfaction and gustation. Marcel Dekker; New York: 2003. [Google Scholar]
- Yamamoto T, Matsuo R, et al. Taste responses of cortical neurons in freely ingesting rats. J Neurophysiol. 1989;61(6):1244–58. doi: 10.1152/jn.1989.61.6.1244. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yuyama N, et al. Cortical neurons responding to tactile, thermal and taste stimulations of the rat's tongue. Brain Res. 1981;221:202–206. doi: 10.1016/0006-8993(81)91075-1. [DOI] [PubMed] [Google Scholar]
- Yaxley S, Rolls ET, et al. Satiety does not affect gustatory activity in the nucleus of the solitary tract of the alert monkey. Brain Res. 1985;347(1):85–93. doi: 10.1016/0006-8993(85)90891-1. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Sugai T, et al. Cortical spatial aspects of optical intrinsic signals in response to sucrose and NaCl stimuli. Neuroreport. 2004;15(1):17–20. doi: 10.1097/00001756-200401190-00005. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Functional neuroimaging of the olfactory system in humans. Int J Psychophysiol. 2000;36(2):165–81. doi: 10.1016/s0167-8760(99)00110-5. [DOI] [PubMed] [Google Scholar]
- Zeki S. Localization and globalization in conscious vision. Ann Rev Neurosc. 2001;24:57–86. doi: 10.1146/annurev.neuro.24.1.57. [DOI] [PubMed] [Google Scholar]
- Zeman A. Consciousness. Brain. 2001;124:1263–1289. doi: 10.1093/brain/124.7.1263. [DOI] [PubMed] [Google Scholar]
- Zou Z, Horowitz LF, et al. Genetic tracing reveals a stereotyped sensory map in the olfactory cortex. Nature. 2001;414:173–179. doi: 10.1038/35102506. [DOI] [PubMed] [Google Scholar]