Abstract
It is commonly perceived that the human immune system is naïve to the newly emerged H5N1 virus. In contrast, most adults have been exposed to influenza A H1N1 and H3N2 viruses through vaccination or infection. Adults born before 1968 have likely been exposed to H2N2 viruses. We hypothesized that CD4+ T cells generated in response to H1N1, H3N2 and H2N2 influenza A viruses also recognize H5N1 epitopes. Tetramer Guided Epitope Mapping and antigen specific class II tetramers were used to identify H5N1 specific T cell epitopes and to detect H5N1 specific T cell responses. 15 out of 15 healthy subjects tested had robust CD4+ T cell responses against matrix protein, nucleoprotein and neuraminidase of the influenza A/Viet Nam/1203/2004 (H5N1) virus. These results are not surprising, since the matrix protein and nucleoprotein of influenza A viruses are conserved, while the neuraminidase of the H5N1 virus is of the same subtype as the circulating H1N1 influenza strain. However, H5N1 hemagglutinin reactive CD4+ T cells were also detected in 14 out of 14 subjects examined, despite the fact that hemagglutinin is less conserved. Most were cross-reactive to H1, H2 or H3 hemagglutinin epitopes. H5N1 reactive T cells were also detected ex vivo, exhibited a memory phenotype, and were capable of secreting IFN-γ, TNF-α, IL-5, and IL-13. These data demonstrate the presence of H5N1 cross-reactive T cells in healthy Caucasian subjects implying that exposure to influenza A H1N1, H3N2 or H2N2 viruses through either vaccination or infection may provide partial immunity to H5N1 virus.
Keywords: T cells, MHC, Epitopes, Viral, Vaccination
Introduction
In recent years, highly pathogenic influenza A H5N1 viruses have caused disease outbreaks in poultry and wild birds in Asia, Africa and Europe. Sporadic human cases have been reported in different countries, mostly in Asia (1–6). As of October 17, 2007, a total of 331 human cases have been confirmed, with a fatality rate of close to 60% (http://www.who.int/csr/disease/avian_influenza).
At present, most of the reported human cases have occurred in rural areas and direct contact with infected poultry was the probable route of transmission. However, the ability of influenza A viruses to undergo genetic reassortment with gene segments from other influenza A strains and the high mutation rate of their RNA genomes have raised concerns that strains of H5N1 could adapt the ability to infect humans directly, eventually causing a human pandemic (7,8). Although the majority of the human population has not been exposed directly to H5N1 viruses, most adults in developed countries have been exposed to other influenza A viruses, including influenza A H1N1, and H3N2 viruses through either vaccination or infection. In addition, many adults born before 1968 have been exposed to H2N2 (9). Given the homology that exists between the viral proteins of different influenza A subtypes, we hypothesized that CD4+ T cells generated in response to other influenza A viruses could also recognize H5N1 viral proteins. In this study we examined whether healthy adults in the U.S. had measurable CD4+ T cell responses directed against the Matrix Protein 1 (M1), Nucleoprotein (NP), Neuraminidase (NA) and Hemagglutinin (HA) of the highly pathogenic A/Viet Nam/1203/2004 (H5N1) virus (10,11). All subjects examined had CD4+ T cells directed against M1, NP and NA of the H5N1 viruses. Moreover, H5N1 HA reactive CD4+ T cells were also detected. Thus the immune repertoire of most healthy subjects may afford some protection from the H5N1 virus.
Materials and Methods
Human Subjects
The study was approved by the Benaroya Research Institute Institutional Review Board. A total of 22 subjects were recruited with informed consent for these studies. All subjects were healthy volunteers of Caucasian descent. A two-step protocol was used to determine the class II HLA genotype for each subject. Low-resolution class II typing was carried out using an SSP UniTray typing kit (Invitrogen,). High-resolution class II typing was accomplished by DNA sequencing with allele specific primers based on low-resolution typing results. Subjects having DR0101, DR0404, DR0701 and DR1101 haplotypes were selected for detailed studies as reported in this manuscript. In addition, subjects with DR0301 and DR1501 were also examined. These alleles were selected based on their prevalence in the US Caucasian population and the availability of these soluble MHC molecules in our laboratory.
Production of class II tetramers
Soluble HLA class II molecules were used in this study. The production and purification of these recombinant class II molecules have been previously described (12). For production of class II tetramers, soluble class II molecules were labeled with biotin by using Biotin ligase according to the manufacturer's instruction (Avidity). Biotinylated class II molecules were dialyzed into phosphate storage buffer (pH 6.0) and loaded with pooled peptide mixtures or individual peptides by combining 0.5 mg/ml class II, 10 mg/ml of peptide (2 mg/ml of each individual peptide for pools), and 0.2% η-octyl-D-glucopyranoside. After incubating at 37°C for 48 hours, tetramers were cross-linked using PE-Streptavidin (Biosource) (13).
Peptides and recombinant H5 HA protein
Overlapping peptides that covered the entire A/Viet Nam/1203/2004 (H5N1) M1, NP, NA and HA proteins were purchased from Mimotopes. These peptides were 20 aa in length sharing a 12 aa overlap with the adjacent peptides. There were a total of 30 M1 peptides, a total of 61 NP peptides, 55 NA peptides and 70 HA peptides. In TGEM experiments, peptides spanning the sequence of each protein were divided up into different pools, with 5 consecutive peptides in each pool. There were a total of 6 pools for M1, 12 pools for NP (with 6 peptides in pool 12), 11 pools for NA, and 14 pools for HA. H1, H2, H3 and H5 HA peptides 12aa to 20aa in length were also purchased from Mimotopes. Recombinant H5 HA protein derived from A/Viet Nam/1203/2004 (H5N1) was provided by Biodefense and Emerging Infections Research Resource Repository (BEI Resources).
TGEM
The TGEM procedure was as previously described (14,15). Briefly, CD4+ T cells were isolated from PBMC using the Miltenyi “no-touch” CD4+ T cell isolation kit. Non-CD4+ cells recovered from the magnetic column were incubated in 48 well plates (3 × 106 cells per well) for 1 hour and then washed, leaving adherent cells for use as APC. After adding 2 million CD4+ T cells per well, each well was stimulated with pooled influenza A peptides (10 μg/ml). Cells were fed with 20U/ml IL-2 in fresh medium starting at day 7, and were re-fed as needed. After 14 days incubation, 100 μl of resuspended cells were stained with PE-conjugated pooled peptide tetramers for 60 min at 37°C. Subsequently, cells were stained with CD4-PerCP, CD3-allophycocyanin and CD25-FITC mAbs (eBioscience) and analyzed by flow cytometry. Cells from pools that gave positive staining were analyzed again with individual peptide tetramers produced by loading empty class II molecules with each of the individual peptides from the positive peptide pool.
Other cell culture and tetramer staining assays
An approach that involved magnetic bead enrichment for tetramer positive cells, as previously described (16), was used to determine the frequencies of H5N1 reactive T cells. In brief, six million of PBMC in a volume of 100 μl was stained with 20 μg/ml tetramers at room temperature for 2 hrs. During the last 20 min, the cells were stained with FITC conjugated anti-CD45RA (eBioscience), allophycocyanin conjugated anti-CD4 (eBioscience), PerCP conjugated anti-CD14 (BD Pharmingen) and PerCP conjugated anti-CD19 (BD Pharmingen). Subsequently, cells were washed and then incubated with anti-PE magnetic bead (Miltenyi Biotec) at 4°C for another 20 min. The cells were washed again and a tenth of the fraction was saved for later analysis. The other fraction was passed through a Miltenyi magnetic column. The bound fraction was flushed out and collected. Cells in both the bound fraction and the pre-column fraction were stained with ViaProbe (BD Biosciences) for 10 min before flow cytometry. For analysis, cells were gated on
FCS/SSC, CD4+, CD14− and CD19− population. Dead cells were also gated out using Viaprobe. The frequency was calculated as the total number of tetramer positive cells in the bound fraction divided by 10 × the total number of CD4+ T cells in the pre-column fraction
For assays using fractionated memory T cells, CD4+ cells were isolated as described above. Cells were then stained with FITC-conjugated anti-CD45RA and PE-Cy5-conjugated anti-CD4 Abs, and the CD4+CD45RA+ and the CD4+CD45RA− fractions were sorted. 2.5 × 106 CD4+CD45RA− cells were stimulated with 10 μg/ml of HA peptide. Cells were fed with IL-2 starting at day 7, and were analyzed with tetramers on day 14.
For experiments using H5 HA proteins (rather than peptides), CD4+ T cells and CD14+ cells at a 10 to 1 ratio were incubated with H5 HA protein at 100 μg/ml for 2 hr at 37°C in a conical tube. Cells were washed once, and then plated out at a density of 2.5 × 106 cells per well in a 48 well plate. Cells were analyzed with tetramers on day 14.
For cross-reactivity experiments, cells were plated into 4 different wells. Cells in each individual well were stimulated with H5 HA peptide, or with HA peptides from the corresponding region of H1, H2, H3 HA (all at 10 μg/ml). Cells were analyzed with tetramers on day 14.
Cytokine Assays
For ex vivo staining of PBMC for cytokines, 4 million PBMC were activated with a panel of tetramers (2.5 μg/ml each) and CD28/CD49d (1 μg/ml), Belfedin A (5 μg/ml) was added 2 hrs later. Cells were then incubated for another 16 hrs at 37°C, and permeabilized for intracellular cytokine staining (ICS) according to protocol as suggested by BD BioSciences. Briefly, cells were treated with 20 mM EDTA, washed, and then subsequently followed by washes with BD FACS Lyse buffer and BD FACS PERM II buffer. Cells were then stained with the appropriate allophycocyanin conjugated cytokine antibody, PerCP conjugated anti-CD4 and FITC conjugated anti-CD45RA. Cells were again washed and resuspended in FACS buffer with 1% paraformaldehyde and 1% FBS before analysis. Cells were gated on size scattering, and the CD4+ tetramer positive population was examined for cytokine staining.
For cytokine staining of in vitro stimulated PBMC, cells were stimulated with the peptide of interest as described above. On day 14, 200,000 cells in 100 μl were transferred to wells in a 96 well flat bottom plate which were precoated with 0.5 μg of the corresponding tetramers of interest. After an overnight culture, 25 μl of the supernatants were harvested and assayed for cytokines using the Meso Scale Discovery (MSD) Cytokine Multiplex kit according to the manufacturer's instruction. The MSD plate was read using the MSD SECTOR Imager 2400.
Results
Detection of CD4+ T cells that are specific for internal viral proteins of A/Viet Nam/1203/2004
In general, there is a perception that the human immune system is naive to the newly emerged H5N1 virus. However, the internal proteins of influenza A viruses, such as M1 and NP are highly conserved amongst different subtypes. As the general population has been exposed to various influenza A viruses, it seemed likely that T cells in the peripheral blood of human subjects would recognize the internal proteins of H5N1 viruses, such as A/Viet Nam/1203/2004.
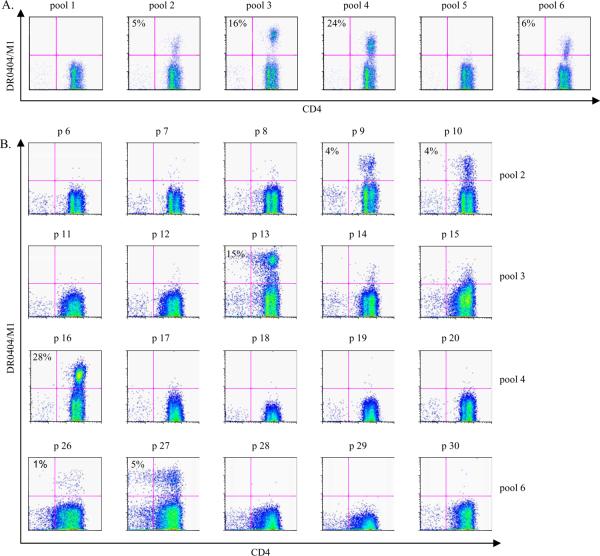
To test this possibility, the Tetramer Guided Epitope Mapping (TGEM) approach (14,15) was used to identify CD4+ T cell epitopes within the M1 protein of A/Viet Nam/1203/2004. In one representative experiments, CD4+ T cells from a DRA1/DRB1*0404 (DR0404) subject were stimulated with the 6 different pooled overlapping peptides derived from A/Viet Nam/1203/2004 M1 peptides. Fourteen days later, cells were stained with the corresponding DR0404/M1 pooled peptide tetramers. M1 peptides that gave positive staining were observed within pools 2, 3, 4 and 6 (Figure 1A). Single peptide loaded tetramers for each individual peptide within the positive pools were used to stain the cells again. As shown in Figure 1B, tetramers loaded with individual peptides p9, p10, p13, p16, p26 and p27 gave clear positive staining for this DR0404 subject. Positive staining with a specific peptide identifies that peptide as a T cell epitope, and confirms the presence of CD4+ T cells specific for that particular epitope within the PBMC of the healthy subject studied. These experiments identified M165–84 (p9), M173–92 (p10), M197–116 (p13), M1121–140 (p16), M1201–220 (p26), and M1209–228 (p27) as peptides containing DR0404 restricted H5N1 M1 epitopes for the subject studied. The sequences of these peptides are shown in Table I.
Figure 1.
Identification of DR0404 restricted M1 epitopes. CD4+ T cells from a DR0404 subject were stimulated with 6 peptide pools derived from the M1 protein of A/Viet Nam/1203/2004. A. Cells were stained with the corresponding DR0404 pooled M1 peptide tetramers at day 14. Pools 2, 3, 4 and 6 gave positive staining. B. Cells stimulated with peptide pools that gave positive staining were stained with DR0404 individual peptide tetramers at day 17. The percentage of tetramer positive cells is indicated. Background staining in these experiments was 0.3% or lower. Peptides p9, p10, p13, p16, p26 and p27, which correspond to M165–84, M173–92, M197–116, M1121–140, M1201–220 and M1209–228 respectively, gave staining significantly above background and were identified as H5 M1 epitopes.
Table I. HLA-DR restricted A/Viet Nam/1203/2004 M1 and NP epitopes
| DR0101 restricted H5N1 M1 epitopes | DR0101 restricted H5N1 NP epitopes | ||
| M117–36 | SGPLKAEIAQKLEDVFAGKN | NP65–84 | RMVLSAFDERRNRYLEEHPS |
| NP73–92 | ERRNRYLEEHPSAGKDPKKT | ||
| NP161–180 | PRMCSLMQGSTLPRRSGAAG | ||
| NP185–204 | GVGTMVMELIRMIKRGINDR | ||
| NP297–316 | SLVGIDPFRLLQNSQVFSLI | ||
| NP401–420 | ASAGQISVQPTFSVQRNLPF | ||
| NP441–460 | RTEIIRMMESARPEDVSFQG | ||
| DR0404 restricted H5N1 M1 epitopes | DR0404 restricted H5N1 NP epitopes | ||
| M165–84 | TLTVPSERGLQRRRFVQNAL | NP321–340 | NPAHKSQLVWMACHSAAFED |
| M173–92 | GLQRRRFVQNALNGNGDPNN | NP401–420 | ASAGQISVQPTFSVQRNLPF |
| M197–116 | VKLYKKLKREITFHGAKEVA | NP433–452 | TEGRTSDMRTEIIRMMESAR |
| M1121–140 | TGALASCMGLIYNRMGTVTT | NP441–460 | RTEIIRMMESARPEDVSFQG |
| M1201–220 | EAMEIANQARQMVQAMRTIG | ||
| M1209–228 | ARQMVQAMRTIGTHPNSSAG | ||
| DR0701 restricted H5N1 M1 epitopes | DR0701 restricted H5N1 NP epitopes | ||
| M19–28 | TYVLSIIPSGPLKAEIAQKL | NP137–156 | MIWHSNLNDATYQRTRALVR |
| M133–52 | AGKNTDLEALMEWLKTRPIL | NP145–164 | DATYQRTRALVRTGMDPRMC |
| M141–60 | ALMEWLKTRPILSPLTKGIL | NP257–276 | IFLARSALILRGSVAHKSCL |
| M197–116 | VKLYKKLKREITFHGAKEVA | NP265–284 | ILRGSVAHKSCLPACVYGLA |
| M1161–180 | SHRQMATITNPLIRHENRMV | NP321–340 | NPAHKSQLVWMACHSAAFED |
| M1169–188 | TNPLIRHENRMVLASTTAKA | ||
| M1177–196 | NRMVLASTTAKAMEQMAGSS | ||
| DR1101 restricted H5N1 M1 epitopes | DR1101 restricted NP H5N1 epitopes | ||
| M197–116 | VKLYKKLKREITFHGAKEVA | NP105–124 | VRELILYDKEEIRRIWRQAN |
| M1169–188 | TNPLIRHENRMVLASTTAKA | NP113–132 | KEEIRRIWRQANNGEDATAG |
| M1201–220 | EAMEIANQARQMVQAMRTIG | NP185–204 | GVGTMVMELIRMIKRGINDR |
| M1209–228 | ARQMVQAMRTIGTHPNSSAG | NP193–212 | LIRMIKRGINDRNFWRGENG |
| NP377–396 | NTLELRSRYWAIRTRSGGNT | ||
| NP385–404 | YWAIRTRSGGNTNQQRASAG | ||
Similar experiments were also carried out to identify DR0404 restricted H5 NP specific T cells. A total of 61 NP peptides in 12 different pools were screened with DR0404/NP pooled peptide tetramers. Positive staining was observed with pools 9, 11 and 12. Subsequent staining identified NP peptides NP321–340 (from pool 9), NP401–420, NP433–452 (from pool 11) and NP441–460 (from pool 12) as DR0404 restricted NP epitopes. The sequences of these peptides are summarized in Table I. Experiments were repeated with other DR0404 subjects, and identical M1 and NP epitopes were identified. Thus the TGEM approach was successful in identifying DR0404 restricted T cells in multiple individuals with the DR0404 haplotype that recognize epitopes within the M1 and NP proteins of the A/Viet Nam/1203/2004 virus.
These results were not surprising, given the high degree of homology between the M1 and NP proteins of H5N1 and circulating strains of influenza A virus. Examination of the H5 M1 and NP amino acid (aa) sequences identified above and the corresponding M1 and NP regions in both the A/New Caledonia/20/99 (H1N1) and A/Panama/2007/99 (H3N2) viruses indicated that these sequences are either highly conserved or completely identical. The observed responses were not unique to subjects having the DR0404 allele, as CD4+ T cell responses to H5N1 M1 and NP proteins were also observed in 6 additional subjects with either DRA1/DRB1*0101(DR0101), DRA1/DRB1*0701(DR0701) or DRA1/DRB1*1101 (DR1101) alleles. These data are also summarized in Table I.
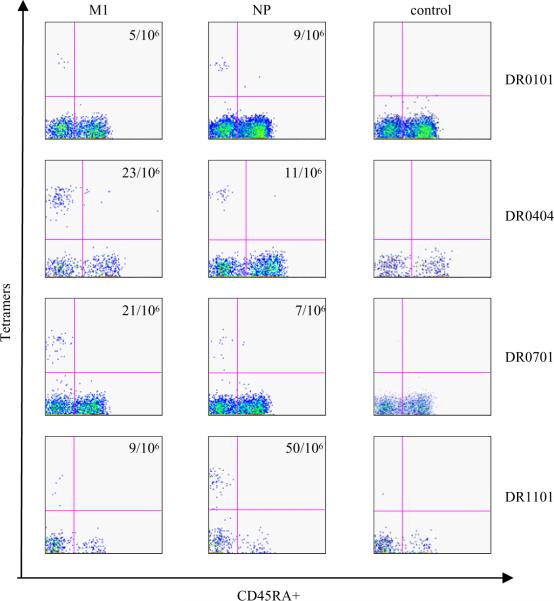
Both H5N1 M1 and NP reactive T cells were also detected ex vivo by tetramers. For these experiments, PBMC were stained with a panel of PE conjugated tetramers. For DR0404 subjects, the M1 tetramers used were DR0404/M173–92, DR0404/M1121–140 and DR0404/M1209–228; the NP tetramers used were DR0404/NP321–340, DR0404/NP401–420 and DR0404/NP433–452. For DR0701 subjects, the M1 tetramers used were DR0701/M133–52, DR0701/M197–116 and DR0701/M1177–196; the NP tetramers used were DR0701/NP145–164, DR0701/NP257–276 and DR0701/NP321–340. For DR0101 subjects, the M1 tetramer used was DR0101/ M117–36; and the NP tetramers used were DR0101/NP73–92, DR0101/NP297–316 and DR0101/NP401–420. For DR1101 subjects, the M1 tetramers used were DR1101/M1169–188 and DR1101/M1201–220; the NP tetramers used were DR1101/NP113–132 and DR1101/NP185–204. Cells were treated with anti-PE magnetic beads and tetramer positive cells were enriched uisng a Miltenyi column. The cells were then co-stained with anti-CD4 and anti-CD45RA antibodies before flow cytometry analyses. Representative results of these experiments are shown in Figure 2. The results also indicated that these M1 and NP reactive T cells were CD45RA−, suggesting that these are memory CD4+ T cells. Amongst the 7 different subjects examined, the frequencies of M1 reactive T cells ranged from 4/106 to 33/106; frequencies of NP reactive T cells ranged from 5/106 to 50/106. The observed frequencies of these H5N1 T cells were similar to the frequencies of the H3N2 influenza T cells as reported in the literature (17). In summary, a total of 15 different subjects were examined by either the TGEM approach or the ex vivo tetramer staining approach, and all had measurable H5N1 M1 and NP T cell responses.
Figure 2.
Ex vivo staining of H5 M1 and H5 NP reactive T cells. PBMC from DR0101, DR0404 DR0701 and DR1101 subjects were stained with a panel of HLA- matched DR0101/M1, DR0101/NP, DR0404/M1, DR0404/NP, DR0701/M1, DR0701/NP, DR1101/M1, DR1101/NP tetramers or the appropriate control tetramers. The panel of tetramers being used and the ex vivo staining protocol were as stated in the text. The DR0101 control tetramers was DR0101/GAD65555–567, the DR0404 control tetramers was DR0404/GAD65555–567, and both the DR0701 and DR1101 control tetramers were empty tetramers. The frequency of antigen specific T cell per million CD4+ T cells is as indicated.
CD4+ T cells which are specific for NA of A/Viet Nam/1203/2004
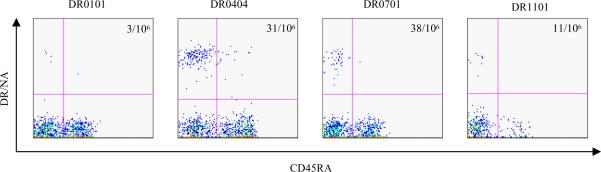
In contrast to the internal viral proteins, the viral envelope protein, NA, of influenza A viruses is less conserved, and is classified into 9 different subtypes (18,19). However, the NA gene of the A/New Caledonia/20/1999 virus (H1N1) (a current circulating influenza virus) and the NA gene of A/Viet Nam/1203/2004 (H5N1) virus belong to the same subtype and there is an 80% similarity in NA aa sequences between these two viruses. We used the TGEM approach to identify CD4+ T cell epitopes within the NA gene and to examine whether CD4+ T cells from healthy human subjects can recognize the NA protein of the A/Viet Nam/1203/2004 virus. A total of 55 NA peptides in 11 different peptide pools were screened by TGEM. Tetramer staining identified NA peptides NA201–220 (from pool 6), NA345–364 (from pool 9) and NA409–428 (from pool 11), as DR0404 restricted NA epitopes. The sequences of these peptides are listed in Table II. In addition to these DR0404 restricted NA epitopes, DR0101, DR0701 and DR1101 restricted NA epitopes were also identified in subsequent experiments (Table II). In addition, NA reactive T cells were also detected ex vivo by tetramer staining in 6 out of 7 individuals tested. Representative results are shown in Figure 3.
Table II. HLA-DR restricted H5N1 NA CD4+ T cell epitopes
| DR0101 restricted H5N1 NA epitopes | DR0701 restricted H5N1 NA epitopes | ||
| NA105–124 | SHLECRTFFLTQGALLNDKH | NA73–92 | PINGWAVYSKDNSIRIGSKG |
| NA105–124 | SHLECRTFFLTQGALLNDKH | ||
| NA113–132 | FLTQGALLNDKHSNGTVKDR | ||
| DR0404 restricted H5N1 NA epitopes | DR1101 restricted H5N1 NA epitopes | ||
| NA201–220 | NNILRTQESECACVNGSCFT | NA225–244 | GPSNGQASHKIFKMEKGKVV |
| NA345–364 | TNSRSGFEMIWDPNGWTETD | NA233–252 | HKIFKMEKGKVVKSVELDAP |
| NA409–428 | GRPKESTIWTSGSSISFCGV | NA393–412 | TGLDCIRPCFWVELIRGRPK |
| NA401–420 | CFWVELIRGRPKESTIWTSG | ||
Figure 3.
Ex vivo staining of NA reactive T cells. PBMC from DR0101, DR0404, DR0701 and DR1101 subjects were stained with a panel of HLA- matched NA tetramers. For the DR0101 subject, the tetramer was DR0101/NA105–124. For the DR0404 subject, the tetramers were DR0404/NA345–364 and DR0404/NA409–428. For the DR0701 subject, the tetramers were DR0701/NA73–92 and DR0701/NA105–124. For the DR1101 subject, the tetramers were DR1101/NA225–244 and DR1101/NA393–412. The frequency of antigen specific T cell per million CD4+ T cells is as indicated.
CD4+ T cells that are specific for HA of A/Viet Nam/1203/2004
Some degree of cross-recognition is to be expected between the M1, NP, and NA proteins given the homology of these proteins amongst the different influenza A subtypes. However, cross-recognition of hemagglutinin might be less likely given the fact that this protein is the least conserved among different subtypes (19–21). For example, amino acid sequence homology comparisons indicated there is only 63% homology between H5 and H1 HA proteins (comparison of the A/Viet Nam/1203/2004 strain and the A/New Caledonia/20/99 H1N1 strain), 74% homology between H5 and H2 (compared with the A/Singapore/1/57 H2N2 strain), and 41% homology between H5 and H3 (compared with the A/Panama/2007/99 H3N2 strain).
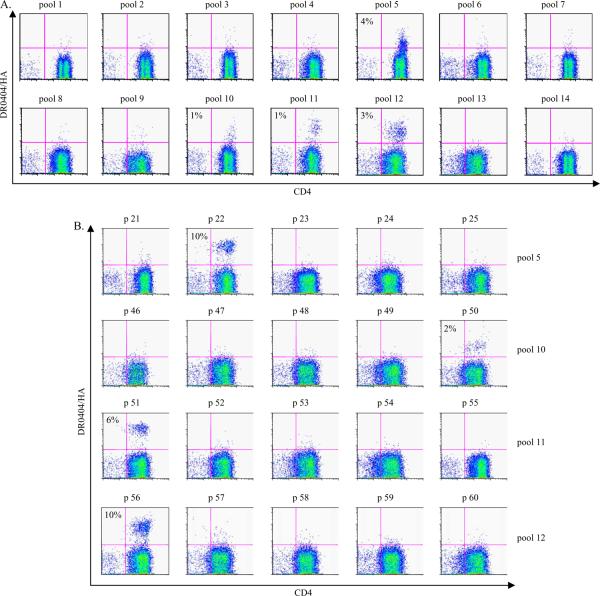
The general population has had exposure to H1N1 and H3N2 viruses, while the population born before 1968 has had exposure to H2N2 viruses (9). We reasoned that repeated exposure to influenza viruses in general would enhance HA T cell cross reactivity. Thus, to maximize our chance of detecting H5 HA reactive T cells, the subjects recruited for this portion of the study were all over 40 years old. To determine whether CD4+ T cells directed against the various influenza A viruses of H1, H2 and H3 subtypes can recognize H5 HA epitopes, the TGEM approach was used to identify H5 HA specific T cells from healthy human subjects. Results from an experiment with a DR0404 subject are shown in Figure 4. Positive tetramer staining was observed for peptide pools 5, 10, 11 and 12 and HA169–188 (p22 from pool 5), HA393–412 (p50 from pool 10), HA401–420 (p51 from pool 11) and HA441–460 (p56 from pool 12) were confirmed as DR0404 restricted H5 HA epitopes. Identical results were observed in another DR0404 subject. The results of the DR0404 restricted HA epitopes in these experiments are also summarized in Table III.
Figure 4.
Identification of DR0404 restricted HA epitopes. CD4+ T cells from a DR0404 subject were stimulated with 14 peptide pools derived from the HA protein of A/Viet Nam/1203/2004. A. Cells were stained with the corresponding DR0404 pooled HA peptide tetramers at day 14. Pools 5, 10, 11 and 12 were identified as positive pools. B. Cells stimulated with peptide pools that gave positive staining were stained with DR0404 individual peptide tetramers at day 17. The percentage of tetramer positive cells is indicated. Background staining in these experiments was 0.3% or lower. Peptides p22, p50, p51 and p56, which correspond to HA169–188, HA393–412, HA401–420 and HA441–460 respectively, were identified as T cell epitopes.
Table III. HLA-DR restricted A/Viet Nam/1203/2004 HA T cell epitopes
| Epitope identified by TGEM | Minimal epitope | HLA restriction | ||
|---|---|---|---|---|
| HA49–68 | LEKKHNGKLCDLDGVKPLIL | ND | DR0701 | |
| HA89–108 | VPEWSYIVEKANPVNDLCYP | HA91–104 | EWSYIVEKANPVND | DR0101 |
| HA153–172 | YQGKSSFFRNVVWLIKKNST | HA157–169 | SSFFRNVVWLIKK | DR0101 |
| HA169–188 | KNSTYPTIKRSYNNTNQEDL | HA174–188 | PTIKRSYNNTNQEDL | DR0404 |
| HA249–268 | LKPNDAINFESNGNFIAPEY | HA253–265 | DAINFESNGNFIA | DR0701 |
| HA265–284 | APEYAYKIVKKGDSTIMKSE | HA267–279 | EYAYKIVKKGDST | DR1101 |
| HA297–316 | PMGAINSSMPFHNIHPLTIG | HA305–316 | MPFHNIHPLTIG | DR0701 |
| HA305–324 | MPFHNIHPLTIGECPKYVKS | HA305–316 | MPFHNIHPLTIG | DR0701 |
| HA313–332 | LTIGECPKYVKSNRLVLATG | HA319–331 | PKYVKSNRLVLAT | DR1101 |
| HA393–412 | GVTNKVNSIIDKMNTQFEAV | ND | DR0301 | |
| HA393–412 | GVTNKVNSIIDKMNTQFEAV | HA400–412 | SIIDKMNTQFEAV | DR0404 |
| HA401–420 | IIDKMNTQFEAVGREFNNLE | HA400–412 | SIIDKMNTQFEAV | DR0404 |
| HA401–420 | IIDKMNTQFEAVGREFNNLE | HA408–420 | QFEAVGREFNNLE | DR1101 |
| HA433–452 | GFLDVWTYNAELLVLMENER | ND | DR1501 | |
| HA441–460 | NAELLVLMENERTLDFHDSN | HA443–458 | ELLVLMENERTLDFHD | DR0101/DR0404 |
Similar experiments lead to identification of DR0101, DR0301, DR0701, DR1101 and DR1501 restricted H5 HA responses in 12 additional healthy subjects. These epitopes are shown in Table III. The epitopes identified in these subjects were confirmed in additional subjects with identical haplotypes. Also, by stimulating T cells with shorter peptides (in the corresponding regions) and staining with tetramers generated using these shorter peptides, H5 HA T cell epitopes that were 12 to 16 aa long were also identified. These data are also summarized in Table III.
Because the experiments described above were carried out using synthetic H5 HA peptides rather than protein, it is possible that some of the epitopes identified are not naturally processed. To investigate this possibility, PBMC from several subjects were stimulated with recombinant H5 HA protein and the resulting H5 HA specific T cell responses were evaluated using H5 HA tetramers generated with the 20 aa peptides as listed in Table III. Using this approach, all of the epitopes shown in Table III (with the exception of HA49–68 which not tested) were confirmed as naturally processed epitopes (data not shown).
In a separate series of experiments, CD4+ cells were fractionated into CD45RA+ and CD45RA− populations, and these two distinct populations were stimulated with H5 HA peptide. Two weeks after the initial culture, HA specific responses were assayed by tetramers staining. Responses to all the H5HA epitopes shown in Table III (with the exception of HA49–68 which was not tested) were detected in the CD45RA− population, suggesting that the observed responses originate primarily from cross-reactive memory T cells rather than naïve T cells. Representative results of these experiments are shown in Figure 5.
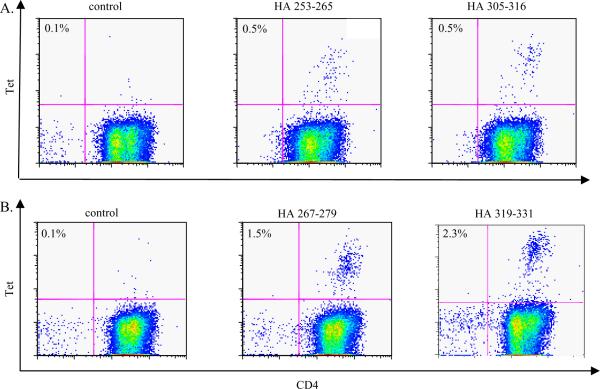
Figure 5.
H5N1 HA CD4+ T cells are memory CD4+ T cells. CD4+ T cells were sorted into CD45RA+ and CD45RA− fractions using a FACSVantage. The CD4+CD45RA− fraction was stimulated with H5 HA peptides. A. Cells from the CD45RA− fraction of a DR0701 subject were stimulated with HA253–265 (middle panel) or HA305–316 peptide (right panel). At day 14, these cells were stained with the corresponding DR0701 tetramers. The negative control was cells which have been stimulated with a M133–52 peptide and stained with DR0701/ HA253–265 tetramers. B. Cells from the CD45RA− fraction of a DR1101 subject were stimulated with HA267–279 (middle panel) or HA319–331 peptide (right panel). At day 14, these cells were stained with the corresponding DR1101 tetramers. The negative control was cells that have been stimulated with M197–116, and stained with DR1101/ HA267–279 tetramers.
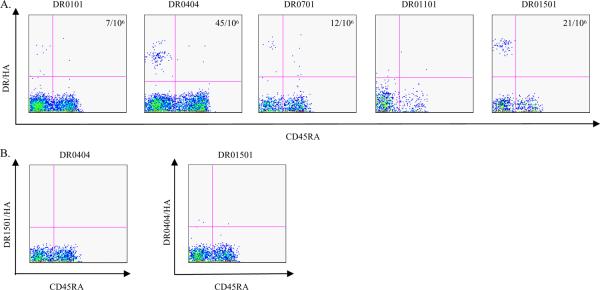
H5 HA reactive T cells were also detected by H5 HA specific tetramers ex vivo in 7 out of 12 subjects examined. The majority of these T cells also exhibited a memory T cell phenotype, with frequencies ranging from 4/106 to 45/106 in the 7 subjects with measurable ex vivo responses. Representative data for these experiments are shown in Figure 6.
Figure 6.
Ex vivo staining of H5 HA reactive T cells. A. PBMC from DR0101, DR0404, DR0701, DR1101 and DR1501 subjects were stained with a panel of HLA-matched DR0101/HA, DR0404/HA, DR0701/HA, DR1101/HA and DR1501/HA tetramers. The tetramers used were as followed, DR0101/ HA91–104, DR0101/ HA157–169 and DR0101/ HA443–458 for DR0101 subjects, DR0404/ HA174–188, DR0404/ HA400–412 and DR0404/ HA443–458 for DR0404 subjects, DR0701/ HA49–68, DR0701/ HA253–265 and DR0701/ HA305–316 for DR0701 subjects, D1101/ HA267–279, DR1101/ HA319–331 and DR1101/ HA408–420 for DR1101 subjects, and DR1501/ HA433–452 for DR1501 subjects. B. As a negative control for the tetramer staining, PBMC from a DR0404 subject was stained with the same DR1501/HA tetramers used in A, and PBMC from a DR1501 subject was stained with the set of DR0404/HA tetramers described as in A. The frequency of antigen specific T cell per million CD4+ T cells is as indicated. No significant staining was observed in the DR1101 subject in A. and the two negative controls in B.
Cross-reactivity of HA specific CD4+ T cells
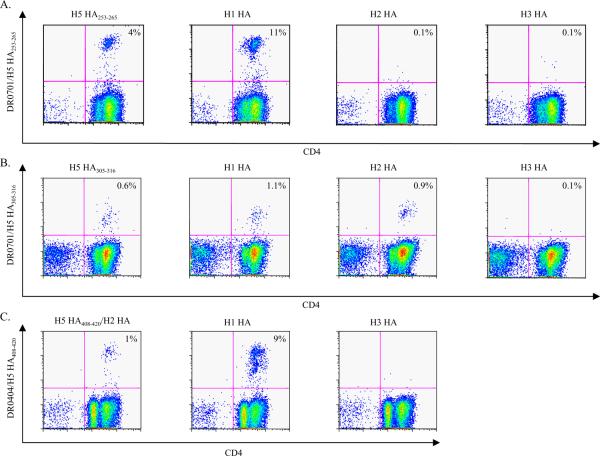
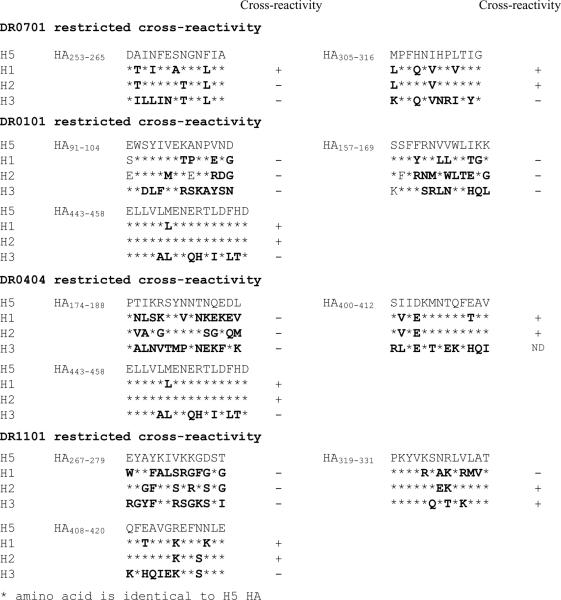
To demonstrate more directly that HA responsive T cells can cross-recognize the HA protein of different influenza A subtypes, H5 HA epitopes and the corresponding HA peptide regions of H1 (A/New Caledonia/20/99), H2 (A/Singapore/1/57) and H3 (A/Panama/2007/99) were used to stimulate PBMC from healthy DR0701 and healthy DR0404 subjects. These cells were then analyzed by staining with H5 HA tetramers. A positive staining of an H1, H2, or H3 stimulated culture would indicate a response elicited by a circulating influenza strain that cross reacts with H5. Representative results are shown in Figure 7. For the DR0701 subject, these results indicated that cross-reactive H5 HA253–265 specific T cells were elicited by HA from A/New Caledonia/20/99 viruses and that cross-reactive H5 HA305–316 specific T cells were elicited by HA from both A/New Caledonia/20/99 and A/Singapore/1/57 viruses. For the DR0404 subject, cross-reactive H5 HA408–420 specific T cells were elicited by HA from both A/New Caledonia/20/99 and A/Singapore/1/57 viruses (aa sequences were exactly identical between A/Viet Nam/1203/2004 (H5N1) and A/Singapore/1/57 (H2N2) in this region).
Figure 7.
Cross-reactivity of H5 HA253–265 and HA305–316 reactive T cells. A. CD4+ T cells from a DR0701 subject were stimulated with H5 HA253–265, or with peptides in the corresponding region of H1N1, H2N2 or H3N2 HA (sequences are as indicated in Table IV). Fourteen days post-stimulation, cells were stained with DR0701/ H5 HA253–265 tetramers. B. CD4+ T cells from a DR0701 subject were stimulated with H5 HA305–316, or with peptides in the corresponding region of H1N1, H2N2 or H3N2 HA (sequences are as indicated in Table IV). Fourteen days post-stimulation, cells were stained with DR0701/ H5 HA305–316 tetramers. C. CD4+ T cells from a DR0404 subject were stimulated with H5 HA408–420, or with peptides in the corresponding regions of H1N1 or H3N2 HA (see Table IV). Fourteen days post-stimulation, cells were stained with DR0404/ H5 HA408–420 tetramers. The aa sequences of H5 HA408–420 and the corresponding region of H2 HA are exactly identical.
Similar experiments were also carried out with other H5 HA epitopes. Cross-reactive H5HA specific T cells that elicited by H1, H2 or H3 peptides were also observed for DR0101, and DR1101 CD4+ T cell epitopes. These results are summarized in Table IV. The cross-reactivity of the DR0301 and DR1501 HA reactive T cells was not determined.
Table IV. Cross recognition of H5 HA reactive T cells
 |
Functional responses of M1, NP, NA and HA reactive T cells
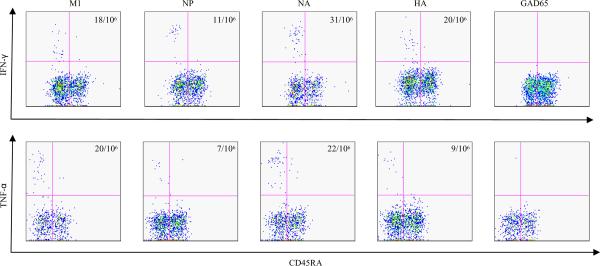
To evaluate the functional responses of H5N1 reactive T cells, the cytokine profile of these T cells were examined by ICS of tetramer positive T cells ex vivo. For these assays, PBMC were activated with a panel of tetramers, and Belfedin A was added 2 hrs later. Cells were then incubated for another 16 hours, and permeabilized for ICS. Representative results of IFN-γ staining of M1, NP, NA and HA reactive T cells are shown in Figure 8. These results demonstrate the functional responses of H5N1 cross- reactive T cells upon challenge with H5N1 Ags.
Figure 8.
Ex vivo cytokine analysis of H5N1 reactive T cells. DR0404 restricted H5N1 M1, NP, NA, and HA T cells were analysis for secretion of IFN-γ and TNF-α after an overnight culture of the PBMC from a DR0404 subject with a panel of M1, NP, NA or HA specific tetramers. The DR0404 M1, NP, NA and HA tetramers used were as previously described. The DR0404/GAD65555–567 tetramers were used as a negative control. The frequencies of IFN-γ and TNF-α secreting cells per million CD4+ T cells are as indicated.
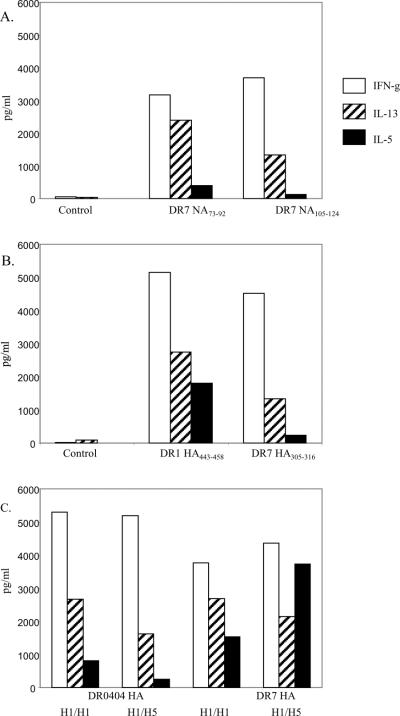
Although similar data were also obtained for ex vivo TNF-α staining (Figure 8), it was not possible to detect IL-5 and IL-13 using this approach. To establish whether H5N1 reactive T cells are capable of producing other cytokines such as IL-5 and IL-13, an in vitro stimulation assay was developed. For these assays, CD4+ T cells were stimulated with individual NA or HA peptides. At day 14, cells were transferred to new wells which were pre-coated with the corresponding peptide specific tetramers. Supernatants were harvested after an overnight culture and assayed for the presence of IFN-γ, IL-5 and IL-13. As shown in Figure 9A and 9B, T cells generated by H5N1 NA and H5N1 HA stimulation secreted IFN-γ, IL-5 and IL-13 during the overnight culture. As shown in Figure 9C, T cells generated by H1 HA stimulation produced similar levels of IFN-γ, IL-5 and IL-13 during the overnight culture regardless of whether these cells were restimulated with H1 HA peptide or with the corresponding H5 HA peptides. Apparently, CD4+ T cells which have been previously activated by H1 HA (in vivo or in vitro) can give a functional response when rechallenged with H5 HA. Together these results suggest that cross-reactive T cells can provide help for cellular and humoral responses to diverse strains of influenza A.
Figure 9.
Cytokine profiles of NA and HA reactive T cells. A. Cytokine profiles of NA reactive T cells. PBMC from a DR0701 subject were stimulated with the NA73–92 (middle panel) or the NA105–124 (right panel) peptide. On day 14, cells were transferred to a well that were precoated with the corresponding tetramers, and supernatants were harvested after an overnight culture. Cytokines were assayed by the Meso Scale multiplex kit. Cells had been stimulated with the NA105–124 peptide were also reactivated with an irrelevant tetramer as a negative control (panel on the left). B. Cytokine profiles of HA reactive T cells. PBMC from a DR0101 subject (middle panel) and a DR0701 subject (right panel) were stimulated with the H5 HA443–458 peptide and the H5 HA305–316 peptide respectively. Cytokines were assayed by the Meso Scale multiplex kit as described above. Cells from the DR0101 subjects were also restimulated with an irrelevant tetramer as a negative control (left panel). C. Cross-reactivity of H1 and H5 HA reactive T cells. PBMC from a DR0404 subject were stimulated with the H1 HA peptide SVIEKMNTQFTAV (Table IV). Cells were reactivated with either the H1 HA peptide or the H5 HA400–412 peptide and then assayed for cytokines (first set and second set of data respectively). PBMC from a DR0701 subject were stimulated with the H1 HA peptide DTIIFEANGNLIA (Table IV). Cells were reactivated with either the H1 HA peptide or the H5 HA253–265 peptide and then assayed for cytokines (third and fourth set of data respectively).
Discussion
In this study, we report the detection of CD4+ T cells that recognize H5N1 influenza viral antigens in the peripheral blood of healthy Caucasian subjects. Although the results being shown focused on subjects with DR0101, DR0404, DR0701 and DR1101 haplotypes, we have also examined subjects that have DR0301 and DR1501 haplotypes. In total, we examined 22 healthy subjects, and T cell responses directed against H5N1 viral proteins were observed in every subject. These findings demonstrated the presence of H5N1 cross-reactive T cells in the Caucasian populations. These A/Viet Nam/1203/2004 (H5N1) responsive CD4+ T cells recognized peptides derived from the well conserved M1, NP, and NA proteins and even the more weakly conserved HA protein.
In concept, our results are in agreement with published reports describing cross-reactivity between different influenza strains. Jameson et al demonstrated that CD4+ and CD8+ human cytotoxic T cell lines generated against A/PR/8/34 (H1N1) could recognize B-LCL infected with various strains of swine and avian influenza A viruses (22). Most of the T cell lines they characterized recognized internal viral proteins, such as M1and NP. There were also lines that recognized NS1, PB1 and PB2. Jameson et al also observed a CD4+ A/PR/8/34 NA specific line that could lyse B-LCL infected with other avian H1N1 viruses. However, cross-reactivity with H5N1 infected cells was not observed. Working with a CD8+ CTL line specific for NP, Boon et al. observed that these CTL recognized NP from other heterosubtypic variants (23). Collectively, these data suggest that most healthy adults in the US have T cells that are directed against the internal proteins of different influenza A subtypes, including the H5N1 subtype which is completely naïve to the US population.
In this current study, we used a more extensive approach to demonstrate that T cells directed against the NA of H5N1 were present in non-exposed Caucasian subjects. This can be accounted for by the fact that most healthy subjects have been exposed to the H1N1 viruses through natural infection or through vaccination. H1N1 and H3N2 viruses are the major influenza A viruses that have circulated the globe since 1968. As the NA protein of H1N1 and H5N1 is of the same NA subtype, it is not unexpected that T cells that are directed against the NA protein of the H1N1 virus would be cross-reactive to the NA protein of the H5N1 virus.
In addition to demonstrating the presence of H5N1 M1, NP, and NA reactive T cells, this study also detected H5N1 HA specific T cells after in vitro expansion in 14 out of 14 healthy Caucasian subjects examined. This finding was less expected because HA is the least conserved protein among different influenza subtypes. The H5 HA epitopes detected were located in both the HA1 and HA2 domains of HA and exhibited a memory T cell phenotype. Since it is unlikely that the subjects studied had been exposed to H5N1 viruses, these observations support the hypothesis that the H5 HA responsive T cells are derived from cross-reactive T cells. Indeed, of the eleven H5 HA T cell epitopes examined (Table IV), five showed cross-reactivity to H1 and H2 HA, one had cross reactivity to H1 HA only and one had cross-reactivity to H2 and H3 HA. The higher degree of cross-reactivity with H5 HA observed for H1 and H2 HA as compared with H3 HA is probably due to the higher degree of sequence homology between H5 HA and H1/H2 HA.
Interestingly, none of the H5 HA epitopes identified were uniquely cross-reactive to H2 HA. Thus, exposure to H2N2 viruses is probably not essential for cross-reactivity to H5 HA. We also noted that cross-reactivity occurred between antigenic peptides that have two or more mismatched amino acids. This illustrates the flexibility of the TcR-MHC/peptide interaction which leads to the cross-reactivity observed. Four of the H5N1 HA T cell epitopes that were detected did not show cross-reactivity to any of the corresponding H1, H2 or H3 HA region under the experimental conditions tested. As these cross-reactivity experiments were carried out with HA peptides selected from only one representative strain of each subtype, it is possible that the H5 HA responsive T cells can recognize the corresponding region of other strains of the H1, H2 or H3 subtypes. Alternatively, it is possible that these H5 HA reactive T cells recognize other unrelated proteins.
Though H5 HA responses were detected after in vitro expansion in all 14 subjects examined, it is notable the HA responses could not be detected ex vivo by tetramers in 5 out of the 12 subjects examined. In contrast, M1 and NP reactive T cells were detected ex vivo in 7 out of 7 subjects examined, and NA reactive T cells were detected in 6 out of 7 subjects examined. These observations suggested that H5 HA reactive T cells may be present at lower frequencies in some portion of the population, while H5 M1, NP and NA reactive T cells are more prevalent.
In general, these experimental results verify a recent literature survey by Bui et al (24). In this comprehensive database search of influenza T cell epitopes, Bui et al identified T cell epitopes that are highly conserved amongst different influenza A subtypes as analyzed by amino acid sequence comparisons. Specifically, they observed that nearly 45% of the published influenza A T cell epitopes (mostly from A/PR/8/34 and A/X-31 strains) have greater than 80% aa sequence homology to A/Viet Nam/1194/2004 or A/Hong Kong/156/97 (H5N1). Because influenza A internal proteins such as M1 and NP are so well conserved amongst different subtypes, it is likely that memory CD4+ T cells elicited by previous exposure to influenza A viruses of the H1, H2 or H3 subtypes can respond to the M1 and NP of H5N1. It is also likely that T cells recognizing other internal viral proteins such as Acidic Polymerase (PA) and Basic Polymerases 1 and 2 (PB1 and PB2) are present, since these internal viral proteins are also well conserved.
Our results also demonstrated that H5N1 cross-reactive T cells yield a productive functional response, secreting cytokines such as TNF-α, IFN-γ, IL-5 and IL-13. It is of particular interest that H5 HA cross-reactive T cells that had been generated by stimulation with the H1 HA peptides produced significant amounts of IFN-γ, IL-5 and IL-13 when rechallenged with H5 HA peptides. These data strongly suggest that vaccination with the current influenza vaccine, which has an H1N1 component and an H3N2 component, can activate NA and HA specific T cells that are cross-reactive to other strains such as the H5N1 virus, thus increasing the pool size of H5N1 NA and HA reactive T cells. In addition, vaccination with live attenuated vaccine can be expected to modulate a more polyclonal pool of influenza A specific T cells compared to subunit vaccines. Subunit influenza vaccines are deprived of internal viral proteins, while live attenuated vaccines include internal viral proteins, which are well conserved. Thus the attenuated vaccines should increase the pool size of T cells that are directed against multiple internal viral proteins of multiple influenza strains including H5N1 viruses.
Humoral immunity is well established as the primary protective mechanism against influenza infection. However, multiple experiments in different animal models have demonstrated the role of T cell mediated protection in influenza infection (25–27). Experiments with murine models also demonstrated that CD4+ T cells can mediate protection through B cell dependant and B cell independent mechanisms, leading to improved viral clearance and a more rapid and effective antibody response (28–30). While we are cautious to directly extrapolate these findings to humans, a few observations clearly support the possibility of cell mediated cross-protection in human influenza infection. For example, Epstein demonstrated in a retrospective study that prior infections of H1N1 protected subjects from subsequent H2N2 infection in the adult population (31). Similar cross-protection was not observed in children. The author suggested that repeated exposures to the influenza A virus were essential to generate cross-protective immunity through cross-reactive T cells or cross-reactive antibodies. In addition, it is interesting to note that the fatality rate for H5N1 infections is 68% for those that are 39 years of age or younger and only 41% for those that are 40 or older. This is in contrast to the usual pattern seen for other influenza infections, in which fatality rates are highest amongst older age groups. The incidence of disease is also lower for those over 40 years old (32,33). Our data indicates that previous exposure to H1N1, H2N2 or H3N2 influenza viruses can generate CD4+ T cells that are cross-reactive toward H5N1 epitopes. It is likely that older adults have a larger or more varied influenza reactive CD4+ and CD8+ T cell pool (as a consequence of repeated exposures to influenza) that is more cross-reactive to H5N1 viruses. It seems plausible that these preexisting cross-reactive T cells could lead to improved viral clearance directly or by providing help for the generation of H5 reactive antibodies. Thus, it is tempting to conclude that the immune repertoire of healthy subjects, especially those with repeated exposures to other influenza A viruses, have partial immunity to the H5N1 virus. This speculation is supported by a recent finding that healthy subjects do have detectable NA antibodies that recognize H5 NA (34). In any case, our results indicate that healthy subjects exhibit memory CD4+ T cell responses directed against multiple H5N1 viral proteins. These findings raise the prospect that widespread administration of the currently available influenza vaccines could reduce the severity of a human H5N1 influenza pandemic.
Non-standard Abbreviations
- HA
Hemagglutinin
- M1
Matrix Protein 1
- NP
Nucleoprotein
- NA
Neuraminidase
- PB
Basic Polymerase
Footnotes
This work was supported in part by NIH contract HHSN266200400028C.
References
- 1.Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus AD, Fouchier RA. Global patterns of influenza a virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- 2.Webster RG, Peiris M, Chen H, Guan Y. H5N1 outbreaks and enzootic influenza. Emerg. Infect. Dis. 2006;12:3–8. doi: 10.3201/eid1201.051024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo PC, Lau SK, Yuen KY. Infectious diseases emerging from Chinese wet-markets: zoonotic origins of severe respiratory viral infections. Curr. Opin. Infect. Dis. 2006;19:401–407. doi: 10.1097/01.qco.0000244043.08264.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen KY. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 5.Wong SS, Yuen KY. Avian influenza virus infections in humans. Chest. 2006;129:156–168. doi: 10.1378/chest.129.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong MD, Hien TT. Avian influenza A (H5N1) J. Clin. Virol. 2006;35:2–13. doi: 10.1016/j.jcv.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webby RJ, Webster RG. Are we ready for pandemic influenza? Science. 2003;302:1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- 8.Fauci AS. Pandemic influenza threat and preparedness. Emerg. Infect. Dis. 2006;12:73–77. doi: 10.3201/eid1201.050983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schafer JR, Kawaoka Y, Bean WJ, Suss J, Senne D, Webster RG. Origin of the pandemic 1957 H2 influenza A virus and the persistence of its possible progenitors in the avian reservoir. Virology. 1993;194:781–788. doi: 10.1006/viro.1993.1319. [DOI] [PubMed] [Google Scholar]
- 10.Govorkova EA, Rehg JE, Krauss S, Yen HL, Guan Y, Peiris M, Nguyen TD, Hanh TH, Puthavathana P, Long HT, Buranathai C, Lim W, Webster RG, Hoffmann E. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J. Virol. 2005;79:2191–2198. doi: 10.1128/JVI.79.4.2191-2198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, Nguyen DC, Szretter KJ, Chen LM, Thawatsupha P, Chittaganpitch M, Waicharoen S, Nguyen DT, Nguyen T, Nguyen HH, Kim JH, Hoang LT, Kang C, Phuong LS, Lim W, Zaki S, Donis RO, Cox NJ, Katz JM, Tumpey TM. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 2005;79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danke NA, Kwok WW. HLA class II-restricted CD4+ T cell responses directed against influenza viral antigens postinfluenza vaccination. J. Immunol. 2003;171:3163–3169. doi: 10.4049/jimmunol.171.6.3163. [DOI] [PubMed] [Google Scholar]
- 13.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J. Clin. Invest. 1999;104:R63–R67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novak EJ, Liu AW, Gebe JA, Falk BA, Nepom GT, Koelle DM, Kwok WW. Tetramer-guided epitope mapping: rapid identification and characterization of immunodominant CD4+ T cell epitopes from complex antigens. J. Immunol. 2001;166:6665–6670. doi: 10.4049/jimmunol.166.11.6665. [DOI] [PubMed] [Google Scholar]
- 15.Kwok WW, Gebe JA, Liu A, Agar S, Ptacek N, Hammer J, Koelle DM, Nepom GT. Rapid epitope identification from complex class-II-restricted T-cell antigens. Trends Immunol. 2001;22:583–588. doi: 10.1016/s1471-4906(01)02038-5. [DOI] [PubMed] [Google Scholar]
- 16.Scriba TJ, Purbhoo M, Day CL, Robinson N, Fidler S, Fox J, Weber JN, Klenerman P, Sewell AK, Phillips RE. Ultrasensitive detection and phenotyping of CD4+ T cells with optimized HLA class II tetramer staining. J. Immunol. 2005;175:6334–6343. doi: 10.4049/jimmunol.175.10.6334. [DOI] [PubMed] [Google Scholar]
- 17.A revision of the system of nomenclature for influenza viruses: a WHO memorandum. Bull. World Health Organ. 1980;58:585–591. [PMC free article] [PubMed] [Google Scholar]
- 18.Webster RG, Laver WG, Air GM, Schild GC. Molecular mechanisms of variation in influenza viruses. Nature. 1982;296:115–121. doi: 10.1038/296115a0. [DOI] [PubMed] [Google Scholar]
- 19.Air GM. Sequence relationships among the hemagglutinin genes of 12 subtypes of influenza A virus. Proc. Natl. Acad. Sci. U. S. A. 1981;78:7639–7643. doi: 10.1073/pnas.78.12.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwaan GF, Olsen B, Osterhaus AD. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005;79:2814–2822. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jameson J, Cruz J, Terajima M, Ennis FA. Human CD8+ and CD4+ T lymphocyte memory to influenza A viruses of swine and avian species. J. Immunol. 1999;162:7578–7583. [PubMed] [Google Scholar]
- 22.Boon AC, de Mutsert G, van Baarle D, Smith DJ, Lapedes AS, Fouchier RA, Sintnicolaas K, Osterhaus AD, Rimmelzwaan GF. Recognition of homo- and heterosubtypic variants of influenza A viruses by human CD8+ T lymphocytes. J. Immunol. 2004;172:2453–2460. doi: 10.4049/jimmunol.172.4.2453. [DOI] [PubMed] [Google Scholar]
- 23.Bui HH, Peters B, Assarsson E, Mbawuike I, Sette A. Ab and T cell epitopes of influenza A virus, knowledge and opportunities. Proc. Natl. Acad. Sci. U. S. A. 2007;104:246–251. doi: 10.1073/pnas.0609330104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg. Infect. Dis. 2006;12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swain SL, Agrewala JN, Brown DM, Jelley-Gibbs DM, Golech S, Huston G, Jones SC, Kamperschroer C, Lee WH, McKinstry KK, Roman E, Strutt T, Weng NP. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol. Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo SH, Webster RG. Cross-reactive, cell-mediated immunity and protection of chickens from lethal H5N1 influenza virus infection in Hong Kong poultry markets. J. Virol. 2001;75:2516–2525. doi: 10.1128/JVI.75.6.2516-2525.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topham DJ, Tripp RA, Sarawar SR, Sangster MY, Doherty PC. Immune CD4+ T cells promote the clearance of influenza virus from major histocompatibility complex class II -/- respiratory epithelium. J. Virol. 1996;70:1288–1291. doi: 10.1128/jvi.70.2.1288-1291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mozdzanowska K, Furchner M, Zharikova D, Feng J, Gerhard W. Roles of CD4+ T-cell-independent and -dependent antibody responses in the control of influenza virus infection: evidence for noncognate CD4+ T-cell activities that enhance the therapeutic activity of antiviral antibodies. J. Virol. 2005;79:5943–5951. doi: 10.1128/JVI.79.10.5943-5951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J. Immunol. 2006;177:2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 30.Epstein SL. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J. Infect. Dis. 2006;193:49–53. doi: 10.1086/498980. [DOI] [PubMed] [Google Scholar]
- 31.Epidemiology of WHO-confirmed human cases of avian influenza A(H5N1) infection. Wkly. Epidemiol. Rec. 2006;81:249–257. [PubMed] [Google Scholar]
- 32.Update: WHO-confirmed human cases of avian influenza A(H5N1) infection. Wkly. Epidemiol. Rec. 2007;82:41–48. [PubMed] [Google Scholar]
- 33.Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS. Med. 2007;4:e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]