Abstract
Celiac disease is one of the most prevalent autoimmune gastrointestinal disorders but as the case of Ms. J illustrates, diagnosis is often delayed or missed. Based on serology studies, the prevalence of celiac disease in many populations is estimated to be approximately 1% and has been increasing steadily over the last 50 years. Evaluation for celiac disease is generally straightforward, and uses commonly available serologic tests, however the signs and symptoms of celiac disease are nonspecific and highly heterogeneous making diagnosis difficult. While celiac disease is often considered a mild disorder treatable with simple dietary changes, in reality celiac disease imparts considerable risks including reduced bone mineral density, impaired quality of life, and increased overall mortality. In addition, the gluten free diet is highly burdensome and can profoundly affect patients and their families. For these reasons, care of individuals with celiac disease requires prompt diagnosis and ongoing multidisciplinary management.
Dr Ship
Ms. J is a 46 F recently diagnosed with celiac disease. She lives in the greater Boston area and has private insurance.
Ms. J has generally been in good health. She has been anemic since her first pregnancy, 20 years ago. She was pregnant three times subsequently, but miscarried each time, always in the second trimester. She transferred to a new physician about 5 years ago. Her hematocrits were then in the low 30s. Her indices were normal, and although her ferritin was low (7.2ng/mL), her iron rose into the normal range with supplementation. She reported heavy menses at the time, which was thought to be the cause of her anemia.
In March of 2010, Ms J. presented for routine care and was found to have a hematocrit of 26 with an mean corpuscular volume (MCV) of 78. She reported inability to tolerate iron due to constipation. She also reported much lighter menses and intermittent epigastic discomfort for which a trial of a proton pump inhibitor was recommended. Given these findings, her internist sent her for an endoscopy and – because of a family history of colon cancer – a colonoscopy.
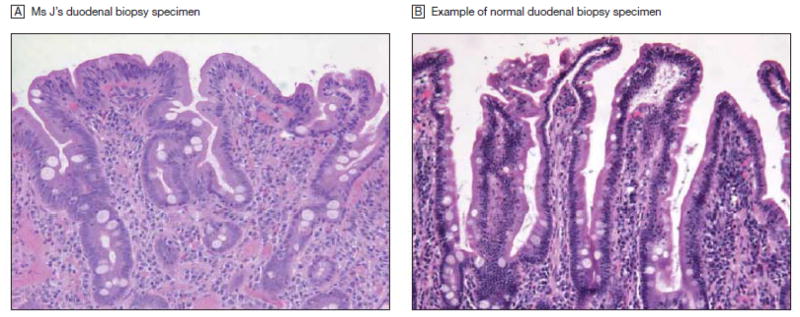
She was seen by a gastroenterologist and had a colonoscopy, which was normal, and an endoscopy which showed villous shortening and an increased number of intraepithelial lymphocytes, consistent with celiac disease. (See Figure 1) Further testing revealed a normal tTG IgA at 14 units (range 0 – 19) but an elevated Anti-DGP (IgA/IgG) at 104 units (range 0 – 19).
Figure 1. Duodenal Biopsy Specimens.
A. Duodenal biopsy specimen from Ms J’s upper endoscopy showing villous shortening and an increased number of intraepithelial lymphocytes, consistent with celiac disease. B, Biopsy specimen of normal duodenum for comparison. Magnification × 200; hematoxylin-eosin stain.
Ms. J was diagnosed with celiac disease and was instructed to follow a gluten free diet. Since then she reports a loss of 15 pounds and notices that her joint pain is entirely better. Ms. J also noted significant improvement in her energy level. Her daughter was tested for celiac disease and had a negative result.
Ms. J’s past medical history is notable for hypertension and mild, situational depression. Her medications include hydrochlorothiazide 25 mg daily, lisinopril 10 mg daily and iron, 6 tablets daily. She has no drug allergies. She does not smoke or drink alcohol.
MS J
When I first was told that I had celiac disease I didn’t really know what to think because I hadn’t really heard of it before. They told me that I was never going to be able to have any type of wheat, rye, and barley products again. At first I thought it’s just a temporary thing and then when I realized that I could never really have any of that food again for the rest of my life, I was in denial. I was like “oh wow” but I started the diet right away. Since I’ve been on it, I dropped 15 lbs, my iron level has gone up, and my joints don’t hurt any more. I just feel overall better than I did.
It’s very difficult to be on a gluten free diet. I find it very hard to go out to eat. We used to go out to eat as a family once a week now it’s very difficult because not all restaurants have gluten free menus and the ones that do have gluten free you don’t know what goes on in the kitchen. I did cheat once when I was on vacation. I will tell you that after being off of the gluten for 2 or 3 month and then cheating I felt really bad the next day. Shopping at the grocery store is also very difficult. It’s very expensive especially in this type of economy. One loaf of bread is $7!
This really is a lifestyle change and that the hardest thing is to know that I can never eat these items for the rest of my life. Some underlying questions I have are: What kind of damage has it done not being diagnosed earlier? Is it irreversible or is it reversible? Also I would like to know why I had the miscarriages. Is there a connection?
AT THE CROSSROADS: QUESTIONS FOR DR LEFFLER
What is the epidemiology and pathophysiology of celiac disease?
Which symptoms should prompt a clinician to test for celiac disease?
How is the diagnosis of celiac disease made? What is the specificity and sensitivity of the tissue transglutaminase antibody testing? When is a small intestinal biopsy indicated?
Are there any populations who should be screened for celiac disease? Should family members be tested?
Once diagnosed, what treatment is possible other than avoidance of gluten? Is adherence to the gluten free diet ever “optional”? What are the harms of cheating?
What testing should patients with celiac disease undergo? Until what age?
What does the future hold?
What do you recommend for our patient?
Throughout the article, the following notation is used to describe the evidence supporting statements:
Level of evidence A: recommendation based on evidence from multiple randomized trials or meta-analyses;
Level of evidence B: recommendation based on evidence from a single randomized trial or nonrandomized studies;
Level of evidence C: recommendation based on expert opinion, case studies, or standards of care
Dr. Leffler
What is the epidemiology and pathophysiology of celiac disease?
Ms J: “What kind of disease is this?”
Celiac disease has long been considered to be a rare disorder of childhood. Significant advances in the understanding of celiac disease have refuted this and the currently accepted prevalence of celiac disease is approximately one to two percent of the general population in many regions of the world including North and South America, Europe, North Africa, the Middle East and India.1, 2 The increased diagnosis of celiac disease is related both to improved testing and to true increases in Celiac disease prevalence.3 Although inflammation is induced by the foreign protein gluten, celiac disease is best understood as a complex autoimmune disorder rather than an allergy as auto-antibodies to tissue transglutaminase (tTG) are central to the disease process. Gluten, the major protein in wheat, rye, barley and related grains, is poorly digested and reaches the intestinal lumen in large polypeptides. In individuals with celiac disease, gluten peptides pass though the mucosa of the small intestine into the submucosa. In the submucosa, gluten peptides are modified by the common enzyme tTG and become able to bind with high affinity to human leukocyte antigen (HLA) DQ2 and DQ8 molecules on antigen presenting cells stimulating both cell mediated and humoral immune reactions.4 (See Figure 2)
Figure 2. Antigen Presentation and Production of Antibodies to Gluten Peptides and Tissue Transglutaminase (tTG).
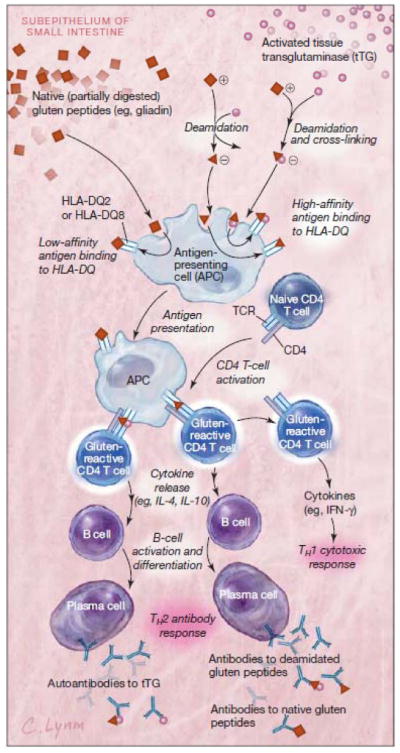
In the subepithelium of the small intestine, native (partially digested) gluten peptides are deamidated by the enzyme tTG. While tTG is ubiquitous, it is predominantly stored intracellularly in an inactive state and released in the presence of inflammation and activated by higher levels of extracellular calcium ions. Deamidation leads to change in shape and charge of the gluten peptides, permitting high-affinity binding to HLA-DQ2 and -DQ8 on APCs such as dendritic cells and macrophages. Only HLA-DQ2 and -DQ8 are able to bind gluten peptides strongly enough to trigger an inflammatory reaction, so the presence of at least 1 of these molecules is a prerequisite for development of celiac disease. Naive T cells that have been activated by deamidated gluten presented by APCs are then able to stimulate both a TH1 cytotoxic and TH2 humoral antibody response. The TH2 response leads to production of antibodies against native gluten peptide, deamidated gluten peptide, and tTG. Antibodies to the self-protein tTG are produced because tTG is often still complexed with deamidated gluten peptides during presentation by APCs. This directed anti-self immune response is the major autoimmune component of celiac disease. TCR indicates T-cell receptor; IFN, interferon.
Which symptoms should prompt a clinician to test for celiac disease?
Ms J: “I didn’t have any diarrhea or constipation; I think that’s what threw my PCP off”
Although discoveries in the pathophysiology of celiac disease have led to accurate serologic testing, the diversity of signs and symptoms creates significant difficulties for clinicians. Infants and children typically present with predominant symptoms of malabsorption including diarrhea and failure to thrive, however, the list of signs and symptoms associated with celiac disease in older children and adults is vast and new associations are reported regularly. (See Table 1)
Table 1.
Presenting Complaints and Pretest Probability of Celiac Disease*
| Pretest Probability | Risk Factors | Evidence Notes | Comments |
|---|---|---|---|
| High: Testing for celiac disease always warranted. Negative serologic test may not adequately rule out celiac disease |
Risk of celiac disease in first and second degree relatives is ~ 8% and 4% respectively25, 73 and increases to 20% in symptomatic family members74 Testing for celiac disease in patients with classic symptoms is felt to be cost effective29 |
Risk in these populations is generally 10% or higher | |
| Medium: Testing for celiac disease generally warranted. Negative Serologic test adequately rules out celiac disease |
|
Two separate studies of cost effectiveness of celiac testing in irritable bowel syndrome conclude that testing is generally warranted in this population.76, 87 | Risk in these populations is generally 4–10% Guidelines published by the American College of Gastroenterology in 2009 recommend celiac disease testing for all patients with diarrhea and presumed irritable bowel syndrome88 |
| Low: Testing for celiac disease warranted only after excluding more likely etiologies or with coexistent risk factors. Negative Serologic test adequately rules out celiac disease |
|
Risk in these populations is generally <4% Few cost effectiveness analyses in celiac disease testing have been performed but limited data suggest routine serologic testing becomes cost effective at a prevalence of >4% |
Celiac disease is rare in individuals of pure East Asian, South-East Asian, Sub-Saharan African and Inuit descent.2 Recommendations do not apply to these groups.
Testing for celiac disease in all patients who present with any of the dozens of possible signs and symptoms would quickly approach population screening, an approach not currently supported by available evidence, as discussed below. Deciding when to test for celiac disease and when to refer for further evaluation is challenging, contributing to an average of 11 years of symptoms prior to diagnosis5, 6 and often a complete to failure to test for celiac disease at all.
Fortunately, types of presentation can be divided into three approximate categories based on risk of celiac disease as described in Table 1 and can be helpful in guiding effective celiac disease testing and referral. It is worth noting that the individuals at highest risk tend to be complicated enough to warrant referral to a gastroenterologist regardless of concern for celiac disease. Indeed, in the case of Ms. J, the combination of difficult to treat iron deficiency anemia and upper gastrointestinal symptoms led to referral to gastroenterology where the diagnosis of celiac disease was promptly made.
How is the diagnosis of celiac disease made? What is the specificity and sensitivity of antibodies to tissue transglutaminase (tTG)? When is a biopsy indicated?
Prior to the 1980’s, the lack of non-invasive testing for celiac disease severely limited diagnosis rates. In the mid 1980’s Anti-Gliadin Antibody (AGA) testing became available, however the positive predictive value in moderate risk groups is less than 30%, precluding efficient diagnosis.1 In the early 1990’s Endomysial Antibody (EMA) testing ( sensitivity and specificity of greater than 95%) became available, however, use was curtailed by cost and interpretability issues.1
In 1997, tissue transglutaminase (tTG) was determined to be the major auto-antigen in celiac disease7, and shortly thereafter assays for anti-tTG antibodies were developed. Current tTG tests are based on IgA antibodies to recombinant human tTG, and in most studies sensitivity and specificity are above 90% and 95%, respectively,1, 8 which equates to a positive predictive value of approximately 75% and a negative predictive value of 99% in moderate risk populations given a 5% pretest probability.
While IgA-tTG testing is generally accepted to be the initial test of choice for celiac disease in most situations, the case of Ms. J, illustrates some important caveats. First, approximately 5% of individuals with celiac disease will be seronegative and all serologic tests appear to be less sensitive in children under the age of two years.9 For this reason, in cases where the pretest probability is high, as in individuals such as Ms. J with iron deficiency anemia and gastorointestinal symptoms or any of the other conditions listed in the first row of Table 1, IgA-tTG is not sufficiently specific to rule out celiac disease and upper endoscopy with duodenal biopsy should be strongly considered. Conversely, while well described, tTG negative celiac disease remains uncommon and there are multiple other causes of small intestinal villous atrophy.10 In order to confirm the diagnosis of celiac disease, patients with intestinal damage suggestive of celiac disease, but who are negative for IgA-tTG, should have further evaluation including a total IgA level, testing for antibodies to deamidated gliadin peptide (DGP), testing for Celiac disease related HLA DQ2 and HLA DQ8 (the absence of which excludes celiac disease) and assessment of clinical and histologic improvement on a gluten free diet (GFD).1
Finally, symptomatic response to a GFD is neither a sensitive or specific test for celiac disease for a number of reasons. First, conditions including food allergy and, much more commonly, gluten intolerance will improve on a GFD.11–13 On the other hand, at least 10% of patients with celiac disease will not fully respond to dietary modification alone either due to inadvertent gluten exposure or coexisting conditions including irritable bowel syndrome.14 Finally patients and clinicans should be aware that both serology and histology will normalize on a GFD, so testing for celiac disease should be completed prior to dietary modification.
Given the high accuracy of modern serologic testing, a common question is why small intestinal biopsy remains necessary for celiac disease diagnosis. Although some suggest that biopsy should no longer be required,15 biopsy remains the gold standard for diagnosis for a number of reasons.16, 17 First, although the sensitivity of IgA-tTG is high, in most risk groups the positive predictive value of a positive test is only around 75% and spurious positive tTG titers can be seen in cirrhosis18, congestive heart failure19 and after enteric infections.20 Additionally, while celiac disease is often considered to be a ‘benign’ diagnosis with little harm in false positive diagnosis, in reality misdiagnosis of celiac disease is deleterious on multiple levels. First, as discussed below, the burden of a GFD is substantial. Second, celiac disease is also associated with complications including refractory celiac disease21 and malignancies22, so that while overall outcomes in celiac disease are generally good,22, 23 mortality rates may be persistently elevated24 and concern regarding complications can lead to extensive medical testing. Patients with confirmed celiac disease commonly experience episodes of recurrent symptoms, and intestinal histology at diagnosis is often vital in evaluation of potential causes.14 False positive diagnosis can also lead directly to increased healthcare costs as individuals with celiac disease are routinely recommended to have tests such as vitamin levels and bone mineral density evaluation which may not be otherwise necessary.16 Finally, as celiac disease is hereditary with risk an approximate 8% risk in first degree family members and 4% risk in second degree family members25, a single false positive diagnosis can precipitate a string of unnecessary tests in patients’ relatives. For all these reasons, the diagnosis of celiac disease requires duodenal biopsy consistent with celiac disease and either positive serologic testing and/or response to a GFD.16
Are there any populations that should be screened for celiac disease? Should family members be tested?
Ms J.: “No one else in my family has been tested except for my daughter”
Screening for celiac disease has been a controversial issue for many years.26, 27 The World Health Organization criteria for screening of noncommunicable diseases can be summarized by the following requirements:28 (1) The disease must be common and well defined. (2) Screening tests must be safe, simple and highly accurate. (3) Both disease testing and treatment must be culturally acceptable and equitable. (4) Treatment for the disease must be available. (5) Early clinical detection must be difficult. (6) If not recognized, the disease could result in severe complications difficult to manage. (7) The overall program for testing and treatment should be cost-effective.
It is tempting to conclude that there is sufficient data to support population screening for celiac disease for the following reasons; First, celiac disease is clearly difficult to detect based on the heterogeneity of presentation as described above. Second, celiac disease is common and causes significant morbidity. Third, modern serologic tests for celiac disease are among the most accurate available for any autoimmune or inflammatory disorder. Fourth, treatment with a GFD is effective in the majority of patients. Fifth, if not recognized complications including osteoporosis, growth impairment, fertility issues and malignancy can occur. Finally, IgA-tTG testing is also relatively inexpensive, costing approximately the same as a lipid panel in most areas. In addition, Markov models suggests that given the mortality associated with untreated symptomatic celiac disease, screening would be cost effective.29
Currently, however, available data regarding the morbidity of undiagnosed and untreated celiac disease is based almost entirely on patients with clinically diagnosed, symptomatic, celiac disease. A large proportion of individuals detected in a mass screening effort would be expected to be minimally symptomatic or asymptomatic, and data suggest that this group may not have the same risks as those with clinically evident celiac disease.30, 31 The few prospective studies that have evaluated screening for celiac disease in adults have not conclusively found screening to be beneficial.32, 33 A final complexity is that celiac disease can present at any age, so the timing and testing intervals would need to be determined in order to balance delayed diagnosis and cost. For these reasons, the currently accepted strategy for celiac diagnosis is aggressive case finding in individuals presenting with signs and symptoms suggestive of celiac disease. Fortunately, although diagnosis is often markedly delayed5, 6, studies suggest that with proper clinician education, case finding can be highly effective.34, 35
Testing of all close relatives of patients with celiac disease should be considered separately from population screening because of the increased risk of celiac disease in first and second degree relatives.16 In children and adolescents, due to the risk of permanent impairment of growth and development, guidelines recommend testing for celiac disease every two to three years or at onset of new symptoms in children with a family history of celiac disease or comorbid conditions.17, 36 In adults, on the other hand, testing is reserved for those with signs or symptoms suggestive of celiac disease,16 although with the growing number of celiac associations, clinicians should maintain a very low threshold for testing.
There are two celiac testing strategies for individuals at risk due to family history. The most common strategy is serologic testing, identical to that for any symptomatic patient. However, serology and intestinal biopsy are both diet dependent and only rule out currently active celiac disease. A second and increasingly popular strategy is to test for the presence of either HLA DQ2 or HLA DQ8, which are prerequisite for the development of celiac disease.16 HLA DQ2 or HLA DQ8 are found in approximately 40% of the general population and are not disease causative, limiting the concerns associated with conventional genetic testing. Although the positive predictive value of HLA typing for celiac disease is extremely low, the negative predictive value is nearly 100%, making this an attractive option individual or parents of individuals who would otherwise need repeated serologic testing.16
Once diagnosed, what treatment is possible other than avoidance of gluten? Is adherence to this ever “optional”? What are the harms of cheating?
Ms J: “It’s very difficult to be on a GFD…It’s very difficult to go out to eat. Shopping…now takes an hour and a half rather than 20 minutes and is very expensive and confusing…you have to really read the labels and be vigilantf… I did cheat once and I felt really bad”
Currently, the only accepted therapy for celiac disease is strict adherence to a GFD.16 While the ability to treat a disease without medications is attractive, as Ms. J reports, adherence to a GFD is quite difficult. The cost of the gluten free diet is two to three times that of a standard diet37 and while a GFD is subsidized in many European countries, elsewhere expense represents a significant hardship. Second, although a balanced GFD can be quite healthy, in many individuals the loss of common whole grains and inconsistent fortification results in failure to meet recommended daily intake of many nutrients.38, 39 With proper counseling and motivation, cost can be minimized and nutritional value maximized by the use of raw ingredients. Further, the need to avoid foods is a profound social stress for children and adults that continues to be troubling years after adoption of a GFD.40–42
Even for those making substantial effort to maintain a strict GFD, the degree of adherence necessary is challenging. It has been shown that as little as the amount of gluten found in 1/30 of a slice of bread is enough to cause intestinal damage,43, 44 and to make matters worse, gluten is an ubiquitous ingredient/contaminant in many foods and even medications.45, 46 For these reasons, an absolute GFD is probably not attainable and the addition of purposeful gluten consumption or “cheating” on top of unavoidable exposure should not be endorsed. A strict GFD should be strongly encouraged as non-adherence is common at all ages,42, 47 associated with a lax attitude toward gluten exposure,48 and even inadvertent gluten exposure commonly results in recurrent symptoms.14, 49
For clinically evident celiac disease, there is evidence for the beneficial effect of a GFD on symptoms50 and quality of life32, 33 and the overall, standardized mortality rate has been shown to decrease from over 2.8 (95% CI 2.51–3.11) to 1.22 (95% CI 1.13–1.32) after one year of treatment.22, 24 However, as discussed in the section on screening above, limited data suggests that asymptomatic patients may safely continue a regular diet with proper monitoring,31 and for patients in whom celiac disease was diagnosed based on screening alone, and who have no evident symptoms or nutritional abnormalities, discussion of the risks and benefits of immediate adoption of the GFD vs. monitoring on a regular diet is reasonable.
What testing should patients with celiac disease undergo? Until what age?
Ms J: “What am I supposed to do now?”
While there is general consensus on establishing the diagnosis of celiac disease and dietary treatment, data on optimal monitoring strategies are limited, and subsequently guidelines vary (see Table 2). With the discovery of the GFD in the 1940’s celiac disease changed from a highly morbid childhood disease with a reported case fatality rate of nine percent51 to one with a very modestly increased standardized mortality rate of 1.39 (95% CI 1.33–1.45).24 This suggested that there was little to be gained by further research on treatment, and over the following decades most efforts focused on epidemiology, pathophysiology, and diagnosis. In recent years, studies of patients followed into adulthood or diagnosed as adults have suggested the need for closer monitoring and a number of consensus guidelines have been developed as noted in Table 2. Overall, given the limitations of available data, ongoing care of the patient with celiac disease should be individualized. Most available guidelines suggest regular celiac disease evaluation by a physician and dietitian, which should include testing of celiac serologies and nutrient levels. Although repeat intestinal biopsy to assess healing was considered routine in the past, the ability to follow serology titers has decreased the need for this practice. Currently, repeat intestinal biopsy is not recommended by most guidelines in adult or pediatric patients who are responding clinically and serologically to treatment. Further recommendations can be found in Table 2.
Table 2.
Monitoring of Individuals with Celiac Disease
| Strength of Recommendation | Recommendation | Comment |
|---|---|---|
| Recommended for All Patients/Suggested by 5–6 of the 6 available Guidelines | While most guidelines suggest laboratory testing of nutritional status, specific recommendations vary greatly. Ferritin, vitamin B12, folate and 25- OH vitamin D are considered routine. Other tests to consider include zinc, calcium, copper, thiamin, albumin, vitamins B6, A, E and K | |
| Consider in Most Patients/Suggested by 2–4 of the 6 available Guidelines | Routine celiac monitoring by a MD can be considered optional if the patient is followed by an expert celiac RD. Timing of follow up by RD or MD is variable but a common schedule is at diagnosis, 3–6 months post diagnosis for the first year or until in clinical remission and then annually thereafter |
|
| Not Routinely Necessary/Suggested in one of the 6 available Guidelines | Most publications do not focus on nutritional therapy and although suggested in only the ADA guideline, recommendation of a multivitamin and calcium/vitamin D is common Vaccination recommended due to association of celiac disease with impaired spleen function |
Recommended in the American Gastroenterology Association Technical Review on the diagnosis and management of Celiac Disease, 200616
Recommended in the North American Society for Pediatric Gastroenterology,
Hepatology and Nutrition Guidelines for the diagnosis and treatment of celiac disease in children, 200536
Recommended in the National Institute of Health Consensus Report on Celiac Disease, 200458
Recommended in the American Dietetics Association Celiac Disease Evidence Based Nutrition Practice Guideline, 200957
Recommended in the Primary Care Society for Gastroenterology: The Management of Adults with Celiac Disease in Primary Care, 2006104
Recommended in the World Gastroenterology Organization Practice Guideline for Celiac Disease, 2007105
What does the future hold?
Ms. J: “What are they going to do, are they going to give you a pill?”
Knowing both the instigating antigen (gluten) and the end result of immune disregulation (enteropathy and autoantibody production), our understanding of the pathophysiology of celiac disease is substantially more complete than for other autoimmune/inflammatory disorders.52 Advances in diagnosis have propelled celiac disease from an uncommon disorder to one of the most common and fastest growing gastroenterological disorders. Although the gluten free diet is safe, it is clearly not an optimal treatment, imparting significant burden to patients, and failing to achieve either complete symptom resolution or intestinal healing in up to 30 percent of patients.14, 53 The combination of comprehensive understanding of celiac disease and the need for adjunctive or alternative treatments to the GFD has spurred much recent work in the area of celiac therapeutics. Therapies currently in testing include enzymes to degrade gluten in the stomach prior to immune presentation in the small intestine, molecules to enhance the tight junctions between enterocytes barring gluten entry, methods of detoxifying gluten in wheat or during food processing and reinduction of immune tolerance through ‘vaccination’ with gluten peptides among other possibilities.54 It is too early to tell which of these first generation therapies will hold substantial benefit for the millions with celiac disease, however it appears likely that our understanding of the pathophysiology of celiac disease will lead to a durable cure and likely pave the way for improved treatments of many other autoimmune/inflammatory disorders.
What do you recommend for our patient?
Ms. J: “It would have been helpful if I had had a chance to speak with a dietitian”
Based on symptoms, positive celiac serology and intestinal histology, Ms. J meets criteria for celiac disease. She should be informed that she has a lifelong autoimmune disorder which necessitates strict avoidance of all foods containing any amount of wheat, rye and barley. Ms. J should also be referred to a dietitian skilled in celiac disease and to a regional celiac advocacy group. It is likely that Ms. J has had celiac disease for many years, and untreated celiac disease may have contributed to her recurrent miscarriages.55, 56 Fortunately, with adherence to a gluten free diet, proper support and nutritional supplementation, she can expect to achieve clinical remission. Based on expert opinion, I would also recommend monitoring of vitamin levels including 25-OH vitamin D, iron and other nutrients based on symptoms.16, 57 Due to the high prevalence of nutritional deficiencies, I would also suggest routine supplementation with a multivitamin and additional vitamin D and calcium with a goal 25-OH vitamin D level of between 20 and 40 ng/ml depending on known bone density and coexisting risk factors for osteopenia, as well as aggressive repletion of any other vitamins or minerals for which she is found to be deficient. Aside from nutritional status, thyroid stimulating hormone and liver function tests should be checked at diagnosis to assess for related autoimmunity.57, 58
Her daughter is over the age of 18, so routine celiac serologic or HLA testing is unnecessary but I would encourage her to inform her relatives that celiac disease is now in the family and that they should discuss serologic testing with their primary care physicians based on any symptoms they may be experiencing. Ms. J’s iron deficiency may indeed be multifactorial from both menses and celiac disease and I would monitor this closely over the next few months, however should she continue to have symptomatic anemia without rapid improvement I would consider parenteral repletion. I would recommend she visit her dietitian and physician approximately six and 12 months after diagnosis to monitor symptoms and recheck celiac serologies. I would also recommend evaluation of bone mineral density approximately one year after adoption of a gluten free diet. Afterward, visits for celiac disease can be continued on an annual basis with both a dietician and physician knowledgeable about celiac disease.
Ms. J’s Questions
Could my miscarriages have been related to celiac disease?
Currently the typical newly diagnosed patient with celiac disease is a woman around the age of 40 years who has had symptoms of celiac disease for over a decade. Given that active celiac disease has nutritional and direct inflammatory consequences on fertility,59, 60 the reproductive life of many patients is irreversibly affected. In particular, the risk of miscarriage appears higher in women with untreated celiac disease compared to the general population.61 For these reasons, clinicians should maintain a very low threshold for celiac disease testing in this population.
Has my body sustained any irreversible damage from celiac disease over the years?
The small intestinal mucosa has enormous regenerative capacity in both health and disease. Even individuals with longstanding, severe celiac enteropathy can expect to achieve complete or near complete intestinal healing with gluten avoidance and nutritional support, although the length of time to healing varies from less than one year to more than five years and healing is associated with younger age at diagnosis and improved GFD adherence.53, 62
Outside of the intestine, however, healing is not always assured. A number of extraintestinal manifestations of celiac disease such as dermatitis herpetiformis, anemia, and joint pain, typically improve significantly or resolve within the first year of treatment, as was seen in Ms. J.63 One of the most common associations with celiac disease is reduced bone mineral density (BMD) which is seen in at more than 50% of patients at diagnosis.64 Although there is often a significant improvement in BMD over the first year of treatment with a GFD, up to 21% of patients will have persistent osteoporosis.64 There are multiple neurologic manifestations of celiac disease, some of including peripheral neuropathy and headaches which resolve, while case studies suggest that other manifestations including ataxia, may stabilize but rarely improve.65 Finally, there is a potential increased risk of secondary autoimmune disorders related to longstanding untreated celiac disease, and once triggered, these will not respond to gluten withdrawal.66
QUESTIONS AND DISCUSSION
Question: I have the sense that everyone who has anything going on is claiming to be gluten sensitive and is trying a GFD. This seems to be an epidemic that is potentially hurting a lot of people rather than helping them
Dr. Leffler: Americans love fad diets and there is certainly a component of that with the increased popularity of the GFD. In some ways it has been beneficial because it has greatly increased food availability for people with celiac disease. While there is certainly a placebo component to adopting a gluten free diet, non-celiac gluten intolerance does appear to be a real phenomenon and studies have shown an HLA predisposition for response to gluten withdrawal12 and a recent double blind randomized controlled trial demonstrated that gluten can exacerbate gastrointestinal symptoms in people without celiac disease who are on a gluten free diet.11 No matter why people choose to follow a gluten free diet, given nutritional concerns including lack of fiber and B vitamins38 they should be seeing a dietician to help maintain a nutritionally sound diet.
Question: Given that celiac disease is hard to treat, is serology of any use in following patients who are not doing as well as we would like? Conversely, does resolution of symptoms always reflect intestinal healing?
Dr. Leffler: While current serologic tests are excellent at detecting untreated celiac disease, there is clear evidence that they are not accurate indicators of disease activity or adherence to the gluten free diet.67 Skilled dietician evaluation remains the gold standard for this GFD assessment, although a short standardized survey is freely available and with a predicted accuracy of 88% is significantly more accurate than the 65% reported for IgA-tTG.48 However, lacking more sensitive non-invasive tests of intestinal health, it is still recommended to check celiac serologies on a yearly basis.16 A persistently positive test should prompt evaluation for a number of potential issues, the most common being gluten exposure.14
Conversely, like many inflammatory disorders, celiac disease activity can fluctuate over time and intestinal inflammation may persist, even in individuals with normalized celiac serologies and resolution of symptoms.1, 53
Question: How do we follow patients with celiac disease who are asymptomatic for the development of severe complications, specifically lymphoma?
Dr. Leffler: This is a very common concern from patients. For the routine celiac patients without refractory celiac disease, whether diagnosed with symptoms and doing well on a gluten free diet or silent and being monitored on a normal diet, the risk of developing lymphoma is actually very low, with a rate of approximately eight cases per 10,000 patient years.22 and there is no indication for routine small bowel imaging or other testing for malignancy. The main complication of celiac disease, --refractory celiac disease -- is symptomatic by definition,21 so for patients who are feeling well, beyond regular nutritional and celiac blood testing and a consideration of bone mineral density evaluation, no further testing is needed.
Figure 3. Pathophysiology of Celiac Disease and Potential Nondietary Therapies Being Tested in Phase 1 or 2 Clinical Trials.
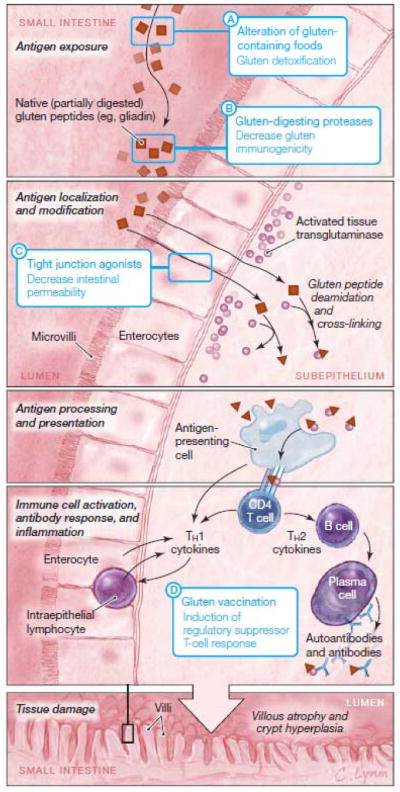
Gluten peptides are poorly digested by mammalian digestive enzymes and reach the small intestinal mucosa as large polypeptides. Gluten peptides are able to cross the mucosa into the subepithelium by transcellular and/or paracellular pathways. In the subepithelium, gluten peptides are deamidated by tissue transglutaminase (Figure 2) and trigger cytotoxicity leading to mucosal damage and humoral immunity leading to antibody production. Detailed understanding of the pathophysiology of celiac disease has allowed for creation of highly targeted potential nondietary therapies (blue boxes). These indude (A) alteration of gluten-containing foods through the use of alternative or genetically modified wheat varieties or through specialized food processing techniques; (B) degradation of gluten proteins in the stomach and small intestinal lumen by selected proteases; (C) preventing gluten passage into the subepithelium of the small intestine through the use of tight junction agonists; and (D) re-induction of tolerance to gluten though immune desensitization.
Acknowledgments
We would like to thank the patient for sharing her story.
Funding/Support: Clinical Crossroads receives no external support.
Footnotes
This conference took place at the Medicine Grand Rounds at the Beth Israel Deaconess Medical Center, Boston, Mass, on 10th February, 2011.
Discussant Financial Disclosures:
Dr. Leffler receives support from an NIH K23 Career Development Award DK082619 and has served as a consultant for the following companies: Alba Pharmaceuticals, Alvine Pharmaceuticals, Prometheus Laboratories, Shire Pharmaceuticals
References
- 1.Leffler DA, Schuppan D. Update on serologic testing in celiac disease. Am J Gastroenterol. 2010 Dec;105(12):2520–2524. doi: 10.1038/ajg.2010.276. [DOI] [PubMed] [Google Scholar]
- 2.Accomando S, Cataldo F. The global village of celiac disease. Dig Liver Dis. 2004 Jul;36(7):492–498. doi: 10.1016/j.dld.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased Prevalence and Mortality in Undiagnosed Celiac Disease. Gastroenterology. 2009 Apr 10;137(1):88–93. doi: 10.1053/j.gastro.2009.03.059. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002 Sep;2(9):647–655. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 5.Cranney A, Zarkadas M, Graham ID, et al. The Canadian Celiac Health Survey. Dig Dis Sci. 2007 Apr;52(4):1087–1095. doi: 10.1007/s10620-006-9258-2. [DOI] [PubMed] [Google Scholar]
- 6.Green PHR, Stavropoulos SN, Panagi SG, et al. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001 Jan;96(1):126–131. doi: 10.1111/j.1572-0241.2001.03462.x. [DOI] [PubMed] [Google Scholar]
- 7.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997 Jul;3(7):797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 8.Volta U, Fabbri A, Parisi C, et al. Old and new serological tests for celiac disease screening. Expert Rev Gastroenterol Hepatol. 2010 Feb;4(1):31–35. doi: 10.1586/egh.09.66. [DOI] [PubMed] [Google Scholar]
- 9.Maglio M, Tosco A, Paparo F, et al. Serum and intestinal celiac disease-associated antibodies in children with celiac disease younger than 2 years of age. J Pediatr Gastroenterol Nutr. 2009 Jan;50(1):43–48. doi: 10.1097/MPG.0b013e3181b99c8f. [DOI] [PubMed] [Google Scholar]
- 10.Robert ME. Gluten sensitive enteropathy and other causes of small intestinal lymphocytosis. Semin Diagn Pathol. 2005 Nov;22(4):284–294. doi: 10.1053/j.semdp.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten Causes Gastrointestinal Symptoms in Subjects Without Celiac Disease: A Double-Blind Randomized Placebo-Controlled Trial. Am J Gastroenterol. 2011 Mar;106(3):508–514. doi: 10.1038/ajg.2010.487. [DOI] [PubMed] [Google Scholar]
- 12.Wahnschaffe U, Schulzke JD, Zeitz M, Ullrich R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007 Jul;5(7):844–850. doi: 10.1016/j.cgh.2007.03.021. quiz 769. [DOI] [PubMed] [Google Scholar]
- 13.Verdu EF. Editorial: Can gluten contribute to irritable bowel syndrome? Am J Gastroenterol. 2011;106(3):516–518. doi: 10.1038/ajg.2010.490. [DOI] [PubMed] [Google Scholar]
- 14.Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol. 2007 Apr;5(4):445–450. doi: 10.1016/j.cgh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010 Aug;123(8):691–693. doi: 10.1016/j.amjmed.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006 Dec;131(6):1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Fasano A, Araya M, Bhatnagar S, et al. Federation of International Societies of Pediatric Gastroenterology, Hepatology, and Nutrition consensus report on celiac disease. J Pediatr Gastroenterol Nutr. 2008 Aug;47(2):214–219. doi: 10.1097/MPG.0b013e318181afed. [DOI] [PubMed] [Google Scholar]
- 18.Villalta D, Crovatto M, Stella S, Tonutti E, Tozzoli R, Bizzaro N. False positive reactions for IgA and IgG anti-tissue transglutaminase antibodies in liver cirrhosis are common and method-dependent. Clin Chim Acta. 2005 Jun;356(1–2):102–109. doi: 10.1016/j.cccn.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Peracchi M, Trovato C, Longhi M, et al. Tissue transglutaminase antibodies in patients with end-stage heart failure. Am J Gastroenterol. 2002 Nov;97(11):2850–2854. doi: 10.1111/j.1572-0241.2002.07033.x. [DOI] [PubMed] [Google Scholar]
- 20.Ferrara F, Quaglia S, Caputo I, et al. Anti-transglutaminase antibodies in non-coeliac children suffering from infectious diseases. Clin Exp Immunol. 2009 Feb;159(2):217–223. doi: 10.1111/j.1365-2249.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut. 2010 Apr;59(4):547–557. doi: 10.1136/gut.2009.195131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West J, Logan RF, Smith CJ, Hubbard RB, Card TR. Malignancy and mortality in people with coeliac disease: population based cohort study. BMJ. 2004 Sep 25;329(7468):716–719. doi: 10.1136/bmj.38169.486701.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solaymani-Dodaran M, West J, Logan RF. Long-term mortality in people with celiac disease diagnosed in childhood compared with adulthood: a population-based cohort study. Am J Gastroenterol. 2007 Apr;102(4):864–870. doi: 10.1111/j.1572-0241.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- 24.Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F. Small-intestinal histopathology and mortality risk in celiac disease. Jama. 2009 Sep 16;302(11):1171–1178. doi: 10.1001/jama.2009.1320. [DOI] [PubMed] [Google Scholar]
- 25.Dube C, Rostom A, Sy R, et al. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterology. 2005 Apr;128(4 Suppl 1):S57–67. doi: 10.1053/j.gastro.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Evans KE, McAllister R, Sanders DS. Should we screen for coeliac disease? No. BMJ. 2009;339:b3674. doi: 10.1136/bmj.b3674. [DOI] [PubMed] [Google Scholar]
- 27.Fasano A. Should we screen for coeliac disease? Yes. BMJ. 2009;339:b3592. doi: 10.1136/bmj.b3592. [DOI] [PubMed] [Google Scholar]
- 28.Strong K, Wald N, Miller A, Alwan A. Current concepts in screening for noncommunicable disease: World Health Organization Consultation Group Report on methodology of noncommunicable disease screening. J Med Screen. 2005;12(1):12–19. doi: 10.1258/0969141053279086. [DOI] [PubMed] [Google Scholar]
- 29.Shamir R, Hernell O, Leshno M. Cost-effectiveness analysis of screening for celiac disease in the adult population. Med Decis Making. 2006 May-Jun;26(3):282–293. doi: 10.1177/0272989X06289012. [DOI] [PubMed] [Google Scholar]
- 30.Lohi S, Maki M, Montonen J, et al. Malignancies in cases with screening-identified evidence of coeliac disease: a long-term population-based cohort study. Gut. 2009 May;58(5):643–647. doi: 10.1136/gut.2007.140970. [DOI] [PubMed] [Google Scholar]
- 31.van Koppen EJ, Schweizer JJ, Csizmadia CG, et al. Long-term health and quality-of-life consequences of mass screening for childhood celiac disease: a 10-year follow-up study. Pediatrics. 2009 Apr;123(4):e582–588. doi: 10.1542/peds.2008-2221. [DOI] [PubMed] [Google Scholar]
- 32.Johnston SD, Rodgers C, Watson RG. Quality of life in screen-detected and typical coeliac disease and the effect of excluding dietary gluten. Eur J Gastroenterol Hepatol. 2004 Nov;16(12):1281–1286. doi: 10.1097/00042737-200412000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Mustalahti K, Lohiniemi S, Collin P, Vuolteenaho N, Laippala P, Maki M. Gluten-free diet and quality of life in patients with screen-detected celiac disease. Eff Clin Pract. 2002 May-Jun;5(3):105–113. [PubMed] [Google Scholar]
- 34.Virta LJ, Kaukinen K, Collin P. Incidence and prevalence of diagnosed coeliac disease in Finland: results of effective case finding in adults. Scand J Gastroenterol. 2009;44(8):933–938. doi: 10.1080/00365520903030795. [DOI] [PubMed] [Google Scholar]
- 35.Catassi C, Kryszak D, Louis-Jacques O, et al. Detection of Celiac disease in primary care: a multicenter case-finding study in North America. Am J Gastroenterol. 2007 Jul;102(7):1454–1460. doi: 10.1111/j.1572-0241.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 36.Hill ID, Dirks MH, Liptak GS, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005 Jan;40(1):1–19. doi: 10.1097/00005176-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Lee AR, Ng DL, Zivin J, Green PH. Economic burden of a gluten-free diet. J Hum Nutr Diet. 2007 Oct;20(5):423–430. doi: 10.1111/j.1365-277X.2007.00763.x. [DOI] [PubMed] [Google Scholar]
- 38.Thompson T, Dennis M, Higgins LA, Lee AR, Sharrett MK. Gluten-free diet survey: are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J Hum Nutr Diet. 2005 Jun;18(3):163–169. doi: 10.1111/j.1365-277X.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 39.Kinsey L, Burden ST, Bannerman E. A dietary survey to determine if patients with coeliac disease are meeting current healthy eating guidelines and how their diet compares to that of the British general population. Eur J Clin Nutr. 2008 Nov;62(11):1333–1342. doi: 10.1038/sj.ejcn.1602856. [DOI] [PubMed] [Google Scholar]
- 40.Whitaker JK, West J, Holmes GK, Logan RF. Patient perceptions of the burden of coeliac disease and its treatment in the UK. Aliment Pharmacol Ther. 2009 May 15;29(10):1131–1136. doi: 10.1111/j.1365-2036.2009.03983.x. [DOI] [PubMed] [Google Scholar]
- 41.Zarkadas M, Cranney A, Case S, et al. The impact of a gluten-free diet on adults with coeliac disease: results of a national survey. J Hum Nutr Diet. 2006 Feb;19(1):41–49. doi: 10.1111/j.1365-277X.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- 42.Olsson C, Hornell A, Ivarsson A, Sydner YM. The everyday life of adolescent coeliacs: issues of importance for compliance with the gluten-free diet. J Hum Nutr Diet. 2008 Aug;21(4):359–367. doi: 10.1111/j.1365-277x.2008.00867.x. [DOI] [PubMed] [Google Scholar]
- 43.Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr. 2007 Jan;85(1):160–166. doi: 10.1093/ajcn/85.1.160. [DOI] [PubMed] [Google Scholar]
- 44.Akobeng AK, Thomas AG. Systematic review: tolerable amount of gluten for people with coeliac disease. Aliment Pharmacol Ther. 2008 Jun 1;27(11):1044–1052. doi: 10.1111/j.1365-2036.2008.03669.x. [DOI] [PubMed] [Google Scholar]
- 45.Schuppan D, Dennis MD, Kelly CP. Celiac disease: epidemiology, pathogenesis, diagnosis, and nutritional management. Nutr Clin Care. 2005 Apr-Jun;8(2):54–69. [PubMed] [Google Scholar]
- 46.Thompson T, Lee AR, Grace T. Gluten contamination of grains, seeds, and flours in the United States: a pilot study. J Am Diet Assoc. 2010 Jun;110(6):937–940. doi: 10.1016/j.jada.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Hall NJ, Rubin G, Charnock A. Systematic review: adherence to a gluten-free diet in adult patients with coeliac disease. Aliment Pharmacol Ther. 2009 Aug 15;30(4):315–330. doi: 10.1111/j.1365-2036.2009.04053.x. [DOI] [PubMed] [Google Scholar]
- 48.Leffler DA, Dennis M, Edwards George JB, et al. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin Gastroenterol Hepatol. 2009 May;7(5):530–536. 536 e531–532. doi: 10.1016/j.cgh.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 49.Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol. 2002 Aug;97(8):2016–2021. doi: 10.1111/j.1572-0241.2002.05917.x. [DOI] [PubMed] [Google Scholar]
- 50.Murray JA, Watson T, Clearman B, Mitros F. Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. Am J Clin Nutr. 2004 Apr;79(4):669–673. doi: 10.1093/ajcn/79.4.669. [DOI] [PubMed] [Google Scholar]
- 51.Di Sant’Agnese PA. Idiopathic celiac disease. II. Course and prognosis. Pediatrics. 1953 Mar;11(3):224–237. [PubMed] [Google Scholar]
- 52.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007 Oct 25;357(17):1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 53.Rubio-Tapia A, Rahim MW, See JA, Lahr BD, Wu TT, Murray JA. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010 Jun;105(6):1412–1420. doi: 10.1038/ajg.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009 Dec;137(6):1912–1933. doi: 10.1053/j.gastro.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Tursi A, Giorgetti G, Brandimarte G, Elisei W. Effect of gluten-free diet on pregnancy outcome in celiac disease patients with recurrent miscarriages. Dig Dis Sci. 2008 Nov;53(11):2925–2928. doi: 10.1007/s10620-008-0242-x. [DOI] [PubMed] [Google Scholar]
- 56.Tata LJ, Card TR, Logan RF, Hubbard RB, Smith CJ, West J. Fertility and pregnancy-related events in women with celiac disease: a population-based cohort study. Gastroenterology. 2005 Apr;128(4):849–855. doi: 10.1053/j.gastro.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 57.Dennis CKARLM, Sandquist D, Sharrett M, Thompson T. [Accessed April 24, 2011, 2011.];American Dietetics Association Celiac Disease Evidence Based Nutrition Practice Guideline. http://www.adaevidencelibrary.com/topic.cfm?cat=3677.
- 58.NIH Consensus Development Conference on Celiac Disease. NIH Consens State Sci Statements. 2004 Jun 28–30;21(1):1–23. [PubMed] [Google Scholar]
- 59.Ludvigsson JF, Montgomery SM, Ekbom A. Celiac disease and risk of adverse fetal outcome: a population-based cohort study. Gastroenterology. 2005 Aug;129(2):454–463. doi: 10.1016/j.gastro.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 60.Shah S, Leffler D. Celiac disease: an underappreciated issue in women’s health. Womens Health (Lond Engl) 2010 Sep;6(5):753–766. doi: 10.2217/whe.10.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soni S, Badawy SZ. Celiac disease and its effect on human reproduction: a review. J Reprod Med. 2010 Jan-Feb;55(1–2):3–8. [PubMed] [Google Scholar]
- 62.Lanzini A, Lanzarotto F, Villanacci V, et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment Pharmacol Ther. 2009 Jun 15;29(12):1299–1308. doi: 10.1111/j.1365-2036.2009.03992.x. [DOI] [PubMed] [Google Scholar]
- 63.Hernandez L, Green PH. Extraintestinal manifestations of celiac disease. Curr Gastroenterol Rep. 2006 Oct;8(5):383–389. doi: 10.1007/s11894-006-0023-7. [DOI] [PubMed] [Google Scholar]
- 64.Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003 Mar;124(3):795–841. doi: 10.1053/gast.2003.50106. [DOI] [PubMed] [Google Scholar]
- 65.Hadjivassiliou M, Sanders DS, Grunewald RA, Woodroofe N, Boscolo S, Aeschlimann D. Gluten sensitivity: from gut to brain. Lancet Neurol. 2010 Mar;9(3):318–330. doi: 10.1016/S1474-4422(09)70290-X. [DOI] [PubMed] [Google Scholar]
- 66.Cosnes J, Cellier C, Viola S, et al. Incidence of autoimmune diseases in celiac disease: protective effect of the gluten-free diet. Clin Gastroenterol Hepatol. 2008 Jul;6(7):753–758. doi: 10.1016/j.cgh.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 67.Leffler DA, Edwards George JB, Dennis M, Cook EF, Schuppan D, Kelly CP. A prospective comparative study of five measures of gluten-free diet adherence in adults with coeliac disease. Aliment Pharmacol Ther. 2007 Nov 1;26(9):1227–1235. doi: 10.1111/j.1365-2036.2007.03501.x. [DOI] [PubMed] [Google Scholar]
- 68.Cataldo F, Marino V, Ventura A, Bottaro G, Corazza GR. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and “Club del Tenue” Working Groups on Coeliac Disease. Gut. 1998 Mar;42(3):362–365. doi: 10.1136/gut.42.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zone JJ. Skin manifestations of celiac disease. Gastroenterology. 2005 Apr;128(4 Suppl 1):S87–91. doi: 10.1053/j.gastro.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 70.Sabel’nikova EA, Parfenov AI, Krums LM, et al. Prevalence of celiac disease in patients with chronic diarrhea. Eksp Klin Gastroenterol. 2004;(3):31–34. 102–103. [PubMed] [Google Scholar]
- 71.Catassi C, Fasano A. Celiac disease as a cause of growth retardation in childhood. Curr Opin Pediatr. 2004 Aug;16(4):445–449. doi: 10.1097/01.mop.0000133637.64414.20. [DOI] [PubMed] [Google Scholar]
- 72.Howard MR, Turnbull AJ, Morley P, Hollier P, Webb R, Clarke A. A prospective study of the prevalence of undiagnosed coeliac disease in laboratory defined iron and folate deficiency. J Clin Pathol. 2002 Oct;55(10):754–757. doi: 10.1136/jcp.55.10.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003 Feb 10;163(3):286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 74.Rostami K, Mulder CJ, van Overbeek FM, et al. Should relatives of coeliacs with mild clinical complaints undergo a small-bowel biopsy despite negative serology? Eur J Gastroenterol Hepatol. 2000 Jan;12(1):51–55. doi: 10.1097/00042737-200012010-00010. [DOI] [PubMed] [Google Scholar]
- 75.Ford AC, Chey WD, Talley NJ, Malhotra A, Spiegel BM, Moayyedi P. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med. 2009 Apr 13;169(7):651–658. doi: 10.1001/archinternmed.2009.22. [DOI] [PubMed] [Google Scholar]
- 76.Spiegel BM, DeRosa VP, Gralnek IM, Wang V, Dulai GS. Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: a cost-effectiveness analysis. Gastroenterology. 2004 Jun;126(7):1721–1732. doi: 10.1053/j.gastro.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 77.Rubio-Tapia A, Murray JA. The liver in celiac disease. Hepatology. 2007 Nov;46(5):1650–1658. doi: 10.1002/hep.21949. [DOI] [PubMed] [Google Scholar]
- 78.Ransford RA, Hayes M, Palmer M, Hall MJ. A controlled, prospective screening study of celiac disease presenting as iron deficiency anemia. J Clin Gastroenterol. 2002 Sep;35(3):228–233. doi: 10.1097/00004836-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 79.Sanders DS, Evans KE, Hadjivassiliou M. Fatigue in primary care. Test for coeliac disease first? Bmj. 2010;341:c5161. doi: 10.1136/bmj.c5161. [DOI] [PubMed] [Google Scholar]
- 80.van der Windt DA, Jellema P, Mulder CJ, Kneepkens CM, van der Horst HE. Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review. Jama. 2010 May 5;303(17):1738–1746. doi: 10.1001/jama.2010.549. [DOI] [PubMed] [Google Scholar]
- 81.Hadjivassiliou M, Grunewald RA, Kandler RH, et al. Neuropathy associated with gluten sensitivity. J Neurol Neurosurg Psychiatry. 2006 Nov;77(11):1262–1266. doi: 10.1136/jnnp.2006.093534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hadjivassiliou M, Grunewald R, Sharrack B, et al. Gluten ataxia in perspective: epidemiology, genetic susceptibility and clinical characteristics. Brain. 2003 Mar;126(Pt 3):685–691. doi: 10.1093/brain/awg050. [DOI] [PubMed] [Google Scholar]
- 83.Cheng J, Malahias T, Brar P, Minaya MT, Green PH. The association between celiac disease, dental enamel defects, and aphthous ulcers in a United States cohort. J Clin Gastroenterol. 2009 Mar;44(3):191–194. doi: 10.1097/MCG.0b013e3181ac9942. [DOI] [PubMed] [Google Scholar]
- 84.Ludvigsson JF, Olen O, Bell M, Ekbom A, Montgomery SM. Coeliac disease and risk of sepsis. Gut. 2008 Aug;57(8):1074–1080. doi: 10.1136/gut.2007.133868. [DOI] [PubMed] [Google Scholar]
- 85.Corazza GR, Zoli G, Di Sabatino A, Ciccocioppo R, Gasbarrini G. A reassessment of splenic hypofunction in celiac disease. Am J Gastroenterol. 1999 Feb;94(2):391–397. doi: 10.1111/j.1572-0241.1999.00865.x. [DOI] [PubMed] [Google Scholar]
- 86.Green PH, Yang J, Cheng J, Lee AR, Harper JW, Bhagat G. An association between microscopic colitis and celiac disease. Clin Gastroenterol Hepatol. 2009 Nov;7(11):1210–1216. doi: 10.1016/j.cgh.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 87.Mein SM, Ladabaum U. Serological testing for coeliac disease in patients with symptoms of irritable bowel syndrome: a cost-effectiveness analysis. Aliment Pharmacol Ther. 2004 Jun 1;19(11):1199–1210. doi: 10.1111/j.1365-2036.2004.01958.x. [DOI] [PubMed] [Google Scholar]
- 88.Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009 Jan;104( Suppl 1):S1–35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 89.Sanders DS, Patel D, Khan FB, et al. Case-finding for adult celiac disease in patients with reduced bone mineral density. Dig Dis Sci. 2005 Mar;50(3):587–592. doi: 10.1007/s10620-005-2479-y. [DOI] [PubMed] [Google Scholar]
- 90.Zipser RD, Patel S, Yahya KZ, Baisch DW, Monarch E. Presentations of adult celiac disease in a nationwide patient support group. Dig Dis Sci. 2003 Apr;48(4):761–764. doi: 10.1023/a:1022897028030. [DOI] [PubMed] [Google Scholar]
- 91.Siniscalchi M, Iovino P, Tortora R, et al. Fatigue in adult coeliac disease. Aliment Pharmacol Ther. 2005 Sep 1;22(5):489–494. doi: 10.1111/j.1365-2036.2005.02619.x. [DOI] [PubMed] [Google Scholar]
- 92.Nachman F, Vazquez H, Gonzalez A, et al. Gastroesophageal reflux symptoms in patients with celiac disease and the effects of a gluten-free diet. Clin Gastroenterol Hepatol. 2011 Mar;9(3):214–219. doi: 10.1016/j.cgh.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 93.Ludvigsson JF, Montgomery SM, Ekbom A. Risk of pancreatitis in 14,000 individuals with celiac disease. Clin Gastroenterol Hepatol. 2007 Nov;5(11):1347–1353. doi: 10.1016/j.cgh.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Collin P, Reunala T. Recognition and management of the cutaneous manifestations of celiac disease: a guide for dermatologists. Am J Clin Dermatol. 2003;4(1):13–20. doi: 10.2165/00128071-200304010-00002. [DOI] [PubMed] [Google Scholar]
- 95.Neuhausen SL, Steele L, Ryan S, et al. Co-occurrence of celiac disease and other autoimmune diseases in celiacs and their first-degree relatives. J Autoimmun. 2008 Sep;31(2):160–165. doi: 10.1016/j.jaut.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garud S, Leffler D, Dennis M, et al. Interaction between psychiatric and autoimmune disorders in coeliac disease patients in the Northeastern United States. Aliment Pharmacol Ther. 2009 Apr 15;29(8):898–905. doi: 10.1111/j.1365-2036.2009.03942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abenavoli L, Proietti I, Zaccone V, Gasbarrini G, Addolorato G. Celiac disease: from gluten to skin. Expert Rev Clin Immunol. 2009 Nov;5(6):789–800. doi: 10.1586/eci.09.46. [DOI] [PubMed] [Google Scholar]
- 98.Lionetti E, Francavilla R, Pavone P, et al. The neurology of coeliac disease in childhood: what is the evidence? A systematic review and meta-analysis. Dev Med Child Neurol. 2010 Aug;52(8):700–707. doi: 10.1111/j.1469-8749.2010.03647.x. [DOI] [PubMed] [Google Scholar]
- 99.Ludvigsson JF, Reutfors J, Osby U, Ekbom A, Montgomery SM. Coeliac disease and risk of mood disorders--a general population-based cohort study. J Affect Disord. 2007 Apr;99(1–3):117–126. doi: 10.1016/j.jad.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 100.Hu WT, Murray JA, Greenaway MC, Parisi JE, Josephs KA. Cognitive impairment and celiac disease. Arch Neurol. 2006 Oct;63(10):1440–1446. doi: 10.1001/archneur.63.10.1440. [DOI] [PubMed] [Google Scholar]
- 101.Zelnik N, Pacht A, Obeid R, Lerner A. Range of neurologic disorders in patients with celiac disease. Pediatrics. 2004 Jun;113(6):1672–1676. doi: 10.1542/peds.113.6.1672. [DOI] [PubMed] [Google Scholar]
- 102.Giordano L, Valotti M, Bosetti A, Accorsi P, Caimi L, Imberti L. Celiac disease-related antibodies in Italian children with epilepsy. Pediatr Neurol. 2009 Jul;41(1):34–36. doi: 10.1016/j.pediatrneurol.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 103.Moccia M, Pellecchia MT, Erro R, et al. Restless legs syndrome is a common feature of adult celiac disease. Mov Disord. 2010 May 15;25(7):877–881. doi: 10.1002/mds.22903. [DOI] [PubMed] [Google Scholar]
- 104.Gastroenterology PCSf. [Accessed, April 15, 2011.];The Managment of Adults with Coeliac Disease in Primary Care. http://www.coeliac.org.uk/sites/files/coeliac/PCSG_the_management_of_adults_with_coeliac_disease_in_primary_care.pdf.
- 105.Bai J, Fried EZM, Corazza GR, Schuppan D, Farthing MJG, Catassi C, Greco L, Cohen H, Krabshuis JH. [Accessed April 24, 2011.];World Gastroenterology Organisation Practice Guidelines: Celiac Disease. http://www.worldgastroenterology.org/assets/downloads/en/pdf/guidelines/04_celiac_disease.pdf.