Abstract
Tooth caries is a carbohydrate-modified bacterial infectious disease, and recurrent caries is a frequent reason for restoration failure. The objective of this study was to develop a novel antibacterial composite using tetracalcium phosphate (TTCP) fillers and bis(2-methacryloyloxy-ethyl) dimethyl-ammonium bromide, which is a quaternary ammonium dimethacrylate (QADM). QADM was synthesized using 2-(N,N-dimethylamino)ethyl methacrylate and 2-bromoethyl methacrylate and incorporated into a resin. The resin was filled with 40% TTCP and 30% glass particles. The following QADM mass fractions in the composite were tested: 0%, 6%, 12%, and 18%. Streptococcus mutans biofilms were formed on the composites and the colony-forming units (CFUs), metabolic activity, and lactic acid production were measured. The TTCP-QADM composite had flexural strength and elastic modulus similar to those of two commercial composites (p > 0.1). Increasing the QADM content in TTCP composite greatly decreased the bacteria growth and biofilm matrix production. There were significantly more dead bacteria with increasing QADM content. TTCP composite containing 18% QADM had biofilm CFU, metabolic activity, and acid production about half of those without QADM. Inversely linear relationships were established between QADM mass fraction and S. mutans biofilm CFU, metabolic activity, and acid production, with correlation coefficients R2 ≥ 0.98. In conclusion, TTCP-QADM composites were developed and the effect of QADM mass fraction on the antibacterial properties of the composite was determined for the first time. The novel TTCP-QADM composites possessing a strong antibacterial capability, together with calcium phosphate ion release and good mechanical properties, are promising for dental restorations to reduce biofilm growth and recurrent caries.
Keywords: resin composite, tetracalcium phosphate, antibacterial, quaternary ammonium salt, Streptococcus mutans biofilm, tooth caries inhibition
INTRODUCTION
Dental composites are increasingly used due to their esthetics and direct-filling capability.1–4 Extensive studies have been undertaken to improve the resin compositions, filler particles, and cure conditions.5–7 Calcium phosphate (CaP) particles have been used as fillers in resin composites. 8–11 These resin-based CaP composites can release calcium (Ca) and phosphate (PO4) ions, which can form hydroxyapatite [Ca10(PO4)6(OH)2], the putative mineral in teeth. Hence, the CaP composites were able to remineralize enamel and dentin lesions.8,9,11 In previous studies, amorphous calcium phosphate (ACP) and dicalcium phosphate anhydrous (DCPA) particles were filled into resins.8,10–12 Tetracalcium phosphate [TTCP, Ca4(PO4)2O] is another important CaP compound used in bone cements and tissue engineering scaffolds.13,14 TTCP particles with a mean size of about 16 μm were previously used.9,13,14 In a recent study, TTCP was ball-milled to yield submicron particles.15 The fine TTCP particles in the composite not only increased the ion release due to a higher surface area but also improved the composite mechanical properties, with strength significantly higher than those of previous CaP composites.15
In addition to Ca and PO4 ion release, another desirable feature is for the composite to be antibacterial. Recent reports showed evidence that the main challenge facing composite restorations is recurrent caries.16–18 Secondary caries at the tooth-restoration margins is the most frequent reason for replacement of existing restorations.19 Replacement of existing dental restorations accounts for more than half of all operative work. Replacement dentistry costs about $5 billion annually in the United States.20 Acidogenic bacteria such as Streptococcus mutans (S. mutans) and their biofilms, with exposure to fermentable carbohydrates, are responsible for caries.21–25 However, resin composites in general are not antibacterial and do not deter bacteria colonization and plaque formation. Composites were actually shown to accumulate more dental plaque on their surfaces than other restorative materials.26–28
Therefore, studies were performed to synthesize antibacterial resin composites. Quaternary ammonium salts (QAS) are widely used in water treatment, surface coatings, and the food industry due to their low toxicity and potent antimicrobial activity.29 Novel QAS-containing composites have been previously fabricated.30–37 Antibacterial QAS monomers such as 12-methacryloyloxydodecylpyridinium bromide (MDPB) were able to copolymerize with other monomers to polymerize and form the composite.31,35 These composites effectively decreased the attachment of S. mutans and plaque accumulation and reduced the lesion depth of secondary root caries.36 In another study, a QAS chloride was used to synthesize an antibacterial bonding agent.32 Moreover, in a recent study, a quaternary ammonium dimethacrylate was incorporated into a resin, which significantly decreased the S. mutans colonization. 38 This quaternary ammonium dimethacrylate is referred to as QADM in this article. QADM has not been previously incorporated into the TTCP composite, and the effect of QADM mass fraction on biofilm response has not been investigated.
Accordingly, the objectives of this study were to develop a TTCP-QADM composite to impart a strong antibacterial capability to the Ca and PO4 releasing composite and to investigate the effect of QADM mass fraction on the mechanical and antibacterial properties of the composite. The hypotheses were: (1) Incorporating QADM into the TTCP composite will achieve a strong antibacterial activity; (2) Increasing the QADM mass fraction will monotonically decrease the S. mutans biofilm viability, metabolic activity, and acid production; (3) QADM addition will not weaken the composite, and the mechanical properties of the TTCPQADM composite will match those of commercial composites without antibacterial capabilities.
MATERIALS AND METHODS
TTCP particles
TTCP was synthesized from a solid-state reaction between CaHPO4 and CaCO3 (J.T. Baker Chemical, Phillipsburg, NJ), which were mixed and heated at 1500°C for 6 h in a furnace (Lindberg, Watertown, WI).13,14 The heated mixture was quenched to room temperature and ground in a blender (Dynamics Corp., New Hartford, CT). The powder was then sieved to obtain TTCP particles with sizes ranging from about 1.5 μm to 60 μm, with a mean size of 16 μm. This TTCP powder was similar to that of previous studies. 9,13,14 This TTCP powder was further ground in 95% ethanol via a ball mill using 120 balls at 300 rpm (Bel-Alert, Pequannock, NJ) for 72 h to obtain a fine TTCP powder, following a previous study.15 As verified with a particle size analyzer (SA-CP3, Shimazu, Kyoto, Japan), this method produced a fine TTCP powder with a particle size range of 0.2–3.0 μm, and a mean particle size of 0.8 μm.39
QADM resin composite
A resin of bisphenol glycidyl dimethacrylate (BisGMA) and triethylene glycol dimethacrylate (TEGDMA) at 1:1 mass ratio was rendered light-curable with 0.2% camphorquinone and 0.8% ethyl 4-N,N-dimethylaminobenzoate. This resin is referred to as BisGMA-TEGDMA. To obtain an antibacterial capability, bis(2-methacryloyloxy-ethyl) dimethyl-ammonium bromide was synthesized as described in a recent study.38 Bis(2-methacryloyloxy-ethyl) dimethyl-ammonium bromide is a quaternary ammonium dimethacrylate, which is referred to as QADM in the present study. The synthesis of this QADM used a modified Menschutkin reaction in which a tertiary amine group was reacted with an organo-halide. A total of 10 mmol of 2-(N,N-dimethylamino)ethyl methacrylate (DMAEMA, Sigma-Aldrich, St. Louis, MO) and 10 mmol of 2-bromoethyl methacrylate (BEMA, Monomer-Polymer and Dajec Labs, Trevose, PA) were combined with 3 g of ethanol in a 20-mL scintillation vial. After stirring at 60°C for 24 h, the solvent was removed and the QADM was obtained in the form of a clear, colorless, and viscous liquid. This method is desirable because the reaction products were generated at quantitative amounts and required no further purification.38 The QADM thus obtained was mixed with the photo-activated BisGMA-TEGDMA resin at the following QADM/(BisGMA-TEGDMA + QADM) mass fractions: 0%, 20%, 40%, and 60%. QADM fractions higher than 60% were not used because QADM slightly decreased the strength of the composite, and a goal of this study was to develop antibacterial composite with mechanical properties matching commercial composites without antibacterial activity. A barium boroaluminosilicate glass of a mean particle size of 1.4 μm (Caulk/Dentsply, Milford, DE) was silanized with 4% 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine. The TTCP particles and glass particles were mixed into the resin at mass fractions of 40% TTCP and 30% glass. This yielded a total filler level of 70%, following a previous study.39 Because each composite had 30% of resin matrix, the QADM mass fractions of 0%, 20%, 40%, and 60% in the resin matrix resulted in the corresponding QADM mass fraction in the composite to be 0%, 6%, 12%, and 18%.
For mechanical testing, the composite paste was placed into rectangular molds of (2 × 2 × 25) mm. For biofilm experiments, the paste was placed into disk molds of 9 mm in diameter and 2 mm in thickness. The specimens were photo-cured (Triad 2000, Dentsply, York, PA) for 1 min on each side. Four experimental composites were thus fabricated and designated as: (1) TTCP+0%QADM, (2) TTCP+6%QADM, (3) TTCP+12%QADM, and (4) TTCP+18%QADM.
A commercial composite with a low level of fluoride (F) release (Heliomolar, Ivoclar, Ontario, Canada) was included as a control (referred to as “CompositeF”). The fillers were silica and ytterbium-trifluoride with a filler level of 66.7%. Heliomolar is indicated for class I and class II restorations in the posterior region, class III and class IV anterior restorations, class V restorations, and pit and fissure sealing. Another commercial composite, Renamel (Cosmedent, Chicago, IL) was used as a nonreleasing control. It is referred to as “CompositeNoF.” It consisted of nanofillers of 20–40 nm with 60% fillers in a multifunctional methacrylate ester resin.40 Renamel is indicated for class III, IV, and V restorations. The control specimens were photo-cured in the same manner as described above.
Mechanical testing
The composite bars were immersed in distilled water for 1 day at 37°C. Flexural strength and elastic modulus were measured using a three-point flexural test with a 10 mm span at a crosshead-speed of 1 mm/min on a computer-controlled Universal Testing Machine (5500R, MTS, Cary, NC). The flexural strength (S) of the composite was calculated as: S = 3PmaxL/(2bh2), where Pmax is the maximum load on the load–displacement curve, L is flexure span, b is specimen width, and h is specimen thickness. The elastic modulus (E) was calculated as: E = (P/d)(L3/[4bh3]), where the load P divided by the corresponding displacement d is the slope of the load–displacement curve in the linear elastic region. Six specimens were tested for each group (n = 6).
Live/dead assay of S. mutans biofilms
S. mutans is a cariogenic bacterium and the primary causative agent of dental caries.21 The use of S. mutans bacteria (ATCC 700610, UA159, American Type Culture, Manassas, VA) was approved by the University of Maryland. The growth medium consisted of brain heart infusion (BHI) broth (BD, Franklin Lakes, NJ), which was supplemented with 0.2% sucrose. To prepare the inoculation medium, 15 μL of stock bacteria was added into 15 mL of growth medium and incubated at 37°C with 5% CO2 for 16 h, during which the S. mutans were suspended in the BHI broth. This S. mutans culture was then diluted by 10-fold in the growth medium to form the inoculation medium.41
The composite disks were sterilized in an ethylene oxide sterilizer (Anprolene AN 74i, Andersen, Haw River, NC). Six specimens were used for each material in each experiment (n = 6). Each disk was placed in a well of a 24-well plate, inoculated with 1.5 mL of the inoculation medium, and incubated at 5% CO2 and 37°C for 3 days to form mature biofilms.41 The growth medium was changed every 24 h, by transferring the disks to a new 24-well plate with fresh growth medium. After 3 days, the biofilms on the disks were washed with phosphate buffered saline (PBS) and stained using the BacLight live/dead bacterial viability kit (Molecular Probes, Eugene, OR). Live bacteria were stained with Syto 9 to produce green fluorescence, and bacteria with compromised membranes were stained with propidium iodide to produce a red fluorescence. Disks were examined using an epifluorescence microscope (Eclipse TE2000-S, Nikon, Melville, NY). Four representative images were taken for each disk, with three disks yielding 12 images for each material.
Disk specimens with S. mutans incubated for 3 days were prepared for examination with scanning electron microscopy (SEM). Each specimen with adherent biofilm was rinsed with PBS, and then immersed in 1% glutaraldehyde in PBS for 4 h at 4°C. The specimens were rinsed with PBS, subjected to graded ethanol dehydrations, and rinsed twice with 100% hexamethyldisilazane. The specimens were then sputter-coated with gold and examined via SEM (Quanta 200, FEI Company, Hillsboro, OR).
MTT metabolic activity
A new set of disks was placed in a 24-well plate, inoculated with 1.5 mL of the inoculation medium, and cultured for 3 days. Each disk was transferred to a new 24-well plate for the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. It is a colorimetric assay that measures the enzymatic reduction of MTT, which is a yellow tetrazole, into formazan. This MTT assay for S. mutans biofilms was described recently.42 Briefly, 1 mL of MTT dye (0.5 mg/mL MTT in PBS) was added to each well and incubated at 37°C in 5% CO2 for 1 h. During this process, metabolically active bacteria metabolized the MTT and reduced it to purple formazan inside the living cells. After 1 h, the disks were transferred to a new 24-well plate, 1 mL of dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals, and the plate was incubated for 20 min with gentle mixing at room temperature in the dark. After brief mixing via pipetting, 200 μL of the DMSO solution from each well was transferred to a 96-well plate, and the absorbance at 540 nm (OD540) was measured via the microplate reader. A higher absorbance indicates a higher formazan concentration, which in turn indicates more metabolic activity in the biofilm present on the composite disk.
Lactic acid production and viable cell counts
A new set of composite disks with biofilms at 3 days was prepared as described above. The disks were rinsed in cysteine peptone water (CPW) to remove loose bacteria. Each disk was placed in a new 24-well plate with 1.5 mL of buffered peptone water (BPW) supplemented with 0.2% sucrose. BPW medium was used so that the mature biofilm would remain stable during the 3-h culture for the acid assay. BPW has a relatively high buffer capacity and the pH would not become significantly acidic, because a low pH would hinder bacterial acid production. Disks with biofilms were incubated at 5% CO2 and 37°C for 3 h to allow the biofilms to produce acid. After 3 h, the BPW solutions were stored for lactate analysis. Lactate concentrations in the BPW solutions were determined using an enzymatic (lactate dehydrogenase) method.43 A microplate reader (SpectraMax M5) was used to measure the absorbance at 340 nm (optical density OD340) for the collected BPW solutions. Standard curves were prepared using a standard lactic acid (Supelco Analytical, Bellefonte, PA).
After the disks were treated for acid production, colony-forming unit (CFU) counts were used to quantify the total number of viable bacteria present on each disk. When biofilms are properly dispersed and diluted, each viable bacterium results in a single colony on an agar plate. The disks were transferred into tubes with 2 mL CPW. Biofilms were harvested by sonication (3510R-MTH, Branson, Danbury, CT) for 3 min, and then vortexing at maximum speed for 20 s using a vortex mixer (Fisher, Pittsburgh, PA). This removed and dispersed the biofilms from the disk. The bacterial suspensions were serially diluted, spread onto BHI agar plates, and incubated for 3 days at 5% CO2 and 37°C. At 3 days, the number of colonies that grew were counted and used, along with the dilution factor, to calculate total CFUs on each disk.
Statistical analysis
One-way analysis of variance was performed to detect the significant effects of the variables. Tukey’s multiple comparison test was used to compare the data at a p-value of 0.05.
RESULTS
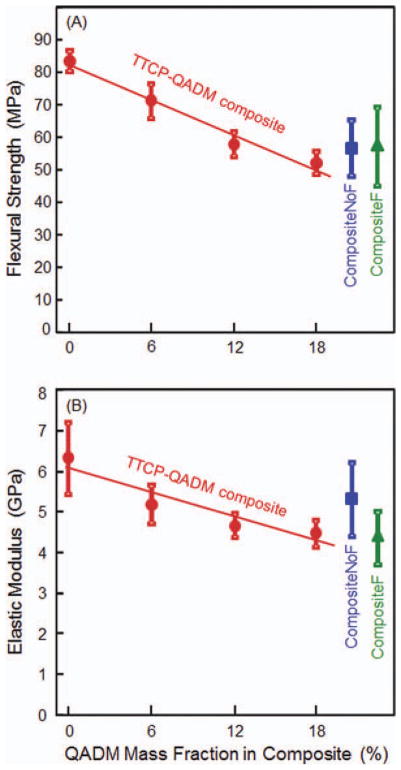
Figure 1 plots the mechanical properties (mean ± SD; n = 6): (A) flexural strength and (B) elastic modulus, as a function of QADM mass fraction in the composite. Values for the two commercial control composites are included near the right axis. Increasing the QADM mass fraction significantly decreased the strength of the TTCP composite (p < 0.05). However, the TTCP composite with 6% to 18% of QADM had strengths that were not significantly different from those of the commercial composites (p > 0.1). In (B), the modulus of TTCP composite without QADM was significantly higher than those at 12% and 18% QADM (p < 0.05). However, each TTCP-QADM composite had modulus that was not significantly different from those of the commercial composites (p > 0.1). To show the decreasing trend of strength and modulus with increasing QADM, linear best fit was performed for the data. This yielded a correlation coefficient R2 = 0.974 for flexural strength, and R2 = 0.884 for elastic modulus.
FIGURE 1.
Composite mechanical properties: (A) flexural strength and (B) elastic modulus. The photo-activated TTCP-QADM composite was filled with 40% TTCP and 30% glass particles by mass. The resin matrix was BisGMA-TEDGMA-QADM. Flexural strength and elastic modulus for the two commercial control composites are included near the right axis. Each value is the mean of six measurements, with the error bar showing one standard deviation (mean ± SD; n = 6). The linear correlation coefficient R2 = 0.974 for strength and R2 = 0.884 for elastic modulus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
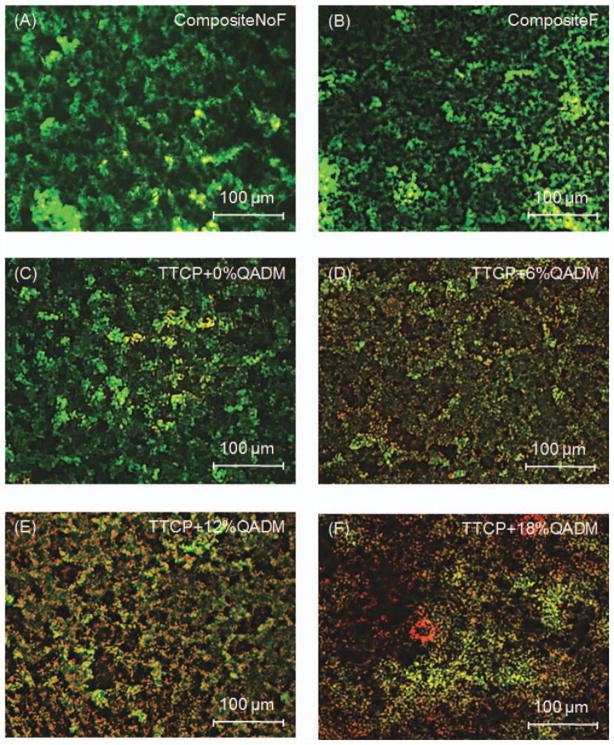
Typical fluorescence photos from the live/dead assay are shown in Figure 2: (A) CompositeNoF, (B) CompositeF, (C) TTCP+0%QADM, (D) TTCP+6%QADM, (E) TTCP+12%QADM, and (F) TTCP+18%QADM. The live bacteria were stained green, and the compromised bacteria were stained red. When the live and dead bacteria were close to each other or on the top of each other, the red staining was mingled with green, thus producing a yellow or orange color. Incorporation of QADM decreased the S. mutans colonization for all tested mass fractions. The surface coverage of the adherent live bacteria was affected by the QADM content, and the live bacteria coverage decreased with increasing QADM. Examination on the entire composite disk, with three disks per material, indicated that the biofilms on CompositeNoF, CompositeF, and TTCP+0%QADM were qualitatively similar. They had predominantly viable bacteria that completely covered the disks, with a few patchy areas and occasional dead bacteria which were slightly more on CompositeF and TTCP+0%QADM. The areas of red/yellow/orange staining noticeably increased on the disks with increasing QADM content. The TTCP composite with 18% QADM had the highest amount of dead bacteria.
FIGURE 2.
Live and dead staining images of S. mutans biofilms at 3 days on: (A) CompositeNoF, (B) CompositeF, (C) TTCP+0%QADM, (D) TTCP+6%QADM, (E) TTCP+12%QADM, and (F) TTCP+18%QADM. Live and dead bacteria had green and red fluorescence, respectively. However, when the live and compromised bacteria were closely associated or overlapping each other, the red color was mingled with green, resulting in yellow and orange colors. The commercial composites did not suppress bacteria colonization and growth. The TTCP-QADM composite was antibacterial, resulting in more compromised bacterial with increasing QADM mass fraction. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Representative SEM micrographs of biofilms grown on the composite disks at 3 days are shown in Figure 3: (A and B) CompositeNoF control; (C) and (D) TTCP composite with an intermediate 12% QADM. Thick and homogeneous coverage of biofilms was observed on CompositeNoF in (A). A great amount of bacteria was embedded in the biofilm matrix, as show at a higher magnification in (B), where the arrow indicates bacteria cells, and “M” indicates the biofilm matrix. In contrast, there were noticeably thinner biofilms with less bacteria coverage on TTCP+12%QADM. As shown in (C), the coverage of biofilms was heterogeneous on TTCP+12%QADM, with “L” indicates areas of less bacteria coverage. In some areas on TTCP+12%QADM, there were individual bacteria that attached to the composite surface without a biofilm matrix. An example of this is shown at a higher magnification in (D), where the arrow indicates the individual bacteria cells, and “R” indicates the bare resin composite surface not covered with a biofilm. Examination on other composites showed that CompositeF and TTCP+0%QADM (not shown) had features similar to CompositeNoF, where relatively thick and homogeneous biofilms covered the composite disks. TTCP+18%QADM had featured similar to TTCP+12%QADM but with even less biofilm coverage. TTCP+6%QADM had slightly more biofilm than TTCP+12%QADM. These observations suggest that while the control composites were completely covered with a thick biofilm matrix, adding QADM into the TTCP composite greatly reduced the biofilm amount.
FIGURE 3.
SEM images of biofilms on composites at 3 days. (A) CompositeNoF control at a low magnification. (B) CompositeNoF at a higher magnification. (C) TTCP+12%QADM at a low magnification. (D) TTCP+12%QADM at a higher magnification. CompositeNoF had thick and homogeneous biofilm coverage. TTCP+12%QADM had thinner biofilms and patchy coverage. Arrow in (B) indicates the bacteria cells, and “M” indicates the biofilm matrix. “L” in (C) indicates areas of less biofilm coverage. Arrow in (D) indicates individual cells. “R” indicates the resin composite not covered with biofilm. CompositeF and TTCP+0%QADM were similar to CompositeNoF and not included here. TTCP+18%QADM had slightly less biofilm coverage, while TTCP+6%QADM had slightly more biofilm coverage, than TTCP+12%QADM shown in (C) and (D). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
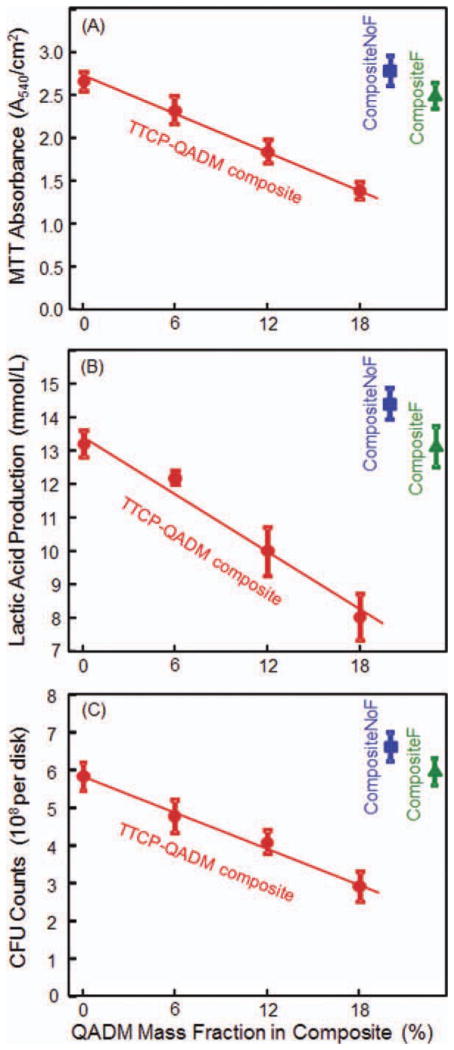
The biofilm metabolic activity was measured using the MTT assay and plotted in Figure 4(A). Although Composite-NoF had the highest absorbance, it was not significantly different from those of CompositeF and TTCP+0%QADM. Increasing the QADM mass fraction in the TTCP composite significantly decreased the MTT absorbance of the S. mutans biofilms adherent on the composite disks (p < 0.05). Again, the trend appeared to be linear within the tested range, yielding R2 = 0.995.
FIGURE 4.
Biofilm metabolic activity, acid production, and CFU counts. Each value is mean ± SD; n = 6. Values for the two commercial control composites are included near the right axis. In (A), a lower MTT absorbance means a reduced metabolic activity in the S. mutans biofilm adherent on the composite, which in turn indicates a stronger antibiofilm activity of the composite. In (B), lactic acid was decreased with increasing QADM mass fraction. In (C), CFU counts were reduced with increasing QADM mass fraction. MTT, lactic acid, and CFU appeared to be inversely and linearly proportional to QADM mass fraction, with R2 of 0.995, 0.980, and 0.990, respectively. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The lactic acid production by the S. mutans biofilms adherent on the composites is plotted in Figure 4(B). The biofilms on CompositeNoF produced the most acid, which was slightly but significantly (p < 0.05) higher than that on CompositeF and the TTCP+0%QADM composite. The lactic acid production by the biofilms was significantly decreased with increasing QADM in the TTCP composite. Among the four TTCP-QADM composites, the acid amount for each composite was significantly different from the rest (p < 0.05). An inversely linear relationship was established between the acid production of the biofilms and the QADM mass fraction in the TTCP composite, with R2 = 0.980.
The CFU counts were plotted in Figure 4(C). Composite-NoF had the highest CFU, closely followed by CompositeF and TTCP+0%QADM; all three were not significantly different (p > 0.1). Increasing the QADM mass fraction significantly decreased the CFU counts (p < 0.05). The CFU of TTCP+18%QADM was about 1/2 that of TTCP+0%QADM. For the TTCP-QADM composites, the CFU appeared to be inversely proportional to the QADM mass fraction, with a linear correlation coefficient R2 = 0.990.
DISCUSSION
Extensive studies have been performed to improve the fillers, coupling agents1 and monomer systems,44 minimize polymerization shrinkage,5,45 enhance the hydrolytic performance, 46 and reduce the degradation and fatigue failure of the composites.7 Novel polymerization strategies have been developed,47 and new nanocomposites with various types of nanofillers have been fabricated.12,48 Furthermore, efforts were made to render the composites capable of releasing Ca, PO4, and F ions for caries inhibition.8–10 Previous Ca-PO4 composites released Ca ions to a concentration of 0.3–1.0 mmol/L, and PO4 to concentrations of 0.1–0.7 mmol/L.9,49 These composites effectively remineralized the tooth lesions by increasing the mineral content of decayed enamel and dentin structures.9,11,49 Similar levels of Ca and PO4 ion releases were achieved with the photo-cured TTCP composite measured using the same technique, while its mechanical properties were significantly better due to the glass particle reinforcement.39 However, there is still a need to add a potent antibacterial feature to the CaP composite.
Polymerizable QAS monomers are strong antibacterial agents that can be incorporated into dental composites.30– 34,37,50 The QAS monomer can be copolymerized with the resin by forming a covalent bond with the polymer network. The QAS is immobilized in the composite and not released or lost over time, thus achieving a lasting antibacterial property. A QAS bromide monomethacrylate, MDPB, was extensively studied, and its antibacterial effect showed no decrease after the composite was immersed in water for 3 months.30 A durable antimicrobial activity was also maintained when QAS bromides and chlorides at different chain lengths were incorporated into a glass ionomer cement.34 The previous QAS monomers used in dental composites were usually monomethacrylates.31,32 Dimethacrylate QAS monomers were recently synthesized and incorporated into a resin composite.38 The synthetic method for QADM was fairly straightforward when compared with the synthesis of other QAS monomers. A potential advantage is that, compared with a QAS monomethacrylate, QADM has reactive groups on both ends of the molecule, which could be incorporated into the resin with less of a negative impact on the mechanical and physical properties of the composite. However, the previous study38 did not incorporate QADM into the CaP composites. This study showed a statistically significant antimicrobial activity for the TTCP composite containing QADM. Further study is needed to examine whether a lasting antibacterial property can be obtained by performing longer term antibacterial experiments and to investigate the clinical significance of the antimicrobial activity.
Increasing the QADM mass fraction produced a monotonic increase in the antibacterial potency of the TTCP composite. Live/dead assay on the biofilms on the composite disks showed a trend of increasing amounts and areas of compromised bacteria with higher QADM content. Compared with the TTCP composite with 0% QADM, the composite with 6% QADM decreased the CFU counts, the MTT metabolic activity, and the lactic acid production by about 15%. Further increasing the QADM to 18% decreased the CFU counts, the MTT metabolic activity, and the lactic acid by ~ 50%, compared with those with no QADM. There seemed to be a linear relationship between the QADM mass fraction in the composite and the biofilm properties, with relatively high correlation coefficient R2 values. SEM examination revealed thick and continuous biofilm coverage on the commercial composites, in contrast to the TTCP composites with QADM, where the biofilms became thinner and patchy. The biofilm matrix synthesized by the bacteria is termed the extracellular polymeric substances (EPS), in which the biofilm cells are embedded.51 The EPS was found to consist of primarily polysaccharides, proteins, lipids, and nucleic acids.51 The EPS was shown to be highly beneficial for bacteria survival by providing a three-dimensional scaffold for mechanical integrity, stability, as well as enhanced bacterial adhesion and cell–cell interactions.51 The SEM examination showed a substantial production of a thick and continuous EPS by the biofilms on the commercial composites. In contrast, incorporating QADM into TTCP composite greatly reduced the EPS formation, resulting in areas of discontinuous biofilm coverage on the composite with individual bacteria cells without EPS. Therefore, this study demonstrated for the first time that QADM in TTCP composite is effective in reducing CFU counts, EPS synthesis, metabolic activity, and acid production of S. mutans biofilms. Furthermore, the antibacterial potency is shown to be linearly proportional to the QADM concentration in the composite.
Regarding the antibacterial mechanisms, previous studies suggested that QAS materials can cause bacteria lysis by binding to the cell membrane and causing cytoplasmic leakage. 37 When the negatively charged bacterial cell contacts the positively charged sites of the QAS resin, it disturbs the electric balance of the cell membrane, and the bacteria could explode under its own osmotic pressure.52 Therefore, increasing the QAS content in the composite would increase the concentration of the positively charged sites on the surface of the composite. Hence, this mechanism would suggest an increase in the antibacterial potency of the composite with increasing QADM content. In a previous study,38 a single mass fraction of QADM was used. Similarly in other studies, the effect of antibacterial monomer mass fraction on biofilm response was not investigated.31–33 This study investigated the effects of QADM mass fraction on the antibacterial properties of TTCP composite for the first time and demonstrated that the QADM mass fraction was related linearly to biofilm properties including CFU counts, metabolic activity, and lactic acid production. Further study is needed to examine if the incorporation of QADM into the resin would affect the Ca and PO4 ion release of the TTCP composite.
Previous studies reported flexural strengths of about 30–40 MPa for resin-based Ca-PO4 composites,9,49 and it was concluded that such low strengths were “inadequate to make these composites acceptable as bulk restoratives.”8 In comparison, the TTCP composite with 0%, 6%, 12%, and 18% of QADM of this study had higher flexural strengths 83, 71, 59, and 51 MPa, respectively. This is likely because the TTCP-QADM composite contained 30% of silanized glass particles for reinforcement. Even with 18% QADM, the composite strength still matched that of the two commercial composites without antibacterial properties. Therefore, the TTCP-QADM composite could still possess a strong antibacterial activity while simultaneously possessing strength and elastic modulus matching those of the commercial control composites. It should be noted that the TTCP composites had larger filler particles and a higher filler level than those of the control composites. Further study is needed to synthesize TTCP particles with smaller sizes, such as nanoparticles, for use in dental composite. Regarding the commercial composites, Heliomolar is indicated for class I and class II restorations in the posterior region, class III and class IV anterior restorations, and class V restorations. Renamel is indicated for class III, IV, and V restorations. The new TTCPQADM composites, with strength and elastic modulus higher than or equal to these commercial composites, may also be suitable for these applications. This study focused on the composite processing methods and the effect of QADM content on the composite properties. Further studies are needed to perform mechanical testing including fracture toughness and wear, both before and after the composites are exposed to biofilms. In addition, multispecies biofilms should be used to better simulate the oral cavity with longer term bacterial exposure to investigate the behavior of the TTCP-QADM composites.
SUMMARY
A new composite was developed containing submicron-sized TTCP particles and bis(2-methacryloyloxy-ethyl) di-methyl-ammonium bromide, a QADM. The rationale was to combine the Ca and PO4 ion release of the TTCP with the antimicrobial activity of the QADM.
The effect of QADM mass fraction in the composite on antibacterial properties was investigated for the first time. Increasing the QADM content decreased the biofilm viability, growth, and EPS production. Inversely linear relationships were established between the QADM mass fraction and the CFU counts, metabolic activity, and lactic acid production of S. mutans biofilms. The TTCP composite with 18% QADM had CFU counts, metabolic activity, and acid production about 1/2 those without QADM.
The antibacterial TTCP-QADM composite with glass particle reinforcement had flexural strength and elastic modulus higher than or equal to those of two commercial composites without an antibacterial activity. Further study is needed to make TTCP nanoparticles and use filler levels to be the same as those of the commercial controls so that their mechanical properties can be directly compared.
The photo-cured TTCP-QADM composite with a strong antibacterial capability together with Ca and PO4 release, and mechanical properties matching those of commercial composites, is promising for dental restorations that can reduce biofilm growth and recurrent caries.
Acknowledgments
The authors are indebted to Drs. Joseph M. Antonucci, Alison M. Kraigsley, Nancy J. Lin, and Sheng Lin-Gibson of the Polymers Division, National Institute of Standards and Technology (NIST), for fruitful discussions. They are very grateful to Esstech (Essington, PA) and Dr. Sibel Antonson at Ivoclar Vivadent (Amherst, NY) for generously donating the materials. This study was supported by NIH (HX), Maryland Nano-Biotechnology Initiative Award (HX), the University of Maryland Dental School, and theWest China College of Stomatology.
Contract grant sponsor: NIH; contract grant numbers: R01 DE14190, R01 DE17974
Contract grant sponsors: Maryland Nano-Biotechnology Initiative Award, the University of Maryland Dental School, and the West China College of Stomatology
References
- 1.Ferracane JL. Current trends in dental composites. Crit Rev Oral Biol Med. 1995;6:302–318. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- 2.Bayne SC, Thompson JY, Swift EJ, Jr, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567–577. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 3.Lim BS, Ferracane JL, Sakaguchi RL, Condon JR. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dent Mater. 2002;18:436–444. doi: 10.1016/s0109-5641(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 4.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: Methods development. Dent Mater. 2003;19:1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 5.Lu H, Stansbury JW, Bowman CN. Impact of curing protocol on conversion and shrinkage stress. J Dent Res. 2005;84:822–826. doi: 10.1177/154405910508400908. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, Ling L, Wang R, Burgess JO. Formation and characterization of a novel fluoride-releasing dental composite. Dent Mater. 2006;22:1014–1023. doi: 10.1016/j.dental.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87:710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physiological evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res B. 2000;53:381–391. doi: 10.1002/1097-4636(2000)53:4<381::aid-jbm12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–566. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 10.Xu HHK, Sun L, Weir MD, Antonucci JM, Takagi S, Chow LC. Nano dicalcium phosphate anhydrous-whisker composites with high strength and Ca and PO4 release. J Dent Res. 2006;85:722–727. doi: 10.1177/154405910608500807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langhorst SE, O’Donnell JNR, Skrtic D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: Quantitative microradiographic study. Dent Mater. 2009;25:884–891. doi: 10.1016/j.dental.2009.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu HHK, Weir MD, Sun L, Moreau JL, Takagi S, Chow LC, Antonucci JM. Strong nanocomposites with Ca, PO4 and F release for caries inhibition. J Dent Res. 2010;89:19–28. doi: 10.1177/0022034509351969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow LC. Calcium phosphate cements: Chemistry, properties, and applications. Mater Res Symp Proc. 2000;599:27–37. [Google Scholar]
- 14.Xu HHK, Weir MD, Simon CG. Injectable and strong nano-apatite scaffolds for cell/growth factor delivery and bone regeneration. Dent Mater. 2008;24:1212–1222. doi: 10.1016/j.dental.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu HHK, Weir MD, Sun L. Calcium and phosphate ion releasing composite: Effect of pH on release and mechanical properties. Dent Mater. 2009;25:535–542. doi: 10.1016/j.dental.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: Clinical, chemistry, and physical behavior considerations. Dent Mater. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 18.NIDCR (National Institute of Dental and Craniofacial Research) announcement # 13-DE-102, Dental Resin Composites and Caries, March 5, 2009.
- 19.Mjör IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. Int Dent J. 2000;50:361–366. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 20.Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. Quality of dental restorations. FDI Commision Projects 2–95. Int Dent J. 2001;51:117–158. doi: 10.1002/j.1875-595x.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 21.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Featherstone JDB. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887–899. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 23.Deng DM, ten Cate JM. Demineralization of dentin by Streptococcus mutans biofilms grown in the constant depth film fermentor. Caries Res. 2004;38:54–61. doi: 10.1159/000073921. [DOI] [PubMed] [Google Scholar]
- 24.Totiam P, Gonzalez-Cabezas C, Fontana MR, Zero DT. A new in vitro model to study the relationship of gap size and secondary caries. Caries Res. 2007;41:467–473. doi: 10.1159/000107934. [DOI] [PubMed] [Google Scholar]
- 25.Cenci MS, Pereira-Cenci T, Cury JA, ten Cate JM. Relationship between gap size and dentine secondary caries formation assessed in a microcosm biofilm model. Caries Res. 2009;43:97–102. doi: 10.1159/000209341. [DOI] [PubMed] [Google Scholar]
- 26.Svanberg M, Mjör IA, Ørstavik D. Mutans streptococci in plaque from margins of amalgam, composite, and glass-ionomer restorations. J Dent Res. 1990;69:861–864. doi: 10.1177/00220345900690030601. [DOI] [PubMed] [Google Scholar]
- 27.Zalkind MM, Keisar O, Ever-Hadani P, Grinberg R, Sela MN. Accumulation of Streptococcus mutans on light-cured composites and amalgam: An in vitro study. J Esthet Dent. 1998;10:187–190. doi: 10.1111/j.1708-8240.1998.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 28.Beyth N, Domb AJ, Weiss E. An in vitro quantitative antibacterial analysis of amalgamand composite resins. J Dent. 2007;35:201–206. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Kourai H, Yabuhara T, Sjirai A, Maeda T, Nagamune H. Syntheses and antimicrobial activities of a series of new bis-quaternary ammonium compounds. Eur J Med Chem. 2006;41:437–444. doi: 10.1016/j.ejmech.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RRB. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73:1437–1443. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 31.Imazato S. Review: Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19:449–457. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 32.Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, Ma S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J Dent. 2009;37:289–296. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent Mater. 2011;27:487–496. doi: 10.1016/j.dental.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res. 2011;90:535–540. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imazato S. Bioactive restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent Mater J. 2009;28:11–19. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 36.Thome T, Mayer MPA, Imazato S, Geraldo-Martins VR, Marques MM. In vitro analysis of inhibitory effects of the antibacterial monomer MDPB-containing restorations on the progression of secondary root caries. J Dent. 2009;37:705–711. doi: 10.1016/j.jdent.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent Mater. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu HHK, Moreau JL. Dental glass-reinforced composite with calcium and phosphate ion release for caries inhibition. J Biomed Mater Res B. 2010;92:332–340. doi: 10.1002/jbm.b.31519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee Y, Lu H, Oguri M, Powers JM. Changes in gloss after simulated generalized wear of composite resins. J Prosthet Dent. 2005;94:370–376. doi: 10.1016/j.prosdent.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Cheng L, Weir MD, Xu HHK, Kraigsley AM, Lin N, Lin-Gibson S, Zhou X. Antibacterial and physical properties of calcium-phosphate and calcium-fluoride nanocomposites with chlorhexidine. 2011 doi: 10.1016/j.dental.2012.01.006. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraigsley AM, Lin-Gibson S, Lin NJ. Effects of polymer degree of conversion on oral biofilm metabolic activity and biomass. 2011 Submitted for publication. [Google Scholar]
- 43.van Loveren C, Buijs JF, ten Cate JM. The effect of triclosan toothpaste on enamel demineralization in a bacterial demineralization model. J Antimicrob Chemother. 2000;45:153–158. doi: 10.1093/jac/45.2.153. [DOI] [PubMed] [Google Scholar]
- 44.Peutzfeldt A. Resin composites in dentistry: The monomer systems. Eur J Oral Sci. 1997;105:97–116. doi: 10.1111/j.1600-0722.1997.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 45.Ferracane JL. Placing dental composites—A stressful experience. Oper Dent. 2008;33–3:247–257. doi: 10.2341/07-BL2. [DOI] [PubMed] [Google Scholar]
- 46.Ferracane JL. Hygroscopic and hydrolytic effects on dental polymer networks. Dent Mater. 2006;22:211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Cramer NB, Stansbury JW, Bowman CN. Recent advances and developments in composite dental restorative materials. J Dent Res. 2011;90:402–416. doi: 10.1177/0022034510381263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen MH. Update on dental nanocomposites. J Dent Res. 2010;89:549–560. doi: 10.1177/0022034510363765. [DOI] [PubMed] [Google Scholar]
- 49.Skrtic D, Antonucci JM, Eanes ED. Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dent Mater. 1996;12:295–301. doi: 10.1016/s0109-5641(96)80037-6. [DOI] [PubMed] [Google Scholar]
- 50.Lee SB, Koepsel RR, Morley SW, Matyjaszewski K, Sun Y, Russell AJ. Permanent, nonleaching antibacterial surface. 1. Synthesis by atom transfer radical polymerization. Biomacromolecules. 2004;5:877–882. doi: 10.1021/bm034352k. [DOI] [PubMed] [Google Scholar]
- 51.Flemming HC, Wingender J. The biofilm matrix. Nat Rev. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 52.Namba N, Yoshida Y, Nagaoka N, Takashima S, Matsuura-Yoshimoto K, Maeda H, Van Meerbeek B, Suzuki K, Takashida S. Antibacterial effect of bactericide immobilized in resin matrix. Dent Mater. 2009;25:424–430. doi: 10.1016/j.dental.2008.08.012. [DOI] [PubMed] [Google Scholar]