Abstract
Memories are the gifts from friends and family that stay with us forever, unless a person develops Alzheimer's disease. Leon J. Thal left many, many memories, along with his desire to create a world where people did not lose them to the ravages of dementing illnesses. Working from the bench to the clinic, he was an incomparable leader, scientist, and educator to whom many, including myself, owe much. The present description of a clinical, genetic, and pathologic study of the oldest old contains much of Leon's influence. With data from >950 subjects, a brain repository, and our collection of DNA, the investigators of the 90+ Study are receptive to collaborations. Through our collective efforts, we will continue the scientific work that Leon so strongly supported.
Keywords: Oldest old, Dementia, Alzheimer's disease
1. Introduction
The extreme elderly are the fastest-growing segment of our population. Currently numbering around 2 million, by 2050, about 10 million Americans will be aged ≥90 years [1]. Those aged >90 years will comprise almost 6% of the population in Japan, 4% in Italy, and 2.5% in the United States. The striking increase in the number of the oldest-old worldwide presents a public health challenge to promote the quality as well as quantity of life. Up to 50% of people in this age group will have dementia [2], and a greater percentage will endure functional disabilities and frailty (Fig. 1).
Fig 1.
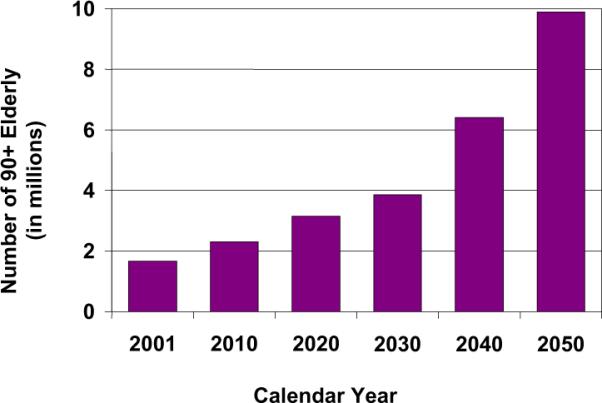
Projected United States population growth among 90+-year-olds.
Initiated in 2003, the goal of the 90+ Study (based in Laguna Woods, CA) is to perform prospective clinical, pathologic, and genetic investigations in people aged ≥90 years. With a committed cohort of elderly research participants, we have assembled possibly the largest prospective study of oldest-old subjects in the world (n = 945), and we anticipate enrolling approximately 600 additional subjects. The survivors of the Leisure World Cohort Study, begun in 1981 (average age at enrollment, 73 years), comprise the population-based sample for our studies in the oldest-old.
2. The public health impact of dementia in the oldest-old
Only a handful of publications have reported age-specific and sex-specific incidence rates for dementia at ages >90. Although several investigations suggested a decline or leveling of dementia incidence in the oldest-old [3,4] particularly for men [3–6], our study indicates a doubling of the incidence with every 5 years of age for both men and women, similar to findings for younger ages [7]. We believe that ours is the first investigation to demonstrate this consistent doubling in both sexes, similar to subjects between ages 65 and 85 years. For the rapidly growing number of people in their 90s and beyond, the risk of developing dementia appears extraordinarily high. By the middle of the century, we will have as many people with dementia in this age group as we currently have in the United States at all ages.
3. Risk factors and protective factors for dementia
Dementia risk factors and protective factors identified in younger subjects may not be relevant for the oldest-old. For example, whereas physical activity and estrogen therapy were associated with a decreased risk of dementia in the younger elderly [8–11], our preliminary investigations, with data from the 1980s, associate these factors with increased longevity [12,13], but not dementia, in people age 90 and older. Studies of risk factors in the oldest-old are scarce, but such data are necessary to identify potential factors that may lead to cost-effective interventions relevant to the health of the rapidly growing number of nonagenarians and centenarians.
4. Motor and physical performance
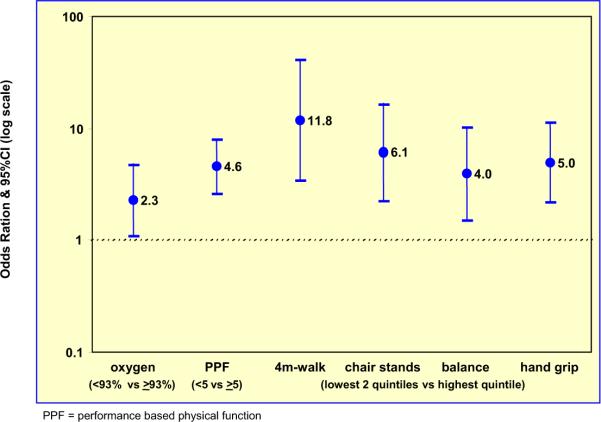
In 90+-year-olds, motor abilities (such as speed, power, and dexterity) and physical performance measures (such as timed walking and hand grip) decline with age and show a wide spectrum of abilities. Increasingly, it appears that these motor and physical declines may be associated with declines in cognition [14], and an increased risk of dementia, disability, and death. One recent study showed that the 3-year risk of dementia was increased in individuals with poor physical performance as measured by walking time, chair-stands time, standing balance, and hand grip (mean age, 73 years) [15]. Our cross-sectional preliminary study found a similar relationship in our 90+-year-old subjects (Fig. 2). The association between motor, physical-performance, and cognitive/functional declines in advanced age may reflect common pathways that may involve neurodegenerative, vascular, or other age-associated mechanisms.
Fig 2.
Odds ratios of dementia for several variables of interest in the 90+ Study.
5. Pathologic changes in the brains of the oldest-old
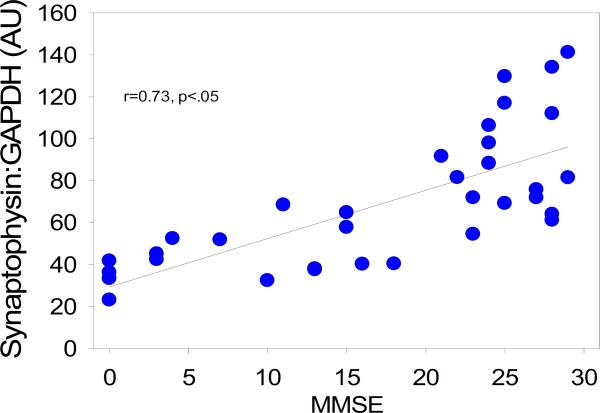
Several investigations, including our own [16], noted that the oldest-old participants who die with dementia frequently do not have the high amounts of amyloid or neurofibrillary pathology generally associated with dementia [17,18]. On the other end of the spectrum, studies found that very elderly subjects who die without overt signs of dementia frequently have significant AD pathology [17,19–21]. We have seen a handful of these cases, including a nondemented 97-year-old woman with an apolipoprotein E 2/2 genotype [22]. In our preliminary studies, we found no relationship between amyloid staging and cognition, and a weak relationship between tangle staging and cognitive status. However, brain weights in our study [16] and in others [19] appear relevant to the preservation of cognition. Of all measures studied to date, presynaptic proteins correlate most strongly with cognitive function and a diagnosis of dementia in the oldest-old [23]. Unlike amyloid and other pathologic measures, the synaptophysin protein was significantly reduced in demented subjects, and correlated with the Mini-Mental State Examination and other neuropsychological test scores, particularly those sensitive to executive function (Fig. 3).
Fig 3.
Correlation between frontal cortex synaptophysin protein level and Mini-Mental State Examination score in the oldest-old.
6. Results from the 90+ Study: factors associated with longevity
In the 13,978 members of the Leisure World Cohort, longevity was associated with:
Postmenopausal estrogen therapy: The risk of death decreased with both increasing duration and decreasing years since last use [12].
Body mass index: At study enrollment (average age, 73), body mass index (BMI) showed a reverse J-shaped relationship with mortality. Both being under-weight and obese increased the risk of mortality. Being overweight or obese at age 21 also increased mortality [24].
Alcohol: Those who drank ≥2 drinks/day had a 15% reduced risk of death; the decreased risk was not limited to one type of alcohol [25].
Caffeine: Caffeine intake exhibited a U-shaped mortality curve [26].
Physical activities: Mortality was incrementally reduced with amount of time spent in physical activities up to 45 minutes/day (a 10% to 25% reduction), after which a constant benefit was observed (a 21% to 25% reduction) [13].
Nonphysical activities: Mortality was reduced with ≥1 hours/day spent in these activities, and the magnitude of the reduction increased with additional time spent in nonphysical activities.
Intake of antioxidant vitamins (A, C, or E) was not associated with longevity [27].
7. Results from the 90+ Study: risk factors for prevalent dementia
We explored the associations between prevalent dementia and the above variables, collected as part of the baseline survey for the Leisure World Cohort Study (1981 to 1985). After adjusting for age and sex, prevalent dementia in 90+-year-olds was not related to estrogen therapy, BMI, intake of supplemental vitamins A, C, and E, total intake of vitamin A, consumption of alcohol or caffeine, or participation in physical activities. Somewhat counterintuitively, a higher total intake of vitamin C was associated with prevalent dementia (odds ratio, 1.43; 95% confidence interval, 1.06 to 1.93). A trend toward a lower prevalence of dementia was seen in participants who spent ≥3 hours a day in nonphysical activities (odds ratio, 0.60; 95% confidence interval, 0.35 to 1.06). It is premature, however, to draw conclusions from these results. Studies of risk factors in incident cases of dementia are underway.
8. Translational research, and sharing our scientific resources
The call for translational research is a call for us to develop new approaches to investigating the challenges of human health. It is overly simplistic to look at a single vitamin, pathologic feature, or lifestyle factor in relation to AD. We require new paradigms and collaborations to take advantage of the wealth of information and recent scientific and technological advances. As we work to develop new quantitative approaches and new designs for clinical trials in this age group, we invite collaborations. We have built significant scientific resources for sharing data, including large repositories of well-characterized brain tissues, extracted DNA, cell lines, and clinical data. The 90+ Autopsy Study has enrolled 155 participants, and 91 autopsies have been completed. Samples of DNA have been collected from >500 participants. To request samples or propose a collaboration with our study, contact our Resource Sharing Coordinator via e-mail (mcorrada@uci.edu). As Leon Thal knew, it will take a village to meet this challenge.
Acknowledgments
The author was supported by NIA/NIH, R01-AG-21055, Clinical and Pathological Studies in the Oldest Old (C. Kawas, Principal Investigator); by NIA/NIH P50-AG-16573, Alzheimer's Disease Research Center of the University of California at Irvine (C. Cotman, Principal Investigator); and by the Al and Trish Nichols Chair in Clinical Neuroscience.
References
- [1].US Census Bureau Population projections (middle series) United States. 2002 Available at: www.census.gov.
- [2].Evans DA, Funkenstein HH, Albert MS, Sher PA, Cook NR, Chown MJ, et al. Prevalence of Alzheimer's disease in a community population of older persons: higher than previously reported. JAMA. 1989;262:2551–6. [PubMed] [Google Scholar]
- [3].Miech RA, Breitner JCS, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90's for men, later for women. The Cache County Study. Neurology. 2002;58:209–18. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- [4].Ruitenberg A, Ott A, van Swieten JC, Hofman A, Breteler MM. Incidence of dementia: does gender make a difference? Neurobiol Aging. 2001;22:575–80. doi: 10.1016/s0197-4580(01)00231-7. [DOI] [PubMed] [Google Scholar]
- [5].Edland SD, Rocca WA, Petersen RC, Cha RH, Kokmen E. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch Neurol. 2002;59:1589–93. doi: 10.1001/archneur.59.10.1589. [DOI] [PubMed] [Google Scholar]
- [6].Hall CB, Verghese J, Sliwinski M, Chen Z, Katz M, Derby C, et al. Dementia incidence may increase more slowly after age 90: results from the Bronx. Aging Study. 2005;65:882–6. doi: 10.1212/01.wnl.0000176053.98907.3f. [DOI] [PubMed] [Google Scholar]
- [7].Berlau D, Corrada M, Paganini-Hill A, Brookmeyer R, Kawas C. Incidence of dementia continues to increase after age 90: results from the 90+ Study. Neurology. 2007;68(Suppl 1):S01.003. [Google Scholar]
- [8].Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–95. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- [9].Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–9. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- [10].Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- [11].Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES Project. Alzheimer Dis Assoc Disord. 2004;18:57–64. doi: 10.1097/01.wad.0000126614.87955.79. [DOI] [PubMed] [Google Scholar]
- [12].Paganini-Hill A, Corrada MM, Kawas CH. Increased longevity in older users of postmenopausal estrogen therapy: the Leisure World Cohort Study. Menopause. 2006;13:12–8. doi: 10.1097/01.gme.0000172880.40831.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kawas C, Corrada M, Paganini-Hill A. Low weight in early adulthood and later life exercise increase chances of surviving to age 90. Neurology. 2003;60(Suppl 2):S58.002. [Google Scholar]
- [14].Wilson RS, Bienias JL, Evans DA, Bennett DA. The Religious Orders Study: overview and relation between change in cognitive and motor speed. Aging Neuropsychol Cogn. 2004;11:280–303. [Google Scholar]
- [15].Wang L, Larson EB, Bowen JD, van Belle G. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006;166:1115–20. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- [16].Corrada M, Head E, Kim R, Kawas C. Braak and Braak staging and dementia in the oldest-old: Preliminary results from the 90+ Study. Neurology. 2005;64(Suppl 1):A276. [Google Scholar]
- [17].Polvikoski T, Sulkava R, Myllykangas L, Notkola I, Niinistro L, Verkkoniemi A, et al. Prevalence of Alzheimer's disease in very elderly people: a prospective neuropathological study. Neurology. 2001;56:1690–6. doi: 10.1212/wnl.56.12.1690. [DOI] [PubMed] [Google Scholar]
- [18].Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch Neurol. 2000;57:713–9. doi: 10.1001/archneur.57.5.713. [DOI] [PubMed] [Google Scholar]
- [19].Katzman R, Terry RD, DeTeresa R, Brown T, Davies P, Fuld P, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–44. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- [20].Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, et al. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer's disease. Neurology. 1988;38:1682–7. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- [21].Silver MH, Newell K, Brady C, Hedley-White ET, Perls TT. Distinguishing between neurodegenerative disease and disease-free aging: correlating neuropsychological evaluations and neuropathological studies in centenarians. Psychosom Med. 2002;64:493–501. doi: 10.1097/00006842-200205000-00014. [DOI] [PubMed] [Google Scholar]
- [22].Berlau DJ, Kahle-Wrobleski K, Head E, Goodus M, Kim R, Kawas C. Dissociation of neuropathologic findings and cognition: case report of an apolipoprotein E epsilon 2/epsilon 2 genotype. Arch Neurol. 2007;64:1193–6. doi: 10.1001/archneur.64.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Head E, Corrada M, Nistor M, Kawas C. Higher levels of synaptophysin, but not GAP-43 protein are associated with intact cognitive function in the oldest-old: the 90+ Study. Neurology. 2006;66(Suppl 2):P05.974, A279. [Google Scholar]
- [24].Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163:938–49. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Paganini-Hill A, Kawas C, Corrada M. Type of alcohol consumed, changes in intake over time and mortality: the Leisure World Cohort Study. Age Ageing. 2007;36:203–9. doi: 10.1093/ageing/afl184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Paganini-Hill A, Kawas C, Corrada M. Non-alcoholic beverage and caffeine consumption and mortality: the Leisure World Cohort Study. Prev Med. 2007;44:305–10. doi: 10.1016/j.ypmed.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Corrada M, Paganini-Hill A, Kawas C. Intake of antioxidant vitamins, calcium, beverages and mortality: the 90+ Study. Neurology. 2004;62(Suppl 5) [Google Scholar]