Abstract
Alzheimer’s disease (AD) is the most common type of dementia in the US and much of the world with rates increasing exponentially from age 65. Increases in life expectancy in the last century have resulted in a large number of people living to old ages and will result in a quadrupling of AD cases by the middle of the century. Preventing or delaying the onset of AD could have a huge impact in the number of cases expected to develop. The oldest-old are the fastest growing segment of the population and are estimated to account for 12% of the population over 65. Establishing accurate estimates of dementia and AD rates in this group is crucial for public health planning. Prevalence and incidence estimates above age 85 are imprecise and inconsistent because of the lack of very old individuals in most studies. Moreover, risk and protective factors in our oldest citizens have been studied little, and clinical-pathological correlations appear to be poor. We introduce The 90+ Study, established to address some of the unanswered questions about AD and dementia in the oldest-old. Our preliminary results show that close to half of demented oldest-old do not have known cerebral pathology to account for their cognitive deficits. Furthermore, the APOE-e4 allele appears to be a risk factor for AD only in the women in our study. In addition to the challenge of preventing and treating AD, the oldest-old will require major investigative energy to better understand the concomitants of longevity, the causes of dementia, and the factors that promote successful aging in oldest citizens.
Keywords: Aging, Alzheimer’s disease, dementia, longevity, nonagenarians, oldest-old
INTRODUCTION
In 1906, at a meeting of South-West Germany Psychiatrists, Alois Alzheimer first described the clinical and pathologic features of a 51-year-old woman with rapidly developing mental impairment [1]. Describing his observations as ‘a peculiar, little known disease process of the presenium’, he later recognized that the changes he saw in Auguste D were similar to the pathological findings in elderly individuals with “senile psychosis”. Although he published this observation in 1911 [2], the medical community did not regard Alzheimer’s disease (AD) as one of the leading causes of functional impairment and death in the elderly until the latter part of the last century [3]. One hundred years after AD was first described, we face a profound and growing epidemic of the disease throughout much of the world in people living to their 70’s, 80’s, and beyond.
THE INCIDENCE AND PREVALENCE OF AD: PROJECTIONS FOR THE 21st CENTURY
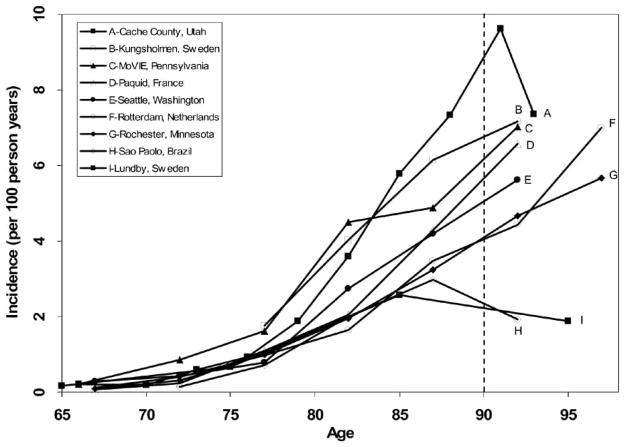
Supported by clinical and pathological data, Alzheimer’s disease is clearly the most common cause of senile dementia throughout much of the world for individuals between the ages of 65 and 85 [4]. Fig. 1 shows age-specific incidence rates of Alzheimer’s disease throughout the world. Between the ages of 65 and 85 years, studies are consistent in showing an exponential increase in the risk of developing AD. Above age 85, prevalence and incidence estimates are somewhat inconsistent, and confidence limits are very wide because of the relative paucity of individuals in this age range in most study populations. For many years, the numbers of people with AD in the US and much of the world have been rapidly growing, primarily because people are living longer -by about 30 years - than they did in Alzheimer’s day. In the United States, it is currently estimated that there are between 2 and 4 million people afflicted with AD [5, 6]. The number of dementia cases worldwide is estimated at around 24 million, most suffering from AD [7]. Projections for the middle of this century show a quadrupling of the numbers of AD cases in the US, and thus, a quadrupling of an already huge public health care burden unless we find a way to prevent or delay the onset of this disease [6].
Fig. 1.
Age-Specific Incidence Rates of Alzheimer’s Disease in Studies with Oldest-Old Participants
RISK AND PREVENTIVE FACTORS AND THE PUBLIC HEALTH IMPACT OF DELAYING DISEASE ONSET
As a strategy to develop therapeutic approaches, considerable research has been conducted in the past few decades to investigate risk and protective factors for Alzheimer’s disease. These studies have identified several putative risk and protective factors. For example, the e4 allele of the Apolipoprotein E gene (APOE) has emerged as a clear genetic susceptibility factor for Alzheimer’s dementia [8], although it has not resulted in effective therapeutic strategies, thus far. Numerous observational studies have reported putative protective effects in relation to dietary intake and nutritional supplementation (fish oils [9], folate [10] and vitamins E and C [11]); mental [12] and physical exercise [13]; and medications including estrogen therapy [14], non-steroidal anti-inflammatory drugs [15], and statins [16]. Unfortunately, the limited randomized clinical trials to date have not supported a role for these agents in the prevention of AD [17, 18], but more studies need to be done.
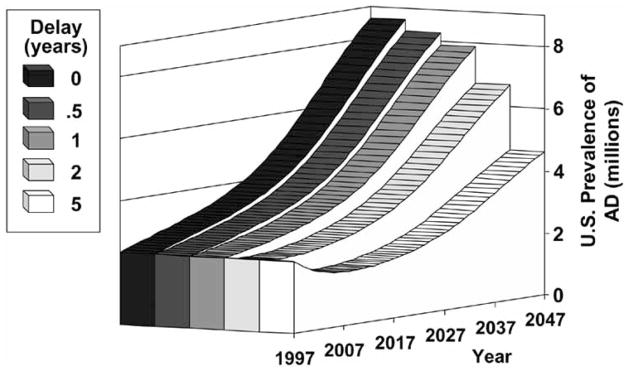
Randomized prevention trials are the most reliable study designs to control for biases and the effects of confounding variables inherent to observational studies. Such trials need to be large to detect moderate effects and to account for the high mortality and attrition rates in elderly subjects. The resources needed to conduct prevention-related research are large, but the costs are small in comparison with the long-term economic and social costs of delaying disability even modestly in an increasingly aging population [6]. Brook-meyer and colleagues calculated projections of Alzheimer’s disease in the United States and the impact of delaying disease onset by 6 months, 1, 2, and 5 years, as shown in Fig. 2 [6]. If interventions could delay onset of the disease by 2 years, after 50 years there would be nearly 2 million fewer cases than currently projected. This potential effect size would be predicted if any of the putative protective factors in observational studies were to be confirmed in clinical trials with protective effects of similar magnitude.
Fig. 2.
Potential Effect on Prevalence of Interventions to Delay Onset of Alzheimer’s Disease. Adapted from Brookmeyer, Gray, & Kawas [6]
INCREASES IN LIFE EXPECTANCY
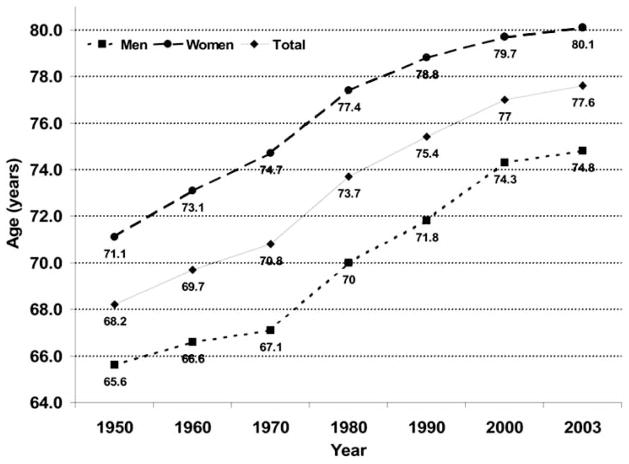
Over the past century, life expectancy has increased by about 30 years [19]. Remarkably, progress in this area continues today with an increase of almost six months over the past three years alone (Fig. 3). Although the gap is closing, women continue to significantly outlive men. By age 70 approximately 1½ women are alive for every man; after age 90, the ratio is more than three women for each man. Reasons for this difference in longevity by gender are unknown, but notably, in all species where there is a difference in longevity, it appears to be the case that the female lives longer. A once rare group, centenarians in the United States now number 50,454 [20] as compared to 4,447 in 1950 [21]. The limits of the human lifespan are poorly understood, but it is likely that life expectancy will continue to increase in the coming decades, providing an ever-growing number of the oldest-old.
Fig. 3.
Estimated Life Expectancy at Birth in the United States, 1950–2003. (Source: National Vital Statistics Report [19]).
THE OLDEST-OLD IN THE POPULATION
Currently there are approximately 2 million people aged 90 or older in the United States. This number is expected to increase strikingly to almost 10 million by the middle of the century (Fig. 4). Projected by the United Nations, there will be over 14 million people aged 90+ in China, almost 8 million in India, and about 6 million in Brazil [22]. While at present, the oldest-old represent about 0.5% of the population in the United States [20], by the middle of the century they will represent 2.5% of the U.S. population [23]. In countries such as Japan, the statistics are even more impressive with almost 5.5% of the population anticipated to be aged 90 or over by the middle of this century [22]. Similar trends in Italy, France, and Germany will result in 3.9, 3.1, and 2.8% of their populations over age 90. It should be noted that these changes in the population structure are related to declines in birth rates, perhaps even more so than changes in mortality. For example, a significant amount of the projected changes in countries like Japan reflect the results of family planning, which halved birth rates in a short period of time [24]. Nonetheless, the absolute number of people greater than 90 continues to increase.
Fig. 4. Oldest-Old Population in the United States from 1950 to 2050.
(Source: US Census Bureau 1950–2000, Population Projections US Census Bureau 2002 [23])
Not only are the oldest-old a growing percentage of the total population, but they are also an increasingly larger percentage of the elderly population (those aged 65 and older). In the US in 1950, those aged 90 and older constituted 1.8% of the elderly, in 1990 they were 3.5% of the elderly, by 2050 those aged 90 and older are estimated to constitute 12% of the elderly population [23]. While the population as a whole is aging, the elderly population itself is aging as well.
This aging of the population also creates a public health problem in terms of the number of people available to care for these oldest-old individuals. In the United States, the ratio of potential caregivers (people aged 20 to 64) to care receivers (people aged 90+) is expected to decrease dramatically in the next few decades. For example, in 2000, for every person aged 90+ there were 98 people aged 20 to 64. By the year 2050, however, it is estimated that there will be 22 people in the potential caregiver age range for every 90+ year old. And this does not account for the millions of people with AD, other dementias, and chronic diseases who are below age 90 and also require caregiving. The disparity between the public health burden and the number of those to provide care is growing ever wider.
STUDIES OF DEMENTIA AND ALZHEIMER’S DISEASE IN THE OLDEST-OLD
While it is easy to assume that Alzheimer’s disease continues to affect an increasing percentage of people in their tenth and eleventh decades, this has not been clearly demonstrated. Estimates of dementia and AD in 85-year-olds suggest that between 1/5 and 1/2 of the population suffer from dementia, primarily of the AD type [25–28]. Although the risk of dementia and AD double with every five years of life from age 65 until age 85 [29], we do not know if this exponential rise continues into the tenth and eleventh decades. Although some studies have suggested persistent rise in incidence and prevalence, other studies have suggested a leveling off, or even a decline, in the risk of developing this degenerative cognitive disorder (Fig. 1). If the risk of developing Alzheimer’s disease and other dementing illnesses doubles with every five years of life after the age of 85, the public health burden will be quite different than if the incidence of these disorders levels off or decreases. Important for public health planning, the prevalence and incidence of dementing and other illnesses in the oldest-old remain unknown.
CLINICAL-PATHOLOGICAL CORRELEATIONS IN THE OLDEST-OLD
In the oldest-old, clinical pathological correlations in patients with dementia have been inconsistent. Several investigators have noted that close to half of demented individuals in the tenth decade are likely to have no obvious pathology to explain their cognitive and functional loss [30, 31]. Conversely, approximately one of every three non-demented individuals at age 85 have been reported to have senile plaques and neurofibrillary tangles at autopsy, suggesting a pathological diagnosis of AD despite the lack of clear clinical dementia syndrome [31–33]. Initial results in a small group of centenarians also showed that about one third of non-demented participants meet pathological criteria for AD [34]. Not all studies, however agree on this issue. For example, in a small sample of non-demented, optimally healthy 85+ year olds, AD lesions were infrequent [35]. Thus, clinical pathological correlations and dementia etiology in the oldest-old is an area that requires further investigation.
RISK AND PROTECTIVE FACTORS FOR DEMENTIA AND AD IN THE OLDEST-OLD
Although APOE has emerged as a clear genetic susceptibility factor for Alzheimer’s dementia in younger subjects, it is not clear if it continues to play a significant role as individuals reach their tenth decade. Some studies of participants aged 85+ found the presence of an e4 allele to be related to prevalent AD [36–38] but this was not the case in a study of Finnish centenarians [39]. Furthermore, it is not known if agents that potentially confer protection in younger subjects such as folate, non-steroidal anti-inflammatory drugs, statins, antioxidants, estrogen therapy, and physical and cognitive activities are operative for individuals in extreme old age. These will be crucial areas of investigation during the coming century as we deal with the growing number of individuals in this age group.
THE 90+ STUDY
Recognizing the dearth of knowledge about a rapidly growing segment of the population, we established The 90+ Study in 2003. All participants of The 90+ Study are survivors from the Leisure World Cohort Study originally recruited between 1981–1985 by investigators from the University of Southern California (USC) [40–42]. The original group included 13,978 people (8,877 women; 5,101 men) with an average age of 73 years. Over the next 25 years, these participants completed follow-up mail surveys with questions about their diets, vitamins, medications, health, and activities.
In collaboration with Dr. Annlia Paganini-Hill of USC, we traced the subjects of the original cohort, inviting qualified participants to join this longitudinal prospective epidemiologic study. As of January 1, 2003, there were 1,150 participants of the original Leisure World Cohort who were alive and aged 90 or older. This group makes up approximately 1% of the entire population of 90+ year olds in the US. Of the original 1,150 eligible people, we have enrolled 938 subjects in The 90+ Study.
Our primary goals in The 90+ Study are to prospectively examine factors associated with longevity and dementia, to determine dementia prevalence and incidence rates and types of dementia, to measure rates of cognitive and functional decline, and to develop DNA and brain tissue banks for future collaborations and studies in the oldest-old. Thus far, we have collected DNA samples in approximately 500 of the participants, and have established cell lines for more than 300 individuals. Remarkable for an epidemiological study, 13% of the participants have agreed to brain autopsy, and 48 autopsies have been completed.
PRELIMINARY RESULTS FROM THE 90+ STUDY
Overall Descriptives
The average age at first evaluation of the 938 participants in The 90+ Study was 94 years (range: 90–106) and 77% were women. While many of them still lived at home alone (28%) a significant portion lived in assisted living or other group quarters (29%) and 15% lived in a nursing home.
A history of high blood pressure was present in almost half of the participants (47%), heart disease (coronary artery disease, myocardial infarction, atrial fibrillation, or congestive heart failure) in 40%, thyroid disease in about one quarter (26%), and clinical stroke in 15%. Depression was reported by 19% and anxiety only by 8% of 90+ participants. While diabetes was relatively infrequent (6%) a history of cancer was reported by more than one third (39%) of 90+ year olds.
More than half of 90+ participants reported having suffered a fall in the previous year (53%) and most participants reported using an assistive device for walking (71%). Half of all 90+ year olds used the help of a paid caregiver, either full- or part-time (51%).
In terms of cognitive performance, about one third of all participants were considered to have normal cognition (32%), another third met criteria for dementia (32%), and the remaining third had cognitive deficits that were not severe enough to meet criteria for dementia (36%). The most common type of dementia, as judged by a neurological examiner, was AD (71%).
Apolipoprotein E as a Risk Factor for Dementia or AD
Previously published studies of Caucasian populations have reported allelic frequencies for the APOE gene at about 8%, 76%, and 16% for the alleles e2, e3, and e4, respectively. In addition, the presence of the e4 allele has been described as a strong risk factor for Alzheimer’s disease and dementia in general. Most studies, however, have included very small numbers of very old subjects. In our study, we found the APOE-e4 allele to be a risk factor in women, but not in men, with clinically-diagnosed AD1. Our study was done in the first 403 participants examined in-person in The 90+ Study. Dementia was determined by the neurological examiner applying DSM-IV criteria for dementia [43] and NINCDS-ADRDA criteria for AD [44].
APOE genotype was available for 342 of the 403 participants (85%). Most participants were women (70%) and Caucasian (100%). The average age was 94.7 years (range: 90 to 105). The APOE allelic frequencies were e2 = 8.2%, e3 = 81.6%, e4 = 10.2%. In the overall sample, the presence of an e4 allele was not associated with the prevalence of dementia or AD (Table 1). When stratified analyses were performed according to sex, the presence of an e4 allele was significantly associated with the prevalence of dementia and AD in women but not in men.
Table 1.
Association of the Apolipoprotein E - e4 allele with Prevalent Dementia and Alzheimer’s Disease, The 90+ Study
| Sex | APOE | Dementia | AD | ||
|---|---|---|---|---|---|
| N (%) | OR (95% CI) | N (%) | OR (95% CI) | ||
| All | e4 − | 75 (27.4) | -- | 53 (21.0) | -- |
| e4 + | 23 (33.8) | 1.53 (0.84–2.78)1 | 18 (28.6) | 1.87 (0.96–3.64)1 | |
| Men | e4 − | 14 (16.7) | -- | 9 (11.4) | -- |
| e4 + | 1 (5.3) | 0.28 (0.03–2.24)2 | 1 (5.3) | 0.42 (0.05–3.58)2 | |
| Women | e4 − | 61 (32.1) | -- | 44 (25.4) | -- |
| e4 + | 22 (44.9) | 2.21 (1.12–4.35)2 | 17 (38.6) | 2.64 (1.24–5.61)2 | |
APOE = Apolipoprotein E; e4 − = no e4 alleles; e4 + = 1 or more e4 alleles; OR = odds ratio; 95%CI = 95% Confidence Interval
Logistic regression adjusted for age and gender
Logistic regression adjusted for age
Our study showed the e4 allelic frequency to be lower in this sample of oldest-old participants with an equivalent increase in the e3 allelic frequency, when compared to studies of younger subjects. Moreover, the e4 allele appears to be a risk factor for dementia and AD only in oldest-old women. Additional clinical and pathological studies in relation to APOE in the oldest-old will be of interest.
Clinical and Pathological Agreement
Braak & Braak [45] staging of β-amyloid plaques and neurofibrillary tangles (NFT) has been associated with the presence and severity of dementia in older individuals, but the few studies in the oldest-old do not consistently support this finding. Our initial results2 showed a poor correlation between a clinical diagnosis of dementia and pathological abnormalities.
Preliminary results in the first 29 participants to come to autopsy are shown below. All available data was reviewed to arrive at a consensus clinical diagnosis, using DSM-IV criteria for dementia [43] and NINCDS-ADRDA criteria for AD [44]. Plaque and tangle staging was done with Braak & Braak criteria [45]. The 29 participants (7 men and 22 women) died between the ages of 92 and 105 (mean: 96.3). Nineteen of these participants were diagnosed as having dementia at the time of their last evaluation, which was on average 3.7 months before death (range: 0.2–16.1). The interval from last evaluation to death and age at death were similar in the demented and non-demented groups. Brain weight was lower in the demented group as were cognitive scores at last evaluation.
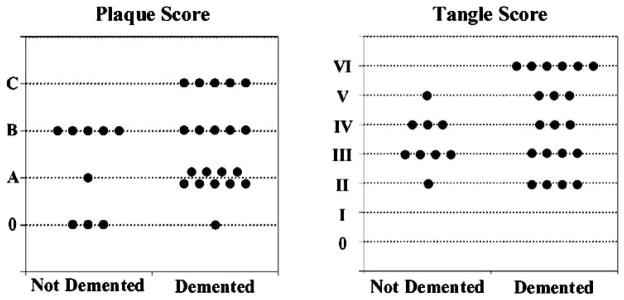
Fig. 5 shows the distribution of plaque and tangle scores for the demented and non-demented groups. Great overlap is seen in the distributions of plaques and tangle scores of the two groups and statistical analyses showed no differences in the plaque and tangle scores of demented and non-demented participants. Of interest, all participants had some degree of NFT and none had a tangle stage less than II.
Fig. 5.
Distribution of Braak & Braak Plaque and Tangle Scores for Demented and Non-Demented Participants, The 90+ Study
When participants were classified according to agreement between clinical and pathological diagnosis, we had three similar sized groups (Fig. 6). None of the participants in the non-demented group had sufficient pathology to meet any of the standard pathological criteria, and thus are designated as “Not-Demented Insufficient Pathology” (NDIP). In the demented group, there were two distinct groups. The first group did not have sufficient pathology to meet any of the pathological criteria, designated here as “Demented Insufficient Pathology” (DIP). The second group met pathological criteria for Alzheimer’s disease, designated here as “Demented Alzheimer Pathology” (DAP). Thus, 9 out of 20 (45%) demented participants did not have pathology that would account for their dementia.
Fig. 6.
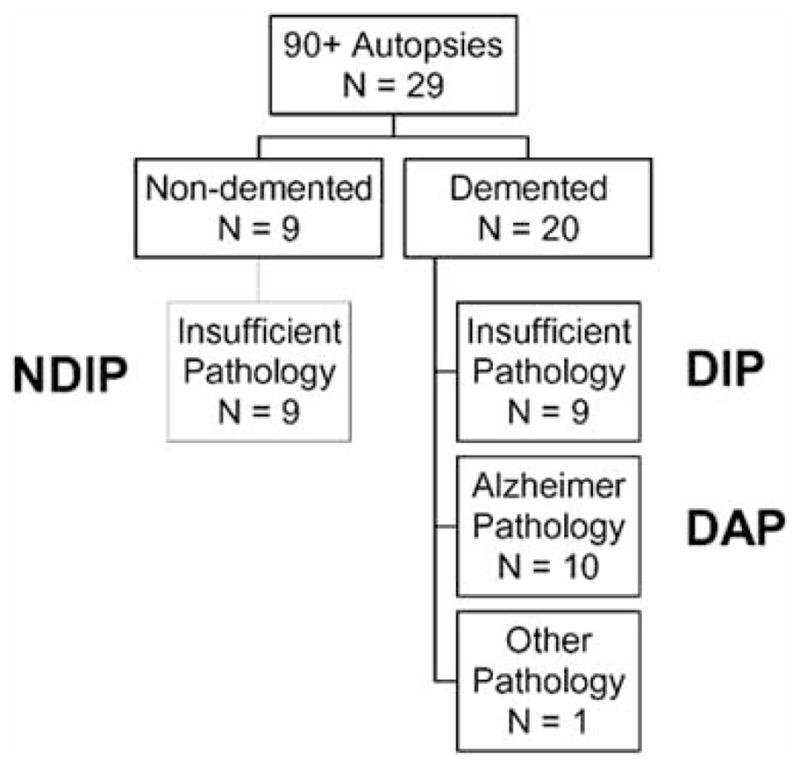
Classification of Autopsied Participants by Clinical and Pathological Diagnosis, The 90+ Study
NDIP = Not Demented Insufficient Pathology; DIP = Demented Insufficient Pathology; DAP = Demented Alzheimer Pathology
To identify features that could help distinguish the groups, we compared clinical characteristics and past medical histories, including hypertension, diabetes, thyroid disease, cardiac diseases (myocardial infarction, arrhythmia, congestive heart failure), stroke, and transient ischemic attack (TIA)) (Table 2). Clinical dementia diagnoses were more varied in the DIP group, perhaps reflecting atypical features and the difficulty of diagnosing dementia in this group. Mini-Mental scores [46] were very different in the 3 groups, with the DAP group having more severe dementia (median score = 0) as compared to the DIP group (median score = 17). There was also a difference in duration of disease, with the DIP group dying sooner after a diagnosis than those in the DAP group, even though age at death was not different. A very interesting finding was that the brain weight of the two demented groups was very similar, and lower, than the NDIP group, even when the NDIP and the DIP groups had similar levels of pathology. There were no significant differences in any of the medical histories except for a self-report of TIA’s, which were more frequent in the DIP group than the DAP group.
Table 2.
Characteristics of Autopsied Participants by Clinical Pathological Groups, The 90+ Study
| Not Demented Insufficient Pathology (NDIP) | Demented Insufficient Pathology (DIP) | Demented AD Pathology (DAP) | NDIP vs DIP | DIP vs DAP | |
|---|---|---|---|---|---|
| p-value1 | |||||
| Clinical Diagnosis | 6 Normal 3 CIND |
5 AD 2 VaD 1 AD/VaD 1 Unknown |
9 AD 1 AD/VaD |
||
| Median (IQR) | |||||
| MMSE Score | 27 (24–28) | 17 (11–22) | 0 (0–4) | <0.01 | <0.01 |
| Age at death (years) | 96 (95–97) | 94 (93–96) | 95 (92–96) | >0.2 | >0.20 |
| Age at onset (years) | - | 90 (89–91) | 88 (84–92) | - | >0.20 |
| Age at diagnosis (years) | - | 92 (91–95) | 90 (87–93) | - | 0.08 |
| Diagnosis to death (years) | - | 2 (1–4) | 6 (4–9) | - | <0.01 |
| Brain weight (g) | 1199 (1169–1253) | 1086 (946–1192) | 1056 (982–1156) | 0.05 | >0.20 |
| Plaque stage | 2 (0–2) | 1 (1–2) | 2 (1–3) | >0.2 | 0.10 |
| Tangle stage | 3 (3–4) | 3 (2–3) | 6 (5–6) | 0.14 | <0.001 |
| Number (%) | |||||
| Female | 5 (56%) | 7 (78%) | 10 (100%) | >0.2 | >0.20 |
| Presence of APOE-e4 allele | 0 | 1 (14%) | 2 (25%) | >0.2 | >0.20 |
| History of TIA’s | 0 | 6 (67%) | 1 (14%) | 0.01 | 0.06 |
| Strokes in autopsy (single or multiple) | 6 (67%) | 2 (22%) | 2 (20%) | >0.2 | >0.2 |
CIND=Cognitive Impairment not demented; AD = Alzheimer’s disease; VaD=Vascular Dementia; AD/VaD = Mixed AD and Vascular Dementia; MMSE=Mini-Mental State Exam; TIA’s=Transient Ischemic Attacks; IQR = Interquartile Range; APOE = Apolipoprotein E
p-values are from Fisher’s exact test for proportions and from Wilcoxon Ranks Sum test for continuous variables
There are several possible interpretations for our findings. First, early AD may take less pathology in 90+ year olds and the DIP group may represent early AD. Perhaps if these individuals had lived longer, they would have developed sufficient pathology to meet current AD pathologic criteria. Another interpretation may be that the DIP group represents a dementia etiology not yet identified. Finally, there are always concerns that we may be misclassifying the DIP group as demented because they were not performing or functioning as well due to various physical problems. Against this last interpretation is the observation that there were no major differences in vision, hearing, or medical histories, and that brain weight in the DIP group was similar to the DAP group. This difference in brain weight suggests to us the presence of a degenerative process.
INVESTIGATIONS IN THE OLDEST-OLD
In addition to The 90+ Study there are other studies in the US and other countries that have been established specifically to investigate the oldest-old.
The New England Centenarian Study [47] recruits people who have reached the age of 100 in an eight-town area surrounding Boston, Massachusetts. In the Oregon Brain Aging Study [35], participants are aged 85 and over, live in the community, function independently, and have no chronic medical conditions. The Vantaa 85+ Study [31] recruited all people at least 85 years of age living in the city of Vantaa, Finland. Two Swedish studies have used a similar approach to recruiting: one recruited all 95+ year olds registered in the city of Göteborg [48], the other recruited all 90+ year old inhabitants of the Kungsholmen district [49]. As we can see, these studies have selected different methods for investigating extreme aging. Hopefully the variety of approaches and populations being studied will yield important information to begin to fill in the gap in knowledge regarding longevity, cognition, and function in the oldest-old. In recognition of this rapidly growing segment of the population even more studies designed to understand these pioneers of aging are likely to be initiated in the near future.
CONCLUSION
In the century since the first description of Alzheimer’s disease, we have been successful in extending life expectancy by about 30 years. As a result, more and more people live to older ages and develop diseases such as Alzheimer’s disease. In the coming century, we continue to face the challenge of developing effective therapies for this devastating and ever more prevalent disorder. Hopefully, current and future research will find ways to delay or prevent AD, one of the most frequent obstacles to successful aging. But the coming decades also bring a new 100-year challenge: the rapidly growing number of people who experience a century of life. Even with the development of an effective treatment or preventive strategy for AD, these approaches may not be very effective in the oldest-old because half of the cases of dementia in this age group may be due to etiologies other than AD. The oldest-old will require major investigative energy to better understand the concomitants of longevity, the causes of dementia, and factors that promote successful aging in oldest citizens. It is a major public health and medical research challenge for this century.
Acknowledgments
This research was funded by grants from the National Institutes of Health (R01CA32197 and R01AG21055) and the Al and Trish Nichols Chair in Clinical Neuroscience.
Footnotes
Corrada M, Paganini-Hill A, Maalouf M, Zuniga R, Kawas C. APOE genotype distribution and its association with prevalent dementia in the oldest-old: The 90+ Study. Abstract presented at The 9th International Conference on Alzheimer’s Disease and Related Disorders, July 17 - 22, Philadelphia, PA, (2004).
Corrada M, Head E, Kim R, Kawas C. Braak and Braak staging and dementia in the oldest-old: Preliminary results from The 90+ Study. Abstract presented at The 57th Annual American Academy of Neurology Meeting, April 9 – 16, Miami, FL, (2005).
References
- 1.Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde” (A characteristic disease of the cerebral cortex) Clin Anat. 1995;8:429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer A, Forstl H, Levy R. An English Translation of Alzheimer’s 1911 paper “Uber eigenartige Krankheitsfalle des spateren Alters” (On certain peculiar diseases of old age) Hist Psychiatry. 1991;2:71–101. doi: 10.1177/0957154X9100200505. [DOI] [PubMed] [Google Scholar]
- 3.Katzman R. The prevalence and malignancy of Alzheimer’s disease: a major killer. Arch Neurol. 1976;33:217–218. doi: 10.1001/archneur.1976.00500040001001. [DOI] [PubMed] [Google Scholar]
- 4.Fratiglioni L, De Ronchi D, Aguero-Torres H. Worldwide prevalence and incidence of dementia. Drugs Aging. 1999;15:365–375. doi: 10.2165/00002512-199915050-00004. [DOI] [PubMed] [Google Scholar]
- 5.Evans DA, Scherr PA, Cook NR, Albert MS, Funkenstein HH, Smith LA, et al. Estimated prevalence of Alzheimer’s disease in the United States. Milbank Q. 1990;68:267–289. [PubMed] [Google Scholar]
- 6.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 9.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 10.Corrada MM, Kawas CH, Hallfrisch J, Muller D, Brookmeyer R. Reduced risk of Alzheimer’s disease with high folate intake: the Baltimore Longitudinal Study of Aging. Alzh Dement. 2005;1:11–18. doi: 10.1016/j.jalz.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 12.Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 13.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 14.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 15.Szekely CA, Thorne JE, Zandi PP, Ek M, Messias E, Breitner JC, et al. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer’s disease: a systematic review. Neuroepidemiology. 2004;23:159–169. doi: 10.1159/000078501. [DOI] [PubMed] [Google Scholar]
- 16.Rockwood K, Kirkland S, Hogan DB, MacKnight C, Merry H, Verreault R, et al. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol. 2002;59:223–227. doi: 10.1001/archneur.59.2.223. [DOI] [PubMed] [Google Scholar]
- 17.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 18.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 19.Arias E. National Vital Statistics Report. National Center for Health Statistics; Hyattsville, Maryland: 2004. United States Life Tables, 2002. [PubMed] [Google Scholar]
- 20.US Census Bureau. Census 2000 Summary File 2, United States. 2001. [Google Scholar]
- 21.Krach CA, Velkoff VA. Centenarians in the United States. U.S. Government Printing Office; Washington, DC: 1999. [Google Scholar]
- 22.United Nations Population Division. World Population Prospects: The 2004 Revision Population Database. 2004 http://esa.un.org/unpp.
- 23.US Census Bureau. Population Projections (Middle Series), United States. 2002. [Google Scholar]
- 24.Davies AM. Epidemiology and the challenge of aging. In: Brody JA, Maddox GL, editors. Epidemiology and Aging: An International Perspective. Springer Publishing Co; New York: 1988. pp. 3–23. [Google Scholar]
- 25.Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, et al. Prevalence of Alzheimer’s disease in a community population of older persons: Higher than previously reported. JAMA. 1989;262(18):2551–2556. [PubMed] [Google Scholar]
- 26.Skoog I, Nilsson L, Palmertz B, Andreasson LA, Svanborg A. A population-based study of dementia in 85-year-olds. N Engl J Med. 1993;328(3):153–158. doi: 10.1056/NEJM199301213280301. [DOI] [PubMed] [Google Scholar]
- 27.Heeren TJ, Lagaay AM, Hijmans W, Rooymans HG. Prevalence of dementia in the ‘oldest old’ of a Dutch community. J Am Geriatr Soc. 1991;39:755–759. doi: 10.1111/j.1532-5415.1991.tb02696.x. [DOI] [PubMed] [Google Scholar]
- 28.Ankri J, Poupard M. Prévalence et incidence des démences au grand âge. Analyse de la littérature. Rev Epidemiol Sante Publique. 2003;51:349–360. (full text in English on www.e342med.com/resp) [PubMed] [Google Scholar]
- 29.Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728–733. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- 30.Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch Neurol. 2000;57:713–719. doi: 10.1001/archneur.57.5.713. [DOI] [PubMed] [Google Scholar]
- 31.Polvikoski T, Sulkava R, Myllykangas L, Notkola IL, Niinisto L, Verkkoniemi A, et al. Prevalence of Alzheimer’s disease in very elderly people: a prospective neuropathological study. Neurology. 2001;56:1690–1696. doi: 10.1212/wnl.56.12.1690. [DOI] [PubMed] [Google Scholar]
- 32.Katzman R, Terry RD, DeTeresa R, Brown T, Davies P, Fuld P, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 33.Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, et al. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988;38:1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 34.Silver MH, Newell K, Brady C, Hedley-White ET, Perls TT. Distinguishing between neurodegenerative disease and disease-free aging: correlating neuropsychological evaluations and neuropathological studies in centenarians. Psychosom Med. 2002;64:493–501. doi: 10.1097/00006842-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Green MS, Kaye JA, Ball MJ. The Oregon brain aging study: neuropathology accompanying healthy aging in the oldest old. Neurology. 2000;54:105–113. doi: 10.1212/wnl.54.1.105. [DOI] [PubMed] [Google Scholar]
- 36.Sulkava R, Kainulainen K, Verkkoniemi A, Niinisto L, Sobel E, Davanipour Z, et al. APOE alleles in Alzheimer’s disease and vascular dementia in a population aged 85+ Neurobiol Aging. 1996;17:373–376. doi: 10.1016/0197-4580(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 37.Gessner R, Reischies FM, Kage A, Geiselmann B, Borchelt M, Steinhagen-Thiessen E, et al. In an epidemiological sample the apolipoprotein E4 allele is associated to dementia and loss of memory function only in the very old. Neurosci Lett. 1997;222:29–32. doi: 10.1016/s0304-3940(97)13334-1. [DOI] [PubMed] [Google Scholar]
- 38.Skoog I, Hesse C, Aevarsson O, Landahl S, Wahlstrom J, Fredman P, et al. A population study of apoE genotype at the age of 85: relation to dementia, cerebrovascular disease, and mortality. J Neurol Neurosurg Psychiatry. 1998;64:37–43. doi: 10.1136/jnnp.64.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sobel E, Louhija J, Sulkava R, Davanipour Z, Kontula K, Miettinen H, et al. Lack of association of apolipoprotein E allele epsilon 4 with late-onset Alzheimer’s disease among Finnish centenarians. Neurology. 1995;45:903–907. doi: 10.1212/wnl.45.5.903. [DOI] [PubMed] [Google Scholar]
- 40.Paganini-Hill A, Ross RK. Reliability of recall of drug usage and other health-related information. Am J Epidemiol. 1982;116:114–122. doi: 10.1093/oxfordjournals.aje.a113386. [DOI] [PubMed] [Google Scholar]
- 41.Paganini-Hill A, Chao A, Ross RK, Henderson BE. Exercise and other factors in the prevention of hip fracture: the Leisure World study. Epidemiology. 1991;2:16–25. doi: 10.1097/00001648-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Paganini-Hill A, Hsu G, Chao A, Ross RK. Comparison of early and late respondents to a postal health survey questionnaire. Epidemiology. 1993;4:375–379. doi: 10.1097/00001648-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 43.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 44.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 45.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 46.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 47.Perls TT, Bochen K, Freeman M, Alpert L, Silver MH. Validity of reported age and centenarian prevalence in New England. Age Ageing. 1999;28:193–197. doi: 10.1093/ageing/28.2.193. [DOI] [PubMed] [Google Scholar]
- 48.Borjesson-Hanson A, Edin E, Gislason T, Skoog I. The prevalence of dementia in 95 year olds. Neurology. 2004;63:2436–2438. doi: 10.1212/01.wnl.0000147260.52841.27. [DOI] [PubMed] [Google Scholar]
- 49.von Strauss E, Fratiglioni L, Viitanen M, Forsell Y, Winblad B. Morbidity and comorbidity in relation to functional status: a community-based study of the oldest old (90+ years) J Am Geriatr Soc. 2000;48:1462–1469. doi: 10.1111/j.1532-5415.2000.tb02638.x. [DOI] [PubMed] [Google Scholar]