Abstract
Key decisions one makes in a lifetime include whether and how often to reproduce, what role to play in the community and, under certain conditions, whether to live or die. Similar decisions are also made at the level of cells: whether to divide, what fate to assume in the multicellular context of metazoan development and, under certain conditions, whether to live or to die. The pro-apoptotic gene hid plays an important role in the execution of cell death in Drosophila. Here, we review the various levels of control that exist to regulate Hid according to the life-or-death choice of a cell.
Keywords: Drosophila, Apoptosis, hid
Drosophila Hid belongs to a family of four pro-apoptotic proteins, Hid (Head involution defective), Grim, Reaper and Sickle, which are collectively known as RHG proteins (Reviewed in [1–3]). RHG proteins act by binding to and neutralizing IAPs (Inhibitor of Apoptosis Proteins) and also by lowering the level of the latter. This results in caspase activation and apoptosis (Fig. 1). Binding to IAPs is mediated by an N-terminal IAP-binding motif (IBM). Outside the IMB domain of RHG proteins, limited sequence similarities have been noted within a “Grim Helix 3” motif of ~15 amino acids and a “Trp-block” of ~30 amino acids that includes the GH3 motif [1, 4–6]. The GH3 domain of Grim is required for its apoptotic function and can induce apoptosis when overexpressed in Drosophila cell culture [4]. The regions of Rpr and Hid that contain the GH3 domains are important for localization of these proteins to the mitochondria and to induce cell killing [7–9]. The ability to bind and antagonize IAPs to induce apoptosis is shared between Drosophila RHG proteins and their mammalian counterparts, Smac (Second Mitochondria-derived Activator of Caspases)/DIABLO and Omi/HtrA2 proteins. Ectopic expression of Drosophila RHG genes can induce apoptosis in Drosophila and in mammalian cell culture (reviewed in [1]).
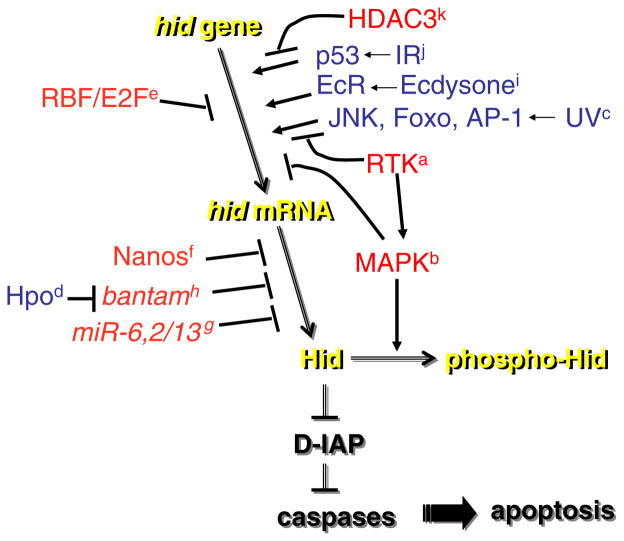
Fig. 1.
A summary of mechanisms that regulate hid. Hid neutralizes Drosophila IAP to cause caspase activation and apoptosis. Multiple mechanisms regulate hid expression. We have not distinguished between direct and indirect action of activators (blue) or repressors (red) in the diagram. Regulation of mitochondrial localization and regulation of Hid where the mechanism is not understood are not depicted. See text for details and reference to the superscript on each mechanism. (Color figure online)
The genes encoding the RHG proteins are linked at a single ~300 kb locus on chromosome III. Homozygotes of a chromosomal deletion H99 that removes rpr, hid and grim lack most cell death that occurs during normal Drosophila development and die as embryos [10]. While rpr and grim are expressed only in cells that are destined to die, hid mRNA is expressed in more cells than are destined to die (for example [11, 12]). Thus, in addition to transcriptional regulation, post-transcriptional repression of hid may play a role in keeping hid-expressing cells alive. This review focuses on published studies that illustrate different mechanisms by which hid is regulated.
Developmental regulation of hid
Hid expression, sub-cellular localization and activity are regulated during embryogenesis, larval development and metamorphosis. In these contexts, Hid is regulated by EGFR/RAS signaling, Hippo tumor suppressor pathway, microRNAs and the hormone Ecdysone.
Cell–cell interaction in the regulation of hid transcript levels
During Drosophila development, receptor tyrosine kinase (RTK) signaling through EGFR represses hid to allow the survival of pupal retinal cells, larval eye imaginal disc cells and embryonic midline glia [13–17]. In each case, short range cell–cell signaling via EGFR allows the survival of immediate neighbors while more distant cells are culled by apoptosis, thus allowing for precise regulation of cell number. Genetic analysis places EGFR acting upstream of hid to prevent apoptosis. Studies during embryogenesis and eye development point to possible mechanisms by which EGFR inhibits hid [17–19] (Fig. 1a, b). EGFR signaling results in the activation of RAS, which can signal through a number of different effectors. Of these, genetic evidence implicates RAF/MAPK and PI3 Kinase/Akt, but not Rel, as downstream effectors of RAS in inhibition of Hid [17–19]. RAF/MAPK appears to inhibit hid at both transcript and post-translational levels. Gain of function mutations in RAS or RAF and overexpression of Pointed, a transcription factor targeted by RAS/MAPK, result in reduced hid mRNA levels in the embryo [18]. It is not known how PI3 Kinase/Akt repress hid during eye development, but there is a precedent for a transcriptional repression mechanism in the case of UV irradiation where RAS represses hid transcription by acting through PI3-Kinase and Akt to inhibit Foxo [20] (c in Fig. 1).
A recent study implicates the Hpo tumor suppressor pathway in developmental programmed cell death (PCD) in the eye (d in Fig. 1). PCD during eye development specifically removes inter-ommatidial cells of the eye disc that are initially produced in excess via mitotic proliferation and later culled during the pupal stages. These are the same cells that rely on EGFR signaling to survive. In mutants in the Hpo pathway, inter-ommatidial cells show a reduction in hid transcript levels and fail to undergo PCD. Thus Hpo normally acts to promote hid expression [21], which is then opposed by EGF signaling. How Hpo promotes hid expression remains to be determined, but bantam miRNA may provide a link. Hpo signaling is known to repress ban [22, 23], while ban is known to inhibit Hid expression as discussed below.
hid transcripts are also induced upon cell competition during organ formation. Cell competition occurs when cells of different growth rates (due to difference in ribosomal protein gene dosage) or cells expressing different levels of the proto-oncogene homolog MYC are juxtaposed. In the wing imaginal disc, the faster growing or higher MYC expressing ‘winners’ cause cell death in the ‘losers’ by inducing hid transcripts [24]. Cell competition requires p53 and the initiator caspase Dronc, but exactly how these activities result in hid transcript accumulation in loser cells remains to be determined.
RBF1 and E2F limit hid expression in a context-dependent manner (e in Fig. 1)
The Drosophila genome encodes two homologs of the transcription factor E2F, E2F1 and E2F2 (reviewed in [25, 26]). Each E2F protein must form a complex with a Dp co-factor to bind DNA with high affinity. There is a single Dp homolog in Drosophila. Genetic analysis indicates that E2F represses hid transcription [27, 28]. Mutations in Dp, which are expected to disable both E2F1 and E2F2, result in elevated hid mRNA. This phenotype is restricted to the narrow band of ‘Zone of Non-Proliferating Cells’ at the dorsal/ventral boundary of wing imaginal discs. In Drosophila S2 cells, E2F1 binds to the hid enhancer and represses hid expression while E2F2 has a relatively minor effect. The hid enhancer contains E2F consensus sequences that are occupied by E2F1 and, to a lesser extent, E2F2 in Chromatin IP experiments in eye-antennal discs [29]. These results suggest that E2F1 in complex with Dp, rather than E2F2, represses hid in the ZNC. The relationship between hid and E2F/Dp may be different, however, outside the ZNC; hid expression appears normal outside the ZNC in the wing disc and in extracts of eye discs in Dp mutants.
RB proteins bind to E2F/Dp complexes and repress transcription. Of the two Drosophila RB-like proteins, RBF1 associates with E2F1 and RBF2 associates with E2F2 [30, 31]. Genetic evidence indicates that RBF1 cooperates with E2F to repress hid. RBF1 mutants show elevated hid mRNA in extracts of whole eye discs or in mutant clones [29]. A hid mutant was isolated in a forward genetic screen for suppressors of apoptosis in RBF1 mutants. A transcriptional reporter linked to the hid enhancer shows elevated expression in rbf1 mutants throughout eye and wing imaginal discs. These findings support a model wherein RBF1, via E2F binding sites in the hid enhancer, limits hid expression in eye and wing discs. This model remains to be reconciled with the above-described findings that Dp mutants express hid normally in the eye disc and in the wing disc outside the ZNC [27, 28]. Nonetheless, the emerging view from these studies is that where E2F1/Dp or RBF1 are found to have an effect on hid expression, the effect is inhibitory. The role of the second Drosophila RBF homolog, RBF2, in hid expression has not been addressed.
Regulation of Hid protein expression
The 3′UTR of hid is over 2 kilo-bases long and mediates the repression of Hid protein expression by at least two different mechanisms. In embryos from nanos mutant mothers, more primordial germ cells (called “pole cells”) accumulate Hid protein and undergo apoptosis in a hid-dependent manner. nos encodes an RNA binding protein. The 3′UTR of hid includes a Nos response element (NRE). Deletion of the NRE allows Hid protein accumulation and apoptosis in the pole cells of embryos from wild type mothers. These data suggest a model where Nos normally binds to hid 3′UTR to repress Hid translation, thereby preventing apoptosis in the future germline [32] (f in Fig. 1).
In addition to Nos, several miRNAs have been implicated in limiting Hid expression via sequences in the 3′UTR (g, h in Fig. 1). Embryos in which miR-6 and miR-2/13 have been neutralized with antisense oligo injection display widespread apoptosis, in part due to the elevated levels of Hid protein. Increased expression after miR-6 depletion in embryos is also seen for a GFP reporter with hid 3′UTR [33]. Similar neutralization of bantam miRNA in embryos results in elevated apoptosis in the embryo and smaller imaginal discs in the larvae [33]. These results are consistent with the phenotype of ban mutants and the effects of ban overexpression, which indicate that ban promotes cell proliferation while inhibiting apoptosis [34, 35]. A ban target that is relevant for apoptosis is Hid. Ectopic ban also represses apoptosis caused by overexpression of Hid or a Hid mutant refractory to phosphorylation and inhibition by MAPK, without altering hid transcript levels; thus repression of hid by ban is post-transcriptional, at least under overexpression conditions [35]. The hid 3′UTR contains five putative ban target sites. Ectopic ban represses a GFP reporter in which GFP coding sequences are followed by the hid 3′UTR. Furthermore, this repression is dependent on the presence of ban target sites in the hid 3′UTR.
ban has also been found to repress hid in two other contexts: after exposure to ionizing radiation (IR) and in mutants in the Drosophila Rb homolog, RBF1. We discuss RBF1 mutants here and regulation after IR exposure in a later section. In the eye imaginal discs of RBF1 mutants, hid mRNA is elevated throughout the disc, but Hid protein and apoptosis are elevated only near the Morphogenetic Furrow (MF), a narrow column of cells in which photo-receptor differentiation commences [29]. Simultaneously mutating ban allows apoptosis to occur in RBF1 mutant clones that are far from the MF. Thus ban may limit Hid protein accumulation in eye imaginal cells.
The primary mechanism of action of miRNAs in animal cells was initially thought to be translational repression of the target mRNAs, but this view is being modified as the targets are also found to become destabilized [36]. In the regulation of hid by miRNAs, relative contribution of translational repression and mRNA destabilization remains to be investigated.
Regulation of Hid expression in the embryo may also occur at the level of the translational machinery itself [37]. During apoptosis and upon heat shock, cap-dependent translation is diminished. However, hid mRNA associates with polysome fractions after heat shock, suggesting that hid can be translated in a cap-independent manner in response to cellular stress. The hid 5′UTR contains an internal ribosome entry site (IRES). Mutants in the cap-binding protein eIF4E have increased apoptosis. These data suggest a model wherein hid translation is repressed via the mRNA cap, but the repression is bypassed in response to stress by cap-independent translation of hid.
Sub-cellular localization of Hid
Once expressed, Hid protein may be limited in its activity by phosphorylation and the requirement for mitochondrial localization. Hid contains at least two functional domains; an N-terminal domain for binding to IAPs and a C-terminal mitochondrial localization domain. Hid co-localizes with mitochondrial markers when expressed in human cells [38]. In Drosophila eye imaginal discs, Hid protein levels are elevated in RBF1 mutants as discussed above [29]. Elevated Hid is peri-nuclear, suggestive of mitochondrial localization. Elevated Hid is diffused throughout the cytoplasm if cells are double mutants for RBF1 and the initiator caspase, Dronc. This suggests that caspase activity is required for mitochondrial localization of Hid protein. The mechanistic basis for this requirement remains to be understood.
The requirement for Dronc in mitochondrial localization of Hid can explain the finding that caspase activity is needed for mitochondrial fragmentation that results from Hid overexpression in S2 cells. In S2 cells, Hid co-localizes with Cytochrome C and, when overexpressed, induces mitochondrial fragmentation and Cytochrome C release [9]. Caspase activity, although insufficient on its own, is necessary for mitochondrial fragmentation or Cytochrome C release. These results agree with the requirement for Dronc in Hid localization in the eye disc, suggesting that caspase activity is needed for mitochondrial localization of Hid in both S2 cells and larval imaginal discs. Deletion of 20 amino acids from the C-terminus, which includes a GH3 motif [6], compromised Hid’s ability to co-localize with Cytochrome C, to disrupt the mitochondria and to induce caspase activation.
Taken together, these results suggest that caspase activity is necessary to localize Hid to the mitochondria and that mitochondrial localization of Hid allows efficient mitochondrial fragmentation and caspase activation.
Phosphorylation by MAPK (b in Fig. 1)
Genetic analysis identified EGFR signaling through RAS as an inhibitor of Hid function. We discussed in preceding paragraphs how RAS might act through MAPK and PI3K-Akt axis to suppress Hid transcript levels. Additional data indicate that RAS suppresses Hid by MAPK-dependent phosphorylation in embryos and during eye development. Hid contains 5 MAPK consensus sequences. Mutating either three or all five of these sites, to generate ‘3A’ or ‘5A’ mutants, respectively, renders Hid more efficient at killing S2 cells and refractory to rescue by constitutively active RAS or MAPK [19]. Constitutively active RAS or MAPK are also less able to rescue the small eye phenotype produced by ectopic expression of Hid-3A and 5A mutants than by wild type Hid. Repression of Hid by phosphorylation also operates in the midline glia of the embryo where MAPK is required for cell survival in response to survival signals from the neighboring axon [17]. MAPK becomes dispensable for midline glia cell survival in hid mutants, suggesting that the key requirement for MAPK is to repress hid. Ectopic expression of the Hid-5A mutant in the wild type background phenocopied MAPK mutants and resulted in the ablation of midline glial cells. Hid-5A mutant is expected to be refractory to phosphorylation by MAPK. Therefore, the role of MAPK in glial cell survival is likely to be through phosphorylation and inhibition of Hid.
Hid expression in response to ecdysone (i in Fig. 1)
Metamorphosis accompanies extensive death of polyploid larval cells such as those in the salivary gland. hid transcripts increase in dying cells in response to the steroid hormone Ecdysone that acts through its receptor, EcR. EcR can bind to and directly activate the transcription of several loci. EcR’s ability to induce hid transcription, however, is likely to be indirect because it requires new protein synthesis; hid induction by Ecdysone is abolished upon treatment with a protein synthesis inhibitor, cycloheximide [39].
In sum, during Drosophila development, tumor suppressor homolog Hpo induces the expression of hid to cull extra cells in the final stages of eye development while Ecdysone induces hid to kill larval cells during metamorphosis. In addition, cap-independent translation may induce Hid expression in response to heat stress. On the other hand, hid is antagonized by pro-survival signaling through EGFR/RAS, by miRNAs and by E2F1/RBF1. What is still missing from this picture is the mechanistic understanding of how hid transcription is regulated during normal embryo and larval development. As the name implies, head involution defective is required for proper sculpting of the embryonic head through PCD. During embryogenesis, hid transcripts are found in a dynamic and complex pattern that overlaps significantly but not entirely with regions of cell death [11]. We have yet to fully understand how transcription of hid is regulated to produce this pattern during embryogenesis.
Regulation of hid in response to DNA damage
Heterozygotes of a chromosomal deficiency, H99, that removes rpr, hid and grim are viable and are able to undergo developmental cell death. H99 heterozygotes, however, are unable to induce apoptosis efficiently in response to external stimuli such as IR. Thus, the ability to induce apoptosis beyond the normal developmental level is sensitive to the dosage of pro-apoptotic genes [40]. More specifically, damaged-induced apoptosis appears sensitive to hid gene dosage. Heterozygotes of strong loss-of-function alleles of hid are also viable but are unable to induce apoptosis in response to external stimuli such as IR, suggesting that the level of hid gene products determines whether a cell lives or dies in response to damage [40]. In contrast, mutants with reduced rpr can still induce apoptosis in response to IR [28]. The importance of skl and grim in damage-induced apoptosis remains to be addressed. We discuss here mechanisms that are known to regulate hid expression or activity after exposure to radiation.
Changes in hid expression after radiation exposure
hid transcript levels increase after exposure to IR or UV, with the maximal increase ranging from 1.7- to 4-fold [28, 40, 41]. The increase in hid transcripts after IR exposure in embryos and larvae requires p53 and its presumptive activator, Chk2 kinase (j in Fig. 1). Despite the requirement for p53 in inducing hid, p53 has not been shown to directly activate transcription from the hid promoter. In contrast, p53 has been shown to bind consensus sequences in the rpr promoter and activate transcription after IR exposure [42].
The ability of cells to induce rpr and hid transcripts in response to IR is also subject to epigenetic regulation during embryogenesis. An IRER (Irradiation-Responsive Enhancer Region) resides upstream of the rpr gene and ~250 kb away from the hid gene [43]. Deletions that removed the IRER also abolished the IR-induced induction of hid and rpr transcripts. Interestingly, grim, which lies between hid and rpr, is not even induced by IR, suggesting that higher-order chromosome arrangements may allow the IRER to regulate hid. The requirement for IRER in the induction of skl by IR remains to be investigated.
As embryogenesis progresses beyond stage 12, the IRER acquires a more ‘closed’ chromatin state. Concomitantly, the induction of rpr and hid expression or apoptosis in response to IR becomes less robust [43]. Histone deacetylase 3 (HDAC3) and several other chromatin remodelers are required for the onset of changes in chromatin structure and accompanying reduction in IR-responsiveness of hid and rpr transcript levels (k in Fig. 1). This mode of epigenetic regulation may not be limited to embryos; mutants in HDAC3, but not HDAC1, show elevated hid mRNA expression and elevated spontaneous apoptosis in larval imaginal discs [44].
Expression of rpr and hid may also be linked in other ways. Overexpression of rpr in wing imaginal discs results in apoptosis but cells can be prevented from dying by co-expression of the viral caspase inhibitor, p35. hid transcripts are induced in such ‘undead’ cells but the mechanism remains to be investigated [45].
Drosophila imaginal discs also undergo p53-independent apoptosis after exposure to IR [41]. In this case, hid is induced 18 h after IR exposure in p53 null mutants, as opposed to 2–4 h after exposure to similar IR doses in wild type. What mediates hid induction in response to IR in p53 mutants remains to be investigated.
hid transcripts also increase in response to UV exposure, but the mechanism appears to be different for this radiation type (c in Fig. 1). Although p53 plays a critical role in inducing hid mRNA and plays a pro-death role after IR exposure, it has a pro-repair and thus a protective role after UV-C exposure [46]. Instead, it is through the actions of Jun N-terminal Kinase (JNK) signaling and transcription factors, Foxo and AP-1 (Drosophila Fos), that hid transcript levels increase in UV-irradiated pupal retina [20]. JNK mutants fail to increase hid mRNA levels and to undergo apoptosis in pupal retina after UV irradiation. Ectopic expression of nuclear Foxo or an active form of JNK in eye and wing imaginal discs induces hid mRNA. Induction of hid by active JNK is reduced upon reduction of foxo gene dosage. The pro-apoptotic action of Foxo is opposed by RTKs (EGFR and IGFR) acting through Akt (a in Fig. 1). RTK signaling also provides a pro-survival function by repressing hid during normal eye development as discussed in a preceding paragraph.
Regulators of hid under normal conditions are important for radiation-induced apoptosis
Two factors known to regulate hid under normal growth conditions also alter the ability of cells to undergo apoptosis. First, mutants in Dp show elevated hid transcripts in the ZNC region of wing imaginal discs as described in a preceding paragraph. The ZNC of mutants in Dp or its partner E2F1 undergoes apoptosis more readily after IR exposure [27]. Second, ban miRNA represses Hid protein accumulation under normal growth conditions. ban mutant larvae undergo more IR-induced apoptosis and are more sensitive to killing by IR. IR sensitivity of ban mutants is rescued by a reduction in the hid gene dosage, suggesting that hid is an important target of ban in radiation responses [47]. These results are consistent with the finding that the reduction of hid gene dosage by half can reduce IR-induced apoptosis. In other words, altering the level of hid gene products under normal growth conditions can alter the ability to cells to undergo apoptosis in response to DNA damage.
Transcription factor association at the hid locus
It is important to note that changes in hid transcript level could result from changes in transcriptional regulation at the hid promoter, changes in hid mRNA turnover or both. Many studies described here examined the level of hid transcripts and did not differentiate between transcriptional changes and altered mRNA stability. Association of a protein with the hid locus would be one indication that regulation occurs through a direct effect on hid transcription. We list below known instances of transcription factor association at the hid locus.
E2F, Foxo and Fos have been shown to bind to the hid locus. Three E2F consensus sites have been reported, at −1.4 kb, −165 bp and +2.2 kb relative to hid transcription [27, 29]. In ChIP assays, the −1.4 kb site associates with E2F1 in S2 cells and with E2F1 and, to a lesser extent, E2F2 in eye-antennal discs. In reporter assays in S2 cells and in eye and wing imaginal discs, this site appears to mediate repression of hid transcription. The −165 bp site also associates with E2F1 in S2 cells in ChIP assays, but to a lesser extent than does the −1.4 kb site. The +2.2 kb site is not found to associate with E2F1 or E2F2 [29]. The functional significance of −165 bp or +2.2 kb sites remains to be investigated.
The first intron of hid contains several Foxo and AP-1 consensus sites that are occupied by the respective transcription factors in ChIP assays of S2 cells [20]. Therefore, regulation of hid by these proteins in UV-irradiated retina may be via direct transcriptional regulation.
Conclusions
There are as many ways to activate Hid as there are to repress it. Developmental signals, hormonal changes during metamorphosis and cell–cell competition can increase hid transcripts. Exposure to genotoxins causes further induction of hid transcripts via p53 or JNK. Pro-apoptotic activities that promote hid transcript accumulation are counterbalanced by pro-survival activities that repress hid not only at the transcriptional level but also at post-transcriptional and post-translational levels. These include E2F1/RBF1 under normal growth conditions, epigenetic modification of the enhancer, RAS/MAPK and PI3 K/Akt pathways in response to RTK signaling, and miRNAs. The balance between pro-apoptotic and pro-survival activities operating within a cell could produce a read-out in terms of Hid activity. The accumulation of Hid activity above a certain level would result in cell death. Interconnectivity between hid-activating and hid-inhibiting activities could further provide feedback loops to control apoptosis. For example, in larval wing discs, IR exposure results in p53-dependent hid transcript accumulation as well as p53-dependent ban activation [47]. ban represses hid post-transcriptionally, thus negatively feeding back on p53-dependent activation and limiting apoptosis in the disc. It is through understanding mechanisms that regulate Hid in the context of each other that we may understand how cells reach life-or-death decisions.
References
- 1.Bergmann A, Yang AY, Srivastava M. Regulators of IAP function: coming to grips with the grim reaper. Curr Opin Cell Biol. 2003;15:717–724. doi: 10.1016/j.ceb.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Hay BA, Guo M. Caspase-dependent cell death in Drosophila. Annu Rev Cell Dev Biol. 2006;22:623–650. doi: 10.1146/annurev.cellbio.21.012804.093845. [DOI] [PubMed] [Google Scholar]
- 3.Steller H. Regulation of apoptosis in Drosophila. Cell Death Differ. 2008;15:1132–1138. doi: 10.1038/cdd.2008.50. [DOI] [PubMed] [Google Scholar]
- 4.Claveria C, Caminero E, Martinez AC, Campuzano S, Torres M. GH3, a novel proapoptotic domain in Drosophila grim, promotes a mitochondrial death pathway. EMBO J. 2002;21:3327–3336. doi: 10.1093/emboj/cdf354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wing JP, Schwartz LM, Nambu JR. The RHG motifs of Drosophila reaper and grim are important for their distinct cell death-inducing abilities. Mech Dev. 2001;102:193–203. doi: 10.1016/s0925-4773(01)00316-1. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L. The ‘unique key’ feature of the Iap-binding motifs in RHG proteins. Cell Death Differ. 2005;12:1148–1151. doi: 10.1038/sj.cdd.4401637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freel CD, Richardson DA, Thomenius MJ, et al. Mitochondrial localization of reaper to promote inhibitors of apoptosis protein degradation conferred by GH3 domain-lipid interactions. J Biol Chem. 2008;283:367–379. doi: 10.1074/jbc.M708931200. [DOI] [PubMed] [Google Scholar]
- 8.Olson MR, Holley CL, Gan EC, Colon-Ramos DA, Kaplan B, Kornbluth S. A GH3-like domain in reaper is required for mitochondrial localization and induction of IAP degradation. J Biol Chem. 2003;278:44758–44768. doi: 10.1074/jbc.M308055200. [DOI] [PubMed] [Google Scholar]
- 9.Abdelwahid E, Yokokura T, Krieser RJ, Balasundaram S, Fowle WH, White K. Mitochondrial disruption in Drosophila apoptosis. Dev Cell. 2007;12:793–806. doi: 10.1016/j.devcel.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 10.White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- 11.Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- 12.Sen A, Kuruvilla D, Pinto L, Sarin A, Rodrigues V. Programmed cell death and context dependent activation of the EGF pathway regulate gliogenesis in the Drosophila olfactory system. Mech Dev. 2004;121:65–78. doi: 10.1016/j.mod.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Miller DT, Cagan RL. Local induction of patterning and programmed cell death in the developing Drosophila retina. Development. 1998;125:2327–2335. doi: 10.1242/dev.125.12.2327. [DOI] [PubMed] [Google Scholar]
- 14.Sawamoto K, Taguchi A, Hirota Y, Yamada C, Jin M, Okano H. Argos induces programmed cell death in the developing Drosophila eye by inhibition of the Ras pathway. Cell Death Differ. 1998;5:548. doi: 10.1038/sj.cdd.4400398. [DOI] [PubMed] [Google Scholar]
- 15.Baker NE, Yu SY. The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell. 2001;104:699–708. doi: 10.1016/s0092-8674(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 16.Yu SY, Yoo SJ, Yang L, et al. A pathway of signals regulating effector and initiator caspases in the developing Drosophila eye. Development. 2002;129:3269–3278. doi: 10.1242/dev.129.13.3269. [DOI] [PubMed] [Google Scholar]
- 17.Bergmann A, Tugentman M, Shilo BZ, Steller H. Regulation of cell number by MAPK-dependent control of apoptosis: a mechanism for trophic survival signaling. Dev Cell. 2002;2:159–170. doi: 10.1016/s1534-5807(02)00116-8. [DOI] [PubMed] [Google Scholar]
- 18.Kurada P, White K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell. 1998;95:319–329. doi: 10.1016/s0092-8674(00)81764-x. [DOI] [PubMed] [Google Scholar]
- 19.Bergmann A, Agapite J, McCall K, Steller H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell. 1998;95:331–341. doi: 10.1016/s0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- 20.Luo X, Puig O, Hyun J, Bohmann D, Jasper H. Foxo and Fos regulate the decision between cell death and survival in response to UV irradiation. EMBO J. 2007;26:380–390. doi: 10.1038/sj.emboj.7601484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 22.Thompson BJ, Cohen SM. The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell. 2006;126:767–774. doi: 10.1016/j.cell.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Nolo R, Morrison CM, Tao C, Zhang X, Halder G. The bantam microRNA is a target of the hippo tumor-suppressor pathway. Curr Biol. 2006;16:1895–1904. doi: 10.1016/j.cub.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 24.de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- 25.Dimova DK, Dyson NJ. The E2F transcriptional network: old acquaintances with new faces. Oncogene. 2005;24:2810–2826. doi: 10.1038/sj.onc.1208612. [DOI] [PubMed] [Google Scholar]
- 26.DeGregori J, Johnson DG. Distinct and overlapping roles for E2F family members in transcription, proliferation and apoptosis. Curr Mol Med. 2006;6:739–748. doi: 10.2174/1566524010606070739. [DOI] [PubMed] [Google Scholar]
- 27.Moon NS, Frolov MV, Kwon EJ, et al. Drosophila E2F1 has context-specific pro- and antiapoptotic properties during development. Dev Cell. 2005;9:463–475. doi: 10.1016/j.devcel.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Moon NS, Di Stefano L, Morris EJ, Patel R, White K, Dyson NJ. E2F and p53 induce apoptosis independently during Drosophila development but intersect in the context of DNA damage. PLoS Genet. 2008;4:e1000153. doi: 10.1371/journal.pgen.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka-Matakatsu M, Xu J, Cheng L, Du W. Regulation of apoptosis of rbf mutant cells during Drosophila development. Dev Biol. 2009;326:347–356. doi: 10.1016/j.ydbio.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du W, Vidal M, Xie JE, Dyson N. RBF, a novel RB-related gene that regulates E2F activity and interacts with cyclin E in Drosophila. Genes Dev. 1996;10:1206–1218. doi: 10.1101/gad.10.10.1206. [DOI] [PubMed] [Google Scholar]
- 31.Stevaux O, Dimova D, Frolov MV, Taylor-Harding B, Morris E, Dyson N. Distinct mechanisms of E2F regulation by Drosophila RBF1 and RBF2. EMBO J. 2002;21:4927–4937. doi: 10.1093/emboj/cdf501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato K, Hayashi Y, Ninomiya Y, et al. Maternal Nanos represses hid/skl-dependent apoptosis to maintain the germ line in Drosophila embryos. Proc Natl Acad Sci USA. 2007;104:7455–7460. doi: 10.1073/pnas.0610052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leaman D, Chen PY, Fak J, et al. Antisense-mediated depletion reveals essential and specific functions of microRNAs in Drosophila development. Cell. 2005;121:1097–1108. doi: 10.1016/j.cell.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Hipfner DR, Weigmann K, Cohen SM. The bantam gene regulates Drosophila growth. Genetics. 2002;161:1527–1537. doi: 10.1093/genetics/161.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. Bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 36.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vazquez-Pianzola P, Hernandez G, Suter B, Rivera-Pomar R. Different modes of translation for hid, grim and sickle mRNAs in Drosophila. Cell Death Differ. 2007;14:286–295. doi: 10.1038/sj.cdd.4401990. [DOI] [PubMed] [Google Scholar]
- 38.Haining WN, Carboy-Newcomb C, Wei CL, Steller H. The proapoptotic function of Drosophila Hid is conserved in mammalian cells. Proc Natl Acad Sci USA. 1999;96:4936–4941. doi: 10.1073/pnas.96.9.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang C, Lamblin AF, Steller H, Thummel CS. A steroid-triggered transcriptional hierarchy controls salivary gland cell death during Drosophila metamorphosis. Mol Cell. 2000;5:445–455. doi: 10.1016/s1097-2765(00)80439-6. [DOI] [PubMed] [Google Scholar]
- 40.Brodsky MH, Weinert BT, Tsang G, et al. Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol. 2004;24:1219–1231. doi: 10.1128/MCB.24.3.1219-1231.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wichmann A, Jaklevic B, Su TT. Ionizing radiation induces caspase-dependent but Chk2- and p53-independent cell death in Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:9952–9957. doi: 10.1073/pnas.0510528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM. Drosophila p53 binds a damage response element at the reaper locus. Cell. 2000;101:103–113. doi: 10.1016/S0092-8674(00)80627-3. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Lin N, Carroll PM, et al. Epigenetic blocking of an enhancer region controls irradiation-induced proapoptotic gene expression in Drosophila embryos. Dev Cell. 2008;14:481–493. doi: 10.1016/j.devcel.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu CC, Bornemann DJ, Zhitomirsky D, Miller EL, O’Connor MB, Simon JA. Drosophila histone deacetylase-3 controls imaginal disc size through suppression of apoptosis. PLoS Genet. 2008;4:e1000009. doi: 10.1371/journal.pgen.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wells BS, Yoshida E, Johnston LA. Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol. 2006;16:1606–1615. doi: 10.1016/j.cub2006.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jassim OW, Fink JL, Cagan RL. Dmp53 protects the Drosophila retina during a developmentally regulated DNA damage response. EMBO J. 2003;22:5622–5632. doi: 10.1093/emboj/cdg543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaklevic B, Uyetake L, Wichmann A, Bilak A, English CN, Su TT. Modulation of ionizing radiation-induced apoptosis by bantam microRNA in Drosophila. Dev Biol. 2008;320:122–130. doi: 10.1016/j.ydbio.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]