Abstract
Background
Prospective and case-control studies have demonstrated that memory loss and executive dysfunction occur early in Alzheimer disease (AD).
Objective
To investigate these observations by the study of persons at risk for autosomal dominant forms of AD.
Methods
Neuropsychological and genetic tests were performed on 51 nondemented at-risk members of 10 Mexican families with two distinct presenilin-1 (PS1) mutations. Test scores were compared between PS1 mutation carriers (MCs; n = 30) and noncarriers (NCs; n = 21) by analyses of variance, co-varying for family and specific mutation. Regression analyses were performed, taking into account age relative to the median age at dementia diagnosis in the family (adjusted age), gender, Beck Depression Inventory (BDI) scores, education, and number of APOE ε4 alleles. Subjects were divided into age tertiles and scores compared within these groups. Composite scores for Verbal Memory, Executive Function/Working Memory, Language, and Visuospatial Function were created, and these scores compared between MCs and NCs.
Results
MCs performed worse than NCs on the Mini-Mental State Examination, Trails Making Tests A and B, Delayed Recall of a 10-Word List, and Wechsler Adult Intelligence Scale WAIS Block Design. In multiple linear regression analyses, BDI score, gender, and number of APOE ε4 alleles did not consistently affect test scores. The differences seen between MCs and NCs were due to differences in the oldest tertile. MCs had lower Visuospatial and Executive Function/Working Memory but not Verbal Memory or Language composite scores.
Conclusions
This study is consistent with findings in sporadic Alzheimer disease of early problems with memory, visuospatial function, and particularly with executive function in PS1 mutation carriers. Depression, gender, and presence of an APOE ε4 allele did not demonstrate large influences on neuropsychological performance.
Despite advances in our understanding of imaging and biochemical biomarkers1 for Alzheimer disease (AD), neuropsychological testing remains the cornerstone for diagnosing and characterizing the cognitive losses that occur early in dementia. In early AD, deficits in verbal episodic memory are most consistently reported,2 but early declines in cognitive processing speed3 and executive function4 have also been documented. Despite this, early AD can still be a challenge to identify confidently as the onset of AD is gradual and it can be challenging to differentiate observed deficits from the changes of normal aging, medical co-morbidities associated with aging, and coexisting depression. Persons in whom the development of AD can be reliably predicted provide a unique opportunity for characterizing the early stages of the disorder.
In a minority of AD cases, an early-onset form of the disease is inherited in a highly penetrant autosomal dominant fashion through mutations in the genes coding for presenilin-1 (PS1),5 presenilin-2 (PS2),6 and the amyloid precursor protein (APP).7 The neuropathologic changes in these forms of AD are similar to those found in the sporadic form of the illness.8 However, investigators have reported atypical neuropathology in some cases of familial AD,9 and the clinical manifestations can be distinctive. Autosomal dominant AD has a younger age at onset10 and a more rapid course11 and can feature para-paresis,9 early myoclonus, and seizures.8
Several groups have described the neuropsychological deficits occurring in persons with PS1 mutations affected by the disease. Consistent with results in late-onset sporadic AD, deficits in delayed recall on both verbal and visual episodic memory tasks are common findings.12 Additionally, executive function can be affected, with multiple investigators describing persons with presentations consistent with frontal lobe degeneration.13 Because of the highly penetrant autosomal dominant nature of the disease in these families, persons known to be at 50% risk for the inheritance of a pathogenic mutation who are not yet demented may be studied to determine the earliest neuropsychological changes that occur in this illness. In the current cross-sectional study, we performed genetic and neuropsychological testing on nondemented at-risk members of families with known pathogenic PS1 mutations and compared the performance of mutation carriers (MCs) and noncarriers (NCs) in an effort to establish the earliest detectable cognitive changes.
Methods
Case selection
We examined 77 persons from 10 Mexican families with an autosomal dominant pattern of inheritance of early-onset dementia consistent with AD. Two families had participated in the study in which the condition was linked to chromosome 14.5 The other eight families were identified by the authors14 when a symptomatic patient was clinically diagnosed with probable AD of presenile onset with a family history of an autosomal dominant pattern of inheritance. The PS1 gene was sequenced in a commercial laboratory for symptomatic members of three families, and a PS1 mutation was confirmed in each. In the remaining seven families, persons at risk by virtue of being a first-degree relative of an index patient suspected to carry a PS1 mutation gave their consent for genetic testing in a research laboratory (see below). This testing confirmed a PS1 mutation in at least one member of each of the 10 families.
Written informed consent was obtained from all subjects according to the Declaration of Helsinki. This protocol was approved by the institutional review boards at the University of California, Irvine, and the National Institute of Neurology and Neurosurgery in Mexico City (INNN). All potential subjects were aware of their 50% chance of inheriting a pathogenic mutation. During the consent process, it was emphasized that they would be tested for this, but within the protocol, they could not be told the result. They were also informed that the presenilin-1 test was commercially available, but no potential subjects had opted to pursue this at the time of this study.
Nine of the 10 families had the same missense mutation in exon 13 of the PS1 gene, causing an alanine-to-glutamic-acid substitution at amino acid 431 (A431E)15 in the presenilin-1 protein. This mutation was shown by autopsy to correlate with the neuropathology of AD in another Mexican American kindred15 and was not found in 28 control subjects from Mexico without a family history of dementia.14 Analyses of GT and CA dinucleotide alleles flanking the PS1 gene revealed that all persons with the A431E mutation also shared these genotypes. This suggests a common founder for this PS1 mutation.14 Members of the final family had also participated in the study in which the PS1 gene was localized to chromosome 14.16 They were subsequently found to have a cytosine-to-guanine transversion at nucleotide 703 in the gene for PS1, causing a leucine-to-valine missense substitution (L235V).
Potential subjects were identified by virtue of being first-degree relatives of someone affected by early-onset dementia with a family history consistent with an autosomal dominant mode of inheritance. No subject in this analysis had presented for clinical assessment for cognitive difficulties. All potential subjects underwent a clinical interview in which they were asked whether or not they felt they had difficulties with their memory or thinking. Those that indicated they did were additionally asked if they thought these problems interfered with their normal activities. Seven subjects answering “yes” to both these questions or for whom a clear history of functional decline was provided by an appropriate informant were excluded.
As appropriate norms for many of the neuropsychological tests described below are not available, cognitive performance per se was not used to exclude subjects from the analyses in this study. To increase the likelihood that the subjects in this analysis were either presymptomatic or in the earliest stage of the disease, we excluded subjects on the basis of age. Previous reports have suggested that the age at PS1-related disease onset is fairly consistent within a given family.17 We therefore determined a median age at dementia diagnosis within each family by obtaining histories about affected family members. We then excluded 13 persons at or beyond this family-specific age. An additional six subjects were excluded because of incomplete data. Neuropsychological and genetic data on the remaining 51 at-risk subjects were analyzed.
Neuropsychological assessment
Subjects were assessed at the INNN or in their homes. Subjects underwent a 30-minute interview, blood draw for genetic testing, 30 minutes of computerized testing, and 2 hours of neuropsychological testing in one session.
All subjects spoke Spanish as their primary language. Each underwent a battery consisting of standardized English-language tests that were translated into Spanish. The English versions have been validated and demonstrated to be reliable. All 51 subjects underwent the following: Mini-Mental State Examination (MMSE),18 Trails Making Test A and B, the Wechsler Memory Scale–Revised (WMS-R; Information subscale, Orientation sub-scale, Mental Control subscale, Logical Memory Immediate Recall, Digit Span Forwards, Digit Span Backwards, Visual Reproduction Immediate, and Associative Learning Immediate Recall), the Rey–Osterrieth Figure (Copy, Immediate Recall, Delayed Recall), a 10-Word Learning List with Immediate Recall, Boston Naming Test, Category Fluency (fruit and animals), Letter Fluency (“F” and “A”), and the Wechsler Adult Intelligence Scale Block Design Subtest.19 Forty-eight subjects were tested for delayed recall of the 10-Word List. A Spanish version of the Beck Depression Inventory (BDI)20 was also administered to all subjects. All persons administering and scoring tests were blinded to subjects’ genetic status.
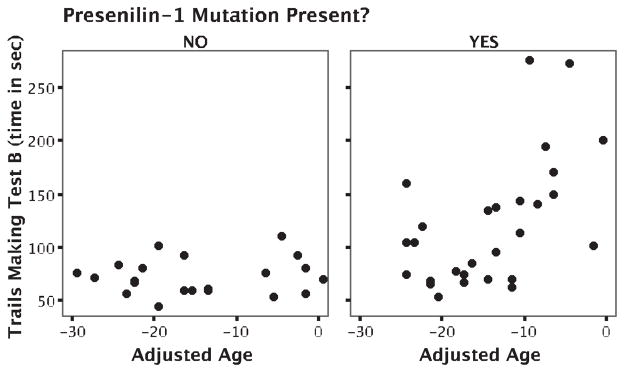
Figure.
Time to complete Trails Making Test B in PS1 mutation carriers and noncarriers. Subjects’ ages are in number of years younger than the median age at dementia diagnosis in their family.
Genetic testing
Whole-blood samples were taken and genomic DNA isolated.21 The exon in which the PS1 mutation was present was sequenced in each at-risk individual. DNA from subjects from families suspected to harbor the A431E mutation was amplified using the standard PCR protocol with primers specific for exon 13. The amplification products were sequenced using a Beckman CEQ. For confirmation, an additional amplification was digested with the restriction enzyme BsrDI and run on a 2% agarose gel. The substitution causing the A431E mutation eliminates the BsrDI site, giving three band sizes: 204, 61, and 143 bp. Normal individuals were also analyzed at the same time to determine if complete digestion had occurred. DNA from members of the family with the L235V mutation was amplified for exon 8 of the PS1 gene, and these products were used for direct sequencing.
APOE genotype was determined by amplification followed by HhaI digestion.22 The products were run on an 8% polyacrylamide gel and visualized by ethidium bromide stain on an ultraviolet transilluminator.
Statistical analysis
Scores on the two categorical verbal fluency tests were combined to form a single score, as were the two letter fluency tests. Immediate recall scores on the two paragraphs of the WMS Logical Memory (A and B) were also combined to form a single score. Three subjects were missing data on the Delayed Recall of the 10-Word List, and one was missing the score on the Boston Naming Test. The mean score on that item for their mutation status group was provided for the composite score analysis (see below).
Analyses were performed using SPSS version 11.0 (Chicago, IL). Subjects were separated into those carrying (MCs) and not carrying (NCs) PS1 mutations. T tests were initially performed, comparing neuropsychological scores between MCs and NCs. Because of the possibility of type 1 errors due to the multiple dependent variables being compared, we used these t tests as a screen to identify variables for further analyses. Intrafamilial factors (environmental, socioeconomic, and non-PS1 genetic variables) might also be expected to influence performance on neuropsychological tests. Therefore, for those test scores in which MCs and NCs differed by t test, one-way analyses of variance (ANOVAs) covarying for family of membership and the type of PS1 mutation in the family were also performed. Regression analyses were also performed for these same variables, taking into account age relative to the median age at disease onset in the family (“adjusted age”; see below), gender, BDI scores, years of education, and number of APOE ε4 alleles.
To further explore the influence of mutation type (A431E, L235V) on cognition controlling for the presence or absence of such a mutation, subjects were divided into those carrying and not carrying PS1 mutations, and t tests were performed comparing the neuropsychological scores of persons from families harboring the A431E mutation with those harboring the L235V mutation. For those tests for which scores differed between persons from families with A431E mutations and those with L235V mutations, two-way ANOVAs analyzing the interaction of mutation type and mutation status (MC vs NC) were performed.
To create composite scores for different cognitive functions, z scores for each test for each subject were calculated with reference to the means and standard deviations of NCs. We then calculated composite scores by averaging the z scores of individual tests: Language (Boston Naming Test, Category Fluency, Letter Fluency), Visuospatial (WAIS Block Design, Rey–Osterrieth Copy, Visual Reproduction Immediate), Verbal Memory (10-Word List Delayed Recall, Paired Associate Immediate Recall, Logical Memory Immediate Recall), and Executive Function/Working Memory (Trails Making Test B Time, Mental Control, Digit Span Backwards). The internal consistency of the four composite scores was evaluated computing as many Cronbach α coefficients, and these were all ≥0.76. These composite scores were compared between MCs and NCs by t tests.
As cognitive deficits would be expected to manifest increasingly as PS1 MCs age, subjects were divided into tertiles according to their age in relation to the family-specific age at dementia diagnosis in their family. The age at which dementia is typically diagnosed within the families was determined as above. The “adjusted age” of each subject was determined by calculating the age of each subject in relation to this family-specific age. Subjects were then divided into equally sized young, older, and oldest groups according to this adjusted age. T tests were performed comparing test scores in these three age groups. It was not possible to perform further statistical analyses on these groups because of their relatively small sizes. Ninety-five percent confidence levels were used to determine significance in all analyses.
Results
Overall, PS1 MCs (n = 30) and NCs (n = 21) were not significantly different with regard to absolute age, age relative to the mean age at AD diagnosis in their family, gender, education, depression level as measured with the BDI, and type of mutation present in their family (table 1). Years of education approached significance in this population (p = 0.06), with a trend for MCs to achieve fewer years of education. There were no differences between MCs and NCs in the proportion reporting a history of any medical illness, a history of any specific medical disorder, or the use of psychotropic medications (by χ2 analysis and, when appropriate, Fisher exact test, data not shown). The frequency of the APOE ε4 allele was 11.8%, which is slightly greater than the 7 to 8% frequency previously reported in Mexican mestizos.23
Table 1.
Demographic characteristics of study group
| PS1 mutation carriers, n = 30 | PS1 mutation noncarriers, n = 21 | p | |
|---|---|---|---|
| Absolute age, y | 28.9 (18–47) | 29.3 (18–43) | 0.87 |
| Relative age, y before median age at AD diagnosis in family | 14.9 (1–25) | 15.1 (0–30) | 0.93 |
| Sex, % F | 60 (n = 18) | 76 (n = 16) | χ2, 0.23 |
| Education, y | 11.1 (6–17) | 12.7 (7–18) | 0.06 |
| Type of mutation in family (A431E vs L235V) | 83% A431E (n = 25) | 71% A431E (n = 15) | χ2, 0.31 |
| 27% L235V (n = 5) | 29% L235V (n = 6) | ||
| Beck Depression Inventory score | 11.4 (0–42) | 7.9 (0–35) | 0.21 |
Numbers or ranges are shown in parentheses.
AD = Alzheimer disease.
PS1 MCs performed more poorly than NCs on the MMSE, Trails Making Test A and B (Time), Delayed Recall of the 10-Word List, and the WAIS Block Design Test. When ANOVAs co-varying for family of membership and type of mutation in the family were performed on those variables that differentiated MCs and NCs by t test, MCs still performed more poorly on the MMSE, on the Trails Making Test A and B, on Delayed Recall of the 10-Word List, and on the WAIS Block Design Test (table 2). Family of membership and mutation type had no significant effect on these outcomes.
Table 2.
Mean scores on neuropsychological tests in PS1 mutation carriers and noncarriers
| Test | +PS1 mutation, n = 30 | −PS1 mutation, n = 21 | p |
|---|---|---|---|
| MMSE | 28.0 (1.6) | 29.2 (0.8) | 0.004 |
| TMT A, s | 49.9 (18.1) | 39.0 (12.9) | 0.028 |
| TMT B, s | 123.0 (57.9) | 72.5 (16.9) | <0.001 |
| 10-Word List, no. of words recalled after delay, n = 48 | 6.3 (1.9) (n = 29) | 7.5 (1.3) (n = 19) | 0.029 |
| WAIS Block Design | 32.5 (7.7) | 37.0 (6.0) | 0.029 |
SDs are in parentheses. Significance values are for analyses of variance taking into account family and type of mutation present in family (A431E, L235V).
MMSE = Mini-Mental State Examination; TMT = Trails Making Test; WAIS = Wechsler Adult Intelligence Scale.
To look at the effects of mutation type (A431E vs L235V), subjects were divided into those carrying and not carrying PS1 mutations. T tests comparing scores of persons from families with the A431E mutation (n = 40; 25 MCs and 15 NCs) with those from the family with the L235V (n = 11; 5 MCs and 6 NCs) mutation showed that NCs from the L235V family tended to score more poorly than NCs from families with the A431E mutation on Verbal Categorical and Letter Fluency. Conversely, MCs from the L235V family tended to score more highly on the WMS Mental Control task than MCs from families with the A431E mutation. On the two-way ANOVAs, mutation type continued to have an influence on performance of the WMS Mental Control task and Categorical Fluency, although the interaction terms were not significant.
When multiple linear regression analyses taking into account relative age, gender, BDI scores, years of education, and number of APOE ε4 alleles were performed, significant differences were still seen between PS1 MCs and NCs on the MMSE (p = 0.005), time to complete the Trails Making Test B (p = 0.003), and Delayed Recall of the 10-Word List (p = 0.041). Relative age had a significant impact on MMSE score, time to perform the Trails Making Test B (figure), and the WAIS Block Design score. Years of education had a significant impact on time to complete the Trails Making Test A and B. BDI score had a significant effect on time to perform the Trails Making Test A. Gender and number of APOE ε4 alleles did not have significant effects on test scores.
When t tests comparing neuropsychological performance between PS1 MCs and NCs were performed separately for the three age groups, MCs did not display worse performance than their non-mutation-carrying kin on any test in the youngest two groups. There were 17 subjects (10 PS1 MCs and 7 NCs) in the oldest tertile. Mean absolute age in this group was 38.5 years, and age in number of years prior to the typical age of dementia diagnosis in their family was 5.6 years. MCs in this group performed more poorly on the MMSE, time to complete the Trails Making Test A and B, WMS Digits Forward, the 10-Word List Immediate and Delayed Recall, and the WAIS Block Design Test (table 3).
Table 3.
Mean scores on neuropsychological tests in PS1 mutation carriers and noncarriers in the tertile closest to the age of dementia diagnosis in their family (mean age 38.5 years)
| Test | +PS1 mutation, n = 10 | −PS1 mutation, n = 7 | p |
|---|---|---|---|
| MMSE | 26.6 (1.6) | 29.0 (1.0) | 0.002 |
| TMT A, s | 61.9 (16.6) | 38.4 (12.6) | 0.005 |
| TMT B, s | 176.2 (59.9) | 77.0 (19.9) | <0.001 |
| WMS Digits Forwards | 4.2 (0.6) | 5.4 (.5) | 0.001 |
| 10-Word List, no. of words recalled immediately | 8.6 (1.2) | 9.7 (.5) | 0.014 |
| 10-Word List, no. of words recalled after delay | 5.1 (2.4) | 7.8 (1.5) | 0.014 |
| WAIS Block Design | 25.8 (7.0) | 35.0 (6.4) | 0.014 |
SDs are in parentheses. Significance values are for t tests comparing the group means.
MMSE = Mini-Mental State Examination; TMT = Trails Making Test; WAIS = Wechsler Adult Intelligence Scale.
When the four composite scores were compared between PS1 MCs and NCs by t tests, MCs tended to perform worse on the Executive Function/Working Memory Tests (p < 0.001) and the Visuospatial Tests but not on Verbal Memory Tests (p = 0.059) or Language Tests (p = 0.930).
Discussion
In this study, PS1 MCs in the early stage of the illness performed more poorly on average than their non-mutation-carrying kin on the MMSE, Delayed Recall of a 10-Word List, and the WAIS Block Design Test and took longer on the Trails Making Test A and B. These differences were maintained when family of membership and mutation type were included as co-variates. This suggests that impairment on these tests occurs consistently across families and these two PS1 mutations. This demonstrates that executive function and delayed verbal memory are affected relatively early in PS1-related AD. The cognitive changes in this form of AD found in this cross-sectional study are broadly consistent with what has been previously found in early sporadic AD of the elderly using prospective and case-control methods.2
Consistent with studies in older populations,24 PS1 MCs had worse verbal memory function as measured by Delayed Recall of a 10-Word List. However, the Verbal Memory composite score did not differentiate the two groups. As prior studies25 have suggested that delayed recall is affected preferentially to immediate recall in early AD, this might be because only the Immediate Recall portions of the other two tests composing this score (Paired Associates and Logical Memory) were administered. This is consistent with previous studies of persons at risk for autosomal dominant AD in which verbal memory was affected early19 and in those that later developed more global deterioration.26 This early impairment of a form of episodic memory likely reflects the early involvement of medial temporal lobe structures as has been demonstrated to occur in both sporadic and autosomal dominant27 forms of AD.
Time to perform the Trails Making Test A and in particular B was longer in the PS1 MCs than NCs. These tasks involve multiple cognitive processes (attention, psychomotor speed, visuospatial abilities, etc.), but Trails B is felt to be especially sensitive to deficits in executive function. Deficits in executive function and full-blown frontotemporal behavioral presentations have been described in association with PS1 mutations.13 Unfortunately, there was a paucity of tests that specifically measure executive function in our battery, but it is worth noting that of those tests that had an executive component, PS1 mutation carriers had impaired performance on some (WAIS Block Design) but not all (Category and Letter Fluency) of them. Other investigators have demonstrated that verbal fluency, particularly category fluency,28 is affected early in the course of AD. Whether spared verbal fluency reflects spared dominant frontal lobe involvement or is due to linguistic, cultural, or educational cofounders in this Mexican cohort is uncertain. Further evidence for deficits in frontal lobe function is that MCs had lower Executive Function/Working Memory composite scores. Further studies of this population with tests more sensitive to and specific for executive dysfunction that are relatively free of a linguistic component (Stroop Interference Test, Wisconsin Card Sort Test) would help delineate the nature of the deficits that occur early in PS1 MCs.
Deficits in visuospatial function are often found to occur early in AD. However, tests presumed to measure this are diverse, and the presence or absence of such deficits depends critically on the test used.29 MCs performed more poorly on the Block Design Subtest of the WAIS and the Visuospatial Composite score. The Block Design Test requires both perceptual and executive abilities, and performance on this test has been shown to be affected early in the course of AD,30 an AD variant with frontal features,31 early onset AD,32 and AD associated with Down syndrome.33 Our finding of early deficits in the WAIS Block Design in PS1 MCs is consistent with these studies.
There appear to be differences in clinical presentation between persons affected by the A431E and L235V PS1 mutations, with the A431E mutation causing a more severe form of the disease with a younger age at onset sometimes accompanied by spastic quadriparesis (unpublished observations). There could thus be differences in the nature of the cognitive deficits produced by these mutations. The analyses in which cognitive scores of the PS1 MCs and NCs from families with the A431E mutation were compared with those from the L235V family suggested that persons without mutations in the L235V family performed worse at Verbal Fluency and those with mutations from the A431E family performed more poorly on the Mental Control task than their respective comparison groups. The lack of significant interactions between mutation status and type, however, militates against the likelihood of measurable differences in the cognitive deficits caused by these distinct mutations. However, our study, in which the L235V PS1 mutation was represented by one family of 11 subjects of different ages, is likely to be underpowered to pick up such cognitive differences should they exist.
When ANOVAs comparing MCs and NCs were performed co-varying for the L235V and A431E mutations, carriers of the L235V mutation were found to perform more poorly on Immediate Recall of verbal paired associates and on Categorical Fluency. It does not seem that the L235V mutation is associated with more severe aphasia as this does not seem to be the case clinically and no differences were seen on other language measures such as the Boston Naming Test. Though a direct effect of the mutation on these outcomes cannot be ruled out, it is possible that non-PS1 genetic factors or environmental factors account for these differences. Of note, the family harboring the L235V mutation resides in a geographically and economically distinct part of Mexico.
The presence of the APOE ε4 allele did not significantly influence cognitive performance. A recent study demonstrated that persons with both a PS1 mutation and the APOE ε4 allele tended to have a younger age at disease onset than PS1 MCs without an ε4 allele.34 Therefore, though the ε4 allele likely exerts an effect on disease progression in PS1 mutation carriers, this effect is relatively small and could not be detected in our study.
Despite the clear status of the older PS1 MCs as showing early cognitive decline, it is possible that concurrent depression in this group could be a variable confounding their cognitive performance. Depression can influence performance on neuropsychological tests and has also been shown to be associated with both early sporadic AD35 and early familial AD.36 Our behavioral assessments were limited to asking subjects if they had ever seen a psychiatrist or psychologist and by having them fill out the BDI. Five of 30 MCs (16.7%) and 2 of 21 NCs (9.5%) reported seeing a mental health professional in the past (two-sided Fisher exact test, p = 0.685). On the BDI, PS1 MCs tended to score higher than NCs, particularly in the oldest tertile (13.0 vs 6.7; p = 0.082). Though a self-rated assessment such as the BDI has limitations in an AD population in which insight into affective state can be diminished,37 because of the pre or early stage of illness in our subjects, we feel that it is an adequate measure of mood. In the multiple regression analysis, subjective depression as measured with the BDI influenced only time to perform the Trails Making Test A (p = 0.005). Though this might reflect psychomotor retardation, it could also reflect the chance association of two co-dependent variables. The lack of an effect of BDI scores on the remaining cognitive outcomes makes it unlikely that depression was a major influence on neuropsychological function in our study.
We expected the age of MCs in relation to the typical age of dementia diagnosis in their family to affect cognitive function. This relative age had a significant impact on MMSE score, time to perform the Trials Making Test B, and the WAIS Block Design score. When subjects were divided into subgroups according to relative age, PS1 mutation carriers performed no worse than NCs on any test in the youngest two tertiles. However, in the oldest group of subjects, deficits were seen on tests of Psychomotor Speed, Attention and Divided Attention, Verbal Memory, and a visuospatial test of Executive Function. These subjects were on average 5.5 years younger than the typical age at dementia diagnosis in their family. It therefore seems that significant cognitive deficits are not present from a young age in these subjects despite the life-long presence of the PS1 mutation. This differs from the results of a study of persons at risk for frontotemporal dementia via a pathogenic mutation in the tau gene (FTD-17)38 in which deficits in verbal fluency, the Stroop Interference Test, Digit-Symbol Substitution, and the Trails Making Test B were found early in MCs and did not correlate with age. This might represent fundamental differences between the pathogenesis of these two illnesses in that FTD-17 might be a neuro-developmental disorder, whereas PS1-related AD occurs as a result of the accumulation of β-amyloid deposits over years.
This study has several limitations. We did not systematically collect information regarding subjects’ cognitive, social, occupational, or activities of daily living performance from unrelated surrogates, largely because of unavailability. Such collateral history would have allowed more objective assessment of subjects’ functional abilities. Instead, we restricted our study population to those who were younger than the median age at disease diagnosis in their family and were not yet functionally impaired by their own report. This provided for a study population that, though not completely “presymptomatic” or “preclinical,” was restricted to persons in the earliest stages of the disorder.
Though a large number of subjects from families with the A431E substitution participated in this study, five of these families were represented by only one to three subjects. Because of possible co-variance contributing to performance on neuropsychological tests that is associated with family of membership, it would be optimal to balance groups in regard to this factor in a study such as this. Nine of the 10 families in this report had the same nucleotide substitution in the PS1 gene, and genetic analyses suggest a common founder for this mutation.14 Their common genetic background would be expected to control for some of the intellectual differences between families. When ANOVAs co-varying for family of membership and specific mutations were performed, cognitive differences were still found between PS1 MCs and NCs.
There are important factors that limit the general-izability of these findings. The specific versions of the tests that were employed, though thoroughly studied in their English forms in English-speaking populations, have not been validated in a Mexican population. This does not affect the internal validity of our study, but it can reduce the applicability of our findings in identifying early AD in Mexicans as important socioeconomic, educational, and other differences may exist between our study group and the general population. Furthermore, the deficits revealed with these tests in the PS1 MCs in this study may not directly equate to the kind of deficits one might encounter in persons from another cultural or language group.
Despite the limitations of retrospective case-control studies and prospective longitudinal studies, they give important information in that they directly characterize the more common late-onset form of AD. The extent to which cognitive changes in PS1-related AD can be generalized to “sporadic” AD of late onset is uncertain as PS1-related AD can have important clinical differences from sporadic AD. As prior cognitive studies have shown similar deficits in concentration, slowed psychomotor performance, and verbal abilities including verbal memory in familial AD and sporadic AD39 and the frontal deficits that we observed have been described by others in sporadic AD,31 we believe that the study of persons at risk for the inheritance of PS1 mutations can provide valuable insights into early AD.
In our study of persons in the preclinical stage of PS1-related AD, we found early deficits in executive function, delayed recall of verbal material, visuospatial function, and performance on the MMSE in PS1 MCs. These were not evident until subjects were within 5 or 10 years of the typical age at dementia diagnosis in their family. In this study, we did not find that APOE genotype, specific PS1 mutation, gender, or degree of subjective depression had a substantial effect on neuropsychological test scores in this population.
Acknowledgments
The authors thank Jennifer Dunkin, PhD, V.A. Elderkin-Thompson, PhD, and Po Lu, PhD, for comments on this manuscript and Dan Tartre for help with data collection.
Supported by Alzheimer’s Disease Research Center Grants AG-16570, AG-10133, AG-16573, and PHS R01 AG-21055 from the National Institute on Aging, an Alzheimer’s Disease Research Center of California Grant, and the Sidell Kagan Foundation. Dr. Ringman is supported by Alzheimer’s Association New Investigator Research Grant 01-2797 (PHS K08 AG-22228) and the Shirley and Jack Goldberg Trust.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Small DH. Alzheimer’s disease biomarkers: their value in diagnosis and clinical trials. Front Biosci. 2002;7:986–988. doi: 10.2741/A824. [DOI] [PubMed] [Google Scholar]
- 2.Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry. 2001;58:853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- 3.Pirozzolo FJ, Christensen KJ, Ogle KM, Hansch EC, Thompson WG. Simple and choice reaction time in dementia: clinical implications. Neurobiol Aging. 1981;2:113–117. doi: 10.1016/0197-4580(81)90008-7. [DOI] [PubMed] [Google Scholar]
- 4.Baddeley AD, Baddeley HA, Bucks RS, Wilcock GK. Attentional control in Alzheimer’s disease. Brain. 2001;124:1492–1508. doi: 10.1093/brain/124.8.1492. [DOI] [PubMed] [Google Scholar]
- 5.Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 6.Sherrington R, Froelich S, Sorbi S, et al. Alzheimer’s disease associated with mutations in presenilin 2 is rare and variably penetrant. Hum Mol Genet. 1996;5:985–988. doi: 10.1093/hmg/5.7.985. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy AM, Newman S, McCaddon A, et al. Familial Alzheimer’s disease. A pedigree with a mis-sense mutation in the amyloid precursor protein gene (amyloid precursor protein 717 valine to glycine) Brain. 1993;116:309–324. doi: 10.1093/brain/116.2.309. [DOI] [PubMed] [Google Scholar]
- 8.Lampe TH, Bird TD, Nochlin D, et al. Phenotype of chromosome 14-linked familial Alzheimer’s disease in a large kindred. Ann Neurol. 1994;36:368–378. doi: 10.1002/ana.410360308. [DOI] [PubMed] [Google Scholar]
- 9.Verkkoniemi A, Kalimo H, Paetau A, et al. Variant Alzheimer disease with spastic paraparesis: neuropathological phenotype. J Neuropathol Exp Neurol. 2001;60:483–492. doi: 10.1093/jnen/60.5.483. [DOI] [PubMed] [Google Scholar]
- 10.Bird TD, Sumi SM, Nemens EJ, et al. Phenotypic heterogeneity in familial Alzheimer’s disease: a study of 24 kindreds. Ann Neurol. 1989;25:12–25. doi: 10.1002/ana.410250104. [DOI] [PubMed] [Google Scholar]
- 11.Farlow M, Murrell J, Ghetti B, Unverzagt F, Zeldenrust S, Benson M. Clinical characteristics in a kindred with early-onset Alzheimer’s disease and their linkage to a G to T change at position 2149 of the amyloid precursor protein gene. Neurology. 1994;44:105–111. doi: 10.1212/wnl.44.1.105. [DOI] [PubMed] [Google Scholar]
- 12.Lopera F, Ardilla A, Martinez A, et al. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA. 1997;277:793–799. [PubMed] [Google Scholar]
- 13.Raux G, Gantier R, Thomas-Anterion C, et al. Dementia with prominent frontotemporal features associated with L113P presenilin 1 mutation. Neurology. 2000;55:1577–1578. doi: 10.1212/wnl.55.10.1577. [DOI] [PubMed] [Google Scholar]
- 14.Murrell JR, Faber K, Alonso ME, et al. The A431E presenilin 1 gene mutation associated with familial Alzheimer’s disease in individuals of Mexican descent: evidence for a founder effect. J Neuropathol Exp Neurol. 2003;62:543. [Google Scholar]
- 15.Cochran EJ, Murrell JR, Fox J, Ringman J, Ghetti B. A novel mutation in the presenilin-1 (PS-1) gene (A431E) associated with early-onset Alzheimer’s disease (AD) J Neuropathol Exp Neurol. 2001;60(5):544. [Google Scholar]
- 16.Schellenberg GD, Bird TD, Wijsman EM, et al. Genetic linkage evidence for a familial Alzheimer’s disease locus on chromosome 14. Science. 1992;258:668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- 17.Fox NC, Kennedy AM, Harvey RJ, et al. Clinicopathological features of familial Alzheimer’s disease associated with the M139V mutation in the presenilin 1 gene. Pedigree but not mutation specific age at onset provides evidence for a further genetic factor. Brain. 1997;120:491–501. doi: 10.1093/brain/120.3.491. [DOI] [PubMed] [Google Scholar]
- 18.Folstein M, Folstein S, McHugh P. “Mini-Mental State”: a practical method for grading the mental state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Olavarrieta C, Ostrosky-Solis F, Garcia de la Cadena C, Rodriguez Y, Alonso E. Neuropsychological changes in subjects at risk of inheriting Alzheimer’s disease. Neuroreport. 1997;8:2449–2453. doi: 10.1097/00001756-199707280-00008. [DOI] [PubMed] [Google Scholar]
- 20.Beck A, Ward C, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 21.Madisen L, Hoar DI, Holroyd CD, Crisp M, Hodes ME. DNA banking: the effects of storage of blood and isolated DNA on the integrity of DNA. Am J Med Genet. 1987;27:379–390. doi: 10.1002/ajmg.1320270216. [DOI] [PubMed] [Google Scholar]
- 22.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 23.Gamboa R, Hernandez-Pacheco G, Hesiquio R, et al. Apolipoprotein E polymorphism in the Indian and Mestizo populations of Mexico. Hum Biol. 2000;72:975–981. [PubMed] [Google Scholar]
- 24.Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology. 2000;55:1847–1853. doi: 10.1212/wnl.55.12.1847. [DOI] [PubMed] [Google Scholar]
- 25.Welsh K, Butters N, Hughes J, Mohs R, Heyman A. Detection of abnormal memory decline in mild cases of Alzheimer’s disease using CERAD neuropsychological measures. Arch Neurol. 1991;48:278–281. doi: 10.1001/archneur.1991.00530150046016. [DOI] [PubMed] [Google Scholar]
- 26.Fox NC, Warrington EK, Seiffer AL, Agnew SK, Rossor MN. Presymptomatic cognitive deficits in individuals at risk of familial Alzheimer’s disease. A longitudinal prospective study. Brain. 1998;121:1631–1639. doi: 10.1093/brain/121.9.1631. [DOI] [PubMed] [Google Scholar]
- 27.Fox NC, Warrington EK, Freeborough PA, et al. Presymptomatic hippocampal atrophy in Alzheimer’s disease. A longitudinal MRI study. Brain. 1996;119:2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- 28.Canning SJ, Leach L, Stuss D, Ngo L, Black SE. Diagnostic utility of abbreviated fluency measures in Alzheimer disease and vascular dementia. Neurology. 2004;62:556–562. doi: 10.1212/wnl.62.4.556. [DOI] [PubMed] [Google Scholar]
- 29.Binetti G, Cappa SF, Magni E, Padovani A, Bianchetti A, Trabucchi M. Visual and spatial perception in the early phase of Alzheimer’s disease. Neuropsychology. 1998;12:29–33. doi: 10.1037//0894-4105.12.1.29. [DOI] [PubMed] [Google Scholar]
- 30.Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol. 2001;58:411–416. doi: 10.1001/archneur.58.3.411. [DOI] [PubMed] [Google Scholar]
- 31.Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- 32.Fujimori M, Imamura T, Yamashita H, et al. Age at onset and visuocognitive disturbances in Alzheimer disease. Alzheimer Dis. 1998;12:163–166. doi: 10.1097/00002093-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Devenny DA, Krinsky-McHale SJ, Sersen G, Silverman WP. Sequence of cognitive decline in dementia in adults with Down’s syndrome. J Intellect Disabil Res. 2000;44:654–665. doi: 10.1046/j.1365-2788.2000.00305.x. [DOI] [PubMed] [Google Scholar]
- 34.Pastor P, Roe CM, Villegas A, et al. Apolipoprotein epsilon4 modifies Alzheimer’s disease onset in an E280A PS1 kindred. Ann Neurol. 2003;54:163–169. doi: 10.1002/ana.10636. [DOI] [PubMed] [Google Scholar]
- 35.Berger AK, Fratiglioni L, Forsell Y, Winblad B, Backman L. The occurrence of depressive symptoms in the preclinical phase of AD: a population-based study. Neurology. 1999;53:1998–2002. doi: 10.1212/wnl.53.9.1998. [DOI] [PubMed] [Google Scholar]
- 36.Ringman JM, Diaz-Olavarrieta C, Rodriguez Y, et al. Female preclinical presenilin-1 mutation carriers unaware of their genetic status have higher levels of depression than their non-mutation carrying kin. J Neurol Neurosurg Psychiatry. 2004;75:500–502. doi: 10.1136/jnnp.2002.005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starkstein SE, Chemerinski E, Sabe L, et al. Prospective longitudinal study of depression and anosognosia in Alzheimer’s disease. Br J Psychiatry. 1997;171:47–52. doi: 10.1192/bjp.171.1.47. [DOI] [PubMed] [Google Scholar]
- 38.Geschwind DH, Robidoux J, Alarcon M, et al. Dementia and neurodevelopmental predisposition: cognitive dysfunction in presymptomatic subjects precedes dementia by decades in frontotemporal dementia. Ann Neurol. 2001;50:741–746. doi: 10.1002/ana.10024. [DOI] [PubMed] [Google Scholar]
- 39.Rosselli MC, Ardila AC, Moreno SC, et al. Cognitive decline in patients with familial Alzheimer’s disease associated with E280a presenilin-1 mutation: a longitudinal study. J Clin Exp Neuropsychol. 2000;22:483–495. doi: 10.1076/1380-3395(200008)22:4;1-0;FT483. [DOI] [PubMed] [Google Scholar]