Abstract
BACKGROUND
During pregnancy, maternal uterine spiral arteries (SAs) are remodelled from minimal-flow, high-resistance vessels into larger diameter vessels with low resistance and high flow. Fetal extravillous trophoblasts (EVT) have important roles in this process. Decidual natural killer cells (dNK cells) are the major maternal immune component of the decidua and accumulate around SAs before trophoblast invasion. A role for dNK cells in vessel remodelling is beginning to be elucidated. This review examines the overlapping and dissimilar mechanisms used by EVT and dNK cells in this process and how this may mirror another example of tissue remodelling, namely cancer development.
METHODS
The published literature was searched using Pubmed focusing on EVT, dNK cells and SA remodelling. Additional papers discussing cancer development are also included.
RESULTS
Similarities exist between actions carried out by dNK cells and EVT. Both interact with vascular cells lining the SA, as well as with each other, to promote transformation of the SA. EVT differentiation has previously been likened to the epithelial–mesenchymal transition in cancer cells, and we discuss how dNK–EVT interactions at the maternal–fetal interface can also be compared with the roles of immune cells in cancer.
CONCLUSIONS
The combined role that dNK cells and EVT play in SA remodelling suggests that these interactions could be described as a partnership. The investigation of pregnancy as a multicellular system involving both fetal and maternal components, as well as comparisons to similar examples of tissue remodelling, will further identify the key mechanisms in SA remodelling that are required for a successful pregnancy.
Keywords: extravillous trophoblast, decidual natural killer cells, spiral artery remodelling, pregnancy, placenta
Introduction
A successful pregnancy is dependent on successful placentation, and placental development is not only reliant on the fetal trophoblast but also the maternal cells present in the decidua. To support the demands of the growing fetus, decidual spiral arteries (SAs) must be transformed into wide diameter, non-vasoactive vessels capable of transporting nutritional and oxygen requirements to the fetus. Disruption of this complex process is proposed to lead to poor SA transformation, which is associated with pregnancy disorders, such as intrauterine growth restriction (IUGR) and pre-eclampsia (Brosens et al., 1967; Pijnenborg et al., 1991; Cartwright et al., 2010). There have been extensive studies on SA remodelling, particularly with regard to the role of fetal trophoblast invading the decidua; however, recent interest has turned to the contribution of maternal cells within the decidua.
During placental development, trophoblast cells at the tips of the branched placental villi differentiate into extravillous trophoblast (EVT). These grow from the villi in columns to form the trophoblast shell, from where they invade into the decidua as far as the inner third of the myometrium, where they are known as interstitial EVT. Trophoblast also form plugs in the decidual SAs during the first trimester of pregnancy, which disappear by the second trimester. These plugs, made up of what are termed endovascular EVT, are thought to arise either from EVT migration retrograde to flow from the trophoblast shell inside the arterial lumen or intravasation from the decidua (Knofler, 2010; Pijnenborg et al., 2011). Aspects of reciprocal signalling between maternal and fetal components of the decidua have been characterized in recent years (Dimitriadis et al., 2010; Chazara et al., 2011). However, dissecting the cell types in the maternal decidua which provoke responses in trophoblast, and vice versa to lead to SA remodelling, is challenging. A number of studies have contributed to current knowledge stating that both endovascular and interstitial EVT are important in mediating the loss of arterial smooth muscle and endothelial cells (Pijnenborg et al., 2006; Harris, 2011), likely through a combination of apoptosis (Ashton et al., 2005; Harris et al., 2005; Keogh et al., 2007; Hamzic et al., 2008; James et al., 2011), destruction of the extracellular matrix (ECM) and induction of vascular smooth muscle cell (VMC) de-differentiation (Harris, 2011). However, evidence indicates that initiation of remodelling occurs in the presence of leukocytes but before the appearance of EVT (Smith et al., 2009; Hazan et al., 2010). It has been postulated that decidual NK (dNK) cells, with their secretion of numerous cytokines, enzymes and other factors, may play a role in the initiation of SA remodelling, as well as interacting with EVT to aid completion of this process (Harris, 2010). This review aims to first examine the evidence that dNK cells and EVT play a role individually in the remodelling of SAs and draw parallels between SA remodelling and tissue remodelling in cancer. We will then discuss how the functions ascribed to both EVT and dNK cells may lead to the concept that these cells may work in a ‘partnership’ to promote SA remodelling; both by the same mechanisms to produce the same outcome and by interacting to regulate each other's behaviour.
Search method
This review was prepared by systematically searching the published literature using PubMed with the search terms extravillous trophoblast and natural killer cells, and focusing on the literature describing SA remodelling. No restrictions were placed on year published; however, only English language literature was included. Additional papers discussing cancer development were also included in this review.
Decidual NK cells
Uterine NK cells are present in the normal human endometrium and the decidua. They increase in number in the late secretory phase of the menstrual cycle and accumulate in the decidua before the appearance of fetal trophoblast and during early pregnancy (Bulmer and Lash, 2005). Throughout pregnancy, dNK cells have been detected in both the decidua basalis and parietalis where trophoblast are present and absent, respectively (Bulmer and Lash, 2005). By the end of the first trimester, dNK cells comprise 70% of the leukocytes in the uterine environment (Kopcow et al., 2010) and these cells are presumed to be proliferative, owing to the expression of the cell proliferation marker Ki67, as well as large numbers of metaphase cells detected in the endometrium and early stage decidua (Pace et al., 1989; Peel, 1989). The importance of dNK cells has been demonstrated in placentation of both mouse and human (Manaster and Mandelboim, 2010) and here we will focus on the characteristics of these cells pertinent to SA remodelling and interaction with trophoblast.
dNK cells in murine pregnancy
Many of the studies implicating dNK cells in SA remodelling have been carried out in the mouse. In the murine decidua, vessel transformation requires a widening of the vessel lumen, an increase in the vessel length and a decrease in the thickness of the muscular wall (Ashkar et al., 2000; Zhang et al., 2011). The importance of dNK cells in mice was first demonstrated in the Tge26 strain of mouse, which lacks NK cells. Mice deficient in NK and T cells displayed abnormally high vessel wall to vessel lumen ratios, hypertensive vascular changes, irregular decidual organization, small yet morphologically intact placentae and 50% fetal death by Day 10 of gestation (Guimond et al., 1997). Engraftment of bone marrow from severe combined immunodeficient mice, which lack T and B-lymphocytes but not NK cells, restored the NK population in the Tge26 mice. This reduced decidual abnormalities, restored fetal viability and demonstrated that the decidual changes were NK associated (Guimond et al., 1998). The importance of cytokine signalling in remodelling was also demonstrated in these studies as interleukin (IL)-12 p40 null mice also showed abnormal decidual vasculature (Croy et al., 1997). IL-12, along with IL-18, has more recently been shown to be a crucial signalling molecule in the murine decidua inducing production of interferon (IFN)-γ by NK cells and subsequent decidual artery modification through IFN-γ (Zhang et al., 2003).
IFN-γ signalling in the mouse is a key pathway inducing arterial modification during pregnancy. Peak IFN-γ expression is co-incident with the peak of NK cells in the mouse pregnant uterus (Ashkar and Croy, 1999). Its role was demonstrated using transgenic murine models, which lacked IFN-γ or its receptor, and these mice presented pregnancies with reduced modification of decidual arteries and necrotic decidua (Ashkar and Croy, 1999). Using models which are completely NK-cell deficient, Ashkar et al. (2000) engrafted bone marrow from mice possessing either NK cells unable to produce IFN-γ, components of the IFN-γ signalling pathway or the IFN-γ receptor. These mice demonstrated different degrees of vessel modification and decidual disorganization, leading the authors to conclude that NK-derived IFN-γ was essential for normal pregnancy. This was further confirmed by the normal decidual morphology seen in NK-deficient mice treated with recombinant IFN-γ (Monk et al., 2005). It has been suggested that IFN-γ may be able to promote cell adhesion, smooth muscle cell proliferation and caspase-dependent apoptosis (Boehm et al., 1997; Boehm et al., 1998; Murphy et al., 2009). Whether NK cells have a similar role in humans regarding IFN-γ has not yet been elucidated.
dNK cells in human pregnancy
The evidence for a role of dNK cells in murine decidual artery modification has led to renewed interest in human dNK cells. In human pregnancy, the major subset of dNK cells expresses the surface markers CD56brightCD16−CD160−, as opposed to peripheral blood (pb) NK cells, which are predominantly CD56dim/−CD16brightCD160+ (King et al., 1998, Searle et al., 1999). CD56 is also known as neural cell adhesion molecule. Although similar in surface marker expression to the small population of CD56brightCD16− pbNK cells, dNK cells display a large number of differentially expressed genes compared with both peripheral blood subsets and are a distinct subset of NK cells (Koopman et al., 2003). Unique phenotypic differences of dNK include the surface expression of CD69, CD9, NKp44 and the absence of L-selectin (Koopman et al., 2003; El Costa et al., 2009). Other differences of dNK cells compared with pbNK cells include increased expression of the receptors NKG2C, NKG2E, NKG2A, KIR2DL4, CD31, CXCR3 (chemokine C-X-C motif) and CXCR4 (Hanna et al., 2003; Koopman et al., 2003; Tabiasco et al., 2006; Manaster and Mandelboim, 2010).
Owing to their unique expression profile, dNK cells are generally considered to have a cytokine-secreting role rather than having the predominantly cytotoxic defence role of pbNK cells (Tabiasco et al., 2006). Although dNK cells express cytotoxic proteins, including perforin, granzymes A and B, and granulysin and thus have cytolytic capacity, this cytotoxic machinery does not cause death of the invading trophoblast except potentially when responding to infection (Le Bouteiller et al., 2011). The pattern of inhibitory and activating receptors expressed on the surface of dNK cells may contribute to the reduced cytotoxicity which they display. For example, engagement of the NKp46 receptor on pbNK but not dNK cells induces IFN-γ release (El Costa et al., 2008) and the co-expression of the NKG2A inhibitory receptor on dNK blocks perforin polarization and target cell killing via NKp46 engagement (El Costa et al., 2008; El Costa et al., 2009). The ligand for NKG2A (HLA-E, expressed on trophoblast) may be responsible for this effect. An alternative reason for decreased cytotoxicity may involve signalling of the 2B4 inhibitory receptor (Vacca et al., 2006; Manaster and Mandelboim, 2010).
The role of dNK cells in SA remodelling has been indicated by immunohistochemical studies of serial sections from staged samples of first trimester human placentation. It is accepted that trophoblasts are involved in SA modifications in human pregnancy; however, Craven et al. (1998) demonstrated that some initial changes occur prior to any cellular contact with the invading EVT. It was subsequently shown that the initial loss of VSMCs and breaks in the endothelial cell (EC) layer takes place in the presence of lymphocytes but in the absence of invading EVT (Smith et al., 2009). These studies described a sequence of temporal stages of SA remodelling with classification into four distinct stages. In Stage I of this process, intact VSMCs and ECs were found with no indication of either leukocytes or EVTs in close proximity. During Stage II, there was evidence of disruption and partial loss of VSMCs, some breaks in the EC layer and extensive disorganization and separation of layers. Both dNK cells (identified by CD56 positivity) and macrophages (identified by CD68 positivity) were found to be infiltrating the vessel walls during this stage of actively remodelling vessels, but not trophoblast. This was also concurrent with a proportion of VSMC and EC undergoing apoptosis, detected by terminal deoxynucleotidyltransferase-mediated dUTP nick-end labelling. In Stage III, there was substantial loss of both VSMCs and ECs, and EVT were present by this stage, with some adhering to the vessel walls. Some apoptosis induction could be attributed to the invading EVT (Smith et al., 2009). By Stage IV, the endothelium and VSMC were lost, fibrinoid deposition was present and endovascular EVT lined the vessel.
The histological study of Smith et al. (2009) therefore has demonstrated further evidence of trophoblast-independent SA remodelling. The direct influence of dNK cell-derived soluble factors on SA remodelling has also been demonstrated. For example, myometrial SAs (obtained from non-pregnant, premenopausal women undergoing hysterectomy) were cultured with dNK cell culture supernatant. These arteries showed evidence of disruption, with altered VSMC alignment and rounded nuclei compared with control arteries, where VSMCs were aligned in compact layers. A uterine priming phenomenon may therefore be occurring where dNK cells are able to generate factors that initiate destabilization of vascular structures and thus SA transformation, prior to EVT interaction. The factors secreted by dNK cells include chemokines and cytokines, such as IL-8, tumour necrosis factor (TNF)α, IFN-γ and leukaemia inhibitory factor (LIF; Saito et al., 1993; Lash et al., 2006). Decidual NK cells can also generate a number of vasoactive factors capable of stimulating VSMC destabilization and disorganization, including angiopoietin (Ang)-1, Ang-2, IFN-γ and vascular endothelial growth factor (VEGF)-C, whose corresponding receptors VEGF-R2 and Tie-2 are expressed by SAs (Lash et al., 1999, 2006; Hu et al., 2006; Kalkunte et al., 2009; Schiessl et al., 2009).
Circumstantial evidence from disease also suggests a role in the human for dNK cells in SA remodelling. Reduced numbers of dNK cells have been demonstrated in patients with pre-eclampsia and IUGR (Williams et al., 2009), which are conditions associated with poor SA remodelling and reduced trophoblast invasion in the decidua (Stallmach et al., 1999). However, the association of dNK cells with pre-eclampsia has not been consistently demonstrated (Bachmayer et al., 2006). Uterine NK cells have also been associated with aberrant angiogenesis in recurrent spontaneous abortion (Quenby et al., 2009).
The actions of dNK cells in promotion of successful placentation in normal pregnancy are therefore beginning to be elucidated. Decidual NK cells are not the only myeloid cell type in the decidua which has been implicated in placentation and vessel remodelling. Macrophages, T-lymphocytes and dendritic cells are also found within the maternal decidua. Macrophages comprise ∼20% of the decidual leukocyte population, and have been associated with phagocytosis of apoptotic cells (Abrahams et al., 2004; Bulmer et al., 2010), cytokine production (Li et al., 2009; Svensson et al., 2011) and regulation of trophoblast invasion (Renaud et al., 2007). T cells make up ∼10% of the decidual leukocyte population. The main function of T cells in the decidua (particularly CD4+ and regulatory T-cells) is thought to be the promotion of tolerance to the fetus (Guerin et al., 2009; Piccinni, 2010). A predominantly Th2 phenotype was originally presumed to be beneficial to pregnancy; however, because a variety of different phenotypes of T cells are present in the decidua the complex interactions of this cell type have not been completely defined (Bulmer et al., 2010). CD8+ T cells have been associated with cytokine production and regulation of trophoblast invasion (Scaife et al., 2006). Natural killer T cells are also present and may act in a similar manner to dNK cells, although little is known at present (Piccinni, 2010). Finally, dendritic cells make up a small percentage of the decidual leukocyte population, being present at ∼1–2% (Bulmer et al., 2010). Dendritic cells are thought not to interact with trophoblast directly (Huang et al., 2008) but may modulate responses of T and NK cells (Dietl et al., 2006). They may also be important for decidualization, as studies in mice (Krey et al., 2008; Plaks et al., 2008) and some evidence in humans (Barrientos et al., 2009) has demonstrated. Therefore, as for dNK cells, roles for these three cell types (macrophoges, dendritic cells and T-lymphocytes) in SA and decidual remodelling are being elucidated, although it is beyond the scope of this review to discuss these in great detail (Dietl et al., 2006; Bulmer et al., 2010; Hazan et al., 2010; Blois et al., 2011).
The extravillous trophoblast
The role of EVT in SA remodelling has been widely investigated (Harris, 2010; Whitley and Cartwright, 2010). Interstitial EVT, which are found surrounding the SAs within the decidua, are proposed to remodel the arteries by destroying the arterial media and mediating loss of the vascular smooth muscle outer layers, partly by inducing vascular cell apoptosis (Harris et al., 2006; Keogh et al., 2007). Endovascular trophoblasts, present in the lumen, temporarily replace the ECs constituting the inner layer of the SAs (Brosens et al., 1967; Kam et al., 1999).
EVT differentiation and invasion
The differentiation status of the trophoblast, from villous cytotrophoblast into EVT, involves a switch from a proliferative to an invasive, cytokine-secreting phenotype. Further differentiation into endovascular trophoblast expressing endothelial-like markers then occurs, and these differentiation stages are important in regulating how remodelling takes place (Zhou et al., 1997). The control of the differentiation into EVT has been attributed to both an intrinsic programme (McMaster et al., 1994) as well as the hypoxic environment provided by endovascular trophoblast plugs in the first trimester, which is detected in EVT by hypoxia-sensitive molecules, such as hypoxia inducible-factor (HIF) 1α and Id1 (James et al., 2006). The differentiation into the invasive EVT phenotype is linked to changes in the expression of a number of genes, which can be broadly categorized as a loss of epithelial phenotype and the gain of an invasive, more mesenchymal phenotype. This phenotypic change includes increased production of cytokines, proteases and adhesion molecules, allowing the EVT to migrate and invade into the uterine environment, and interact with different decidual cell types (Knofler, 2010).
The phenotypic adhesion molecule change demonstrated by EVT has been termed ‘integrin switching’, and enables the expression of molecules which are typically expressed by the endothelium that they replace. This process also allows EVT to invade and interact with the ECM. These changes in gene expression include an up-regulation of α1β1 and α5β1 integrin (Damsky et al., 1992), VE-(endothelial) cadherin, platelet endothelial adhesion molecule, vascular endothelial adhesion molecule, α4-integrins and αV-β3 and a decrease in E-cadherin and connexin-40 (Zhou et al., 1997; Wright et al., 2006; Arimoto-Ishida et al., 2009; James et al., 2010). The importance of decidual secreted factors in controlling this differentiation has been demonstrated (Godbole et al., 2011). For example, blocking epidermal growth factor receptor (EGFR/HER1) activation in an EVT cell line incubated with decidual cell-conditioned media prevented both differentiation mediated by EGF and heparin-binding EGF (HB-EGF; Wright et al., 2010) and the differentiation-associated decrease in connexin-40 (Wright et al., 2006) and transforming growth factor (TGF)-α signalling can also decrease the expression of α1 and α6-integrins (Leach et al., 2004). Other decidual molecules which play an important role in the switch to a pro-invasive phenotype include insulin-like growth factor-binding protein-1, TGF-β (Irving and Lala, 1995) and IL-8 (Jovanovic et al., 2010).
In addition to changes in the expression of adhesion molecules, EVT up-regulate a range of proteases to aid invasion through the ECM. Included in these are matrix metalloproteinase (MMP)-2 (Staun-Ram et al., 2009), MMP-3 (Husslein et al., 2009) and MMP-9 (LaMarca et al., 2005; Naruse et al., 2009a, b). Up-regulation of cathepsins (Varanou et al., 2006) and urokinase plasminogen activator (uPA), which can activate several MMPs, has also been described (Liu et al., 2003). In addition, cellular motility—a key aspect of the invasive process—is up-regulated by external factors. Hepatocyte growth factor (HGF) has been implicated in trophoblast migration, by interacting with its receptor, the c-met proto-oncogene, a tyrosine kinase receptor expressed by EVT (Kauma et al., 1999). HGF promotes EVT motility by induction of inducible nitric oxide synthase, via the phosphatyklinositol-3-kinase signalling pathway (Cartwright et al., 1999; Cartwright et al., 2002; Harris et al., 2008). Furthermore, EGF can activate RhoA (a member of the Ras homologue gene family), causing actin rearrangement in trophoblasts, thus aiding trophoblast migration (Han et al., 2010). The factors affecting invasiveness of EVT are therefore of key importance in the remodelling process (Knofler, 2010).
EVT and vascular cell interactions
The acquisition of this altered phenotype of adhesion molecules and invasive capability described above enables the actions of EVT in SA remodelling. One additional role EVT play in SA remodelling is through induction of vascular cell apoptosis. SA remodelling is characterized by loss of the outer layer of VSMCs, the ECM and the inner layer of ECs, which is replaced by endovascular EVT by the end of the first trimester (Robertson et al., 1967; Pijnenborg et al., 2006). Various in vitro studies investigating EVT-dependent remodelling of SAs have suggested that apoptotic mechanisms are involved (Whitley and Cartwright, 2009). Apoptosis has been described in vascular cells in a mouse model of trophoblast invasion (Red-Horse et al., 2006) and has been detected in VSMC of arteries undergoing remodelling in human first trimester decidua (Smith et al., 2009). Most mechanisms investigated in vitro have involved the TNF family of cytokines. EVT have been shown to produce the pro-apoptotic cytokine Fas-ligand, which can bind to its receptor on vascular cells to induce caspase-dependent death. There is evidence that Fas-ligand is involved in the induction of apoptosis of both ECs (Ashton et al., 2005; James et al., 2011) and VSMC (Harris et al., 2006; Harris et al., 2007) caused by endovascular and interstitial EVT. A related cytokine, TNF-alpha-related apoptosis-inducing ligand (TRAIL) was also found to be expressed by EVT and involved in the induction of VSMC death (Keogh et al., 2007).
Apoptosis of SAs has not, however, been consistently described (Bulla et al., 2005). One difficulty in the detection of apoptotic vascular cells in decidual SAs may be related to rapid phagocytosis, as the ability of EVT to phagocytose apoptotic ECs has been demonstrated (Chen et al., 2005). Successful physiological change of vessels and EVT invasion is also reliant on other mechanisms, such as elastolytic processes carried out by EVT, which ensure the breakdown of the elastic fibres found at the basal sections of decidual SAs and amongst all the myometrial segments of the SAs. MMPs produced by EVT are proposed to aid migration through breaks in the internal elastic lamina in vivo and disruption of the ECM around SAs may aid EVT remodelling (Naruse et al., 2009a, b; Smith et al., 2009). MMP-12 is an elastolytic protease expressed by trophoblast which is capable of degrading several ECM components, including collagen type IV, laminin, fibronectin, vitronectin and heparin sulphate proteoglycans. Furthermore, in other examples of vessel remodelling MMP-12 is capable of activating pro-MMP-2 and pro-MMP-3, as well as processing α1-anti-trypsin and latent TGF-β (Lagente et al., 2009), and this may be a mechanism by which MMP-12 production by EVT may influence VSMC disruption and de-differentiation. Therefore, the role of the EVT in the remodelling of SAs is crucial.
Cell parallels: the tumour microenvironment
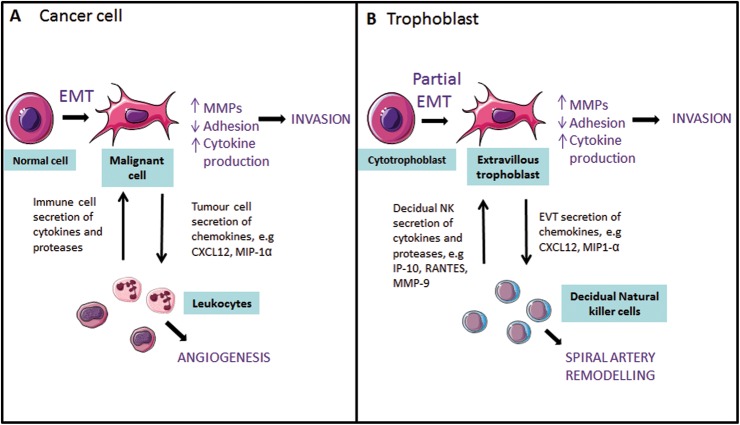
The invasion and migration of EVT into the maternal decidua shares similarities with the characteristic properties of malignant cells and metastasizing cells at the invasive front of a tumour (Fig. 1). There are, however, two major differences displayed by EVT: the strict restrictions temporally and spatially to the pregnant decidua and myometrium and the loss of proliferative ability. The differentiation from cytotrophoblast into EVT has been likened to the common, but uncontrolled, epithelial–mesenchymal transition (EMT) seen in cancer (Denker, 1993; Kalluri and Weinberg, 2009). This transition involves the change from a column of cells in contact by a range of adhesion molecules to individual cells capable of migrating long distances, and is made possible by changes in cytoskeletal proteins and the production of proteases which break down the ECM. The acquisition of these properties allows cells to migrate to different regions of the body, and also occurs in embryo development and wound healing. However, the transition undergone by EVT and cancer cells is thought to be an intermediate phenotype of the EMT (Kalluri and Weinberg, 2009).
Figure 1.
Similarities between the EMT in cancerous cells and cytotrophoblast differentiation into extravillous trophoblast. (A) During malignant transformation, tumour cells undergo an EMT into invasive cells, which secrete increased levels of proteases and cytokines, leading to increased invasion. Secretion of chemokines, such as chemokine (C-X-C motif) ligand-12 (CXCL12) and MIP-1α, leads to chemoattraction of leukocytes which play a role in tumour angiogenesis, and may secrete further cytokines which act upon the tumour cells. (B) During cytotrophoblast differentiation, trophoblast undergoes a transformation into invasive EVT and up-regulates proteases and cytokines. Interactions between decidual natural killer (NK) cells and EVT, for example the secretion of chemokines and proteases, are proposed to contribute to SA remodelling.
The initiation of EMT in cancer cells is proposed to be dependent on signals from the tumour stroma such as EGF, HGF and TGF-β (Kalluri and Weinberg, 2009). Similarly, as cytotrophoblast differentiates into EVT, strikingly similar molecules from the decidua induce this change, and many of the same transcription factors are activated in both tumour cells and EVT, which orchestrate the phenotypic changes in EMT. For example, tumour cell EGFR phosphorylation has wide-ranging effects including the down-regulation of adhesion molecules (Kalluri and Weinberg, 2009) and up-regulation of MMP-2 (Xu et al., 2010), giving the combined effect of increased invasion. In placental development, the phosphorylation of EGFR1 in cytotrophoblast by HB-EGF up-regulates the pro-migratory EGFR2 receptor (Wright et al., 2010), down-regulates the inter-cellular adhesion molecule connexin 40 (Wright et al., 2006) and increases MMP-2 secretion (Staun-Ram et al., 2009). EGFR phosphorylation can also activate the transcription factor Snail, a molecule which in many cancer types is also induced by TGF-β1 and TGF-β2 signalling and leads to the most common change in EMT, a loss of E-cadherin expression and increased invasive capability (Medici et al., 2008). In placental development, Snail is regulated by hypoxia and in turn regulates the repression of E-cadherin expression, which up-regulates integrins critical for ECM remodelling and therefore decidual invasion (Arimoto-Ishida et al., 2009). Finally, HGF has been identified in cancer as a critical molecule promoting metastasis (Birchmeier et al., 2003), while EVT motility is significantly stimulated by HGF (Cartwright et al., 2002). The signalling pathways described here are by no means the only ones showing similarity between these two processes and are described extensively elsewhere (Ferretti et al., 2007).
However, the close similarity with the EMT is not the only parallel seen between EVT and tumour cells. For example, it is interesting that, similar to EVT, many tumours express HLA-G (Amiot et al., 2011). The expression of soluble HLA-G has been demonstrated to induce cytokine and chemokine production (e.g. IL-8, IL-6, TNF-α, IFN-γ and IL-6) in both dNK and pbNK cells via internalization of the KIR2DL4 receptor (Rajagopalan et al., 2006; Li et al., 2009). This may contribute to tissue remodelling in both the maternal decidua and in tumours. Both soluble and membrane-bound HLA-G are also proposed to act in both situations as a method of avoiding immune surveillance (Poehlmann et al., 2006; Amiot et al., 2011). However, the role of HLA-G in both cancer and trophoblast is still largely unknown (Apps et al., 2008), and other parallels with the immune system may be important in tissue remodelling in both examples.
One such example may be the role of immune cells in cancer, which has recently been identified as an important aspect of tumour biology (Mantovani et al., 2008). The tumour microenvironment is commonly infiltrated by several types of leukocyte including macrophages, neutrophils and NK cells, and these cells of the innate immune system are proposed to infiltrate tumours by chemoattraction to pro-inflammatory cytokines secreted by the cancer cells. These immune cells have been shown to contribute to tissue remodelling within cancer, including promotion of metastasis of tumour cells and angiogenesis (de Visser et al., 2006). NK cells were so named in recognition of their cytotoxicity towards tumour cells, and the role of NK cells in a variety of tumours has been long studied and clinical trials exploiting their effects are underway (Ljunggren and Malmberg, 2007; Vivier et al., 2011). In mouse models, early studies showed that the depletion of NK cells by antibodies increased tumour growth (Seaman et al., 1987). In humans, low pbNK cytotoxic activity is associated with increased risk of cancer (Imai et al., 2000) and NK cell presence in tumour is positively associated with prognosis (Villegas et al., 2002; Qiu et al., 2009; Levy et al., 2011). Engagement of receptors on the NK cell, particularly NKp46, NKp30, NKp44 and NKG2D, is important for tumour cell cytotoxicity (Vivier et al., 2011).
As described earlier in this review, pbNK and dNK cells display different phenotypes. Tumour-associated NK cells have also been found to express a unique phenotype, possibly related to the microenvironment in which they are found. NK cells appear to be important in immediate rejection of tumour cells by cytotoxicity, rather than the control of established tumours, which may be more attributed to T lymphocytes (Levy et al., 2011). NK cells are found in low numbers in tumours, and so most studies have been performed on pbNK rather than on isolated tumour NK cells. However, NK cells isolated from ovarian cancer display reduced cytotoxicity in a classical tumour cytotoxicity assay and decreased CD16 expression, an effect which could be mirrored in pbNK incubated with cancer cells (Carlsten et al., 2009). Additionally, NK cells isolated from lung cancer showed a lower cytotoxicity compared with those from normal lung or peripheral blood, and increased expression of the activation marker CD69 and the receptor NKp44 (Carrega et al., 2008). The receptor for HLA-E, NKG2A, is also more frequently expressed by NK cells which are associated with tongue cancer (Katou et al., 2007). These are all characteristics of dNK cells (although dNK expression of NKp44 is disputed; Kusumi et al., 2006; Male et al., 2011). Therefore, whether the phenotype of NK cells in an established tumour microenvironment changes, to be more similar to the cytokine secreting, low cytotoxic phenotype of dNK cells, is unknown. Whether NK cells contribute with tumour cells to remodelling in cancer is also unknown. However, the role of macrophages and neutrophils in tissue remodelling in cancer has been more intensively studied in cancer biology.
The most common infiltrating immune cell into cancer is often the macrophage (Dirkx et al., 2006). Tumour-associated macrophages are increased in many cancer types including colon (Forssell et al., 2007), breast (Bingle et al., 2006) and melanoma (Hussein, 2006) and are often associated with poor prognosis (Salvesen and Akslen, 1999; Mantovani et al., 2011). Subpopulations of macrophages which have different phenotypes and promote tumour progression in different ways have been identified. In general, it has been suggested that the macrophages found in tumours are predominantly of a ‘M2’ phenotype, also known as ‘alternatively activated’ and associated with processes such as wound healing, as opposed to pathogen killing and inflammatory cytokine production (Mantovani et al., 2008; Mantovani et al., 2011). However, the phenotype of macrophages in cancer may not be neatly divided into these two categories and, instead, they share overlapping features and at least six phenotypes have been identified, which promote features including tumour cell survival, metastasis and immunosuppression (Qian and Pollard, 2010). One main contribution by macrophages to the growth of tumours is thought to be angiogenesis. In an important study, macrophages were shown to be essential for the initiation of angiogenesis in breast cancer (Lin et al., 2007). One subset of macrophages, which are identified by the presence of the Tie2 marker, expresses a number of angiogenic factors and enhances tumour growth in mouse models (Venneri et al. 2007; Qian and Pollard, 2010). Factors secreted by macrophages include VEGF, fibroblast growth factor-2 and IL-8, amongst other pro-angiogenic chemokines (Nesbit et al. 2001; Bingle et al. 2006; Lamagna et al., 2006; Venneri et al., 2007). Macrophages can also promote tumour invasion and metastasis, through the secretion of remodelling proteases including MMP12 and MMP-9 (Luo et al., 2006; Yang et al., 2007). The phenotype and actions shown by tumour-associated macrophages therefore display similarities with the difference in phenotype and activities between pbNK and dNK cells.
It is then possible to draw parallels between cancer cells and immune cells interacting with vasculature within the tumour, and EVT and dNK cell interactions with vascular cells to remodel SAs. EVT secrete cytokines for which dNK cells express receptors, and the resulting cross-talk can induce dNK cell migration: these include the ligand CXCL12 (Hanna et al., 2003) and macrophage inflammatory protein (MIP)-1α (Drake et al., 2001). By a similar mechanism, secretion of chemokines by tumour cells has been demonstrated to chemoattract immune cells: examples include IL-8 and CXCL12-mediated chemoattraction of neutrophils and macrophages in breast cancer (Yao et al., 2007; Welford et al., 2011), IL1-β-mediated infiltration of macrophages in lung cancer (Kimura et al., 2007), CXCL1-induced chemoattraction of neutrophils to endometrial adenocarcinoma (Wallace et al., 2009) and MIP1-α-mediated infiltration of macrophages (Wu et al., 2008). During placentation, therefore, expression of chemokines by EVT may augment the decidual stroma's role of homing dNK cells further to the SAs.
Macrophages and neutrophils can also promote the invasion and metastasis of cancer cells. This is achieved through the secretion of proteases, including MMP-9 and MMP-12, which break down the ECM and facilitate cell migration (Luo et al., 2006; Ardi et al., 2007; Yang et al., 2007). In the decidua, dNK cells are thought to fulfil a similar role through their production of MMP-2, 9, tissue inhibitor of metalloproteinase-2 (Naruse et al., 2009b) and uPA (Naruse et al., 2009a), which may facilitate migration of EVT. Additionally, similar signalling pathways may be activated in EVT as in tumour cells in response to immune cells. For example, IL-8, which is produced both by the tumour microenvironment and immune cells, induces production of MMP-2 in melanoma (Luca et al., 1997) and MMP-9 in prostate cancer (Inoue et al., 2000) which increases tumour invasion. In the decidua, IL-8 is produced by dNK cells and this increases EVT MMP-2 expression and invasion (De Oliveira et al., 2010), as does CXCL10 expression via the CXCR1 and CXCR3 receptors, respectively (Hanna et al., 2006).
However, dNK cells are also proposed to play a role in the inhibition of EVT invasion to regulate excessive decidual remodelling. This is potentially achieved by production of the cytokines TNFα, TGF-β1 and IFN-γ. Additionally, differences in the repertoire of cytokines produced by dNK cells have been demonstrated at different gestational ages (Lash et al., 2010a, b, c). It is therefore possible that the role of dNK cells alters as gestation progresses. Decidual NK cells have been shown to be more stimulatory as gestation advances, possibly as EVT at lower gestational ages are more intrinsically invasive and therefore less responsive to external factors (Lash et al., 2010a, b, c).
The remodelling partnership: dNK cells and EVT
Similar to the mechanisms employed by immune cells and cancer cells to promote tumour growth, dNK cells and EVT may also be interacting to carry out SA remodelling. As described, the panel of surface receptors expressed by dNK is distinct from those in pbNK cells (Koopman et al., 2003; Sharkey et al., 2008; El Costa et al., 2009). Receptors displaying differing levels of expression between pbNK and dNK include the natural cytotoxicity receptors NKp46, NKp30 and Nkp44 (El Costa et al., 2009) and ILT2 as well as the inhibitory receptors NKG2A and NKG2C and the killer cell immunoglobulin-like receptors (KIR; El Costa et al., 2009)]. Interactions exist between these dNK cell receptors and EVT major histocompatibility complex (MHC) molecules. The receptor NKG2A/CD94 on dNK cells binds to the MHC class I molecule, HLA-E on the invading trophoblast (King et al., 1998; Hanna et al., 2006) and engagement of this receptor and ligand pairing is thought to override cytotoxic signals from the NKp46 natural cytotoxicity receptor expressed by dNK (El Costa et al., 2008). Engagement of the NKp44 receptor by trophoblast can also induce secretion of various cytokines (Hanna et al., 2006). The interaction between dNK KIR expression and fetal trophoblast HLA-C expression is interesting, as particular combinations are associated with an increased risk of pre-eclampsia, fetal growth restriction and recurrent spontaneous abortion (Hiby et al., 2004; Hiby et al., 2010). The HLA-C-specific KIR receptor genotype can be divided into two groups dependent on the presence (A) or absence (B) of activatory KIR receptors. HLA-C has two allotypes, C1 and C2. Increased risk of pregnancy complication is found when mothers which have the AA KIR genotype are paired with fetal trophoblast cells with a C2 allotype (Hiby et al., 2004), potentially related to a premature halting of trophoblast-mediated vessel transformation directed by dNK cells (Parham, 2004). Decidual NK–EVT interactions are crucial in the maintenance of a normal pregnancy and SA remodelling. We will now discuss the additional mechanisms by which interactions between dNK cells and EVT may achieve SA remodelling. The combined role that these cell types play could be described as a ‘partnership’.
Contribution to artery remodelling: dNK cell actions on EVT
Studies investigating the effect of dNK cells on EVT have established that cytokines produced by dNK cells can promote trophoblast invasion. For example, Hanna et al. (2006) demonstrated that dNK cells are able to produce IL-8 and INF-γ inducible protein (IP)-10, even in the presence of low concentrations of IL-15. Corresponding to this, it was also found that invasive EVT expressed CXCR1 and CXCR3, the receptors for IL-8 and IFN-γ-induced protein-10, respectively. This suggested that cross-talk between dNK cells and EVT is taking place via stimulation of these receptor pathways. Both in vitro and in vivo trophoblast migration experiments revealed that dNK cells are able to stimulate recruitment and migration of EVT by their production of IL-8 and IP-10, ensuring their recruitment to the SAs (Hanna et al., 2006). The same authors demonstrated that dNK cells produce the RANTES chemokine (regulated on activation, normal T-cell expressed and secreted), which regulates macaque trophoblast migration (Thirkill et al., 2005). We have also demonstrated that HGF production by dNK cells induces motility in an EVT cell line (Fraser et al., 2011). EVT invasion is dependent on proteases and their inhibitors produced by the EVT themselves. IL-8 promotes the up-regulation of MMP-2 and MMP-9 and the invasion of an EVT cell line (De Oliveira et al., 2010; Jovanovic et al., 2010), and also reduces EVT apoptosis (Knofler, 2010).
As previously described, EVT differentiation into an invasive phenotype is required for successful trophoblast invasion. Adhesion molecules altered in this differentiation, such as integrins expressed by EVT, can also be altered by chemokines which are secreted by dNK cells; for example, IL-8 increases expression of integrins α1 and β5 (Jovanovic et al., 2010). LIF is produced by dNK cells (Reister et al., 2006) and has been associated with Stat3 activation (Poehlmann et al., 2005) which can regulate trophoblast invasion (Corvinus et al., 2003) and is involved in the EMT. In addition, the ability of dNK-cell secreted factors to indirectly induce a key step in trophoblast differentiation in the rat has been described, as factors including VEGF increase oxygen delivery to the site of placentation and thus the differentiation of trophoblast via the hypoxic response element HIF 1α (Chakraborty et al., 2011).
Further effects on EVT by dNK cells include the ability to promote the ‘endothelial-like’ properties of endovascular trophoblast. Decidual NK cell conditioned media not only induced increased migration of primary and cell line EVT but also promoted their formation into capillary tubes and increased expression of the endothelial-like adhesion molecule, ICAM (Hu et al., 2010). Additionally, VEGF-C expression by dNK cells has been demonstrated to be important in increasing EVT resistance to cytotoxicity, as well as promoting the assembly of EVT into networks of tube-like structures (Kalkunte et al., 2009). Therefore the signalling from dNK cells to EVT may also promote the differentiation of EVT, a hypothesis which is also reinforced by the promotion of EVT migration from placental explants by dNK cell-conditioned medium, implying a switch from villous cytotrophoblast to EVT (Lash et al., 2010a, b, c).
Contribution to artery remodelling: EVT actions on dNK cells
EVT may also influence dNK cells in order to promote SA remodelling. Previously described in this review is the secretion of CXCL12 by EVT within the decidua and SAs, inducing migration of dNK cells (Hanna et al., 2003). MIP-1α production by cytotrophoblast has also been demonstrated, and this is a chemotactic factor to dNK cells and may therefore function to position dNK cells in the vicinity of SAs (Drake et al., 2001). The expression of GnRH-induced CXCL chemokines by EVT has also been demonstrated to be chemotactic to dNK cells (Cavanagh et al., 2009).
The unique properties of the HLA-G molecule expressed by EVT may also influence the actions of dNK cells in SA remodelling. HLA-G has only been found to bind to two receptors on dNK (KIR2DL4 and LILR-1) owing to differences in contact residues which bind to KIRs on HLA-G compared with the classical MHC molecules such as HLA-C (Gonen-Gross et al., 2010) Interactions between HLA-G-expressing cells and dNK cells can reduce the expression of some cytokines by dNK cells, including TNF-α (Rieger et al., 2002; Ntrivalas et al., 2006). A further mechanism that has been demonstrated is via the actions of soluble HLA-G (sHLA-G), which is proposed to have a different role to its membrane-bound form, and is secreted by EVT in the decidual environment. It has been suggested that by binding to KIR2DL4 on dNK cells, sHLA-G alters mRNA levels of numerous cytokines in NK cells, including IL-6, IL-8 and IFN-γ (Rajagopalan et al., 2006). This study was carried out using pbNK cells; however, endometrial NK cells have also been demonstrated to secrete IFN-γ and increase proliferation in response to sHLA-G (van der Meer et al., 2007). Additionally, the homodimer form of sHLA-G has been demonstrated to increase secretion of IL-6, IL-8 and TNF-α in dNK cells (Li et al., 2009), and is therefore proposed to promote a phenotype of dNK cells capable of participating in SA remodelling. The importance of the HLA-G–KIR2DL4 interaction in the decidua is however still unknown, with the suggestion that it is not necessary for reproduction in humans (Gomez-Lozano et al., 2003; Nowak et al., 2011).
Finally, EVT recruitment of dNK cells may be achieved by an indirect mechanism. It has also been proposed that EVT may alter chemokine secretion by cells making up SAs, which may alter dNK cell recruitment (Hazan et al., 2010). However, expression of MMP-12 by VSMC and EVT may act to indirectly inhibit dNK cell recruitment by the cleavage and inactivation of leukocyte chemotactic proteins (Harris et al., 2010; Harris, 2011), and so the picture is far from clear.
Contribution to artery remodelling: similar mechanisms
Some of the repertoire of angiogenic factors, cytokines and enzymes which contribute to SA remodelling are secreted by both EVT and dNK cells, and may perform the same and possibly redundant functions in SA remodelling. For example, both cell types express the proteases MMP-2 and MMP-9, as described above (Naruse et al., 2009b). The expression of a number of similar angiogenic factors and cytokines has been highlighted by recent studies using multiplex arrays and enzyme-linked immunosorbent assay to examine factors secreted from isolated primary EVT and dNK cells (Lash et al., 2006; Engert et al., 2007; Lash et al., 2010a, b, c; Naruse et al., 2010). These include the expression of VEGF-C by trophoblast which is proposed to disrupt the VSMC layer of SAs (Lash et al., 2011) and expression of VEGF-C by dNK cells may perform a similar function (Kalkunte et al., 2009). The angiopoietins (Ang1 and Ang 2) have well described roles in blood vessel stabilization or breakdown, and are also expressed by both cell types (Lash et al., 2006; Schiessl et al., 2009). EVT and dNK cells also secrete the angiogenic factors IL-8, platelet-derived growth factor BB homodimer, placenta growth factor and FGFb (Saito et al., 1993; Lash et al., 2006; De Oliveira et al., 2010; Lash et al., 2010a). While the functions of these proteins secreted by dNK cells and EVT have not been described in all cases, it is possible that the actions of these on SAs induce similar responses, and may even be redundant. The unique and overlapping secreted factors of dNK cells and EVT are summarized in Table I. Discrepancies exist in the literature between the presence and absence of factors, and may be related to sampling method or gestation age of samples examined.
Table I.
Factors secreted by human primary dNK cells and EVT at a stage when SA remodelling is taking place during pregnancy
Data were obtained by measuring factors secreted from isolated cells and immunohistochemistry studies of the placental bed. ✓, detected; NE, not expressed; NI , not investigated. See text for descriptions of all factors.
Apoptotic signalling has been implicated in SA remodelling by trophoblast cells and various studies have demonstrated the induction of apoptotic death of vascular cells by invasive trophoblast contact, as described above (Ashton et al., 2005; Chen et al., 2005; Harris et al., 2005). There may also be a similar pathway used by dNK cells. Apoptotic markers can be detected in SA VSMC when dNK cells but not EVT are present (Smith et al., 2009). Additionally, recent evidence from our group has revealed that dNK cells induce vascular cell apoptosis via a FasL pathway (Fraser et al., 2009) similar to EVT (Ashton et al., 2005; Keogh et al., 2005). This implies that the induction of apoptosis in vascular cells is a pathway used by more than one cell type in the decidua, and that cytokine and angiogenic factor signalling is not the only similarity between dNK cells and EVT.
These observations demonstrate many similarities between dNK cells and EVTs at the maternal–fetal interface. These interactions still need to be fully elucidated but they indicate that dNK cells and EVT may work in partnership, either by acting via similar mechanisms and/or by actions on each other, during the first trimester of pregnancy. However, recent data suggest that in co-culture, EVT and dNK cells decrease secretion of some angiogenic factors and chemokines (Lash et al., 2011), and therefore there is still much to be elucidated regarding the redundancy of remodelling mechanisms.
When the partnership goes wrong
In some placental disorders, it has been postulated that the EMT is disrupted in trophoblast, in a similar yet opposite manner to the EMT disruption leading to uncontrolled cancer growth (Kokkinos et al., 2010). This could lead to the inadequate trophoblast invasion seen in pre-eclampsia and IUGR. For example, Genbacev et al. described an inability of cytotrophoblasts to differentiate into an invasive phenotype, as well as increased trophoblast apoptosis in pre-eclamptic pregnancies (Genbacev et al., 1999, 2000). Disruptions between cancer cell and immune cell signalling are also proposed to lead to disruptions in tumour growth and therapies which block signalling between these cell types are now being investigated (Squadrito and Palma, 2011; Tseng et al., 2011). It is therefore possible that, mirroring the interactions between innate immune cells and tumour cells, the interaction between dNK cells and trophoblast can also be disrupted, playing a role in placental disorders.
The proposed pathological consequences of abnormal placental growth and SA remodelling include pre-eclampsia, recurrent spontaneous abortion and IUGR (Cartwright et al., 2010). Both EVT and dNK cells individually have been implicated in the cause of these disorders. An association between pre-eclampsia and impaired EVT trophoblast invasion and reduced SA remodelling has long been evident (Brosens et al., 1972; Pijnenborg et al., 1991), with numerous studies investigating the mechanisms of this impairment (Kaufmann et al., 2003; Whitley and Cartwright, 2009). Furthermore, increased dNK cell numbers has been linked to recurrent spontaneous abortion (Quenby et al., 2009), although this is controversial (Tang et al., 2011), and clinical trials aimed at decreasing dNK cell number in high-risk patients are underway (Tang et al., 2009). There is also evidence for decreased dNK cells in third trimester pre-eclamptic and fetal-growth restricted pregnancies (Williams et al., 2009). These associations between dNK cell numbers and disease indicate the possible therapeutic potential of investigating the functions of dNK signalling further.
To fully resolve all the cellular and molecular interactions associated with placentation and SA remodelling, studies which link the two cell types in disorders of SA remodelling may be required. The HLA-C/KIR interaction described in this review is one such mechanism (Hiby et al., 2004; Hiby et al., 2010; Chazara et al., 2011), and this finding has recently been mirrored in the mouse. Expression of the murine classical MHC molecule H2-K on mouse trophoblast giant cells has been detected, and antigenic dissimilarity between paternal MHC and maternal NK is associated with increased decidual vessel dilation, fetal growth and placental weight, which may have implications for the study of reproductive pathologies in human pregnancy (Madeja et al., 2011). Additional mechanisms other than MHC have also been investigated. It has been demonstrated that trophoblast co-cultured with decidual immune cells (60% dNK cells in this study) produce increased soluble-Flt, a protein associated with pre-eclampsia (Matsubara et al., 2005). Moreover, dNK cells expressing granulysin, a cytotoxic protein, are increased in patients who have undergone a first trimester spontaneous abortion as compared with a first trimester termination of pregnancy. Granulysin-expressing-dNK cells are capable of inducing apoptosis in EVT, and therefore this may be an aspect of disrupted signalling leading to pregnancy disorders (Nakashima et al., 2008). The expression of MMP-12 by trophoblast and other cell types, which inhibits dNK cell recruitment, is decreased in pre-eclampsia, further implying a connection between dNK cells and pathological conditions. Additionally, our studies of pregnancies with a high risk of developing pre-eclampsia show that dNK cells isolated from these patients are less able to induce EVT motility and fail to induce vascular cell apoptosis as compared with dNK cells isolated from low-risk pregnancies (Fraser et al., 2009). As such, further studies of the interactions between dNKs and EVT in patients with pathologies associated with altered SA remodelling will be of great interest to the field.
Conclusions and future perspectives
Although many disorders of pregnancy, such as pre-eclampsia, present symptoms in the second or third trimester, the remodelling of SAs in the first trimester is a key process in setting up a successful pregnancy. The role of EVT in this process has been extensively studied, however not in conjunction with dNK cells, and the role of dNK cells in SA remodelling has only recently been explored (Hanna et al., 2006; Harris, 2011). What is becoming apparent is that pregnancy disorders are likely to involve the actions of both fetal and maternal cells. The interactions between fetal and maternal cells are also complex, involving both the cytotoxic- and chemokine-producing capacity of dNK cells, and their roles alters as gestation progresses. The investigation of pregnancy as a multicellular system involving both fetal and maternal components, as well as comparison to other examples of tissue remodelling including cancer progression, is identifying key mechanisms in this process and will allow development of future therapeutic targets for pregnancy disorders.
Authors' roles
A.E.W., R.F. and J.E.C. conceived the manuscript. A.E.W. and R.F. drafted the manuscript and A.E.W. drafted figures and tables. A.E.W., R.F. and J.E.C. revised the manuscript and approved the final version.
Funding
A.E.W. was supported by the Wellcome Trust (project reference 091550). R.F. was a recipient of a PhD studentship from the Division of Basic Medical Sciences, St. George's, University of London.
Conflict of interest
None declared.
Acknowledgements
The authors would like to acknowledge the support of the Wellcome Trust (project reference 091550).
References
- Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G. Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol. 2004;51:275–282. doi: 10.1111/j.1600-0897.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- Amiot L, Ferrone S, Grosse-Wilde H, Seliger B. Biology of HLA-G in cancer: a candidate molecule for therapeutic intervention? Cell Mol Life Sci. 2011;68:417–431. doi: 10.1007/s00018-010-0583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Gardner L, Moffett A. A critical look at HLA-G. Trends Immunol. 2008;29:313–321. doi: 10.1016/j.it.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Arimoto-Ishida E, Sakata M, Sawada K, Nakayama M, Nishimoto F, Mabuchi S, Takeda T, Yamamoto T, Isobe A, Okamoto Y, Lengyel E, Suehara N, Morishige K, Kimura T. Up-regulation of alpha5-integrin by E-cadherin loss in hypoxia and its key role in the migration of extravillous trophoblast cells during early implantation. Endocrinology. 2009;150:4306–4315. doi: 10.1210/en.2008-1662. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Croy BA. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod. 1999;61:493–502. doi: 10.1095/biolreprod61.2.493. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton SV, Whitley GS, Dash PR, Wareing M, Crocker IP, Baker PN, Cartwright JE. Uterine spiral artery remodeling involves endothelial apoptosis induced by extravillous trophoblasts through Fas/FasL interactions. Arterioscler Thromb Vasc Biol. 2005;25:102–108. doi: 10.1161/01.ATV.0000148547.70187.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmayer N, Rafik Hamad R, Liszka L, Bremme K, Sverremark-Ekstrom E. Aberrant uterine natural killer (NK)-cell expression and altered placental and serum levels of the NK-cell promoting cytokine interleukin-12 in pre-eclampsia. Am J Reprod Immunol. 2006;56:292–301. doi: 10.1111/j.1600-0897.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- Barrientos G, Tirado-Gonzalez I, Klapp BF, Karimi K, Arck PC, Garcia MG, Blois SM. The impact of dendritic cells on angiogenic responses at the fetal-maternal interface. J Reprod Immunol. 2009;83:85–94. doi: 10.1016/j.jri.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Bingle L, Lewis CE, Corke KP, Reed MW, Brown NJ. Macrophages promote angiogenesis in human breast tumour spheroids in vivo. Br J Cancer. 2006;94:101–107. doi: 10.1038/sj.bjc.6602901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Blois SM, Klapp BF, Barrientos G. Decidualization and angiogenesis in early pregnancy: unravelling the functions of DC and NK cells. J Reprod Immunol. 2011;88:86–92. doi: 10.1016/j.jri.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Boehm U, Guethlein L, Klamp T, Ozbek K, Schaub A, Futterer A, Pfeffer K, Howard JC. Two families of GTPases dominate the complex cellular response to IFN-gamma. J Immunol. 1998;161:6715–6723. [PubMed] [Google Scholar]
- Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967;93:569–579. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- Bulla R, Villa A, Bossi F, Cassetti A, Radillo O, Spessotto P, De Seta F, Guaschino S, Tedesco F. VE-cadherin is a critical molecule for trophoblast-endothelial cell interaction in decidual spiral arteries. Exp Cell Res. 2005;303:101–113. doi: 10.1016/j.yexcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Lash GE. Human uterine natural killer cells: a reappraisal. Mol Immunol. 2005;42:511–521. doi: 10.1016/j.molimm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. Int J Dev Biol. 2010;54:281–294. doi: 10.1387/ijdb.082763jb. [DOI] [PubMed] [Google Scholar]
- Carlsten M, Norell H, Bryceson YT, Poschke I, Schedvins K, Ljunggren HG, Kiessling R, Malmberg KJ. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J Immunol. 2009;183:4921–4930. doi: 10.4049/jimmunol.0901226. [DOI] [PubMed] [Google Scholar]
- Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, Ratto GB, Mingari MC, Moretta L, Ferlazzo G. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112:863–875. doi: 10.1002/cncr.23239. [DOI] [PubMed] [Google Scholar]
- Cartwright JE, Tse WK, Whitley GS. Hepatocyte growth factor induced human trophoblast motility involves phosphatidylinositol-3-kinase, mitogen-activated protein kinase, and inducible nitric oxide synthase. Exp Cell Res. 2002;279:219–226. doi: 10.1006/excr.2002.5616. [DOI] [PubMed] [Google Scholar]
- Cartwright JE, Fraser R, Leslie K, Wallace AE, James JL. Remodelling at the maternal-fetal interface: relevance to human pregnancy disorders. Reproduction. 2010;140:803–813. doi: 10.1530/REP-10-0294. [DOI] [PubMed] [Google Scholar]
- Chakraborty D, Rumi MA, Konno T, Soares MJ. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc Natl Acad Sci USA. 2011;108:16295–16300. doi: 10.1073/pnas.1109478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazara O, Xiong S, Moffett A. Maternal KIR and fetal HLA-C: a fine balance. J Leukoc Biol. 2011;90:703–716. doi: 10.1189/jlb.0511227. [DOI] [PubMed] [Google Scholar]
- Chen Q, Stone PR, McCowan LM, Chamley LW. Interaction of Jar choriocarcinoma cells with endothelial cell monolayers. Placenta. 2005;26:617–625. doi: 10.1016/j.placenta.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Corvinus FM, Fitzgerald JS, Friedrich K, Markert UR. Evidence for a correlation between trophoblast invasiveness and STAT3 activity. Am J Reprod Immunol. 2003;50:316–321. doi: 10.1034/j.1600-0897.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- Craven CM, Morgan T, Ward K. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta. 1998;19:241–252. doi: 10.1016/s0143-4004(98)90055-8. [DOI] [PubMed] [Google Scholar]
- Croy BA, Ashkar AA, Foster RA, DiSanto JP, Magram J, Carson D, Gendler SJ, Grusby MJ, Wagner N, Muller W, Guimond MJ. Histological studies of gene-ablated mice support important functional roles for natural killer cells in the uterus during pregnancy. J Reprod Immunol. 1997;35:111–133. doi: 10.1016/s0165-0378(97)00054-5. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Fitzgerald ML, Fisher SJ. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest. 1992;89:210–222. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira LG, Lash GE, Murray-Dunning C, Bulmer JN, Innes BA, Searle RF, Sass N, Robson SC. Role of interleukin 8 in uterine natural killer cell regulation of extravillous trophoblast cell invasion. Placenta. 2010;31:595–601. doi: 10.1016/j.placenta.2010.04.012. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Dietl J, Honig A, Kammerer U, Rieger L. Natural killer cells and dendritic cells at the human feto-maternal interface: an effective cooperation? Placenta. 2006;27:341–347. doi: 10.1016/j.placenta.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Nie G, Hannan NJ, Paiva P, Salamonsen LA. Local regulation of implantation at the human fetal-maternal interface. Int J Dev Biol. 2010;54:313–322. doi: 10.1387/ijdb.082772ed. [DOI] [PubMed] [Google Scholar]
- Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80:1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- El Costa H, Casemayou A, Aguerre-Girr M, Rabot M, Berrebi A, Parant O, Clouet-Delannoy M, Lombardelli L, Jabrane-Ferrat N, Rukavina D, et al. Critical and differential roles of NKp46- and NKp30-activating receptors expressed by uterine NK cells in early pregnancy. J Immunol. 2008;181:3009–3017. doi: 10.4049/jimmunol.181.5.3009. [DOI] [PubMed] [Google Scholar]
- El Costa H, Tabiasco J, Berrebi A, Parant O, Aguerre-Girr M, Piccinni MP, Le Bouteiller P. Effector functions of human decidual NK cells in healthy early pregnancy are dependent on the specific engagement of natural cytotoxicity receptors. J Reprod Immunol. 2009;82:142–147. doi: 10.1016/j.jri.2009.06.123. [DOI] [PubMed] [Google Scholar]
- Engert S, Rieger L, Kapp M, Becker JC, Dietl J, Kammerer U. Profiling chemokines, cytokines and growth factors in human early pregnancy decidua by protein array. Am J Reprod Immunol. 2007;58:129–137. doi: 10.1111/j.1600-0897.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update. 2007;13:121–141. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472–1479. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- Fraser R, Whitley GS, Johnstone AP, Thilaganathan B, Cartwright JE. Decidual natural killer cells from pregnancies at higher risk of pre-eclampsia fail to induce endothelial apoptosis: a defect in spiral artery remodelling? Placenta. 2009;30:27. [Google Scholar]
- Fraser R, Whitley GS, Johnstone AP, Thilaganathan B, Cartwright JE. Impaired decidual natural killer cell regulation of trophoblast invasion and vascular remodelling in pregnancies at higher risk of pre-eclampsia. Reprod Sci. 2011;18:272A. [Google Scholar]
- Genbacev O, DiFederico E, McMaster M, Fisher SJ. Invasive cytotrophoblast apoptosis in pre-eclampsia. Hum Reprod. 1999;14(2):59–66. doi: 10.1093/humrep/14.suppl_2.59. [DOI] [PubMed] [Google Scholar]
- Genbacev O, McMaster MT, Fisher SJ. A repertoire of cell cycle regulators whose expression is coordinated with human cytotrophoblast differentiation. Am J Pathol. 2000;157:1337–1351. doi: 10.1016/S0002-9440(10)64648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbole G, Suman P, Gupta SK, Modi D. Decidualized endometrial stromal cell derived factors promote trophoblast invasion. Fertil Steril. 2011;95:1278–1283. doi: 10.1016/j.fertnstert.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Gomez-Lozano N, de Pablo R, Puente S, Vilches C. Recognition of HLA-G by the NK cell receptor KIR2DL4 is not essential for human reproduction. Eur J Immunol. 2003;33:639–644. doi: 10.1002/eji.200323741. [DOI] [PubMed] [Google Scholar]
- Gonen-Gross T, Goldman-Wohl D, Huppertz B, Lankry D, Greenfield C, Natanson-Yaron S, Hamani Y, Gilad R, Yagel S, Mandelboim O. Inhibitory NK receptor recognition of HLA-G: regulation by contact residues and by cell specific expression at the fetal-maternal interface. PLoS One. 2010;5:e8941. doi: 10.1371/journal.pone.0008941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. 2009;15:517–535. doi: 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod. 1997;56:169–179. doi: 10.1095/biolreprod56.1.169. [DOI] [PubMed] [Google Scholar]
- Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187:217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzic E, Cartwright JE, Keogh RJ, Whitley GS, Greenhill D, Hoppe A. Live cell image analysis of cell-cell interactions reveals the specific targeting of vascular smooth muscle cells by fetal trophoblasts. Exp Cell Res. 2008;314:1455–1464. doi: 10.1016/j.yexcr.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Han J, Li L, Hu J, Yu L, Zheng Y, Guo J, Zheng X, Yi P, Zhou Y. Epidermal growth factor stimulates human trophoblast cell migration through Rho A and Rho C activation. Endocrinology. 2010;151:1732–1742. doi: 10.1210/en.2009-0845. [DOI] [PubMed] [Google Scholar]
- Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, Katz G, Haimov-Kochman R, Fujii N, Yagel S, et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16-human natural killer cells. Blood. 2003;102:1569–1577. doi: 10.1182/blood-2003-02-0517. [DOI] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Harris LK, Baker PN, Keogh RJ, Cartwright JE, Whitley GS, Aplin JD. Trophoblast-induced smooth muscle cell apoptosis during spiral artery remodelling involves Fas/Fas ligand interactions. Placenta. 2005;26 A.77 (Abstract) [Google Scholar]
- Harris LK, Keogh RJ, Wareing M, Baker PN, Cartwright JE, Aplin JD, Whitley GS. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism. Am J Pathol. 2006;169:1863–1874. doi: 10.2353/ajpath.2006.060265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LK, Keogh RJ, Wareing M, Baker PN, Cartwright JE, Whitley GS, Aplin JD. BeWo cells stimulate smooth muscle cell apoptosis and elastin breakdown in a model of spiral artery transformation. Hum Reprod. 2007;22:2834–2841. doi: 10.1093/humrep/dem303. [DOI] [PubMed] [Google Scholar]
- Harris LK, McCormick J, Cartwright JE, Whitley GS, Dash PR. S-nitrosylation of proteins at the leading edge of migrating trophoblasts by inducible nitric oxide synthase promotes trophoblast invasion. Exp Cell Res. 2008;314:1765–1776. doi: 10.1016/j.yexcr.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Harris LK. Review: trophoblast-vascular cell interactions in early pregnancy: how to remodel a vessel. Placenta. 2010a;31(Suppl.):S93–S98. doi: 10.1016/j.placenta.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Harris LK, Smith SD, Keogh RJ, Jones RL, Baker PN, Knofler M, Cartwright JE, Whitley GS, Aplin JD. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am J Pathol. 2010b;177:2103–2115. doi: 10.2353/ajpath.2010.100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LK. IFPA gabor than award lecture: transformation of the spiral arteries in human pregnancy: key events in the remodelling timeline. Placenta. 2011;32(Suppl. 2):S154–S158. doi: 10.1016/j.placenta.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Hazan AD, Smith SD, Jones RL, Whittle W, Lye SJ, Dunk CE. Vascular-leukocyte interactions: mechanisms of human decidual spiral artery remodeling in vitro. Am J Pathol. 2010;177:1017–1030. doi: 10.2353/ajpath.2010.091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Morgan L, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Dutz JP, MacCalman CD, Yong P, Tan R, von Dadelszen P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-gamma. J Immunol. 2006;177:8522–8530. doi: 10.4049/jimmunol.177.12.8522. [DOI] [PubMed] [Google Scholar]
- Hu Y, Eastabrook G, Tan R, MacCalman CD, Dutz JP, von Dadelszen P. Decidual NK cell-derived conditioned medium enhances capillary tube and network organization in an extravillous cytotrophoblast cell line. Placenta. 2010;31:213–221. doi: 10.1016/j.placenta.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Huang SJ, Chen CP, Schatz F, Rahman M, Abrahams VM, Lockwood CJ. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol. 2008;214:328–336. doi: 10.1002/path.2257. [DOI] [PubMed] [Google Scholar]
- Hussein MR. Tumour-associated macrophages and melanoma tumourigenesis: integrating the complexity. Int J Exp Pathol. 2006;87:163–176. doi: 10.1111/j.1365-2613.2006.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husslein H, Haider S, Meinhardt G, Prast J, Sonderegger S, Knofler M. Expression, regulation and functional characterization of matrix metalloproteinase-3 of human trophoblast. Placenta. 2009;30:284–291. doi: 10.1016/j.placenta.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- Irving JA, Lala PK. Functional role of cell surface integrins on human trophoblast cell migration: regulation by TGF-beta, IGF-II, and IGFBP-1. Exp Cell Res. 1995;217:419–427. doi: 10.1006/excr.1995.1105. [DOI] [PubMed] [Google Scholar]
- James JL, Stone PR, Chamley LW. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Hum Reprod Update. 2006;12:137–144. doi: 10.1093/humupd/dmi043. [DOI] [PubMed] [Google Scholar]
- James JL, Whitley GS, Cartwright JE. Pre-eclampsia: fitting together the placental, immune, cardiovascular pieces. J Pathol. 2010;221:363–378. doi: 10.1002/path.2719. [DOI] [PubMed] [Google Scholar]
- James JL, Whitley GS, Cartwright JE. Shear stress and spiral artery remodelling: the effects of low shear stress on trophoblast-induced endothelial cell apoptosis. Cardiovasc Res. 2011;90:130–139. doi: 10.1093/cvr/cvq396. [DOI] [PubMed] [Google Scholar]
- Jovanovic M, Stefanoska I, Radojcic L, Vicovac L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1. Reproduction. 2010;139:789–798. doi: 10.1530/REP-09-0341. [DOI] [PubMed] [Google Scholar]
- Kalkunte SS, Mselle TF, Norris WE, Wira CR, Sentman CL, Sharma S. Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J Immunol. 2009;182:4085–4092. doi: 10.4049/jimmunol.0803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam EP, Gardner L, Loke YW, King A. The role of trophoblast in the physiological change in decidual spiral arteries. Hum Reprod. 1999;14:2131–2138. doi: 10.1093/humrep/14.8.2131. [DOI] [PubMed] [Google Scholar]
- Katou F, Ohtani H, Watanabe Y, Nakayama T, Yoshie O, Hashimoto K. Differing phenotypes between intraepithelial and stromal lymphocytes in early-stage tongue cancer. Cancer Res. 2007;67:11195–11201. doi: 10.1158/0008-5472.CAN-07-2637. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- Kauma SW, Bae-Jump V, Walsh SW. Hepatocyte growth factor stimulates trophoblast invasion: a potential mechanism for abnormal placentation in preeclampsia. J Clin Endocrinol Metab. 1999;84:4092–4096. doi: 10.1210/jcem.84.11.6120. [DOI] [PubMed] [Google Scholar]
- Keogh RJ, Harris LK, Freeman A, Baker PN, Aplin JD, Whitley GS, Cartwright JE. Fetal-derived trophoblast use the apoptotic cytokine tumor necrosis factor-alpha-related apoptosis-inducing ligand to induce smooth muscle cell death. Circ Res. 2007;100:834–841. doi: 10.1161/01.RES.0000261352.81736.37. [DOI] [PubMed] [Google Scholar]
- King A, Burrows T, Verma S, Hiby S, Loke YW. Human uterine lymphocytes. Hum Reprod Update. 1998;4:480–485. doi: 10.1093/humupd/4.5.480. [DOI] [PubMed] [Google Scholar]
- Knofler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol. 2010;54:269–280. doi: 10.1387/ijdb.082769mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinos MI, Murthi P, Wafai R, Thompson EW, Newgreen DF. Cadherins in the human placenta—epithelial-mesenchymal transition (EMT) and placental development. Placenta. 2010;31:747–755. doi: 10.1016/j.placenta.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Konishi H, Nakatsuka M, Chekir C, Noguchi S, Kamada Y, Sasaki A, Hiramatsu Y. Advanced glycation end products induce secretion of chemokines and apoptosis in human first trimester trophoblasts. Hum Reprod. 2004;19:2156–2162. doi: 10.1093/humrep/deh389. [DOI] [PubMed] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopcow HD, Eriksson M, Mselle TF, Damrauer SM, Wira CR, Sentman CL, Strominger JL. Human decidual NK cells from gravid uteri and NK cells from cycling endometrium are distinct NK cell subsets. Placenta. 2010;31:334–338. doi: 10.1016/j.placenta.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey G, Frank P, Shaikly V, Barrientos G, Cordo-Russo R, Ringel F, Moschansky P, Chernukhin IV, Metodiev M, Fernandez N, et al. In vivo dendritic cell depletion reduces breeding efficiency, affecting implantation and early placental development in mice. J Mol Med (Berl) 2008;86:999–1011. doi: 10.1007/s00109-008-0379-2. [DOI] [PubMed] [Google Scholar]
- Kusumi M, Yamashita T, Fujii T, Nagamatsu T, Kozuma S, Taketani Y. Expression patterns of lectin-like natural killer receptors, inhibitory CD94/NKG2A, and activating CD94/NKG2C on decidual CD56bright natural killer cells differ from those on peripheral CD56dim natural killer cells. J Reprod Immunol. 2006;70:33–42. doi: 10.1016/j.jri.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Lagente V, Le Quement C, Boichot E. Macrophage metalloelastase (MMP-12) as a target for inflammatory respiratory diseases. Expert Opin Ther Targets. 2009;13:287–295. doi: 10.1517/14728220902751632. [DOI] [PubMed] [Google Scholar]
- Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol. 2006;80:705–713. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- LaMarca HL, Ott CM, Honer Zu Bentrup K, Leblanc CL, Pierson DL, Nelson AB, Scandurro AB, Whitley GS, Nickerson CA, Morris CA. Three-dimensional growth of extravillous cytotrophoblasts promotes differentiation and invasion. Placenta. 2005;26:709–720. doi: 10.1016/j.placenta.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lash GE, Cartwright JE, Whitley GS, Trew AJ, Baker PN. The effects of angiogenic growth factors on extravillous trophoblast invasion and motility. Placenta. 1999;20:661–667. doi: 10.1053/plac.1999.0427. [DOI] [PubMed] [Google Scholar]
- Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF, Robson SC, Bulmer JN. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol. 2006;80:572–580. doi: 10.1189/jlb.0406250. [DOI] [PubMed] [Google Scholar]
- Lash GE, Naruse K, Innes BA, Robson SC, Searle RF, Bulmer JN. Secretion of angiogenic growth factors by villous cytotrophoblast and extravillous trophoblast in early human pregnancy. Placenta. 2010a;31:545–548. doi: 10.1016/j.placenta.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Lash GE, Otun HA, Innes BA, Percival K, Searle RF, Robson SC, Bulmer JN. Regulation of extravillous trophoblast invasion by uterine natural killer cells is dependent on gestational age. Hum Reprod. 2010b;25:1137–1145. doi: 10.1093/humrep/deq050. [DOI] [PubMed] [Google Scholar]
- Lash GE, Robson SC, Bulmer JN. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010c;31(Suppl.):S87–S92. doi: 10.1016/j.placenta.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Lash GE, Naruse K, Robson A, Innes BA, Searle RF, Robson SC, Bulmer JN. Interaction between uterine natural killer cells and extravillous trophoblast cells: effect on cytokine and angiogenic growth factor production. Hum Reprod. 2011;26:2289–2295. doi: 10.1093/humrep/der198. [DOI] [PubMed] [Google Scholar]
- Le Bouteiller P, Siewiera J, Casart Y, Aguerre-Girr M, El Costa H, Berrebi A, Tabiasco J, Jabrane-Ferrat N. The human decidual NK-cell response to virus infection: what can we learn from circulating NK lymphocytes? J Reprod Immunol. 2011;88:170–175. doi: 10.1016/j.jri.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Leach RE, Kilburn B, Wang J, Liu Z, Romero R, Armant DR. Heparin-binding EGF-like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev Biol. 2004;266:223–237. doi: 10.1016/j.ydbio.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Lee BN, Hammill H, Popek EJ, Cron S, Kozinetz C, Paul M, Shearer WT, Reuben JM. Production of interferons and beta-chemokines by placental trophoblasts of HIV-1-infected women. Infect Dis Obstet Gynecol. 2001;9:95–104. doi: 10.1155/S1064744901000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy EM, Roberti MP, Mordoh J. Natural killer cells in human cancer: from biological functions to clinical applications. J Biomed Biotechnol. 2011;2011:676198. doi: 10.1155/2011/676198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Houser BL, Nicotra ML, Strominger JL. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc Natl Acad Sci USA. 2009;106:5767–7572. doi: 10.1073/pnas.0901173106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- Madeja Z, Yadi H, Apps R, Boulenouar S, Roper SJ, Gardner L, Moffett A, Colucci F, Hemberger M. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc Natl Acad Sci USA. 2011;108:4012–4017. doi: 10.1073/pnas.1005342108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male V, Sharkey A, Masters L, Kennedy PR, Farrell LE, Moffett A. The effect of pregnancy on uterine NK cell KIR repertoire. Eur J Immunol. 2011;41:3017–3027. doi: 10.1002/eji.201141445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010;63:434–444. doi: 10.1111/j.1600-0897.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011;41:2522–2525. doi: 10.1002/eji.201141894. [DOI] [PubMed] [Google Scholar]
- Matsubara K, Nagamatsu T, Fujii T, Kozuma S, Taketani Y. Lymphokine-activated killer cells induced from decidual lymphocytes reduce the angiogenic activity of trophoblasts by enhancing the release of soluble fms-like tyrosine kinase-1 from trophoblasts: an implication for the pathophysiology of preeclampsia. J Reprod Immunol. 2005;68:27–37. doi: 10.1016/j.jri.2005.07.003. [DOI] [PubMed] [Google Scholar]
- McMaster MT, Bass KE, Fisher SJ. Human trophoblast invasion. Autocrine control and paracrine modulation. Ann N Y Acad Sci. 1994;734:122–131. doi: 10.1111/j.1749-6632.1994.tb21740.x. [DOI] [PubMed] [Google Scholar]
- Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk JM, Leonard S, McBey BA, Croy BA. Induction of murine spiral artery modification by recombinant human interferon-gamma. Placenta. 2005;26:835–838. doi: 10.1016/j.placenta.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Murphy SP, Tayade C, Ashkar AA, Hatta K, Zhang J, Croy BA. Interferon gamma in successful pregnancies. Biol Reprod. 2009;80:848–859. doi: 10.1095/biolreprod.108.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A, Shiozaki A, Myojo S, Ito M, Tatematsu M, Sakai M, Takamori Y, Ogawa K, Nagata K, Saito S. Granulysin produced by uterine natural killer cells induces apoptosis of extravillous trophoblasts in spontaneous abortion. Am J Pathol. 2008;173:653–664. doi: 10.2353/ajpath.2008.071169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse K, Lash GE, Bulmer JN, Innes BA, Otun HA, Searle RF, Robson SC. The urokinase plasminogen activator (uPA) system in uterine natural killer cells in the placental bed during early pregnancy. Placenta. 2009a;30:398–404. doi: 10.1016/j.placenta.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Naruse K, Lash GE, Innes BA, Otun HA, Searle RF, Robson SC, Bulmer JN. Localization of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitors for MMPs (TIMPs) in uterine natural killer cells in early human pregnancy. Hum Reprod. 2009b;24:553–561. doi: 10.1093/humrep/den408. [DOI] [PubMed] [Google Scholar]
- Naruse K, Innes BA, Bulmer JN, Robson SC, Searle RF, Lash GE. Secretion of cytokines by villous cytotrophoblast and extravillous trophoblast in the first trimester of human pregnancy. J Reprod Immunol. 2010;86:148–150. doi: 10.1016/j.jri.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Nowak I, Majorczyk E, Ploski R, Senitzer D, Sun JY, Kusnierczyk P. Lack of KIR2DL4 gene in a fertile Caucasian woman. Tissue Antigens. 2011;78:115–119. doi: 10.1111/j.1399-0039.2011.01711.x. [DOI] [PubMed] [Google Scholar]
- Pace D, Morrison L, Bulmer JN. Proliferative activity in endometrial stromal granulocytes throughout menstrual cycle and early pregnancy. J Clin Pathol. 1989;42:35–39. doi: 10.1136/jcp.42.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel S. Granulated metrial gland cells. Adv Anat Embryol Cell Biol. 1989;115:1–112. doi: 10.1007/978-3-642-74170-8. [DOI] [PubMed] [Google Scholar]
- Piccinni MP. T cell tolerance towards the fetal allograft. J Reprod Immunol. 2010;85:71–75. doi: 10.1016/j.jri.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van Assche A. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Brosens I. Deep placentation. Best Pract Res Clin Obstet Gynaecol. 2011;25:273–285. doi: 10.1016/j.bpobgyn.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008;118:3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlmann TG, Fitzgerald JS, Meissner A, Wengenmayer T, Schleussner E, Friedrich K, Markert UR. Trophoblast invasion: tuning through LIF, signalling via Stat3. Placenta. 2005;26(Suppl. A):S37–S41. doi: 10.1016/j.placenta.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Poehlmann TG, Schaumann A, Busch S, Fitzgerald JS, Aguerre-Girr M, Le Bouteiller P, Schleussner E, Markert UR. Inhibition of term decidual NK cell cytotoxicity by soluble HLA-G1. Am J Reprod Immunol. 2006;56:275–285. doi: 10.1111/j.1600-0897.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Xiao-Jun W, Zhi-Wei Z, Gong C, Guo-Qiang W, Li-Yi Z, Yuan-Fang L, Rajiv-Prasad K. The prognostic significance of peripheral T-lymphocyte subsets and natural killer cells in patients with colorectal cancer. Hepatogastroenterology. 2009;56:1310–1315. [PubMed] [Google Scholar]
- Quenby S, Nik H, Innes B, Lash G, Turner M, Drury J, Bulmer J. Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum Reprod. 2009;24:45–54. doi: 10.1093/humrep/den348. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, van der Meer A, Joosten I, Long EO. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 2006;4:e9. doi: 10.1371/journal.pbio.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reister F, Kingdom JC, Ruck P, Marzusch K, Heyl W, Pauer U, Kaufmann P, Rath W, Huppertz B. Altered protease expression by periarterial trophoblast cells in severe early-onset preeclampsia with IUGR. J Perinat Med. 2006;34:272–279. doi: 10.1515/JPM.2006.052. [DOI] [PubMed] [Google Scholar]
- Renaud SJ, Macdonald-Goodfellow SK, Graham CH. Coordinated regulation of human trophoblast invasiveness by macrophages and interleukin 10. Biol Reprod. 2007;76:448–454. doi: 10.1095/biolreprod.106.055376. [DOI] [PubMed] [Google Scholar]
- Saito S, Nishikawa K, Morii T, Enomoto M, Narita N, Motoyoshi K, Ichijo M. Cytokine production by CD16-CD56bright natural killer cells in the human early pregnancy decidua. Int Immunol. 1993;5:559–563. doi: 10.1093/intimm/5.5.559. [DOI] [PubMed] [Google Scholar]
- Salvesen HB, Akslen LA. Significance of tumour-associated macrophages, vascular endothelial growth factor and thrombospondin-1 expression for tumour angiogenesis and prognosis in endometrial carcinomas. Int J Cancer. 1999;84:538–543. doi: 10.1002/(sici)1097-0215(19991022)84:5<538::aid-ijc17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Scaife PJ, Bulmer JN, Robson SC, Innes BA, Searle RF. Effector activity of decidual CD8+ T lymphocytes in early human pregnancy. Biol Reprod. 2006;75:562–567. doi: 10.1095/biolreprod.106.052654. [DOI] [PubMed] [Google Scholar]
- Schiessl B, Innes BA, Bulmer JN, Otun HA, Chadwick TJ, Robson SC, Lash GE. Localization of angiogenic growth factors and their receptors in the human placental bed throughout normal human pregnancy. Placenta. 2009;30:79–87. doi: 10.1016/j.placenta.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Seaman WE, Sleisenger M, Eriksson E, Koo GC. Depletion of natural killer cells in mice by monoclonal antibody to NK-1.1. Reduction in host defense against malignancy without loss of cellular or humoral immunity. J Immunol. 1987;138:4539–4544. [PubMed] [Google Scholar]
- Searle RF, Jones RK, Bulmer JN. Phenotypic analysis and proliferative responses of human endometrial granulated lymphocytes during the menstrual cycle. Biol Reprod. 1999;60:871–878. doi: 10.1095/biolreprod60.4.871. [DOI] [PubMed] [Google Scholar]
- Sharkey AM, Gardner L, Hiby S, Farrell L, Apps R, Masters L, Goodridge J, Lathbury L, Stewart CA, Verma S, et al. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J Immunol. 2008;181:39–46. doi: 10.4049/jimmunol.181.1.39. [DOI] [PubMed] [Google Scholar]
- Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol. 2009;174:1959–1971. doi: 10.2353/ajpath.2009.080995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallmach T, Hebisch G, Orban P, Lu X. Aberrant positioning of trophoblast and lymphocytes in the feto-maternal interface with pre-eclampsia. Virchows Arch. 1999;434:207–211. doi: 10.1007/s004280050329. [DOI] [PubMed] [Google Scholar]
- Staun-Ram E, Goldman S, Shalev E. p53 Mediates epidermal growth factor (EGF) induction of MMP-2 transcription and trophoblast invasion. Placenta. 2009;30:1029–1036. doi: 10.1016/j.placenta.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Squadrito ML, Palma MD. Macrophage regulation of tumor angiogenesis: Implications for cancer therapy. Mol Aspects Med. 2011;32:123–145. doi: 10.1016/j.mam.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol. 2011;187:3671–3682. doi: 10.4049/jimmunol.1100130. [DOI] [PubMed] [Google Scholar]
- Tabiasco J, Rabot M, Aguerre-Girr M, El Costa H, Berrebi A, Parant O, Laskarin G, Juretic K, Bensussan A, Rukavina D, et al. Human decidual NK cells: unique phenotype and functional properties—a review. Placenta. 2006;27(Suppl. A):S34–S39. doi: 10.1016/j.placenta.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Tang AW, Alfirevic Z, Turner MA, Drury J, Quenby S. Prednisolone trial: study protocol for a randomised controlled trial of prednisolone for women with idiopathic recurrent miscarriage and raised levels of uterine natural killer (uNK) cells in the endometrium. Trials. 2009;10:102. doi: 10.1186/1745-6215-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AW, Alfirevic Z, Quenby S. Natural killer cells and pregnancy outcomes in women with recurrent miscarriage and infertility: a systematic review. Hum Reprod. 2011;26:1971–1980. doi: 10.1093/humrep/der164. [DOI] [PubMed] [Google Scholar]
- Thirkill TL, Lowe K, Vedagiri H, Blankenship TN, Barakat AI, Douglas GC. Macaque trophoblast migration is regulated by RANTES. Exp Cell Res. 2005;305:355–364. doi: 10.1016/j.yexcr.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Tseng D, Vasquez-Medrano DA, Brown JM. Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas. Br J Cancer. 2011;104:1805–1809. doi: 10.1038/bjc.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca P, Pietra G, Falco M, Romeo E, Bottino C, Bellora F, Prefumo F, Fulcheri E, Venturini PL, Costa M, et al. Analysis of natural killer cells isolated from human decidua: evidence that 2B4 (CD244) functions as an inhibitory receptor and blocks NK-cell function. Blood. 2006;108:4078–4085. doi: 10.1182/blood-2006-04-017343. [DOI] [PubMed] [Google Scholar]
- Varanou A, Withington SL, Lakasing L, Williamson C, Burton GJ, Hemberger M. The importance of cysteine cathepsin proteases for placental development. J Mol Med. 2006;84:305–317. doi: 10.1007/s00109-005-0032-2. [DOI] [PubMed] [Google Scholar]
- Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–28. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, Di Serio C, Naldini L, De Palma M, Tozer GM, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest. 2011;121:1969–1973. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley GS, Cartwright JE. Trophoblast-mediated spiral artery remodelling: a role for apoptosis. J Anat. 2009;215:21–26. doi: 10.1111/j.1469-7580.2008.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley GS, Cartwright JE. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta. 2010;31:465–474. doi: 10.1016/j.placenta.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PJ, Bulmer JN, Searle RF, Innes BA, Robson SC. Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: a comparison with late normal pregnancy. Reproduction. 2009;138:177–184. doi: 10.1530/REP-09-0007. [DOI] [PubMed] [Google Scholar]
- Wright JK, Dunk CE, Perkins JE, Winterhager E, Kingdom JC, Lye SJ. EGF modulates trophoblast migration through regulation of Connexin 40. Placenta. 2006;27(Suppl. A):S114–S121. doi: 10.1016/j.placenta.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Wright JK, Dunk CE, Amsalem H, Maxwell C, Keating S, Lye SJ. HER1 signaling mediates extravillous trophoblast differentiation in humans. Biol Reprod. 2010;83:1036–1045. doi: 10.1095/biolreprod.109.083246. [DOI] [PubMed] [Google Scholar]
- Xu Z, Jiang Y, Steed H, Davidge S, Fu Y. TGFbeta and EGF synergistically induce a more invasive phenotype of epithelial ovarian cancer cells. Biochem Biophys Res Commun. 2010;401:376–381. doi: 10.1016/j.bbrc.2010.09.059. [DOI] [PubMed] [Google Scholar]
- Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W, Zhu YF, Wang SM. Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer. 2007;121:1949–957. doi: 10.1002/ijc.22930. [DOI] [PubMed] [Google Scholar]
- Zhang JH, He H, Borzychowski AM, Takeda K, Akira S, Croy BA. Analysis of cytokine regulators inducing interferon production by mouse uterine natural killer cells. Biol Reprod. 2003;69:404–411. doi: 10.1095/biolreprod.103.015529. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol. 2011;8:1–11. doi: 10.1038/cmi.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]