Abstract
Background
The aim of this study was to evaluate the clinical usefulness of a semiquantitative procalcitonin kit for assessing severity of sepsis and early determination of mortality in affected patients.
Methods
This was a prospective, observational study including 206 septic patients enrolled between June 2008 and August 2009. Disseminated intravascular coagulation (DIC), Sequential Organ Failure Assessment (SOFA), Acute Physiology and Chronic Health Evaluation (APACHE) II scores were measured, along with semiquantitative procalcitonin concentrations. Patients were divided into three groups based on their semiquantitative procalcitonin concentrations (group A, <2 ng/mL; group B ≥ 2 ng/mL < 10 ng/mL; group C ≥ 10 ng/mL).
Results
A significant difference in DIC, SOFA, and APACHE II scores was found between group A and group C and between group B and group C (P < 0.01). Patients with severe sepsis and septic shock had significantly higher procalcitonin concentrations than did patients with less severe disease. The rate of patients with septic shock with high procalcitonin concentrations showed an upward trend. There was a significant (P < 0.01) difference between the three groups with regard to numbers of patients and rates of severe sepsis, septic shock, DIC, and mortality.
Conclusion
Semiquantitative procalcitonin concentration testing can be helpful for early assessment of disease severity in patients with sepsis. Furthermore, it may also help in predicting early mortality in septic patients. Based on the level of semiquantitative procalcitonin measured in patients with suspected sepsis, a timely decision can be reliably made to transfer them to a tertiary hospital with an intensive care unit for optimal care.
Keywords: sepsis, semiquantitative procalcitonin, Sequential Organ Failure Assessment, Acute Physiology and Chronic Health Evaluation II, mortality, procalcitonin
Introduction
Sepsis is one of the most significant causes of mortality in the intensive care unit. Recent international guidelines for management of severe sepsis and septic shock1 recommend early diagnosis and treatment of sepsis because any delay may lead to a more serious outcome, ie, fatal organ failure and death.2 A diagnosis of sepsis is based on evidence of infection along with the presence of systemic inflammatory response syndrome, defined by the presence of two or more of the following: elevated or lowered body temperature, abnormal white blood cell count, elevated heart rate, and high respiratory rate.3 It has been documented that quantitative evaluation of procalcitonin is useful for discriminating between patients with bacterial infection and those with systemic inflammatory response syndrome caused by another illness.4–8 Similarly, many studies have reported that quantitative procalcitonin values may help to discriminate between patients with severe sepsis infection and septic shock and those with less severe conditions.4–13 Quantitative procalcitonin concentration is significantly correlated with both the Sequential Organ Failure Assessment (SOFA)14 score and the Acute Physiology and Chronic Health Evaluation (APACHE) II15 score,13,16,17 and significant differences in quantitative procalcitonin concentrations have been found between patients with and without septic shock.13 A quantitative procalcitonin concentration is also useful as an early prognostic indicator; among patients with septic shock, those who died in the intensive care unit had significantly higher quantitative procalcitonin concentrations at all assay time points than those who survived to be discharged from an intensive care unit.18
Due to the cost of procalcitonin analysis, hospitals with the capability to measure quantitative procalcitonin concentrations are limited to larger facilities with intensive care units. However, in hospitals and medical clinics without intensive care units, it is important to diagnose sepsis early and to make an estimate of severity in support of an early decision to transfer patients to a hospital with an intensive care unit so that they may receive optimal care. Therefore, semiquantitative procalcitonin measurement may benefit patients in hospitals and medical clinics without intensive care units.
A semiquantitative procalcitonin test kit is easy to use at the bedside. Research indicates that semiquantitative procalcitonin concentration ranges are well correlated with those of quantitative procalcitonin measurements.19,20 However, to the best of our knowledge, no study has reported on the usefulness of a semiquantitative procalcitonin kit for estimating severity and determining the prognosis of sepsis. Therefore, the aim of this study was to evaluate the clinical usefulness of a semiquantitative procalcitonin kit for assessing severity of sepsis and early determination of mortality in affected patients.
Materials and methods
Study population
This prospective observational study was conducted in the Department of General Medicine and Emergency Department of Toyooka Public Hospital, Hyogo, Japan, a 500-bed teaching hospital that has been designated as the major emergency center of the North Hyogo province by the Japanese government. The local ethics committee approved the study and waived the requirement for written informed consent. Patients were evaluated for the presence of systemic inflammatory response syndrome and sepsis as defined by American College of Chest Physicians/Society of Critical Care Medicine guidelines.3 Systemic inflammatory response syndrome was defined as a systemic inflammatory response to an unspecified stimulus manifested by the presence of two or more of the following: body temperature < 36°C or >38°C, heart rate > 90 beats per minute, respiratory rate > 20 breaths per minute or PaCO2 < 32 Torr (mmHg), and white blood cell count > 12,000 cells/mm3, <4000 cells/mm3, or >10% band forms (immature white blood cells). Sepsis was diagnosed when patients met criteria for systemic inflammatory response syndrome and an infectious source was documented or strongly suspected on the basis of clinical appearance.3 We excluded patients younger than 18 years of age as well as patients with do not resuscitate status, those with liver cirrhosis, those receiving warfarin, and individuals with evidence of traumatic injury. The patients included were consecutively enrolled from June 2008 to August 2009. Semiquantitative procalcitonin concentrations and C-reactive protein were measured on admission. SOFA and APACHE II scores were determined on the day of admission. Blood pressure, body temperature, pulse rate, respiratory rate, white blood cell count, Glasgow Coma Scale,21 serum albumin level, and antithrombin activity level were also measured. The maximum C-reactive protein value was defined as the highest level recorded during the first 48 hours of hospital admission.
The final diagnosis was determined by the physician based on clinical course in the hospital. Systemic inflammatory response syndrome, severe sepsis, and septic shock were diagnosed based on the criteria of the Consensus Conference of the American Society of Chest Physicians/Society of Critical Care Medicine.3 A diagnosis of disseminated intravascular coagulation (DIC) was based on the diagnostic criteria established by the Japanese Association for Acute Medicine.22 All patients were followed up for 28 days after enrollment in the study, and 28-day all-cause mortality was assessed.
Procalcitonin measurement
Procalcitonin was measured using the semiquantitative Procalcitonin- Q® test kit (Brahms Ag Diagnostica, Berlin, Germany). Procalcitonin-Q is a rapid immunochromatographic test performed by pipetting 200 μL of serum onto a test strip, with results available in 30 minutes. The manufacturer’s reference scale categorizes four procalcitonin level intervals, ie, <0.5 ng/mL, ≥0.5 ng/mL and <2 ng/mL, ≥2 ng/mL and <10 ng/mL, and ≥10 ng/mL.
Previous reports have shown that quantitative procalcitonin concentrations above 2 ng/mL discriminate between patients with systemic inflammatory response syndrome, severe bacterial infections, and sepsis, and patients with systemic inflammatory response syndrome, sepsis, and organ failure.17,23 It is also suspected that a semiquantitative procalcitonin concentration above 2 ng/mL indicates the presence of bacterially induced systemic inflammatory response syndrome.24 Therefore, patients were divided into three groups based on semiquantitative procalcitonin concentrations: group A comprised patients showing semiquantitative procalcitonin results < 2 ng/mL; group B had semiquantitative procalcitonin results ≥ 2 ng/mL and <10 ng/mL; and group C had semiquantitative procalcitonin results ≥ 10 ng/mL.
Statistical analysis
The statistical significance of differences were determined using the analysis of variance or the Chi-square test. Calculations were performed with R, version 2.12.1 (R Foundation for Statistical Computing, Vienna, Austria), and P values < 0.05 were considered to be statistically significant.
Results
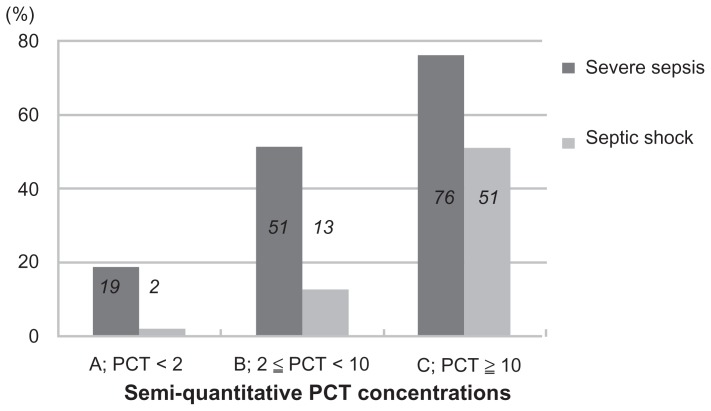
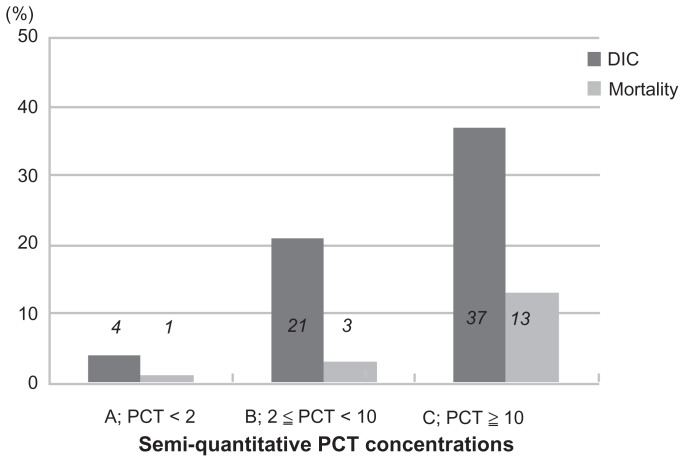
A total of 206 patients were enrolled in the study, were of mean age 75.8 ± 13.5 (range 23–103) years, and had a male-to- female ratio of 113:93. Table 1 shows the infection sites in the 206 patients. The enrolled patients were divided into three groups as follows: group A, comprising 84 patients (46 with a procalcitonin concentration < 0.5 ng/mL and 38 with procalcitonin ≥0.5 and <2 ng/mL); group B, including 39 patients; and group C with 83 patients. The clinical data for each group are shown in Table 2. Systolic blood pressure and mean blood pressure significantly decreased in groups B and C as compared with group A. Furthermore, group C patients showed increased respiratory rates compared with groups A and B. The antithrombin activity level was significantly decreased in groups B and C as compared with group A. Table 3 shows mean DIC, SOFA, and APACHE II scores in each procalcitonin group. A significant difference in DIC, SOFA, and APACHE II scores was found between group A and group C and between group B and group C. Figure 1 shows a comparison of semiquantitative procalcitonin concentration with the rate of severe sepsis and septic shock. In group C, the rates of patients with severe sepsis and septic shock were extremely high, at 76% and 51%, respectively. Figure 2 shows a comparison of semiquantitative procalcitonin concentration with the rate of DIC and 28-day all-cause mortality. The mortality rate, at 13% in group C, is obviously high.
Table 1.
Infection sites in patients
| Infection site | Patients (n) |
|---|---|
| Respiratory system | |
| Pneumonia | 104 |
| Empyema thoracis | 3 |
| Urinary system | |
| Pyelonephritis | 64 |
| Prostatitis | 1 |
| Hepatobiliary system | |
| Liver abscesses | 3 |
| Cholangitis | 3 |
| Cholecystitis | 2 |
| Soft tissue system | |
| Cellulitis | 5 |
| N ecrotizing fasciitis | 5 |
| Gastrointestinal system | |
| Colon diverticulitis | 3 |
| Appendicitis | 3 |
| Enterocolitis | 2 |
| Others | |
| Iliopsoas muscle abscess | 2 |
| Purulent meningitis | 2 |
| Acute tonsillitis | 2 |
| Infective endocarditis | 1 |
| Acute sinusitis | 1 |
Table 2.
Clinical data for study patient groups according to procalcitonin level
| PCT level | P value | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Group A <2 | Group B ≥2, <10 | Group C ≥10 | A vs B | A vs C | B vs C | |
| n | 84 | 39 | 83 | |||
| Gender (male/female) | 42/42 | 26/13 | 38/47 | |||
| Age (years) | 75.3 ± 15.1 | 76.1 ± 13.4 | 76.4 ± 12.2 | 1.00 | 1.00 | 1.00 |
| Systolic BP (mmHg) | 125.6 ± 22.6 | 117.2 ± 23.0 | 102.1 ± 21.6 | 0.049 | <0.001 | 0.001 |
| Diastolic BP (mmHg) | 71.1 ± 14.6 | 63.1 ± 14.7 | 57.8 ± 15.0 | 0.03 | <0.001 | 0.049 |
| Mean BP (mmHg) | 88.8 ± 15.2 | 81.1 ± 16.5 | 72.6 ± 16.0 | 0.014 | 0.001 | <0.001 |
| Respiratory rate (per minute) | 22.6 ± 4.7 | 23.8 ± 4.9 | 26.0 ± 5.9 | 0.32 | <0.001 | 0.05 |
| Heart rate (beats per minute) | 92.3 ± 22.7 | 101.2 ± 19.5 | 102.8 ± 21.8 | 0.11 | <0.001 | 0.82 |
| Temperature (°C) | 37.9 ± 1.7 | 38.5 ± 1.0 | 38.1 ± 2.1 | 0.34 | 0.51 | 0.51 |
| GCS score | 13.8 ± 2.4 | 13.4 ± 2.4 | 12.9 ± 3.1 | 0.41 | 0.08 | 0.62 |
| WBC (×1000/μL) | 13.1 ± 6.2 | 14.7 ± 7.5 | 13.5 ± 8.1 | 0.84 | 0.85 | 0.85 |
| Maximum CRP (mg/dL) | 15.0 ± 8.5 | 17.8 ± 7.8 | 19.5 ± 9.5 | 0.25 | 0.001 | 0.33 |
| Albumin (g/dL) | 2.87 ± 0.55 | 2.65 ± 0.54 | 2.49 ± 0.48 | 0.09 | <0.001 | 0.14 |
| AT activity (%) | 82.3 ± 14.9 | 74.3 ± 14.5 | 61.7 ± 16.3 | 0.01 | <0.001 | <0.001 |
Abbreviations: AT, antithrombin; BP, blood pressure; GCS, Glasgow Coma Scale; WBC, white blood cells; CRP, C-reactive protein; vs, versus.
Table 3.
DIC score, SOFA score, and APACHE II score for each group divided by procalcitonin level
| PCT level | P value | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Group A <2 | Group B ≥2, <10 | Group C ≥10 | A vs B | A vs C | B vs C | |
| DIC score | 1.52 ± 1.06 | 1.80 ± 1.24 | 2.99 ± 2.19 | 1.00 | <0.001 | 0.001 |
| APACHE II score | 12.7 ± 6.3 | 15.7 ± 5.8 | 20.5 ± 7.8 | 0.074 | <0.001 | 0.001 |
| SOFA score | 1.9 ± 1.8 | 3.1 ± 2.3 | 5.2 ± 3.1 | 0.057 | <0.001 | <0.001 |
Abbreviations: DIC, disseminated intravascular coagulation; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; vs, versus.
Figure 1.
Comparison of semiquantitative procalcitonin concentrations with rates of severe sepsis and septic shock.
Figure 2.
Comparison of semiquantitative procalcitonin concentrations with rate of disseminated intravascular coagulation and mortality.
Abbreviations: DIC, disseminated intravascular coagulation.
Table 4 shows the numbers of patients and rates of severe sepsis, septic shock, DIC, and mortality in each procalcitonin group. The results were significantly different between three groups. For numbers of patients and rates of severe sepsis, septic shock, DIC, and mortality, the value in group C was higher than expected and the value in group A was lower than expected from the adjusted residual error. All mortality was due to infection. No other cause of death was documented. Almost all patients with septic shock were in group C (procalcitonin ≥ 10). Approximately 60% of patients with DIC were in group C, and there were few patients with DIC in group A. Moreover, the mortality rate was highest in group C, which also had the highest procalcitonin level.
Table 4.
Numbers of patients and rates of severe sepsis, septic shock, disseminated intravascular coagulation, and mortality for each group according to procalcitonin level
| PCT level | P value | |||
|---|---|---|---|---|
|
|
||||
| Group A <2 | Group B ≥2, <10 | Group C ≥10 | ||
| Severe sepsis | 15 (19%) | 20 (51%) | 63 (76%) | <0.0001 |
| Septic shock | 2 (2%) | 5 (13%) | 42 (51%) | <0.0001 |
| DIC | 3 (4%) | 8 (21%) | 31 (37%) | <0.0001 |
| Mortality | 1 (1%) | 1 (3%) | 11 (13%) | 0.003 |
Abbreviations: DIC, disseminated intravascular coagulation; PCT, procalcitonin.
Discussion
Our results show that semiquantitative procalcitonin concentration is an important discriminator for severity of sepsis. Furthermore, it may help in predicting early mortality in patients with sepsis. The results for systolic blood pressure, mean blood pressure, and respiratory rates may reflect severity of circulatory failure and respiratory failure. Antithrombin activity levels have been reported to be of prognostic value for the prediction of death in patients with severe sepsis or septic shock.25,26 Our results suggest that the semiquantitative procalcitonin concentration is indirectly a predictor of death in these patients.
In the present study, SOFA and APACHE II scores were significantly increased in group C as compared with groups A and B. Higher SOFA and APACHE II scores have been associated with significantly higher quantitative procalcitonin concentrations in several previous studies.13,16,17 Our results show that semiquantitative procalcitonin concentration is an important discriminator that can evaluate sepsis severity as well as quantitative procalcitonin concentration. In group C, rates of patients with severe sepsis and septic shock were extremely high. This result using semiquantitative procalcitonin measurement is similar to that found with quantitative procalcitonin measurement, where significant differences are apparent in quantitative procalcitonin concentrations between patients with and without septic shock.13 The rate of patients with DIC also showed an upward trend with increasing semiquantitative procalcitonin concentrations. These results strongly suggest that semiquantitative procalcitonin concentration is a predictor of severity in patients with sepsis. While septic shock is a clinical diagnosis, severe sepsis (sepsis with acute organ failure) is a complicated diagnosis that can utilize several different organ failure assessments. The procalcitonin assay correlates with the presence of severe sepsis and can be helpful in the early triage of this patient population.
The mortality rate, which was 13% in group C, is obviously high. Also, the value in group C was higher than expected. Semiquantitative procalcitonin concentration may be of great initial prognostic value. In hospitals and medical clinics without intensive care units, it is important to diagnose sepsis early and to make an estimate of severity in support of an early decision to transport patients to a hospital with an intensive care unit so they may receive optimal care. Our study may contribute to making a timely decision to transfer them to a tertiary hospital with an intensive care unit.
This study had several limitations. First, the timing of onset of infection was not considered, although it is known that serum procalcitonin begins to rise within 2–4 hours and shows peak levels at 6 hours.27 Therefore some patients may have been measured before procalcitonin began to rise. Second, because we used clinical and microbiological evidence, it might have been difficult to ascertain the precise cause of sepsis in all patients, and this might have introduced some misclassification bias. Finally, the conduct of this study was confined to departments in single centers, so case numbers were limited, and multicenter validation would have increased the generalizability of such findings.
In conclusion, semiquantitative procalcitonin concentration testing can be helpful for early assessment of disease severity in patients with sepsis. Furthermore, it may help in predicting early mortality in patients with sepsis. Based on the level of semiquantitative procalcitonin measured in patients with suspected sepsis, a timely decision can be reliably made to transfer them to a tertiary hospital with an intensive care unit for optimal care.
Acknowledgments
We thank Daisuke Sugiyama, Department of Internal Medicine Related, Kobe University Graduate School of Medicine, for conducting the statistical analysis of this study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 2.Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis. 2007;7:210–217. doi: 10.1016/S1473-3099(07)70052-X. [DOI] [PubMed] [Google Scholar]
- 3.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [No authors listed] [PubMed] [Google Scholar]
- 4.Aikawa N, Fujishima S, Endo S, et al. Multicenter prospective study of procalcitonin as indicator of sepsis. J Infect Chemother. 2005;11:152–159. doi: 10.1007/s10156-005-0388-9. [DOI] [PubMed] [Google Scholar]
- 5.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delèvaux I, André M, Colombier M, et al. Can procalcitonin measurement help in differentiating between bacterial infection and other kinds of inflammatory processes? Ann Rheum Dis. 2003;62:337–340. doi: 10.1136/ard.62.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gendrel D, Raymond J, Coste J, et al. Comparison of procalcitonin with C-reactive protein, interleukin 6 and interferon-alpha for differentiation of bacterial vs viral infections. Pediatr Infect Dis J. 1999;18:875–881. doi: 10.1097/00006454-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Ugarte H, Silva E, Mercan D, De Mendonça A, Vincent JL. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med. 1999;27:498–504. doi: 10.1097/00003246-199903000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006;34:1996–2003. doi: 10.1097/01.CCM.0000226413.54364.36. [DOI] [PubMed] [Google Scholar]
- 10.Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36:941–952. doi: 10.1097/CCM.0B013E318165BABB. [DOI] [PubMed] [Google Scholar]
- 11.Schneider HG, Lam QT. Procalcitonin for the clinical laboratory: a review. Pathology. 2007;39:383–390. doi: 10.1080/00313020701444564. [DOI] [PubMed] [Google Scholar]
- 12.Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Crit Infect Dis. 2004;39:206–217. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 13.Kushimoto S, Shibata Y, Koido Y, Kawai M, Yokota H, Yamamoto Y. The clinical usefulness of procalcitonin measurement for assessing the severity of bacterial infection in critically ill patients requiring corticosteroid therapy. J Nihon Med Sch. 2007;74:236–240. doi: 10.1272/jnms.74.236. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 16.Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit Care. 1999;3:45–50. doi: 10.1186/cc306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endo S, Aikawa N, Fujishima S, et al. Usefulness of procalcitonin serum level for the discrimination of severe sepsis from sepsis: a multicenter prospective study. J Infect Chemother. 2008;14:244–249. doi: 10.1007/s10156-008-0608-1. [DOI] [PubMed] [Google Scholar]
- 18.Clec’h C, Ferriere F, Karoubi P, et al. Diagnostic and prognostic value of procalcitonin in patients with septic shock. Crit Care Med. 2004;32:1166–1169. doi: 10.1097/01.ccm.0000126263.00551.06. [DOI] [PubMed] [Google Scholar]
- 19.Prat C, Dominguez J, Rodrigo C, et al. Use of quantitative and semi-quantitative procalcitonin measurements to identify children with sepsis and meningitis. Eur J Clin Microbiol Infect Dis. 2004;23:136–138. doi: 10.1007/s10096-003-1066-4. [DOI] [PubMed] [Google Scholar]
- 20.Kordek A, Podraza W, Czajka R. Reliability of semiquantitative determination of procalcitonin serum concentrations in neonates. Diagn Microbiol Infect Dis. 2006;56:31–34. doi: 10.1016/j.diagmicrobio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 22.Gando S, Iba T, Eguchi Y, et al. Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) Study Group. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34:625–631. doi: 10.1097/01.ccm.0000202209.42491.38. [DOI] [PubMed] [Google Scholar]
- 23.Dorizzi RM, Polati E, Sette P, Ferrari A, Rizzotti P, Luzzani A. Procalcitonin in the diagnosis of inflammation in intensive care units. Clin Biochem. 2006;39:1138–1143. doi: 10.1016/j.clinbiochem.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Meisner M, Brunkhorst FM, Reith HB, Schmidt J, Lestin HG, Reinhart K. Clinical experiences with a new semiquantitative solid phase immunoassay for rapid measurement of procalcitonin. Clin Chem Lab Med. 2000;38:989–995. doi: 10.1515/CCLM.2000.147. [DOI] [PubMed] [Google Scholar]
- 25.Fourrier F, Chopin C, Goudemand J, et al. Septic shock, multiple organ failure, and disseminated intravascular coagulation. Compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest. 1992;101:816–823. doi: 10.1378/chest.101.3.816. [DOI] [PubMed] [Google Scholar]
- 26.Mesters RM, Mannucci PM, Coppola R, Keller T, Ostermann H, Kienast J. Factor VIIa and antithrombin III activity during severe sepsis and septic shock in neutropenic patients. Blood. 1996;88:881–886. [PubMed] [Google Scholar]
- 27.Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79:1605–1608. doi: 10.1210/jcem.79.6.7989463. [DOI] [PubMed] [Google Scholar]