Abstract
Epoxyeicosatrienoic acids (EETs) are cytochrome P450 metabolites of arachidonic acid that are produced by the vascular endothelium in responses to various stimuli such as the agonists acetylcholine (ACH) or bradykinin or by shear stress which activates phospholipase A2 to release arachidonic acid. EETs are important regulators of vascular tone and homeostasis. In the modulation of vascular tone, EETs function as endothelium-derived hyperpolarizing factors (EDHFs). In models of vascular inflammation, EETs attenuate inflammatory signaling pathways in both the endothelium and vascular smooth muscle. Likewise, EETs regulate blood vessel formation or angiogenesis by mechanisms that are still not completely understood. Soluble epoxide hydrolase (sEH) converts EETs to dihydroxyeicosatrienoic acids (DHETs) and this metabolism limits many of the biological actions of EETs. The recent development of inhibitors of sEH provides an emerging target for pharmacological manipulation of EETs. Additionally, EETs may initiate their biologic effects by interacting with a cell surface protein that is a G-protein coupled receptor (GPCR). Since GPCRs represent a common target of most drugs, further characterization of the EET receptor and synthesis of specific EET agonists and antagonist can be used to exploit many of the beneficial effects of EETs in vascular diseases, such as hypertension and atherosclerosis. This review will focus on the current understanding of the contribution of EETs to the regulation of vascular tone, inflammation and angiogenesis. Furthermore, the therapeutic potential of targeting the EET pathway in vascular disease will be highlighted.
Keywords: epoxyeicosatrienoic acid, cytochrome P450, endothelial cell, hyperpolarization, angiogenesis, inflammation
1. Introduction
Epoxyeicosatrienoic acids (EETs) play a pivotal role in numerous cellular processes involved in vascular function, including vasodilation and inflammation. The multifunctional nature of EETs underlies the importance of these compounds in cardiovascular disease. This review will focus on the current understanding of the contribution of EETs to the regulation of vascular tone, inflammation and angiogenesis. Furthermore, the therapeutic potential of targeting the EET pathway in vascular disease will be highlighted.
2. Biochemistry
In endothelial cells, arachidonic acid is metabolized by the cyclooxygenase (COX), lipoxygenase and cytochrome P450 (CYP) epoxygenase pathways (Rosolowsky and Campbell, 1993, 1996). The epoxygenase pathway leads to the formation of four regioisomeric EETs, 14,15-EET, 11,12-EET, 8,9-EET and 5,6-EET (Figure 1) (Capdevila et al., 1981; Zeldin, 2001). The EETs are released by endothelial cells in response to receptor agonists such as ACH or bradykinin to function as autocrine and paracrine hormones (Campbell et al., 1996a; Fisslthaler et al., 2001; Gauthier et al., 2005; Huang et al., 2005; Nithipatikom et al., 2000). The relative abundance of each EET regioisomer differs among vascular beds depending on which CYP isoforms are expressed, as each CYP isoform generates its own unique profile of EET regioisomers. While many CYP isozymes have been identified in blood vessels, endothelial CYP2C8/2C9 and CYP2J2 function in humans to produce mainly 14,15-EET, with lesser amounts of 11,12-EET (Wu et al., 1996; Zeldin, 2001; Zeldin et al., 1995). No EET production is detected in smooth muscle (Campbell et al., 2006).
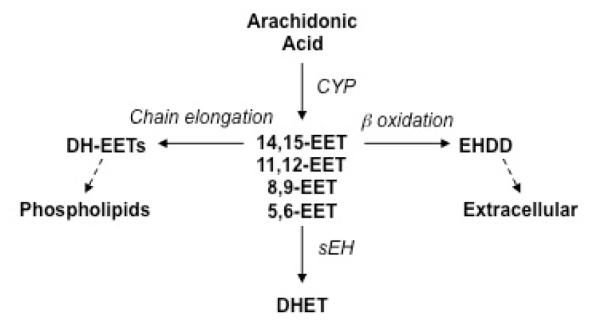
Figure 1.
Overview of EET Biosynthesis and Metabolism. Arachidonic acid is metabolized by cytochrome P450 (CYP) epoxygenases to produce epoxyeicosatrienoic acids (EETs). There are four EET regioisomers which vary with the placement of the epoxide group. They include 5,6-, 8,9-, 11,12- and 14,15-EET. EETs are esterified to cellular membrane phospholipids following chain elongation, metabolized by β–oxidation to shorter carbon chain molecules or metabolized by soluble epoxide hydrolase (sEH) to their corresponding vicinal diols, the dihydroxyeicosatrienoic acids (DHETs). DH-EET = dihomo-EET; EHDD = epoxyheptadecadienoic acid
The EETs are rapidly taken up by vascular cells and metabolized by soluble epoxide hydrolase (sEH) to form dihydroxyeicosatrienoic acids (DHETs) (Figure 1) (Spector et al., 2004; Spector and Norris, 2007). The DHETS are generally less bioactive than their corresponding EETs. Alternatively, EETs undergo secondary metabolism or β-oxidation, forming 16-carbon epoxy-derivatives that accumulate in the extracellular fluid, and they can be chain-elongated to form 22-carbon 1α-1β-dihomo derivatives that are incorporated into phospholipids (Figure 1) (Fang et al., 2002). Understanding the chemical and biochemical mechanisms that contribute to EET function will play an important part in the future design of drugs that act through the EET pathway. This topic is discussed in more detail below.
3. EETs and Vascular Tone
One of the early descriptions of a biological action of the EETs was as vasodilators in the intestinal microcirculation (Proctor et al., 1987). This original observation eventually led to the identification of EETs as endothelium-dependent hyperpolarizing factors (EDHFs) (Figures 2 & 3) (Archer et al., 2003; Campbell et al., 1996a; Coats et al., 2001; Gauthier et al., 2005; Huang et al., 2005; Miura et al., 2001; Popp et al., 1998). There is a plethora of studies in humans and animals that support this observation and the topic is the focus of a number of recent reviews (Campbell and Fleming, 2010; Imig and Hammock, 2009; Revermann, 2010; Sudhahar et al., 2010). Key aspects are highlighted below.
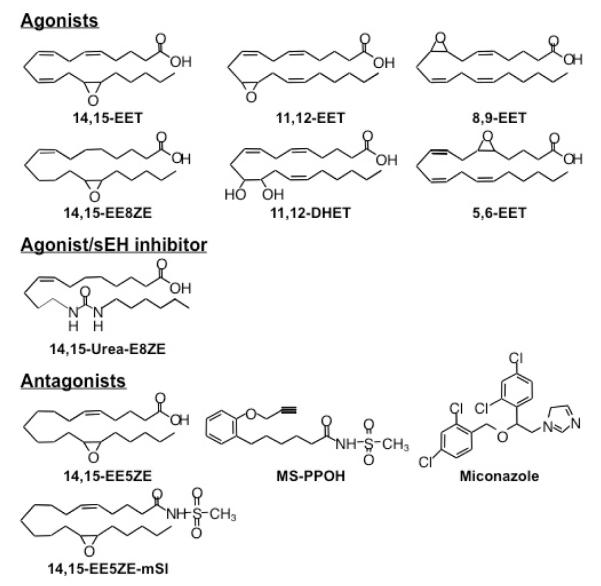
Figure 2.
Chemical structures of epoxyeicosatrienoic acid (EET) agonists, an EET agonist/sEH inhibitor and EET antagonists. mSI = methylsulfonamide; EE8ZE = epoxyeicosa-8Z-enoic acid; EE5ZE = epoxyeicosa-5Z-enoic acid; E8ZE = eicosa-8Z-enoic acid; eEH = soluble epoxide hydrolase
Figure 3.

Proposed mechanisms of epoxyeicosatrienoic acid (EET)-mediated hyperpolarization and relaxation. Stimulation by bradykinin, acetylcholine, or shear activates phospholipase in endothelial cell membranes to elicit the release of arachidonic acid. Arachidonic acid is metabolized by cytochrome P450 (CYP) epoxygenases of the 2C and 2J families to EETs. EETs diffuse to the smooth muscle cell to induce membrane hyperpolarization via activation of large conductance, calcium-sensitive potassium (BKCa) channels. This causes K efflux, an increase in membrane potential (Em) or hyperpolarization and relaxation. EETs also act intracellularly in endothelial cells to promote Ca efflux through transient receptor potential (TRP) channels. Calcium activates small conductance (SK) and intermediate conductance (IK) KCa to cause hyperpolarization. Endothelial hyperpolarization spreads to the smooth muscle cell via gap junctions. R = putative EET receptor; Gs = stimulatory guanine nucleotide-binding protein.
I. Experimental Evidence
In response to chemical or physical stimuli such as ACH, thrombin, bradykinin, and fluid sheer stress, vascular endothelial cells regulate the tone of the underlying smooth muscle by producing and releasing various relaxing and contracting factors (Cohen and Vanhoutte, 1995; Feletou and Vanhoutte, 2006; Fleming and Busse, 2006; Furchgott and Vanhoutte, 1989; Furchgott and Zawadzki, 1980). The relaxing factors are the COX metabolite, prostacyclin (PGI2), endothelium-derived relaxing factor (EDRF) identified as nitric oxide (NO), and EDHFs. EDHF was originally described as an endothelial factor that hyperpolarized the membrane of the underlying vascular smooth muscle. Hyperpolarization was mediated by the opening of potassium (K) channels because it was inhibited by increased extracellular K concentration and K channel blockers (Adeagbo and Triggle, 1993; Cohen and Vanhoutte, 1995). Inhibitors of NO synthase or COX do not block hyperpolarizations. PGI2, NO, and EDHF serve the same function to dilate the blood vessels in response to agonists, and to physical forces. These dilators antagonize the activity of vasoconstrictors and maintain organ blood flow. The activity of NO differs from EDHF along the vasculature. Endothelium-dependent dilation to NO is greatest in large arteries, whereas EDHF has its greatest effect in small arteries and arterioles (Nagao et al., 1992; Nishikawa et al., 1999). While a number of compounds have been proposed as mediators of EDHF activity, there is convincing evidence that EETs and EDHF have identical properties. Both are produced by the endothelium and open K channels, hyperpolarize, and relax smooth muscle. Like EDHF, EETs are more potent in relaxing small coronary arteries than large arteries (Campbell et al., 1996b; Oltman et al., 1998). While the primary role of the EETs is vasodilation in most vascular beds, EETs cause vasoconstriction in the pulmonary artery (Kerseru et al., 2008; Moreland et al., 2007; Zhu et al., 2000). The exact mechanism of the vasoconstriction has not been elucidated and remains a focus for future studies.
In a number of different vascular beds from a variety of species, endothelium-dependent vasodilator responses to arachidonic acid, ACH or bradykinin that are not blocked by NO synthase and COX inhibitors are blocked by inhibitors of CYP, the enzyme responsible for EET synthesis. EETs were more completely characterized as EDHFs using bovine and porcine coronary arteries (Campbell et al., 1996a; Fisslthaler et al., 1999). The four regioisomeric EETs relaxed bovine coronary arteries in a concentration-related manner. Significant relaxations to the EETs occurred with nanomolar concentrations and were blocked by the K channel blockers TEA and charybdotoxin, and high K. When membrane potential of coronary arterial vascular smooth muscle was measured, 11,12-EET caused membrane hyperpolarization. This hyperpolarization was also blocked by iberiotoxin. Similarly, others showed that in human coronary and internal mammary arteries, 11,12-EET relaxed and hyperpolarized the smooth muscle, and these effects were blocked by the K channel blocker iberiotoxin (Archer et al., 2003; Larsen et al., 2005). Additionally, arachidonic acid, bradykinin and methacholine hyperpolarized the smooth muscle of endothelium-intact coronary arteries, and CYP inhibitors blocked the hyperpolarizations. Antisense oligonucleotides against CYP2C inhibited the relaxations and hyperpolarizations to bradykinin, whereas sense and scrambled oligonucleotides were without effect (Fisslthaler et al., 1999). This inhibition by the antisense oligonucleotides was accompanied by a reduction of the expression of CYP2C. Arachidonic acid and methacholine stimulated the release of EETs from perfused coronary arteries and coronary endothelial cells. Identical results were obtained with human internal mammary arteries (Archer et al., 2003). Related studies were performed with bradykinin in bovine and porcine coronary arteries and human internal mammary arteries (Archer et al., 2003; Campbell et al., 2001; Hecker et al., 1994; Weston et al., 2005). CYP inhibitors attenuated relaxations and hyperpolarization by bradykinin. Taken collectively, these studies indicate that the hyperpolarizations and relaxations to methacholine and bradykinin are mediated by the EETs. Furthermore, in arteries from experimental animals and humans, physical forces such as shear stress and increased flow caused endothelium-dependent relaxations that were reduced, but not blocked, by inhibitors of NOS and COX (Gauthier and Campbell, 2006; Huang et al., 2005; Miura et al., 2001; Popp et al., 1998). The non-NO, non-PG–dependent relaxations were blocked by CYP inhibitors and were associated with the release of EETs. While the non-NO, non-PG mediated relaxations to ACH were greater in vessels from females compared to males, few studies have measured responses in the presence of CYP inhibitors or EET antagonists (McCulloch and Randall, 1998; Scotland et al., 2005; Tagawa et al., 1997; White et al., 2000; Woodman and Boujaoude, 2004). Additional studies are necessary to establish the exact role of gender in EET-mediated regulation of vascular tone.
For EETs to be considered important in the regulation of vascular tone, EETs should function as EDHF in intact animals. In the microcirculation of anesthetized dogs, bradykinin and ACH caused non-NO, non-PG mediated relaxations that were blocked by high extracellular K and iberiotoxin indicating a role of an EDHF in mediating the dilation (Nishikawa et al., 1999; Nishikawa et al., 2000; Watanabe et al., 2005; Widmann et al., 1998). They were also blocked by the CYP inhibitors, miconazole and metyrapone. Similarly, following COX and NOS inhibition, arachidonic acid induced dilation of coronary microvessels. These dilations were blocked by K channel blockers and a CYP inhibitor suggesting that a CYP metabolite of arachidonic acid functions as EDHF in the canine coronary circulation. Using intravital microscopy of cremaster arterioles from conscious hamsters to measure changes in vessel diameter, high concentrations of ACH induced a dilation that was not altered by NOS and COX inhibitors (Watanabe et al., 2005). However, the relaxations were completely blocked by the elevation of extracellular K. Likewise, treatment with the CYP inhibitor, sulfaphenazole attenuated the non-NO, non-PG, ACH-induced relaxations. EET-mediated dilations were also demonstrated in vivo in canine coronary and kidney arterioles, hamster cheek pouch arterioles, rat cremaster arterioles, and rat mesenteric and hind limb, and sciatic nerve circulations (Chu et al., 2000; Loeb et al., 1997; Matsuda et al., 2004; Nishikawa et al., 1999; Oltman et al., 2001; Oltman et al., 1998; Parkington et al., 2002; Thomsen et al., 2000; Welsh and Segal, 2000).
The topic of EDHF in the regulation of vascular tone in humans was recently reviewed (Bellien et al., 2008a) with a careful evaluation of data implicating a specific role of EETs. To summarize, ex vivo studies in isolated arteries and a small number of in vivo studies in humans provide evidence that EETs may function as EDHF (Archer et al., 2003; Bellien et al., 2010; Bellien et al., 2008b; Hatoum et al., 2005; Kemp and Cocks, 1997; Larsen et al., 2008; Larsen et al., 2005; Lenasi, 2009; Lenasi and Strucl, 2008; Taddei et al., 2006; Virdis et al., 2010). In the isolated vessel studies, the existence of a CYP related EDHF has been clearly reported for the human coronary arterioles, internal mammary artery and radial artery. While in vivo data for EET-mediated vasodilation in humans is understandably limited, there is strong evidence to support an in vivo role of EETs as EDHF. Intraarterial infusions of bradykinin-induced increases in forearm blood flow in human subjects treated with the COX inhibitor, aspirin and the NOS inhibitor, N (G)-monomethyl-L-arginine (LNA). These non-PG, non-NO increases in blood flow were blocked by potassium chloride infusion or TEA and inhibited by CYP inhibitor miconazole (Halcox et al., 2001; Honing et al., 2000). Forearm blood flow response to ACH or bradykinin has been compared in normotensive and essential hypertensive patients (Taddei et al., 2006). In normotensive subjects, vasodilation to ACH and bradykinin was blunted by NOS inhibition but not by the CYP 2C9 inhibitor sulfaphenazole. In contrast, in the hypertensive patients, the vasodilator responses to the agonists were reduced compared with the normotensive group. More interestingly, the relaxation responses in the hypertensive group were resistant to NO inhibition but were blunted by CYP inhibition. These findings suggest that EETs sustain endothelium-dependent vasodilation in hypertension. A similar compensatory role for EETs may exist in patients with congestive heart failure (Katz and Krum, 2001). Forearm blood flow responses to ACH were decreased by COX and NOS inhibition in the normal subjects but not in patients with congestive heart failure. While the effect of a CYP antagonist was not tested, the results again suggest the presence of a non-PG, non-NO relaxing factor that participates in the regulation of forearm blood flow in humans. More recently, EETs regulate arterial stiffness during changes in blood flow (Bellien et al., 2010). Heating the skin of the hand increases blood flow by promoting the release of vasorelaxing factors which decrease isometric tone and wall stiffness. The NOS inhibitor, LNA partially inhibited the increase in blood flow. The remaining blood flow increase was completely prevented with TEA and the CYP inhibitor fluconazole. Therefore, in human peripheral conduit arteries, the adaptation of smooth muscle tone and arterial stiffness during blood flow variations is regulated by the vascular endothelium through the release of both NO and CYP-mediated EDHF.
In summary, EETs derived from endothelial CYP epoxygenases are diffusible vasodilator factors that act by hyperpolarizing vascular smooth muscle cells. Experiments performed both in vitro and in vivo in animals and humans support the contribution of EETs to endothelium dependent relaxations.
II. Mechanism of Action
All four EET regioisomers, 14,15-, 11,12-, 8,9- and 5,6-EET, equipotently relax bovine and canine coronary arteries (Campbell et al., 1996a; Rosolowsky and Campbell, 1993). This suggests that the position of the epoxy group is not critical for relaxation in these arteries. Alternatively, 5,6-EET is more active than the other EET regioisomers in relaxing rat-tail arteries, rabbit and pig cerebral arteries and rat renal arteries (Carroll et al., 1987; Ellis et al., 1990; Leffler and Fedinec, 1997; Pomposiello et al., 2003). Therefore, regioisomer specificity of EET-induced relaxations varies with species and vascular bed. Structural modifications aimed specifically to characterize the molecular components critical for EET dilator activity and to identify analogs with EET-specific antagonist properties have been reported for 14,15-EET (Figure 3) (Chen et al., 2009; Falck et al., 2009; Falck et al., 2003a; Gauthier et al., 2002; Gauthier et al., 2004; Gauthier et al., 2003a; Yang et al., 2007; Yang et al., 2008). 14,15-EET analogs, with modifications in the epoxy and carboxyl groups, deletions of the double bonds, and variations in the carbon chain length, were synthesized and tested for their ability to cause relaxation. Specific structural features were identified for full agonist activity: an acidic group at carbon-1, a 20 carbon backbone, a Δ 8 double bond, and a 14(S),15(R)-cis epoxide (Falck et al., 2003a). Thus, the basic full agonist was 14(S),15(R)-(cis)-epoxyeicosa-8Z-enoic acid (14,15-EE-8ZE). Addition of a methylsulfonamide (mSI) group to carboxyl of 14,15-EET reduced its metabolism by β-oxidation and incorporation into membrane phospholipids (Gauthier et al., 2003a). More recently, Falck et al have developed chimeric analogues which are capable of functioning as stable 14,15-EET surrogates and inhibitors of sEH (Falck et al., 2009). Having analogues that limit 14,15-EET auto-oxidation and metabolism to inactive DHETs by sEH will increase the potential therapeutic applications of these drugs.
Analogs with low agonist activity were tested for their ability to inhibit EET-induced relaxations (Falck et al., 2003a; Gauthier et al., 2003b). 14,15-epoxyeicosa-5Z-enoic acid (14,15-EE-5Z-E) inhibited the relaxations to 14,15-, 11,12-, 8,9-, and 5.6-EET; however, it was most active in inhibiting 14,15-EET. The non-PG–mediated relaxations to arachidonic acid were also blocked by 14,15-EE-5Z-E (Figure 2). In contrast, it did not alter the relaxations to the NO donor sodium nitroprusside, the PGI2 analog iloprost, or the K channel openers bimakalim and NS1619. Thus, 14,15-EE 5Z-E is a selective EET antagonist that does not inhibit other endothelial factors or K channels (Gauthier et al., 2002). 14,15-EE-5ZE-mSI inhibited relaxations to 14,15- and 5,6-EET but not to 11,12- or 8,9-EET (Gauthier et al., 2003a). 14,15-EE-5ZE-mSI did not alter the relaxations to sodium nitroprusside, iloprost, bimakalim, or NS1619 and did not affect the metabolism of arachidonic acid. Thus, 14,15-EE-5Z-E-mSI is a selective antagonist of 14,15- and 5,6-EET. 14,15-EE-5ZE-mSI inhibited the indomethacin-resistant relaxations to arachidonic acid; however, the inhibition was less than what occurred with 14,15-EE-5Z-E. The combination of 14,15-EE-5Z-E-mSi and the CYP inhibitor MS-PPOH inhibited arachidonic acid–induced relaxations to the same extent as 14,15-EE-5ZE alone. Thus, 11,12- and/or 8,9-EET must contribute to the relaxation response to arachidonic acid. The discovery of EET antagonists provided vital pharmacological tools to examine the role of EETs as EDHFs. Thus, in the presence of COX and NOS inhibitors, ACH- and bradykinin-mediated relaxations and hyperpolarization were inhibited by 14,15-EE-5Z-E (Archer et al., 2003; Gauthier et al., 2002; Gauthier et al., 2003a).
EETs activate smooth muscle large conductance, calcium-activated potassium (BKCa) channels (Campbell et al., 1996a; Li and Campbell, 1997; Li et al., 1997). This leads to K efflux, an increase in the membrane potential (Em) or hyperpolarization. The hyperpolarization inhibits activation of voltage-activated calcium channels reducing calcium entry and causing relaxation. EETs activate BKCa channels in nanomolar concentrations in cell-attached patches in which the cell cytosol is in contact with the channel. However, when this association is disrupted using inside-out patches, the EETs are without effect (Li et al., 1997). The addition of GTP, but not ATP, to the cytoplasmic side of the inside-out patch restores the ability of the EET to activate BKCa channels. This can be blocked by the guanine nucleotide-binding (G) protein inhibitor GDP-β-S. It is also blocked by the addition of an antibody against Gsα but not by anti-Giα or anti-Gβγ antibodies. Activation of Gsα by ADP ribosylation with cholera toxin also increases BKCa channel activity in cell-attached patches (Li et al., 1999). In inside out patches, BKCa channel activity is increased by Gsα-GTP; however, Gsα-GDP and Gβγ do not alter channel activity (Kume et al., 1992; Scornik et al., 1993). Other evidence implicates Gs in EET action. 11,12-EET increases tissue plasminogen activator (tPA) activity and protein expression in endothelial cells (Node et al., 2001). This is associated with a 3.5-fold increase in GTP binding to Gsα but not Giα. These studies indicate that a G protein with the characteristics of Gsα mediates the EET activation of BKCa channels, and this occurs by a membrane-delimited mechanism (i.e. the action is confined to the membrane and does not require compounds from other parts of the cell) (Li et al., 1997). In addition, in intact smooth muscle cells, EETs promote endogenous ADP ribosylation of Gsα to increase BKCa channel activation (Li et al., 1999). It is thought that EETs activate the BKCa channel by a Gsα-mediated, membrane-delimited mechanism, which is sustained by ADP ribosylation of Gsα.
Others have suggested that EET-mediated vascular smooth muscle cell relaxation by endothelial-mediated hyperpolarization (Feletou and Vanhoutte, 2006; Fleming and Busse, 2006). Agonists such as ACH and bradykinin increase endothelial EET synthesis. EETs act in an autocrine manner to increase intracellular calcium and activate endothelial apamin-sensitive, small conductance (SKCa) and charybdotoxin-sensitive, intermediate conductance (IKCa) KCa channels. This results in hyperpolarization of the endothelial cell. Smooth muscle cell relaxation occurs because of the hyperpolarizing current spreading from the endothelium to smooth muscle through gap junctions. Fleming and coworkers provide evidence in CYP 2C9-overexpressing human umbilical vein endothelial cells that EETs regulate intracellular calcium by inducing the translocation of “transient receptor potential” (TRP) channel proteins to caveolin-1-rich areas of the endothelial cell membrane (Fleming et al., 2007; Loot et al., 2008). NO- and PGI2-independent flow-induced vasodilatation of the murine carotid artery is inhibited by CYP inhibitor as well as to a TRP4 channel blocker (Loot et al., 2008). The exact molecular mechanisms by which EETs induce the membrane translocation of TRP channels remains to be elucidated, although protein kinase A (PKA) may be involved in this process.
The evidence that EET action is coupled to a G protein, and EETs promote GTP binding to membranes suggests that EETs act through a GPCR. This concept is supported by the following studies. First, as indicated above, 14(S),15(R)-cis-EE-8Z-E-enoic acid was the simplest structure with full agonist activity (Falck et al., 2003a). The requirement for a specific stereoisomer of the epoxide suggested a specific binding site for the EET. Second, using vascular smooth muscle cells, 14,15-EET was tethered to silica beads so that it could not enter the cell. Tethered-14,15-EET inhibited aromatase activity to a similar extent as (untethered) 14,15-EET (Snyder et al., 2002). Thus, 14,15-EET acted on the cell surface and not intracellularly. Finally, a high-affinity EET binding site was described in intact cells and membrane preparations from guinea pig mononuclear cells and human U937 cells. By use of 3H-14,15-EET as a radioligand, specific and saturable binding with a K of 5.7 nM was determined in guinea pig monocytes and a K d of 13.84 nM in U937 cells (Wong d et al., 1993; Wong et al., 2000; Wong et al., 1997). This binding site was further defined in the cell membranes by Yang et al. (Yang et al., 2008) by use of 20-125iodo-14,15-epoxyeicosa-8Z-enoic acid (20-125I-14,15-EE-8Z-E). 20-125I-14,15-EE-8Z-E bound U937 membranes in a specific, saturable, and reversible manner with high affinity. EET analogs, but not prostaglandins or lipoxygenase metabolites, displaced the 14,15-EET radioligands from their binding site. The binding was also inhibited by GTPγs suggesting G protein coupling. More recently, 20-125I-14,15-EE-5Z-E was characterized as an antagonist. It showed specific, saturable, reversible binding to U937 membranes and bound with higher affinity than the agonist radioligand. 20-125I-14,15-EE-5Z-E had a Kd of 1.11 nM, whereas 20-125I-14,15-EE-8ZE had a Kd of 11.8 nM (Yang et al., 2008). A series of CYP and sEH inhibitors were tested for their ability to displace 20-125I-14,15-EE-5Z-E from its binding site. The CYP inhibitors miconazole and MS-PPOH inhibited binding of the ligand suggesting that some CYP inhibitors are also EET antagonists (Chen et al., 2009). Identifying the EET receptor remains a subject of active research and an area that has obvious future therapeutic implications since many clinically useful drugs target receptors in their mechanism of action.
III. Therapeutic Potential
One likely limitation to the vasodilator effect of EETs is their metabolism by sEH or β-oxidation to the less biologically active metabolites (Imig and Hammock, 2009). The development of metabolically stable EET agonists or blocking EET metabolism to enhance the action of endogenous EETs are current approaches. For example, Falck and coworkers have developed urea analogs of the EETs with agonist activity that also inhibit sEH (Falck et al., 2009). These compounds have not been tested in vivo. Using isolated blood vessels EET-mediated relaxation responses were enhanced in the presence of a sEH inhibitor (Hercule et al., 2009; Larsen et al., 2005; Olearczyk et al., 2009). This result provided motivation to explore a possible contribution of sEH to the development of hypertension and a potentially novel use of sEH inhibitors in the treatment of the cardiovascular diseases. sEH can be inhibited in vitro by a variety of urea, carbamate, and amide derivatives. Furthermore, novel inhibitors of sEH were developed such as 12-(3-adamantane-1-yl-ureido)-dodecanoic acid (AUDA) and AUDA butyl ester. The topic of sEH inhibitors is the subject of a recent review by Imig and Hammock (Imig and Hammock, 2009). The first experimental evidence that a sEH inhibitor increases EET concentrations and lowers blood pressure in an animal model of hypertension was reported by Yu et al (Yu et al., 2000). EET hydrolysis was increased in kidneys of the spontaneously hypertensive rat (SHR) model compared to WKY controls. When the sEH inhibitor DCU was administered to 8-week-old SHRs daily for 4 days, systolic blood pressure decreased. Imig and colleagues extended this observation and were the first to demonstrate that sEH contributes significantly to the elevated blood pressure in angiotensin II (ANG II)-dependent hypertension (Imig et al., 2002). Kidney sEH protein levels were elevated in the rat model of ANG II hypertension compared to normotensive rats. Administration of the selective sEH inhibitor, N-cyclohexyl-N-dodecyl urea (NCND) for 4 days lowered arterial blood pressure by 30 mmHg. A different sEH inhibitor, N-adamantyl-N’-dodecylurea (ADU), lowered systolic blood pressure, normalized vascular endothelial function, and attenuated left-ventricular hypertrophy in the deoxycorticosterone acetate (DOCA) plus high salt model of hypertension in rats (Imig et al., 2005). In mice with a target disruption of the sEH gene, the increase in blood pressure to DOCA-salt was blunted compared to wild type mice (Manhiani et al., 2009). Furthermore, treatment of the wild type, DOCA-salt mice with the sEH inhibitor had a blood pressure lowering effect. Finally, Arete Therapeutics Inc has developed an orally administered sEH inhibitor, AR9281 that is currently in a Phase II clinical program for the treatment of pre-diabetic patients with impaired glucose tolerance, mild obesity and mild to moderate hypertension. The Phase I clinical program for AR9281 demonstrated the drug was safe and well tolerated in healthy volunteers. Results from the Phase II clinical trial will lay the groundwork for the future use of sEH inhibitors and EET agonists in the treatment of vascular disease.
4. EETs and Inflammation
Endothelial cells release a number or pro- and anti-inflammatory mediators (Pober and Sessa, 2007). A strong link between inflammation and endothelial dysfunction has been established. Inflammation is a risk factor for cardiovascular diseases, like hypertension and atherosclerosis. EETs have anti-inflammatory properties implicating their potential therapeutic use in the treatment of inflammation (figure 4). The role of CYP epoxygenases, sEH and cardiovascular inflammation has been recently reviewed (Deng et al., 2010). The following sections will summarize specific effects of EETs on cell adhesion and platelet activation providing updated evidence on the role of EETs in inflammation.
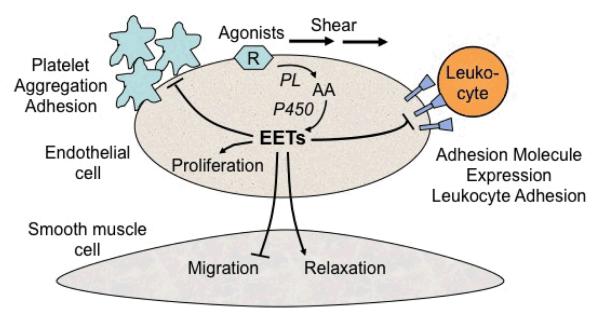
Figure 4.
A simplifed schematic of vascular effects of epoxyeicosatrienoic acids (EETs). Arrows denote stimulation, straight line without arrow denotes inhibition. Agonists or shear stress activate phospholipase (PL) in endothelial cell membranes. This releases arachidonic acid (AA) which is metabolized by cytochrome P450 (CYP) to EETs. EETs can diffuse to the vascular smooth muscle cell to cause relaxation and inhibit cell migration. EETs inhibit adhesion molecule expression on endothelial cells which decreases leukocyte adherence. EETs prevent platelet aggregation and adhesion. EETs promote angiogenesis by stimulating endothelial cell proliferation.
I. Cell Adhesion
Macrophage- and leukocyte-derived cytokines such as tumor necrosis factor α (TNF-α) and interleukin 1α (IL-1α) activate endothelial cells and promote the surface expression of cell adhesion molecules (CAMs) including vascular cell adhesion molecule 1 (VCAM-1), E-selectin and intercellular adhesion molecule 1 (ICAM-1). These events are integral to the inflammatory response.
A. Experimental Evidence
Node et al. reported that EETs are potent inhibitors of CAM expression induced by TNF-α, IL-1α and bacterial lipopolysaccharide (LPS) (Node et al., 1999). Although, EETs inhibited the expression of VCAM-1, E-selectin and ICAM-1, the effect on VCAM-1 was the most pronounced. 11,12-EET was the most potent isomer causing 72% inhibition of TNF-α induced VCAM-1 expression. The IC50 for 11,12-EET-induced inhibition of VCAM-1 was 20 nM. 8,9-EET and 5,6-EET were less active whereas 14,15-EET was without activity. Interestingly, 14,15-EET increased adherence of monocytes to endothelial cells suggesting a clear difference in activity between the EET regioisomers. The anti-inflammatory effect of EETs to decrease endothelial-leukocyte adhesion has been confirmed in a number of subsequent cell and animal models (Falck et al., 2003b; Fleming et al., 2001b; Liu et al., 2005; Moshal et al., 2008; Pratt et al., 2002).
B. Mechanism of Action
The mechanism of action of EETs to inhibit monocyte and leukocyte adhesion is independent of membrane hyperpolarization. Inhibition of KCa channels with iberiotoxin or charybdotoxin blocked EET-induced vasodilatation but did not block EET-induced inhibition of VCAM-1 expression (Node et al., 1999). Instead, EETs exert their anti-inflammatory effects in the vasculature by inhibiting cytokine-induced nuclear factor-κB (NF-κB). The proinflammatory transcription factor, NF-κB is essential for the induction of numerous inflammatory mediators such as CAMs, COX-2 and inducible (i)NOS. NF-κB is normally bound to an inhibitory protein IκB and maintained as an inactive NF-κB– IκB complex in the cytoplasm. Cytokines like TNF-α activate IκB kinase (IKK), which phosphorylates Ser32 and Ser36 of IκB. Following polyubiquitination of the diphosphorylated IκB, the protein is degraded by the 26S proteasome. The free NF-κB subunits RelA (p65) and p50 are translocated to the nucleus where they bind to target genes that encode pro-inflammatory proteins and consequently regulate their transcription. Node et al showed that 11,12-EET repressed VCAM-1 expression by inhibiting κB cis-acting elements in the promoter region of the VCAM-1 gene. In cells stimulated with TNF-α, the nuclear accumulation of Rel A was prevented by the coadministration of 11,12-EET. Stimulation of endothelial cells with TNF-α caused a rapid and almost complete disappearance of IκB-α that was prevented by cotreatment with 11,12-EET, but not 14,15-EET. Elevated concentrations of homocysteine contribute to inflammation and endothelial dysfunction by a mechanism that involves activation of NF-κB. This pathway induces matrix metalloproteinase (MMP)-9 expression and activity. MMPs participate in extracellular matrix degradation and may regulate CAM adhesion. Incubation of murine aortic endothelial cells with increasing concentration of homocysteine decreased CYP 2J2 expression and activated MMP-9 (Moshal et al., 2008). Homocysteine induced MMP-9 activation by increasing NF-κB–DNA binding. CYP transfection or exogenous addition of 8,9-EET (1 μM) attenuated homocysteine-induced MMP-9 activation. 8,9-EET also increased IκB-α levels and attenuated the nuclear accumulation of Rel A. Exogenous 11,12-EET (up to 3 μM) had no effect on MMP activation. Activation of the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) in cultured endothelial cells suppresses the NF-κB-mediated expression of inflammatory proteins such as VCAM-1and ICAM-1. In bovine aortic endothelial cells, all 4 EET regioisomers blocked TNF-α mediated NF-κB activation and this was prevented in cells pretreated with GW9662, an antagonist of PPAR© (Liu et al., 2005). Competition and direct binding assays revealed that EETs bind to the ligand-binding domain of PPARγ with Kd in the μM range (Cowart et al., 2002). The relationship between PPARγ and NF-κB in EET-induced changes in CAM expression needs further study.
C. Therapeutic Potential
Inflammation is a common characteristic of numerous cardiovascular diseases such as hypertension and atherosclerosis. Just as sEH inhibitors increase vasodilatory effects of EETs, these inhibitors have potential in the treatment of certain inflammatory disorders by enhancing anti-inflammatory effects of EETs. As described above, a number of studies have looked at the effect of sEH inhibitors in animal models of hypertension (Imig and Hammock, 2009). One consequence of chronic hypertension is renal vascular and glomerular injury. The response to injury involves upregulation of inflammatory proteins. In rats with ANG II-induced hypertension, sEH inhibitors decreased collagen expression in glomeruli and tubular cells. Macrophage infiltration was also reduced (Imig et al., 2002). Similarly, in diabetic Goto–Kakizaki rats the sEH inhibitor, AUDA, blocked inflammation independently of lowering blood pressure (Olearczyk et al., 2009). Targeted disruption of the sEH gene prevented both renal inflammation and injury in DOCA-salt hypertensive mice (Manhiani et al., 2009). Reduction in renal inflammation and injury was also seen in wild type DOCA-salt mice treated with a sEH inhibitor. Macrophage infiltration, renal NF-κB activation and monocyte chemmoattractant protein-1 excretion were all reduced in the sEH knockout mice. Finally, inhibition of sEH enhanced the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor, MK886 in a LPS-challenged murine model of inflammation (Liu et al., 2010). The relative effectiveness of sEH inhibitors versus EET agonists versus stable EET agonists with sEH inhibitory activity is an area in need of study.
Atherosclerosis is a dynamic and progressive process with endothelial dysfunction and inflammation of the vascular wall. A recent study investigated the role of sEH inhibition in the hyperlipidemic ApoE-/- mouse following cuff placement around the femoral artery or ligation of left common carotid artery (Ulu et al., 2008). AUDA treatment reduced neointimal formation only in the femoral cuff model, which typically exhibits a more pronounced inflammatory phenotype than does the carotid artery ligation model. In the femoral cuff model, mRNA expressions for the cytokine, Gro-β and for the pro-inflammatory enzyme, COX2, were significantly lower in sEH–/–/ApoE–/– mice compared with ApoE–/– mice. However, there were no differences in macrophage infiltration or the cytokines, TNF-α, Gro-α and MCP-1 in the two groups.
II. Platelet Activation
Inflammatory disorders are often associated with platelet activation (Smyth et al., 2009). It is well established that endothelial NO and PGI2 inhibit platelet aggregation and activation. There is also experimental evidence to support EET-mediated anti-platelet effects (Figure 4).
A. Experimental Evidence
Platelet aggregation of washed human platelets induced by arachidonic acid was inhibited by EET isomers (1 to 10 pM) with no evidence of stereospecificity (Fitzpatrick et al., 1986). However, 11,12-EET (up to 10 μM) had no effect on platelet aggregation stimulated with collagen, ADP or a thrombin-receptor activating peptide (VanRollins, 1995). Using an in vivo model of platelet aggregation in pial arterioles of mice, 14,15-EET, but not 8,9-EET, delayed platelet aggregation (Krotz et al., 2010; Krotz et al., 2003). The dose of 14,15-EET (0.3 mg/kg) producing the effect had no effect on blood flow. The effect of EETs on adhesion of washed human platelets to confluent HUVEC was examined under both static and flow conditions (Krotz et al., 2010). 11,12-EET (1 μM) decreased endothelial cell adhesion of platelets and also decreased P-selectin expression. In an endothelial cell line that overexpressed CYP2C9, bradykinin increased the production of 11,12-, 8,9- and 14,15-EET compared to controls. Media from the cell incubations with bradykinin inhibited platelet adhesion to human umbilical vein endothelial cells (HUVECs). Platelet adhesion and rolling was assessed in the dorsal skinfold chamber model in the microcirculation of hamsters (Krotz et al., 2010). The CYP2C9 inhibitor, sulfaphenazole, enhanced platelet adhesion to the endothelium. The addition of 11,12-EET (10 μM) reversed the effect of CYP inhibition. The view of EETs as important endogenous anti-platelet factors remains to be elucidated in future studies. Studies will need to clarify whether the endothelium in vivo releases EETs in amounts sufficient to control platelet activation.
B. Mechanism of Action
The exact mechanism whereby EETs inhibit platelet aggregation has not been established. In human platelets, inhibition of aggregation by EETs effect was not associated with a decrease in the production of the pro-aggregatory, thromboxane (TX) A2 (Fitzpatrick et al., 1986). However, in mice, 14,15-EET, but not 8,9-EET, decreased serum TXB2 concentrations (VanRollins, 1995). In human platelets, 11,12-EET caused a concentration-dependent increase in NOS activity and stimulated nitrite production (Zhang et al., 2008a). While NO inhibits platelet aggregation, there is no direct evidence that NO mediates the inhibition of aggregation by EETs. Platelets express KCa channels and EET-mediated inhibition of platelet adhesion likely targets these channels. Cultured HUVECs expressing CYP2C9 and stimulated by bradykinin released a factor that hyperpolarized human platelets (Krotz et al., 2010; Krotz et al., 2003). The inhibitor of CYP2C9, sulfaphenazole blocked this hyperpolarization. Hyperpolarization was also prevented by pretreatment of platelets with charybdotoxin or apamin, inhibitors of SKCa and IKCa channels. EET-mediated inhibition of platelet adhesion to endothelial cells was prevented by charybdotoxin but not apamin. Exogenous EETs (1 μM) were also tested for direct effects on platelet membrane hyperpolarization. Pronounced hyperpolarizations occurred with 11,12-EET and 8,9-EET. 14,15-EET was less active. These studies suggest that the EETs hyperpolarize the platelet membrane resulting in reduced adhesion.
C. Therapeutic Potential
There are no studies in which sEH inhibitors or EET analogs have been tested for anti-platelet effects in disease models. However, there is evidence the sEH inhibitors increase the action of aspirin (Liu et al., 2010). In certain cardiovascular diseases, the COX product, TXA2, increases platelet activation. Low dose aspirin is an effective anti-platelet drug that limits adverse cardiovascular events by blocking TXA2 synthesis and activity. In the LPS-induced mouse model of inflammation, the-AUCB, metabolism of arachidonic acid by COX is increased. A sEH inhibitor, t decreased TXA2 but not PGE2 in the plasma of LPS-treated mice. Aspirin treatment inhibited both TXA2 and PGE2. If t-AUCB was co-administered with aspirin, there was a greater reduction in PGE2 and TXA2 than with aspirin alone suggesting that EETs increase the activity of aspirin. While it still needs to be directly tested experimentally, it is possible that sEH inhibitors (or EET analogs) may be effective anti-platelet drugs on their own. Alternatively, since sEH inhibitors increase aspirin effects, these two drugs could be combined allowing the use of aspirin in doses that limit aspirin-related side effects.
5. EETs and Angiogenesis
Angiogenesis is a process involving a tightly regulated interaction between a numbers of different signaling molecules which results in the sprouting of endothelial cells from existing blood vessels. While complex, angiogenesis is crucial for all tissue growth, expansion and repair. Arachidonic acid metabolites contribute to angiogenesis by modulating endothelial cell proliferation or migration and capillary formation. EETs are regarded as pro-angiogenic compounds (Michaelis and Fleming, 2006) (Figure 4) and therapeutic manipulation of their effects may be important in diseases like ischemic heart disease, ischemic stroke and atherosclerosis.
I. Evidence
The first report that EETs may be mitogens was performed in cultured rat glomerular mesengial cells (Harris et al., 1992). Following a 24 hour exposure to exogenously administered 14,15- or 8,9-EET (1-10 μM), mesengial cells had a significant increase in 3H-thymidine incorporation. Subsequent studies in various types of vascular endothelial cells confirmed EETs as pro-angiogenic mediators. Munzenmaier and Harder initially observed that CYP epoxygenase products were necessary for cerebral microvascular endothelial cells to form tubular structures in vitro (Munzenmaier and Harder, 2000). In porcine coronary artery endothelial cells, CYP 2C8 overexpression increased 11,12-EET production and enhanced endothelial cell number (Michaelis et al., 2005b). Furthermore, in human umbilical vein endothelial cells, overexpression of CYP2C9 increased cell number and proliferation (Michaelis et al., 2005b). The specific CYP2C9 inhibitor, sulfaphenazole prevented the proliferative response. Using an in vivo model of angiogenesis, a single high concentration (150 μM) of 14,15-EET increased functional vasculature in a Matrigel plug (Medhora et al., 2003). More recently, the ability of all four EETs to regulate endothelial cell proliferation in vitro and angiogenesis in vivo was investigated (Pozzi et al., 2005; Yang et al., 2009). All four EETs induced significant increases in pulmonary murine endothelial cell proliferation with 5,6-EET eliciting the greatest effect. None of the DHETs stimulated endothelial cell proliferation. 5,6-and 8,9-EET, but not 11,12- or 14, 15-EET, increased cell migration and capillary tube formation. To test the angiogenic activity of 5,6- and 8,9-EET in vivo, inert sponges were implanted subcutaneously in the back of adult mice and were injected every other day with either vehicle, 5,6-EET, or 8,9-EET (50 μM). After 14 days, sponges injected with 5,6- or 8,9-EET showed increased vessel density compared with sponges injected with vehicle only, demonstrating clearly in vivo de novo vascularization. Stimulation of endothelial cells with vascular endothelial growth factor (VEGF) induced the expression of CYP2C and the generation of 11,12-EET. Pretreatment with the CYP inhibitor, miconazole prevented the increase in 11,12-EET. VEGF-induced endothelial cell tube formation which was prevented by the EET antagonist, 14,15-EEZE. Hypoxia enhances cell proliferation and EETs increase during hypoxia. Exposure of CYP 2C8- or 2C9-transfected HUVECs to hypoxia increased endothelial cell migration and tube formation (Michaelis et al., 2005a; Michaelis et al., 2005b). These effects were blocked by the EET antagonist, 14,15-EEZE. Similar findings were obtained in porcine coronary artery endothelial cells. Bovine retinal endothelial cells expressed CYP2C protein under basal conditions (Michaelis et al., 2008). Hypoxia enhanced CYP2C protein expression and EET formation. Treatment with CYP 2C antisense or the EET antagonist suppressed hypoxia-induced cell migration and in vitro tube formation.
In contrast to the studies in endothelial cells, exogenously administered EETs inhibit rat aortic smooth muscle cell migration in response to growth factors (Sun et al., 2002). Overexpression of CYP2J2 in these cells attenuated migration, and this effect was prevented by the CYP inhibitors, SKF525A and clotrimazole, but not by the KCa channel blocker, charybdotoxin. In these studies, EETs had no effect on proliferation as measured by 3H-thymidine incorporation.
The ability of EETs to stimulate endothelial cell proliferation and decrease vascular smooth muscle cell migration may relate to tissue-specific effects of EETs. While these differing responses validate the underlying complexity of angiogenesis, it also supports the idea that EETs might be protective not only by inhibiting complications of smooth muscle cell migration in atherosclerosis, but also by promoting endothelial cells proliferation and neovascularization in ischemic tissues.
II. Mechanism
Among the various mitogenic signaling pathways, the activation of extracellular-signal-regulated kinases (ERK), p38 mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) play important roles in endothelial cell function. Numerous studies have investigated signaling pathways involved in EET-mediated angiogenesis (Deng et al., 2010; Spector, 2009; Spector and Norris, 2007). EET-induced proliferation in a renal epithelial cell line was dependent on the activation of Src kinase and initiation of a tyrosine kinase phosphorylation cascade (Chen et al., 1998). An essential step in the signaling mechanism of EETs involved transactivation of epidermal growth factor-like (EGF) receptor. 14,15-EET induces EGF receptor activation and downstream signaling by induction of pro-heparin binding (HB)-EGF processing through activation of a metalloproteinase that causes release of soluble HB-EGF. The released HB-EGF then binds to EGF receptor and activates its intrinsic receptor tyrosine kinase, leading to autophosphorylation. Transactivation of the EGF receptor following the cleavage of HB-EGF is a common event in cell activation and the mitogenic response to a variety of substances, including agonists acting through GPCRs. In human endothelial cells overexpressing CYP 2C9, EETs also stimulated proliferation via a mechanism that involved activation of the EGF receptor (Michaelis et al., 2003). PI3K/Akt is a downstream target of the EGF receptor and all four EET regioisomers increased Akt phosphorylation and cell proliferation in murine endothelial cells (Pozzi et al., 2005). Incubation of human coronary artery endothelial cells with 11,12-EET activated ERK1/2 and the p38 MAPK (Fleming et al., 2001a). Activation could be observed in response to 11,12-EET at concentrations as low as 3 nM, whereas 14,15-EET had no effect. The KCa channel blockers charybdotoxin and apamin had no effect on the activation of ERK1/2 by 11,12-EET. The phosphorylation of Erk1/2 and p38 MAP kinase was also enhanced in actively proliferating endothelial cells that overexpressed CYP 2C. In pulmonary murine microvascular endothelial cells, all three major pathways involved in endothelial cell-mediated mitogenic function, namely PI3K/Akt, p38 MAPK, and ERK1/2, were activated by the EETs (Pozzi et al., 2005). 8,9-EETs was the most potent activator of p38 MAPK. Proper cell growth involves a series of cell-cycle regulatory proteins, cyclins that exert their function by binding to and activating a number of specific cyclin-dependent kinases (CDKs). 11,12-EET activation of the PI3-K/Akt elicited the subsequent phosphorylation and inhibition of forkhead transcription factors, FOXO1 and FOXO3b (Potente et al., 2003). This caused a down-regulation of the CDK inhibitor, p27Kip1 and increase in cyclin D1 expression. Overexpression of CYP2C9 in endothelial cells increased EET production that was associated with activation of MAPK phosphatase-1, decrease in c-Jun N-terminal kinase (JNK) activity. and increase in cyclin D1 expression. 11,12-EET (1 μM) also induced the expression of MAPK phosphatase-1(Potente et al., 2002).
EETs activate the cAMP/PKA pathway in endothelial cells (Imig et al., 1999). In relationship to angiogenesis, enhanced proliferation in HUVECs overexpressing the CYP2C9 enzyme was blocked by a PKA inhibitor (Michaelis et al., 2005a). These cells had increased concentrations of cAMP, COX-2 protein and 11, 12-EET. CYP2C9 overexpression stimulated endothelial tube formation, which was attenuated by the COX-2 inhibitor celecoxib. Thus, COX-2 may also contribute to CYP2C9-induced angiogenesis. In the rat aortic vascular smooth muscle cells, 11,12-EET inhibited migration and increased intracellular cAMP levels and PKA activity (Sun et al., 2002). Inhibitors of cAMP and PKA reversed the antimigratory effects of 11, 12-EET.
Mechanisms to explain EET-mediated effects on angiogenesis are dependent on not only the cell type used but also the specific EET isomer. More comprehensive investigation of the signaling pathways using EET-specific mimetics is a necessary focus for future studies.
III. Therapeutic Potential
Recognizing EETs as signaling molecules that contribute to endothelial cell proliferation and vascular smooth muscle cell migration suggests that therapeutic manipulation of the EET pathway may aid in the treatment of diseases in which angiogenesis is involved. For example, angiogenesis is the key step for recovery after ischemia. Studies suggest that EETs are involved in the healing process of ischemic stroke (Iliff and Alkayed, 2009; Iliff et al., 2010; Simpkins et al., 2009; Zhang et al., 2007; Zhang et al., 2008b). Cerebral ischemia was induced by a permanent occusion of middle cerebral artery (MCA) occlusion in the stroke-prone spontaneously hypertensive rats (SHRSP) (Simpkins et al., 2009). Rats treated with the sEH inhibitor, AUDA, for 6 weeks had marked reduction in percentage of the hemisphere damaged by ischemia compared to untreated rats. MCA vessel wall thickness, wall to lumen ratio and collagen deposition was reduced by AUDA in the SHRSP compared to WKY rats. Microvessel density in the untreated adult SHRSP was 33% lower than in the adult WKY rats. However, AUDA increased the microvessel density by 20% in the SHRSP providing further support that therapeutic manipulation of EETs with sEH inhibitors may provide cerebral protection by reducing the area at risk.
In contrast to beneficial effects of angiogenesis in ischemia is the view that smooth muscle cell migration and proliferation contribute to pathological processes in atherosclerosis. EETs inhibit vascular smooth muscle cell migration in cultured cells but it is not known if an increase in EETs with sEH inhibitors would offer protection in an in vivo atherosclerotic model by specifically attenuating vascular smooth muscle cell migration. In ANG II–treated apoE-deficient mice treated with a sEH inhibitor, there was a reduction in atherosclerotic lesion (Ulu et al., 2008). This indicated that while EETs may limit lesion development, the mechanism is independent of effects on vascular smooth muscle cell migration or proliferation. This idea was challenged using the carotid ligation technique in SHRSP (Simpkins et al., 2010). Unlike the ANG-II ApoE-mice, treatment with the sEH inhibitor reduced vascular remodeling responses in the SHRSP model compared to WKY controls. A similar reduction in vascular remodeling occurred in mice lacking the sEH gene and subjected to carotid artery ligation. Neointimal area to medial ratio was reduced by the sEH inhibitor of wild-type mice. Interestingly, in a different model of vascular remodeling in which a wire is used to mechanically denude the femoral artery endothelium, sEH inhibitor treatment of rats, or in mice lacking the sEH gene, there was no reduction in neointimal hyperplasia indicating the importance of the endothelium as the source of EETs. While not yet proven, this contrasting result in the two different models suggests a role of the endothelium in EET-mediated protective effects. Having stable EET analogs with sEH inhibition versus sEH inhibitors or EET agonists to use for future in vivo studies will provide a better understanding of the mechanisms of vascular remodeling in atherosclerosis.
6. Conclusion
A considerable and diverse body of evidence supports the role of EETs in vascular function. Several key points can be made. EETs are endothelial-derived CYP metabolites of arachidonic acid and function as autocrine and paracrine mediators to regulate vascular tone, cell adhesion, platelet activation and angiogenesis. There are four regioisomers of EET: 5,6-, 8,9-, 11,12-, and 14,15-EET. EETs are hydrolyzed by sEH to corresponding DHETs and this results in a decrease in EET activity. As vasodilators, EETs act on vascular smooth muscle to cause K activation, hyperpolarization and relaxation through a G-protein coupled mechanism. Regioisomer specificity of EET-induced relaxations varies with species and vascular bed. The ability of EETs (primarily 11,12-EET) to inhibit monocyte- and leukocyte-adhesion to endothelial cells occurs through a signaling pathway that inhibits NF-κB activation. Platelet aggregation and platelet activation are attenuated by EETs by mechanisms that are still undefined. Endothelial cell proliferation is enhanced whereas vascular smooth muscle migration is inhibited by EETs. Mechanisms for EET-mediated angiogenesis are diverse, complex and dependent on cell type and the EET regioisomer involved. Comparisons of the threshold concentration of exogenously applied EETs that exerts a specific biological response are shown in Figure 5. Some of the effects of EETs occur at low physiological concentrations (hyperpolarization and relaxation of vascular smooth muscle) while others require much greater concentrations (monocyte adhesion and smooth muscle cell migration).
Figure 5.
Vascular effects of exogenous EETs. The threshold concentration of EETs that produce the vascular effects are indicated on a concentration line. BKCa, large conductance, calcium-activated potassium; VCAM-1, vascular cell adhesion molecule 1; tPA, tissue plasminogen activator; ENaC, epithelial sodium channel; TRP, transient receptor potential; PMN, polymorphonuclear leukocyte
The current data suggests that a GPCR for EETs exist. Identifying and characterizing the putative EET receptor(s) is an important focus of future studies. This will aid in clarification of mechanism of action of the EETs and novel drug design. Structural modifications of 14,15-EET provided important information regarding the molecular components critical for dilator activity and resulted in the identification of 14,15-EET-specific analogs with antagonist properties. Structural requirements for the activity of other EET regioisomers are needed. Inhibitors of sEH have been used in a number of different in vitro and in vivo systems as a way to increase EET concentrations and prolong biological activity. The development of EET analogs that combine agonist activity with sEH inhibition are predicted to further advance the therapeutic applications of EETs in cardiovascular disease.
Acknowledgements
Support was provided by National Institutes of Health Grants HL-51055 (WBC) and HL-093181 (SLP).
Non-standard Abbreviations
- EET
epoxyeicosatrienoic acid(s)
- sEH
soluble epoxide hydrolase
- DHET
dihydroxyeicosatrienoic acids
- PPARα
peroxisome proliferator-activated receptor
- G
guanine nucleotide-binding
- GPCR
G-protein coupled receptor
- ACH
acetylcholine
- COX
cyclooxygenase
- CYP
cytochrome P450
- EDHFs
endothelium-dependent hyperpolarizing factors
- PGI2
prostacyclin
- EDRF
endothelium-derived relaxing factor
- NO
nitric oxide
- K
potassium
- LNA
N (G)-monomethyl-L-arginine
- EE-8Z-E
epoxyeicosa-8Z-enoic acid
- EE-5Z-E
epoxyeicosa-5Z-enoic acid
- mSI
methylsulfonamide
- Em
membrane potential
- BKCa
large conductance, calcium-activated potassium
- SKCa
small conductance, calcium-activated potassium
- IKCa
intermediate conductance, calcium-activated potassium
- TRP
transient receptor potential
- tPA
tissue plasminogen activator
- PKA
protein kinase A
- AUDA
12-(3-adamantane-1-yl-ureido)-dodecanoic acid
- SHR
spontaneously hypertensive rat
- NCND
N-cyclohexyl-N-dodecyl
- ADU
N-adamantyl-N’-dodecylurea
- DOCA
deoxycorticosterone acetate
- ANG II
angiotensin II
- TNF-α
tumor necrosis factor α
- IL-1α
interleukin 1α
- CAMs
cell adhesion molecules
- VCAM-1
vascular cell adhesion molecule 1
- ICAM-1
intercellular adhesion molecule 1
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-κB
- MMP
matrix metalloproteinase
- HUVECs
human umbilical vein endothelial cells
- TX
thromboxane
- VEGF
vascular endothelial growth factor
- EGF
epidermal growth factor-like
- ERK
extracellular-signal-regulated kinases
- MAPK
mitogen-activated protein kinase
- PI3K/Akt
phosphoinositide 3-kinase/protein kinase B
- HB
heparin binding
- CDKs
cyclin-dependent kinases
- JNK
c-Jun N-terminal kinase
- MCA
middle cerebral artery
- SHRSP
stroke-prone spontaneously hypertensive rats
- ENaC
epithelial sodium channel
- PMN
polymorphonuclear leukocyte
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Adeagbo ASO, Triggle CR. Varying extracellular [K]: a functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J Cardiovasc Pharmacol. 1993;21:423–429. [PubMed] [Google Scholar]
- Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, et al. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BKca channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- Bellien J, Favre J, Iacob M, Gao J, Thuillez C, Richard V, et al. Arterial stiffness is regulated by nitric oxide and endothelium-derived hyperpolarizing factor during changes in blood flow in humans. Hypertension. 2010;55:674–680. doi: 10.1161/HYPERTENSIONAHA.109.142190. [DOI] [PubMed] [Google Scholar]
- Bellien J, Thuillez C, Joannides R. Contribution of endothelium-derived hyperpolarizing factors to the regulation of vascular tone in humans. Fundam Clin Pharmacol. 2008a;22:363–377. doi: 10.1111/j.1472-8206.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- Bellien J, Thuillez C, Joannides R. Role of endothelium-derived hyperpolarizing factor in the regulation of radial artery basal diameter and endothelium-dependent dilatation in vivo. Clin Exp Pharmacol Physiol. 2008b;35:494–497. doi: 10.1111/j.1440-1681.2008.04903.x. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Falck JR, Gauthier K. Role of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factor in bovine coronary arteries. Med Sci Monitor. 2001;7:578–584. [PubMed] [Google Scholar]
- Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pfluegers Arch. 2010;459:881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996a;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Holmes BB, Falck JR, Capdevila JH, Gauthier KM. Adenoviral expression of cytochrome P450 epoxygenase in coronary smooth muscle cells: Regulation of potassium channels by endogenous 14(S),15(R)-EET. Am J Physiol. 2006;290:H64–H71. doi: 10.1152/ajpheart.00516.2005. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Pratt PF, Gebremedhin D, Harder DR. Epoxyeicosatrienoic acids are endothelium-derived hyperpolarizing and vasodilating factors in bovine coronary arteries. In: Vanhoutte PM, editor. Endothelium-Derived Hyperpolarizing Factor. Harwood Academic; Amsterdam: 1996b. pp. 81–89. [Google Scholar]
- Capdevila J, Chacos N, Werringloer J, Prough RA, Estabrook RW. Liver microsomal cytochrome P450 and the oxidative metabolism of archidonic acid. Proc Natl Acad Sci USA. 1981;78:5362–5366. doi: 10.1073/pnas.78.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MA, Schwartzman M., Capdevila, J., Falck JR, McGiff JC. Vasoactivity of arachidonic acid epoxides. Europ JPharmacol. 1987;138:281–283. doi: 10.1016/0014-2999(87)90445-6. [DOI] [PubMed] [Google Scholar]
- Chen J-K, Falck JR, Reddy KM, Capdevila J, Harris RC. Epoxyeicosatrienoic acids and their sulfonimide derivatives stimulate tyrosine phosphorylation and induce mitogenesis in renal epithelial cells. J Biol Chem. 1998;273:29254–29261. doi: 10.1074/jbc.273.44.29254. [DOI] [PubMed] [Google Scholar]
- Chen Y, Falck JR, Tuniki VR, Campbell WB. 20-125Iodo-14,15-epoxyeicosa-5Z-enoic acid: a high affinity radioligand used to characterize the epoxyeicosatrienoic acid antagonist binding site. J Pharmacol Exp Ther. 2009;331:1137–1145. doi: 10.1124/jpet.109.157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ZM, Croft KD, Kingsbury DA, Falck JR, Reddy KM, Beilin LJ. Cytochrome P450 metabolites of arachidonic acid may be important mediators in angiotensin II-induced vasoconstriction in the rat mesentery in vivo. Clin Sci (Lond) 2000;98:277–282. [PubMed] [Google Scholar]
- Coats P, Johnston F, MacDonald J, McMurray JJV, Hillier C. Endothelium-derived hyperpolarizing factor: Identification and mechanism of action in human subcutaenous resistance arteries. Circulation. 2001;103:1702–1708. doi: 10.1161/01.cir.103.12.1702. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization: Beyond nitric oxide and cyclic GMP. Circulation. 1995;92:3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- Cowart LA, Wei S, Hsu MH, Johnson EF, Krishna UM, Falck JR, et al. The CYP4A isoforms hydroxylate epoxyeicosatrienoic acids to form high affinity peroxisome proliferator-activated receptor ligands. J Biol Chem. 2002;20:35105–35112. doi: 10.1074/jbc.M201575200. [DOI] [PubMed] [Google Scholar]
- Deng Y, Theken KN, Lee CR. Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J Mol Cell Cardiol. 2010;48:331–341. doi: 10.1016/j.yjmcc.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EF, Police RJ, Yancy L, McKinney JS, Amruthesh SC. Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am J Physiol. 1990;259:H1171–H1177. doi: 10.1152/ajpheart.1990.259.4.H1171. [DOI] [PubMed] [Google Scholar]
- Falck JR, Kodela R, Manne R, Atcha KR, Puli N, Dubasi N, et al. 14,15-Epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates containing epoxide bioisosteres: Influence upon vascular relaxation and soluble epoxide hydrolase inhibition. J Med Chem. 2009;52:5069–5075. doi: 10.1021/jm900634w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck JR, Krishna UM, Reddy YK, Kumar PS, Reddy KM, Hittner SB, et al. Comparison of the vasodilatory properties of 14,15-EET analogs: Structural requirements for dilation. Am J Physiol. 2003a;284:H337–H349. doi: 10.1152/ajpheart.00831.2001. [DOI] [PubMed] [Google Scholar]
- Falck JR, Reddy LM, Reddy YK, Bondlela M, Krishna UM, Ji Y, et al. 11,12-Epoxyeicosatrienoic acid (11,12-EET): Structural determinants for inhibition of TNF-alpha-induced VCAM-1 expression. Bioorganic Med Chem Letters. 2003b;13:4011–4014. doi: 10.1016/j.bmcl.2003.08.060. [DOI] [PubMed] [Google Scholar]
- Fang X, Weintraub NL, Oltman CL, Stoll LL, Kaduce TL, Harmon S, et al. Human coronary endothelial cells convert 14,15-EET to a biologically active chain-shortened epoxide. Am J Physiol Heart Circ Physiol. 2002;283:H2306–2314. doi: 10.1152/ajpheart.00448.2002. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolaizing factor. Where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Popp R, Michaelis UR, Kiss L, Fleming I, Busse R. Cyclic stretch enhances the expression and activity of coronary endothelium-derived hyperpolarizing factor synthase. Hypertension. 2001;38:1427–1432. doi: 10.1161/hy1201.096532. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, et al. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick FA, Ennis MD, Baze ME, Wynalda MA, McGee JE, Liggett W. Inhibition of cyclooxygenase activity and platelet aggregation by epoxyeicosatrienoic acids. J Biol Chem. 1986;261:15334–15338. [PubMed] [Google Scholar]
- Fleming I, Busse R. Endothelium-derived epoxyeicosatrienoic acids and vascular function. Hypertension. 2006;47:629–633. doi: 10.1161/01.HYP.0000208597.87957.89. [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Michaelis UR, Kiss L, Popp R, Busse R. The coronary endothelium-derived hyperpolarizing factor (EDHF) stimulates multiple signalling pathways and proliferation of vascular cells. Pfluegers Arch. 2001a;442:511–518. doi: 10.1007/s004240100565. [DOI] [PubMed] [Google Scholar]
- Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, et al. Endothelium-derived hyperpolarizing factor synthase (cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001b;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, et al. Epoxyeicosatrienoic acids regulate Trp-channel-dependent Ca signaling and hyperpolariztion in endothelial cells. Arterio Thromb Vasc Biol. 2007;27:2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JW. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Campbell WB. Epoxyeicosatrienoic acids mediate flow-induced dilation of bovine small coronary arteries. Hypertension. 2006;48:e68. [Google Scholar]
- Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, et al. 14,15-Epoxyeicosa-5(Z)-enoic acid: A selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Edwards EM, Falck JR, Reddy DS, Campbell WB. 14,15-Epoxyeicosatrienoic acid represents a transferable endothelium-dependent relaxing factor in bovine coronary arteries. Hypertension. 2005;45:666–671. doi: 10.1161/01.HYP.0000153462.06604.5d. [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Falck JR, Reddy LM, Campbell WB. 14,15-EET analogs: Characterization of structural requirements for agonist and antagonist activity in bovine coronary arteries. Pharmacol Res. 2004;49:515–524. doi: 10.1016/j.phrs.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Jagadeesh SG, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic-mSI: A 14,15- and 5,6-EET antagonist in bovine coronary arteries. Hypertension. 2003a;42:555–561. doi: 10.1161/01.HYP.0000091265.94045.C7. [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Pratt P, Falck JR, Campbell WB. Inhibition of bradykinin-induced relaxations by an epoxeicosatrienoic acid antagonist: 14,15-epoxyeicosa-5Z-monoenoic acid. In: Vanhoutte PM, editor. EDHF 2002. Taylor and Francis; New York: 2003b. pp. 325–331. [Google Scholar]
- Halcox JPJ, Narayanan S, Crames-Joyce L, Mincemoyer R, Quyyumi AA. Characterization of endothelium-derived hyperpolarizing factor in the human forearm microcirculation. Am J Physiol. 2001;280:H2470–H2477. doi: 10.1152/ajpheart.2001.280.6.H2470. [DOI] [PubMed] [Google Scholar]
- Harris RC, Homma T, Jacobson HR, Capdevila J. Epoxyeicosatrienoic acids activate Na/H exchange and are mitogenic in cultured rat glomerular mesangial cells. J Cell Physiol. 1992;144:429–437. doi: 10.1002/jcp.1041440310. [DOI] [PubMed] [Google Scholar]
- Hatoum OA, Gauthier KM, Binion DG, Miura H, Telford G, Otterson MF, et al. Novel mechanism of vasodilation in inflammatory bowel disease. Arterioscler Thromb Vasc Biol. 2005;25:2355–2361. doi: 10.1161/01.ATV.0000184757.50141.8d. [DOI] [PubMed] [Google Scholar]
- Hecker M, Bara AT, Bauersachs J, Busse R. Characterization of endothelium-derived hyperpolarizing factor as a cytochrome P450-derived arachidonic acid metabolite in mammals. J Physiol. 1994;481:407–414. doi: 10.1113/jphysiol.1994.sp020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercule HC, Schunck W-H, Gross V, Seringer J, Leung FP, Weldon SM, et al. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol. 2009;29:54–60. doi: 10.1161/ATVBAHA.108.171298. [DOI] [PubMed] [Google Scholar]
- Honing MLH, Smits P, Morrison PJ, Rabelink TJ. Bradykinin-induced vasodilation of human forearm resistance vessels is primarily mediated by endothelium-dependent hyperpolarization. Hypertension. 2000;35:1314–1318. doi: 10.1161/01.hyp.35.6.1314. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res. 2005;96:376–383. doi: 10.1161/01.RES.0000155332.17783.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Alkayed NJ. Soluble Epoxide Hydrolase Inhibition: Targeting Multiple Mechanisms of Ischemic Brain Injury with a Single Agent. Future Neurol. 2009;4:179–199. doi: 10.2217/14796708.4.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Jia J, Nelson J, Goyagi T, Klaus J, Alkayed NJ. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat. 2010;91:68–84. doi: 10.1016/j.prostaglandins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nature Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD, Inscho EW, Deichmann PC, Reddy KM, Falck JR. Afferent arteriolar vasodilation to the sulfonimide analog of 11,12-epoxyeicosatrienoic acid involves protein kinase A. Hypertension. 1999;33:408–413. doi: 10.1161/01.hyp.33.1.408. [DOI] [PubMed] [Google Scholar]
- Imig JD, Zhao X, Capdevila J, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- Imig JD, Zhao X, Zaharis CZ, Olearczyk JL, Pollock DM, Newman JW, et al. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46:975–981. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz SD, Krum H. Acetylcholine-mediated vasodilation in the forearm circulation of patients with heart failure: Indirect evidence for the role of endothelium-derived hyperpolarizing factor. Am J Cardiol. 2001;87:1089–1092. doi: 10.1016/s0002-9149(01)01466-7. [DOI] [PubMed] [Google Scholar]
- Kemp BK, Cocks TM. Evidence that mechanisms dependent and independent of nitric oxide mediate endothelium-dependent relaxation to bradykinin in human small resistance-like coronary arteries. Br J Pharmacol. 1997;120:757–762. doi: 10.1038/sj.bjp.0700928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerseru B, Barbosa-Sicard E, Popp R, Fisslthaler B, Dietrich A, Gudermann T, et al. Epoxyeicosatrienoic acids and the soluble epoxide hydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstriction response. FASEB J. 2008;22:4306–4315. doi: 10.1096/fj.08-112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotz F, Hellwig N, Burkle MA, Lehrer S, Riexinger T, Mannell H, et al. A sulfaphenazole-sensitive EDHF opposes platelet-endothelium interactions in vitro and in the hamster microcirculation in vivo. Cardiovasc Res. 2010;85:542–550. doi: 10.1093/cvr/cvp301. [DOI] [PubMed] [Google Scholar]
- Krotz F, Riexinger T, Buerkle MA, Nithipatikom K, Gloe T, Sohn HY, et al. Membrane potential-dependent inhibition of platelet adhesion to endothelial cells by epoxyeicosatrienoic acids. Arterioscler Thromb Vasc Biol. 2003;24:595–600. doi: 10.1161/01.ATV.0000116219.09040.8c. [DOI] [PubMed] [Google Scholar]
- Kume H, Graziano MP, Kotlikoff MI. Stimulatory and inhibitory regulation of calcium-activated potassium channels by guanine nucleotide-binding proteins. Proc Natl Acad Sci USA. 1992;89:11051–11055. doi: 10.1073/pnas.89.22.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, et al. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: Interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102:59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, et al. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BKca channels: Implications for soluble epoxide hydrolase inhibition. Am J Physiol. 2005;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, Fedinec AL. Newborn piglet cerebral microvascular responses to epoxyeicosatrienoic acids. Am J Physiol. 1997;273:H333–H338. doi: 10.1152/ajpheart.1997.273.1.H333. [DOI] [PubMed] [Google Scholar]
- Lenasi H. The role of nitric oxide- and prostacyclin-independent vasodilatation in the human cutaneous microcirculation: effect of cytochrome P450 2C9 inhibition. Clin Physiol Funct Imaging. 2009;29:263–270. doi: 10.1111/j.1475-097X.2009.00862.x. [DOI] [PubMed] [Google Scholar]
- Lenasi H, Strucl M. The effect of nitric oxide synthase and cyclooxygenase inhibition on cutaneous microvascular reactivity. Eur J Appl Physiol. 2008;103:719–726. doi: 10.1007/s00421-008-0769-8. [DOI] [PubMed] [Google Scholar]
- Li P, Chen C-L, Bortell R, Campbell WB. 11,12-Epoxyeicosatrienoic acid stimulates endogenous mono-ADP-ribosylation in bovine coronary arterial smooth muscle. Circ Res. 1999;85:349–356. doi: 10.1161/01.res.85.4.349. [DOI] [PubMed] [Google Scholar]
- Li P-L, Campbell WB. Epoxyeicosatrienoic acids activate potassium channels in coronary smooth muscle through guanine nucleotide binding protein. Circ Res. 1997;80:877–884. doi: 10.1161/01.res.80.6.877. [DOI] [PubMed] [Google Scholar]
- Li P-L, Zou A-P, Campbell WB. Regulation of potassium channels in coronary arterial smooth muscle by endothelium-derived vasodilators. Hypertension. 1997;29:262–267. doi: 10.1161/01.hyp.29.1.262. [DOI] [PubMed] [Google Scholar]
- Liu JY, Yang J, Inceoglu B, Qiu H, Ulu A, Hwang SH, et al. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol. 2010;79:880–887. doi: 10.1016/j.bcp.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang Y, Schmelzer K, Lee T-S, Fang X, Zhu Y, et al. The antiinflammatory effect of laminar flow: the role of PPARγ. epoxyeicosatrienoic acids and soluble epoxide hydrolase. Proc Nat Acad Sci USA. 2005;102:16747–16752. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb AL, Godeny I, Longnecker DE. Anesthetics alter relative contributions of NO and EDHF in rat cremaster muscle microcirculation. Am J Physiol. 1997;273:H618–627. doi: 10.1152/ajpheart.1997.273.2.H618. [DOI] [PubMed] [Google Scholar]
- Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilation. Cardiovas Res. 2008;80:445–452. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, et al. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol. 2009;297:F740–748. doi: 10.1152/ajprenal.00098.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Hayashi K, Wakino S, Kubota E, Honda M, Tokuyama H, et al. Role of endothelium-derived hyperpolarizing factor in ACE inhibitor-induced renal vasodilation in vivo. Hypertension. 2004;43:603–609. doi: 10.1161/01.HYP.0000118053.42262.71. [DOI] [PubMed] [Google Scholar]
- McCulloch AI, Randall MD. Sex differences in the relative contributions of nitric oxide and EDHF to agonist-stimulated endothelium-dependent relaxations in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1998;123:1700–1706. doi: 10.1038/sj.bjp.0701781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Daniels J, Mundey K, Fisslthaler B, Busse R, Jacobs ER, et al. Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2003;284:H215–224. doi: 10.1152/ajpheart.01118.2001. [DOI] [PubMed] [Google Scholar]
- Michaelis UR, Falck JR, Schmidt R, Busse R, Fleming I. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce the expression of cyclooxygenase-2 in endothelial cells. Arterioscler Thromb Vasc Biol. 2005a;25:321–332. doi: 10.1161/01.ATV.0000151648.58516.eb. [DOI] [PubMed] [Google Scholar]
- Michaelis UR, Fisslthaler B, Barbosa-Sicard E, Falck JR, Fleming I, Busse R. Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis. J Cell Sci. 2005b;118:5489–5498. doi: 10.1242/jcs.02674. [DOI] [PubMed] [Google Scholar]
- Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR) FASEB J. 2003;17:770–772. doi: 10.1096/fj.02-0640fje. [DOI] [PubMed] [Google Scholar]
- Michaelis UR, Fleming I. From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: Epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol Ther. 2006;111:584–595. doi: 10.1016/j.pharmthera.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Michaelis UR, Xia N, Barbosa-Sicard E, Falck JR, Fleming I. Role of cytochrome P450 2C epoxygenases in hypoxia-induced cell migration and angiogenesis in retinal endothelial cells. Invest Ophthalmol Vis Sci. 2008;49:1242–1247. doi: 10.1167/iovs.07-1087. [DOI] [PubMed] [Google Scholar]
- Miura H, Wachtel RE, Liu Y, Loberiza J, F. R, Saito T, Miura M, et al. Flow-induced dilation of human coronary arterioles: Important role of Ca2+-activated K+ channels. Circulation. 2001;103:1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- Moreland KT, Procknow JD, Sprague RS, Iverson JL, Lonigro AJ, Stephenson AH. Cyclooxygenase (COX)-1 and COX-2 participate in 5,6-epoxyeicosatrienoic acid-induced contraction of rabbit intralobar pulmonary arteries. J Pharmacol Exp Ther. 2007;321:446–454. doi: 10.1124/jpet.106.107904. [DOI] [PubMed] [Google Scholar]
- Moshal KS, Zeldin DC, Sithu SD, Sen U, Tyagi N, Kumar M, et al. Cytochrome P450 (CYP) 2J2 gene transfection attenuates MMP-9 via inhibition of NF-kappabeta in hyperhomocysteinemia. J Cell Physiol. 2008;215:771–781. doi: 10.1002/jcp.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: Role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol. 2000;278:H1163–1167. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- Nagao T, Illiano S, Vanhoutte PM. Heterogenous distribution of endothelium-dependent relaxations resistant to N-nitro-L-arginine in rats. Am J Physiol. 1992;263:H1090–H1094. doi: 10.1152/ajpheart.1992.263.4.H1090. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Stepp DW, Chilian WM. In vivo location and mechanism of EDHF-mediated vasodilation in canine coronary microcirculation. Am J Physiol. 1999;277:H1252–H1259. doi: 10.1152/ajpheart.1999.277.3.H1252. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol. 2000;279:H459–H465. doi: 10.1152/ajpheart.2000.279.2.H459. [DOI] [PubMed] [Google Scholar]
- Nithipatikom K, Pratt PF, Campbell WB. Determination of EETs using microbore liquid chromatography with fluorescence detection. Am J Physiol. 2000;279:H857–H862. doi: 10.1152/ajpheart.2000.279.2.H857. [DOI] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K, Ruan X-L, Dai J, Yang S-X, Graham L, Zeldin DC, et al. Activation of Gas mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem. 2001;276:15983–15989. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- Olearczyk JJ, Quigley JE, Mitchell BC, Yamamoto T, Kim IH, Newman JW, et al. Administration of a substituted adamantyl urea inhibitor of soluble epoxide hydrolase protects the kidney from damage in hypertensive Goto-Kakizaki rats. Clin Sci (Lond) 2009;116:61–70. doi: 10.1042/CS20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltman CL, Kane NL, Fudge JL, Weintraub NL, Dellsperger KC. Endothelium-derived hyperpolarizing factor in coronary microcirculation: Responses to arachidonic acid. Am J Physiol. 2001;281:H1553–H1560. doi: 10.1152/ajpheart.2001.281.4.H1553. [DOI] [PubMed] [Google Scholar]
- Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res. 1998;83:932–939. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Chow JA, Evans RG, Coleman HA, Tare M. Role for endothelium-derived hyperpolarizing factor in vascular tone in rat mesenteric and hindlimb circulations in vivo. J Physiol. 2002;542:929–937. doi: 10.1113/jphysiol.2002.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- Pomposiello SI, Quilley J, Carroll MA, Falck JR, McGiff JC. 5,6-epoxyeicosatrienoic acid mediates the enhanced renal vasodilation to arachidonic acid in the SHR. Hypertension. 2003;42:548–554. doi: 10.1161/01.HYP.0000090095.87899.36. [DOI] [PubMed] [Google Scholar]
- Popp R, Fleming I, Busse R. Pulsatile stretch in coronary arteries elicits release of endothelium-derived hyperpolarizing factor: A modulator of arterial compliance. Circ Res. 1998;82:696–703. doi: 10.1161/01.res.82.6.696. [DOI] [PubMed] [Google Scholar]
- Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem. 2003;278:29619–29625. doi: 10.1074/jbc.M305385200. [DOI] [PubMed] [Google Scholar]
- Potente M, Michaelis UR, Fisslthaler B, Busse R, Fleming I. Cytochrome P450 2C9-induced endothelial cell proliferation involves induction of mitogen-activated protein (MAP) kinase phosphatase-1, inhibition of the c-Jun N-terminal kinase, and up-regulation of cyclin D1. J Biol Chem. 2002;277:15671–15676. doi: 10.1074/jbc.M110806200. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Zent R, et al. Characterization of 5,6-and 8,9-epoxyeicosatrienoic acids as potent in vivo angiogenic lipids. J Biol Chem. 2005;280:27138–27146. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- Pratt PF, Rosolowsky M, Campbell WB. Effect of epoxyeicosatrienoic acids on polymorphonuclear leukocyte function. Life Sci. 2002;70:2521–2533. doi: 10.1016/s0024-3205(02)01533-3. [DOI] [PubMed] [Google Scholar]
- Proctor KG, Falck JR, Capdevila J. Intestinal vasodilation by epoxyeicosatrienoic acids: Arachidonic acid metabolites produced by a cytochrome P450 monooxygenase. Circ Res. 1987;60:50–59. doi: 10.1161/01.res.60.1.50. [DOI] [PubMed] [Google Scholar]
- Revermann M. Pharmacological inhibition of the soluble epoxide hydrolase-from mouse to man. Curr Opin Pharmacol. 2010;10:173–178. doi: 10.1016/j.coph.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Rosolowsky M, Campbell WB. Role of PGI2 and EETs in the relaxation of bovine coronary arteries to arachidonic acid. Am J Physiol. 1993;264:H327–H335. doi: 10.1152/ajpheart.1993.264.2.H327. [DOI] [PubMed] [Google Scholar]
- Rosolowsky M, Campbell WB. Synthesis of hydroxyeicosatetraenoic acids (HETEs) and epoxyeicosatrienoic acids (EETs) by cultured bovine coronary artery endothelial cells. Biochim Biophys Acta. 1996;1299:267–277. doi: 10.1016/0005-2760(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Scornik FS, Codina J, Birnbaumer L, Toro L. Modulation of coronary smooth muscle Kca channels by Gsa independent of phosphorylation by protein kinase A. Am J Physiol. 1993;265:H1460–H1465. doi: 10.1152/ajpheart.1993.265.4.H1460. [DOI] [PubMed] [Google Scholar]
- Scotland RS, Madhani M, Chauhan SD, Moncada S, Andresen J, Nilsson H, et al. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double knockout mice: Key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation. 2005;111:796–803. doi: 10.1161/01.CIR.0000155238.70797.4E. [DOI] [PubMed] [Google Scholar]
- Simpkins AN, Rudic RD, Roy S, Tsai HJ, Hammock BD, Imig JD. Soluble epoxide hydrolase inhibition modulates vascular remodeling. Am J Physiol Heart Circ Physiol. 2010;298:H795–806. doi: 10.1152/ajpheart.00543.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins AN, Rudic RD, Schreihofer DA, Roy S, Manhiani M, Tsai HJ, et al. Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol. 2009;174:2086–2095. doi: 10.2353/ajpath.2009.080544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, et al. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7:1759–1766. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- Snyder GD, Krishna UM, Falck JR, Spector AA. Evidence for a membrane site of action for 14,15-EET on expression of aromatase in vascular smooth muscle. Am J Physiol. 2002;283:H1936–H1942. doi: 10.1152/ajpheart.00321.2002. [DOI] [PubMed] [Google Scholar]
- Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50(Suppl):S52–56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): Metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- Sudhahar V, Shaw S, Imig JD. Epoxyeicosatrienoic acid analogs and vascular function. Curr Med Chem. 2010;17:1181–1190. doi: 10.2174/092986710790827843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Sui X-X, Bradbury A, Zeldin DC, Conte MS, Liao JK. Inhibition of vascular smooth muscle cell migration by cytochrome P450 epoxygenase-derived eicosanoids. Circ Res. 2002;90:1020–1027. doi: 10.1161/01.res.0000017727.35930.33. [DOI] [PubMed] [Google Scholar]
- Taddei S, Varsari D, Cipriano A, Ghiadoni L, Glaetta F, Franzoni F, et al. Identification of a cytochrome P450 2C9-derived endothelium-derived hyperpolarizing factor in human essential hypertensive patients. J Am Coll Cardiol. 2006;48:508–515. doi: 10.1016/j.jacc.2006.04.074. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Shimokawa H, Tagawa T, Kuroiwa-Matsumoto M, Hirooka Y, Takeshita A. Short-term estrogen augments both nitric oxide-mediated and non-nitric oxide-mediated endothelium-dependent forearm vasodilation in postmenopausal women. J Cardiovasc Pharmacol. 1997;30:481–488. doi: 10.1097/00005344-199710000-00012. [DOI] [PubMed] [Google Scholar]
- Thomsen K, Rubin I, Lauritzen M. In vivo mechanisms of acetylcholine-induced vasodilation in rat sciatic nerve. Am J Physiol Heart Circ Physiol. 2000;279:H1044–1054. doi: 10.1152/ajpheart.2000.279.3.H1044. [DOI] [PubMed] [Google Scholar]
- Ulu A, Davis BB, Tsai HJ, Kim IH, Morisseau C, Inceoglu B, et al. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in apolipoprotein e-knockout mouse model. J Cardiovasc Pharmacol. 2008;52:314–323. doi: 10.1097/FJC.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRollins M. Epoxygenase metabolites of docosahexaenoic and eicosapentaenoic acids inhibit platelet aggregation at concentrations below those affecting thromboxane synthesis. J Pharmacol Exp Ther. 1995;274:798–804. [PubMed] [Google Scholar]
- Virdis A, Cetani F, Giannarelli C, Banti C, Ghiadoni L, Ambrogini E, et al. The sulfaphenazole-sensitive pathway acts as a compensatory mechanism for impaired nitric oxide availability in patients with primary hyperparathyroidism. Effect of surgical treatment. J Clin Endocrinol Metab. 2010;95:920–927. doi: 10.1210/jc.2009-1669. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Yashiro Y, Mizuno R, Ohhashi T. Involvement of NO and EDHF in flow-induced vasodilation in isolated hamster cremasteric arterioles. J Vasc Res. 2005;42:137–147. doi: 10.1159/000083652. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Role of EDHF in conduction of vasodilation along hamster cheek pouch arterioles in vivo. Am J Physiol Heart Circ Physiol. 2000;278:H1832–1839. doi: 10.1152/ajpheart.2000.278.6.H1832. [DOI] [PubMed] [Google Scholar]
- Weston AH, Feletou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: Involvement of potassium channel activation and epoxyeicosatrienoic acids. Brit J Pharmacol. 2005;145:775–784. doi: 10.1038/sj.bjp.0706256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RM, Rivera CO, Davison CA. Nitric oxide-dependent and-independent mechanisms account for gender differences in vasodilation to acetylcholine. J Pharmacol Exp Ther. 2000;292:375–380. [PubMed] [Google Scholar]
- Widmann MD, Weintraub NL, Fudge JL, Brooks LA, Dellsperger KC. Cytochrome P-450 pathway in acetylcholine-induced canine coronary microvascular vasodilation in vivo. Am J Physiol. 1998;274:H283–H289. doi: 10.1152/ajpheart.1998.274.1.H283. [DOI] [PubMed] [Google Scholar]
- Wong PY, Lin KT, Yan YT, Ahern D, Iles J, S.Y. S, et al. 14(R), 15(S)-Epoxyeicosatrienoic acid receptor in guinea pig mononuclear cell membranes. J Lipid Mediat Cell Signal. 1993;6:199–208. [PubMed] [Google Scholar]
- Wong PY-K, Lai P-S, Falck JR. Mechanism and signal transduction of 14(R), 15 (S)-epoxyeicosatrienoic acid (14,15-EET binding in guinea pig monocytes. Prostag Other Lipid Med. 2000;62:321–333. doi: 10.1016/s0090-6980(00)00079-4. [DOI] [PubMed] [Google Scholar]
- Wong PY-K, Lai P-S, Shen S-Y, Belosludtsev YY, Falck JR. Post-receptor signal transduction and regulation of 14(R), 15(S)-epoxyeicosatrienoic acid (14,15-EET) binding in U-937 cells. J Lipid Med Cell Signal. 1997;16:155–169. doi: 10.1016/s0929-7855(97)00005-9. [DOI] [PubMed] [Google Scholar]
- Woodman OL, Boujaoude M. Chronic treatment of male rats with daidzein and 17 beta-oestradiol induces the contribution of EDHF to endothelium-dependent relaxation. Br J Pharmacol. 2004;141:322–328. doi: 10.1038/sj.bjp.0705603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem. 1996;271:3460–3468. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
- Yang S, Wei S, Pozzi A, Capdevila JH. The arachidonic acid epoxygenase is a component of the signaling mechanisms responsible for VEGF-stimulated angiogenesis. Arch Biochem Biophys. 2009;489:82–91. doi: 10.1016/j.abb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Holmes BB, Gopal VR, Kishore RVK, Sangras B, Yi X-Y, et al. Characterization of 14,15-epoxyeicosatrienoyl-sulfonamides as 14,15-epoxyeicosatrienoic acid agonists: Use for studies of metabolism and ligand binding. J Pharmacol Exp Ther. 2007;321:1023–1031. doi: 10.1124/jpet.107.119651. [DOI] [PubMed] [Google Scholar]
- Yang W, Tuniki VR, Anjaiah S, Falck JR, Hillard CJ, Campbell WB. Characterization of epoxyeicosatrienoic acid binding site in U937 membranes using a novel radiolabeled agonist, 20-125I-14,15-epoxyeicosa-8(Z)-enoic acid. J Pharmacol Exp Ther. 2008;324:1019–1027. doi: 10.1124/jpet.107.129577. [DOI] [PubMed] [Google Scholar]
- Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- Zeldin DC, Dubois RN, Falck JR, Capdevila JH. Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch Biochem Biophys. 1995;322:7686. doi: 10.1006/abbi.1995.1438. [DOI] [PubMed] [Google Scholar]
- Zhang L, Cui Y, Geng B, Zeng X, Tang C. 11,12-Epoxyeicosatrienoic acid activates the L-arginine/nitric oxide pathway in human platelets. Mol Cell Biochem. 2008a;308:51–56. doi: 10.1007/s11010-007-9611-6. [DOI] [PubMed] [Google Scholar]
- Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, et al. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27:1931–1940. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, et al. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008b;39:2073–2078. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Bousamra M, 2nd, Zeldin DC, Falck JR, Townsley M, Harder DR, et al. Epoxyeicosatrienoic acids constrict isolated pressurized rabbit pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2000;278:L335–343. doi: 10.1152/ajplung.2000.278.2.L335. [DOI] [PubMed] [Google Scholar]