Abstract
DJ-1, which is linked to recessively inherited Parkinson's disease when mutated, is a multi-functional protein with anti-oxidant and transcription regulatory activities. However, the mechanism(s) through which DJ-1 and the genes it regulates provide neuroprotection is not fully understood. Here, we show that wild-type DJ-1 induces the expression of thioredoxin 1 (Trx1), a protein disulfide oxidoreductase, whereas pathogenic mutant isoforms L166P and M26I cannot. Conversely, DJ-1 knockdown in SH-SY5Y cells and DJ-1 knockout in mice result in significant decrease in Trx1 protein and mRNA expression levels. The importance of Trx1 in the cytoprotective function of DJ-1 is confirmed using a pharmacological inhibitor of Trx reductase, 1-chloro-2,4-dinitrobenzene, and Trx1 siRNA. Both approaches result in partial loss of DJ-1-mediated protection. Additionally, knockdown of Trx1 significantly abrogates DJ-1-dependent, hydrogen peroxide-induced activation of the pro-survival factor AKT. Promoter analysis of the human Trx1 gene identified an antioxidant response element (ARE) that is required for DJ-1-dependent induction of Trx1 expression. The transcription factor Nuclear factor erythroid-2 related factor 2 (Nrf2), which is a critical inducer of ARE-mediated expression, is regulated by DJ-1. Overexpression of DJ-1 results in increased Nrf2 protein levels, promotes its translocation into the nucleus and enhances its recruitment onto the ARE site in the Trx1 promoter. Further, Nrf2 knockdown abolishes DJ-1-mediated Trx1 induction and cytoprotection against hydrogen peroxide, indicating the critical role of Nrf2 in carrying out the protective functions of DJ-1 against oxidative stress. These findings provide a new mechanism to support the antioxidant function of DJ-1 by increasing Trx1 expression via Nrf2-mediated transcriptional induction.
INTRODUCTION
Parkinson disease's (PD) is the most prevalent neurodegenerative movement disorder characterized by progressive loss of doparminergic neurons in the substantia nigra (SN) and marked reduction in striatal dopamine content (1). Several genetic forms have been identified to date causing both dominantly and recessively inherited PD, but environmental factors may also contribute to disease risk. Mounting evidence suggests that oxidative stress is involved in the progression of PD pathology (2,3). Mitochondrial dysfunction and decreased antioxidant capacity are observed in PD brains and constitute a fundamental property of toxins used to model the disease in laboratory animals (4,5).
Loss of function mutations in DJ-1 (PARK7), including exonic deletions and point mutations, cause autosomal recessive PD (6). DJ-1 is a multifunctional protein that acts as an antioxidant and a transcriptional regulator. It becomes more acidic after oxidative stress, and its oxidized forms have been detected in the brains of patients with sporadic PD (7,8). Overexpression of DJ-1 protects cells against oxidative damage induced by H2O2, MPP+ or 6-hydroxydopamine, while deficiency of DJ-1 sensitizes cells and mice to such insults (9–11). In addition, DJ-1 regulates the expression of several genes including that of the androgen receptor (AR) by binding to the transcriptional inhibitor PIASxα, and upregulates tyrosine hydroxylase expression by inhibiting the sumoylation of pyrimidine tract-binding protein-associated splicing factor (12,13). DJ-1 also increases the expression of several antioxidants, such as glutamate-cysteine ligase and manganese superoxide dismutase through interacting with peroxisome proliferator-activated receptor-γ co-activator 1 α (PGC-1α), leading to decreased cellular reactive oxygen species levels (14,15).
The transcription factor Nuclear factor erythroid-2 related factor 2 (Nrf2), which is a master regulator for antioxidant proteins and detoxifying enzymes, is stabilized by DJ-1 (16). As a result, in primary mouse embryonic fibroblasts (MEFs) derived from DJ-1-knockout mice, the expression of Nrf2 target genes including NAD(P)H quinone oxidoreductase 1 (NQO1) is suppressed (16). Under normal conditions in unstressed cells, Nrf2 is bound to kelch-like ECH-associated protein (Keap1) in the cytoplasm and degraded through the ubiquitin-proteasome pathway. But upon exposure to oxidative stress, it is released from Keap1 and translocates to the nucleus to activate its target genes (17,18) through binding to conserved antioxidant response elements (AREs).
Thioredoxin 1 (Trx1) is a cellular redox protein that plays a central role in maintaining the redox state and modulates redox-regulated gene expression, consequently protecting cells from oxidative stress. Upon reduction of oxidized proteins, Trx1 forms a disulfide bond between its two cysteine residues of the CXXC motif in its active site. Oxidized Trx1 is then reduced reversibly by thioredoxin reductase (TrxR) and nicotinamide adenine dinucleotide phosphate (NADPH). Thus, the Trx system, which includes Trx1, TrxR and NADPH, exerts a powerful protein disulfide reductase function (19). As a result, loss of Trx1 in many cell types leads to oxidative stress and increased vulnerability to various stresses. In addition, Trx1 acts as a potent survival factor through regulating the activity of apoptosis signal-regulating kinase-1 (ASK1) (20). Considering the state of oxidative stress in neurodegenerative diseases including PD, several lines of evidence implicate Trx1 as an important modulator of cell susceptibility in these conditions and their models. For example, over-expression of Trx1 suppresses H2O2, 1-methyl-4-phenylpyridinium (MPP+) and paraquat induced toxicity in cultured cells (21,22), and Trx1 transgenic mice exhibit resistance of caspase-12 activation and dopaminergic neuron loss after exposure to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (23). On the other hand, brain thioredoxin levels decrease significantly following MPTP intoxication of mice (5). Further, Trx1 suppresses Parkin-associated endothelin receptor-like receptor (Pael-R)-induced neurotoxicity and extends longevity in Drosophila (24).
In this study, we investigated the role of DJ-1 in the regulation of Trx1 expression using cellular models and DJ-1 knockout mice. The results demonstrate that the cytoprotective activity of DJ-1 is mediated by the transcriptional induction of the Trx1 gene through the Nrf2 pathway.
RESULTS
DJ-1 induces Trx1 expression
Since DJ-1 is well established to protect against oxidative stress (14,25,26), we examined whether it modulates the expression of Trx1, a major antioxidant protein. HeLa cells were transiently transfected with Flag-tagged wild-type (WT) and pathogenic mutants (M26I and L166P) of DJ-1, and cell lysates were subjected to western blot analyses with anti-Flag and anti-Trx1 antibody. Trx1 levels increased by 2.6-fold in cells expressing WT Flag-DJ-1 compared with empty-vector transfected cells, while M26I or L166P mutant forms of DJ-1 could not up-regulate Trx1 expression (Fig. 1A). Conversely, knocking down DJ-1 expression using siRNA in SH-SY5Y cells significantly reduced endogenous Trx1 protein levels by 50% (Fig. 1B). Similarly, DJ-1 knockdown in SK-N-BE(2)C and HeLa cells resulted in >50% decreased Trx1 protein levels (data not shown). To further confirm this effect, we compared Trx1 expression in the SN of DJ-1 null and WT mice using western blotting and quantitative real-time polymerase chain reaction (RT-PCR). Consistent with the cell-based data, DJ-1 null mice had significantly lower Trx1 protein and mRNA expression compared with WT mice (Fig. 1C and D). These findings indicate that DJ-1 induces Trx1 mRNA and protein expression in the brain.
Figure 1.
DJ-1 induces Trx1 protein and mRNA levels. (A) WT DJ-1, but not pathogenic mutants M26I or L166P, induces Trx1 expression. Flag-tagged WT or mutant DJ-1 was co-transfected with pCMV-β-Gal (internal control) into HeLa cells for 48 h. The expression of endogenous Trx1 and Flag-DJ-1 was assessed using anti-Trx1 and anti-Flag antibodies, respectively. Trx1 levels were quantified relative to empty-vector control transfected cells set to 1.0. Quantification of band intensities relative to β-actin from three different experiments is shown below. **analysis of variance (ANOVA) P< 0.01. (B) Knockdown of DJ-1 reduces Trx1 expression in SH-SY5Y cells. Cells were transfected with siRNA to DJ-1 (+) or scrambled sequence (−) for 72 h. Endogenous expression of DJ-1 and Trx1 was assessed by western blotting. Quantification of band intensities relative to β-actin from three experiments is shown below. **t-test P< 0.01. (C) Trx1 protein level is decreased in SN of DJ-1 null mice. Levels of Trx1 in DJ-1 null mice were calculated relative to band density in WT SN set to 1.0 and normalized to β-actin band density. Quantification of eight experiments is shown below. **t-test P< 0.01. (D) Trx1 mRNA level is decreased in SN of DJ-1 null mice. The expression of endogenous Trx1 in SN of WT and DJ-1 null mice was determined by quantitative real-time polymerase chain reaction (RT-PCR) analysis. Relative levels of Trx1 mRNA were normalized to β-actin mRNA in three independent experiments. *t-test P< 0.05.
Trx1 is required for the cytoprotective function of DJ-1
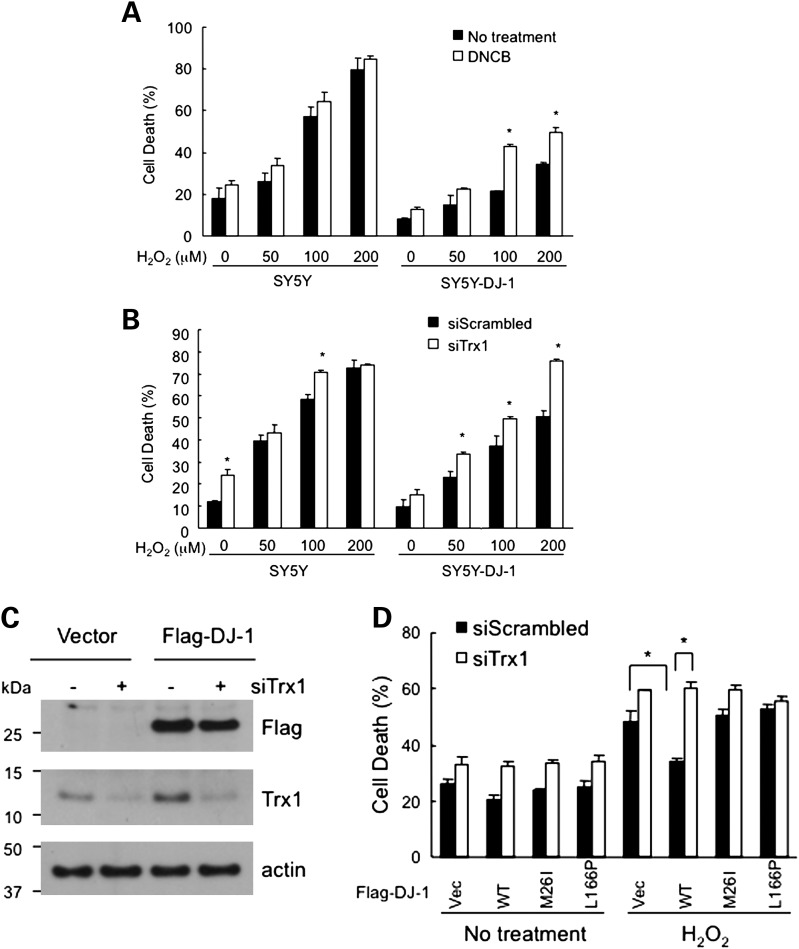
To determine whether Trx1 plays a role in the cytoprotective activity of DJ-1, we used a pharmacological inhibitor of Trx reductase (TrxR) and silenced Trx1 expression with siRNA. Both these manipulations exacerbated oxidative stress-induced toxicity in DJ-1 overexpressing cells. SH-SY5Y cells stably expressing Flag-DJ-1 (SY5Y-DJ-1) or empty-vector (SY5Y) were pretreated with 1-chloro-2,4-dinitrobenzene (DNCB), an irreversible inhibitor of TrxR, for 1 h and then challenged with various concentrations of H2O2. As expected, SY5Y-DJ-1 cells were relatively protected against H2O2 compared with SY5Y cells, but DNCB pretreatment significantly attenuated DJ-1-mediated cytoprotection (Fig. 2A). To test the effect of Trx1 knockdown on the cytoprotection of DJ-1, Trx1 siRNA was transfected into SY5Y and SY5Y-DJ-1 cells for 72 h resulting in significant suppression of endogenous Trx1 expression in both cell lines (Fig. 2C). Following H2O2 challenge, the protection afforded by WT DJ-1 in SY5Y-DJ-1 cells was significantly lost with Trx1 knockdown (Fig. 2B). The modest increase in cell death seen with siRNA-mediated inhibition of Trx1 in native SY5Y cells could be due to the substantial expression of DJ-1 endogenously in these cells (Fig. 1B) or perhaps other regulators of Trx1. The ability of WT DJ-1 to protect against H2O2-induced cell death through Trx1 was also tested in transiently transfected HeLa cells and compared with DJ-1 mutants. As expected, WT DJ-1, but not M26I or L166P mutant isoforms, protected these cells, and this cytoprotective activity was lost when Trx1 was knocked down (Fig. 2D). These results collectively imply that Trx1 plays an important role in the cytoprotective function of DJ-1.
Figure 2.
Trx1 is required for DJ-1-mediated cytoprotection. (A) Pharmacological inhibition of TrxR partially impairs the cytoprotective activity of DJ-1. SY5Y and SY5Y-DJ-1 cells stably expressing Flag-DJ-1 were pretreated with 5 μm DNCB, an irreversible inhibitor of Trx reductase, for 1 h and then treated with H2O2 at the indicated concentrations for 24 h. DJ-1 overexpressing cells were generally protected, but treatment with DNCB resulted in greater sensitivity to H2O2. (B) Knockdown of Trx1 abolishes the protective effect of DJ-1 against H2O2-induced toxicity. SY5Y and SY5Y-DJ-1 cells were transfected with Trx1 siRNA. After 72 h, cells were treated with the indicated concentrations of H2O2 for 24 h, and cell death was assessed by the lactate dehydrogenase (LDH) release. Experiments were done in triplicates and repeated three times. *ANOVA P< 0.05. (C) Knockdown of Trx1 using siRNA. SY5Y and SY5Y-DJ-1 cells were transfected with Trx1 siRNA for 72 h. Endogenous Trx1 levels were examined by western blotting. (D) Knockdown of Trx1 abolishes the protective effect of WT DJ-1 against H2O2-induced toxicity, while mutant DJ-1 isoforms cannot protect cells and are not influenced by Trx1 knockdown. HeLa cells were transiently transfected with Trx1 siRNA, WT or pathogenic mutants of DJ-1 (M26I, L166P), and GFP. After 48 h, cells were treated with 1 mm H2O2 for 12 h. Dead cells were identified by PI staining and counted as percent of GFP-positive cells. Experiments were done in triplicates. *ANOVA P< 0.05.
Trx1 modulates DJ-1-induced AKT phosphorylation
DJ-1 is crucial for AKT activation upon oxidative injury in the mouse MPTP model (26). We, therefore, set out to investigate the role of Trx1 in this process. HeLa cells were stably transfected with DJ-1 shRNA or control vector, and western blot analysis with anti-DJ-1 antibody confirmed significant knockdown of endogenous DJ-1 expression (Fig. 3A, no H2O2 lanes). Challenging these cells with 1 mm H2O2 for various times demonstrated the expected robust activation of AKT in control cells but marked repression of this response in DJ-1 knockdown cells (Fig. 3A and B). Knockdown of DJ-1 also reduced Trx1 protein level (Fig. 3A) consistent with the data in Figure 1. These findings were confirmed in a separate cell line stably transfected with a different DJ-1 shRNA (Supplementary Material, Fig. S1). To directly examine the role of Trx1 in AKT-mediated protective effects of DJ-1, HeLa cells were co-transfected with Flag-DJ-1 and Trx1 siRNA for 72 h followed by treatment with 1 mm H2O2 for 30 min. DJ-1 overexpression enhanced H2O2-induced-AKT activation, whereas simultaneously knocking down Trx1 essentially abolished this AKT response (Fig. 3C and D). Taken together, these results indicate that Trx1 mediates DJ-1-dependent AKT activation upon oxidative stress.
Figure 3.
Trx1 is required for DJ-1-mediated AKT activation. (A) DJ-1 knockdown prevents H2O2-induced AKT activation. HeLa cells stably transfected with DJ-1 shRNA1 or control vector were treated with 1 mm H2O2 for various times. Cell lysates were examined by western blot analyses with anti-pAKT (S473), AKT, DJ-1 and Trx1 antibodies. (B) Quantification of the data in (A). Relative levels of phospho-AKT were normalized to AKT from three independent experiments. **ANOVA P < 0.001. (C) Trx1 knockdown prevents DJ-1-mediated AKT activation following oxidative stress. HeLa cells were transfected with Flag-DJ-1 for 24 h and subsequently with siRNA to Trx1 or scrambled sequence for 48 h and treated with 1 mm H2O2 for 30 min. Western blot analyses were performed with pAKT (S473), AKT, Trx1 and Flag antibodies. (D) Quantification of the data in (C). Relative levels of phospho-AKT were normalized to AKT from three independent experiments. *ANOVA P < 0.01; **P< 0.001.
DJ-1 activates Trx1 transcription through ARE
To understand the precise mechanism by which DJ-1 increases Trx1 mRNA expression, we studied the promoter of the human Trx1 gene. Serial 5′ deletions of the Trx1 promoter region fused to the luciferase reporter gene were tested in SY5Y and SY5Y-DJ-1 cells. Expression of DJ-1 greatly activated the reporter gene in pTrx1(-1148)-Luc, pTrx1(-980)-Luc or pTrx1(-463)-Luc. In contrast, significantly weaker induction of luciferase activity was observed with shorter constructs pTrx1(-352)-Luc and pTrx1(-217)-Luc, suggesting that the region of the Trx1 gene between positions −463 and −352 relative to transcription start site is required for most of the DJ-1 response (Fig. 4A). This region contains a sequence that is homologous to the consensus ARE, suggesting involvement of this site in DJ-1-dependent induction of Trx1. This possibility was verified by mutating this ARE consensus sequence and demonstrating significant loss of DJ-1-dependent activation of this promoter (Fig. 4B). To determine whether this transcriptional induction activity of DJ-1 is an important mechanism in protecting dopamine neurons and is lost by pathogenic mutants linked to PD, the ability of M26I and L166P mutants to induce Trx1 promoter activity was compared with WT DJ-1 in HeLa cells. While WT DJ-1 significantly enhanced the activity of this promoter, the two mutants were ineffective (Fig. 4C), consistent with the inability of these mutants to induce Trx1 protein levels in Figure 1A. These data imply that intact DJ-1 activates transcription of the Trx1 gene through an ARE consensus sequence.
Figure 4.
DJ-1 leads to Trx1 promoter activation through the ARE sequence. (A) Identification of the Trx1 promoter region responsible for the response to DJ-1. SY5Y and SY5Y-DJ-1 cells were transfected with serial deletion mutants of the pTrx1-Luc vector, as indicated in the left panel together with pCMV-β gal as internal control for 36 h. Data are representative of three independent experiments. (B) Mutation in the ARE consensus sequence partially abrogates DJ-1-induced Trx1 transcription. WT and mutated sequences are indicated in the upper panel. (C) DJ-1 mutants fail to activate transcription from the Trx1 promoter. WT and pathogenic mutants M26I and L166P of DJ-1 were transfected with pCMV-β-gal into HeLa cells for 36 h. Values shown in (A)–(C) represent the ratio of luciferase activity of DJ-1 overexpressing cells to that of vector-control cells. Data are representative of three independent experiments. *ANOVA P< 0.05 compared with all other groups.
Nrf2 is required for DJ-1-mediated Trx1 induction and DJ-1-dependent cytoprotection
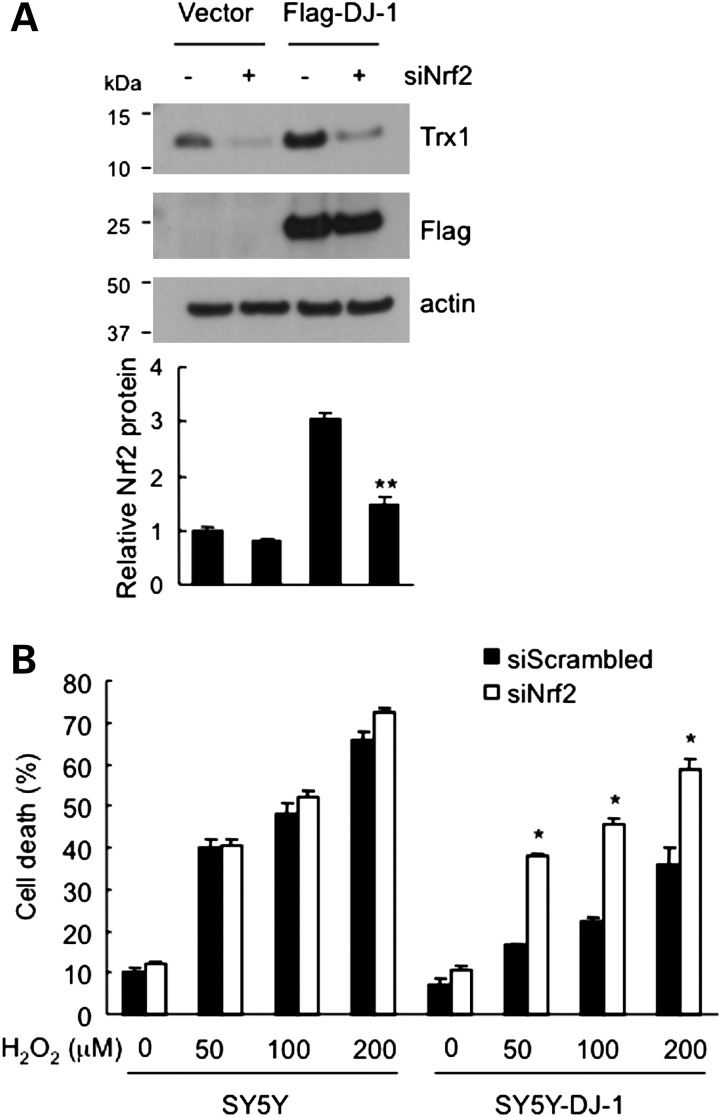
Since the transcription factor Nrf2 is a critical inducer of ARE-mediated expression, and DJ-1 activates the Trx1 promoter through an ARE consensus sequence, we determined whether Nrf2 is involved in DJ-1-mediated Trx1 induction. First, knocking down Nrf2 with siRNA in SY5Y or SY5Y-DJ-1 cells abrogated DJ-1-mediated upregulation of Trx1 expression (Fig. 5A). And second, Nrf2 knockdown exacerbated oxidative stress-induced cell death in DJ-1 expressing cells (Fig. 5B). The cytoprotection achieved with DJ-1 alone was partially lost by knocking down Nrf2 compared with scrambled sequence siRNA. These results indicate that Nrf2 mediates DJ-1-dependent Trx1 induction and cytoprotection.
Figure 5.
Nrf2 mediates DJ-1-dependent Trx1 expression and cytoprotection. (A) Nrf2 knockdown reduces Trx1 expression. Flag-DJ-1 and empty-vector-engineered cells were transfected with siRNA-targeting Nrf2 (siNrf2), and western blots were performed 72 h later. Quantification of band intensities relative to β-actin from three experiments is shown below. **ANOVA P < 0.01 compared with Flag-DJ-1 and scrambled siRNA-transfected group. (B) Nrf2 knockdown diminishes the protective effect of DJ-1 against H2O2-induced toxicity. Cells were transfected with Nrf2 siRNA for 48 h and then treated with the indicated H2O2 concentrations for 24 h. Cell death was assessed by LDH assay. *ANOVA P< 0.05.
WT DJ-1 but not mutants upregulates Nrf2 protein levels
DJ-1 has been suggested to increase Nrf2 stability (16), but the significance of this finding to the pathogenesis of DJ-1-linked PD is unclear. First, we compared Nrf2 expression in SY5Y-DJ-1 and SY5Y cells using quantitative real-time PCR and western blot analysis. While levels of Nrf2 mRNA were not changed in the presence of DJ-1, its protein levels were elevated in SY5Y-DJ-1 cells (Fig. 6A and B). Next, to investigate the effect of DJ-1 mutants on Nrf2 expression, HeLa cells were transfected with DJ-1 siRNA and then transfected again with Flag-tagged WT or pathogenic mutants of DJ-1. Consistent with the induction of the Nrf2 protein with DJ-1 (Fig. 6B), endogenous Nrf2 levels decreased by knocking down DJ-1. Reintroduction of WT DJ-1 restored Nrf2 level, whereas M26I or L166P mutant isoforms of DJ-1 could not (Fig. 6C). These results indicate that DJ-1 increases Nrf2 protein expression and this function is lost by PD causing mutations.
Figure 6.
DJ-1 increases Nrf2 protein levels. (A) No change of Nrf2 mRNA in the presence of DJ-1. Real-time PCR analyses of Nrf2 mRNA in SY5Y and SY5Y-DJ-1 cells. Relative mRNA levels were normalized to GAPDH levels. (B) Increase in the Nrf2 protein in the presence of DJ-1. Western blot analysis of Nrf2 in SY5Y and SY5Y-DJ-1 cells. Quantification of band intensities relative to β-actin from seven experiments is shown on the right. **t-test P < 0.01. (C) WT but not mutant DJ-1 increases Nrf2 levels. HeLa cells were transfected with siRNA-targeting DJ-1 (siDJ-1 +) or scrambled siRNA (−) for 24 h and then transfected with Flag-DJ-1 (WT or mutants) for 48 h. Nrf2 levels decreased when DJ-1 was knocked down. Reconstitution with WT DJ-1, but not M26I or L166P mutants, recovered Nrf2 expression. Quantification of Nrf2 band intensities relative to β-actin from three experiments is shown on the right. **ANOVA P < 0.001.
To test the impact of DJ-1 on the interaction between Nrf2 and its inhibitor Keap1 (16), HeLa cells were transfected with Flag-Nrf2 and Myc-DJ-1 followed by immunoprecipitation with Myc antibody. In addition, these cells were transfected with Flag-Nrf2, Flag-DJ-1 and HA-Keap1 followed by immunoprecipitation with HA antibody. These experiments, which included the use of a chemical cross-linker, showed that DJ-1 does not interact directly with either Nrf2 or Keap1, nor does it alter the binding between these two proteins (Fig. 7A and B). Further, DJ-1 does not alter the ubiquitination state of Nrf2 (Fig. 7B). These findings suggest that the DJ-1 mediated increase in Nrf2 levels does not involve Keap1.
Figure 7.
DJ-1 does not impact the interaction between Nrf2 and Keap1. (A) DJ-1 does not interact with Nrf2. HeLa cells were transfected with Flag-Nrf2 and Myc-DJ-1 for 48 h. Cells were washed twice with PBS, and dithiobis[succinimidylpropionate] (DSP, Thermo Scientific) crosslinker solution was added to a final concentration of 1–2 mm at room temperature for 30 min. Stop solution was then added to a final concentration of 10–20 mm and cells incubated for another 15 min. Lysates were immunoprecipitated with anti-Myc antibody, and western blot analysis was performed with anti-Flag and anti-Myc. No interaction between DJ-1 and Nrf2 is detected. (B) DJ-1 does not bind to Keap1, does not influence the interaction between Nrf2 and Keap1 and does not affect Nrf2 ubiquitination. HeLa cells were transfected with Flag-Nrf2, HA-Keap1 or Flag-DJ-1 for 48 h. Crosslinking was done as in (A). Cell lysates were immunoprecipitated with anti-HA antibody and western blot analysis was performed with anti-Flag and anti-HA.
DJ-1 stimulates translocation of Nrf2 into the nucleus and enhances its recruitment to the Trx1 promoter
Considering the critical role of Nrf2 in cell survival under stress conditions, it is regulated at several stages, including its protein level, nuclear translocation and DNA binding (18,27,28). To determine whether DJ-1 regulates translocation of Nrf2, SY5Y-DJ-1 and SY5Y cell lysates were fractionated and subjected to western blotting for Nrf2. In the presence of DJ-1 overexpression, Nrf2 levels increased in the nucleus and decreased in the cytoplasm (Fig. 8A), suggesting that DJ-1 promotes Nrf2 to move into the nucleus. On the other hand, M26I or L166P mutant isoforms of DJ-1 could not stimulate the translocation of Nrf2 to the nucleus in HeLa cells (Fig. 8B).
Figure 8.
DJ-1 stimulates nuclear translocation of Nrf2 and enhances its recruitment to the Trx1 promoter. (A) Overexpression of DJ-1 enhances nuclear translocation of Nrf2. SY5Y (−) and SY5Y-DJ-1 (+) cell lysates were fractionated to nuclear and cytoplasmic fractions. Western blotting was done with the following antibodies: anti-Nrf2, anti-Lamin B1 for nuclear marker, anti-tubulin for cytoplasmic marker and anti-Flag. Nrf2 levels in nuclear or cytoplasmic fractions were calculated relative to total Nrf2 band density set to 1.0. Quantification of band intensities relative to Lamin B1 for nuclear fraction and relative to tubulin for cytoplasmic fraction from three experiments is shown below. *ANOVA P< 0.05. (B) Pathogenic mutants of DJ-1 fail to stimulate the nuclear translocation of Nrf2. HeLa cells were transiently transfected with Flag-DJ-1 WT, M26I or L166P. Cell lysates were fractionated to nuclear and cytoplasmic fractions and subjected to western blotting as in (A). *ANOVA P< 0.05. Vec, vector control. (C) Nrf2 binds strongly to the Trx1 promoter, whereas DJ-1 does so only marginally. HeLa cells were transfected with Flag-Nrf2 or Flag-DJ-1. Lysates were immunoprecipitated with anti-Flag antibody. (D) WT but not mutant isoforms of DJ-1 enhances Nrf2 recruitment to the Trx1 promoter. HeLa cells were transfected with Flag-DJ-1 (WT or mutants), and ChIP was performed with anti-Nrf2 antibody or immunoglobulin G (IgG) control. (E) Knockdown of DJ-1 eliminates recruitment of Nrf2 to the Trx1 promoter. HeLa cells were transfected with siRNA-targeting DJ-1 (siDJ-1 +) or scrambled siRNA (−) for 72 h. ChIP was performed with anti-Nrf2 antibody or immunoglobulin G (IgG) control. For (B)–(D), ChIP-enriched DNA was analyzed by PCR amplification of the Trx1 promoter spanning the ARE or the TH promoter as a negative control.
Upon translocating to the nucleus, Nrf2 activates its target genes through binding to ARE sites (29). First, to determine whether Nrf2 and DJ-1 bind to the human Trx1 promoter, chromatin immunoprecipitation (ChIP) assay was performed in Flag-Nrf2 or Flag-DJ-1 overexpressing HeLa cells. PCR analysis of chromatin coimmunoprecipitated using Flag-agarose showed that Nrf2 indeed binds strongly to the ARE site in the Trx1 promoter, whereas DJ-1 barely binds (Fig. 8C). Secondly, to investigate the impact of DJ-1 on Nrf2 recruitment to the Trx1 promoter, we performed ChIP assay in Myc-DJ-1 overexpressing cells and found that WT DJ-1 but not its pathogenic mutants enhances Nrf2 recruitment to the ARE region of the Trx1 promoter (Fig. 8D). Conversely, knocking down DJ-1 with siRNA abolished Nrf2 binding to the Trx1 promoter (Fig. 8E). These results collectively suggest that DJ-1 is required for recruiting Nrf2 onto its target sequence in the Trx1 promoter without itself binding to this DNA segment to a significant degree.
DISCUSSION
The present findings demonstrate that DJ-1 protects cells from oxidative stress by inducing Trx1 expression via the transcription factor Nrf2. DJ-1 activates Nrf2 by increasing its protein levels, promoting its nuclear translocation and enhancing its binding to the ARE consensus sequence in the Trx1 promoter. Thus, the increased Trx1 levels through Nrf2 help cells cope with oxidative stress in the presence of DJ-1.
Trx1 is one of the antioxidant enzymes involved in the control of cellular redox balance that has neuroprotective effects against ischemia, excitotoxicity and MPTP by alleviating oxidative stress (22,30,31). Several inducers of Trx1, such as neurotropin, geranylderanylacetone and sulforaphane, have been tested for their cytoprotective effect, and their ability to ameliorate various forms of stress-induced damage in mice reported (32–34). However, little information has been available prior to this report on the role of Trx1 in relation to PD. In addition to its function as an antioxidant, Trx1 is well known to regulate apoptosis by inhibiting ASK1 (20). DJ-1 blocks ASK1 activation through two mechanisms: one by preventing the translocation of the death protein Daxx from the nucleus to the cytoplasm and thus preventing its interaction with and activation of ASK1 (25), and the second is by preventing the dissociation of ASK1 from Trx1 (35). Based on the results of the present study, we suggest a new mechanism through which DJ-1 can exert its protective function against oxidative stress, namely by inducing Trx1 expression. Moreover, Trx1 has an important role in regulating phosphatase and tensin homolog (PTEN), which catalyzes the dephosphorylation of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), a step that results in inhibition of the AKT signaling pathway (36). Trx1 binds to PTEN in a redox-dependent manner to inhibit its lipid phosphatase activity resulting in AKT activation (37). Evidence exists that DJ-1 also activates the PI3K/AKT pathway in response to oxidative stress in vitro and in vivo (26,38). DJ-1 binds directly to PTEN and inhibits it leading to AKT activation (39,40). Activated AKT promotes cell survival by phosphorylating proapoptotic factors BAD and caspase-9 thereby inhibiting their functions (41). Our data show that knocking down Trx1 significantly impairs the ability of DJ-1 to activate AKT, and suggest that the cytoprotective function of DJ-1 is carried out in part through Trx1-mediated AKT activation.
The promoter region of the human Trx1 gene contains consensus-binding sites for many transcription factors, such as forkhead box O3, Nrf2 and cAMP response element-binding protein (42,43). The present results demonstrate a functional ARE site and show that Nrf2 is required for DJ-1-mediated Trx1 expression. In agreement with this finding, several lines of evidence suggest a functional link between Nrf2 and Trx1. In Nrf2-deficient MEFs, Trx1 is nearly undetectable, whereas in MEFs deficient in the Nrf2 inhibitor Keap1, Trx1 level is considerably increased compared with WT cells (44). In addition, the upregulation of Trx and TrxR observed by treating cells with 4-hydroxy-2-nonenal is largely abolished in Nrf2 siRNA-transfected photoreceptor-derived cells (45). Thus, Nrf2 is an important factor in upregulating Trx1 expression under stress conditions.
DJ-1 has been suggested to increase the expression of Nrf2 target antioxidant genes by stabilizing the Nrf2 protein in a liver cell line and MEFs (16). Upregulation of NQO1 using the prototypical Nrf2 activator tert-butylhydroquinone (tBHQ) is repressed by DJ-1 siRNA. However, the use of higher concentration of tBHQ to drive Nrf-2 activation to a greater degree can overcome the effect of DJ-1 deficiency (16). Similarly, exposure of a human lung epithelial cell line to cigarette smoke, which causes oxidative modification of DJ-1 and enhances its proteasomal degradation, as well as disruption of DJ-1 in the mouse lung result in decreased Nrf2 stability and impaired antioxidant gene induction (46). Knocking down Keap1 or using the Nrf2 activator sulforaphane restores Nrf2-dependent antioxidant responses such as NQO1 and glutamate-cysteine ligase modifier subunit even in DJ-1 deficient cells (46). Additionally, activation of Nrf2 in primary neuronal cultures and in the mouse brain using tBHQ or overexpressing Nrf2 with an adenoviral vector can activate the Nrf-2-ARE pathway in the absence of DJ-1 (47). These reports are consistent with our present finding that Nrf2 activation is downstream of DJ-1 and, therefore, direct activation of Nrf2 is sufficient to turn on antioxidant genes in the absence of DJ-1.
Nrf2 is bound to Keap1 in the cytoplasm under normal conditions but is released and translocated to the nucleus to activate its target genes upon exposure to oxidative stress (18). DJ-1 has been reported to upregulate Nrf2 protein levels but not mRNA by preventing the interaction between Keap1 and Nrf2, without binding to either component, and preventing the ubiquitination and proteasomal clearance of Nrf2 (16,46). Our data confirm that DJ-1 increases only Nrf2 protein, but not mRNA, and co-immunoprecipitation using chemical cross-linking did not detect binding between DJ-1 and either Nrf2 or Keap1. However, we find no experimental evidence that DJ-1 blocks the interaction between Nrf2 and its inhibitor Keap1 nor do we detect prevention of Nrf2 ubiquitination in this context (16). We, therefore, conclude that DJ-1 increases Nrf2 protein levels indirectly through mechanism(s) other than Keap1. Whether DJ-1 interacts with other proteins to regulate Nrf2 protein stability remains a question. More recently, CR6-interacting Factor 1 (CRIF1), similar to Keap1, was found to negatively regulate Nrf2 protein stability by promoting its proteasome-mediated degradation (48). However, CRIF1-mediated regulation of Nrf2 is different than that carried out by Keap1 in that the former can do so in both normal and oxidative stress conditions, whereas the latter is active only under oxidative stress. It is conceivable that another mechanism may be operational by which DJ-1 enhances the Nrf2 pathway without oxidative stress, and perhaps DJ-1 may inhibit CRIF1-mediated Nrf2 degradation. Likewise, several recent studies report that phosphorylation or acetylation of Nrf2 may regulate its intracellular localization and activity (28,49). The possibility that DJ-1 may regulate the posttranslational modifications of Nrf2 cannot be excluded considering that DJ-1 interacts with transcriptional repressors such as PIASxα and DJ-1-binding protein, and inhibits the recruitment of the histone deacetylase complex (12,50).
In summary, the current findings provide evidence that, in addition to the multiple functional properties of DJ-1, another mechanism through which it provides cytoprotection is by inducing Trx1 expression. Increased Nrf2 levels and nuclear translocation are required for Trx1 gene induction in this process. These observations demonstrate the importance of the DJ-1/Nrf2/Trx1 axis in the cellular response to oxidative stress-induced cell death and suggest that this axis may be a therapeutic target for disorders associated with oxidative stress such as PD.
MATERIALS AND METHODS
Materials
Expression vectors encoding Flag-tagged WT or L166P DJ-1 were previously described (25). M26I mutant DJ-1 was derived from Flag-WT DJ-1 using site-directed mutagenesis (Invitrogen). Myc-DJ-1 was generated by moving DJ-1 cDNA from Flag-WT DJ-1 into Myc-tagged plasmid. pSilencer2.1 U6 plasmid (Ambion) was used to clone shRNA for DJ-1. Luciferase vectors containing serial deletions of the human Trx1 promoter were a kind gift from Junji Yodoi (Tokyo University). ARE mutant of human Trx1 promoter was made from full-length promoter as previously described (51). Flag-Nrf2 was kindly provided by Ken Itoh (Hirosaki University School of Medicine, Japan). H2O2 and DNCB were purchased from Sigma. DJ-1 null mice were a kind gift from Ted Dawson (Johns Hopkins University).
Cell culture, transfection and treatments
Human neuroblastoma cell line SH-SY5Y, SY5Y-DJ-1 cells stably expressing Flag-DJ-1, and human HeLa cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. SY5Y-DJ-1 cells, which are described previously (25), are 100% positive for Flag-DJ-1 determined by immunocytochemistry using Flag antibody. SH-SY5Y and SY5Y-DJ-1 cells were transfected with Lipofectamine 2000 reagent (Invitrogen) according to the supplier's instructions. HeLa cells were transfected with pRNAT-U6.1/Neo plasmid (GenScript Corporation) expressing two different shRNAs for DJ-1 or pRNAT-U6.1/Neo alone as negative control using polyethylenimine reagent as described previously (52). Cells were selected with 1 mg/ml G418 (Sigma) to obtain two separate lines stably expressing DJ-1 shRNA.
Real-time PCR
Total RNA was isolated from cells and mouse tissues using Trizol reagent (Invitrogen), and cDNA was obtained using Superscript RT kit (Invitrogen) according to the manufacturer's instructions. Negative reverse transcriptase controls were included in each assay. Quantitative real-time PCR was performed by the iCycler iQ RT–PCR detection system (Bio-Rad). PCR primer sequences were as follows: mouse Trx1 (5′-ATGGTGAAGCTGATCGAGAGC-3′, 5′-GGCATATTCAGTAATAGAGGC-3′), mouse β-actin (5′-CAGTTCGCCATGGATGACGAT-3′, 5′-ATCTGGGTCATCTTTTCACGGTTG-3′), human Nrf2 (16) or human GAPDH (5′-ATTCCATGGCACCGTCAAGGCT-3′, 5′-TCAGGTCCACCACTGACACGTT-3′). PCR amplification was performed using the SYBR Green PCR master mix (Applied BioSystem). All quantifications were normalized to the level of endogenous control β-actin or GAPDH. The relative levels of mRNA were compared and expressed as fold of control levels. Data shown were representative of three separate experiments.
Western blot analyses
Cells and brain tissues were washed in ice-cold phosphate-buffered saline (PBS) and lysed with 1% sodium dodecyl sulfate containing protease and phosphatase inhibitors (Roche, Basel, Swiss). Lysates were sonicated for 10 s three times and their protein contents were determined using a bicinchoninic acid assay kit. Antibodies used for western blot analyses and their sources are as follows: anti-Trx1, peroxidase-conjugated anti-Myc, anti-Flag and anti-Lamin B1 from Santa Cruz Biotechnology; DJ-1 (691, a kind gift from Benoit I. Giasson) and anti-human DJ-1 from Stressgen; anti-Nrf2 from Epitomics; anti-pAKT (S473) and anti-AKT from Cell Signaling; anti-β-actin and anti-α-tubulin from Sigma. For fractionation of nuclear and cytoplasmic extracts, cells were grown in 100 mm dishes and fractionated using Nuclear Extract kit (Active motif) according to the manufacturer's instructions. Specific western blot signals were detected using an enhanced chemiluminescence kit (PerkinElmer LAS, Inc). Quantification of immunoreactivity was performed using ImageJ (NIH, USA).
RNA interference
Cells were transfected with siRNAs specific for human DJ-1 (D-005984-00; Dharmacon), human Trx1 (D-006340-01; Dhamacon), Nrf2 (Ambion) or non-targeting sequence (D-001206-13; Dharmacon), at a final concentration of 50 nm using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Twenty-four hours after transfection, the medium was replaced and cells were harvested at 72 h of post-transfection period.
Cell death assay
Forty-eight hours after siRNA transfection, cells were incubated with the indicated H2O2 concentrations and duration in serum-free media for 24 h. For DNCB treatment, cells were pretreated with 5 μm DNCB for 1 h and incubated with H2O2 in serum-free media for 24 h. Cell death was assessed by measuring the release of lactate dehydrogenase (LDH) from damaged cells using the Cytotoxicity Detection Kit (Roche) according to the manufacturer's instructions.
Propidium iodide staining
HeLa cells plated in 24-well plates were co-transfected with 50 nm siRNA specific for Trx1 or non-targeting sequence, 0.8 μg Flag-tagged WT or mutant DJ-1 (M26I and L116P) and 0.08 μg EGFP-C1 (Clontech) using Lipofectamine 2000 reagent. Forty-eight hours after transfection, cells were incubated with 1 mm H2O2 for 12 h. Following fixation with 4% paraformaldehyde for 20 min, cells were stained with 0.5 µM of propidium iodide (PI) (Molecular Probes) for 5 min at room temperature. Cell death was assessed by counting red fluorescent cells and green fluorescent cells under a Zeiss Axiovert 200 microscope, and the percentage of PI-positive dead cells among green fluorescent protein (GFP)-positive transfected cells was compared across conditions.
Luciferase reporter assay
Cells were plated in 24-well plates and co-transfected with human Trx1 promoter luciferase reporter plasmids (42) and β-galatosidase construct as control for transfection efficiency, and harvested 36 h post-transfection in Glo lysis buffer (Promega, Madison, WI, USA). Luciferase activity was determined in triplicates with Steady-Glo Luciferase Assay System (Promega) using Wallac 1420 multilabel counter (Perkin-Elmer, Waltham, MA, USA) and normalized to β-galactosidase activity measured with the β-Galactosidase Enzyme Assay System (Promega).
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed as previously described (53). Briefly, cells were cross-linked by adding formaldehyde, lysed and sonicated. Lysates were immunoprecipitated by Flag or Nrf2 antibodies, proteins digested with proteinase K and ChIP-enriched DNA was subjected to PCR using the following primers: Trx1 promoter containing ARE (5′-GAACAGAAGGAGGTTACAGAG-3′ and 5′-TGAATCGAACACGCCCACGCT-3′) or TH promoter as negative control (5′-GGAACTTGCAAGGATTTGGA-3′ and 5′-CCATTTCAGCCTCCCAAGTA-3′) (54). Immunoglobulin G (IgG) was used as negative control for immunoprecipitation with anti-Nrf2.
Statistical analysis
Results are presented as means ± SEM from at least three independent experiments. Statistical analyses were performed using Student's t-test or analysis of variance (ANOVA) followed by the Newman–Keuls test. A value of P < 0.05 was accepted as significant.
SUPPLEMENTARY MATERIAL
FUNDING
This project was supported by National Institutes of Health (NIH) grants NS059869 and NS053517 to M.M.M. who is the William Dow Lovett Professor of Neurology. E.J. is supported by NIH grant NS070898.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Drs Ted Dawson for providing DJ-1 null mice, Junji Yodoi for human Trx1 promoter construct, Ken Itoh for Flag-Nrf2 construct and Benoit I. Giasson for DJ-1 antibody.
Conflict of Interest statement. None declared.
References
- 1.Thomas B., Beal M.F. Parkinson's disease. Hum. Mol. Genet. 2007;16(Spec No. 2):R183–R194. doi: 10.1093/hmg/ddm159. doi:10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 2.Beal M.F. Oxidatively modified proteins in aging and disease. Free Radic. Biol. Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. doi:10.1016/S0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 3.Andersen J.K. Oxidative stress in neurodegeneration: cause or consequence? Nat. Med. 2004;10(uppl.):S18–S25. doi: 10.1038/nrn1434. doi:10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 4.Sofic E., Sapcanin A., Tahirovic I., Gavrankapetanovic I., Jellinger K., Reynolds G.P., Tatschner T., Riederer P. Antioxidant capacity in postmortem brain tissues of Parkinson's and Alzheimer's diseases. J. Neural. Transm. Suppl. 2006;71:39–43. doi: 10.1007/978-3-211-33328-0_5. doi:10.1007/978-3-211-33328-0_5. [DOI] [PubMed] [Google Scholar]
- 5.Kojima S., Matsuki O., Nomura T., Yamaoka K., Takahashi M., Niki E. Elevation of antioxidant potency in the brain of mice by low-dose gamma-ray irradiation and its effect on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced brain damage. Free Radic. Biol. Med. 1999;26:388–395. doi: 10.1016/s0891-5849(98)00200-7. doi:10.1016/S0891-5849(98)00200-7. [DOI] [PubMed] [Google Scholar]
- 6.Bonifati V., Rizzu P., van Baren M.J., Schaap O., Breedveld G.J., Krieger E., Dekker M.C., Squitieri F., Ibanez P., Joosse M., et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. doi:10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 7.Choi J., Sullards M.C., Olzmann J.A., Rees H.D., Weintraub S.T., Bostwick D.E., Gearing M., Levey A.I., Chin L.S., Li L. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J. Biol. Chem. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. doi:10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandopadhyay R., Kingsbury A.E., Cookson M.R., Reid A.R., Evans I.M., Hope A.D., Pittman A.M., Lashley T., Canet-Aviles R., Miller D.W., et al. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson's disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. doi:10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- 9.Taira T., Saito Y., Niki T., Iguchi-Ariga S.M., Takahashi K., Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. doi:10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinat C., Shendelman S., Jonason A., Leete T., Beal M.F., Yang L., Floss T., Abeliovich A. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS Biol. 2004;2:e327. doi: 10.1371/journal.pbio.0020327. doi:10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim R.H., Smith P.D., Aleyasin H., Hayley S., Mount M.P., Pownall S., Wakeham A., You-Ten A.J., Kalia S.K., Horne P., et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc. Natl Acad. Sci. USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. doi:10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi K., Taira T., Niki T., Seino C., Iguchi-Ariga S.M., Ariga H. DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx alpha to the receptor. J. Biol. Chem. 2001;276:37556–37563. doi: 10.1074/jbc.M101730200. doi:10.1074/jbc.M101730200. [DOI] [PubMed] [Google Scholar]
- 13.Zhong N., Kim C.Y., Rizzu P., Geula C., Porter D.R., Pothos E.N., Squitieri F., Heutink P., Xu J. DJ-1 transcriptionally up-regulates the human tyrosine hydroxylase by inhibiting the sumoylation of pyrimidine tract-binding protein-associated splicing factor. J. Biol. Chem. 2006;281:20940–20948. doi: 10.1074/jbc.M601935200. doi:10.1074/jbc.M601935200. [DOI] [PubMed] [Google Scholar]
- 14.Zhou W., Freed C.R. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J. Biol. Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. doi:10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- 15.Zhong N., Xu J. Synergistic activation of the human MnSOD promoter by DJ-1 and PGC-1alpha: regulation by SUMOylation and oxidation. Hum. Mol. Genet. 2008;17:3357–3367. doi: 10.1093/hmg/ddn230. doi:10.1093/hmg/ddn230. [DOI] [PubMed] [Google Scholar]
- 16.Clements C.M., McNally R.S., Conti B.J., Mak T.W., Ting J.P. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl Acad. Sci. USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. doi:10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. doi:10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. doi:10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura H., Nakamura K., Yodoi J. Redox regulation of cellular activation. Annu. Rev. Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. doi:10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 20.Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. doi:10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byun H.S., Cho E.W., Kim J.S., Moon M.S., Yum J.J., Kim K.C., Kim I.G. Thioredoxin overexpression in HT-1080 cells induced cellular senescence and sensitization to gamma radiation. FEBS Lett. 2005;579:4055–4062. doi: 10.1016/j.febslet.2005.06.023. doi:10.1016/j.febslet.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 22.Bai J., Nakamura H., Hattori I., Tanito M., Yodoi J. Thioredoxin suppresses 1-methyl-4-phenylpyridinium-induced neurotoxicity in rat PC12 cells. Neurosci. Lett. 2002;321:81–84. doi: 10.1016/s0304-3940(02)00058-7. doi:10.1016/S0304-3940(02)00058-7. [DOI] [PubMed] [Google Scholar]
- 23.Bai J., Nakamura H., Kwon Y.W., Tanito M., Ueda S., Tanaka T., Hattori I., Ban S., Momoi T., Kitao Y., et al. Does thioredoxin-1 prevent mitochondria- and endoplasmic reticulum-mediated neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine? Antioxid. Redox Signal. 2007;9:603–608. doi: 10.1089/ars.2006.1513. doi:10.1089/ars.2006.1513. [DOI] [PubMed] [Google Scholar]
- 24.Umeda-Kameyama Y., Tsuda M., Ohkura C., Matsuo T., Namba Y., Ohuchi Y., Aigaki T. Thioredoxin suppresses Parkin-associated endothelin receptor-like receptor-induced neurotoxicity and extends longevity in Drosophila. J. Biol. Chem. 2007;282:11180–11187. doi: 10.1074/jbc.M700937200. doi:10.1074/jbc.M700937200. [DOI] [PubMed] [Google Scholar]
- 25.Junn E., Taniguchi H., Jeong B.S., Zhao X., Ichijo H., Mouradian M.M. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc. Natl Acad. Sci. USA. 2005;102:9691–9696. doi: 10.1073/pnas.0409635102. doi:10.1073/pnas.0409635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aleyasin H., Rousseaux M.W., Marcogliese P.C., Hewitt S.J., Irrcher I., Joselin A.P., Parsanejad M., Kim R.H., Rizzu P., Callaghan S.M., et al. DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc. Natl Acad. Sci. USA. 2010;107:3186–3191. doi: 10.1073/pnas.0914876107. doi:10.1073/pnas.0914876107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain A.K., Bloom D.A., Jaiswal A.K. Nuclear import and export signals in control of Nrf2. J. Biol. Chem. 2005;280:29158–29168. doi: 10.1074/jbc.M502083200. doi:10.1074/jbc.M502083200. [DOI] [PubMed] [Google Scholar]
- 28.Sun Z., Chin Y.E., Zhang D.D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol. Cell Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. doi:10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen T., Huang H.C., Pickett C.B. Transcriptional regulation of the antioxidant response element. Activation by Nrf2 and repression by MafK. J. Biol. Chem. 2000;275:15466–15473. doi: 10.1074/jbc.M000361200. doi:10.1074/jbc.M000361200. [DOI] [PubMed] [Google Scholar]
- 30.Takagi Y., Hattori I., Nozaki K., Mitsui A., Ishikawa M., Hashimoto N., Yodoi J. Excitotoxic hippocampal injury is attenuated in thioredoxin transgenic mice. J. Cereb. Blood Flow Metab. 2000;20:829–833. doi: 10.1097/00004647-200005000-00009. doi:10.1097/00004647-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Zhou F., Gomi M., Fujimoto M., Hayase M., Marumo T., Masutani H., Yodoi J., Hashimoto N., Nozaki K., Takagi Y. Attenuation of neuronal degeneration in thioredoxin-1 overexpressing mice after mild focal ischemia. Brain Res. 2009;1272:62–70. doi: 10.1016/j.brainres.2009.03.023. doi:10.1016/j.brainres.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Tanito M., Masutani H., Kim Y.C., Nishikawa M., Ohira A., Yodoi J. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Invest. Ophthalmol. Vis. Sci. 2005;46:979–987. doi: 10.1167/iovs.04-1120. doi:10.1167/iovs.04-1120. [DOI] [PubMed] [Google Scholar]
- 33.Hirota K., Nakamura H., Arai T., Ishii H., Bai J., Itoh T., Fukuda K., Yodoi J. Geranylgeranylacetone enhances expression of thioredoxin and suppresses ethanol-induced cytotoxicity in cultured hepatocytes. Biochem. Biophys. Res. Commun. 2000;275:825–830. doi: 10.1006/bbrc.2000.3392. doi:10.1006/bbrc.2000.3392. [DOI] [PubMed] [Google Scholar]
- 34.Hoshino Y., Nakamura T., Sato A., Mishima M., Yodoi J., Nakamura H. Neurotropin demonstrates cytoprotective effects in lung cells through the induction of thioredoxin-1. Am. J. Respir. Cell Mol. Biol. 2007;37:438–446. doi: 10.1165/rcmb.2006-0402OC. doi:10.1165/rcmb.2006-0402OC. [DOI] [PubMed] [Google Scholar]
- 35.Im J.Y., Lee K.W., Junn E., Mouradian M.M. DJ-1 protects against oxidative damage by regulating the thioredoxin/ASK1 complex. Neurosci. Res. 2010;67:203–208. doi: 10.1016/j.neures.2010.04.002. doi:10.1016/j.neures.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X., Senechal K., Neshat M.S., Whang Y.E., Sawyers C.L. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc. Natl Acad. Sci. USA. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. doi:10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meuillet E.J., Mahadevan D., Berggren M., Coon A., Powis G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN's lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN's tumor suppressor activity. Arch. Biochem. Biophys. 2004;429:123–133. doi: 10.1016/j.abb.2004.04.020. doi:10.1016/j.abb.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y., Gehrke S., Haque M.E., Imai Y., Kosek J., Yang L., Beal M.F., Nishimura I., Wakamatsu K., Ito S., et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl Acad. Sci. USA. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. doi:10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim R.H., Peters M., Jang Y., Shi W., Pintilie M., Fletcher G.C., DeLuca C., Liepa J., Zhou L., Snow B., et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7:263–273. doi: 10.1016/j.ccr.2005.02.010. doi:10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y.C., Kitaura H., Taira T., Iguchi-Ariga S.M., Ariga H. Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int. J. Oncol. 2009;35:1331–1341. [PubMed] [Google Scholar]
- 41.Datta S.R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. doi:10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 42.Kim Y.C., Masutani H., Yamaguchi Y., Itoh K., Yamamoto M., Yodoi J. Hemin-induced activation of the thioredoxin gene by Nrf2. A differential regulation of the antioxidant responsive element by a switch of its binding factors. J. Biol. Chem. 2001;276:18399–18406. doi: 10.1074/jbc.M100103200. doi:10.1074/jbc.M100103200. [DOI] [PubMed] [Google Scholar]
- 43.Li X.N., Song J., Zhang L., LeMaire S.A., Hou X., Zhang C., Coselli J.S., Chen L., Wang X.L., Zhang Y., et al. Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes. 2009;58:2246–2257. doi: 10.2337/db08-1512. doi:10.2337/db08-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niso-Santano M., Gonzalez-Polo R.A., Bravo-San Pedro J.M., Gomez-Sanchez R., Lastres-Becker I., Ortiz-Ortiz M.A., Soler G., Moran J.M., Cuadrado A., Fuentes J.M. Activation of apoptosis signal-regulating kinase 1 is a key factor in paraquat-induced cell death: modulation by the Nrf2/Trx axis. Free Radic. Biol. Med. 2010;48:1370–1381. doi: 10.1016/j.freeradbiomed.2010.02.024. doi:10.1016/j.freeradbiomed.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 45.Tanito M., Agbaga M.P., Anderson R.E. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic. Biol. Med. 2007;42:1838–1850. doi: 10.1016/j.freeradbiomed.2007.03.018. doi:10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Malhotra D., Thimmulappa R., Navas-Acien A., Sandford A., Elliott M., Singh A., Chen L., Zhuang X., Hogg J., Pare P., et al. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am. J. Respir. Crit. Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. doi:10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Gan L., Johnson D.A., Johnson J.A. Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur. J. Neurosci. 2010;31:967–977. doi: 10.1111/j.1460-9568.2010.07138.x. doi:10.1111/j.1460-9568.2010.07138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang H.J., Hong Y.B., Kim H.J., Bae I. CR6-interacting factor 1 (CRIF1) regulates NF-E2-related factor 2 (NRF2) protein stability by proteasome-mediated degradation. J. Biol. Chem. 2010;285:21258–21268. doi: 10.1074/jbc.M109.084590. doi:10.1074/jbc.M109.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Z., Huang Z., Zhang D.D. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS ONE. 2009;4:e6588. doi: 10.1371/journal.pone.0006588. doi:10.1371/journal.pone.0006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niki T., Takahashi-Niki K., Taira T., Iguchi-Ariga S.M., Ariga H. DJBP: a novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex. Mol. Cancer Res. 2003;1:247–261. [PubMed] [Google Scholar]
- 51.Ko J.K., Ma J. A rapid and efficient PCR-based mutagenesis method applicable to cell physiology study. Am. J. Physiol. Cell Physiol. 2005;288:C1273–C1278. doi: 10.1152/ajpcell.00517.2004. doi:10.1152/ajpcell.00517.2004. [DOI] [PubMed] [Google Scholar]
- 52.Boussif O., Lezoualc'h F., Zanta M.A., Mergny M.D., Scherman D., Demeneix B., Behr J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. doi:10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang H., Magilnick N., Lee C., Kalmaz D., Ou X., Chan J.Y., Lu S.C. Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-kappaB and AP-1. Mol. Cell Biol. 2005;25:5933–5946. doi: 10.1128/MCB.25.14.5933-5946.2005. doi:10.1128/MCB.25.14.5933-5946.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reddy S.D., Rayala S.K., Ohshiro K., Pakala S.B., Kobori N., Dash P., Yun S., Qin J., O'Malley B.W., Kumar R. Multiple coregulatory control of tyrosine hydroxylase gene transcription. Proc. Natl Acad. Sci. USA. 2011;108:4200–4205. doi: 10.1073/pnas.1101193108. doi:10.1073/pnas.1101193108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.