Abstract
Purpose
To study changes in choroidal thickness with ranibizumab treatment for choroidal neovascularization (CNV).
Design
Prospective case series.
Methods
This prospective study consisted of 60 CNV-affected eyes of 60 patients treated with intravitreal injections of ranibizumab using an on-demand protocol after an initial loading phase. The eyes studied included 20 with age-related macular degeneration (AMD), 20 with polypoidal choroidal vasculopathy (PCV), and 20 with myopic CNV. In the eyes with AMD and PCV, choroidal thickness at the fovea was measured with optical coherence tomography using enhanced depth imaging. In eyes with myopic CNV, the choroidal thickness was measured using standard optical coherence tomography without the enhanced depth imaging technique.
Results
With ranibizumab treatment, central retinal thickness decreased significantly (P < 0.001) and visual acuity improved significantly (P < 0.001). However, central choroidal thickness (167.2 ± 108.3 μm) showed no significant change at 1 month after the loading phase (165.2 ± 107.8 μm, P = 0.120) or at final examination (164.8 ± 107.7 μm, P = 0.115). At baseline, central retinal thickness in eyes with AMD was significantly greater that those with PCV (P = 0.005) or high myopia (P = 0.029). However, central choroidal thickness in eyes with myopic CNV was significantly thinner than in eyes with AMD (P < 0.001) or PCV (P < 0.001). In each type of disease, there was no significant change in central choroidal thickness with ranibizumab treatment.
Conclusion
The effect of ranibizumab on the choroidal thickness is minimal, if any.
Keywords: choroidal thickness, ranibizumab, optical coherence tomography
Introduction
Ranibizumab, a Fab fragment of a recombinant, humanized, monoclonal antibody, blocks all isoforms of vascular endothelial growth factor (VEGF)-A.1 The inhibition of VEGF-A reduces the leakage from choroidal neovascularization (CNV) and further growth of CNV.2,3 The current standard treatment for exudative age-related macular degeneration (AMD) is intravitreal injections of ranibizumab.4 Previous large-scale, prospective, randomized studies of eyes with AMD showed dramatic effects of ranibizumab on CNV and subsequent improvement of visual acuity.5,6 However, most patients with AMD require frequent injections of ranibizumab to maintain the initial visual improvement during the loading phase.7 Mendrinos et al8 recently reported that the retinal arteriolar diameter was reduced by 18.5% at 30 days and by 19.1% at 12 months after the initiation of ranibizumab treatments for exudative AMD. Because VEGF has various physiological effects on eyes, including cell proliferation,9 neuroprotective effects,10 maintenance of the choriocapillaris,11 and increases in ocular blood flow,12 long-term inhibition of VEGF may adversely affect the eye.13
The choroid is a multifunctional structure with one of the highest blood flow rates in the body.14,15 It primarily works to support the function and nutrition of the outer retina, including photoreceptors and retinal pigment epithelium.15 Since Spaide et al16 introduced enhanced depth-imaging optical coherence tomography (EDI-OCT), which is based on spectral-domain OCT technology, many investigators have reported on the role of the choroid in the pathogenesis of various ocular diseases,17–22 including AMD,23–25 polypoidal choroidal vasculopathy (PCV),23,24,26 central serous chorioretinopathy,27,28 and myopic CNV.29,30 It has recently been suggested that choroidal thinning may be involved in the development of CNV associated with AMD and high myopia.23,24,31 VEGF works to induce vessel dilation and to increase ocular blood flow through a mechanism involving increased nitric oxide production.32,33 It is then possible that long-term inhibition of VEGF by ranibizumab might cause the constriction of the choroidal vessels, leading to further thinning of the choroid. Thus far, however, only limited information is available on the effects of ranibizumab on the choroid. In this prospective study, we performed longitudinal measurements of choroidal thickness before and after ranibizumab treatment for CNV associated with AMD, PCV, and high myopia to evaluate the possible adverse effects of ranibizumab on the choroid.
Patients and methods
This prospective study consisted of 60 eyes of 60 patients with CNV treated with intravitreal injections of ranibizumab (Lucentis; Novartis, Bülach, Switzerland). The choroidal thickness of the eyes was examined using OCT at Kyoto University Hospital between May 2010 and August 2011. The underlying pathology of the 60 eyes included 20 consecutive eyes with AMD, 20 with PCV, and 20 with myopic CNV. The inclusion criteria for the study groups were (1) symptomatic neovascular lesions beneath the center of the fovea, (2) the presence of active exudative features involving the macula, (3) treatment by intravitreal injections of ranibizumab after the initial diagnosis, and (4) OCT examination before and after treatments. Exclusion criteria were (1) the obscuration of retinal or choroidal images by media opacity or thick subfoveal hemorrhage, (2) a history of intraocular inflammation, (3) the presence or history of other macular abnormalities (eg, idiopathic CNV, retinal angiomatous proliferation, angioid streaks, other secondary CNV, or central serous chorioretinopathy), (4) a history of previous treatment for CNV, and (5) eyes with a history of pars plana vitrectomy or other intraocular surgeries, except history of cataract surgery. Subjects with systemic diseases or conditions that could affect retinal or choroidal thickness were also excluded, such as those with Vogt-Koyanagi-Harada disease, malignant hypertension, or pregnant women.
At the initial visit, each patient underwent a comprehensive ophthalmologic examination, including measurement of best- corrected visual acuity with a Landolt chart; determination of intraocular pressure; indirect ophthalmoscopy; slit lamp biomicroscopy with a contact lens; OCT examination (Spectralis HRA+OCT; Heidelberg Engineering, Heidelberg, Germany); and fluorescein and indocyanine green angiography (HRA-2; Heidelberg Engineering). The diagnostic criteria for PCV were based on indocyanine green angiography, which showed a branching vascular network that terminates in polypoidal swelling. The diagnosis of myopic CNV was based on a minimum spherical equivalent refractive error of more than −6.0 diopters or an axial length of more than 26.5 mm, characteristic retinal signs of high myopia, and evidence of CNV based on the presence of leakage on fluorescein angiography and/or intraretinal or subretinal fluid on OCT.
At the loading phase, all eyes with PCV and AMD received three successive intravitreal injections of ranibizumab at monthly intervals (at baseline and at 1 and 2 months); eyes with myopic CNV were treated by a single injection of ranibizumab. After the loading phase, each patient was scheduled for an examination each month, at which time they underwent a comprehensive ophthalmologic examination. Each eye was re-treated by an intravitreal injection of ranibizumab as an on-demand protocol, depending on the presence of exudative signs, such as intraretinal edema or subretinal fluid on OCT and/or leakage on fluorescein angiography during follow-up. Injections of ranibizumab were performed under sterile conditions, and prophylactic topical antibiotics were applied for 1 week after the injection.
In eyes with AMD and PCV, the choroidal thickness was measured using the EDI technique, which was performed by placing the OCT instrument close enough to the eye to obtain an inverted image. All images were obtained using an eye-tracking system, and 100 scans were averaged automatically to improve the signal-to-noise ratio. The central choroidal thickness, defined as the vertical distance between the outer surface of the retinal pigment epithelium and the hyperreflective line of the chorioscleral interface, was measured from the horizontal line scan under the center of the fovea. In eyes with myopic CNV, the choroidal thickness was measured with standard OCT without the EDI technique, as identification of the chorioscleral interface in eyes with high myopia is easy and reliable. The central retinal thickness was defined as the distance between the inner surface of the neurosensory retina and the inner surface of the retinal pigment epithelium under the center of the fovea. All measurements were done manually by a retina specialist, blinded to the study parameters, using a built-in caliber OCT machine.
All statistical tests were performed using SPSS version 16.0 software (SPSS Inc, Chicago, IL). All values are presented as mean ± standard deviation. For statistical analysis, best-corrected visual acuity as measured with a Landolt chart was converted to a logarithm of the minimal angle of resolution. Initial measurement values between the three types of diseases were compared using oneway analysis of variance with Bonferroni corrections. Measurements before and after the treatments were analyzed by repeated measures analysis of variance, and multiple comparisons were done using the Bonferroni post-hoc method. A P value of <0.05 was considered statistically significant.
Results
In the current study, 60 eyes of 60 patients (36 men and 24 women; age range, 35–90 years; mean age, 71.1 ± 9.6 years) with subfoveal CNV were treated with ranibizumab. Table 1 shows the initial characteristics of the study population. Table 2 shows the change in mean visual acuity, central retinal thickness, and central choroidal thickness before and after treatments with ranibizumab. Of the 60 eyes studied, 20 had AMD, 20 had PCV, and 20 had myopic CNV. The mean follow-up period was 8.9 ± 3.9 months. The mean refractive error was 1.54 (± 1.75) diopters (D) (range: −1.75–+3.0 D) in the AMD group, −0.56 (± 1.28) (range: −2.75–+2.5 D) in the PCV group, and −10.96 (± 3.32) (range: −21.0–−7 D) in the myopic CNV group. The mean axial length in eyes with myopic CNV was 28.71 ± 1.01 mm. With ranibizumab treatment, central retinal thickness decreased significantly at 1 month after the loading phase (P < 0.001) and at final examination (P < 0.001). In parallel with the reduction of retinal thickness, visual acuity was significantly improved at 1 month after the loading phase (P = 0.005) and at final examination (P < 0.001). However, choroidal thickness (167.2 ± 108.3 μm) showed no significant change at 1 month after the loading phase (165.2 ± 107.8 μm, P = 0.120) or at final examination (164.8 ± 107.7 μm, P = 0.115) (Table 2).
Table 1.
Characteristics of cohort eyes
| Total | Age-related macular degeneration | Polypoidal choroidal vasculopathy | Myopic choroidal neovascularization | P valueb | |
|---|---|---|---|---|---|
| Number of eyes | 60 | 20 | 20 | 20 | |
| Age (years) | 71.1 ± 9.6 | 76.2 ± 6.2 | 71.7 ± 9.2 | 65.6 ± 10.3 | 0.001 |
| Sex (male/female) | 36/24 | 15/5 | 16/4 | 5/15 | 0.001 |
| Refractive error (diopters) (range) | −2.74 ± 5.97 (−21.0–+3.0) | 1.54 ± 1.75 (−1.75–+3.0) | −0.56 ± 1.28 (−2.75–+2.5) | −10.96 ± 3.32 (−21.0–−7.0) | <0.001 |
| Number of injections (range) | 3.2 ± 1.4 (1–7) | 3.5 ± 0.9 (3–6) | 3.9 ± 1.4 (3–7) | 2.2 ± 1.3 (1–5) | <0.001 |
| Follow-up (months) | 8.9 ± 3.9 | 8.4 ± 3.7 | 8.4 ± 3.3 | 9.8 ± 4.6 | 0.422 |
| Visual acuity (logMAR)a | 0.45 ± 0.37 | 0.61 ± 0.41 | 0.24 ± 0.23 | 0.49 ± 0.37 | 0.004 |
| Central retinal thickness (μm) | 436.0 ± 198.5 | 551.0 ± 232.5 | 361.2 ± 161.4 | 395.9 ± 143.9 | 0.004 |
| Central choroidal thickness (μm) | 167.2 ± 108.3 | 217.4 ± 98.6 | 230.8 ± 68.8 | 53.6 ± 38.2 | <0.001 |
Notes:
LogMAR, logarithm of the minimum angle of resolution;
values were compared using one-way analysis of variance with Bonferroni corrections.
Table 2.
Changes in visual acuity, retinal thickness, and choroidal thickness during ranibizumab treatment
| Before treatment | After the loading phaseb,c | At final examinationc | |
|---|---|---|---|
| Visual acuity (logMAR)a | |||
| Total | 0.45 ± 0.37 | 0.33 ± 0.38 (0.005) | 0.28 ± 0.34 (<0.001) |
| Age-related macular degeneration | 0.61 ± 0.41 | 0.58 ± 0.43 (0.663) | 0.45 ± 0.33 (0.030) |
| Polypoidal choroidal vasculopathy | 0.24 ± 0.23 | 0.19 ± 0.29 (0.885) | 0.12 ± 0.25 (0.046) |
| Myopic choroidal neovascularization | 0.49 ± 0.37 | 0.23 ± 0.28 (<0.001) | 0.24 ± 0.31 (<0.001) |
| Central retinal thickness (μm) | |||
| Total | 436.0 ± 198.5 | 262.9 ± 113.8 (<0.001) | 256.7 ± 106.0 (<0.001) |
| Age-related macular degeneration | 551.0 ± 232.5 | 298.4 ± 156.6 (<0.001) | 293.2 ± 147.8 (<0.001) |
| Polypoidal choroidal vasculopathy | 361.2 ± 161.4 | 244.7 ± 92.2 (0.008) | 236.2 ± 76.1 (0.004) |
| Myopic choroidal neovascularization | 395.9 ± 143.9 | 245.7 ± 72.1 (<0.001) | 240.8 ± 71.7 (<0.001) |
| Central choroidal thickness (μm) | |||
| Total | 167.2 ± 108.3 | 165.2 ± 107.8 (0.120) | 164.8 ± 107.7 (0.115) |
| Age-related macular degeneration | 217.4 ± 98.6 | 214.2 ± 99.2 (0.305) | 215.1 ± 98.7 (0.586) |
| Polypoidal choroidal vasculopathy | 230.8 ± 68.8 | 228.7 ± 68.0 (0.876) | 227.4 ± 67.9 (0.138) |
| Myopic choroidal neovascularization | 53.6 ± 38.2 | 52.7 ± 38.5 (1.00) | 51.9 ± 38.2 (0.348) |
Notes:
LogMAR, logarithm of the minimum angle of resolution;
at the loading phase, all eyes with PCV and AMD received three successive intravitreal injections of ranibizumab at monthly intervals (at baseline and at 1 and 2 months); eyes with myopic CNV were treated by a single injection of ranibizumab;
data were analyzed by repeated measures analysis of variance and multiple comparisons were done using the Bonferroni post-hoc method.
At baseline, the central retinal thickness in eyes with AMD was significantly greater than in eyes with PCV (P = 0.005) or myopic CNV (P = 0.029). However, central choroidal thickness in eyes with myopic CNV was significantly lower than in eyes with AMD (P < 0.001) or PCV (P < 0.001). Immediately after the initiation of treatment, OCT examination showed reduced exudative change in eyes with AMD, PCV, and myopic CNV. Retinal thickness was significantly reduced with the reduction of serous retinal detachment and macular edema ( Figures 1–3). Figure 4 shows the change in central retinal thickness in each group. This reduction in retinal thickness was similar in each type of disease. However, OCT examination showed no change in the choroidal structure with the treatments (Figures 1–3). There was no significant change in central choroidal thickness with treatment in any of the disease types (Figure 4).
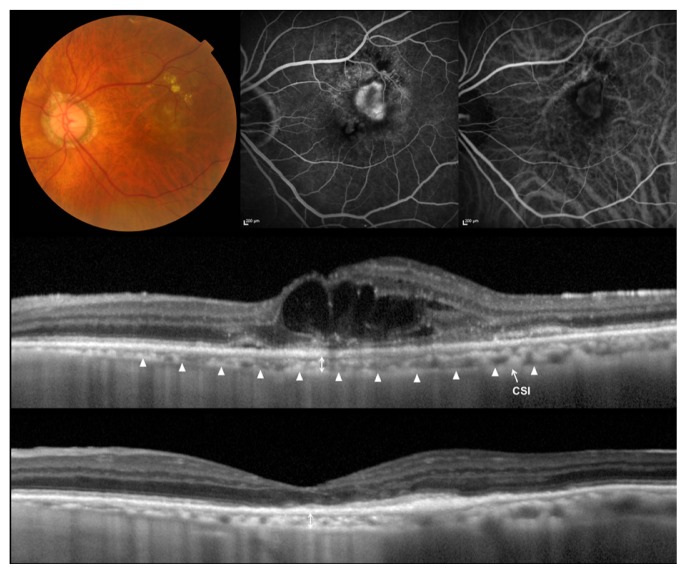
Figure 1.
Choroidal neovascularization (CNV) associated with age-related macular degeneration treated with ranibizumab.
Notes: (Top left) Left eye of an 81-year-old man with CNV associated with age-related macular degeneration. Initial visual acuity in the left eye was 0.2 in a Landolt chart (0.70 in logarithm of the minimal angle of resolution) and refractive error was −1.75 Diopter. (Top middle) Fluorescein angiography shows a minimally classic CNV. (Top right) Indocyanine green angiography shows no polypoidal lesions. (Middle) A horizontal section through the fovea obtained by optical coherence tomography with the enhanced-depth imaging technique before the treatment. Multiple cystoid spaces are seen. Central retinal thickness was 529 μm, and central choroidal thickness was 105 μm. (Bottom) A horizontal section through the fovea after three monthly injections of ranibizumab at the loading phase. Intraretinal fluid was absorbed completely (central retinal thickness, 157 μm), and visual acuity improved to 0.3 in a Landolt chart (0.52 in logarithm of the minimal angle of resolution). The choroidal structure shows no changes (central choroidal thickness, 101 μm). At 9 months, the central choroidal thickness was still 103 μm.
Abbreviation: CSI, chorioscleral interface.
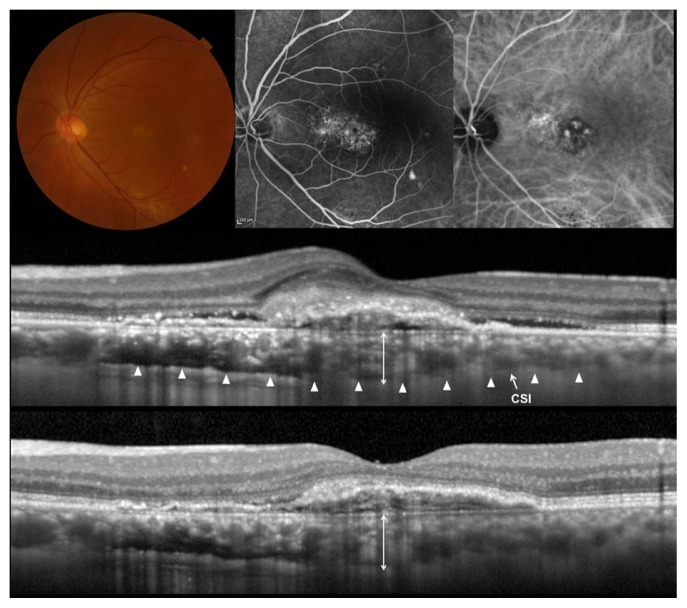
Figure 3.
Myopic choroidal neovascularization (CNV) treated with ranibizumab.
Notes: (Top left) Right eye of a 67-year-old woman with CNV secondary high myopia. Spherical equivalent was −8.75 diopters, and axial length was 27.64 mm. Initial visual acuity in the left eye was 0.3 in a Landolt chart (0.52 in logarithm of the minimal angle of resolution). (Top middle and right) Fluorescein (left) and indocyanine green (right) angiography shows a small CNV. (Middle) A horizontal section through the fovea before treatment obtained by optical coherence tomography without the enhanced-depth imaging technique. CNV accompanied exudative change with surrounding serous retinal detachment. Central retinal thickness was 479 μm and central choroidal thickness was 43 μm. (Bottom) A horizontal section through the fovea after a single injection of ranibizumab. Intraretinal fluid was absorbed completely (central retinal thickness, 250 μm), and visual acuity improved to 0.7 in a Landolt chart (0.15 in logarithm of the minimal angle of resolution). The choroidal structure shows no changes (central choroidal thickness, 40 μm).
Abbreviation: CSI, chorioscleral interface.
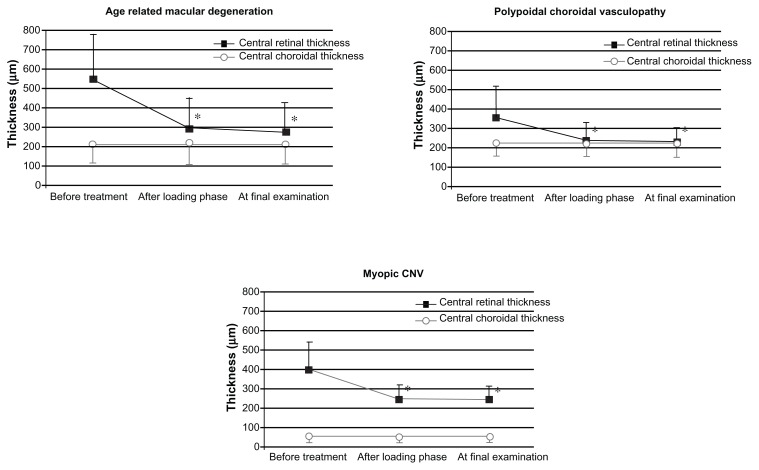
Figure 4.
Changes in mean central retinal thickness and mean central choroidal thickness during ranibizumab treatment for choroidal neovascularization (CNV) in eyes with age-related macular degeneration (top left), polypoidal choroidal vasculopathy (top right), and myopic CNV (second row).
Notes: The black squares indicate central retinal thickness; the open circles indicate central choroidal thickness. *P < 0.01, compared with values before treatment. At the loading phase, all eyes with PCV and AMD received three successive intravitreal injections of ranibizumab at monthly intervals (at baseline and at 1 and 2 months); eyes with myopic CNV were treated by a single injection of ranibizumab.
Discussion
An increasing number of investigators have studied the choroid in association with the pathophysiology of various chorioretinal diseases.17–24,29,34,35 With the use of EDI-OCT, Chung et al23 and Koizumi et al24 separately reported foveal choroidal thinning in eyes with exudative AMD. In addition, with the use of 3-dimensional OCT at 1060 nm, Wood et al36 reported choroidal thinning of the macular area in eyes with early AMD. The thinning of the macular choroid may be partially involved in the development of CNV associated with AMD. In eyes with high myopia, previous reports have shown that the choroid is extremely thin;29,30 the central choroidal thickness in our patients with high myopia was 53.6 ± 38.1 μm, which was much thinner than in patients with AMD and PCV. In addition, Ikuno et al31 reported that eyes with myopic CNV have a thinner choroid than their contralateral eye without CNV; the authors speculated that a thinner choroid is a risk factor for CNV in eyes with high myopia.23,24,31
Treatment with ranibizumab is expected to induce longterm inhibition of all isoforms of VEGF-A in the eye.1 It may well be that physicians are concerned about the adverse effect of ranibizumab on the choroid, especially when they treat eyes with AMD or high myopia. In the current study, choroidal thickness (167.2 ± 108.3 μm) showed no significant change after the loading phase (165.2 ± 107.8 μm) or at final examination (164.8 ± 107.7 μm). In a PubMed search, we could find no reference to changes in choroidal thickness after anti-VEGF treatment. Based on a retrospective analysis of 47 eyes with exudative AMD treated with ranibizumab, Sadda et al37 have recently noted that choroidal thickness appears to decrease slightly over time in eyes with exudative AMD, but that ranibizumab does not appear to accelerate this decline. Previously, Margolis and Spaide18 reported that subfoveal choroidal thickness decreased by 1.56 μm per year in normal subjects. Our patients showed a mean decrease of 2.4 μm (1.4%) in central choroidal thickness during a mean follow-up time of 8.9 months. This decrease in choroidal thickness may not be so different from the change seen in normal subjects.
More recently, Koizumi et al38 reported that in eyes with exudative AMD treated with ranibizumab, mean subfoveal choroidal thickness decreased from 228 μm at baseline to 213 μm at 3 months (P = 0.001), 215 μm at 6 months (P = 0.009), and 213 μm at 12 months (P = 0.015). Although the authors found a statistically significant decrease in the foveal choroidal thickness, the mean reduction was only 15 μm. Recently, Ikuno et al39 reported that the intervisit intraclass correlation coefficient for foveal choroidal thickness was 0.893 (95% CI, 0.864–0.916). However, because measurement of choroidal thickness has high variance due to focal irregularities or indistinctness of the chorioscleral border,23,24,40,41 it might be difficult to detect minimal changes in thickness practically, even with the EDI-OCT technique. Based on the current findings and other recent reports, the effect of ranibizumab on choroidal thickness is estimated to be small, if any.
In contrast to AMD23,24 and high myopia,29,30 previous reports showed increased choroidal thickness in PCV.23,24,26 Recently, Maruko et al26 reported that subfoveal choroidal thickness is decreased after photodynamic therapy in eyes with PCV. In addition, the authors suggested that the choroidal thickening seen in PCV eyes is associated with increased choroidal vascular hyperpermeability.26 After photodynamic therapy, choroidal thickness in eyes with PCV was reduced not only in the macular area, where the photodynamic therapy was performed, but also beyond the vascular arcade. Photodynamic therapy could possibly reduce choroidal thickness by decreasing choroidal vascular hyperpermeability. Our patients with PCV showed no significant reduction in choroidal thickness after ranibizumab treatments. Although VEGF works to increase the vascular permeability in the eye,42 the effect of the ranibizumab on choroidal vascular permeability may be limited.
Papadopoulou et al43 recently reported a reduction in the vessel caliber of the retinal arterioles in eyes with AMD after treatment with ranibizumab. In addition, other investigators have reported the vasoconstriction44 or even occlusion of retinal arterioles45,46 induced by intravitreal injections of bevacizumab, another anti-VEGF agent. Because VEGF is reported to induce vascular dilation and increase retinal blood flow,12 pan-inhibition of VEGF by ranibizumab may cause vasoconstriction of retinal arterioles. However, we found no prominent effects of ranibizumab on choroidal thickness; it is unclear why no effects were seen. One possibility is that the concentration of ranibizumab in the choroid is low due to the blockage of the retinal pigment epithelium. Another possibility is that VEGF has a limited role in the control of blood flow and vascular permeability in the choroid.
Major limitations of the current study are its small sample size, lack of controls, and short follow-up period. Another limitation is that measurement of choroidal thickness was performed manually and only at the fovea. The measurement of choroidal thickness tends to be affected by focal irregularity or indistinctness of the chorioscleral border.23,24,40 The raster scan protocol, which uses many measurement points from an OCT at a longer wavelength, would minimize measurement errors. Software to determine the borders of the choroid automatically is essential to standardize this technique further.41,47,48 Despite these shortcomings, our findings suggest that the effect of ranibizumab on choroidal thickness can be estimated to be minimal, if any. However, we studied only the change in the thickness of the choroid and did not evaluate changes in its function. It is possible that choroidal blood flow changes with ranibizumab treatment. Therefore, it may be necessary to evaluate choroid function during ranibizumab treatment as well.
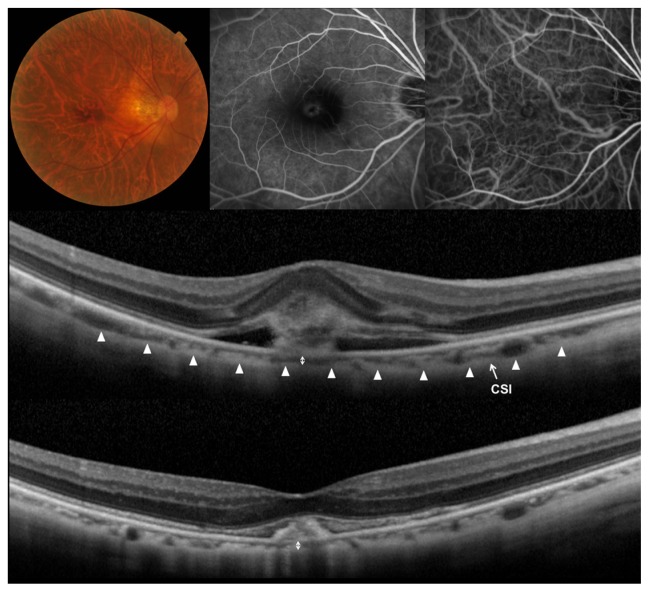
Figure 2.
Polypoidal choroidal vasculopathy treated with ranibizumab.
Notes: (Top left) Left eye of a 58-year-old man with polypoidal choroidal vasculopathy. Initial visual acuity in the left eye was 0.15 in a Landolt chart (0.82 in logarithm of the minimal angle of resolution). (Top middle) Fluorescein angiography shows occult with no classic choroidal neovascularization. (Top right) Indocyanine green angiography shows a branching vascular network terminating in polypoidal lesions. (Middle) A horizontal section through the fovea obtained by optical coherence tomography using the enhanced-depth imaging technique before the treatment shows fibrin exudates with surrounding serous retinal detachment. Central retinal thickness was 295 μm and central choroidal thickness was 270 μm. (Bottom) A horizontal section through the fovea after three monthly injections and an additional injection of ranibizumab. Exudative change was almost absorbed (central retinal thickness, 235 μm), and visual acuity improved to 0.6 in a Landolt chart (0.22 in logarithm of the minimal angle of resolution). The choroidal structure showed no changes, and the central choroidal thickness was 259 μm.
Abbreviation: CSI, chorioscleral interface.
Acknowledgements
This study was supported in part by the Japan Society for the Promotion of Science (JSPS), Tokyo, Japan (Grant-in-Aid for Scientific Research, no 21592256), and by the Japan National Society for the Prevention of Blindness, Tokyo, Japan.
Contributions of the authors were as follows: Conception and design of the study (AAE, AT, NY); analysis and interpretation (AAE, AT, KO, SO, KY, AO, NY); writing of the article (AAE, AT); critical revision of the article (KO, SO, KY, AO, NY); final approval of the article (AAE, AT, KO, SO, KY, AO, NY); and data collection (AAE, AT, KO, SO, KY, AO).
The Institutional Review Board and Ethics Committee of Kyoto University approved this study, which adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from each subject before examination.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen Y, Wiesmann C, Fuh G, et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293(4):865–881. doi: 10.1006/jmbi.1999.3192. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26(8):859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 3.Miller JW, Adamis AP, Shima DT, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994;145(3):574–584. [PMC free article] [PubMed] [Google Scholar]
- 4.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 6.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell P, Korobelnik JF, Lanzetta P, et al. Ranibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trials. Br J Ophthalmol. 2000;94(1):2–13. doi: 10.1136/bjo.2009.159160. [DOI] [PubMed] [Google Scholar]
- 8.Mendrinos E, Mangioris G, Papadopoulou D, Donati G, Pournaras C. One year results of the effect of intravitreal ranibizumab on the retinal arteriolar diameter in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.09-3721. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 9.Claffey KP, Robinson GS. Regulation of VEGF/VPF expression in tumor cells: consequences for tumor growth and metastasis. Cancer Metastasis Rev. 1996;15(2):165–176. doi: 10.1007/BF00437469. [DOI] [PubMed] [Google Scholar]
- 10.Nishijima K, Ng YS, Zhong L, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171(1):53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaauwgeers HG, Holtkamp GM, Rutten H, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155(2):421–428. doi: 10.1016/S0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27(3):284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res. 2000;55:15–35. [PubMed] [Google Scholar]
- 14.Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci. 2000;41(10):3117–3123. [PubMed] [Google Scholar]
- 15.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Fong AH, Li KK, Wong D. Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt-Koyanagi-Harada disease. Retina. 2011;31(3):502–509. doi: 10.1097/IAE.0b013e3182083beb. [DOI] [PubMed] [Google Scholar]
- 18.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147(5):811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Reibaldi M, Boscia F, Avitabile T, et al. Enhanced depth imaging optical coherence tomography of the choroid in idiopathic macular hole: a cross-sectional prospective study. Am J Ophthalmol. 2011;151(1):112–117. doi: 10.1016/j.ajo.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009;147(5):801–810. doi: 10.1016/j.ajo.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Yeoh J, Rahman W, Chen F, et al. Choroidal imaging in inherited retinal disease using the technique of enhanced depth imaging optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2010;248(12):1719–1728. doi: 10.1007/s00417-010-1437-3. [DOI] [PubMed] [Google Scholar]
- 22.Blackburn J, McGwin G., Jr Enhanced depth imaging optical coherence tomography of the choroid in idiopathic macular hole. Am J Ophthalmol. 2011;151(3):560–561. doi: 10.1016/j.ajo.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011;118(5):840–845. doi: 10.1016/j.ophtha.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Koizumi H, Yamagishi T, Yamazaki T, Kawasaki R, Kinoshita S. Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249(8):1123–1128. doi: 10.1007/s00417-011-1620-1. [DOI] [PubMed] [Google Scholar]
- 25.Manjunath V, Goren J, Fujimoto JG, Duker JS. Analysis of choroidal thickness in age-related macular degeneration using spectral-domain optical coherence tomography. Am J Ophthalmol. 2011;152(4):663–668. doi: 10.1016/j.ajo.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maruko I, Iida T, Sugano Y, Saito M, Sekiryu T. Subfoveal retinal and choroidal thickness after verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2011;151(4):594–603. doi: 10.1016/j.ajo.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M, Spaide RF. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010;117(9):1792–1799. doi: 10.1016/j.ophtha.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469–1473. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 29.Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148(3):445–450. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 30.Ikuno Y, Tano Y. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50(8):3876–3880. doi: 10.1167/iovs.08-3325. [DOI] [PubMed] [Google Scholar]
- 31.Ikuno Y, Jo Y, Hamasaki T, Tano Y. Ocular risk factors for choroidal neovascularization in pathologic myopia. Invest Ophthalmol Vis Sci. 2010;51(7):3721–3725. doi: 10.1167/iovs.09-3493. [DOI] [PubMed] [Google Scholar]
- 32.Tilton RG, Chang KC, LeJeune WS, Stephan CC, Brock TA, Williamson JR. Role for nitric oxide in the hyperpermeability and hemodynamic changes induced by intravenous VEGF. Invest Ophthalmol Vis Sci. 1999;40(3):689–696. [PubMed] [Google Scholar]
- 33.Ku DD, Zaleski JK, Liu S, Brock TA. Vascular endothelial growth factor induces EDRF-dependent relaxation in coronary arteries. Am J Physiol. 1993;265(2 Pt 2):H586–H592. doi: 10.1152/ajpheart.1993.265.2.H586. [DOI] [PubMed] [Google Scholar]
- 34.Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of Vogt-Koyanagi-Harada disease. Retina. 2011;31(3):510–517. doi: 10.1097/IAE.0b013e3181eef053. [DOI] [PubMed] [Google Scholar]
- 35.Imamura Y, Iida T, Maruko I, Zweifel SA, Spaide RF. Enhanced depth imaging optical coherence tomography of the sclera in dome-shaped macula. Am J Ophthalmol. 2011;151(2):297–302. doi: 10.1016/j.ajo.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Wood A, Binns A, Margrain T, et al. Retinal and choroidal thickness in early age-related macular degeneration. Am J Ophthalmol. 2011;152(6):1030–1038. doi: 10.1016/j.ajo.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 37.Sadda S. Effect of anti-VEGF therapy on choroidal thickness in patients with neovascular age-related macular degeneration. Paper presented at: The 29th Annual Meeting of the American Society of Retina Specialists; Boston, NE. August 20–24, 2011. [Google Scholar]
- 38.Koizumi H, Yamazaki T, Yamagisgi T, Kinoshitra S. Subfoveal choroidal thickness after ranibizumab therapy for neovascular AMD: 12-month result. Poster session presented at: The 2011 Annual Meeting of American Academy of Ophthalmology; Orlando, FL. October 22–25, 2011. [Google Scholar]
- 39.Ikuno Y, Maruko I, Yasuno Y, et al. Reproducibility of retinal and choroidal thickness measurements in enhanced depth imaging and high-penetration optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):5536–5540. doi: 10.1167/iovs.10-6811. [DOI] [PubMed] [Google Scholar]
- 40.Rahman W, Chen FK, Yeoh J, Patel P, Tufail A, Da Cruz L. Repeatability of manual subfoveal choroidal thickness measurements in healthy subjects using the technique of enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(5):2267–2271. doi: 10.1167/iovs.10-6024. [DOI] [PubMed] [Google Scholar]
- 41.Hirata M, Tsujikawa A, Matsumoto A, et al. Macular choroidal thickness and volume in normal subjects measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):4971–4978. doi: 10.1167/iovs.11-7729. [DOI] [PubMed] [Google Scholar]
- 42.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 43.Papadopoulou DN, Mendrinos E, Mangioris G, Donati G, Pournaras CJ. Intravitreal ranibizumab may induce retinal arteriolar vasoconstriction in patients with neovascular age-related macular degeneration. Ophthalmology. 2009;116(9):1755–1761. doi: 10.1016/j.ophtha.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Soliman W, Vinten M, Sander B, et al. Optical coherence tomography and vessel diameter changes after intravitreal bevacizumab in diabetic macular oedema. Acta Ophthalmol. 2008;86(4):365–371. doi: 10.1111/j.1600-0420.2007.01057.x. [DOI] [PubMed] [Google Scholar]
- 45.Yokoyama K, Choshi T, Kimoto K, Shinoda K, Nakatsuka K. Retinal circulatory disturbances following intracameral injection of bevacizumab for neovascular glaucoma. Acta Ophthalmol. 2008;86(8):927–928. doi: 10.1111/j.1755-3768.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- 46.Kim KS, Chang HR, Song S. Ischaemic change after intravitreal bevacizumab (Avastin) injection for macular oedema secondary to non-ischaemic central retinal vein occlusion. Acta Ophthalmol. 2008;86(8):925–927. doi: 10.1111/j.1755-3768.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 47.Agawa T, Miura M, Ikuno Y, et al. Choroidal thickness measurement in healthy Japanese subjects by three-dimensional high-penetration optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2011;249(10):1485–1492. doi: 10.1007/s00417-011-1708-7. [DOI] [PubMed] [Google Scholar]
- 48.Esmaeelpour M, Povazay B, Hermann B, et al. Three-dimensional 1060-nm OCT: choroidal thickness maps in normal subjects and improved posterior segment visualization in cataract patients. Invest Ophthalmol Vis Sci. 2010;51(10):5260–5266. doi: 10.1167/iovs.10-5196. [DOI] [PubMed] [Google Scholar]