Abstract
Exposure to behavioral stress normally triggers a complex, multi-level response of the hypothalamic-pituitary-adrenal (HPA) axis that helps maintain homeostatic balance. Although the endocannabinoid (eCB) system (ECS) is sensitive to chronic stress, few studies have directly addressed its response to acute stress. Here we show that acute restraint stress enhances eCB-dependent modulation of GABA release measured by whole-cell voltage clamp of inhibitory post-synaptic currents (IPSCs) in rat hippocampal CA1 pyramidal cells in vitro. Both Ca2+-dependent, eCB-mediated depolarization-induced suppression of inhibition (DSI), and muscarinic cholinergic receptor (mAChR) mediated eCB mobilization are enhanced following acute stress exposure. DSI enhancement is dependent on the activation of glucocorticoid receptors (GRs) and is mimicked by both in vivo and in vitro corticosterone treatment. This effect does not appear to involve cyclooxygenase-2 (COX-2), an enzyme that can degrade eCBs; however, treatment of hippocampal slices with the L-type calcium (Ca2+) channel inhibitor, nifedipine, reverses while an agonist of these channels mimics the effect of in vivo stress. Finally, we find that acute stress produces a delayed (by 30 min) increase in the hippocampal content of 2-arachidonoylglycerol, the eCB responsible for DSI. These results support the hypothesis that the ECS is a biochemical effector of glucocorticoids in the brain, linking stress with changes in synaptic strength.
Keywords: DSI, 2-arachidonoylglycerol, GABA inhibition, COX-2, IPSC, muscarinic receptor
INTRODUCTION
Stress activates the hypothalamic-pituitary-adrenal (HPA) axis and induces the release of glucocorticoid hormones that exert widespread effects on the brain and periphery (McEwen, 2008). Appropriate regulation of the HPA axis is critical for health and survival, and several limbic brain structures, including the hippocampus, are involved in the integration of HPA hormonal responses (Herman et al., 2005). The hippocampus has an abundance of glucocorticoid receptors (GRs) and is exquisitely sensitive to glucocorticoids (De Kloet et al., 1998). Exposure to stress reduces the expression of long-term potentiation and concurrently enhances the expression of long-term depression (LTD), which are neural correlates of mnemonic processes (Kim et al., 1996; Chaouloff et al., 2007; Wong et al., 2007). Stress and glucocorticoids probably modulate synaptic plasticity in the hippocampus via changes in postsynaptic ionotropic glutamate receptor trafficking (Kim et al., 1996; Wong et al., 2007; Groc et al., 2008), although changes in presynaptic transmitter release also occur (Karst et al., 2005).
Stress and glucocorticoids could also modulate plasticity within the hippocampus by recruiting the endocannabinoid system (ECS). In the central nervous system (CNS), the ECS comprises the eCB receptor (CB1R), the endocannabinoids (eCBs) N-arachidonylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG), and enzymes that synthesize, transport, and hydrolyze the eCBs. Activation of CB1Rs on presynaptic terminals by 2-AG can modulate synaptic transmission by regulating both GABA and glutamate release (Kano et al., 2009). Stimulation of 2-AG synthesis by increased diacylglycerol lipase alpha (DGLα) activity (Gao et al., 2010; Tanimura et al., 2010) follows postsynaptic depolarization and/or metabotropic receptor activation.
Stress and/or glucocorticoids increase eCB mobilization, and support for the hypothesis that the ECS is an important effector of GR activation in the brain is accumulating (Hill and McEwen, 2010). Exposure to footshock increases both 2-AG and AEA levels in the periaqueductal gray (Hohmann et al., 2005) and bath application of glucocorticoids increases eCB production and CB1R-mediated inhibition of glutamate release in hypothalamic slices (Di et al., 2003, 2005). The rapid behavioral effects of glucocorticoids and/or stress on sexual behavior and emotional memory depend on CB1R activation (Coddington et al., 2007; Campolongo et al., 2009). Taken together, these studies suggest that glucocorticoids can affect behavior via the ECS in limbic brain regions. Our hypothesis is that the ECS is a general effector of GR actions on signaling in the CNS. We tested this hypothesis in the hippocampus, a brain region with a high density of CB1R and well-characterized eCB regulation of GABAergic signaling. We used the Ca2+-dependent, eCB-mediated suppression of inhibition called DSI (Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001) as an assay of ECS function. We find that exposure of rats to a single episode of restraint causes GR-dependent potentiation of the ECS in CA1 pyramidal cells, and increases hippocampal tissue content of 2-AG. The effect of stress on DSI was blocked by an L-type Ca2+ channel inhibitor, and mimicked by an L-channel agonist, suggesting that glucocorticoids enhance Ca2+-dependent 2-AG mobilization.
MATERIALS AND METHODS
Subjects
All animal handling procedures were approved by the University of Maryland School of Medicine IACUC (specific approval for behavioral and brain slice studies; approval #0609001), and by the Animal Care Committee of the University of British Columbia (specific approval for behavioral and eCB measurement studies; approval # A09-0220). Male, Sprague Dawley rats were housed in groups of three. Rats were 5-6 weeks old (Charles River Lab., Wilmington, MA) in the electrophysiological studies, and 9-10 weeks old in the biochemical studies (Charles River, Montreal, Canada). Animal rooms were maintained at 21°C on a reversed 12 h light/dark cycle. All rats were given ad libitum access to food and water. Subjects were randomly assigned to either control or acute stress groups. Acute restraint stress was induced by putting the rat into a Plexiglas cylindrical restrainer (Kent Scientific Corp., Torrington, CT) for 30 min.
Drugs
ω-agatoxin was purchased from Ascent Scientific Ltd. (Bristol, UK). All other drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Tocris (Ellisville, MO). Drugs used for bath application or pretreatment were first dissolved in DMSO (LY 341495, LY 225910, corticosterone); ethanol (nifedipine, FPL 64176, SR 141716A); or water (atropine, meloxicam, carbachol), then added to the bathing saline at the desired final concentrations (the ratios of final concentration to stock concentration were from 1:1000 to 1:10,000). In the electrophysiology studies, RU 38486 in pure DMSO (20 mg/ml) was injected subcutaneously 30 min before acute stress (Stress + RU) or 90 min before control slice preparation (Con + RU) at a dose of 20 mg/kg. In the biochemical studies, RU 38486 (20 mg/kg) was injected subcutaneously in 1:1 saline and propylene glycol vehicle. In some studies, corticosterone (CORT; 10 mg/kg) was dissolved in 1:1 saline and propylene glycol and was injected subcutaneously 1 h before decapitation. DMSO and 1:1 saline and propylene glycol were injected subcutaneously into naïve animals 90 min and 1 h before decapitation, respectively, to serve as vehicle control to each group. In all cases the injection volume was 1 ml/kg.
Hippocampal slice preparation
Rats were killed by decapitation after heavy sedation with isoflurane. Hippocampal slices were prepared from the stressed groups either immediately (≤ 5 min, Stress-immed) or 30 min after removal of the animal from the restrainer (Stress-30 min). Hippocampal slices were prepared between 9 to 11 a.m., which is during the active (dark) phase. Hippocampi were isolated and sectioned into 400-μm-thick slices in ice-cold saline using a Leica VT 1200S Vibratome (Leica Microsystems Inc., Bannockburn, IL). The slices were maintained at room temperature for over 1 h in an interface holding chamber in a humidified atmosphere saturated with 95% O2/5% CO2. The recording chamber (Warner Instr., Hamden, CT) warmed the submerged slices, and experiments were performed at 30 ± 1°C.
Electrophysiology
Whole-cell voltage-clamp recordings of CA1 pyramidal cells were made using the “blind” patch method. Electrode resistances in the bath were 3-5 MΩ. During experiments, series resistance was checked by −2 mV hyperpolarizing voltage steps, and if it exceeded 35 MΩ, increased by > 15%, or if current baselines were unstable, data were discarded. Cell membrane potentials were held at −70 mV. IPSCs were elicited by 100 μs extracellular stimuli (eIPSCs) delivered with concentric bipolar stimulating electrodes (David Kopf Instruments, Tujunga, CA) placed in the stratum (s.) radiatum between CA3 and CA1, 0.5-1 mm away from the recording site. The eIPSCs were evoked at 4-s intervals. Data were collected using an Axopatch 1C amplifier (Molecular Devices, Sunnyvale, CA), filtered at 2 kHz, and digitized at 5 kHz using a Digidata 1200 and pClamp 8 software. Representative continuous traces were collected on a WINDAQ Data Q DI-710 (DATAQ Instr., Inc., Akron, OH) and are used for illustrative purposes only.
The extracellular recording solution contained in mM: 120 NaCl, 3 KCl, 2.5 CaCl2, 2 MgSO4, 1 NaH2PO4, 25 NaHCO3, and 20 glucose, and was bubbled with 95% O2, 5% CO2 (pH 7.4) at 30°C. The solution flowed continuously through the recording chamber at a rate of ~0.6 ml/min. Ionotropic glutamate responses were blocked with 20 μM AP-5 plus 10 μM NBQX. All slices were pretreated with 300 nM agatoxin for ≥ 30 min (usually ≥ 1 h) before being transferred to the recording chamber. ω-Agatoxin IVA (agatoxin) is irreversible within the time frame of our experiments (Wheeler et al., 1994), hence P/Q channels remained inhibited despite the absence of agatoxin in the perfusion solution. Whole-cell pipettes contained (in mM) 90 CsCH3SO3, 50 CsCl, 10 HEPES, 0.2 BAPTA, 0.3 Tris-GTP, 2 Mg-ATP, 1 MgCl2, and 5 QX-314 (pH 7.25). Cells were voltage-clamped at −70 mV, and eCB-dependent eIPSC suppression (DSI) was induced by 0.5-, 1-, 2-, or 3-s voltage steps to 0 mV.
Data analysis
Both the magnitude and duration of DSI were analyzed. The DSI magnitude was determined by the reduction from the mean amplitude of five successive eIPSCs evoked just before the depolarizing pulse was given to the pyramidal cell (baseline), compared to the mean amplitude of four successive eIPSCs taken just after the pulse (DSI period). The reduction ratio of these values (1-DSI period/baseline) was multiplied by 100%. A DSI value of 0% means the eIPSC was not reduced by depolarization, and a value of 100% means it was abolished. Whenever possible, 3 DSI trials were averaged to obtain mean DSI in a given condition, but at least 2 trials were used in every case. In some experiments (Figs. 1A1 and C, Fig. 3, and Supplemental Fig. 1), the mean magnitude of DSI induced by a 2-s step in the control group (either naïve, vehicle-treated, or tested prior to drug application) was normalized to 100% and all other values were expressed relative to this value. The decay time constant (τd) of DSI, which was obtained from single exponential fits of the envelope of the peak amplitudes of the eIPSCs during recovery from DSI, was used to calculate DSI duration. Changes of DSI magnitude were also assessed in some experiments by approximating the integrals of the area under the envelope of the eIPSC amplitudes from the point of maximal IPSC suppression throughout recovery of DSI to baseline. These DSI integrals were obtained by subtracting the amplitude of the normalized eIPSC from the baseline at each time point and multiplying this value by interstimulus interval, i.e. (100-normalized eIPSC%)*4 (example shown in Fig. 2C). The mean of the last five eIPSCs of each DSI trial was taken as baseline, and all the recovery responses in this trial were normalized (×100%) to this mean.
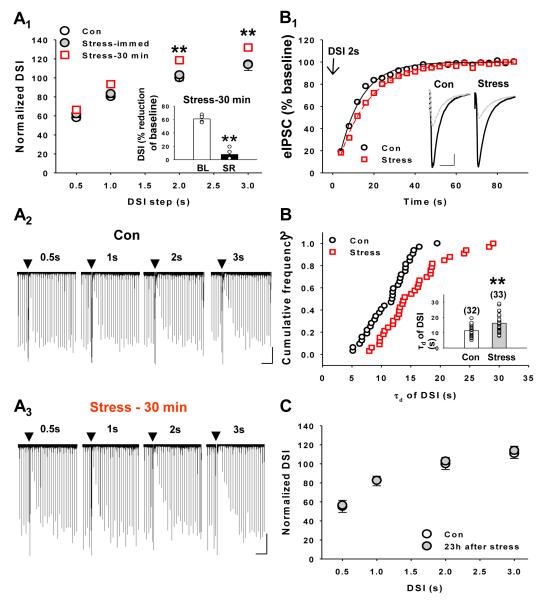
Figure 1. Acute stress enhances hippocampal DSI.
(A1) The magnitudes of DSI induced by 2-s and 3-s DSI steps were enhanced over control levels (Con) by acute stress, if slices were prepared 30 min after the animal’s removal from the restrainer (Stress-30 min), but not if they were prepared immediately after removal (Stress-immed, n=11 cells) *p < 0.05, Stress-30 min (n=32 cells) vs Con (n=33 cells). (Cells from acutely stressed animals studied 30 min after exposure to restraint are referred to as “stressed” cells.) Insert shows that the DSI in Stress-30 min group was blocked by the CB1R antagonist, SR 141716A (“SR”) (**p < 0.01, BL vs SR, n=4 cells). (A2 and A3) Representative whole-cell voltage clamp of DSI trials measured from a control and a stressed cell. Each downward deflection represents an evoked inhibitory post-synaptic current (eIPSC) which is compressed at this slow time scale. At the points indicated by the black triangles, a single voltage step from the holding potential of −70 mV to 0 mV was given for the indicated duration. DSI is the period after the voltage step where the eIPSCs are reduced and then gradually recover to their baseline amplitudes prior to the voltage step. Cal. bar: 20 s, 200 pA. (B1) Time course of mean DSI induced by a 2-s DSI step from control (Con, n=32) and stressed (Stress, n=33) cells; the voltage step ended at time 0. Inner traces were taken before (solid line, average of 5 consecutive eIPSCs just before depolarization) and after (dotted line, average of 4 consecutive eIPSCs just after depolarization) 2-s DSI step, control and stressed cells, respectively. Cal. bar: 50 ms, 200 pA. Both data sets were fit with single exponential functions, solid black or dashed red lines, which were used to determine the time constants of decay, τd, of DSI for the groups. (B2) Frequency distribution of τd following 2-s DSI steps, determined from individual control and stressed cells. Inner histogram shows the group data for τd. Each circle represents one cell. **p < 0.01, Stress vs Con. (C) The magnitude of DSI recovers to control level 23 h after acute stress. A group of animals was untreated (Con, n=17) and DSI was measured from slices as usual (2-s step). Another group was subjected to the usual acute stress protocol and then returned to their home cages for 23 h before slices were made (n=20). There were no differences in the DSI measured from the two groups.
Figure 3. GR activation mediates the enhancement of DSI by restraint stress.
(A) In vivo pretreatment with the GR antagonist RU 38486 (RU, 20 mg/kg), prior to exposure to restraint stress (Stress plus RU, n=14) prevented the enhancement of DSI (Con plus RU, n=9 and Con plus Vehicle, n=5). (B) Subcutaneous injection of corticosterone (10 mg/kg CORT, s.c., n=9) or vehicle (n=13) one h before slice preparation mimicked the stress effect on DSI magnitude. (C) Comparison of the effects of in vitro incubation with CORT (100 nM and 1 μM, n=8 and 9 respectively), or vehicle (n=13) on the magnitude of DSI. See text for statistical comparisons.
Figure 2. Acute stress increases carbachol-induced enhancement of DSI.
(A1) Time course of mean DSI, 0.5-s step, from control (Con, n=6) and stressed cells (Stress, n=6) before (open symbols) and after (filled symbols) 0.5 μM carbachol (CCh) application. (A2 and A3) Representative traces from a control and a stressed cell, respectively. Cal. bar 20 s, 200 pA. (B) Group data, DSI decay time constant τd from control (open bars) and stressed cells (striped bars) before (baseline, BL) and after CCh application (CCh). Each circle represents one cell. (C) Illustration of the estimated DSI integral, 0.5-s DSI step, obtained with data from the stressed cell shown in A3 after CCh application. The dark bars represent the integral of the area below 100%. (D) The difference of the DSI integral (Δ DSI integral) after CCh application between control and stressed cells. Each circle represents one cell. **p < 0.01 Stress vs Con.
Hippocampal lipid extraction and eCB analysis
All rats for this study were injected subcutaneously with RU 38486 (20 mg/kg) or an equivalent amount of propylene glycol/saline vehicle; those rats subjected to restraint stress were placed into restrainers 30 min after injection. Three groups of animals were compared: non-stressed (returned to home cage after injection and killed 60 min later); killed immediately following 30 min restraint stress (Stress-immed); and killed 30 min following the cessation of restraint stress (Stress-30 min). For all groups, brains were removed following rapid decapitation and the hippocampus was rapidly dissected free, frozen in dry ice within 5 min of decapitation, and stored at −80 °C until analysis. The hippocampus was subjected to a lipid extraction process described previously (Hill et al., 2009). Samples were weighedand placed into borosilicate glass culture tubes containing 2 ml of acetonitrile with 84 pmol of [2H8]AEA and 186 pmol of [2H8]2-AG. Tissue was homogenized witha glass rod and sonicated for 30 min. Samples were incubated overnight at −20°C to precipitate proteins, then centrifuged at 1,500 × g to remove particulates. The supernatants were removed to a new glass tubeand evaporated to dryness under N2 gas. The samples were re-suspended in 300 μl of methanol to recapture any lipids adheringto the glass tube, and dried again under N2 gas. Dried extracts were suspended in 20 μl of methanol, and stored at −80°C until analysis. The contents of the two primary eCBs, AEA and 2-AG, were determined using isotope-dilution, liquid chromatography–mass spectrometry (Patel et al., 2005).
Statistical analyses
Data analyses were done using Clampfit 8 (Axon Instruments) or Prism (GraphPad) and graphs were drawn in SigmaPlot 8.0 or in Prism. Two-way analyses of variance (ANOVA, performed by SigmaStat 3.0 or Prism) were used to compare groups when stimulation duration and in vivo or in vitro treatment are the main effects; when in vitro treatments were compared in groups treated differently in vivo; and eCB content in the hippocampus. Bonferroni’s corrected t tests were used to compare groups if significant main effects or interactions were identified by the ANOVA. When only a single stimulation condition was used, one-way ANOVA was used for group comparisons of physiological data followed by Student-Newman-Keuls tests for multiple comparisons. Paired t tests were used for single comparisons within groups, and unpaired t tests were used for comparisons between two groups under the same conditions. The significance level for all tests was p < 0.05 (*), except for the K-S test, for which p ≤ 0.01. Group means ± SEMs are shown in all graphs.
RESULTS
Acute stress prolongs DSI in hippocampal CA1 pyramidal cells
There are two sources of perisomatic GABAA-mediated eIPSCs in hippocampal CA1 pyramidal cells, the cholecystokinin-containing (CCK) and parvalbumin-containing (PV) interneurons (Somogyi and Klausberger, 2005; Freund and Katona, 2007). Of the two, CB1Rs are only on the CCK cells (Freund et al., 2003), which release GABA via Ca2+ influx through N-type (ω-conotoxin GVIA-sensitive) voltage-gated Ca2+ channels (VGCCs). The PV cells release GABA via P/Q-type (agatoxin-sensitive) VGCCs. To isolate the eCB-sensitive eIPSCs from the insensitive ones, agatoxin pretreatment was used to prevent occurrence of eIPSCs from PV cells (see Methods), and whole-cell recordings were made in acute hippocampal slices.
Depolarizing voltage-clamp command pulses delivered to pyramidal cells generate the Ca2+-dependent suppression of inhibition called DSI (Alger, 2002, for review), which is mediated by eCB release (Kano et al., 2009). The magnitude of DSI is dependent on the depolarization duration (Lenz et al., 1998). We used four durations (0.5-s, 1-s, 2-s, 3-s) to vary DSI magnitude and obtain a sensitive assay of eCB function (Figure 1A1). Recordings were made in slices from control (Con, n=32 cells from 27 rats) and two groups of stressed animals: one in which the slices were harvested within 5 min after the end of the stress period (Stress-immed, n=11 cells from 5 rats) and a second in which the slices were harvested 30 min after the end of the stress period (Stress-30 min, n=33 cells from 21 rats). Two-way ANOVA of these results indicates a significant effect of in vivo treatment on the magnitude of DSI (F2,218 = 10; p=0.0001) and a significant interaction between in vivo treatment and duration of depolarization (F6,218 = 2.4; p=0.02). Post hoc tests revealed significant differences between the control and Stress-30 min groups at depolarization durations of 2 and 3 seconds (Figure 1A1). However, there were no significant differences in DSI magnitude in slices from control and Stress-immed groups at any depolarization duration. Thus, DSI enhancement was observed only when the slices were prepared 30 min after the end of the restraint period, and not when they were prepared immediately. DSI observed in the Stress-30 min group was dependent on CB1R activation, since it was abolished by bath application of the CB1R antagonist, SR141716 (SR; 10 μM; Fig 1A1 inset, n=4 cells; 3-s depolarizing step in Stress-30 min group: DSI was 60.9 ± 2.8% before and 7.8 ± 4.8% in the presence of SR, p<0.001, paired t-test). Figs. 1A2 and 1A3 illustrate representative traces obtained with different DSI steps from a control cell and from a Stress-30 min cell, respectively. We used the Stress-30 min protocol (“stressed” rats) to test the effect of stress in all subsequent experiments.
Stress exposure also altered DSI duration. Fig 1B1 shows the eIPSC recovery following a 2-s DSI step in slices from non-stressed and stressed groups. The time constants of decay (τd) of the single exponential fits to the eIPSC recovery from peak DSI back to baseline were calculated and compared. The cumulative frequency distributions of τd showed a significant rightward shift to more prolonged DSI values in the stressed group (Fig. 1B2, K-S test, p <0.01,) and the mean τd of DSI increased from 11.3 ± 0.6 s (Con, n=32) to 15.6 ± 0.9 s (Stress, n=33) (p<0.001, t-test, Fig. 1B2 inset).
We examined the duration of DSI enhancement by a single episode of acute stress. After the termination of restraint stress, rats were put back to their home cages and the slices were prepared 23 h later. Cells from a new group of unstressed rats served as controls and DSI was measured at depolarization durations of 0.5, 1, 2, and 3 seconds (Figure 1C; 23 h after stress, n=20 cells from 6 rats and Con, n=17 cells from 5 rats). Two-way ANOVA of these data demonstrated that in vivo treatment did not significantly affect DSI duration (F1,140 = 0.24; p=0.63); thus DSI in the 23-h delay group was indistinguishable from DSI in never-stressed, control animals.
Acute stress increases mAChR -amplification of DSI
While DSI itself is a purely Ca2+-dependent form of eCB mobilization, it can be amplified by activating muscarinic cholinergic receptors (mAChR) with the cholinergic agonist, carbachol (CCh) (Kim et al., 2002; Ohno-Shosaku et al., 2003). To determine if the synergistic effect of Ca2+ and mAChR activation on eCB mobilization was also enhanced by stress, we used a 0.5-s depolarization together with CCh application (0.5 μM, 4 min), a protocol that markedly enhances and prolongs DSI (Kim et al., 2002). Brief depolarization steps were used in these experiments to maximize the opportunity to observe an increase in DSI, and to preclude the possibility of missing an enhancement because of a ceiling effect, which could occur if maximal DSI was induced with longer depolarizing steps. The τd of DSI in slices from control (n=6 cells from 5 rats) and stressed (n=6 cells from 6 rats) rats was determined before (BL) and after (CCh) the application of CCh in vitro (Figs. 2A1 and 2B; representative traces in Figs. 2A2 and 2A3). Two-way ANOVA of the τd values demonstrated significant effects of both CCh application (F1,20 = 69, p<0.0001) and stress exposure (F1,20 = 6.3, p<0.05) and a nearly significant interaction (F1,20 = 4.1, p=0.056). Thus, CCh significantly prolonged DSI in cells from both control and stressed rats, and tended to have a greater effect on DSI τd in the slices from stressed rats. To determine if the CCh effect was indeed greater in stressed animals, we also tested the DSI integral, which encompasses changes in both peak amplitude and duration of DSI (see Materials and Methods). Fig. 2C shows how the integral is defined using the cell illustrated in Fig. 2A3 after the addition of CCh. The CCh-induced increase in the DSI integral (Δ DSI integral) was significantly larger in stressed cells than in control cells (Fig. 2D, p<0.004 using t-test). Hence, in addition to enhancing ECS actions per se, stress also increases the coupling between mAChRs and the ECS.
The glucocorticoid receptor mediates the prolongation of DSI by stress
Many effects of stress in the CNS occur because the increase in circulating corticosteroid (CORT) concentrations is enough to fully activate CNS glucocorticoid receptors (GR) (Chrousos and Kino, 2007; McEwen, 2008). We tested three predictions of the hypothesis that activation of GR by CORT is responsible the restraint stress-induced increase in eCB signaling. The first prediction was that inhibition of GRs in vivo would prevent the enhancement of DSI by stress. To test this, we pretreated control and stressed rats in vivo with RU 38486 (20 mg/kg), an antagonist of GR. An additional non-stressed group was pretreated with DMSO, the vehicle for RU 38486 in these studies. Vehicle treatment had no effect on DSI responses in slices from non-stressed rats (control data in Figure 1C were used for comparison; F1,80 = 0.01, p=0.9). Two-way ANOVA revealed that there is no main effect of in vivo treatment on DSI magnitude among the three treatment groups [RU 38486 pretreated, non-stressed (Con + RU, n=6 cells from 5 rats) and RU 38486 pretreated, stressed (Stress + RU, n=14 cells from 10 rats) and non-stressed rats pretreated with DMSO (Con + Vehicle, n=5 cells from 3 rats] (Fig. 3A; F2,66 = 0.35, p=0.71). All DSI values were normalized to the 2-s DSI value of the Con + Vehicle group. Thus, GR activation is required for the effects of stress to increase hippocampal DSI.
The second prediction was that elevation of CORT in vivo would enhance eCB responses in unstressed rats. To test this, rats were injected subcutaneously with 10 mg/kg CORT (CORT s.c., n=9 cells from 5 rats) or propylene glycol/saline vehicle (Vehicle s.c., n=13 cells from 5 rats) 60 min prior to the harvest of hippocampal slices and determination of DSI (Fig. 3B). Injection of the propylene glycol/saline vehicle did not significantly affect DSI compared to non-injected controls (control data in Figure 1C were used for comparison; F1,112 = 1.5, p=0.2). Two-way ANOVA demonstrated a significant effect of CORT injection on DSI (F1,60 = 5, p=0.036). The effect of CORT was to increase DSI, mimicking the effects of stress.
A third prediction of the hypothesis is that application of CORT in vitro to hippocampal slices from naïve rats will enhance DSI. To test this prediction, slices from untreated rats were exposed for 20 min to CORT which was then washed out for at least 1 h before electrophysiological recordings were started (protocol modified from Verkuyl et al., 2005). Two concentrations of CORT, 100 nM (n=8 cells from 5 rats) and 1 μM (n=9 cells from 7 rats) were compared to vehicle (n=14 cells from 13 rats). DSI was determined at four depolarization times (Fig. 3C). Two-way ANOVA demonstrated that CORT treatment has a significant effect on DSI (F2,81=44, p=0.02). Thus, in addition to stress exposure and CORT injection, exposure of hippocampal slices to CORT also enhanced DSI.
Acute stress enhances mGluR-dependent hippocampal LTD via a GR-dependent mechanism (Chaouloff et al., 2007), and other transmitter systems, including mGluRs, cholecystokinin receptor (CCK2) and mAChRs can stimulate the ECS (Kano et al., 2009). To determine if acute stress enhances DSI directly by stimulating the ECS, or indirectly by stimulating another neurotransmitter system, we used a cocktail of antagonists. The cocktail included 100 μM LY 341495 (broad spectrum mGluR antagonist at this dose), 2 μM LY 225910 (CCK2 receptor antagonist) and 2 μM atropine (mAChR antagonist). After collecting baseline DSI data, we applied the cocktail for 15 min and tested DSI again. DSI enhancement was observed in slices from stressed animals in the presence of the cocktail (Supplemental Fig. 1, n=8 cells from 7 rats, see legend for details.)
COX-2 inhibition does not mediate the effects of stress on DSI
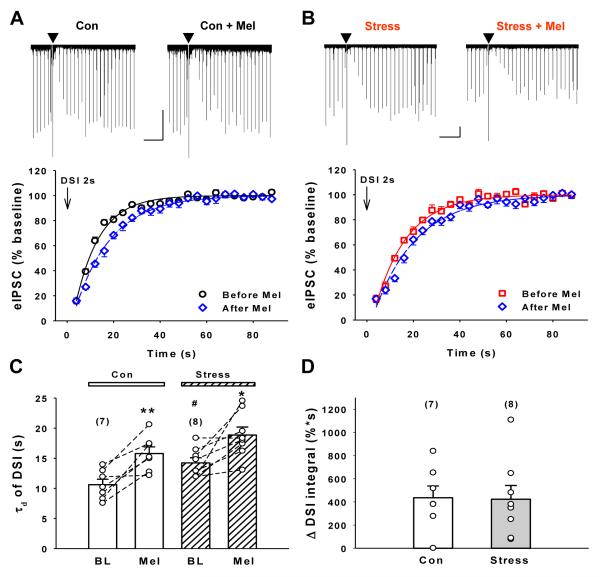
In the hypothalamus, glucocorticoids can shift arachidonic acid metabolism toward eCB synthesis by inhibiting cyclooxygenase-2 (COX-2) (Malcher-Lopes et al., 2008). Both 2-AG and AEA are substrates for COX-2 (Yu et al., 1997; Kozak et al., 2000), and inhibition of COX-2 enhances DSI in the hippocampus (Kim and Alger, 2004; Hashimotodani et al., 2007). These earlier findings suggest the hypothesis that stress and CORT enhance DSI via inhibition of COX-2. If this hypothesis is correct, then enhancement of DSI by a pharmacological inhibitor of COX-2 will be occluded in slices from stressed animals. To test this prediction, a selective COX-2 inhibitor, meloxicam (30 μM), was bath applied for 15-20 min prior to the induction of DSI. Meloxicam (Mel) prolonged DSI equally in hippocampal slices from both control (Fig. 4A, n=7 cells from 7 rats) and stressed rats (Fig. 4B, n=8 cells from 7 rats). The effects of meloxicam on mean τd in slices from control and stressed rats were compared using two-way ANOVA (Fig. 4C). The effects of both stress exposure (F1,26 = 12.4, p=0.0016) and in vitro treatment with meloxicam (F1,26 = 27, p<0.0001) were highly significant; however the interaction was not (F1,26 = 0.07, p=0.8), indicating no difference in the effect of meloxicam between slices from stressed and control rats. Meloxicam also caused similar increases in the DSI integrals above their baseline values in control (Δ DSI integral of 435.8 ± 101.9 %·s) and stressed cells (Δ DSI integral of 423.3 ± 118.9 %·s), p>0.02, t-test; Fig. 4D). Taken together, these data indicate that inhibition of COX-2 is not the mechanism involved in the effects of stress to increase DSI.
Figure 4. A COX-2 inhibitor does not occlude the effect of stress on DSI.
(A and B) Bath-application of the COX-2 inhibitor, meloxicam (Mel), 30 μM, prolonged DSI (2-s step) duration in control (A, n=7) and stressed cells (B, n=8). Representative traces from a control (A) and a stressed (B) cell are shown above. Cal. bar: 20s, 200pA. (C) Group data τd; each circle represents one cell. Meloxicam enhances τd in control, **p < 0.01, and stressed cells *p < 0.05; Stress BLτd is greater than control BLτd; #p < 0.05. (D) Meloxicam increased Δ DSI integral similarly in control and stressed cells. Each circle represents one cell.
Ca2+ influx through L-type VGCCs is required for the stress-induced increase in DSI
CORT increases the amplitude of whole-cell VGCCs in hippocampal CA1 pyramidal cells (Kerr et al., 1992; Joels et al., 2003). Acute stress and glucocorticoid treatment increase Ca2+ influx through L-type Ca2+ channels (L-channels) (Chameau et al., 2007). Although the induction of DSI is mainly dependent on N-channel current, L-channel current can also contribute under certain circumstances (Pitler and Alger, 1992; Lenz et al., 1998; Ohno-Shosaku et al., 2007). To test the possibility that L-channels are involved in the effect of stress on DSI, we bath applied the L-channel blocker, nifedipine (Nif, 10 μM). Nifedipine did not significantly affect the duration of DSI induced by a 2-s depolarization in hippocampal slices from untreated rats (Fig. 5A, n=6 cells from 6 rats), whereas it significantly decreased DSI duration in hippocampal slices from rats exposed to stress (Fig. 5B, n=9 cells from 9 rats). Percent DSI was compared in slices from control and stressed rats before and after the addition of nifedipine (Fig. 5C). Two-way ANOVA demonstrated that in vitro nifedipine treatment produced a significant effect on DSI (F1,13 = 11, p=0.006) while stress did not have a significant main effect (F1,13 = 3.4, p=0.09). There was a significant interaction between stress and in vitro treatment (F1,13 = 14, p=0.002). Comparisons using Bonferroni’s post hoc tests indicated that nifedipine decreased DSI in cells from stressed rats (p < 0.001) but not in cells from unstressed rats. Nifedipine also affected the prolongation of DSI τd (Fig. 5D) Two-way ANOVA demonstrated that neither stress (F1,13 = 4.49, p=0.054) nor nifedipine treatment (F1,13 = 1.29, p=0.277) had significant main effects on τd. However, there was a significant interaction (F1,13 = 5.25, p=0.039) and Bonferroni’s post hoc tests indicated that nifedipine significantly (p<0.05) decreased τd in cells from stressed rats, but not in control cells. Finally, as a direct test of the hypothesis that an increase in L-channel activity can enhance DSI, we bath-applied FPL 64176 (5 μM or 10 μM; n=8 cells from 8 rats), an agonist of L-channels, or its vehicle (n=6 cells from 3 rats), to control slices and used a 1-s DSI step to avoid the possibility that a ceiling effect that might occur with longer step durations. FPL 64176 increased both DSI magnitude (from 33.2 ± 4.3% to 48 ± 4.2% p < 0.001, paired t-test,) and duration (from 8.6 ± 1.1% to 11.1 ± 1.1%, p < 0.02, paired t test) (examples and grouped data shown in Figs. 6A and B; statistical tests done on data in Figs. 6C and D) while treatment of the slices with vehicle had no effect. Taken together, these results support the hypothesis that stress enhances hippocampal DSI by recruiting Ca2+ influx through L-type Ca2+ channels.
Figure 5. L-channel Ca2+ current mediates the stress effect on DSI.
(A and B) Bath application of the L-channel blocker, nifedipine (Nif, 10 μM), did not affect DSI duration in control (A, n=6) while it decreased DSI duration in stressed cells (B, n=9). Representative traces from a control (A) and a stressed (B) cell are shown above. Cal. bar: 20s, 200pA. (C) Group data, %DSI; each circle represents one cell. Nif reduced DSI in stressed cells, but not in control cells; * p<0.05. (D) Group data, DSI τd; each circle represents one cell. Nif reduced τd in stressed cells, but not in control cells, * p<0.05.
Figure 6. L-channel activation increases responses to postsynaptic depolarization.
(A) Bath application of vehicle (ethanol, 1:5000) did not affect the responses of control cells to 1-s postsynaptic depolarization. Cal. bar: 20s, 200pA. (B) Bath application of L-channel activator, FPL 64176 (FPL, 5 μM or 10 μM) increased DSI responses. Cal. bar: 20s, 200pA. Representative traces from a vehicle-treated (A) or an FPL 64176 treated (B) cell are shown above. (C) Group data, %DSI; each circle represents one cell. FPL 64176 enhanced DSI **p < 0.01. (D) Group data, DSI τd; each circle represents one cell. FPL 64176 enhanced DSI τd, *p < 0.05.
Acute stress increases the 2-AG content of the hippocampus
In the studies described thus far, we have used DSI as a bioassay of the effects of stress on the ECS. If acute restraint potentiates activation of the ECS, a possible mechanism would be stress-induced increases in synaptic 2-AG. To test this prediction, we measured eCBs in bulk tissue contents within the hippocampus following stress exposure. There was a significant interaction between stress exposure and RU 38486 treatment (F2, 36 = 5.44; p < 0.01), with post hoc analysis revealing that, compared to control, rats exposed to 30 min of restraint stress exhibited a significant increase in hippocampal 2-AG content (p < 0.001; Stress-immed vs Con, Fig. 7A) and the 2-AG content was still increased 30 min after stress (p < 0.05, Stress-30 min vs Con). The effect of stress on 2-AG content was prevented by in vivo RU 38486 pretreatment (Fig. 7A). Interestingly, there was also a significant interaction between stress and RU 38486 treatment with respect to tissue levels of the other major eCB AEA (F2, 36 = 4.23, p < 0.05), such that in the Stress-immed group, stress exposure significantly reduced hippocampal AEA compared to control (p <0.01, Fig. 7B). AEA content recovered to control concentrations within 30 min after stress exposure (p > 0.05, Stress-30 min vs Con, Fig. 7B). The reduction of AEA was also reversed by RU 38486 pretreatment (Fig. 7B), suggesting a role of GR activation in this change as well. These data suggest the hypothesis that GR activation during stress results in increased 2-AG content and a concomitant facilitation of DSI in the hippocampus.
Figure 7. Acute stress enhances the content of 2-AG that via GR activation.
(A) Acute stress enhanced the content of 2-arachidonoylglycerol (2-AG) in hippocampus, and this was prevented by in vivo RU 38486 pretreatment. Stress increased 2-AG content over basal levels (n=7) both in the Stress-immed (**p < 0.01, n=6) and Stress-30 min (#p < 0.05, n=8) conditions. 2-AG content was greater in the Stress-immed than the Stress-30 min group (&p <0.05). (B) Acute stress transiently decreased the content of anandamide (AEA) which was prevented by in vivo RU 38486 pretreatment. AEA in the Stress-immed group differed from basal AEA content (**p < 0.01), and the Stress-30 min group differed from the Stress-immed group given in vivo vehicle treatment (##p < 0.01), but not if RU 38486 had been given.
DISCUSSION
We find that acute exposure to restraint stress enhances eCB-mediated signaling in the hippocampus of male rats. It is unknown whether this effect occurs in female rats. The magnitude and duration of Ca2+-dependent eCB mobilization (DSI) and its enhancement by mAChR activation are increased in hippocampal slices. Focusing on DSI, we found that the effect of stress took time to develop, not being evident until 30 min after the restraint period ended. DSI enhancement was blocked by the GR antagonist, RU 38486, and mimicked by in vivo or in vitro CORT treatment. The effects of stress on ECS in the hippocampus are likely mediated by increases in circulating CORT since they were blocked by a GR antagonist and mimicked by CORT administration both in vivo and in vitro. The cellular mechanism altered by stress has not been fully determined, but [Ca2+]i is critical for both DSI and mAChR enhancement of DSI (Kano et al., 2009), and using pharmacological tools, we found that L-type Ca2+ channels may be the proximate downstream effectors of stress on the ECS. These results are consistent with the report of Chameau et al. (2007) that stress rapidly upregulates L-channels. Restraint stress also increased the 2-AG content in the hippocampus, as expected if DSI is mediated by 2-AG (Gao et al., 2010; Tanimura et al., 2010).
Several factors could contribute to the stress- and CORT-induced changes in DSI, including the amount of eCB available (owing to changes in synthesis, release, uptake or degradation) or the sensitivity or number of CB1R. Changes in CB1Rs occur after chronic unpredictable stress (Hill et al., 2005, 2008b; Reich et al., 2009) or prolonged glucocorticoid treatment (Hill et al., 2008a). In striatum, the sensitivity of GABA synapses to eCB is impaired following one social defeat stress exposure and is abolished by exposures over 3- or 7-days (Rossi et al., 2008). However, CORT administration for shorter periods (i.e. one day) does not affect CB1R agonist binding parameters in hippocampus, suggesting that longer-lasting increases of CORT are required for the regulation of CB1R expression. Indeed, up-regulation of CB1R cannot fully explain our results, as increased CB1R expression would have affected all eCB-mediated responses, yet significant DSI enhancement was not seen with all DSI-inducing voltage steps. An effect of stress on CB1R signaling downstream from the receptor is not ruled out by our results.
An alternative explanation is that stress and CORT enhance DSI by increasing eCB mobilization. Hippocampal 2-AG content is significantly increased following stress, which is consistent with an increase in 2-AG mobilization, and with earlier reports that foot shock and glucocorticoid application stimulate eCB biosynthesis (both AEA and 2-AG) in the periaqueductal gray matter and hypothalamus, respectively (Di et al., 2003; Hohmann et al., 2005; Malcher-Lopes et al., 2006). The acute effect of stress on tissue 2-AG could be brain region specific, as a single exposure to restraint increases 2-AG content in the medial prefrontal cortex in a temporal and GR-dependent fashion (Hill et al., under review) that is similar to what we find in hippocampus, but does not increase 2-AG content in the amygdala (Hill et al., 2009). Injection of 20 mg/kg CORT in rats does not significantly affect hippocampal 2-AG tissue contents measured 24 h later (Hill et al., 2008a), and our data obtained now show that the effect of stress on DSI does not persist for 23 h either. However, the relationship of bulk measurements of 2-AG to cellular 2-AG mediated signaling processes is not clear. Recent studies with mutant mice lacking functional DGLα (Gao et al., 2010; Tanimura et al., 2010) reveal that a significant fraction of total 2-AG measured in different brain areas is independent of DGLα, and in fact, much of the DGLα-dependent 2-AG could be unrelated to rapid physiological signaling (Gao et al., 2010). Thus, measured increases in the tissue content of 2-AG may not be directly related to the 2-AG that is available for signaling through CB1Rs. These considerations could account in part for the disparity between the bulk increase in 2-AG that is present immediately after cessation of the restraint stress period (Fig. 7), and the physiologically measured increase in DSI, which is not detectable until 30 min later (Fig. 1A1). The proposed existence of multiple functionally distinct “pools” of 2-AG or DGLα creates further complexity in relating bulk measurements of 2-AG with specific CB1R-mediated phenomena (Edwards et al., 2006; Zhang et al., 2011). Finally, stress could interact with the ECS via more than one mechanism.
Stress-enhanced DSI was not affected by inhibiting receptors, such as mGluRs, mAChRs and CCK2Rs, that are affected by stress (Beck et al., 1996; Venero and Borrell, 1999) and that also mediate eCB generation in the hippocampus, suggesting that these receptors are not part of the underlying mechanism. COX-2 hydrolyzes 2-AG in hippocampus, and COX-2 inhibition prolongs DSI (Kim and Alger, 2004; Hashimotodani et al., 2007). In the hypothalamus, glucocorticoids may shift arachidonic acid metabolism toward eCB synthesis via COX-2 inhibition (Malcher-Lopes et al., 2008). However, we found that a COX-2 inhibitor prolonged DSI equally in stressed and control slices, arguing against an involvement of COX-2 in stress-enhanced eCB function. We noted that even when DSI itself was not enhanced by stress (i.e., when the DSI step was only 0.5 or 1.0 s), the ability of CCh to enhance DSI was amplified. This could represent a non-linear interaction between the Ca2+- and GPCR-pathways, an additional action of stress on the GPCR pathway, or an increase Ca2+-sensitivity of the eCB mobilization mechanisms.
Corticosterone, which is released in high concentrations after stress, can easily pass the blood-brain barrier and bind to intracellular receptors in the brain (De Kloet et al., 2005). Activation of the relatively low-affinity GRs will be initiated, since the higher-affinity mineralocorticoid receptors will already be substantially occupied by normally circulating corticosteroid levels. The activation of GRs increases the amplitude of L-currents in hippocampal CA1 region (Chameau et al., 2007). L-channels exist on CA1 pyramidal cells in high densities (Westenbroek et al., 1990) and contribute significantly to the total somatic Ca2+ current measured in these cells. Although the principal carriers of Ca2+ current for hippocampal eCB mobilization are N-channels, L-channel influx can contribute to DSI (Pitler and Alger, 1992; Lenz et al., 1998; Ohno-Shosaku et al., 2007). Our results can best be explained by a glucocorticoid-mediated increase in L-channel Ca2+ influx into the pyramidal cells, and consequent increase in eCB mobilization.
Tasker and colleagues were the first to report that GR activation affects eCB synaptic signaling (Di et al., 2003). They found that bath application of CORT resulted in rapid suppression of glutamate release from terminals innervating parvocellular secretory neurons of the paraventricular nucleus in the hypothalamus. The effect of CORT required CB1R engagement, leading the authors to hypothesize that CORT recruited the ECS to produce synaptic plasticity at this synapse. These investigators showed further that the effect of CORT was consistent with a non-genomic mechanism such that it was membrane delineated and required G protein activation (Di et al., 2003). Although the ECS is also involved in the effects that we observe, several differences between our findings and those of the Tasker group suggest that the cellular mechanisms involved are likely to be different in the two systems. First, the effect of stress on DSI in the hippocampus is not seen immediately after stress offset, when circulating CORT concentrations are at a peak (Diorio et al., 1993) but is seen 30 min later. In the hypothalamus, CORT had an immediate effect on glutamate release. Second, the effect of stress in the hippocampus requires L-type Ca2+ channels; the increase inCa2+ currents mediated by glucocorticoids is a genomic effect that depends on protein synthesis (Karst et al., 1994). Thus the mechanism in the hippocampus may involve genomic GR signaling, as opposed to the non-genomic mechanism of the hypothalamus.
Our biochemical data are consistent with other evidence that 2-AG, and not AEA, is the eCB for DSI in the hippocampus (Kim and Alger, 2004; Hashimotodani et al., 2008; Gao et al., 2010; Tanimura et al., 2010). In fact, we also observed a significant, though transient, decrease in AEA content caused by stress. This is consistent with previous reports of stress-induced reduction in AEA in other brain regions (Patel et al., 2005; Rademacher et al., 2008; Hill et al., 2009). Although the linkage is unclear, recent studies show significant reductions in AEA in DGL knock-out mice (Gao et al., 2010; Tanimura et al., 2010). Our data show an inverse, rather than a direct, relationship between AEA and 2-AG, nevertheless, it may be interesting that the AEA and 2-AG systems seem to be altered by common forms of behavioral experience.
As increases in eCB actions decrease GABA release, the present data may help account for previous reports of reductions in GABA levels within the hippocampus following acute stress exposure (Harvey et al., 2004; Briones-Aranda et al., 2005). Patel and coworkers (Patel et al., 2009) recently reported that, in the amygdala, repeated restraint episodes are required to amplify DSI and increase 2-AG tissue content, hence the hippocampus may be more sensitive in this regard. Stress-induced mobilization of 2-AG could be a general mediator of stress-induced modulation of synaptic transmitter release, and it will be interesting to learn if the ECS is affected by acute restraint stress in other areas.
In conclusion, we show that hippocampal ECS is potentiated by acute stress and GR activation. These findings are consonant with recent behavioral findings that acute stress and/or glucocorticoids modify emotional and motivated behaviors via increased eCB signaling (Coddington et al., 2007; Campolongo et al., 2009). In contrast, prolonged exposure to stress and/or glucocorticoids decreases CB1R expression and eCB levels (Hill et al., 2005, 2008a, 2008b; Reich et al., 2009), suggesting that the relationship between stress and the ECS is biphasic. This is reminiscent of other responses to stress that also exhibit a biphasic response, such as immune functioning, which is facilitated by acute stress, but which is subject to immuno-suppression under chronic stress conditions (Dhabhar, 2003). This shift may reflect the burden of “allostatic load”; the loss of ECS function during chronic stress could contribute to allostatic load and to the development of disease states (McEwen, 2004). Stress-induced mobilization of eCB signaling could represent an adaptive response to acute stress, which would help maintain emotional and behavioral flexibility in the face of aversive stimuli, while the loss of eCB signaling following chronic stress could contribute to the development of stress-related psychiatric disorders, such as depression and anxiety disorders (Gorzalka et al., 2008; Lutz, 2009).
Supplementary Material
Supplemental Figure 1 – Antagonists of receptors that can mobilize eCBs do not affect stress-enhanced DSI. Bath-application of a cocktail of antagonists of mGluRs, CCK2Rs, and mAChRs (100 μM LY 341495 + 2 μM LY 225910 + 2 μM atropine, respectively) to cells from stressed animals did not reduce the magnitude of DSI (n=8). All DSI was normalized to the 2-s DSI value before cocktail application. DSI values before and after cocktail application, respectively, were: 0.5s) 55.9 ± 3.1% and 51.2 ± 4.3%; 1-s) 78.6 ± 6.7% and 79.7 ± 5.2%; 2-s) 100 ± 4.9% and 96.5 ± 7.1%; 3-s) 107.5± 3.9% and 105.9 ± 2.8%. Differences n.s.; p>0.05 paired t-test at each DSI step length, before and during cocktail application.
ACKNOWLEDGEMENTS
We thank Drs. Margaret McCarthy and Jaylyn Waddell for their insightful comments on a draft of this report. This work was supported by NIH RO1 DA 014625 and RO1 MH 077277 (to B.E.A.), NIH RO1 DA 09155 (to C.J.H.) and by an operating grant to B.B.G. from the Canadian Institute for Health Research (CIHR). M.N.H. is the recipient of a CIHR postdoctoral fellowship.
REFERENCES
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Beck SG, Choi KC, List TJ, Okuhara DY, Birnsteil S. Corticosterone alters 5-HT1A receptor-mediated hyperpolarization in area CA1 hippocampal pyramidal neurons. Neuropsychopharmacology. 1996;14:27–33. doi: 10.1016/S0893-133X(96)80056-X. [DOI] [PubMed] [Google Scholar]
- Briones-Aranda A, Rocha L, Picazo O. Alterations in GABAergic function following forced swimming stress. Pharmacol Biochem Behav. 2005;80:463–470. doi: 10.1016/j.pbb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. ECBs in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci U S A. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chameau P, Qin Y, Spijker S, Smit G, Joels M. Glucocorticoids specifically enhance L-type calcium current amplitude and affect calcium channel subunit expression in the mouse hippocampus. J Neurophysiol. 2007;97:5–14. doi: 10.1152/jn.00821.2006. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Hémar A, Manzoni O. Acute stress facilitates hippocampal CA1 metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2007;27:7130–7135. doi: 10.1523/JNEUROSCI.1150-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10:213–219. doi: 10.1080/10253890701292119. [DOI] [PubMed] [Google Scholar]
- Coddington E, Lewis C, Rose JD, Moore FL. ECBs mediate the effects of acute stress and corticosterone on sex behavior. Endocrinology. 2007;148:493–500. doi: 10.1210/en.2006-0740. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Stress, leukocyte trafficking, and the augmentation of skin immune function. Ann NY Acad Sci. 2003;992:205–217. doi: 10.1111/j.1749-6632.2003.tb03151.x. [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Rapid glucocorticoid-mediated eCB release and opposing regulation of glutamate and gamma-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinol. 2005;146:4292–4301. doi: 10.1210/en.2005-0610. [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via eCB release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–47. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA, Kim J, Alger BE. Multiple mechanisms of endocannabinoid response initiation in hippocampus. J Neurophysiol. 2006;95:67–7. doi: 10.1152/jn.00813.2005. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang MY, Strassle BW, Lu P, Mark L, Piesla MJ, Deng K, Kouranova EV, Ring RH, Whiteside GT, Bates B, Walsh FS, Williams G, Pangalos MN, Samad TA, Doherty P. Loss of retrograde eCB signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Hillard CJ. Regulation of eCB signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nature Neurosci. 2008;11:868–870. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal eCB tone and terminates retrograde eCB signaling in the hippocampus. J Neurosci. 2007;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Maejima T, Fukami K, Kano M. Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocanabinoid release. Neuropharmacology. 2008;54:58–67. doi: 10.1016/j.neuropharm.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Harvey BH, Oosthuizen F, Brand L, Wegener G, Stein DJ. Stress-restress evokes sustained iNOS activity and altered GABA levels and NMDA receptors in rat hippocampus. Psychopharmacology (Berl) 2004;175:494–502. doi: 10.1007/s00213-004-1836-4. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psych. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, et al. Suppression of amygdalar eCB signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwen BS. ECBs: The silent partner of glucocorticoids in the synapse. Proc Natl Acad Sci U S A. 2009;106:4579–4580. doi: 10.1073/pnas.0901519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, Ho WS, Shi L, Patel S, Gorzalka BB, Hillard CJ. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus. 2008a;18:221–226. doi: 10.1002/hipo.20386. [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, et al. Regional alterations in the eCB system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem. 2008b;106:2322–2336. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of eCB signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An eCB mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Joels M, Velzing E, Nair S, Verkuyl JM, Karst H. Acute stress increases calcium current amplitude in rat hippocampus: temporal changes in physiology and gene expression. Eur J Neurosci. 2003;18:1315–1324. doi: 10.1046/j.1460-9568.2003.02845.x. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. ECB-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Wadman WJ, Joels M. Corticosteroid receptor-dependent modulation of calcium currents in rat hippocampal CA1 neurons. Brain Res. 1994;649:234–242. doi: 10.1016/0006-8993(94)91069-3. [DOI] [PubMed] [Google Scholar]
- Kerr DS, Campbell LW, Thibault O, Landfield PW. Hippocampal glucocorticoid receptor activation enhances voltage-dependent Ca2+ conductances: relevance to brain aging. Proc Natl Acad Sci U S A. 1992;89:8527–8531. doi: 10.1073/pnas.89.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde eCB effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc Natl Acad Sci U S A. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the eCB, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- Lenz RA, Wagner JJ, Alger BE. N- and L-type calcium channel involvement in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells. J Physiol (Lond) 1998;512:61–73. doi: 10.1111/j.1469-7793.1998.061bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B. ECB signals in the control of emotion. Curr Opin Pharmacol. 2009;9:46–52. doi: 10.1016/j.coph.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, Tasker JG. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via eCB release. J Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcher-Lopes R, Franco A, Tasker JG. Glucocorticoids shift arachidonic acid metabolism toward eCB synthesis: a non-genomic anti-inflammatory switch. Eur J Pharmacol. 2008;583:322–339. doi: 10.1016/j.ejphar.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann NY Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde eCB signalling in the hippocampus. Eur J Neurosci. 2003;18:109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Hashimotodani Y, Ano M, Takeda S, Tsubokawa H, Kano M. ECB signalling triggered by NMDA receptor-mediated calcium entry into rat hippocampal neurons. J Physiol (Lond) 2007;584:407–418. doi: 10.1113/jphysiol.2007.137505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term eCB signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–2709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on eCB content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203:264–269. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, De C V, Musella A, Kusayanagi H, Mataluni G, Bernardi G, Usiello A, Centonze D. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J Neurosci. 2008;28:7284–7292. doi: 10.1523/JNEUROSCI.5346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol (Lond) 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, Sakimura K, Kano M. The eCB 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. Eur J Neurosci. 1999;11:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- Verkuyl JM, Karst H, Joels M. GABAergic transmission in the rat paraventricular nucleus of the hypothalamus is suppressed by corticosterone and stress. Eur J Neurosci. 2005;21:113–121. doi: 10.1111/j.1460-9568.2004.03846.x. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Ahlijanian MK, Catterall WA. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 1990;347:281–284. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of eCB signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Wong TP, Howland JG, Robillard JM, Ge Y, Yu W, Titterness AK. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proc Natl Acad Sci U S A. 2007;104:11471–11476. doi: 10.1073/pnas.0702308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang M, Bisogno T, Di Marzo V, Alger BE. Endocannabinoids generated by Ca2+ or by metabotropic glutamate receptors appear to arise from different pools of diacylglycerol lipase. PLoS ONE. 2011;6:e16305. doi: 10.1371/journal.pone.0016305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 – Antagonists of receptors that can mobilize eCBs do not affect stress-enhanced DSI. Bath-application of a cocktail of antagonists of mGluRs, CCK2Rs, and mAChRs (100 μM LY 341495 + 2 μM LY 225910 + 2 μM atropine, respectively) to cells from stressed animals did not reduce the magnitude of DSI (n=8). All DSI was normalized to the 2-s DSI value before cocktail application. DSI values before and after cocktail application, respectively, were: 0.5s) 55.9 ± 3.1% and 51.2 ± 4.3%; 1-s) 78.6 ± 6.7% and 79.7 ± 5.2%; 2-s) 100 ± 4.9% and 96.5 ± 7.1%; 3-s) 107.5± 3.9% and 105.9 ± 2.8%. Differences n.s.; p>0.05 paired t-test at each DSI step length, before and during cocktail application.