Abstract
Background
The purpose of this open-label study was to investigate the effect of a curcumin-phospholipid (lecithin, Meriva®) formulation (Norflo® tablet) on visual acuity and retinal thickness in patients with acute or chronic central serous chorioretinopathy.
Methods
Visual acuity was assessed by ophthalmologic evaluation, and optical coherence tomography was used to measure retinal thickness. Norflo tablets were administered twice a day to patients affected by central serous chorioretinopathy. The study included 18 eyes from 12 patients who completed a 6-month follow-up period. Visual acuity before and after Norflo treatment was the primary endpoint. The secondary endpoints were neuroretinal or pigment epithelial detachment, as measured by optical coherence tomography.
Results
After 6 months of therapy, 0% of eyes showed reduction in visual acuity, 39% showed stabilization, and 61% showed improvement. The improvement was statistically significant (P = 0.08). After 6 months of therapy, 78% of eyes showed reduction of neuroretinal or retinal pigment epithelium detachment, 11% showed stabilization, and 11% showed an increase.
Conclusion
Our results, albeit preliminary, show that curcumin administered as Norflo tablets is efficacious for the management of central serous chorioretinopathy, a relapsing eye disease, and suggest that bioavailable curcumin is worth considering as a therapeutic agent for the management of inflammatory and degenerative eye conditions, including those that activate the retinal microglia.
Keywords: curcumin, central serous chorioretinopathy, retinal pigment epithelium detachment, Norflo®, Meriva®
Introduction
Central serous chorioretinopathy, first described by Von Graefe in 1866 and also known as recurrent central retinitis,1 is a well characterized disorder leading to serous neurosensory elevation of the retina. The acute form of the disease is associated with focal leakage at the level of the retinal pigment epithelium and hyperpermeability of the choroid, and can be diagnosed on fluorescein and indocyanine green angiography.2 The disorder is self-limiting in the majority of patients, who usually retain excellent vision. However, cases that do not resolve spontaneously can develop pigment epithelium and photoreceptor damage, resulting in permanent visual impairment. The pathophysiology of central serous chorioretinopathy remains poorly understood, but the cascade of events leading to neurosensory detachment includes, or might even be triggered by, changes in choroid permeability.
Curcumin has antioxidant and anti-inflammatory activity, and has shown efficacy in animal models of acute and chronic inflammation of relevance to eye disease. Using cultured corneal cells from the transgenic mouse, it was shown that curcumin targets fibroblast growth factor-2 and inhibits expression of gelatinase B, critically affecting angiogenesis.3 Furthermore, in an animal model of dry eye, curcumin showed anti-inflammatory effects in cultured corneal epithelial cells exposed to hyperosmotic conditions.4 Finally, curcumin has been shown to reduce oxidative stress, the main cause of cataract progression,3,5–15 in a murine model of chemically induced hyperglycemia,16 by inhibiting cell proliferation and production of cytokines, which are the main triggers of the inflammatory response.15 Curcumin is a pleiotropic agent, for which over 100 different targets have been identified. Within an anti-inflammatory context, upregulation of peroxisome proliferator-activated receptor (PPAR)-γ, a ligand-inducible transcription factor, plays a key role, because activation of PPAR-γ controls responses of the microglia and limits inflammation.17–21 Taken together, these observations provided a rationale for studying the effect of curcumin in patients with central serous chorioretinopathy. This condition is triggered and sustained by inflammation, and a previous study had identified ibuprofen and aspirin as promising agents for the treatment of central serous chorioretinopathy,17,18 in which elevated levels of plasminogen activator inhibitor occur.22,23 Curcumin has poor systemic availability, but formulation with phospholipids from lecithin (Meriva®) dramatically increases its oral absorption,24–28 so avoids the use of megadoses that would jeopardize patient compliance.
Materials and methods
A total of 18 eyes from 12 patients (11 men, one woman) referred to our tertiary retinal center in Milan for treatment of central serous chorioretinopathy were enrolled in the study. The age of the patients ranged from 29 to 68 years. All patients provided their written informed consent before entering the study, in accordance with the tenets of the Declaration of Helsinki. Diagnosis of central serous chorioretinopathy was based on optical coherence tomography (OCT) and angiography with fluorescein and indocyanine green. Ophthalmological examination also included slit-lamp examination, Early Treatment Diabetic Retinopathy Study best-corrected visual acuity measurement, intraocular pressure measurement, and fundus examination using a Volk + 90 D lens. Enhanced-depth OCT is a new imaging modality (Spectralis, Heidelberg Engineering, Heidelberg, Germany) that allows reproducible measurement of choroidal thickness and may be useful as an aid for differentiating central serous chorioretinopathy from other retinal pathology, eg, age-related macular degeneration. The most recent version of the Spectralis software incorporates enhanced-depth imaging into the scanning protocol, but was not available for this study. Follow-up is ongoing, but all patients successfully completed at least 6 months of treatment. All patients underwent a full ophthalmological examination at days 0, 30, 90, and 180. The results was compared before and after 180 days of treatment. Ad hoc medical record reporting was performed at each follow-up visit. A black and white Amsler grid was recorded at each visit.
All patients received two Norflo® tablets (Eye Pharma Co, Italy) per day, corresponding to 1.2 g of lecithinized curcumin (Meriva®, Indena, Milano, Italy), during the study period. This therapy was administered to patients who had frequent relapses in the last one year of follow-up, and was started at the time of first diagnosis in naïve patients. We also investigated the percentage of satisfaction and tolerance to additional therapy by means of a detailed questionnaire (included on the clinical record sheet) developed to assess patient tolerance and compliance with Norflo treatment. Visual acuity and neuroretinal or pigment epithelial detachment were assessed before and after treatment.
The Student’s t-test and Wilcoxon Signed-Rank test were used to assess the statistical significance of changes in best-corrected visual acuity and retinal thickness.
Results
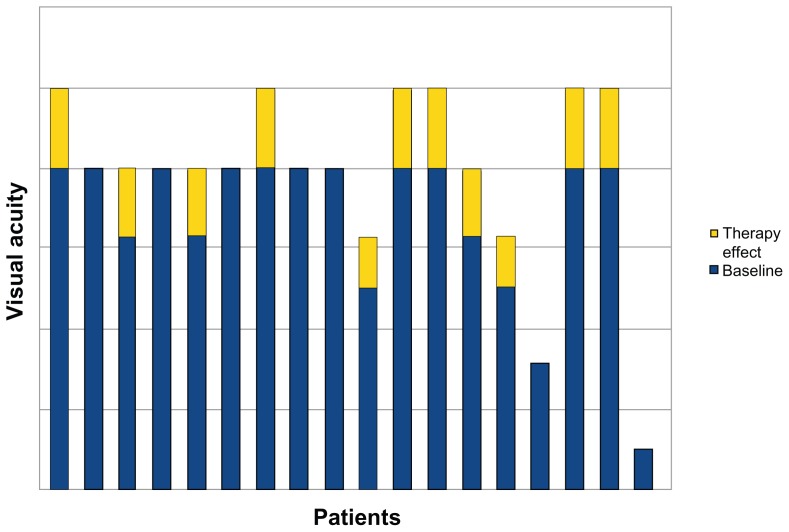
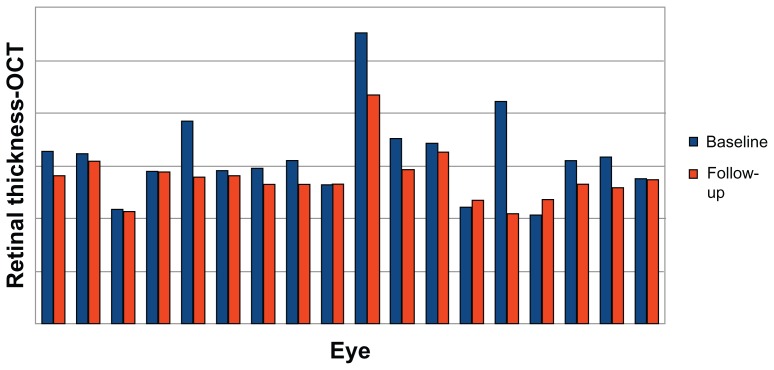
At baseline, mean best-corrected visual acuity was 0.63 ± 0.2 (logMAR 0.2 ± 0.7). After 6 months of treatment, best-corrected visual acuity improved to 0.8 ± 0.2 (logMAR 0.1 ± 0.7); 0% of eyes showed reduction in visual acuity, 39% showed stability, and 61% showed improvement (Figure 1). This improvement was statistically significant (P = 0.08). Average neuroretinal/retinal pigment epithelium detachment at baseline was 317.8 μ ± 80.2 μ. Seventy-eight percent of eyes showed a reduction of neuroretinal/retinal pigment epithelium detachment, 11% showed stability, and 11% showed an increase (Figures 2 and 3). Mean neuroretinal/retinal pigment epithelium detachment decreased to 277 μ ± 49.5 μ in the treatment group, and the difference was statistically significant after 6 months (P = 0.96). No statistically significant differences were observed for near best-corrected visual acuity, and all patients reported improvement in the appearance of the Amsler grid as distortions, blurring, discoloration, or other change. No systemic adverse effects were observed. The significance testing results simply reflect an insufficient sample size and the distribution of data obtained for visual acuity and OCT retinal thickness.
Figure 1.
Visual acuity after 6 months of treatment with the curcumin-lecithin formulation.
Figure 2.
Retinal thickness after 6 months of treatment with the curcumin-lecithin formulation.
Abbreviation: OCT, optical coherence tomography.
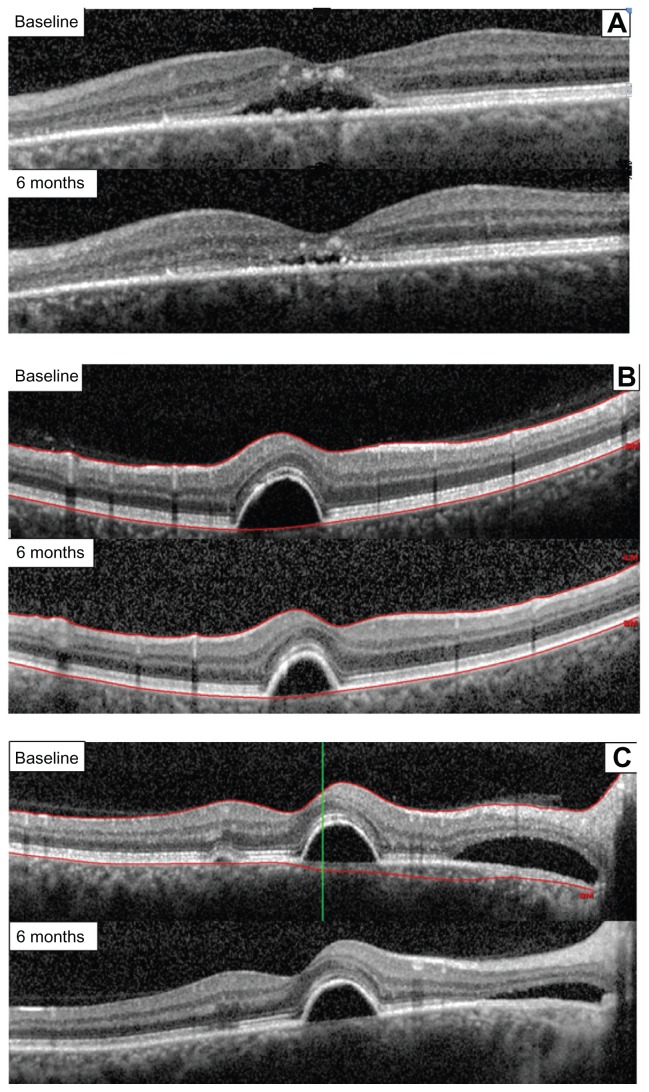
Figure 3.
Retinal thickness after 6 months of treatment with the curcumin-lecithin formulation in three different cases. (A) Case 1, reduction of a subfoveal neuroretinal detachment. (B) Case 2, reduction of a juxtafoveal retinal pigment epithelium detachment and extrafoveal neuroretinal detachment. (C) Case 3, reduction of a juxtafoveal retinal pigment epithelium and extrafoveal neuroretinal detachment.
Discussion
Anti-inflammatory agents, including ibuprofen and aspirin, have been used in the treatment of central serous chorioretinopathy,23,29 providing a rationale for the use of curcumin in this condition. The anti-inflammatory mechanism of curcumin is pleiotropic, and involves inhibition of proinflammatory transcription factors and enzymes at both the functional and genomic levels. Curcumin is also an activator of PPAR-γ,30 a transcription factor, the stimulation and upregulation of which is associated with significant anti-inflammatory activity.7 The main immune cells involved in ocular inflammation are the glia and microglia, in which the function of PPAR-γ, a sensor of metabolism, is critically involved. Therefore, modulation of PPAR-γ has considerable potential in many ocular diseases, including vitreoretinopathy, diabetic retinopathy, glaucoma, and age-related macular degeneration.17
Despite these interesting clues, the clinical activity of curcumin does not correspond to its pharmacological potential, with the dismally low oral bioavailability of the natural product hampering translation of the many promising results observed in in vitro and animal studies into the clinical setting. To overcome the bioavailability problem, a lecithin formulation of curcumin (Meriva®) has been developed, and incorporation into a phospholipid matrix leads to an almost 30-fold increase in absorption, effectively overcoming the megadose issue reported in the literature to have plagued this compound.25–28,31,32
Encouraged by the activity of curcumin in inflammatory eye diseases,17–19,26,33–41 as well as the improvement in absorption associated with Meriva, we investigated the potential of curcumin formulated as Norflo tablets in the management of central serous chorioretinopathy, a chronic relapsing eye disease. Central serous chorioretinopathy is associated with various conditions characterized by exposure to increased levels of endogenous or exogenous glucocorticoids, as shown by its association with Cushing’s syndrome. It is also prevalent in patients with type A behavior, and stressful events and pregnancy might represent other risk factors for the disease, all conditions being characterized by endogenous hypercortisolism. In addition, many cases of central serous chorioretinopathy have been described during or following treatment with glucocorticoids administered by any route for various systemic or ocular conditions.42,43 Twelve patients were enrolled in this open-label study, with examination of 18 eyes. All patients completed at least 6 months of follow-up with no dropouts, indicating the excellent tolerability of the treatment and good quality of life for patients, with follow-up visits consistently showing overall patient well being. Significant reversal of decreased visual acuity and improvement in the histological status of the disease were observed in all patients. In view of the small number of patients in this study, its short follow-up duration, and inclusion of different types of central serous chorioretinopathy, it is theoretically possible that the observed improvement was spontaneous or that resolution was simply coincident with the study treatment. Nevertheless, the reduction in retinal pigment epithelium and neurosensory detachment, as well as improvement in visual acuity, but not necessarily disease resolution, suggests that Meriva may be efficacious in the treatment of central serous chorioretinopathy.
In general, central serous chorioretinopathy has an unpredictable prognosis, so it is difficult to make specific treatment recommendations. However, treatment should be considered after 3 months without spontaneous resolution of acute or chronic central serous chorioretinopathy.43 Resolution of detachment can usually be achieved in acute central serous chorioretinopathy by focal photocoagulation of leaking retinal pigment epithelium lesions, and in chronic central serous chorioretinopathy by photodynamic therapy, but the effect of these therapies on long-term visual outcome is not adequately documented. Reattachment within 4 months of onset is considered an appropriate therapeutic aim because prolonged detachment is associated with atrophy of photoreceptors. These considerations suggest that treatment with lecithinized curcumin could be useful both as prompt therapy aimed at retinal reattachment and as adjunctive therapy to reduce the number of relapses in the advanced stages of the disease.
Conclusion
Because of its anti-inflammatory profile and angiogenesis-modulating properties, curcumin has great potential in the treatment of retinal inflammatory conditions and retinal neovascular proliferative diseases sustained by activity of vascular endothelial growth factor.18,19,26,44–46 Formulation with lecithin has been shown to be beneficial for the absorption of curcumin, and we have shown that lecithinized curcumin (Meriva), administered in the form of two tablets of Norflo per day, could provide beneficial effects in patients suffering from central serous chorioretinopathy, reversing their decline in visual acuity and improving the histological status of their disease. Despite the limitations of this study in terms of number of patients, duration of follow-up, and heterogeneity of central serous chorioretinopathy, the reduction in retinal pigment epithelium and neurosensory detachment, as well as improvement in visual acuity, is very promising. Our results suggest that appropriately formulated curcumin has great potential in the management of central serous chorioretinopathy, and provides a rationale for initiation of larger placebo-controlled studies that might also consider the combination of curcumin with other therapeutic options to treat this disease, eg, low-fluence photodynamic therapy and intravitreal therapy.
Acknowledgments
The author thanks Elia Moretti for the statistical analysis. They also thank Giovanni Appendino, Department of Chemical, Food, Pharmaceutical and Pharmacological Sciences, University of Piemonte Orientale, Novara, and Stefano Togni, Indena SpA, Milan, as well as Aldo Cagnola, Eye Pharma Company, Genoa, for their contribution to the study.
Footnotes
Disclosure
The author reports no conflict of interest in this work.
References
- 1.Graefe AV. Uber centrale recidivierende retinitis. Graefes Arch Clin Exp Ophthalmol. 1866;12:211–215. [German] [Google Scholar]
- 2.Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63(Suppl 3):1–139. [PubMed] [Google Scholar]
- 3.Mohan R, Sivak J, Ashton P, et al. Curcuminoids inhibit the angiogenic response stimulated by fibroblast growth factor-2, including expression of matrix metalloproteinase gelatinase B. J Biol Chem. 2000;275(14):10405–10412. doi: 10.1074/jbc.275.14.10405. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Hu DN, Pan Z, Lu CW, Xue CY, Aass I. Curcumin protects against hyperosmoticity-induced IL-1 beta elevation in human corneal epithelial cell via MAPK pathways. Exp Eye Res. 2010;90(3):437–443. doi: 10.1016/j.exer.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Bengmark S. Curcumin, an atoxic antioxidant and natural NF kappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. JPEN J Parenter Enteral Nutr. 2006;30(1):45–51. doi: 10.1177/014860710603000145. [DOI] [PubMed] [Google Scholar]
- 6.Bright JJ. Curcumin and autoimmune disease. Adv Exp Med Biol. 2007;595:425–451. doi: 10.1007/978-0-387-46401-5_19. [DOI] [PubMed] [Google Scholar]
- 7.Jacob A, Wu R, Zhou M, Wang P. Mechanism of the anti-inflammatory effect of curcumin: PPAR-gamma activation. PPAR Res. 2007;2007:89369. doi: 10.1155/2007/89369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nonn L, Duong D, Peehl DM. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis. 2007;28(6):1188–1196. doi: 10.1093/carcin/bgl241. [DOI] [PubMed] [Google Scholar]
- 9.Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73(9):1434–1445. doi: 10.1016/j.bcp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10(3):511–545. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 11.Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50(11):2191–2193. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- 12.Jobin C, Bradham CA, Russo MP, et al. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163(6):3474–3483. [PubMed] [Google Scholar]
- 13.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75(4):787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Biswas S, Rahman I. Modulation of steroid activity in chronic inflammation: a novel anti-inflammatory role for curcumin. Mol Nutr Food Res. 2008;52(9):987–994. doi: 10.1002/mnfr.200700259. [DOI] [PubMed] [Google Scholar]
- 15.Yadav VS, Mishra KP, Singh DP, Mehrotra S, Singh VK. Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol. 2005;27(3):485–497. doi: 10.1080/08923970500242244. [DOI] [PubMed] [Google Scholar]
- 16.Kumar PA, Suryanarayana P, Reddy PY, Reddy GB. Modulation of alpha-crystallin chaperone activity in diabetic rat lens by curcumin. Mol Vis. 2005;11:561–568. [PubMed] [Google Scholar]
- 17.Malchiodi-Albedi F, Matteucci A, Bernardo A, Minghetti L. PPAR-gamma, microglial cells, and ocular inflammation: new venues for potential therapeutic approaches. PPAR Res. 2008;2008:295784. doi: 10.1155/2008/295784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murata T, He S, Hangai M, et al. Peroxisome proliferator-activated receptor-gamma ligands inhibit choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41(8):2309–2317. [PubMed] [Google Scholar]
- 19.Bonne C. PPAR gamma: a novel pharmacological target against retinal and choroidal neovascularization. J Fr Ophtalmol. 2005;28(3):326–330. doi: 10.1016/s0181-5512(05)81062-9. French. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui AM, Cui X, Wu R, et al. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-gamma. Crit Care Med. 2006;34(7):1874–1882. doi: 10.1097/01.CCM.0000221921.71300.BF. [DOI] [PubMed] [Google Scholar]
- 21.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 22.Iijima H, Iida T, Murayama K, Imai M, Gohdo T. Plasminogen activator inhibitor 1 in central serous chorioretinopathy. Am J Ophthalmol. 1999;127(4):477–478. doi: 10.1016/s0002-9394(98)00378-x. [DOI] [PubMed] [Google Scholar]
- 23.Caccavale A, Imparato M, Romanazzi F, Negri A, Porta A, Ferentini F. A new strategy of treatment with low-dosage acetyl salicylic acid in patients affected by central serous chorioretinopathy. Med Hypotheses. 2009;73(3):435–437. doi: 10.1016/j.mehy.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Cuomo J, Appendino G, Dern AS, et al. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod. 2011;74(4):664–669. doi: 10.1021/np1007262. [DOI] [PubMed] [Google Scholar]
- 25.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 26.Wang YJ, Pan MH, Cheng AL, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15(12):1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 27.Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60(2):171–177. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 28.Marczylo TH, Steward WP, Gescher AJ. Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel ultraperformance liquid chromatography (UPLC) method. J Agric Food Chem. 2009;57(3):797–803. doi: 10.1021/jf803038f. [DOI] [PubMed] [Google Scholar]
- 29.Caccavale A, Romanazzi F, Imparato M, Negri A, Morano A, Ferentini F. Central serous chorioretinopathy: a pathogenetic model. Clin Ophthalmol. 2011;5:239–243. doi: 10.2147/OPTH.S17182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Yang P, Kijlstra A. Distribution, markers, and functions of retinal microglia. Ocul Immunol Inflamm. 2002;10(1):27–39. doi: 10.1076/ocii.10.1.27.10328. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104(6):1322–1331. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- 32.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- 33.Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13(4):318–322. doi: 10.1002/(SICI)1099-1573(199906)13:4<318::AID-PTR445>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Lal B, Kapoor AK, Agrawal PK, Asthana OP, Srimal RC. Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phytother Res. 2000;14(6):443–447. doi: 10.1002/1099-1573(200009)14:6<443::aid-ptr619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 35.Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol. 2007;595:453–470. doi: 10.1007/978-0-387-46401-5_20. [DOI] [PubMed] [Google Scholar]
- 36.Lao CD, Ruffin MT, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330(1–2):155–163. doi: 10.1016/j.ijpharm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 39.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30(2):85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Barry J, Fritz M, Brender JR, Smith PE, Lee DK, Ramamoorthy A. Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: the case of the antioxidant curcumin. J Am Chem Soc. 2009;131(12):4490–4498. doi: 10.1021/ja809217u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41(1):40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouzas EA, Moret P, Pournaras CJ. Central serous chorioretinopathy complicating solar retinopathy treated with glucocorticoids. Graefes Arch Clin Exp Ophthalmol. 1999;237(2):166–168. doi: 10.1007/s004170050213. [DOI] [PubMed] [Google Scholar]
- 43.Wang M, Munch IC, Hasler PW, Prunte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86(2):126–145. doi: 10.1111/j.1600-0420.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 44.Jeong SJ, Koh W, Lee EO, et al. Antiangiogenic phytochemicals and medicinal herbs. Phytother Res. 2011;25(1):1–10. doi: 10.1002/ptr.3224. [DOI] [PubMed] [Google Scholar]
- 45.Arbiser JL, Klauber N, Rohan R, et al. Curcumin is an in vivo inhibitor of angiogenesis. Mol Med. 1998;4(6):376–383. [PMC free article] [PubMed] [Google Scholar]
- 46.Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (Lond) 2007;4:8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]