Abstract
We previously observed that 17β-estradiol (E2) augments ischemic borderzone vascular density 10 days after focal cerebral ischemia-reperfusion in rats. We now evaluated the effect of E2 on vascular remodeling, lesional characteristics, and motor recovery up to 30 days after injury. Peri-lesional vascular density in tissue sections from rats treated with 0.72 mg E2 pellets was higher compared to 0.18 mg E2 pellets or placebo (P) pellets: vascular density index, 1.9 ± 0.2 (0.72 mg E2) vs. 1.4 ± 0.2 (0.18 mg E2) vs. 1.5 ± 0.4 (P), p=0.01. This was consistent with perfusion magnetic resonance imaging (MRI) measurements of lesional relative cerebral blood flow (rCBF): 1.89 ± 0.32 (0.72 mg E2) vs. 1.32 ± 0.19 (P), p=0.04. Post-ischemic angiogenesis occurred in P-treated as well as E2-treated rats. There was no treatment-related effect on lesional size, but lesional tissue was better preserved in E2-treated rats: cystic component as a % of total lesion, 30 ± 12 (0.72 mg E2) vs. 29 ± 17 (0.18 mg E2) vs. 61 ± 29 (P), p=0.008. Three weeks after right middle cerebral artery territory injury, rats treated with 0.72 mg E2 pellets used the left forelimb more than P-treated or 0.18 mg E2-treated rats: limb use asymmetry score, 0.09 ± 0.43 (0.72 mg E2) vs. 0.54 ± 0.12 (0.18 mg E2) vs. 0.54 ± 0.40 (P), p=0.05. We conclude that treatment with 0.72 mg E2 pellets beginning one week prior to ischemia/reperfusion and continuing through the one-month recovery period results in augmentation of lesional vascularity and perfusion, as well as improved motor recovery.
1. Introduction
Ischemic stroke causes substantial morbidity and mortality. Management of patients includes primary prevention, thrombolysis for acute stroke, physical rehabilitation, and secondary prevention. Intravenous recombinant tissue plasminogen activator (IV rt-PA) is indicated for acute thrombolysis in patients meeting specific criteria and improves outcomes after ischemic stroke, albeit in real-world practice, few patients are candidates for IV rt-PA and many survive with substantial injury and neurologic deficits (Albers et al., 2008; Del Zoppo et al., 2009). To address this, a great deal of pre-clinical research effort has gone into the field of ischemic neuroprotection, but although many compounds demonstrate neuroprotection in pre-clinical studies, none have so far translated successfully in clinical trials (Tymianski, 2010). This necessitates continued research into novel medical therapies geared towards neuroprotection and neurorepair which could, perhaps combined with innovative physical rehabilitation strategies, offer patients a chance of improved functional outcome.
We are interested in delineating the mechanisms of post-ischemic cerebral vascular remodeling and determining whether pharmacologic augmentation of neurorepair translates to improved functional recovery. Estrogens, notably 17β-estradiol (E2), mediate neuroprotection in experimental cerebral ischemia, albeit with some variability among different models (Brown et al., 2009; Lebesgue et al., 2009; Strom et al., 2009). Estrogens also mediate at least two components of post-ischemic neurorepair: neurogenesis (Li et al., 2011; Suzuki et al., 2007b) and angiogenesis (Ardelt et al., 2007). Our previous work on E2 in cerebral ischemia-reperfusion in rats demonstrated that E2 treatment with commercially available long-term slow-release E2 pellets implanted one week prior to transient middle cerebral artery occlusion (MCAO) augmented lesional vascular density as well as lesion size, and did not improve motor function recovery 10 days after injury (Ardelt et al., 2007). The goal of the current study was to build upon the previous work and examine E2-mediated tissue protection and repair using a multi-modality approach during long-term (four-week) recovery after transient focal cerebral ischemia. To evaluate vascular remodeling, we employed dual-bolus sequential dynamic contrast enhanced (DCE) and dynamic susceptibility contrast (DSC) MRI (Pike et al., 2009), as well as immunolabeling in tissue sections to characterize lesional blood vessels. To evaluate lesional size and characteristics, we utilized both MRI T2 mapping and histologic tissue analysis. Results were then interpreted in the context of recovery of forelimb function using the cylinder test.
2. Results
Hormonal status
E2-releasing pellets initially yielded supraphysiologic serum levels which declined to physiologic levels by 30 days after implantation (Table 1): pregnancy levels (Watson et al., 1975) in the case of the 0.72 mg E2 pellet and metestrus levels in the case of the 0.18 mg E2 pellet (Brown-Grant et al., 1970). Although both types of pellets yielded supraphysiologic serum E2 levels, levels were 10 times higher in the 0.72 mg group than in the 0.18 mg E2 group during ischemia-reperfusion, while in P-treated rats serum E2 levels were physiologic (metestrus levels) throughout the injury and recovery period (Table 1). This data correlates well with the previous detailed study of these pellets (Strom et al., 2009).
Table 1.
| Serum estradiol (E2), pg/ml* | ||||
|---|---|---|---|---|
| Time from implantation | 1 day | 10 days** | 20 days | 30 days |
| Placebo | 7 ± 1 | nd | nd | 5 ± 4 |
| E2, 0.18 mg | 300 ± 32 | 90 ± 23 | 29 ± 16 | 2 ± 2 |
| E2, 0.72 mg | 974 ± 40 | 877 ± 595 | 426 ± 427 | 48 ± 34 |
Mean ± sd
Average timing of MCAO after pellet implantation.
nd = not determined
n=2 for all except the 30-day time point: n=11 (P); n=7 (E2, 0.18 mg); n=11 (E2, 0.72 mg)
Increases in body weight between ovariectomy/pellet implantation and MCAO (10 ± 4 days) correlated with pellet type and were: P, 22g (15 - 46g); 0.18 mg E2, 10g (9 - 18g); 0.72 mg E2, 0g (-8 - 8g), p<0.001, Friedman test. Increases in body weight between MCAO and euthanasia (30 ± 2 days) also correlated with pellet type: P, 50 ± 21g; 0.18 mg E2, 38 ± 18g; 0.72 mg E2, 16 ± 15g, p<0.001, ANOVA.
Intra-ischemic physiology
Intra-ischemic physiologic parameters were within the normal range and not different between cohorts, with the exception of blood glucose, which was lowest in the 0.72 mg E2 – treated group (Table 2).
Table 2.
| Placebo (P) (n=11) |
Estradiol (E2), 0.18 mg (n=7) |
E2, 0.72 mg (n=11) |
|
|---|---|---|---|
| Occlusion, % baseline | 41 (30-43) | 38 (31-39) | 37 (23-41) |
| Mean arterial pressure, mmHg | 90 ± 8 | 85 ± 7 | 88 ± 11 |
| Rectal temperature, °C | 37.0 (37.0-37.1) | 37.1 (37.0-37.1) | 37.1 (36.8-37.1) |
| Temporalis temperature, °C | 36.9 ± 0.1 | 36.8 ± 0.1 | 36.7 ± 0.2 |
| Blood pH | 7.40 (7.37-7.45) | 7.42 (7.40-7.45) | 7.42 (7.40-7.45) |
| pCO2, mmHg | 36 ± 3 | 35 ± 2 | 36 ± 4 |
| pO2, mmHg | 146 ± 27 | 155 ± 17 | 155 ± 16 |
| Blood glucose, mg/dl | 160 (131-210) | 145 (113-226) | 109 (96-135)* |
| Hematocrit, % | 34 (32-35) | 33 (32-34) | 35 (32-35) |
| Reperfusion, % baseline | 115 (84-153) | 123 (91-126) | 110 (88-125) |
Friedman test, p=0.02, E2, 0.72 mg vs. P and E2, 0.18 mg
Lesional magnetic resonance imaging and histology
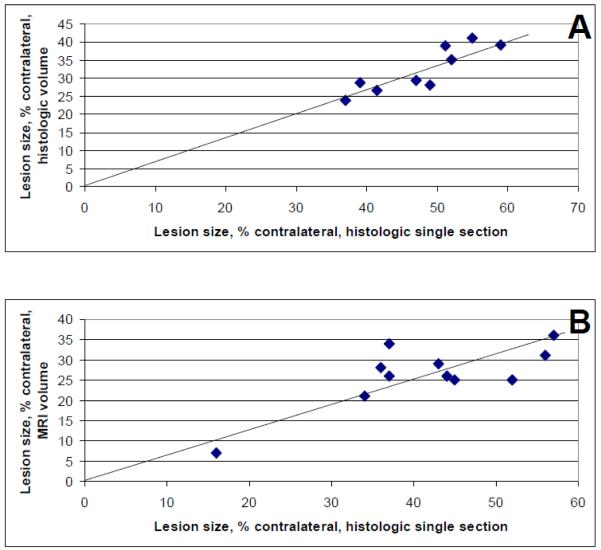
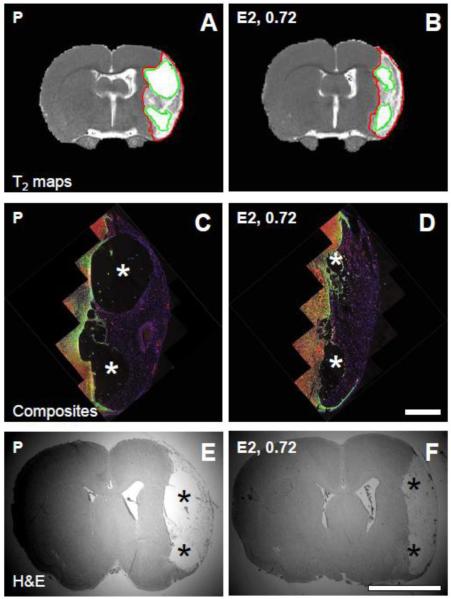
Lesion volumes determined from histologic sections and multi-slice T2 maps in pilot experiments correlated with lesion area determined from representative hematoxylin and eosin-stained tissue sections, one section per animal, with correlation coefficients of 0.9 and 0.8, respectively (Figure 1). Using the histologic single section method of lesional size estimation overestimated the size by 32 ± 6% compared to histologic volume and by 37 ± 14% compared to MRI volume. Since the overestimate was uniform across the range of lesion sizes typical of the rat MCAO model in our lab, this method was used in the study, i.e., representative lesion area in histologic sections at the level of bregma 1.1 expressed as % of contralateral tissue area was used to determine treatment-related effects: there was no difference in lesion size between the three cohorts (Table 3). Ischemic lesion cores were composed of cysts and remnant tissue in all animals, but E2-treated rats in both the 0.18 mg and 0.72 mg cohorts had less cystic and more remnant tissue per total lesion, compared to P-treated rats, in both histologic sections and MR images suggesting better lesional tissue preservation with E2 treatment at either dose (Table 3; Figure 2).
Figure 1.
Lesion size correlations. (A) Lesion size expressed as % contralateral volume was obtained in eight histologic sections spanning bregma 3.24 to -6.24 (Osborne et al., 1987) and correlated with lesion size expressed as % contralateral area in a histologic section at bregma 1.1. The correlation coefficient was 0.9, p=0.002, n=9. (B) Lesion size expressed as % contralateral volume was obtained using magnetic resonance imaging (MRI) and correlated with lesion size expressed as % contralateral area in a histologic section at bregma 1.1. The correlation coefficient was 0.8, p=0.005, n=11.
Table 3.
| Total lesion (% contralateral hemisphere) |
Cystic component (% total lesion) |
|||||
|---|---|---|---|---|---|---|
| P | E2, 0.18 mg | E2, 0.72 mg | P | E2, 0.18 mg | E2, 0.72 mg | |
| H&E | 37±18 (n=11) |
42±8 (n=7) |
41±19 (n=11) |
61±28 (n=11) |
29±17 (n=7) |
30 ±12* (n=11) |
| MRI | 43.2 ± 4.9 (n=3) |
nd | 38.4±5.5 (n=4) |
55.3±3.8 (n=3) |
nd | 32.4±12.5** (n=4) |
H&E: hematoxylin and eosin; MRI: magnetic resonance imaging; nd = not determined
ANOVA, p=0.008 for P vs. E2, 0.18 and P vs. E2, 0.72.
Student’s t-test, p=0.03 for P vs.E2, 0.72.
Figure 2.
Magnetic resonance images and histology. (A) and (B): T2 maps were obtained from placebo (P)-treated (A) and 17β-estradiol (E2, 0.72) - treated (B) rats. Regions of interest (ROIs) derived from T2 threshold analyses were used to define the total ischemic lesion (red outlines) and cystic regions of the lesion (green outlines). Lesions in 0.72 mg E2-treated animals were characterized by smaller cystic components and more preserved tissue compared to P-treated animals. (C) and (D): Low magnification composites of immunolabeled brain sections corresponding to the MR T2 maps were generated to confirm lesion characteristics in P-treated (C) and E2-treated (D) rats. Tissue sections were stained with anti-GFAP antibody (green), anti-CD31 antibody (red), and Hoechst (blue). Asterisks indicate cystic regions; scale bar (C), (D), 1 mm; magnification 4X. (E) and (F): Tissue sections were stained with hematoxylin and eosin (H&E) in order to calculate lesional areas in representative coronal sections of P-treated and E2-treated (F) rats. Asterisks indicate cystic regions; scale bar (E), (F), 5 mm; magnification 0.625X. Panels (A), (C), (E) are from the same animal; (B), (D), (F) are from the same animal.
Thirty days after injury, lesional endothelial cell nuclei contained BrdU which had been incorporated during the first four post-injury days, demonstrating post-injury angiogenesis (Figure 3). Remnant lesional tissue around cysts in P-treated and E2-treated rats was composed of endothelial cells (CD31 and zona occludens-positive) and macrophages (CD11b/c-positive, Iba-1-negative) and was devoid of neuronal markers (Figure 3) and astrocytic markers (Figure 4).
Figure 3.
Lesional characteristics. Lesional endothelial cells in both placebo (P)-treated and 0.72 mg 17β-estradiol (E2) pellet -treated (E2, 0.72) rats incorporated and retained 5-bromo-2′-deoxyuridine (BrdU) administered during the first four days after cerebral ischemia-reperfusion and expressed vascular markers. (A) CD31-labeled blood vessel (red) with a light-blue nucleus (arrow) due to a overlay of blue Hoechst stain and green BrdU immunofluorescence, and an example of a Hoechst-stained, BrdU-negative endothelial cell nucleus (arrow-head) in tissue from a P-treated rat; scale bar, 20 μm; magnification 40X. (B) BrdU-labeled endothelial cells immunoreactive for zona occludens (arrow) in tissue from an E2-treated rat; scale bar, 10 μm; magnification 60X. Lesional tissue from P-treated and E2-treated rats also contained CD11b/c-positive (C) / Iba-1-negative (D) macrophages; scale bar (C), (D), 100 μm; magnification 20X. Lesional tissue (double asterisk) in contrast to uninjured tissue (single asterisk) did not express the neuronal marker microtubule-associated protein-2 (Map2), as shown in a tissue section from a P-treated rat (E), or NeuN, as shown in a tissue section from an E2-treated rat (F); scale bar (E), (F), 200 μm; magnification 10X.
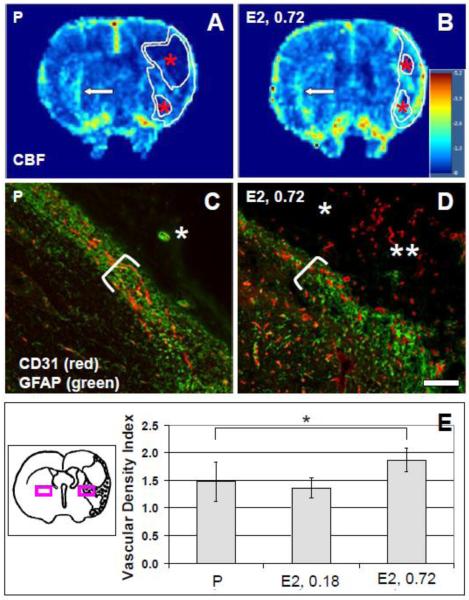
Figure 4.
Lesional vasculature. (A) and (B): Cerebral blood flow (CBF) maps were derived from DSC-MRI in placebo (P)-treated (A) and 17β-estradiol (E2, 0.72)-treated (B) rats; color scales are relative, with contralateral hues approximately set to unity. CBF was higher in lesional regions of interest (ROIs, delineated white overlay) specifically in remnant tissue around cysts (asterisks) of E2-treated animals compared to P-treated animals. To avoid contamination from striatal and thalamic arterial flow, analysis of CBF was performed within the ROI above the horizontal midline. Arrows indicate examples of CBF signal thought to correspond to basal ganglionic arteries. (C) and (D): Peri-lesional striatal ROIs were evaluated in tissue sections labeled with antibodies against CD31 (red) and GFAP (green) in P-treated (P) (C) and E2-treated (E2, 0.72) (D) rats. GFAP expression was enriched in borderzone, and was absent from lesional, tissue. Single asterisks indicate cysts; double asterisks in (D) indicate vascular lesional tissue in the E2-treated rat; brackets delineate glial scars; scale bar (C), (D), 50 μm; magnification 20X. (E): Vascular density in peri-lesional ROIs was quantified and found to be higher in 0.72 mg E2-treated rats compared to 0.18 mg E2 or P-treated rats, *p=0.013, ANOVA. The diagram (not to scale) shows the locations of ischemic ROIs (purple box on the right-hand side) and non-ischemic ROIs (purple box on the left-hand side) used for determination of vascular density indices.
Lesional vasculature and perfusion
Parametric maps obtained with DSC-MRI showed increased lesional rCBF in 0.72 mg E2-treated vs. P-treated, rats (Figure 4). Since this method of CBF determination encompasses macrovascular and microvascular contributions, flow in the ROIs may result from meningeal, cortical, striatal, and thalamic perforating arteries as well as lesional capillaries. The total lesion rCBF was 1.47 ± 0.55 and 0.77 ± 0.08 for 0.72 mg E2-treated and P-treated rats, respectively, p=0.09. rCBF determined specifically in lesional tissue around cysts above the dorso-ventral midline was increased in 0.72 mg E2-treated rats: 1.89 ± 0.32 (0.72 mg E2) vs. 1.32 ± 0.19 (P), p=0.04. rCBF in cyst regions was low and not different between P-treated and E2-treated rats: 0.55 ± 0.15 (0.72 mg E2) and 0.51 ± 0.10 (P). Relative cerebral blood volume (rCBV) values tracked rCBF values: 1.46 ± 0.65 (0.72 mg E2) and 0.74 ± 0.08 (P) for the total lesion, p=0.11; 1.87 ± 0.43 (0.72 mg E2) and 1.24 ± 0.30 (P) for lesional tissue around cysts, p=0.09; and 0.49 ± 0.17 (0.72 mg E2) and 0.48 ± 0.07 (P) for cysts, ns. There were no statistically significant differences in lesional ve and Ktrans e values obtained from DCE-MRI in E2-treated vs. P-treated, rats (data not shown).
In tissue sections, vascular density was highest at the medial border of lesions, compared to other regions, in all animals (Figure 4). Vascular density was highest in 0.72 mg E2-treated, compared to 0.18 mg E2-treated and P-treated, rats.
Functional recovery of left forelimb function
An increase in left forelimb use was observed in 0.72 mg E2 pellet-treated, compared to P-treated and 0.18 mg E2 pellet-treated, rats three weeks after ischemic injury (Figure 5).
Figure 5.
Left forelimb function. Rats were evaluated using the cylinder test before (Pre-MCAO) and weekly for three weeks (Week 1, 2, 3) after transient right middle cerebral artery occlusion (MCAO). The ischemic lesion causes decreased use of the left forelimb, i.e., a positive limb use asymmetry score, derived from counting limb placements, [(right - left) / (right + left + both)]. 0.72 mg 17β-estradiol (E2)-treated, compared to placebo (P)-treated and 0.18 mg E2-treated rats exhibited increased left forelimb use three weeks after experimental ischemic stroke, *p=0.05, ANOVA.
3. Discussion
In our previous investigation of E2-mediated cerebral vascular remodeling in a rat cerebral ischemia-reperfusion model, we observed E2-mediated augmentation of peri-lesional vascular density 10 days after injury, although we did not observe a decrement in ischemic lesion size or improvement in functional recovery (Ardelt et al., 2007). In the current study, we employed MRI, immunolabeling and histology, as well as forelimb use testing to evaluate E2-mediated neuroreparative and neuroprotective effects during the first month of survival after transient cerebral ischemic injury in rats. We found that rats treated with 0.72 mg E2 pellets (in contrast to 0.18 mg E2 or P pellets) starting 10 days prior to cerebral ischemia-reperfusion had recovered function of the affected forelimb by three weeks after injury. Functional improvement was not associated with smaller lesions, but rather, higher lesional vascular density and blood flow.
Estrogens have generally been found to be neuroprotective in cerebral ischemia (Brown et al., 2009), although both beneficial and harmful effects have been observed (Strom et al., 2009). Part of the explanation for differential estrogen effects relates to the estrogenic compound used, its dose and route of administration (which is related to serum E2 levels), and when relative to ovariectomy and injury it is administered (Strom et al., 2009; Strom et al., 2010; Suzuki et al., 2007a). In the current study, we used commercially available long-term slow-release E2 pellets in two different strengths which were implanted 10 days prior to transient MCAO. These pellets initially yielded supra-physiologic serum E2 levels, which gradually declined to metestrus levels (0.18 mg E2 pellet) or pregnancy levels (0.72 mg E2 pellets) by the time of euthanasia (Brown-Grant et al., 1970; Watson et al., 1975), i.e., ischemia-reperfusion took place after a 10-day period of supra-physiologic E2 levels, while neurorepair occurred during the period of subsequent gradual serum E2 decline throughout the 30 days after injury. At the time of euthanasia, although lesional size was similar in all groups, lesional tissue was better preserved in both E2-treated groups compared to the P-treated group. Lesional tissue was composed of vascular elements and macrophages and was devoid of neurons and astrocytes. Peri-lesional vascular density was higher in the 0.72 mg E2-treated rats compared to 0.18 mg E2 or P-treated rats. These observations suggest that higher levels of E2 were necessary to augment and/or maintain the new vessels than to preserve lesional tissue or delay injury evolution. Furthermore, since functional improvement occurred only in the 0.72 mg E2 – treated group, a disconnect between lesional size, tissue integrity and functional recovery is suggested by the data. Similar observations on lesion size and function have been made in other ischemic brain injury models suggesting that mechanisms other than neuroprotection, i.e., post-injury repair, may indeed be important in functional improvement (Li et al., 2004; Millerot-Serrurot et al., 2007).
Functional recovery in this study correlated with increased lesional vascularity and blood flow. BrdU incorporation into endothelial cells suggested that increased vascularity resulted at least in part from post-ischemic angiogenesis, but a combination of increased endothelial survival and angiogenesis, both of which have been attributed to E2 in other organs (Guo et al., 2009; Rubanyi et al., 2002), is possible. It has been previously observed that post-ischemic cerebral angiogenesis is a transient phenomenon with a decline in vascularity between 30 and 90 days after injury (Yu et al., 2007): in our study, in addition to effects on angiogenesis, treatment with 0.72 mg E2 pellets may have prevented or delayed involution of new blood vessels more robustly than treatment with 0.18 mg E2 pellets.
DSC-MRI indicated that lesional rCBF was augmented with 0.72 mg E2 pellets. Since this method of rCBF determination does not differentiate between arterial and capillary contributions, it is likely that the lesional flow increase observed in E2-treated rats is due to both local vasodilation of peri-lesional striatal, cortical, and meningeal arteries (Dorr et al., 2007), as well as newly generated and/or surviving peri-lesional and lesional capillaries which were observed in tissue sections. Further refinement of the data was not possible due to the natural asymmetry of striatal, thalamic and cortical penetrating arteries with respect to location and size (Dorr et al., 2007) and the inability to ensure that perfusion slices were obtained from exactly the same anatomical locations in all animals, i.e., potential contamination of CBF data due to the native arteries could not be avoided even though CBF analysis was limited to ROIs above the horizontal midline, where such contamination is attenuated.
While our study suggests that persistently high serum E2 levels are necessary for both vascular density augmentation and functional recovery in this model, it does not explain whether increased vascularity is mechanistically responsible for improved forelimb function or indicative of another E2-mediated key beneficial effect such as neurogenesis (Li et al., 2011). If E2-mediated vascularity and perfusion augmentation underlie functional recovery, then the mechanism may be facilitation of neural precursor migration and/or synaptogenesis through enhanced growth factor delivery to neurovascular niches at lesional periphery (Dang et al., 2011; Li et al., 2011; Ohab et al., 2006; Zhang et al., 2005). Our study of the lesion cores corroborates the findings of others and suggests that because the core is composed only of blood vessels and macrophages, it is not likely to be directly involved in functional recovery even with E2 treatment (Yu et al., 2007).
The current study shows that supraphysiologic serum E2 levels were required to significantly and durably augment new vessels and improve function. This finding should be interpreted in the context of E2-mediated cerebral or systemic detrimental effects which were not specifically sought here but may have occurred (Strom et al., 2009). Mortality, although infrequent with only two deaths, was observed exclusively in the 0.72 mg E2 group, suggesting that extremely high E2 levels may have triggered fatal complications. This, however, does not significantly impact the main observation of this study: a component of neurorepair, rather than neuroprotection, was associated with improvement in function in this experimental paradigm, suggesting that agents that do not offer neuroprotection but augment repair may be reasonable candidates for further investigation if they have a favorable side effect profile.
In summary, treatment with 0.72 mg E2 slow-release pellets starting prior to cerebral ischemia-reperfusion and continuing for four weeks after injury enhances functional recovery which is associated with lesional vascular remodeling.
4. Methods and Materials
All procedures performed on rats were approved by the University of Alabama Institutional Animal Care and Use Committee (where all authors except MRL were employed when the study commenced) and the University of Chicago Institutional Animal Care and Use Committee (where the animal work was concluded) and were in accordance with NIH guidelines for the use of animals in research.
Animal numbers, endpoints, and blinding
The endpoints of the main study were vascular assessment, lesional characterization, forelimb motor function determination, and serum E2 level at euthanasia. Rats in the main study were randomly assigned (see below) to experimental groups. Based on pilot experiments (see below), sample sizes of 10 per cohort were estimated for the main study. Rats meeting surgical and intra-ischemic physiologic inclusion criteria comprising of normal blood pressure, rectal and temporalis temperature, and arterial blood gas; appropriate degree of occlusion (see below); and demonstration of reperfusion were included in the final analysis. Reasons for exclusion from final analysis of rats meeting above criteria and surviving 24 hours post-operatively were: delayed death (two rats in the 0.72 mg E2 group); lack of histologic lesion (one rat in the P group); unusual lesion location (one rat in the P group); and baseline asymmetrical forelimb use (one rat in the 0.18 mg E2 group). The final groups comprised 11 rats implanted with P pellets, 11 implanted with 0.72 mg E2 pellets, and seven implanted with 0.18 mg E2 pellets. Four animals in the P group (one was excluded due to unusual lesion location) and four in the 0.72 mg E2 group underwent MRI, and three animals in the P and three in the 0.72 mg E2 groups received post-ischemic injections of 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich, St. Louis, MO).
Investigators performing MCAO, behavioral analysis, histology, immunolabeling, and MRI were blinded to hormonal status (pellet assignment and serum E2 level) of the animals until final data analysis.
Randomization of pellet assignment (and MRI or BrdU allocation) was accomplished by selecting a piece of paper with the unique rat number from one box and pellet (or MRI or BrdU) assignment from another box.
Pilot experiments comprising lesional size methodological correlations and determination of post-pellet implantation serum E2 levels were performed in 11 rats for MRI – histology correlations; nine for histologic volume vs. single section area correlations; and 14 for post-implantation serum E2 level determination (two per time-point and pellet type).
Animal care and feed
Rats were maintained in cages of two on a 6AM-6PM light-dark cycle and were provided with water and diet (Teklad 2018SX, Harlan Breeders, Indianapolis, IN) ad lib.
Ovariectomy and pellet implantation
Female Wistar rats (Harlan), 200-225 grams, were anesthetized with isoflurane (in an oxygen, 0.2 L/min, and medical air, 1.0 L/min, carrier), 5% for induction and 1.75% for maintenance. Animals were sterilely prepped and draped, and bilateral abdominal incisions were made. The ovaries were removed, and hemostasis was achieved using silk suture. P or E2-releasing pellets (0.18 mg or 0.72 mg, 60-day release; Innovative Research of America, Sarasota, FL) were implanted in a subcutaneous pocket in the neck. Animals underwent transient MCAO 10 ± 4 days after ovariectomy/pellet implantation.
Transient middle cerebral artery occlusion
All surgical procedures were carried out aseptically. Anesthesia was induced with 5%, and maintained with 1.75%, isoflurane in a carrier of medical air (1.0 L/min) and oxygen (0.2 L/min). PE-50 tubing was inserted into the right femoral artery for real time mean arterial pressure (MAP) monitoring and blood sampling. The skin overlying the vertex of the skull was incised, and the skull overlying the right middle cerebral artery (MCA) territory was exposed and thinned for laser Doppler flow (LDF) monitoring (Moor Instruments, Axminster, UK). The animal was placed in a Plexiglas holder; the head was secured with ear bars, and the right neck was dissected. The common carotid artery, external carotid artery and pterygopalatine artery were tied off, and the occipital artery was cauterized. A 4-0 monofilament suture with a heat-fixed tip was introduced into the common carotid artery and advanced until LDF dropped to less than 45% of baseline (Alkayed et al., 1998). Blood pressure, rectal temperature, and temporalis muscle temperature were monitored and controlled continuously prior to, during, and after occlusion. Blood was obtained for glucose, pH, PO2, pCO2, and hematocrit determination prior to, and one hour into, occlusion. Per surgical protocol, all animals that underwent MCAO had to have normal physiologic parameters including arterial blood gases prior to occlusion. After two hours of occlusion, the 4-0 monofilament suture was removed from the carotid artery with concomitant LDF monitoring to document reperfusion, the wounds were closed, and the animal was awakened. Per surgical protocol, all animals that were included in the final analysis had to have normal physiologic parameters for 15 minutes after reperfusion. Animals meeting all criteria were survived for 30 ± 2 days.
Administration of 5-bromo-2′-deoxyuridine (BrdU)
BrdU was administered intraperitoneally at a dose of 50 mg/kg twice daily for a total of six doses starting 24 hours after cerebral ischemic injury.
Forelimb function evaluation
Prior to, and once weekly for three weeks after experimental ischemia-reperfusion, animals underwent functional forelimb motor and sensory testing with the cylinder test (Li et al., 2004). Animals were placed in a Plexiglas cylinder, 20 cm in diameter, and were videotaped for five minutes. When placed in the cylinder, rats rear up and explore the cylinder wall with their forelimbs. Injury to the right middle cerebral artery territory causes decreased use of the left forelimb. To evaluate pre- and post-ischemic forelimb use, initial paw placement upon each episode of rearing during a 5-minute videotaping session was noted; paw placement was recorded as left, right, or both. Videotaping, recording of paw placement, and calculation of limb use asymmetry score [(right - left) / (right + left + both)] from the videotapes, were independently performed by two investigators, and the results were averaged.
Magnetic resonance imaging
Pilot experiments were first performed to establish scanning protocols and determine MRI – histology lesion size correlations.
Rats in the main study underwent MRI during the fourth week of recovery. Animals were anesthetized with isoflurane (5% in 100% oxygen at 1.0 L/min); the left femoral vein was cannulated; and PE-50 tubing was secured in the vein for contrast injection. Rats were placed in a Plexiglas holder; the head was secured with ear bars; and the cradle was positioned in the small animal MRI system (Bruker BioSpec, 9.4T, 20 cm bore, with a 72 mm RF resonator and active-decoupled 20 mm surface coil for transmit/receive; Bruker Biospin Corp., Billerica, MA). While in the magnet, anesthesia was maintained with 1.5% isoflurane in a 100% oxygen carrier; respiratory rate was monitored; and rectal temperature was monitored and controlled to 37°C using a temperature-controlled water circuit within the holder.
Axial T2-weighted MR images were obtained using the Paravision RARE spin echo sequence: 12 slices, 1.0 mm slice width, 256 × 256 matrix, 117 μm in-plane resolution, TR 2000 ms, TEeffective 52 ms , RARE factor 8, 1 average, acquisition time 1.06 min. A T2-weighted multi-slice multi-echo (MSME) spin echo sequence was implemented, employing 10 echoes with 15 msec spacing, 4 slices, 1 mm slice thickness, 1 average, TR = 5000 ms; matrix 256 × 192, FOV 30 × 30 mm, one average, acquisition time 16 min, zero-filled to 117 μm in-plane resolution. T2 maps were generated by performing a pixel by pixel fit of the decaying image intensity to the function (SI(t) = SI0 e−t/T2).
A dual bolus DCE/DSC perfusion MRI approach was employed as previously described (Pike et al., 2009). The DCE protocol employed a fully relaxed Paravision FLASH sequence followed by 400 rapidly acquired FLASH T1 images as previously described but with a FOV 30 × 30 mm and 234 μm in-plane resolution (Pike et al., 2009). Magnevist (3x diluted, 2.0 μL/g, 0.33 mmol/kg; Berlex Inc., Montville, NJ) was injected into the femoral vein during the series. The DSC-MRI sequence was implemented after the DCE series using T2*-weighted FLASH images as previously described, but with a FOV 30 × 30 mm and 234 μm in-plane resolution (Pike et al., 2009). Macromolecular contrast agents ferumoxide (Feridex; 3x diluted, 3.0 μL/g, 44.8 g iron/g; Berlex) or ferumoxytol (Feraheme; total dose, 1.8 mg Fe; AMAG Pharmaceuticals, Lexington, MA) were injected via the femoral vein during the image series.
Analysis of MR images
a. Lesion area and volume
Lesional size MRI - histology correlations were first characterized (Figure 1). Lesional cross-sectional areas and volumes were determined from T2 maps, using the particle analysis tool in the JIM software package (Xinapse Systems LTD, Northants, UK), to identify regions with T2 values greater than 60 msec. This guided the manual drawing of regions of interest (ROIs) in lesioned and normal brain on the ipsilateral side as well as on the contralateral side. The percent lesion area was calculated using the indirect method, (contralateral side – non-lesioned ipsilateral side)/contralateral side)*100%, in each of four contiguous (1 mm thick) coronal slices through the central region of the lesion; data was averaged over the four slices. Analogous ROIs were drawn on images from multi-slice T2-weighted image sets to determine the volume of the entire lesion.
b. Percent cyst in lesions
Lesions were subdivided into regions with T2 values greater than 60 ms and 100 ms, in order to target total lesion and cystic areas, respectively. The 60 ms threshold effectively distinguished the total lesion from normal parenchyma (T2 ~ 44 ms). Cystic regions had a T2 value approaching that of cerebrospinal fluid (T2 ~ 108 ms). Cyst and lesion areas were averaged over four contiguous T2 map slices. Percent cyst/total lesion was determined by calculating the ratio of the total 100 ms area to the 60 ms area*100%.
c. Perfusion MRI
Mean parameter values for total lesion and cyst were determined from the ROIs described above. Remnant tissue ROIs were non-cystic areas created by subtraction of cyst from total lesion ROIs. The rCBF and rCBV parameter means obtained from these ROIs were restricted to areas above the dorso-ventral midline in order to avoid interference from thalamic and striatal artery flow (Dorr et al., 2007). For calculations of ratios, ROIs were placed in the contralateral cortex.
Perfusion MRI data were processed using the JIM software package as previously described (Pike et al., 2009). Calculation of DCE-MRI parameters followed the general approach previously described (Tofts et al., 1999) using the modified model which includes a blood plasma volume term (vp) (Tofts, 1997). For DCE-MRI, the temporal veins were used to determine the vascular input function (VIF). Parametric maps were generated for Ktrans, the volume transfer constant for the contrast agent (units: min−1), which is largely an index of vascular permeability, and ve, the extravascular extracellular space volume fraction (units: fraction of tissue volume) which quantifies the interstitial space that is accessible to the contrast agent. Calculation of DSC-MRI perfusion parameters followed the model-independent method previously described (Ostergaard et al., 1996). Parametric maps were generated for CBF (ml blood/100 g tissue/min) and CBV (blood volume percentage of total tissue volume).
Euthanasia and processing of brain tissue for histology and immunolabeling
Blood was collected at euthanasia via cardiac puncture for serum E2 measurement with the Ultra-sensitive Estradiol Radioimmunoassay (Diagnostic Systems, Webster, TX) or Elecsys Estradiol Assay (Roche Diagnostics GMBH, Penzberg, Germany), and brains were placed in plastic molds filled with embedding compound and flash-frozen in 2-methylbutane bath on dry ice. Brains were sectioned on a cryostat in the coronal plane: five 5 μm-thick adjacent sections were collected every 100 μm. For immunofluorescent labeling, brain sections were thawed, placed in fixative, blocked with phosphate-buffered saline (PBS) with 1% horse serum, incubated with primary antibodies (Table 4), washed in PBS, and incubated with fluorescently tagged secondary antibodies (Cy3-labeled at 1:100 dilution, Millipore, Billerica, MA or ALEXA 488-labeled at 1:1000 dilution, Invitrogen, Carlsbad, CA). For BrdU detection, slides were incubated with 2N HCl for 60 minutes at 37°C and rinsed in 0.1M Borate buffer prior to the blocking step. All sections were labeled with bis Benzimide H 33258 (Hoechst 33258, Sigma-Aldrich) after immunolabeling to visualize cell nuclei. One tissue section in each group of five adjacent sections was stained with hematoxylin-eosin using standard protocols.
Table 4.
| Primary Antibody (host species) |
Company, Location (catalog number) |
Immunogen (labeled structure) |
Antibody Dilution (fixative) |
Visualization Method |
|---|---|---|---|---|
| BrdU (mouse) |
Roche Applied Science, Indianapolis, IN (11170 376001) |
BrdU (nuclei containing BrdU) |
1:200 (acetone) |
Cy3 or ALEXA 488 |
| CD11b/c (mouse) |
BD Biosciences Pharmigen, San Diego, CA (550299) |
rat peritoneal cells (macrophages, microglia) |
1:100 (acetone) |
Cy3 |
| CD31 (mouse) |
Chemicon, Temecula, CA (MAB1393) |
activated rat microglia (blood vessels) |
1:1000 (acetone) |
Cy3 or chromogenic |
| Glial fibrillary acidic protein; GFAP (rabbit) |
Dako Cytomation, (Glostrup, Denmark) (z0334) |
bovine spinal cord (astrocytes) |
1:1000 (acetone or formalin) |
ALEXA 488 |
| Iba-1 (rabbit) |
Wako Chemicals, Dallas, TX (019-19741) |
Iba-1 C-terminus synthetic peptide, rat sequence (activated macrophages, microglia) |
1:1000 (formalin) |
Cy3 |
| Microtubule associated protein-2; Map2 (rabbit) |
Millipore, Billerica, MA (AB5622) |
purified rat microtubule-associated protein (neuronal processes) |
1:1000 (formalin) |
Cy3 |
| NeuN (mouse) |
Millipore, Billerica, MA (MAB377) |
purified mouse brain nuclear fraction (neurons) |
1:1000 (formalin) |
Cy3 |
| Zona occludens (rabbit) |
Invitrogen Corporation, Carlsbad, CA (71-1500) |
human occludin C- terminal 150 amino acids (blood vessels) |
1:500 (acetone or formalin) |
Cy3 |
Labeling of tissue sections for vascular density determination
Antibodies against CD31 (platelet endothelial cell adhesion molecule-1 or PECAM-1) primarily label vascular endothelium (Parums et al., 1990). Brain sections were fixed with acetone and incubated with anti-CD31 antibodies (Chemicon, Temecula, CA) at a dilution of 1:100. Immunoreactivity was visualized with diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA).
Analysis of histology and immunolabeling
In pilot experiments, lesion volume determined in multiple histologic sections using previously described methods (Osborne et al., 1987) was correlated with lesion area at the level of bregma 1.1 (Figure1). Thereafter, representative hematoxylin/eosin-stained brain sections at the level of bregma 1.1 (one tissue section per brain) were photographed using a 1.25x lens with an in-line 0.5x video adapter on a BX41 microscope fitted with a DP72 digital camera (Olympus America, Center Valley, PA). Ischemic lesion size was determined with the Metamorph imaging analysis program using the indirect method, i.e., [(contralateral side - preserved ipsilateral side)/contralateral side]*100%.
Multiple partially overlapping images of the entire ischemic lesion in a representative coronal brain section (one tissue section per brain) labeled with antibodies against GFAP and CD31 were obtained with a 4x lens using the Olympus microscope and camera. Images were generated at a resolution of 4140 × 3086 pixels, and a composite of each ischemic lesion was generated from individual overlapping 4x images using Power Point (Microsoft Corp., Redmond, WA). Composite images were quantitatively analyzed with Metamorph software to determine the percent cyst per total lesion area. The total area of the ischemic lesion was determined by generating ROIs comprising the area delineated by the GFAP-positive glial scar and the meninges. Cyst areas were determined by drawing ROIs outlining the cystic areas. The percent cyst area was calculated using the following formula: % cyst area = (sum of cyst ROI areas/total lesion ROI area)*100%.
To determine vascular density, peri-lesional and mirror-image ROIs were digitally imaged in CD31-labeled, non-counterstained brain sections using a 20x lens. DAB-positive structures were counted using Metamorph Offline version 4.6r4 software (Molecular Devices Corporation, Sunnyvale, CA) after manual threshold adjustment. The ischemic/non-ischemic ratio was derived for each brain section, and results from three sections per animal were averaged.
Statistics
Statistical analyses were performed with Sigma Stat 3.0 (SPSS, Chicago, IL). Student’s t-test, Mann-Whitney Rank Sum Test, analysis of variance (ANOVA), Friedman test on ranks, and Pearson correlation were utilized as appropriate. Mean ± s.d. and median (25%-75% quartile) are reported.
Highlights.
this is an investigation of how estradiol impacts post-ischemic vascular remodeling and affects functional outcome during 30-day post-ischemic survival in a rat cerebral ischemia-reperfusion model of stroke
the study uses magnetic resonance imaging, histology and immunofluorescent labeling in tissue sections, and a test of forelimb function
the study shows that high-dose estradiol administration does not attenuate lesion size but augments lesional vascularity and blood flow and correlates with improved outcome
Acknowledgments
Supported by NIH/NINDS: 7K08 NS050167-07 (AAA - PI) NIH/NCI: 1R21CA114279-01A2 (MMP - PI) NIH/NIBIB: RO1 EB00422-22 (MMP - Co-I)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers GW, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th Edition) 2008;133:630S–669S. doi: 10.1378/chest.08-0720. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, et al. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–65. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Ardelt AA, et al. Estradiol augments peri-infarct cerebral vascular density in experimental stroke. Exp Neurol. 2007;206:95–100. doi: 10.1016/j.expneurol.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Grant K, et al. Peripheral plasma oestradiol and luteinizing hormone concentrations during the oestrous cycle of the rat. J Endocrinol. 1970;48:295–6. doi: 10.1677/joe.0.0480295. [DOI] [PubMed] [Google Scholar]
- Brown CM, et al. Estradiol is a potent protective, restorative, and trophic factor after brain injury. Semin Reprod Med. 2009;27:240–9. doi: 10.1055/s-0029-1216277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang J, et al. Gonadal steroids prevent cell damage and stimulate behavioral recovery after transient middle cerebral artery occlusion in male and female rats. Brain Behav Immun. 2011;25:715–26. doi: 10.1016/j.bbi.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Del Zoppo GJ, et al. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–8. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr A, et al. Three-dimensional cerebral vasculature of the CBA mouse brain: a magnetic resonance imaging and micro computed tomography study. Neuroimage. 2007;35:1409–23. doi: 10.1016/j.neuroimage.2006.12.040. [DOI] [PubMed] [Google Scholar]
- Guo J, et al. Estrogen-receptor-mediated protection of cerebral endothelial cell viability and mitochondrial function after ischemic insult in vitro. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebesgue D, et al. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids. 2009;74:555–61. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. Estrogen enhances neurogenesis and behavioral recovery after stroke. J Cereb Blood Flow Metab. 2011;31:413–25. doi: 10.1038/jcbfm.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Millerot-Serrurot E, et al. Effect of early decrease in the lesion size on late brain tissue loss, synaptophysin expression and functionality after a focal brain lesion in rats. Neurochem Int. 2007;50:328–35. doi: 10.1016/j.neuint.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, et al. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–16. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne KA, et al. Quantitative assessment of early brain damage in a rat model of focal cerebral ischaemia. J Neurol Neurosurg Psychiatry. 1987;50:402–10. doi: 10.1136/jnnp.50.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard L, et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36:715–25. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- Parums DV, et al. JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol. 1990;43:752–7. doi: 10.1136/jcp.43.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike MM, et al. High-resolution longitudinal assessment of flow and permeability in mouse glioma vasculature: Sequential small molecule and SPIO dynamic contrast agent MRI. Magn Reson Med. 2009;61:615–25. doi: 10.1002/mrm.21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi GM, et al. Effect of estrogen on endothelial function and angiogenesis. Vascul Pharmacol. 2002;38:89–98. doi: 10.1016/s0306-3623(02)00131-3. [DOI] [PubMed] [Google Scholar]
- Strom JO, et al. Dose-related neuroprotective versus neurodamaging effects of estrogens in rat cerebral ischemia: a systematic analysis. J Cereb Blood Flow Metab. 2009;29:1359–72. doi: 10.1038/jcbfm.2009.66. [DOI] [PubMed] [Google Scholar]
- Strom JO, et al. Different methods for administering 17beta-estradiol to ovariectomized rats result in opposite effects on ischemic brain damage. BMC Neurosci. 2010;11:39. doi: 10.1186/1471-2202-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, et al. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007a;104:6013–8. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, et al. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp Neurol. 2007b;500:1064–75. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997;7:91–101. doi: 10.1002/jmri.1880070113. [DOI] [PubMed] [Google Scholar]
- Tofts PS, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–32. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Tymianski M. Can molecular and cellular neuroprotection be translated into therapies for patients? Yes, but not th eway we tried it before. Stroke. 2010;41:S87–S90. doi: 10.1161/STROKEAHA.110.595496. [DOI] [PubMed] [Google Scholar]
- Watson J, et al. Plasma hormones and pituitary luteinizing hormone in the rat during the early stages of pregnancy and after post-coital treatment with tamoxifen (ICI 46,474) J Endocrinol. 1975;65:7–17. doi: 10.1677/joe.0.0650007. [DOI] [PubMed] [Google Scholar]
- Yu SW, et al. Stroke-evoked angiogenesis results in a transient population of microvessels. J Cereb Blood Flow Metab. 2007;27:755–763. doi: 10.1038/sj.jcbfm.9600378. [DOI] [PubMed] [Google Scholar]
- Zhang RL, et al. Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11:408–16. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]