Abstract
Cilia are conserved, microtubule-based cell surface projections that emanate from basal bodies, membrane-docked centrioles. The beating of motile cilia and flagella enables cells to swim and epithelia to displace fluids. In contrast, most primary cilia do not beat but instead detect environmental or intercellular stimuli. Inborn defects in both kinds of cilia cause human ciliopathies, diseases with diverse manifestations such as heterotaxia and kidney cysts. These diseases are caused by defects in ciliogenesis or ciliary function. The signaling functions of cilia require regulation of ciliary composition, which depends on the control of protein traffic into and out of cilia.
Introduction
Ciliated organisms are found in each of the existing eukaryotic clades, including Excavata, such as euglenids, Rhizaria, such as ameboflagellates, Chromalveolata, such as dinoflagellates, Amoebozoa, such as pelobionts, Plantae, such as green algae, and Opisthokonta, such as the fungus Batrachochytrium dendrobatidis and animals. Thus, the eukaryotic cenancestor must have had a cilium, which performed both motor and sensory functions (Satir et al., 2008; Cavalier-Smith, 2010). In multicellular organisms, many cilia have become specialized cellular antennae, known as primary cilia, which regulate processes such as embryogenesis, tumorigenesis, feeding behavior, kidney function, vision, and smell (Davenport et al., 2007; McEwen et al., 2008; Han et al., 2009; Wong et al., 2009; Goetz and Anderson, 2010; Hildebrandt et al., 2011). Unlike primary cilia, motile cilia and flagella are restricted to a handful of human tissues, in which they propel sperm, regulate embryonic left–right patterning, clear airway mucus, and participate in cerebrospinal fluid movement (Bloodgood, 2010).
Cilia are not fully encompassed by the membrane (Fig. 1); yet, their composition is distinct from that of the surrounding cytosol and plasma membrane. Other specialized subcellular domains, such as neuronal axons, generate their unique composition through multiple mechanisms (Winckler and Mellman, 2010). Some proteins are delivered directly to axons by means of specific targeting signals and pathways (selective targeting). Other proteins accumulate in axons by being endocytosed from somatodendritic, but not axonal, membranes (selective removal). Yet, other proteins concentrate inside axons by specifically interacting with axonal components (selective retention), whereas still others cannot enter axons because they are anchored to cytoskeletal or extracellular matrix elements elsewhere in the cell (selective exclusion). These mechanisms act in concert with diffusion barriers at the base of the axon, which restrict entrance and exit of both membrane and soluble proteins (Winckler and Mellman, 2010). Emerging evidence suggests that, similar to axons, selective targeting, exclusion, retention, and diffusion barriers also control ciliary composition (Mazelova et al., 2009a; Dishinger et al., 2010; Hu et al., 2010; Francis et al., 2011).
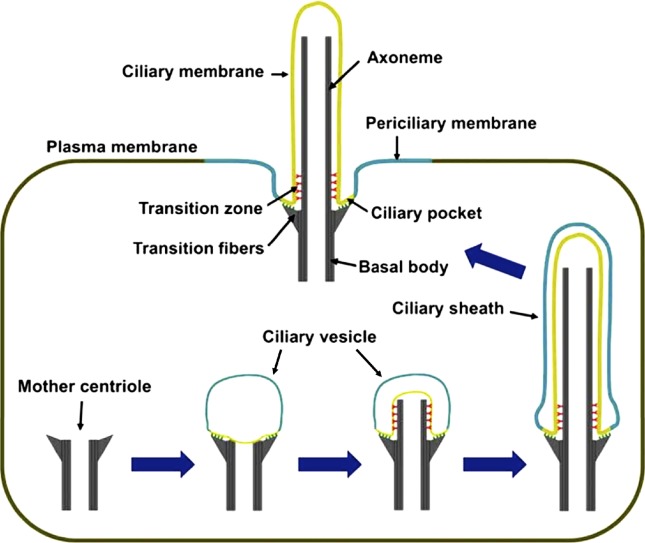
Figure 1.
Ciliogenesis. Cilium formation starts when a mother centriole contacts a ciliary vesicle. Axonemes elongate at their tips and so are constructed from proximal to distal, with the most proximal region giving rise to the transition zone. The ciliary vesicle grows with the axoneme and gives rise to the ciliary sheath, whose fusion with the plasma membrane externalizes the cilium and transforms the outer sheath into the periciliary membrane.
Building the cilium: Where the centriole meets the membrane
The ciliary axoneme is nucleated from the mother centriole, the older of the two centrioles in the centrosome (Bornens, 2012). Because mother centrioles are part of spindle poles during cell division, cilia must disassemble before mitosis and form again only upon entry into G1 (Kobayashi and Dynlacht, 2011).
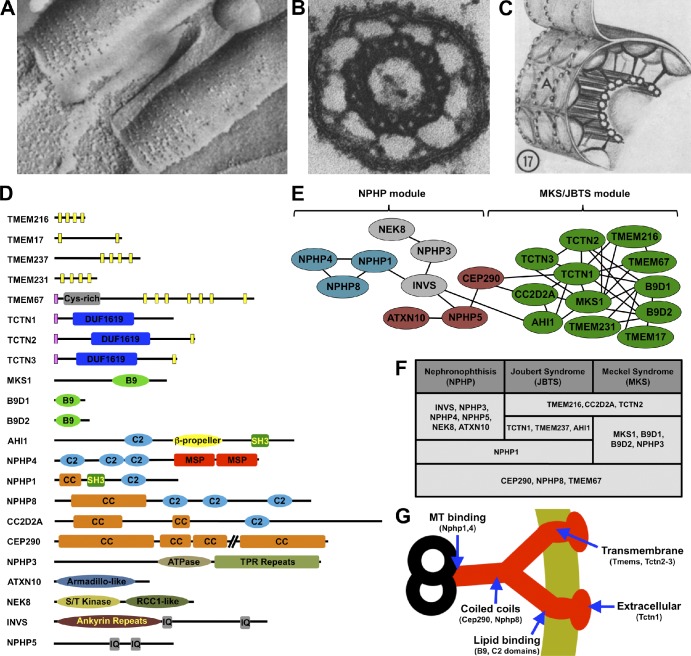
Ciliogenesis begins with the attachment of the distal end of the mother centriole to a vesicle (Fig. 1; Sorokin, 1962, 1968). This attachment is mediated by the centriolar distal appendages, also called transition fibers when they are associated with a cilium (Anderson, 1972; Deane et al., 2001). After docking, a bud emerges from the mother centriole, bending the membrane (Sorokin, 1962). This bud elongates from its tip to form the axoneme, but the base remains structurally distinct and will become the transition zone (Rosenbaum and Child, 1967; Boisvieux-Ulrich et al., 1989). The transition zone starts where the nine microtubule triplets in the basal body become doublets and is characterized by Y links, champagne glass-shaped structures that connect each doublet to the overlying membrane (Gilula and Satir, 1972). This overlying membrane contains the ciliary necklace, a circumferential set of intramembranous particles (Fig. 2, A–C; Gilula and Satir, 1972; Sattler and Staehelin, 1974; Menco, 1980; Hufnagel, 1983; Fisch and Dupuis-Williams, 2011).
Figure 2.
The transition zone. (A) Freeze-etch electron micrograph of tracheal epithelial cilia. The ciliary necklaces are at the ciliary base. (B) Electron micrograph of a cross section through a transition zone of a mollusk gill cilium, showing the microtubule doublets connected to the ciliary membrane by nine Y links. (C) Diagram of the transition zone, showing the Y links connecting the microtubules to the ciliary necklace. The A indicates the convex freeze-fracture face of the membrane. A–C are obtained from Gilula and Satir (1972). (D) Domain structure of transition zone components. The Tectonic proteins (TCTN1–3) share a signal peptide (pink bars) and a cysteine-rich Tectonic domain. Transition zone transmembrane proteins (TMEMs) include TCTN2, TCTN3, TMEM17, TMEM67, TMEM231, and TMEM237 (predicted transmembrane helices are shown as yellow bars). MKS1, B9D1, and B9D2 share B9 domains related to lipid-binding C2 domains. Several transition zone proteins contain C2 domains, coiled-coil (CC) domains, or both. Inversin (INVS) and NPHP5 have calmodulin-binding IQ motifs. NPHP4 contains two major sperm protein (MSP) domains, NPHP3 is an ATPase with tetratricopeptide repeat (TPR) domains, NEK8 is a serine/threonine kinase, and ATXN10 contains Armadillo repeats. (E) The transition zone protein interaction network. Genetic experiments in C. elegans reveal two main functional modules in this network. The first module is mostly comprised of genes, the human homologues of which are implicated in NPHP, whereas the second contains genes associated with MKS and JBTS. These functional modules closely match the results of biochemical experiments in mammalian cells. According to these, proteins in the MKS–JBTS module (green) mostly interact with other proteins within the same module and only rarely with those in the NPHP module (each line designates a reported protein–protein interaction). The NPHP module consists of several interconnected complexes, shown in different colors (Sang et al., 2011; van Reeuwijk et al., 2011). (F) Most known transition zone proteins are encoded by genes mutated in at least one of three related ciliopathies, NPHP, JBTS, and MKS. (G) Schematic of a Y link with a model of its composition. Nphp1 and Nphp4 bind microtubules, so they may connect Y links to microtubule doublets (Mollet et al., 2005). Cep290 and other coiled-coil proteins may form the central portion Y links. C2 and B9 domain-containing proteins are predicted to bind lipids, so they may be membrane proximal. Tctn1 also interacts with transmembrane proteins but is predicted to be on the extracellular face of the ciliary necklace, as it contains a signal peptide.
The transition zone houses a network of ciliopathy proteins that play important roles in Y link and axoneme formation (Fig. 2, D–G; van Reeuwijk et al., 2011). One of these complexes spans the membrane and contains many of the proteins implicated in Meckel syndrome (MKS) and Joubert syndrome (JBTS), two ciliopathies characterized by brain, kidney, and limb defects (Dowdle et al., 2011; Garcia-Gonzalo et al., 2011; Sang et al., 2011; Chih et al., 2012). Another transition zone complex contains Nphp1, 4, and 8, three proteins encoded by genes mutated in nephronophthisis (NPHP), a cystic kidney ciliopathy (Winkelbauer et al., 2005; Fliegauf et al., 2006; Vierkotten et al., 2007; Jiang et al., 2008, 2009; Sang et al., 2011; Won et al., 2011). In Caenorhabditis elegans, homologues of the MKS–JBTS and NPHP complexes have overlapping functions in forming both Y links and transition fibers (Williams et al., 2008, 2010, 2011; Huang et al., 2011; Warburton-Pitt et al., 2012). Nphp8, mutations in which can also cause MKS or JBTS, functionally interacts with members of both C. elegans modules and is required for the transition zone localization of MKS–JBTS proteins (Huang et al., 2011; Williams et al., 2011). Thus, Nphp8 connects both modules, functionally if not structurally. In other organisms, a similar role may be played by Cep290, a protein that is absent from C. elegans but is involved in human NPHP, MKS, and JBTS and is part of the MKS–JBTS complex (Garcia-Gonzalo et al., 2011; Sang et al., 2011). In the green alga Chlamydomonas reinhardtii, Cep290 is essential for Y link formation, indicating that, despite their evolutionary conservation, Y link assembly has species-specific requirements (Craige et al., 2010).
From the transition zone, the axoneme elongates until it reaches a stable length (Ishikawa and Marshall, 2011). As no protein synthesis occurs within the cilium, intraflagellar transport (IFT) must deliver axoneme components to the ciliary tip for assembly (Rosenbaum and Child, 1967). IFT is a bidirectional axoneme trafficking system driven by microtubule motors (Kinesin-2 and cytoplasmic Dynein propel the anterograde and retrograde directions, respectively) that are associated with two subcomplexes, IFT-A and -B (Pedersen and Rosenbaum, 2008). Although Kinesin-2 and IFT-B subunits are essential for axoneme formation, cytoplasmic Dynein and many IFT-A subunits are not, but disruptions in either cause cilia to become short and bulbous (Marszalek et al., 1999; Huangfu et al., 2003; Huangfu and Anderson, 2005; May et al., 2005; Pedersen and Rosenbaum, 2008; Tran et al., 2008; Ocbina et al., 2011; Qin et al., 2011). These data suggest that the IFT-B complex is required for anterograde IFT, which traffics tubulin subunits and other building blocks to the ciliary tip and is thus required for ciliogenesis, and that IFT-A complexes participate in retrograde IFT. These results do not exclude roles for IFT-B in retrograde trafficking or for IFT-A in the anterograde trafficking of some cargo.
Transition fibers may promote ciliogenesis by recruiting IFT components to the ciliary base (Deane et al., 2001; Ishikawa et al., 2005; Graser et al., 2007; Singla et al., 2010). Transition zone proteins are also required for ciliogenesis in some cell types of both C. elegans and mice (Garcia-Gonzalo et al., 2011; Williams et al., 2011). Because some transition zone proteins interact with IFT components, they might also help recruit them to the ciliary base (Zhao and Malicki, 2011). However, cell types that grow axonemes in the absence of specific transition zone components display normal levels of IFT proteins and normal IFT rates, indicating that this may not be the case (Garcia-Gonzalo et al., 2011; Williams et al., 2011).
The elongation of the axoneme requires a parallel expansion of the ciliary membrane. This expansion takes place at the plasma membrane in epithelial cells but occurs intracellularly in mesenchymal cells (Sorokin, 1962, 1968). In the latter case, the ciliary vesicle fuses with secondary vesicles, creating a ciliary sheath with an inner and an outer membrane (Fig. 1). In epithelial cells, ciliary membrane expansion may result from both vesicle fusion at the ciliary base and the lateral diffusion of lipids and proteins from the contiguous plasma membrane. Consistent with the intimate involvement of vesicle trafficking in ciliogenesis, several proteins involved in vesicle budding (AP-1 and Clathrin), targeting (Rab8, Rab11, and TRAPP), tethering (Exocyst), and fusion (SNAREs) participate in ciliogenesis (Yoshimura et al., 2007; Mazelova et al., 2009b; Zuo et al., 2009; Kaplan et al., 2010; Westlake et al., 2011).
Fusion of the ciliary sheath with the plasma membrane exposes the cilium to the extracellular space (Fig. 1). Because this process is topologically equivalent to other forms of exocytosis, externalizing the cilium likely requires exocytic machinery. Upon fusion, the outer membrane of the ciliary sheath becomes the periciliary membrane, a domain that continues to act as the docking region for cilium-bound vesicles and thus plays an important role in the homeostasis of mature cilia (Bouck, 1971; Peters et al., 1983; Papermaster et al., 1985; Nachury et al., 2010).
How are ciliary membrane and axoneme extension coordinated? One regulator of this coordination may be Broad minded (Bromi; Ko et al., 2010). A Bromi mouse mutation causes an expansion of the ciliary membrane, within which the axoneme is curled, consistent with miscoordination of axoneme–membrane attachment or growth. Bromi acts via cell cycle–related kinase (CCRK), whose disruption recapitulates the Bromi phenotype. Interestingly, mutations in the C. reinhardtii CCRK orthologue lead to one flagellum being longer than the other (Tam et al., 2007). Thus, CCRK may have a conserved role in controlling the size of the ciliary axoneme and membrane.
Ciliary protein trafficking
Trafficking of receptors and signal transducers to primary cilia is critical for ciliary function and is disrupted in ciliopathies (Berbari et al., 2008a; Garcia-Gonzalo et al., 2011; Lancaster et al., 2011). Furthermore, ciliary signaling often involves regulated trafficking of select proteins into and out of cilia. A good example is vertebrate Hedgehog signaling. Sonic hedgehog binds to its receptor Patched, causing it to exit the cilium and allowing Smoothened to enter, which in turn affects the ciliary accumulation and activity of Gli transcription factors (Corbit et al., 2005; Haycraft et al., 2005; Rohatgi et al., 2007; Kim et al., 2009). Similarly, regulated trafficking of signaling proteins into or out of C. reinhardtii flagella is required for mating and phototaxis (Pan et al., 2003; Wang et al., 2006; Huang et al., 2007; Lechtreck et al., 2009). In addition, ciliary protein trafficking can modulate signaling sensitivity. For example, retinal photoreceptors adapt to darker environments by increasing Transducin and decreasing Arrestin in their outer segments, which are specialized cilia (Calvert et al., 2006). Thus, understanding how proteins reach the ciliary base and how they enter and accumulate in the cilium is essential to understanding cilium-based intercellular communication.
Trafficking of soluble proteins to the ciliary base.
Cytosolic proteins should be able to reach the ciliary base by diffusion. However, transport to Xenopus laevis centrioles is at least threefold faster than diffusion, and several centriolar components require microtubules and the Dynein–Dynactin complex to reach centrioles (Young et al., 2000; Dammermann and Merdes, 2002; Quintyne and Schroer, 2002; Guo et al., 2006; Kodani et al., 2010). Furthermore, the ciliary localization of Gli2 requires cytoplasmic microtubules, the minus ends of which anchor to the subdistal appendages of the basal body (Mogensen et al., 2000; Kim et al., 2009).
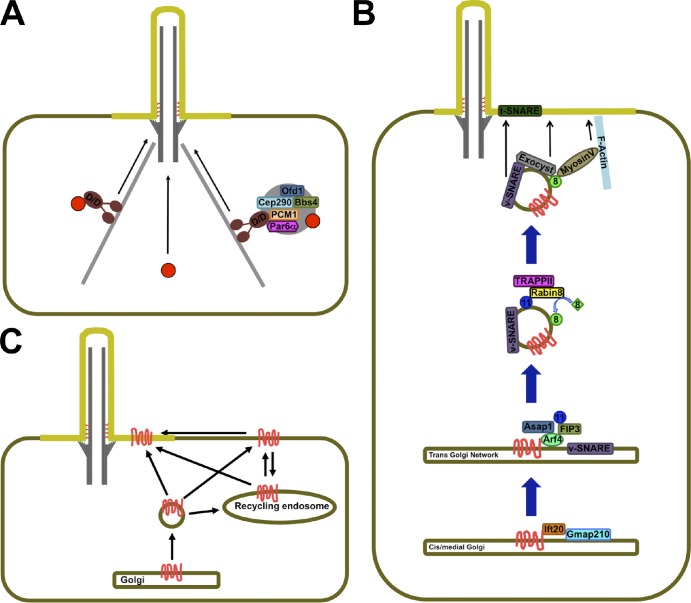
Interestingly, some of the proteins involved in this microtubule-dependent pathway, including PCM-1 and Par6-α, accumulate in centriolar satellites, electron-dense granules that surround centrioles (Kubo et al., 1999; Dammermann and Merdes, 2002; Kodani et al., 2010). These satellites are also observed in the vicinity of ciliary basal bodies (Kubo et al., 1999; Mogensen et al., 2000; Kunimoto et al., 2012). Several ciliopathy proteins, including Ofd1, Cep290, and the BBSome complex, localize to centriolar satellites as well as the cilium (Nachury et al., 2007; Kim et al., 2008, 2009; Lopes et al., 2011). Collectively, these data suggest a role for minus end–directed microtubule traffic of soluble proteins toward the ciliary base and the use of satellites as way stations for some (Fig. 3 A).
Figure 3.
Trafficking to the ciliary base. (A) Soluble proteins (red circles) may reach the ciliary base by diffusion (center) or travel as cargo on minus end–directed microtubule motors, such as the Dynein–Dynactin complex (D/D; left). (right) Trafficking of soluble cargo may also involve centriolar satellites, large protein aggregates that may serve as assembly points, and way stations for cilium-bound proteins. (B) Trafficking of some transmembrane proteins (red) along the Golgi may be aided by IFT20, which may then hand them off to Arf4. Arf4 orchestrates the formation of cargo-containing cilium-bound vesicles that contain active Rab11 (blue), whose effector Rabin8 recruits active Rab8 (green) to the vesicle surface. Rab8 in turn recruits effectors that mediate the vesicle’s approach, tethering, and fusion with the periciliary membrane. (C) Transmembrane proteins (red) may reach the cilium laterally from the plasma membrane or aboard vesicles that fuse with the periciliary membrane. Cilia-bound vesicles may derive from the Golgi or from recycling endosomes, which themselves may receive input from the Golgi and plasma membrane.
Trafficking of soluble proteins into the cilium.
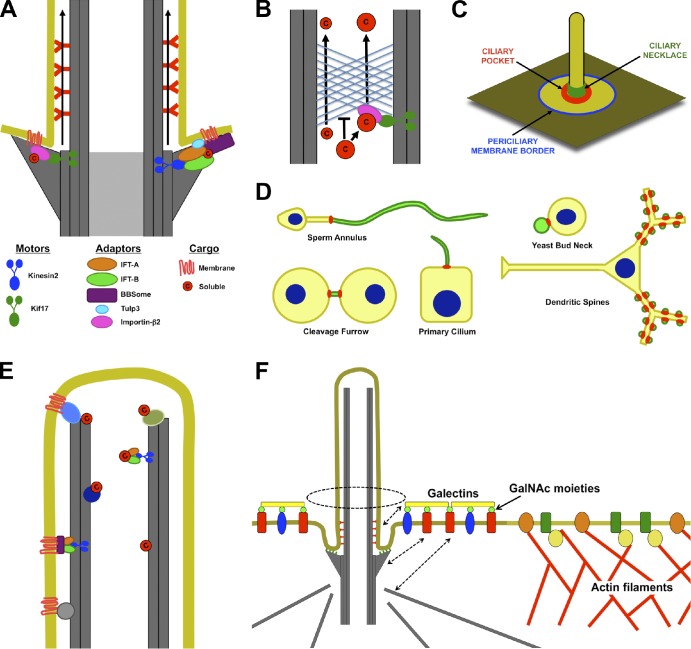
Two large complexes involved in flagellar motility, the outer Dynein arms and the radial spoke complex, both require IFT to enter flagella, suggesting that there is a size limit for entry into the cilium that is overcome using IFT (Fig. 4, A and B; Qin et al., 2004; Hou et al., 2007). However, the molecular mass barrier to ciliary entry may be high; in rods, single (27 kD), tandem (54 kD), and triple GFP (81 kD) all reach the outer segments, and mathematical modeling suggests they diffuse freely through the connecting cilia (Calvert et al., 2010; Najafi et al., 2012).
Figure 4.
Access into and maintenance of distinct ciliary compartments. (A) Both soluble and transmembrane proteins may need to associate directly or indirectly with plus end–directed microtubule motors (Kinesin-2 or Kif17) to enter the cilium. The entrance may be between adjacent transition fibers. (B) Like the nuclear pore, the ciliary base can exclude proteins on the basis of their size. Thus, small proteins may diffuse into the cilium, but large proteins may need to associate with transport machinery to enter the cilium. (C) Potential locations for diffusion barriers preventing entrance of membrane proteins into cilia include the border between the plasma and periciliary membranes (blue), the bottom of the ciliary pocket where transition fibers anchor the basal body to the membrane (red), and the ciliary necklace (green). (D) Septin rings (red) form membrane diffusion barriers that define specific membrane compartments (green), including those of cilia, flagella, the midbody, dendritic spines, and yeast buds. (E) Soluble and membrane proteins may be retained inside cilia by directly or indirectly interacting with microtubules. (F) Selective exclusion and retention can account for differences in periciliary and plasma membrane composition. The cortical actin cytoskeleton is excluded from the region under the periciliary membrane. As a result, membrane proteins that directly or indirectly interact with actin filaments are excluded from the periciliary region. Conversely, certain proteins, such as Galectin-3, that accumulate in the periciliary region may be retained by extracytosolic interactions with the ciliary membrane or by interactions with the basal body or its associated microtubules (dashed arrows). c, cargo; GalNAc, N-acetyl-galactosamine.
Alternatively, different cell types may have different requirements for ciliary entry. Although photoreceptor connecting cilia structurally resemble the transition zones of other cilia, connecting cilia may be optimized for fast protein exchange to adapt to changes in illumination (Calvert et al., 2006). Dextrans of 40 kD and larger fail to enter cilia of nonphotoreceptor cells, whereas dextrans 10 kD or smaller readily enter, suggesting that the cilia of some cell types have low size exclusion limits (Kee et al., 2012).
Given that the basal body lumen is filled with electron-dense material, the only path available for soluble proteins to access the cilium may be between adjacent transition fibers (Fisch and Dupuis-Williams, 2011; Brito et al., 2012). These spaces, ∼60 nm at their widest, could theoretically fit large protein complexes but not vesicles (Nachury et al., 2010). However, the actual exclusion limit for these spaces may be lower and remains unknown. A size exclusion barrier might also reside at the transition zone, where Y links may regulate soluble protein entry (Menco, 1980). In addition to acting as size exclusion filters, these physical barriers may also function as “smart” filters, allowing passage of large proteins only if they contain ciliary localization sequences (CLSs).
Recently, it has been suggested that ciliary and nuclear entry share several characteristics (Fig. 4 B; Dishinger et al., 2010; Kee et al., 2012). The nuclear pore excludes proteins greater than ∼30 kD but allows passage of larger proteins if bound to importins or exportins (Cook et al., 2007). Importins and exportins directly interact with and transiently displace the phenylalanine-glycine repeat nucleoporins, whose natively unfolded repeats form a meshwork that occludes the nuclear pore lumen (Hoelz et al., 2011). In this way, importins and exportins catalyze the nucleocytoplasmic transport of their cargos, which they recognize via NLSs or nuclear export sequences (NESs), respectively. Giving direction to the process is a concentration gradient of the GTP-bound form of the small GTPase Ran, which accumulates inside the nucleus where it stimulates cargo dissociation from importins and cargo association with exportins. The Ran-GTP gradient is in turn generated by the asymmetric distribution of RCC1, the chromatin-associated exchange factor that generates Ran-GTP, and RanGAP, the cytosolic enzyme that generates Ran-GDP (Cook et al., 2007).
Surprisingly, importin binding is also required for some proteins to accumulate inside cilia (Fan et al., 2007; Dishinger et al., 2010; Hurd et al., 2011). One such protein is the kinesin Kif17, whose ciliary localization depends on importin-β2 binding to an NLS-like CLS in Kif17 (Dishinger et al., 2010). Indeed, replacing the CLS in Kif17 with a bona fide NLS supports its ciliary entry, and Kif17 mutants containing the CLS, but lacking the kinesin motor domain, accumulate in the nucleus instead of the cilium (Dishinger et al., 2010). Furthermore, grafting the Kif17 CLS onto a nonciliary kinesin causes the resulting fusion protein to go to the cilium (Dishinger et al., 2010). Therefore, ciliary localization of Kif17 requires both importin binding and an additional activity present in its motor domain, possibly plus end–directed microtubule motility. Kif17 is in turn required for the olfactory cyclic nucleotide–gated (CNG) channel to accumulate in cilia, supporting the hypothesis that kinesin–importin complexes transport other proteins into cilia (Jenkins et al., 2006).
Like importin-β2, Ran is implicated in ciliary trafficking. A GTP-locked form of Ran causes Kif17 to move from the ciliary tip to less distal ciliary positions until it disappears from the cilium altogether (Dishinger et al., 2010). Interestingly, knockdown of RanBP1, a RanGAP cofactor present in the cilium, leads to an accumulation of ciliary Ran-GTP and also causes Kif17 to leave the ciliary tip (Fan et al., 2011). These results can potentially be explained by effects of Ran-GTP on the balance between anterograde and retrograde trafficking of Kif17 along ciliary microtubules or by changes in microtubule dynamics, which Ran-GTP is known to regulate (Keryer et al., 2003; Mishra et al., 2010; Fan et al., 2011; Halpin et al., 2011). Together with the fact that a GDP-locked Ran mutant that disrupts nucleocytoplasmic transport does not affect the ciliary localization of Kif17, these data suggest that the ciliary functions of Ran differ from those in nucleocytoplasmic transport (Dishinger et al., 2010). Future experiments that address how Ran-GTP levels in the cilium are controlled, the function of nucleoporins at the ciliary base (Kee et al., 2012), and whether exportins regulate ciliary exit will clarify the extent to which trafficking through the ciliary base and nuclear pores resemble each other.
Intraciliary retention of soluble proteins.
The steady-state accumulation of a protein within a cilium can be achieved by either promoting its entry or blocking its exit. Analogous to ciliary entry, a protein’s ciliary exit rate reflects two parameters: (1) how frequently it reaches the ciliary base from within the cilium, and (2) how efficiently it crosses the ciliary base. Many ciliary components directly or indirectly interact with microtubules, limiting their access to the ciliary base and retarding their exit (Sloboda and Howard, 2007). Also, a slow rate of exit across the ciliary base may cause some proteins to be retained at the transition zone or in the compartment above it where Inversin localizes (Shiba et al., 2009; Sang et al., 2011).
Trafficking of membrane proteins to the ciliary base.
Transmembrane proteins start their lives in the endoplasmic reticulum, from which they travel to the Golgi apparatus. After reaching the TGN, they are sorted into vesicles bound to different subcellular destinations. The plasma membrane acts as the default destination of TGN-derived vesicles, but targeting sequences on the transmembrane cargo proteins can drive vesicles to other compartments (Gu et al., 2001; Baker et al., 2008). In particular, CLSs on transmembrane proteins help recruit ciliary trafficking components that guide these vesicles to the periciliary membrane. For example, a VxPx motif in the cytosolic tail of Rhodopsin, a light-activated G protein–coupled receptor (GPCR), recruits the small GTPase Arf4 to the TGN, leading to the formation of a complex that mediates Rhodopsin incorporation into cilium-bound vesicles (Fig. 3 B). This complex includes ASAP1, a protein that may couple cargo recruitment and vesicle formation, Rab11, which participates in ciliary vesicle trafficking, and Arfophilin-1/FIP3, which is a dual Arf/Rab11 interactor and may hence allow Rab11 recruitment to Arf4-containing vesicles (Mazelova et al., 2009a).
Like Rhodopsin, ciliary targeting of Polycystin-1 and -2, two transmembrane proteins involved in polycystic kidney disease, is dependent on Arf4-binding CLSs that contain VxPx motifs and, at least for Polycystin-1, also depends on ASAP1 and Rab11 (Geng et al., 2006; Ward et al., 2011). Similarly, the CNGB1b subunit of the olfactory CNG channel contains a VxPx CLS (Jenkins et al., 2006). Therefore, Rhodopsin, Polycystins, and the CNG channel appear to use a common ciliary targeting mechanism. Formation of these cilia-bound vesicles may also involve clathrin coats and AP-1 adaptors, as these are required to move ODR-10, another GPCR, to C. elegans sensory cilia (Dwyer et al., 2001; Kaplan et al., 2010). As clathrin and AP-1 are essential for budding of endosome-bound vesicles from the TGN, endosome- and cilium-bound vesicles may form by similar mechanisms, or some ciliary proteins may travel to cilia via endosomes (Fig. 3 C; Bonifacino and Traub, 2003; Kaplan et al., 2010).
Like Clathrin and AP-1, other proteins may have specialized roles in ciliary trafficking. For example, IFT20, a component of the IFT-B complex involved in ciliogenesis, also localizes to Golgi cisternae, post-Golgi vesicles, and basal bodies (Follit et al., 2006, 2008; Sedmak and Wolfrum, 2010). This localization suggests that IFT20 may have roles in moving proteins from the Golgi to the basal body, and indeed, moderate knockdown of IFT20 inhibits the ciliary localization of Polycystin-2 (Follit et al., 2006). GMAP210, a protein required for the Golgi but not basal body localization of IFT20, also promotes the ciliary localization of Polycystin-2, consistent with a trafficking role for IFT20 in the Golgi (Follit et al., 2008). Similarly, IFT20 is required in rod cells for the efficient transport of Rhodopsin out of the Golgi (Keady et al., 2011). IFT20 interacts with the cytosolic tail of Rhodopsin but not through the VxPx motif (Keady et al., 2011). It will be interesting to see whether IFT20 binds to other ciliary membrane proteins, such as Polycystin-1 and -2, to target them to cilia.
Intriguingly, IFT20 localizes predominantly to the cis- and medial-Golgi cisternae and not the TGN, where most transmembrane proteins are sorted for delivery to post-Golgi compartments (Follit et al., 2006). One possibility is that IFT20 accompanies its cargo as it moves through the cis- and medial-Golgi and hands it off to Arf4 at the TGN (Fig. 3 B). Alternatively, IFT20 might facilitate transport from the cis-Golgi to the cilium, bypassing the TGN, as has been proposed for Polycystin-2 (Hoffmeister et al., 2011).
Post-Golgi trafficking of cilium-bound vesicles involves Rab11, whose active form recruits and activates the Rab8–guanine nucleotide exchange factor Rabin8, which in turn recruits Rab8 and TRAPPII, a vesicle-trafficking complex (Knödler et al., 2010; Westlake et al., 2011). Active Rab8 then recruits effectors, including the Exocyst complex and possibly MyosinV, that tether the vesicle to the periciliary membrane (Fig. 3 B; Deretic et al., 2004; Ishikawa et al., 2005; Roland et al., 2007; Omori et al., 2008; Mazelova et al., 2009b; Jin et al., 2011). The Exocyst complex facilitates the pairing of cognate SNARE proteins, leading to vesicle fusion with the periciliary membrane (Mazelova et al., 2009b). In addition to delivering membrane proteins to the ciliary base, this same pathway drives ciliary membrane expansion during ciliogenesis, indicating that both processes rely on the same machinery (He and Guo, 2009; Mazelova et al., 2009b; Zuo et al., 2009; Follit et al., 2010; Kaplan et al., 2010; Hoffmeister et al., 2011; Ward et al., 2011; Westlake et al., 2011).
Vesicles containing cilium-bound membrane proteins may not always travel directly from the Golgi to the periciliary membrane (Fig. 3 C). A possible way station is recycling endosomes, which contain Rab8, Rab11, and other cilia regulators (Finetti et al., 2009; Kaplan et al., 2010; Kim et al., 2010a). In other instances the cilium is reached via the plasma membrane, as is the case of Smoothened (Milenkovic et al., 2009; Wang et al., 2009).
Trafficking of membrane proteins into the cilium.
One way for peripheral membrane proteins to enter the cilium is by associating with proteins that mask their hydrophobic moieties, thereby allowing them to behave like their cytosolic counterparts. For example, two N-myristoylated proteins, Nphp3 and Cystin, rely on Unc119b, a soluble myristoyl-binding protein, for transport to the cilium (Wright et al., 2011). Interestingly, Unc119b cannot enter the cilium unless bound to its cargo (Wright et al., 2011; Zhang et al., 2011; Nakata et al., 2012). Upon ciliary entry, Arl3 dissociates the Unc119b–cargo complex, allowing the myristoylated cargo to associate with the ciliary membrane (Wright et al., 2011). Similarly, Pde6d, an Unc119b-related Arl3 effector, binds and helps bring prenylated proteins into photoreceptor outer segments (Zhang et al., 2004, 2007). Therefore, recognition and masking of lipid modifications may be a general mechanism for transporting peripheral membrane proteins to cilia (Cevik et al., 2010; Emmer et al., 2010; Evans et al., 2010; Follit et al., 2010; Maric et al., 2011).
Unlike peripheral membrane proteins, transmembrane proteins must enter cilia by moving laterally from the periciliary membrane, as membrane extraction or vesicle entry into cilia seems unlikely. Membrane diffusion barriers separate the ciliary and periciliary membranes in at least some cell types, indicating that lateral transport between these two membranes must be facilitated by machinery that engages CLSs in the cargo (Hu et al., 2010; Chih et al., 2012). For example, ciliary GPCRs, including Mchr1, Sstr3, and Htr6, rely on a CLS in their third intracellular loop to enter cilia (Berbari et al., 2008a). Ciliary entry of these GPCRs requires direct binding of this CLS to the BBSome, a complex including most Bardet–Biedl syndrome–associated proteins (Nachury et al., 2007; Berbari et al., 2008b; Jin et al., 2010; Seo et al., 2011). The BBSome also interacts with the IFT machinery, whose microtubule motors may provide the driving force needed for GPCRs to enter cilia (Ou et al., 2007; Lechtreck et al., 2009).
More generally, direct or indirect association with microtubule motors may be the sine qua non for transmembrane proteins to cross the ciliary base. If so, different combinations of motors, adaptors, and CLSs may control ciliary entry and exit (Fig. 4 A). As motors, Kinesin II and Kif17 mediate ciliary entry, whereas cytoplasmic Dynein 2 functions during exit (Jenkins et al., 2006; Ishikawa and Marshall, 2011). Adaptors for these motors may include IFT proteins, the BBSome, and Tulp3 (Ou et al., 2007; Lechtreck et al., 2009; Mukhopadhyay et al., 2010). Tulp3 interacts with IFT-A components and, like the BBSome, is required for the ciliary accumulation of Sstr3 and Mchr1 (Berbari et al., 2008b; Mukhopadhyay et al., 2010). However, Tulp3 is not known to interact with the CLSs of these receptors, and its role in repressing Hedgehog signaling indicates that it has additional functions (Cameron et al., 2009; Norman et al., 2009; Patterson et al., 2009).
The main adaptor for Kif17 may be importin-β2, which binds to and is required for the ciliary targeting of several membrane proteins (Fan et al., 2007; Hurd et al., 2011). Another Kif17 adaptor may be Ankyrin G, which interacts with a CLS on CNGB1b, the ciliary targeting of which is blocked by dominant-negative Kif17 (Jenkins et al., 2006; Kizhatil et al., 2009). The presence of two separate CLSs in CNGB1b, the VxPx-containing motif and the Ankyrin-binding sequence, raises the possibility that packaging into cilium-bound vesicles and entry from the periciliary membrane represent separate decisions. Thus, cilium-bound cargo proteins and vesicle-trafficking components may be separated at the periciliary membrane, with the nonciliary vesicular components being retrieved from the periciliary membrane by endocytosis. Accordingly, the periciliary membrane, which when invaginated is called the ciliary pocket, is an active site of clathrin-dependent endocytosis (Gadelha et al., 2009; Molla-Herman et al., 2010).
What is the nature of the membrane diffusion barrier at the ciliary base? Two recent studies have identified a Septin ring and members of the MKS–JBTS transition zone complex as components of this barrier (Hu et al., 2010; Chih et al., 2012).
Septins are small GTPases that interact with membranes and can polymerize into filaments and rings (Weirich et al., 2008). These rings form barriers to membrane diffusion in many contexts, from the yeast bud neck to dendritic spines (Fig. 4, C and D; Caudron and Barral, 2009). In sperm, the Septin4-based annulus forms a barrier that controls flagellar composition (Ihara et al., 2005; Kissel et al., 2005; Kwitny et al., 2010). Similarly, Septins 2 and 7 are part of a ring and restrict membrane protein diffusion at the base of primary cilia (Hu et al., 2010; Kim et al., 2010b). Interestingly, the sperm annulus assembles from the flagellar pocket (Molla-Herman et al., 2010; Shang et al., 2010). Hence, the ciliary pocket, or more generally the periciliary membrane, may play an analogous role in the formation of the ciliary Septin ring. Consistent with this hypothesis, the ciliary pocket is a docking site for actin filaments, which interact with Septins and modulate Septin ring formation (Weirich et al., 2008; Molla-Herman et al., 2010; Kim et al., 2011).
Similar to Septins, components of the MKS–JBTS transition zone complex also restrict diffusion of membrane proteins across the ciliary base, thus allowing ciliary membrane proteins to accumulate in cilia while keeping plasma membrane proteins out (Dowdle et al., 2011; Garcia-Gonzalo et al., 2011; Chih et al., 2012). In C. elegans, defects in ciliary composition have only been detected upon disruption of both MKS–JBTS and NPHP complex members (Williams et al., 2011). However, whether these complexes collaborate to form a membrane diffusion barrier at the transition zones of other organisms is not yet clear.
The Septin barrier and the transition zone barrier may be interdependent or even one and the same, as Septin2 is required for the transition zone localization of several MKS–JBTS complex proteins, including B9d1, Cc2d2a, and Tmem231 (Chih et al., 2012). Because Tectonic1, a core MKS–JBTS complex component, is not required for Septin2 to form a ring at the ciliary base, the membrane diffusion barrier may assemble sequentially, with the Septin ring forming first (Garcia-Gonzalo et al., 2011). Where exactly this barrier lies is not yet clear, but it is likely to be in the region of the transition fibers and the Y links. Consistent with this hypothesis, C. elegans cilia lacking Y links and transition fibers fail to exclude plasma membrane proteins from cilia, and C. reinhardtii flagella of Cep290 mutants lack Y links and fail to accumulate flagellar membrane proteins (Craige et al., 2010; Williams et al., 2011). In addition, Cep290 mutant flagella possess increased levels of IFT and BBSome components, suggesting a role for Y links in controlling the ciliary localization of soluble proteins as well (Craige et al., 2010).
Intraciliary and extraciliary retention of membrane proteins.
As with soluble proteins, intraciliary retention is one means by which membrane-associated proteins can accumulate in cilia (Fig. 4 E). For instance, the ciliary levels of CEACAM1, a transmembrane protein, increase when the protein is conjugated to a microtubule-binding domain (Francis et al., 2011). However, the extent to which similar mechanisms operate in vivo is not yet clear.
Analogously, extraciliary retention may explain how some membrane proteins are excluded from cilia. This may be especially important in photoreceptors, where the outer segment acts as the default destination for transmembrane proteins, perhaps reflecting the massive amount of transport through connecting cilia, each of which traffics ∼1,000 Rhodopsin molecules per second (Besharse et al., 1977; Baker et al., 2008; Gospe et al., 2010). For example, nonciliary transmembrane proteins, such as Glut1 or Syntaxin3, use targeting sequences to be kept outside of the outer segment (Baker et al., 2008; Gospe et al., 2010). These results raise the question of why Rhodopsin requires its CLS. Perhaps this CLS increases the efficiency of Rhodopsin transport to the cilium, rather than being strictly required for it.
Another example of ciliary exclusion is the transmembrane protein Podocalyxin, which is restricted from reaching the ciliary base of MDCK cells by its association with the cortical actin cytoskeleton (Francis et al., 2011). When its ties to the actin cytoskeleton are severed, Podocalyxin readily enters the periciliary and ciliary membranes, bringing into question the existence of diffusion barriers at the ciliary base (Francis et al., 2011). One possible explanation is that, in these experiments, Podocalyxin reached the nascent ciliary membrane before diffusion barrier formation, as Podocalyxin was expressed before cilia formed (Breslow and Nachury, 2011). Alternatively, it is possible that Podocalyxin contains a CLS that allows it to enter cilia when detached from actin. Regardless, these data raise the possibility that ciliary barriers depend on the cellular differentiation state or the cell type.
Similar retention mechanisms may also account for why some proteins, such as Galectin-3, localize to the periciliary membrane but are excluded from the ciliary and plasma membranes (Vieira et al., 2006). Because Galectin-3 is secreted from cells and cross-links glycosylated proteins on the cell surface, its retention to the periciliary region may depend on interactions with unidentified periciliary transmembrane proteins, whose localization may in turn depend on associations with structures at the ciliary base (Fig. 4 F; Partridge et al., 2004; Ohtsubo et al., 2005; Huang 2010). Therefore, the distinct periciliary membrane identity could arise from a combination of retention and exclusion mechanisms even in the absence of a diffusion barrier separating it from the plasma membrane (Fig. 4 C). Additional mechanisms, such as selective removal, may also aid in the generation of a distinct periciliary membrane. For instance, Galectin-3–mediated cross-linking can prevent endocytosis of cell surface proteins, which might facilitate their accumulation in the periciliary membrane (Partridge et al., 2004; Winckler and Mellman, 2010).
How to reconcile the evidence for selective exclusion, retention, and diffusion barriers, all of which can control ciliary protein localization? Each of these mechanisms may apply to some, but not all, ciliary proteins, and some of these proteins may be subject to multiple mechanisms of ciliary localization control. This diversity of mechanisms may allow the cilium to maintain a unique composition despite not being bounded by the membrane.
Conclusion
The cilium is a specialized organelle whose function critically depends on its composition. To control which proteins enter and exit cilia, cells regulate protein entrance across its base, the only region not surrounded by a membrane. The ciliary base acts as a selective filter, allowing passage of proteins with specific biophysical properties or that associate with transporters. Cells also control ciliary composition by controlling which proteins reach the ciliary base. Although soluble proteins do so by diffusion or by traveling along microtubules, transmembrane proteins must incorporate into cilium-bound vesicles leaving the Golgi or enter indirectly via the plasma membrane or recycling endosomes. In addition to these selective targeting mechanisms, selective exclusion and retention also control the composition of cilia and may allow the periciliary membrane to maintain its unique identity. Thus, the complex architecture of the cilium allows for multiple independent regulatory mechanisms that control its composition and allow its function to emerge from its form.
Acknowledgments
We thank the members of the Reiter laboratory for helpful discussions.
This work was supported by grants from the National Institutes of Health (AR054396 and GM095941), the Burroughs Wellcome Fund, the Packard Foundation, and the Sandler Family Supporting Foundation.
Footnotes
Abbreviations used in this paper:
- CLS
- ciliary localization sequence
- CNG
- cyclic nucleotide gated
- GPCR
- G protein–coupled receptor
- IFT
- intraflagellar transport
- JBTS
- Joubert syndrome
- MKS
- Meckel syndrome
- NES
- nuclear export sequence
- NPHP
- nephronophthisis
References
- Anderson R.G. 1972. The three-dimensional structure of the basal body from the rhesus monkey oviduct. J. Cell Biol. 54:246–265 10.1083/jcb.54.2.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S.A., Haeri M., Yoo P., Gospe S.M., III, Skiba N.P., Knox B.E., Arshavsky V.Y. 2008. The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J. Cell Biol. 183:485–498 10.1083/jcb.200806009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari N.F., Lewis J.S., Bishop G.A., Askwith C.C., Mykytyn K. 2008a. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl. Acad. Sci. USA. 105:4242–4246 10.1073/pnas.0711027105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari N.F., Johnson A.D., Lewis J.S., Askwith C.C., Mykytyn K. 2008b. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol. Biol. Cell. 19:1540–1547 10.1091/mbc.E07-09-0942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse J.C., Hollyfield J.G., Rayborn M.E. 1977. Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. J. Cell Biol. 75:507–527 10.1083/jcb.75.2.507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood R.A. 2010. Sensory reception is an attribute of both primary cilia and motile cilia. J. Cell Sci. 123:505–509 10.1242/jcs.066308 [DOI] [PubMed] [Google Scholar]
- Boisvieux-Ulrich E., Laine M.C., Sandoz D. 1989. In vitro effects of taxol on ciliogenesis in quail oviduct. J. Cell Sci. 92:9–20 [DOI] [PubMed] [Google Scholar]
- Bonifacino J.S., Traub L.M. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395–447 10.1146/annurev.biochem.72.121801.161800 [DOI] [PubMed] [Google Scholar]
- Bornens M. 2012. The centrosome in cells and organisms. Science. 335:422–426 10.1126/science.1209037 [DOI] [PubMed] [Google Scholar]
- Bouck G.B. 1971. The structure, origin, isolation, and composition of the tubular mastigonemes of the Ochromas flagellum. J. Cell Biol. 50:362–384 10.1083/jcb.50.2.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow D.K., Nachury M.V. 2011. Primary cilia: how to keep the riff-raff in the plasma membrane. Curr. Biol. 21:R434–R436 10.1016/j.cub.2011.04.039 [DOI] [PubMed] [Google Scholar]
- Brito D.A., Gouveia S.M., Bettencourt-Dias M. 2012. Deconstructing the centriole: structure and number control. Curr. Opin. Cell Biol. 24:4–13 10.1016/j.ceb.2012.01.003 [DOI] [PubMed] [Google Scholar]
- Calvert P.D., Strissel K.J., Schiesser W.E., Pugh E.N., Jr, Arshavsky V.Y. 2006. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 16:560–568 10.1016/j.tcb.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Calvert P.D., Schiesser W.E., Pugh E.N., Jr 2010. Diffusion of a soluble protein, photoactivatable GFP, through a sensory cilium. J. Gen. Physiol. 135:173–196 10.1085/jgp.200910322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D.A., Pennimpede T., Petkovich M. 2009. Tulp3 is a critical repressor of mouse hedgehog signaling. Dev. Dyn. 238:1140–1149 10.1002/dvdy.21926 [DOI] [PubMed] [Google Scholar]
- Caudron F., Barral Y. 2009. Septins and the lateral compartmentalization of eukaryotic membranes. Dev. Cell. 16:493–506 10.1016/j.devcel.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. 2010. Origin of the cell nucleus, mitosis and sex: roles of intracellular coevolution. Biol. Direct. 5:7 10.1186/1745-6150-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevik S., Hori Y., Kaplan O.I., Kida K., Toivenon T., Foley-Fisher C., Cottell D., Katada T., Kontani K., Blacque O.E. 2010. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J. Cell Biol. 188:953–969 10.1083/jcb.200908133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B., Liu P., Chinn Y., Chalouni C., Komuves L.G., Hass P.E., Sandoval W., Peterson A.S. 2012. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat. Cell Biol. 14:61–72 10.1038/ncb2410 [DOI] [PubMed] [Google Scholar]
- Cook A., Bono F., Jinek M., Conti E. 2007. Structural biology of nucleocytoplasmic transport. Annu. Rev. Biochem. 76:647–671 10.1146/annurev.biochem.76.052705.161529 [DOI] [PubMed] [Google Scholar]
- Corbit K.C., Aanstad P., Singla V., Norman A.R., Stainier D.Y., Reiter J.F. 2005. Vertebrate Smoothened functions at the primary cilium. Nature. 437:1018–1021 10.1038/nature04117 [DOI] [PubMed] [Google Scholar]
- Craige B., Tsao C.C., Diener D.R., Hou Y., Lechtreck K.F., Rosenbaum J.L., Witman G.B. 2010. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 190:927–940 10.1083/jcb.201006105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann A., Merdes A. 2002. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 159:255–266 10.1083/jcb.200204023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport J.R., Watts A.J., Roper V.C., Croyle M.J., van Groen T., Wyss J.M., Nagy T.R., Kesterson R.A., Yoder B.K. 2007. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 17:1586–1594 10.1016/j.cub.2007.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane J.A., Cole D.G., Seeley E.S., Diener D.R., Rosenbaum J.L. 2001. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 11:1586–1590 10.1016/S0960-9822(01)00484-5 [DOI] [PubMed] [Google Scholar]
- Deretic D., Traverso V., Parkins N., Jackson F., Rodriguez de Turco E.B., Ransom N. 2004. Phosphoinositides, ezrin/moesin, and rac1 regulate fusion of rhodopsin transport carriers in retinal photoreceptors. Mol. Biol. Cell. 15:359–370 10.1091/mbc.E03-04-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishinger J.F., Kee H.L., Jenkins P.M., Fan S., Hurd T.W., Hammond J.W., Truong Y.N., Margolis B., Martens J.R., Verhey K.J. 2010. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat. Cell Biol. 12:703–710 10.1038/ncb2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle W.E., Robinson J.F., Kneist A., Sirerol-Piquer M.S., Frints S.G.M., Corbit K.C., Zaghloul N.A., van Lijnschoten G., Mulders L., Verver D.E., et al. 2011. Disruption of a ciliary B9 protein complex causes Meckel syndrome. Am. J. Hum. Genet. 89:94–110 10.1016/j.ajhg.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer N.D., Adler C.E., Crump J.G., L’Etoile N.D., Bargmann C.I. 2001. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 31:277–287 10.1016/S0896-6273(01)00361-0 [DOI] [PubMed] [Google Scholar]
- Emmer B.T., Maric D., Engman D.M. 2010. Molecular mechanisms of protein and lipid targeting to ciliary membranes. J. Cell Sci. 123:529–536 10.1242/jcs.062968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R.J., Schwarz N., Nagel-Wolfrum K., Wolfrum U., Hardcastle A.J., Cheetham M.E. 2010. The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum. Mol. Genet. 19:1358–1367 10.1093/hmg/ddq012 [DOI] [PubMed] [Google Scholar]
- Fan S., Fogg V., Wang Q., Chen X.W., Liu C.J., Margolis B. 2007. A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin β interactions. J. Cell Biol. 178:387–398 10.1083/jcb.200609096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S., Whiteman E.L., Hurd T.W., McIntyre J.C., Dishinger J.F., Liu C.J., Martens J.R., Verhey K.J., Sajjan U., Margolis B. 2011. Induction of Ran GTP drives ciliogenesis. Mol. Biol. Cell. 22:4539–4548 10.1091/mbc.E11-03-0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finetti F., Paccani S.R., Riparbelli M.G., Giacomello E., Perinetti G., Pazour G.J., Rosenbaum J.L., Baldari C.T. 2009. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat. Cell Biol. 11:1332–1339 10.1038/ncb1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch C., Dupuis-Williams P. 2011. Ultrastructure of cilia and flagella - back to the future! Biol. Cell. 103:249–270 10.1042/BC20100139 [DOI] [PubMed] [Google Scholar]
- Fliegauf M., Horvath J., von Schnakenburg C., Olbrich H., Müller D., Thumfart J., Schermer B., Pazour G.J., Neumann H.P., Zentgraf H., et al. 2006. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J. Am. Soc. Nephrol. 17:2424–2433 10.1681/ASN.2005121351 [DOI] [PubMed] [Google Scholar]
- Follit J.A., Tuft R.A., Fogarty K.E., Pazour G.J. 2006. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell. 17:3781–3792 10.1091/mbc.E06-02-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit J.A., San Agustin J.T., Xu F., Jonassen J.A., Samtani R., Lo C.W., Pazour G.J. 2008. The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet. 4:e1000315 10.1371/journal.pgen.1000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit J.A., Li L., Vucica Y., Pazour G.J. 2010. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J. Cell Biol. 188:21–28 10.1083/jcb.200910096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S.S., Sfakianos J., Lo B., Mellman I. 2011. A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J. Cell Biol. 193:219–233 10.1083/jcb.201009001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadelha C., Rothery S., Morphew M., McIntosh J.R., Severs N.J., Gull K. 2009. Membrane domains and flagellar pocket boundaries are influenced by the cytoskeleton in African trypanosomes. Proc. Natl. Acad. Sci. USA. 106:17425–17430 10.1073/pnas.0909289106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalo F.R., Corbit K.C., Sirerol-Piquer M.S., Ramaswami G., Otto E.A., Noriega T.R., Seol A.D., Robinson J.F., Bennett C.L., Josifova D.J., et al. 2011. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat. Genet. 43:776–784 10.1038/ng.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L., Okuhara D., Yu Z., Tian X., Cai Y., Shibazaki S., Somlo S. 2006. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J. Cell Sci. 119:1383–1395 10.1242/jcs.02818 [DOI] [PubMed] [Google Scholar]
- Gilula N.B., Satir P. 1972. The ciliary necklace. A ciliary membrane specialization. J. Cell Biol. 53:494–509 10.1083/jcb.53.2.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz S.C., Anderson K.V. 2010. The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 11:331–344 10.1038/nrg2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospe S.M., III, Baker S.A., Arshavsky V.Y. 2010. Facilitative glucose transporter Glut1 is actively excluded from rod outer segments. J. Cell Sci. 123:3639–3644 10.1242/jcs.072389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graser S., Stierhof Y.D., Lavoie S.B., Gassner O.S., Lamla S., Le Clech M., Nigg E.A. 2007. Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 179:321–330 10.1083/jcb.200707181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F., Crump C.M., Thomas G. 2001. Trans-Golgi network sorting. Cell. Mol. Life Sci. 58:1067–1084 10.1007/PL00000922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Yang Z., Song W., Chen Q., Wang F., Zhang Q., Zhu X. 2006. Nudel contributes to microtubule anchoring at the mother centriole and is involved in both dynein-dependent and -independent centrosomal protein assembly. Mol. Biol. Cell. 17:680–689 10.1091/mbc.E05-04-0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin D., Kalab P., Wang J., Weis K., Heald R. 2011. Mitotic spindle assembly around RCC1-coated beads in Xenopus egg extracts. PLoS Biol. 9:e1001225 10.1371/journal.pbio.1001225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.G., Kim H.J., Dlugosz A.A., Ellison D.W., Gilbertson R.J., Alvarez-Buylla A. 2009. Dual and opposing roles of primary cilia in medulloblastoma development. Nat. Med. 15:1062–1065 10.1038/nm.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft C.J., Banizs B., Aydin-Son Y., Zhang Q., Michaud E.J., Yoder B.K. 2005. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 1:e53 10.1371/journal.pgen.0010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Guo W. 2009. The exocyst complex in polarized exocytosis. Curr. Opin. Cell Biol. 21:537–542 10.1016/j.ceb.2009.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F., Benzing T., Katsanis N. 2011. Ciliopathies. N. Engl. J. Med. 364:1533–1543 10.1056/NEJMra1010172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelz A., Debler E.W., Blobel G. 2011. The structure of the nuclear pore complex. Annu. Rev. Biochem. 80:613–643 10.1146/annurev-biochem-060109-151030 [DOI] [PubMed] [Google Scholar]
- Hoffmeister H., Babinger K., Gürster S., Cedzich A., Meese C., Schadendorf K., Osten L., de Vries U., Rascle A., Witzgall R. 2011. Polycystin-2 takes different routes to the somatic and ciliary plasma membrane. J. Cell Biol. 192:631–645 10.1083/jcb.201007050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Qin H., Follit J.A., Pazour G.J., Rosenbaum J.L., Witman G.B. 2007. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J. Cell Biol. 176:653–665 10.1083/jcb.200608041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Milenkovic L., Jin H., Scott M.P., Nachury M.V., Spiliotis E.T., Nelson W.J. 2010. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 329:436–439 10.1126/science.1191054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.L. 2010. Regulation of ion channels by secreted Klotho: mechanisms and implications. Kidney Int. 77:855–860 10.1038/ki.2010.73 [DOI] [PubMed] [Google Scholar]
- Huang K., Diener D.R., Mitchell A., Pazour G.J., Witman G.B., Rosenbaum J.L. 2007. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J. Cell Biol. 179:501–514 10.1083/jcb.200704069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Szymanska K., Jensen V.L., Janecke A.R., Innes A.M., Davis E.E., Frosk P., Li C., Willer J.R., Chodirker B.N., et al. 2011. TMEM237 is mutated in individuals with a Joubert syndrome related disorder and expands the role of the TMEM family at the ciliary transition zone. Am. J. Hum. Genet. 89:713–730 10.1016/j.ajhg.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D., Anderson K.V. 2005. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA. 102:11325–11330 10.1073/pnas.0505328102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D., Liu A., Rakeman A.S., Murcia N.S., Niswander L., Anderson K.V. 2003. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 426:83–87 10.1038/nature02061 [DOI] [PubMed] [Google Scholar]
- Hufnagel L.A. 1983. Freeze-fracture analysis of membrane events during early neogenesis of cilia in Tetrahymena: changes in fairy-ring morphology and membrane topography. J. Cell Sci. 60:137–156 [DOI] [PubMed] [Google Scholar]
- Hurd T.W., Fan S., Margolis B.L. 2011. Localization of retinitis pigmentosa 2 to cilia is regulated by Importin beta2. J. Cell Sci. 124:718–726 10.1242/jcs.070839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M., Kinoshita A., Yamada S., Tanaka H., Tanigaki A., Kitano A., Goto M., Okubo K., Nishiyama H., Ogawa O., et al. 2005. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev. Cell. 8:343–352 10.1016/j.devcel.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Marshall W.F. 2011. Ciliogenesis: building the cell’s antenna. Nat. Rev. Mol. Cell Biol. 12:222–234 10.1038/nrm3085 [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Kubo A., Tsukita S., Tsukita S. 2005. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat. Cell Biol. 7:517–524 10.1038/ncb1251 [DOI] [PubMed] [Google Scholar]
- Jenkins P.M., Hurd T.W., Zhang L., McEwen D.P., Brown R.L., Margolis B., Verhey K.J., Martens J.R. 2006. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr. Biol. 16:1211–1216 10.1016/j.cub.2006.04.034 [DOI] [PubMed] [Google Scholar]
- Jiang S.T., Chiou Y.Y., Wang E., Lin H.K., Lee S.P., Lu H.Y., Wang C.K., Tang M.J., Li H. 2008. Targeted disruption of Nphp1 causes male infertility due to defects in the later steps of sperm morphogenesis in mice. Hum. Mol. Genet. 17:3368–3379 10.1093/hmg/ddn231 [DOI] [PubMed] [Google Scholar]
- Jiang S.T., Chiou Y.Y., Wang E., Chien Y.L., Ho H.H., Tsai F.J., Lin C.Y., Tsai S.P., Li H. 2009. Essential role of nephrocystin in photoreceptor intraflagellar transport in mouse. Hum. Mol. Genet. 18:1566–1577 10.1093/hmg/ddp068 [DOI] [PubMed] [Google Scholar]
- Jin H., White S.R., Shida T., Schulz S., Aguiar M., Gygi S.P., Bazan J.F., Nachury M.V. 2010. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 141:1208–1219 10.1016/j.cell.2010.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Sultana A., Gandhi P., Franklin E., Hamamoto S., Khan A.R., Munson M., Schekman R., Weisman L.S. 2011. Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev. Cell. 21:1156–1170 10.1016/j.devcel.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan O.I., Molla-Herman A., Cevik S., Ghossoub R., Kida K., Kimura Y., Jenkins P., Martens J.R., Setou M., Benmerah A., Blacque O.E. 2010. The AP-1 clathrin adaptor facilitates cilium formation and functions with RAB-8 in C. elegans ciliary membrane transport. J. Cell Sci. 123:3966–3977 10.1242/jcs.073908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keady B.T., Le Y.Z., Pazour G.J. 2011. IFT20 is required for opsin trafficking and photoreceptor outer segment development. Mol. Biol. Cell. 22:921–930 10.1091/mbc.E10-09-0792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee H.L., Dishinger J.F., Blasius T.L., Liu C.J., Margolis B., Verhey K.J. 2012. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat. Cell Biol. 14:431–437 10.1038/ncb2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keryer G., Di Fiore B., Celati C., Lechtreck K.F., Mogensen M., Delouvee A., Lavia P., Bornens M., Tassin A.M. 2003. Part of Ran is associated with AKAP450 at the centrosome: involvement in microtubule-organizing activity. Mol. Biol. Cell. 14:4260–4271 10.1091/mbc.E02-11-0773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Krishnaswami S.R., Gleeson J.G. 2008. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum. Mol. Genet. 17:3796–3805 10.1093/hmg/ddn277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kato M., Beachy P.A. 2009. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc. Natl. Acad. Sci. USA. 106:21666–21671 10.1073/pnas.0912180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lee J.E., Heynen-Genel S., Suyama E., Ono K., Lee K., Ideker T., Aza-Blanc P., Gleeson J.G. 2010a. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 464:1048–1051 10.1038/nature08895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.S., Froese C.D., Estey M.P., Trimble W.S. 2011. SEPT9 occupies the terminal positions in septin octamers and mediates polymerization-dependent functions in abscission. J. Cell Biol. 195:815–826 10.1083/jcb.201106131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.K., Shindo A., Park T.J., Oh E.C., Ghosh S., Gray R.S., Lewis R.A., Johnson C.A., Attie-Bittach T., Katsanis N., Wallingford J.B. 2010b. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 329:1337–1340 10.1126/science.1191184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissel H., Georgescu M.M., Larisch S., Manova K., Hunnicutt G.R., Steller H. 2005. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev. Cell. 8:353–364 10.1016/j.devcel.2005.01.021 [DOI] [PubMed] [Google Scholar]
- Kizhatil K., Baker S.A., Arshavsky V.Y., Bennett V. 2009. Ankyrin-G promotes cyclic nucleotide-gated channel transport to rod photoreceptor sensory cilia. Science. 323:1614–1617 10.1126/science.1169789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knödler A., Feng S., Zhang J., Zhang X., Das A., Peränen J., Guo W. 2010. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc. Natl. Acad. Sci. USA. 107:6346–6351 10.1073/pnas.1002401107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H.W., Norman R.X., Tran J., Fuller K.P., Fukuda M., Eggenschwiler J.T. 2010. Broad-minded links cell cycle-related kinase to cilia assembly and hedgehog signal transduction. Dev. Cell. 18:237–247 10.1016/j.devcel.2009.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Dynlacht B.D. 2011. Regulating the transition from centriole to basal body. J. Cell Biol. 193:435–444 10.1083/jcb.201101005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodani A., Tonthat V., Wu B., Sütterlin C. 2010. Par6 alpha interacts with the dynactin subunit p150 Glued and is a critical regulator of centrosomal protein recruitment. Mol. Biol. Cell. 21:3376–3385 10.1091/mbc.E10-05-0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A., Sasaki H., Yuba-Kubo A., Tsukita S., Shiina N. 1999. Centriolar satellites: Molecular characterization, ATP-dependent movement toward centrioles and possible involvement in ciliogenesis. J. Cell Biol. 147:969–980 10.1083/jcb.147.5.969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto K., Yamazaki Y., Nishida T., Shinohara K., Ishikawa H., Hasegawa T., Okanoue T., Hamada H., Noda T., Tamura A., et al. 2012. Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell. 148:189–200 10.1016/j.cell.2011.10.052 [DOI] [PubMed] [Google Scholar]
- Kwitny S., Klaus A.V., Hunnicutt G.R. 2010. The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis. Biol. Reprod. 82:669–678 10.1095/biolreprod.109.079566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Gopal D.J., Kim J., Saleem S.N., Silhavy J.L., Louie C.M., Thacker B.E., Williams Y., Zaki M.S., Gleeson J.G. 2011. Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nat. Med. 17:726–731 10.1038/nm.2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck K.F., Johnson E.C., Sakai T., Cochran D., Ballif B.A., Rush J., Pazour G.J., Ikebe M., Witman G.B. 2009. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J. Cell Biol. 187:1117–1132 10.1083/jcb.200909183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes C.A., Prosser S.L., Romio L., Hirst R.A., O’Callaghan C., Woolf A.S., Fry A.M. 2011. Centriolar satellites are assembly points for proteins implicated in human ciliopathies, including oral-facial-digital syndrome 1. J. Cell Sci. 124:600–612 10.1242/jcs.077156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric D., McGwire B.S., Buchanan K.T., Olson C.L., Emmer B.T., Epting C.L., Engman D.M. 2011. Molecular determinants of ciliary membrane localization of Trypanosoma cruzi flagellar calcium-binding protein. J. Biol. Chem. 286:33109–33117 10.1074/jbc.M111.240895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek J.R., Ruiz-Lozano P., Roberts E., Chien K.R., Goldstein L.S. 1999. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc. Natl. Acad. Sci. USA. 96:5043–5048 10.1073/pnas.96.9.5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May S.R., Ashique A.M., Karlen M., Wang B., Shen Y., Zarbalis K., Reiter J., Ericson J., Peterson A.S. 2005. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev. Biol. 287:378–389 10.1016/j.ydbio.2005.08.050 [DOI] [PubMed] [Google Scholar]
- Mazelova J., Astuto-Gribble L., Inoue H., Tam B.M., Schonteich E., Prekeris R., Moritz O.L., Randazzo P.A., Deretic D. 2009a. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 28:183–192 10.1038/emboj.2008.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J., Ransom N., Astuto-Gribble L., Wilson M.C., Deretic D. 2009b. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J. Cell Sci. 122:2003–2013 10.1242/jcs.039982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen D.P., Jenkins P.M., Martens J.R. 2008. Olfactory cilia: our direct neuronal connection to the external world. Curr. Top. Dev. Biol. 85:333–370 10.1016/S0070-2153(08)00812-0 [DOI] [PubMed] [Google Scholar]
- Menco M. 1980. Qualitative and quantitative freeze-fracture studies on olfactory and respiratory epithelial surfaces of frog, ox, rat, and dog. IV. Ciliogenesis and ciliary necklaces (including high-voltage observations). Cell Tissue Res. 212:1–16 10.1007/BF00234028 [DOI] [PubMed] [Google Scholar]
- Milenkovic L., Scott M.P., Rohatgi R. 2009. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J. Cell Biol. 187:365–374 10.1083/jcb.200907126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R.K., Chakraborty P., Arnaoutov A., Fontoura B.M., Dasso M. 2010. The Nup107-160 complex and gamma-TuRC regulate microtubule polymerization at kinetochores. Nat. Cell Biol. 12:164–169 10.1038/ncb2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen M.M., Malik A., Piel M., Bouckson-Castaing V., Bornens M. 2000. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J. Cell Sci. 113:3013–3023 [DOI] [PubMed] [Google Scholar]
- Molla-Herman A., Ghossoub R., Blisnick T., Meunier A., Serres C., Silbermann F., Emmerson C., Romeo K., Bourdoncle P., Schmitt A., et al. 2010. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J. Cell Sci. 123:1785–1795 10.1242/jcs.059519 [DOI] [PubMed] [Google Scholar]
- Mollet G., Silbermann F., Delous M., Salomon R., Antignac C., Saunier S. 2005. Characterization of the nephrocystin/nephrocystin-4 complex and subcellular localization of nephrocystin-4 to primary cilia and centrosomes. Hum. Mol. Genet. 14:645–656 10.1093/hmg/ddi061 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S., Wen X., Chih B., Nelson C.D., Lane W.S., Scales S.J., Jackson P.K. 2010. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev. 24:2180–2193 10.1101/gad.1966210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury M.V., Loktev A.V., Zhang Q., Westlake C.J., Peränen J., Merdes A., Slusarski D.C., Scheller R.H., Bazan J.F., Sheffield V.C., Jackson P.K. 2007. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 129:1201–1213 10.1016/j.cell.2007.03.053 [DOI] [PubMed] [Google Scholar]
- Nachury M.V., Seeley E.S., Jin H. 2010. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu. Rev. Cell Dev. Biol. 26:59–87 10.1146/annurev.cellbio.042308.113337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi M., Maza N.A., Calvert P.D. 2012. Steric volume exclusion sets soluble protein concentrations in photoreceptor sensory cilia. Proc. Natl. Acad. Sci. USA. 109:203–208 10.1073/pnas.1115109109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K., Shiba D., Kobayashi D., Yokoyama T. 2012. Targeting of Nphp3 to the primary cilia is controlled by an N-terminal myristoylation site and coiled-coil domains. Cytoskeleton (Hoboken). 69:221–234 10.1002/cm.21014 [DOI] [PubMed] [Google Scholar]
- Norman R.X., Ko H.W., Huang V., Eun C.M., Abler L.L., Zhang Z., Sun X., Eggenschwiler J.T. 2009. Tubby-like protein 3 (TULP3) regulates patterning in the mouse embryo through inhibition of Hedgehog signaling. Hum. Mol. Genet. 18:1740–1754 10.1093/hmg/ddp113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocbina P.J., Eggenschwiler J.T., Moskowitz I., Anderson K.V. 2011. Complex interactions between genes controlling trafficking in primary cilia. Nat. Genet. 43:547–553 10.1038/ng.832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo K., Takamatsu S., Minowa M.T., Yoshida A., Takeuchi M., Marth J.D. 2005. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell. 123:1307–1321 10.1016/j.cell.2005.09.041 [DOI] [PubMed] [Google Scholar]
- Omori Y., Zhao C., Saras A., Mukhopadhyay S., Kim W., Furukawa T., Sengupta P., Veraksa A., Malicki J. 2008. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat. Cell Biol. 10:437–444 10.1038/ncb1706 [DOI] [PubMed] [Google Scholar]
- Ou G., Koga M., Blacque O.E., Murayama T., Ohshima Y., Schafer J.C., Li C., Yoder B.K., Leroux M.R., Scholey J.M. 2007. Sensory ciliogenesis in Caenorhabditis elegans: assignment of IFT components into distinct modules based on transport and phenotypic profiles. Mol. Biol. Cell. 18:1554–1569 10.1091/mbc.E06-09-0805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Misamore M.J., Wang Q., Snell W.J. 2003. Protein transport and signal transduction during fertilization in Chlamydomonas. Traffic. 4:452–459 10.1034/j.1600-0854.2003.00105.x [DOI] [PubMed] [Google Scholar]
- Papermaster D.S., Schneider B.G., Besharse J.C. 1985. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest. Ophthalmol. Vis. Sci. 26:1386–1404 [PubMed] [Google Scholar]
- Partridge E.A., Le Roy C., Di Guglielmo G.M., Pawling J., Cheung P., Granovsky M., Nabi I.R., Wrana J.L., Dennis J.W. 2004. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 306:120–124 10.1126/science.1102109 [DOI] [PubMed] [Google Scholar]
- Patterson V.L., Damrau C., Paudyal A., Reeve B., Grimes D.T., Stewart M.E., Williams D.J., Siggers P., Greenfield A., Murdoch J.N. 2009. Mouse hitchhiker mutants have spina bifida, dorso-ventral patterning defects and polydactyly: identification of Tulp3 as a novel negative regulator of the Sonic hedgehog pathway. Hum. Mol. Genet. 18:1719–1739 10.1093/hmg/ddp075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen L.B., Rosenbaum J.L. 2008. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 85:23–61 10.1016/S0070-2153(08)00802-8 [DOI] [PubMed] [Google Scholar]
- Peters K.R., Palade G.E., Schneider B.G., Papermaster D.S. 1983. Fine structure of a periciliary ridge complex of frog retinal rod cells revealed by ultrahigh resolution scanning electron microscopy. J. Cell Biol. 96:265–276 10.1083/jcb.96.1.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H., Diener D.R., Geimer S., Cole D.G., Rosenbaum J.L. 2004. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J. Cell Biol. 164:255–266 10.1083/jcb.200308132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Lin Y., Norman R.X., Ko H.W., Eggenschwiler J.T. 2011. Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components. Proc. Natl. Acad. Sci. USA. 108:1456–1461 10.1073/pnas.1011410108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne N.J., Schroer T.A. 2002. Distinct cell cycle–dependent roles for dynactin and dynein at centrosomes. J. Cell Biol. 159:245–254 10.1083/jcb.200203089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R., Milenkovic L., Scott M.P. 2007. Patched1 regulates hedgehog signaling at the primary cilium. Science. 317:372–376 10.1126/science.1139740 [DOI] [PubMed] [Google Scholar]
- Roland J.T., Kenworthy A.K., Peranen J., Caplan S., Goldenring J.R. 2007. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol. Biol. Cell. 18:2828–2837 10.1091/mbc.E07-02-0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J.L., Child F.M. 1967. Flagellar regeneration in protozoan flagellates. J. Cell Biol. 34:345–364 10.1083/jcb.34.1.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang L., Miller J.J., Corbit K.C., Giles R.H., Brauer M.J., Otto E.A., Baye L.M., Wen X., Scales S.J., Kwong M., et al. 2011. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 145:513–528 10.1016/j.cell.2011.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P., Mitchell D.R., Jékely G. 2008. How did the cilium evolve? Curr. Top. Dev. Biol. 85:63–82 10.1016/S0070-2153(08)00803-X [DOI] [PubMed] [Google Scholar]
- Sattler C.A., Staehelin L.A. 1974. Ciliary membrane differentiations in Tetrahymena pyriformis. Tetrahymena has four types of cilia. J. Cell Biol. 62:473–490 10.1083/jcb.62.2.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak T., Wolfrum U. 2010. Intraflagellar transport molecules in ciliary and nonciliary cells of the retina. J. Cell Biol. 189:171–186 10.1083/jcb.200911095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S., Zhang Q., Bugge K., Breslow D.K., Searby C.C., Nachury M.V., Sheffield V.C. 2011. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS Genet. 7:e1002358 10.1371/journal.pgen.1002358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang P., Baarends W.M., Hoogerbrugge J., Ooms M.P., van Cappellen W.A., de Jong A.A., Dohle G.R., van Eenennaam H., Gossen J.A., Grootegoed J.A. 2010. Functional transformation of the chromatoid body in mouse spermatids requires testis-specific serine/threonine kinases. J. Cell Sci. 123:331–339 10.1242/jcs.059949 [DOI] [PubMed] [Google Scholar]
- Shiba D., Yamaoka Y., Hagiwara H., Takamatsu T., Hamada H., Yokoyama T. 2009. Localization of Inv in a distinctive intraciliary compartment requires the C-terminal ninein-homolog-containing region. J. Cell Sci. 122:44–54 10.1242/jcs.037408 [DOI] [PubMed] [Google Scholar]
- Singla V., Romaguera-Ros M., Garcia-Verdugo J.M., Reiter J.F. 2010. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev. Cell. 18:410–424 10.1016/j.devcel.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda R.D., Howard L. 2007. Localization of EB1, IFT polypeptides, and kinesin-2 in Chlamydomonas flagellar axonemes via immunogold scanning electron microscopy. Cell Motil. Cytoskeleton. 64:446–460 10.1002/cm.20195 [DOI] [PubMed] [Google Scholar]
- Sorokin S. 1962. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 15:363–377 10.1083/jcb.15.2.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S.P. 1968. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell Sci. 3:207–230 [DOI] [PubMed] [Google Scholar]
- Tam L.W., Wilson N.F., Lefebvre P.A. 2007. A CDK-related kinase regulates the length and assembly of flagella in Chlamydomonas. J. Cell Biol. 176:819–829 10.1083/jcb.200610022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran P.V., Haycraft C.J., Besschetnova T.Y., Turbe-Doan A., Stottmann R.W., Herron B.J., Chesebro A.L., Qiu H., Scherz P.J., Shah J.V., et al. 2008. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat. Genet. 40:403–410 10.1038/ng.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reeuwijk J., Arts H.H., Roepman R. 2011. Scrutinizing ciliopathies by unraveling ciliary interaction networks. Hum. Mol. Genet. 20(R2):R149–R157 10.1093/hmg/ddr354 [DOI] [PubMed] [Google Scholar]
- Vieira O.V., Gaus K., Verkade P., Fullekrug J., Vaz W.L., Simons K. 2006. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc. Natl. Acad. Sci. USA. 103:18556–18561 10.1073/pnas.0608291103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierkotten J., Dildrop R., Peters T., Wang B., Rüther U. 2007. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 134:2569–2577 10.1242/dev.003715 [DOI] [PubMed] [Google Scholar]
- Wang Q., Pan J., Snell W.J. 2006. Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell. 125:549–562 10.1016/j.cell.2006.02.044 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhou Z., Walsh C.T., McMahon A.P. 2009. Selective translocation of intracellular Smoothened to the primary cilium in response to Hedgehog pathway modulation. Proc. Natl. Acad. Sci. USA. 106:2623–2628 10.1073/pnas.0812110106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton-Pitt S.R., Jauregui A.R., Li C., Wang J., Leroux M.R., Barr M.M. 2012. Ciliogenesis in Caenorhabditis elegans requires genetic interactions between ciliary middle segment localized NPHP-2 (inversin) and transition zone-associated proteins. J. Cell Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward H.H., Brown-Glaberman U., Wang J., Morita Y., Alper S.L., Bedrick E.J., Gattone V.H., II, Deretic D., Wandinger-Ness A. 2011. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol. Biol. Cell. 22:3289–3305 10.1091/mbc.E11-01-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirich C.S., Erzberger J.P., Barral Y. 2008. The septin family of GTPases: architecture and dynamics. Nat. Rev. Mol. Cell Biol. 9:478–489 10.1038/nrm2407 [DOI] [PubMed] [Google Scholar]
- Westlake C.J., Baye L.M., Nachury M.V., Wright K.J., Ervin K.E., Phu L., Chalouni C., Beck J.S., Kirkpatrick D.S., Slusarski D.C., et al. 2011. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc. Natl. Acad. Sci. USA. 108:2759–2764 10.1073/pnas.1018823108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C.L., Winkelbauer M.E., Schafer J.C., Michaud E.J., Yoder B.K. 2008. Functional redundancy of the B9 proteins and nephrocystins in Caenorhabditis elegans ciliogenesis. Mol. Biol. Cell. 19:2154–2168 10.1091/mbc.E07-10-1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C.L., Masyukova S.V., Yoder B.K. 2010. Normal ciliogenesis requires synergy between the cystic kidney disease genes MKS-3 and NPHP-4. J. Am. Soc. Nephrol. 21:782–793 10.1681/ASN.2009060597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C.L., Li C., Kida K., Inglis P.N., Mohan S., Semenec L., Bialas N.J., Stupay R.M., Chen N., Blacque O.E., et al. 2011. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J. Cell Biol. 192:1023–1041 10.1083/jcb.201012116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B., Mellman I. 2010. Trafficking guidance receptors. Cold Spring Harb. Perspect. Biol. 2:a001826 10.1101/cshperspect.a001826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelbauer M.E., Schafer J.C., Haycraft C.J., Swoboda P., Yoder B.K. 2005. The C. elegans homologs of nephrocystin-1 and nephrocystin-4 are cilia transition zone proteins involved in chemosensory perception. J. Cell Sci. 118:5575–5587 10.1242/jcs.02665 [DOI] [PubMed] [Google Scholar]
- Won J., Marín de Evsikova C., Smith R.S., Hicks W.L., Edwards M.M., Longo-Guess C., Li T., Naggert J.K., Nishina P.M. 2011. NPHP4 is necessary for normal photoreceptor ribbon synapse maintenance and outer segment formation, and for sperm development. Hum. Mol. Genet. 20:482–496 10.1093/hmg/ddq494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.Y., Seol A.D., So P.L., Ermilov A.N., Bichakjian C.K., Epstein E.H., Jr, Dlugosz A.A., Reiter J.F. 2009. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat. Med. 15:1055–1061 10.1038/nm.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K.J., Baye L.M., Olivier-Mason A., Mukhopadhyay S., Sang L., Kwong M., Wang W., Pretorius P.R., Sheffield V.C., Sengupta P., et al. 2011. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev. 25:2347–2360 10.1101/gad.173443.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S., Egerer J., Fuchs E., Haas A.K., Barr F.A. 2007. Functional dissection of Rab GTPases involved in primary cilium formation. J. Cell Biol. 178:363–369 10.1083/jcb.200703047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A., Dictenberg J.B., Purohit A., Tuft R., Doxsey S.J. 2000. Cytoplasmic dynein-mediated assembly of pericentrin and gamma tubulin onto centrosomes. Mol. Biol. Cell. 11:2047–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Liu X.H., Zhang K., Chen C.K., Frederick J.M., Prestwich G.D., Baehr W. 2004. Photoreceptor cGMP phosphodiesterase delta subunit (PDEdelta) functions as a prenyl-binding protein. J. Biol. Chem. 279:407–413 10.1074/jbc.M306559200 [DOI] [PubMed] [Google Scholar]
- Zhang H., Li S., Doan T., Rieke F., Detwiler P.B., Frederick J.M., Baehr W. 2007. Deletion of PrBP/delta impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments. Proc. Natl. Acad. Sci. USA. 104:8857–8862 10.1073/pnas.0701681104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Constantine R., Vorobiev S., Chen Y., Seetharaman J., Huang Y.J., Xiao R., Montelione G.T., Gerstner C.D., Davis M.W., et al. 2011. UNC119 is required for G protein trafficking in sensory neurons. Nat. Neurosci. 14:874–880 10.1038/nn.2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Malicki J. 2011. Nephrocystins and MKS proteins interact with IFT particle and facilitate transport of selected ciliary cargos. EMBO J. 30:2532–2544 10.1038/emboj.2011.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X., Guo W., Lipschutz J.H. 2009. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol. Biol. Cell. 20:2522–2529 10.1091/mbc.E08-07-0772 [DOI] [PMC free article] [PubMed] [Google Scholar]