Abstract
A family of neutral glycosphingolipids containing a 3-O-acetyl-sphingosine galactosylceramide (3-SAG) has been characterized. Seven new derivatives of galactosylceramide (GalCer), designated as fast-migrating cerebrosides (FMCs) by TLC retention factor, have been identified. The simplest compounds – FMC-1 and FMC-2 – of this series have been characterized as the 3-SAG containing nonhydroxy and hydroxy fatty acyl, respectively. The next two – FMC-3 and FMC-4 – add 6-O-acetyl-galactose and the most complex glycosphingolipids, FMC-5, -6 and -7, are 2,3,4,6-tetra-O-acetyl-3-SAG. These hydrophobic myelin lipid biomarkers coappear with GalCer during myelinogenesis and disappear along with GalCer in de- or dys-myelinating disorders. Myelin lipid antigens, including FMCs, are keys to myelin biology, opening the possibility of new and novel immune modulatory tools for treatment of autoimmune diseases including multiple sclerosis.
Keywords: anergy, fast-migrating cerebrosides, glycosphingolipids, multiple sclerosis, myelin, NKT cells
Myelin composition & architecture
Myelination is an essential CNS developmental process that is characterized by lipid as well as protein assembly into the oliogodendrocyte cell membrane, which is elaborated into a spiraling membrane that invests axons. Knowledge of the biochemical and physicochemical properties of myelin lipids, and particularly of their composition, organization, structure and accessibility with respect to the compacted myelin multilayer is central to understanding how and why myelin-specific antigens are selected during the development of inflammatory demyelination in multiple sclerosis (MS).
The multilayer CNS myelin membrane sheaths surrounding axons (Figure 1A) are comprised of lipids and proteins distributed according to charge, lipo- or hydro-philicity, and relative molecular weight [1]. The lipids are assembled with myelin-specific membrane proteins, the most abundant being the integral membrane protein – proteolipid protein and the extrinsic myelin basic protein while myelin-associated glycoprotein, myelin oligodendrocyte glycoprotein, and 2′3′-cyclic-nucleotide 3′-phosphodiesterase are quantitatively minor constituents (Figure 1B). The three main classes of lipids comprising CNS myelin are cholesterol, glycosphingolipids (GSL; derivatives of galactosylceramides [GalCer]), and phospholipids (PL): these myelin lipid classes have long been known to have constant molar proportions of 2:1:2 [2,3].
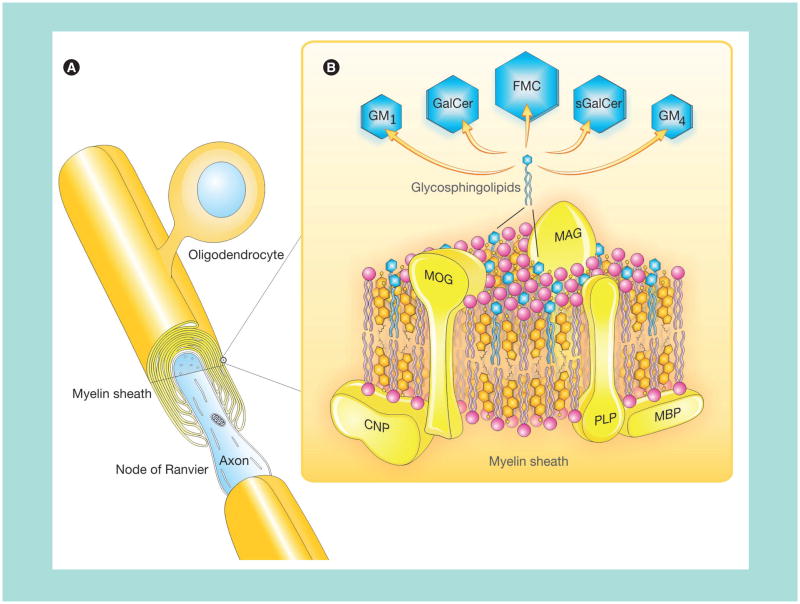
Figure 1. A composite diagram summarizing features of CNS myelin.
(A) Architecture of CNS myelin showing an oligodendrocyte and myelin sheath in CNS. This is a simplified version with fewer myelin sheaths per oligodendrocyte than normal. The cutaway view shows a multilayer membrane (only a few drawn for clarity) formed by oligodendrocytes. (B) Molecular composition of CNS myelin (three-dimensional view). Hypothetical arrangement of protein (20%) and lipids (80%) in the myelin multilayer membrane. Proteins (PLP, MBP, MOG, MAG and CNP) are marked in yellow and the comprising lipids are indicated as follows: cholesterol in orange, phospholipids in pink and the glycosphingolipids in blue.
CNP: 2′3′-cyclic-nucleotide 3′-phosphodiesterase; FMC: Fast-migrating cerebroside; GalCer: Galactosylceramide; GM1: Monosialoganglioside; GM4: Sialosylgalactosylceramide; MAG: Myelin-associated glycoprotein; MBP: Myelin basic protein; MOG: Myelin oligodendrocyte glycoprotein; PLP: Proteolipid protein; sGalCer: Sulfatide.
Adapted and modified from [11] with permission from SAGE Publications.
Galactocerebrosides (e.g., GalCer) are the most abundant glycolipid component of myelin, and constitute a molecular family that differs between members mainly in fatty acid chain length and presence or absence of a fatty acid C2 hydroxyl, with minor changes in the chemical structure of the ceramide moiety. A fifth of the total myelin glycosphingolipid is sulfatide (sGalCer), which has the galactose 3′-OH sulfated. Sulfatide is an amphipatic glycolipid that has a polar sulfated galactosyl head group facing out from the plasma membrane and is anchored in the membrane bilayer by ceramide-containing N-acyl-linked long-chain fatty acid. Other GalCer derivatives are recently discovered acetylated (see later) (Figure 1B) [4–6] or sialylated ones.
The other major myelin lipids are cholesterol and phosphatidylethanolamine. Much of the latter group consists of plasmalogens, in which fatty acids are replaced by an aliphatic long-chain alkenyl ether. Phosphatidylcholine (lecithin) is a major myelin constituent, while sphingomyelin is only a minor component. Sphingomyelin and cholesterol form membrane domains called ‘lipid rafts’ that are sites of important functions such as signal transduction [7,8]. Minor components (0.1–0.3%) of myelin include gangliosides, complex N-acetylneuraminic acid-containing glycosphingolipids, especially monosialoganglioside (GM1) and sialosylgalactosylceramide (GM4) [9].
The lipid composition of the CNS has been intensively studied in MS in an effort to discern a primary lipid defect [10]. In additon, the detection of antilipid antibodies in MS has been a long-time focus with renewed interest in recent years [11].
Fast-migrating cerebrosides: new molecular components of the myelin sheath
The recently discovered derivatives of GalCer, based on their TLC retention factor (Rf), are designated as fast-migrating cerebrosides (FMCs). Although these more hydrophobic neutral glycosphingolipids have largely been over-looked in lipid analysis of the nervous system for the past 30 years, there are several reports that hint at the existence of this molecular species in the CNS. In 1963, Kochetkov et al. purified several minor cerebroside components (O-alkenyl cerebrosides) from the brain by preparative TLC followed by crystallization with methanol, and tentatively proposed a series of compounds with an alkenyl ether grouping at the C3-OH of the sphingosine moiety of GalCer, designating them ‘sphingo-plasmalogenes’ [12]. Subsequent studies did not confirm the proposed structure and these compounds purified from the brain and spinal cord were later characterized as GalCer derivatives with fatty acyl/acetal substituents at different hydroxyls of galactose [13–20].
The difficulties in obtaining a precise characterization of 3-O-acetyl-sphingosine series FMCs are a consequence of their susceptibility to mild alkali and acid, and acid–alkali methanolysis techniques that are commonly used for lipid purification and characterization. The oversight was further compounded by the following obstacles: first, the methylation procedure employed; second, previous lack of availability of a sensitive physical or chemical technique in the 1980s for structural characterization, together with the third point, focus upon the substitution on the galactosyl moiety rather than modification of the sphingoid base of ceramide. We overcame these difficulties and purified FMCs that were labile to both weak acid and alkali by employing a modified neutral permethylation procedure [21].
Structural characterization of FMCs
We have characterized seven novel derivatives of GalCer (known as FMCs) in vertebrate brain, and identified a novel series with a unique 3-O-acetylation of the sphingosine long-chain base. The chemical composition and primary structures of these compounds are summarized in Figure 2. The simplest FMCs (FMC-1 and FMC-2) are 3-O-acetyl sphingosine-GalCer incorporating either nonhydroxy or 2-hydroxy fatty acyl attached to the sphingosine base 3-hydroxyl moiety [4]. The next two compounds of this series, FMC-3 and FMC-4, are analogs of FMC-1 and FMC-2, respectively, with additional acetyl modification at the galactose C6 hydroxyl [5]. More complex (FMC-5 and FMC-6) are 3-O-acetyl-sphingosine-2,3,4,6-tetra-O-acetyl-GalCer with nonhydoxy and 2-hydroxy fatty N-acyl ceramide types, respectively [6]. The most hydrophobic FMC-7 is the hexa-acetylated 3-O-acetyl-spingosine-2,3,4,6- tetra-O-acetyl-GalCer [6]. A list of molecular ions (e.g., [M + Li]+) and assignments for predominant 24-carbon lipoforms examined is compiled in Table 1.
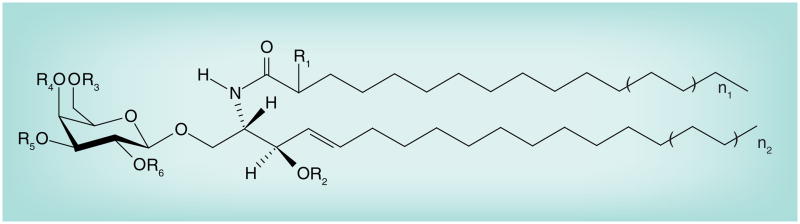
Figure 2. Myelin O-acetyl galactosylceramide series structures.
Galactosylceramide: R1 = H, R2 = H, R3-R6 = H.
FMC-1: R1 = H, R2 = Ac, R3-R6 = H; FMC-2: R1 = OH, R2 = Ac, R3-R6 = H; FMC-3: R1 = H, R2 = Ac, R3 = Ac, R4-R6 = H; FMC-4: R1 = OH, R2 = Ac, R3 = Ac, R4-R6 = H; FMC-5: R1 = H, R2-R6 = Ac; FMC-6: R1 = OH, R2-R6 = Ac; FMC-7: R1 = OAc, R2-R6 = Ac.
AC: Acetyl; FMC: Fast-migrating cerebroside.
Table 1.
Fast-migrating cerebroside structures correlating with nominal, mono-isotopic m/z of predominant lithium adducts in electrospray ionization–mass spectrometry profiles.
| GSL molecular species | [M + Li]+m/z | Ceramide acetyl groups | Hexose acetyl groups | Structure† | Ref. |
|---|---|---|---|---|---|
| Monohexosylceramide | 816, 818 | 0 | 0 | NFA GalCer 24:1/24:0 | [4] |
| Monohexosylceramide | 832, 834 | 0 | HFA GalCer 24:1/24:0 | [4] | |
| Mono-O-acetylated monohexosylceramide | 858, 860 | 1 | 0 | 3-O-acetyl-sphingosine-GalCer (NFA) 24:1/24:0; FMC-1 | [4] |
| Mono-O-acetylated monohexosylceramide | 874, 876 | 1 | 0 | 3-O-acetyl-sphingosine-GalCer (HFA) 24:1/24:0; FMC-2 | [4] |
| Di-O-acetylated monohexosylceramide | 900, 902 | 1 | 1 | 3-O-acetyl-sphingosine-6-O-acetyl-GalCer (NFA) 24:1/24:0; FMC-3 | [5] |
| Di-O-acetylated monohexosylceramide | 916, 918 | 1 | 1 | 3-O-acetyl-sphingosine-6-O-acetyl-GalCer (HFA) 24:1/24:0; FMC-4 | [5] |
| Penta-O-acetylated monohexosylceramide | 1026, 1028 | 1 | 4 | 3-O-acetyl-sphingosine-2,3,4,6-tetra-O-acetyl-GalCer (NFA) 24:1/24:0; FMC-5 | [5,6] |
| Penta-O-acetylated monohexosylceramide | 1042, 1044 | 1 | 4 | 3-O-acetyl-sphingosine-2,3,4,6-tetra-O-acetyl-GalCer (HFA) 24:1/24:0; FMC-6 | [5,6] |
| Hexa-O-acetylated monohexosylceramide | 1084, 1086 | 2 | 4 | 3-O-acetyl-sphingosine-2,3,4,6-tetra-O-acetyl-GalCer (2-O-acetyl-HFA) 24:1/24:0; FMC-7 | [5,6] |
m/z increments observed for: acetyl group = 42; hydroxy fatty acid = 16; unsaturated fatty acid = 2; CH2–CH2 unit = 28.
All FMC species were found to have d18:1 sphingosine as the sole sphingoid component.
Final structures are based upon tandem mass spectrometry and nuclear magnetic resonance spectroscopy; see references.
ESI-MS: Electrospray ionization–mass spectrometry; FMC: Fast-migrating cerebroside; GalCer: Galactosylceramide; GSL: Glycosphingolipid; HFA: 2-hydroxy fatty acid; NFA: Nonhydroxy fatty acid; SAG: 3-O-acetyl-sphingosyl-galactosylceramide.
Occurrence, expression & developmental variation of FMCs
Fast-migrating cerebrosides are myelin constituents, enriched in both CNS and peripheral nervous system (PNS) myelin with an average GalCer concentration value of 20% for CNS and 30% for PNS [4]. Immunohistochemical staining of antibody against the acetyl-cerebrosides of highest Rf, the most hydrophobic derivatives (a mixture of FMC-5, -6 and -7), carried out in gerbil cerebellum tissue showed specific staining of myelin and myelinated fibers [22]. The cellular expression of these FMCs in primary culture of glial cells is noteworthy as well. Our immunocytochemical study, using mixed glial cells and antibodies against a mixture of FMC-5, -6 and -7 and GA1, stained both oligodendrocytes and their progenitors differently [22]. FMC-5, -6 and -7 and GA1 were expressed by both oligodendrocytes and progenitors. These cells were stained with O1 antibody, specific to GalCer, a marker for oligodendrocytes and myelin. Both GA1 antibody and O1 antibody stained oligodendrocyte cell bodies and processes whereas antibody against FMC-5/-6/-7 stained only the cell bodies, and not the processes [22].
Fast-migrating cerebrosides, acetyl-GalCer derivatives, have been identified in rat, bovine and human brain tissue, and determined to vary by species from 15 to 35% of tissue GalCer content [4]. Although an average concentration of FMCs in the human brain has been estimated quantitatively, an occasional individual variation has been observed as well. Similarly, there can be a variation in FMCs concentration from different strains of the same species.
The alkali-labile FMCs are expressed early, at postpartum day (P)10 in the mouse brain, and attain a peak concentration at P25–P30, remaining unchanged thereafter throughout maturity [21]. Their pattern of appearance indicates that they coappear with GalCer during myelinogenesis [21]. Moreover, in addition to GalCer, FMCs are expressed by oligodendrocytes and are markers for them [22]. However, the pathway of FMC synthesis has not been established and their function in oligodendrocyte-mediated myelinogenesis has not been examined [22]. Based on sphingosyl acetylation, there are three possible pathways for FMC synthesis and these can be outlined as follows: GalCer→FMC; ceramide→3-O-acetyl-ceramide→FMC; and sphinganine→3-O-acetyl-sphinganine→3- O-acetyl-dihydroceramide→3-O-acetyl-ceramide→ FMC [4]. In all of these cases, a brain 3-O-acetyltransferase is proposed to catalyze the acetyl group transfer to sphingosine C3 hydroxyl of either GalCer, ceramide or sphingosine. We anticipate that the first pathway is more likely since neither 3-O-acetyl-ceramide nor 3-O-acetylsphinganine has been observed or reported in brain tissue or elsewhere. The total absence of FMCs in ceramide galactosyl-transerase (GalT1) knockout (KO) mutant mice suggests that the responsible FMC synthases (transferases) are specific to GalCer [21].
In order to examine these FMCs in relation to myelin structure and function, their content was determined in the GalT1 KO mutant brain that totally lacks GalCer, and in murine dysmyelinating jimpy and quaking mutants. The myelin of the KO mutant deteriorates within the first weeks of life, and the mice manifest paranodal axon–glia disruption and an average survival of 45 days [23]. This KO model established the critical importance of neutral GSLs, GalCer and its derivatives (including FMCs) in myelination and vertebrate brain function. However, identification of the role of individual neutral GSL components (e.g., GalCer, sulfatide, GM4 and FMCs) awaits further studies and specific gene deletions. The genetic dysmyelinating defect leads to myelin reduction in jimpy and quaking mouse mutants (Table 2) and the extent of reduction is related to the severity of dysmyelination. This is consistent with the indication that FMCs are myelin components.
Table 2.
Galactosylceramide and fast-migrating cerebroside concentrations in murine mutant dysmyelination.
| Murine mutant type | Alteration of | ||
|---|---|---|---|
| NFA GalCer† | HFA GalCer† | FMCs† | |
| Jimpy | 98% reduction | 98% reduction | 90% reduction |
| Quaking | 62% reduction | 21% reduction | 48% reduction |
Compared with wild-type littermate control mice.
FMC: Fast-migrating cerebroside; HFA: 2-hydroxy fatty acid; NFA: Nonhydroxy fatty acid.
Data taken from [21].
A dramatic decline in FMC concentration has also been observed in the human dysmyelinating disorder Krabbé’s leukoencephalopathy, with survival in the infantile type of 2–4 years. The disease results from a deficiency of cerebroside β1-1 glycosidase, which is responsible for degradation of GalCer [24,25]. It has been proposed that psychosine, a cytotoxic lysolipid, accumulates in early development leading to oligodendrocyte degeneration and consequent dysmyelination [26–29]. A significant decrease in GalCer in Krabbé’s disease brain has also been reported [26]. We confirmed the decreased GalCer concentration (75–80%), and observed a greater decrease (95%) in FMCs in Krabbé’s disease brain. Some FMCs contain an additional fatty acid/alkyl group that is acetal-linked to the 3-O-acetylsphingosine GalCer structure, and thus has an increased hydrophobicity that could mediate interaction with other myelin lipid/protein components and could thereby protect against myelin degradation. The extent of loss of FMCs (along with GalCer) in jimpy, quaking mice as well as in Krabbé’s disease brain indicates that there is a myelin deficiency along with a loss of myelin function and premature death.
Demyelination in multiple sclerosis
Multiple sclerosis is the most common human demyelinating disease. Recent progress made in understanding MS pathogenesis has led to a current consensus opinion that the disease is mediated by autoreactive CNS-specific CD4+ T cells, although the role of B cells has recently been re-emphasized. Demyelinated plaques and associated astrocytic proliferation (gliosis) are the results of local inflammation and represent the major pathological characteristics of the disease [30]. The immunopathological events can be divided into an initial T-cell priming, activation phase in the periphery and a subsequent effector phase with migration of leukocytes across the blood–brain barrier (BBB), local reactivation and invasion of the CNS parenchyma [31].
T-cell priming occurs within systemic immune compartments and is initiated by immunization with myelin antigens, including myelin lipids. There are different mechanisms for lipid antigen uptake, depending upon the source of antigen and its structure, such that endogenous lipids are differentially distributed in subcellular compartments and internalized lipids are transported to different endocytotic vesicles depending on the length and chemistry of their alkyl chains and their mode of internalization [32–34]. Lipids with longer alkyl chains are transported to late endosomes [32] corresponding to the localization of CD1b molecules that appear to be specialized for binding long-chain lipids [35,36]. Lipids containing alkyl chains that have multiple unsaturated sites or shorter saturated tails are trafficked to early or recycling endosomes [32], compartments surveyed by CD1c and CD1a that present these types of lipids [37,38].
There are at least three types of accessory proteins involved in lipid uptake by CD1d molecules in the case of exogenous lipids: glycosidases that trim glycolipids to reveal antigenic epitopes; lipid transfer proteins that ensure rapid and efficient transfer of lipid monomers and include Niemann–Pick protein (NPC) type 1 and 2, saposins, GM2-activator (GM2-A), and CD1e; and proteins that bind and deliver extracellular lipids to intracellular sites where loading is facilitated (an example of this type is apoE). The synthetic form of α-GalCer, KRN7000, is loaded into CD1d primarily in endosomes after uptake by endocytosis through a process facilitated by extracellular lipid-carrying proteins, such as apoE [39]. Loading is dependent upon acidic pH and availability of lipid transfer proteins such as saposins and GM2-A protein [40–43].
Interestingly, a recent study demonstrated that α-GalCer analogs with relatively short acyl chains are excluded from intracellular loading within late endosomes even though they are transported efficiently to this site [44]. Thus, there is an extremely strong bias to direct cell surface loading of CD1d by such glycolipid antigens. The difference in the requirement for antigen uptake by antigen-presenting cells (APCs) for presentation of KRN7000 and most or possibly all Th2-biasing type analogs of α-GalCer suggested the possibility that the latter compounds are presented preferentially by nonphagocytic CD1d APCs such as B cells. This structure-dependent sorting of glycolipid ligands for preferential loading into CD1d molecules in either the lipid raft or the nonlipid raft regions may be an important mechanism that steers a proinflammatory or tolerogenic response.
Antigens presented by APCs within secondary lymphoid organs induce the activation and expansion of myelin-specific T cells, and these activated myelin-reactive T cells circulate through the organism searching for their specific antigens to become reactivated [45].
Migration of T cells across the BBB is a complex multistep process and occurs via interactions between adhesion molecules found on the surface of lymphocytes and endothelial cells [46]. First, circulating T cells slow in the bloodstream due to contact between distinct adhesion molecules on their surface and on endothelial cells. In the second step, homeostatic chemokines, such as CCL19 and CCL21 are produced by cells and mediate T-cell activation [47,48]. The third and fourth steps include firm adhesion and final transmigration of the lymphocytes across the BBB. In several studies the ICAM-1 and the VCAM-1 expressed on the CNS microvascular endothelial cells and their respective T-cell ligands were implicated to have crucial roles in the transmigration process. In the fifth step, CD4+ T cells accumulate within enlarged perivascular spaces where they can encounter their specific antigens (e.g., myelin components) presented by the MHC class II on the surface of APCs, such as perivascular dendritic cells [49]. This immune synaptic contact reactivates the T cells. However, for complete activation, differentiation and clonal expansion, a costimulating process involving additional molecules is required [50,51]. This antigen-triggered reactivation enables T cells to transverse the glia limitans and migrate into CNS parenchyma.
Once within the brain parenchyma, T cells can activate local microglia and produce vasoactive substances, chemokines and cyto- and myelinotoxic mediators that further attract peripheral leukocytes and progressively damage brain tissue [52]. Despite this insight into pathophysiology, the cause of MS remains unclear, and definitive treatment of this frequent and chronic disease still eludes us.
Lipid antigens as targets for autoimmune attack
For many years, attention has been focused upon immune responses to myelin-derived proteins as targets for autoimmune attack while myelin lipid antigens have been overlooked for the most part [53–61]. The lipid composition of MS CNS tissue has been intensively studied. Lipids are the most abundant myelin constituents, and relevant antibodies may recognize carbohydrates and lipids as well as proteins. Lipids and glycolipids are also biologically relevant and are suspected to have roles in autoimmune diseases because they are prominent on the microbial surfaces that are presented to host immune responses.
A major CNS myelin glycolipid, cerebroside or GalCer that accounts for 32% on molar basis of myelin lipids, has been considered a target for autoaggressive attack in MS. Menge et al. revived interest in anti-GalCer antibodies as candidate MS immune markers [62], while antibodies in MS to other glycolipids such as gangliosides [63–65], sulfatides [66,67] and PLs [68] have also been previously reported in MS. The pathogenic role of glycosphingolipids and PLs in MS is summarized in Table 3. Considerable interest has been stirred by a study employing lipid-array analysis that found antibodies to sulfatide, sphingomyelin, several PLs, oxidized PLs and oxidized sterols in MS cerebrospinal fluid (CSF) [69]. Another fundamental and exciting recent development has been a growing realization of the importance of the potent lipid bioactive breakdown products [70–79] (lyso-sphingolipids), emphasizing the relevance of lipids as mediators of injury to myelin and related CNS injury, as well as antigens. Our own studies of cross-reactivity of the myelin marker acetyl-cerebrosides (FMCs) with mycoplasma and other complex lipids (see following section) support a role for lipid antigens in the MS mechanism.
Table 3.
Pathogenicity of glycosphingolipids and phospholipids in multiple sclerosis.
| Myelin lipid component | Alteration in MS |
|---|---|
| GalCer/sGalCer | sGalCer more potently induces immune attack than GalCer and is an immunodominant myelin lipid [169] sGalCer synthetic analogs with different fatty acid chain lengths (C16–C24) study indicates that cis-tetracosenosyl (C24:1) is the most active and potentially the immunodominant species [134] Intrathecal anti-sGalCer antibodies are higher in MS than OND controls [66] Anti-sGalCer antibodies are found in all MS subtypes with greater frequency in SPMS than PPMS and RRMS [66] Anti-sGalCer antibodies are increased in MS sera [67,170] Reactivity against GM1, sGalCer, oxidized PL and sterols is increased in MS CSF compared with OND controls [69] sGalCer is observed on oligodendrocytes [171] indicating presence of candidate target autoimmune attack sGalCer-reactive antibodies act upon cultured oligodendrocytes producing oligodendrocyte loss or myelin destruction as judged by Ca2+ influx/depolarization [172] Anti-sGalCer IgM mAb induces dysmyelination of loose whorls in vitro, but not in formed myelin sheaths [173] Anti-GalCer and anti-sGalCer mAbs inhibit differentiation of oligodendrocyte progenitor cultures; both render oligodendrocytes nonfunctional [174] Anti-sGalCer antibodies damage oligodendrocyte microtubules [172] Anti-GalCer antibodies adversely affect cytoskeleton [172] sGalCer and GalCer are essential for proper formation of mouse CNS myelin, but PNS myelin appears normal in the absence of these lipids produced in transgenic mice [175] sGalCer is critical for oligodendroglial–axon interaction [176], distribution of Na+ and K+ channels [177], and myelin maintenance [178] sGalCer activates microglia and promotes glial inflammatory responses [179] T-cell responses to sGalCer and gangliosides are elevated in a CD1-restricted stimulation paradigm [160] sGalCer-specific NKT cells have been observed in EAE CNS [158] sGalCer suppresses mouse EAE [158] sGalCer/PLP in CFA coadministration increases EAE severity [69] MOG/sGalCer coadministration with CFA induces in mouse CNS 3–4% infiltrating T cells made up of sGalCer-reactive T cells and approximately six-times lower CNS iNKT-cell levels [158] iNKT cells outnumber sGalCer-reactive T cells in ratio of 5:1 in peripheral organs in both health and disease [169] |
| Gangliosides | Antiganglioside antibody titers increase in MS serum [63–65,180,181] Anti-GM1, anti-GM3, and anti-GD1a autoantibodies associate with MS progression [63–65,180,181] Anti-GM1, anti-asialo-GM1, and anti-GD1a are more frequent in PPMS than in RRMS or SPMS [65] Anti-GM3 levels are elevated in PPMS and SPMS in comparison with RRMS, HS and OND [64] Anti-GM1 antibodies increase in MS [182,183] and this is not related to brain atrophy or a specific marker for axon damage Responses to mixed gangliosides increase in MS peripheral blood T lymphocytes [181] Proliferative responses to GM3 and GQ1b increase in peripheral blood T cells in PPMS [181] Anti-GM1 IgM titers are higher in RRMS serum during the first MS attack; serum ganglioside patterns are altered indicating GM1 role [180] GM1 and GD1a levels rise and GM3 decreases in serum in the first MS attack [180] GM1 decreases in serum and GD1a increases in chronic MS [180] GD2-like epitopes on oligodendrocytes may be autoantigens because anti-GD2 autoantibodies are cytotoxic and cause demyelination and neuron/axon damage [184] resembling MS lesions [185,186] GM1 serum levels in RRMS increase in the first MS attack [187] |
| PLs | Anti-PL antibodies and particularly anti-CL are common in MS [68,188–195] Serum anti-PL has been observed in definite MS [68] Anti-PL autoantibodies (IgG and/or IgM) are higher in MS than non-MS neurological disease [68]; IgG anti-CLs have been observed in 9% MS; IgM elevated in 44% MS; anti-PS titers are similar to anti-CL, but anti-PC antibodies are usually absent Choline elevates in NAWM (later develop MRI-visible lesions) [196] Oxidized phosphatidylcholine occurs in MS brain and not control [197]; oxidized PC and antioxidized PC in MS CNS suggests neurodegeneration Lysolecithin produces demyelination; myelin integrity is lost in 1–2 days [198] Polyclonal IgM from CD5+ B lymphocytes recognize myelin lipids, particularly PC [199]; intrathecal antilipid IgM correlates with aggressive MS and might be predictive |
CFA: Complete Freund adjuvant; CL: Cardiolipin; CSF: Cerebrospinal fluid; EAE: Experimental autoimmune encephalitis; GalCer: Galactosylceramide; HS: Healthy subject; iNKT cell: Invariant natural killer T cell; mAb: Monoclonal antibody; MOG: Myelin oligodendrocyte protein; MS: Multiple sclerosis; NAWM: Normal appearing white matter; NKT cell: Natural killer T cell; OND: Other neurological disease; PC: Phosphatidylcholine; PL: Phospholipid; PLP: Proteolipid protein; PNS: Peripheral nervous system; PPMS: Primary progressive multiple sclerosis; PS: Phosphatidylserine; RRMS: Relapsing remitting multiple sclerosis; sGalCer: Sulfatide; SPMS: Secondary progressive multiple sclerosis.
Infection affecting CNS: molecular mimicry in MS
For a century, the prominent immunoglobulins in CNS and CSF, whose antigenic specificity is largely unknown, have intrigued MS investigators. Epidemiological studies indicate that MS has both genetic and environmental components; a recurring formulation of environmental factors affecting a complex background of genetic susceptibility. The environmental factors concerned may be infectious agents, and roles for numerous viral [80–83] and bacterial pathogens [84–89] have been postulated. The pathology is generally believed to reflect autoimmune attack upon myelin autoantigens, and many epidemiological and pathological features suggest that MS is either an infectious disease, or is precipitated by infection [90,91]. In this larger context, MS mechanisms are postulated to encompass bacterial, viral or other sources of infection, producing sensitization to a neural antigen, or cross-reactivity and consequent molecular mimicry, affecting lipid antigens of microbes and myelin as well as the often cited peptide mimicries [91–96]. Molecular mimicry is a hypothesis that links insight into the role of infection with the mechanism and generation of autodamaging inflammatory attack in MS. There are three mechanisms that have been proposed for infectious induction of MS: molecular mimicry with activation of autoreactive cells by cross-reactivity between self and foreign antigens; bystander activation with release of cytokines by autoreactive cells driven by relatively nonspecific inflammatory tissue events; and viral or other microbe persistence either with or without epitope spread. The theory that molecular mimicry may be implicated in MS pathogenesis has been supported by identification of sequence homologies between myelin protein genes and viral and bacterial nucleic acids [92,97]. Such homologies suggest an infection trigger to immune response transition, and this is supported not only by the relatively frequent occurrence of molecular mimic examples [90,92,97,98], but also by MS CSF immunoglobulin abnormalities [99–101] indicated by the elevated antibodies to many viruses and bacteria, as well as the enhanced reactivity in MS of lymphocytes to various viral antigens [84–89]. Our initial studies also suggest that myelin O-acetyl-cerebrosides, infection and neurological disease may be linked [6].
Infection affecting PNS: molecular mimicry in Guillan–Barré syndrome & other radiculopathies & neuropathies
Unlike CNS myelin, human peripheral nerve myelin contains sialosylneolactotetraosylceramide (also known as sialosylparagloboside [LM1]), sialosyllactosaminyl-paragloboside and sulphated glucuronyl paragloboside as well as its higher lactosaminyl homologue, sulphated glucuronyl lactosaminyl-paragloboside. Sulphated glucuronyl paragloboside and its homologue, sulphated glucuronyl lactosaminyl-paragloboside, have structures similar to that of LM1, except for a 3′-sulfated glucoronic acid instead of sialic acid on the terminal saccharide chain. LM1, GM1, GM3, GM2 and sialosyllactosaminyl- paragloboside are the major monosialosylgangliosides in human PNS, although only some of these have been identified as dominant autoantigens in peripheral nerve diseases.
Molecular mimicry has also been proposed as a key mechanism for several other immune-mediated inflammatory neurological disorders occurring in PNS. Study of peripheral nerve and root disorders has realized support for a role of glycolipid, and particularly the glycose moiety, as antigenic. For example, in patients suffering from chronic inflammatory demylinating polyradiculoneuropathy, a subacute/chronic inflammatory disorder related to the Guillain–Barré syndrome (GBS), in which serum anti-GM1 and anti-LM1 antibody titers are elevated, sera also show cross-reactivity against Campylobacter jejuni antigens [102]. This strongly suggests that molecular mimicry of PNS myelin gangliosides and microbial glyco-eiptopes on LPS is a mechanism of the autoimmune neuropathy. Since a major difference between the CNS and PNS is the abundance of neolacto-series gangliosides in the PNS, it appears most likely that a molecular mimicry mechanism involving FMCs is not applicable to the demyelinating neuropathy in PNS. Another PNS example is the Miller–Fisher syndrome, a variant form of acute inflammatory GBS neuropathy affecting control of eye movement that manifests molecular mimicry of sialylated epitopes of the liposaccharides (LPS) of C. jejuni and gangliosides in the PNS [103]. Thus, molecular mimicry between infectious agents and GSLs may function in the production of anti-GSL antibodies and this is a plausible mechanism of GBS, Miller–Fisher syndrome [104,105] and multifocal motor neuropathy [106]. In gastric infection, another organ site is implicated for serum anti-GM1 antibody titers are elevated in patients with Helicobacter pylori infection. Coupled with the finding that C. jejuni-positive sera cross-react with H. pylori antigens, this suggests that both of these GI tract-resident bacteria have a common LPS structural feature that resembles GM1 [107]. In addition to C. jejuni and H. pylori, other agents such as cytomegalovirus, Haemophilus influenzae, Epstein–Barr virus and Mycoplasma may contribute to the clinical and immunological heterogeneity of GBS and peripheral neuropathies with paraproteinemia by a similar mechanism [108]. For example, human cytomegalovirus infection has been associated with peripheral neuropathies in patients with antibodies against sulfoglucuronyl glycosphingolipids (SGGLs) [109]. The SGGL epitope is present in potential target antigens implicated in the development of IgM paraproteinemia and peripheral neuropathy although the relationship between infectious agent and anti-GSL antibodies has yet to be defined [109]. It would be interesting to test this hypothesis by immunizing animals directly with LPS fractions isolated from these infectious agents in order to establish whether these animals develop anti-GSL antibodies, and whether this could experimentally induce animal neurological disease.
Myelin acetyl-cerebrosides: cross-reactivity with microbial lipids
The myelin acetyl-cerebrosides resemble acyl-and/or acetyl-carbohydrates of LPS and/or glycolipid found on the external surface of many bacteria [110–117] and thus are candidate lipid antigen mimics targeted by immune responses initially directed at bacterial acyl- or acetyl-conjugated glycosides and subsequently inducing autoimmune inflammation in CNS.
Exploring lipid mimicry pathogenesis for induction of MS autoimmunity, we examined CSF IgGs for cross-reactivity between myelin/microbial lipids focusing on acetylcerebrosides, FMCs and other myelin lipids including GalCer and sGalCer; MfGL-II, a glyco-glycero-choline-containing lipid from the human pathogen Mycoplasma fermentans, and LPS purified from microbes such as Chlamydia pneumoniae, C. jejuni, H. pylori and Escherichia coli. In our first assessment we observed cross-reactivity of anti-FMC-7 antibody with purified glyco-glycero-phosphocholine-containing lipid MfGL-II from M. fermentans [6] that may be relevant to immune triggering of myelin-targeted inflammatory demyelination by the mycoplasma glycolipid [6]. We also observed binding of MS CSF to LPS from E. coli J5, although not to LPS of other screened bacterial species [6].
As an alternative approach to examine cross-reactivity to lipid antigens employing the functionally enigmatic antibodies found in approximately 95% of MS patients, we studied CSF by comparing other inflammatory neurological disease controls that comprised acute meningitis, meningoencephalitis and subacute sclerosing panenecephalitis, other noninflammation neurological diseases, and MS including the main clinical subtypes. Screening for lipid binding in MS and other neurological diseases revealed that the anti-FMC-7 antibody level was significantly higher in primary progressive MS compared with secondary progressive MS; and relapsing–remitting MS in remission compared with a secondary progressive MS cohort [6]. However, the greatest antihydrophobic FMC reactivity was observed in the inflammatory CNS diseases (meningitis, meningoencephalitis and subacute sclerosing panenecephalitis) [6]. In addition, highly elevated antimicrobial (M. fermentans and E. coli) antibodies were found in the other inflammatory neurological disease controls group [6].
Both approaches, although not fully conclusive, suggest a biologically relevant correlation between Mycoplasma or E. coli J5 infection and demyelination via antibody-mediated recognition. The range of reactivities observed with some of the MS samples, binding to FMC-7 (as well as GalCer, sGalCer, MfGL-II and LPS E. coli J5), suggest that the reactivity is relevant to pathogenesis in some MS patients; an interpretation consistent with the recognized heterogeneity [118,119] of both pathology and clinical features of MS. These preliminary data illustrate the potential importance of molecular mimicry between microbial infection and autoimmune disease of the CNS, although further studies are needed to probe the frequency and strength of the cross-reactivity.
Natural killer T cells: glycolipid-reactive T cells in MS
Peripheral T cells that react with glycolipid antigens in healthy subjects participate in regulation of pathological autoimmunity via peripheral tolerance, and comprise pathways that: act directly on autoreactive cells (e.g., ignorance, anergy, phenotypic skewing); or act indirectly through natural killer T (NKT) cells and CD4+CD25+ T regulatory cells [120]. Disturbance of this regulatory control could lead to autoimmune disease. Several types of regulatory cell populations are believed to be involved in suppression and prevention of MS development, and in promotion of recovery of patients with relapsing–remitting MS. Additionally, regulatory roles for CD8+ T cells [121], B cells [122] and NKT cells [123] in suppressing the pathogenic Th1 cells in experimental autoimmune encephalitis (EAE) have been reported.
A significant body of evidence has emerged in recent years indicating an immunosuppressive role for NK and NKT cells. We focus here on NKT cells because they have been recognized to regulate autoimmunity in MS [124,125]. Included in this group are invariant NKT (iNKT) cells that are a separate T-cell lineage characterized by CD1d reactivity, and an invariant Vα24-Jα18 T-cell receptor (TCR) α-chain coexpressed with Vβ11 TCR β-chain (Figure 3). There is also glycolipid– antigen reactivity, expression of NK cell and memory T-cell markers, and potent cytokine-producing capacity [126] including both IFN-γ and IL-4 activity. Human NKT cells express NK-cell markers (Figure 3), including NK receptor (NKR)-PIA (CD161), CD94, CD56, CD57 and CD122, some of which we have found to be altered in MS patients (Table 4) [127]. Human NKR+ T cells have been reported to share functional similarities with murine iNKT cells including rapid MHC-unrestricted cytotoxicity and potent cytokine secretion [128]. A minor proportion of these lymphocytes coexpress a TCR α-chain homologous to the murine Vα14Jα18, Vα24Jα18. However, the majority are heterogeneous, coexpressing other TCRs and various NK receptors. Although the mechanisms that control the expression of NKRs on T cells are not well understood, these receptors are believed to function as costimulatory molecules or to regulate effector T-cell activity [129]. T cells bearing NKRs as well as such innate lymphocyte populations, such as γδ T cells, are front-line immune regulatory cells [130,131].
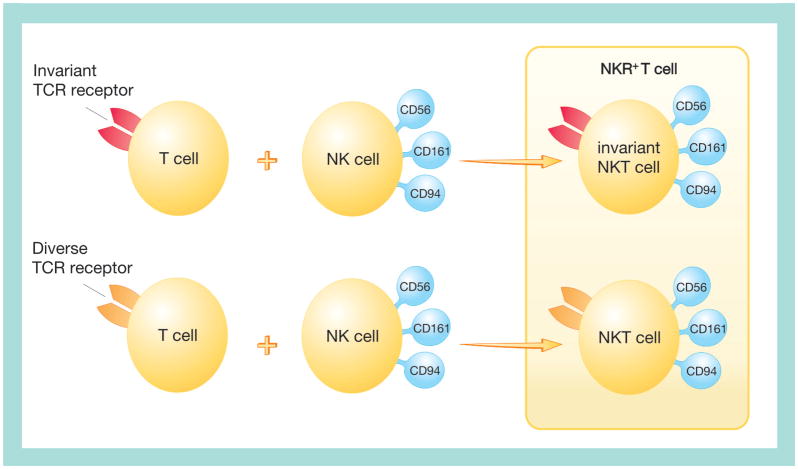
Figure 3. Features of T cells and natural killer† cells included in natural killer receptor-positive T-cell, natural killer T-cell and invariant natural killer T-cell subsets.
As for classical T cells, the NKR+ T cells coexpress a TCR and cell surface receptors that are characteristic of the NK cell lineage. NKR+ T cells constitute heterogeneous cell populations that represent a significant proportion of the total T-cell population. These cells are found in liver, bone marrow, spleen and GI tract and account for 35–50% of lymphocytes in these organs [200,201]. Based on their TCRs, these cells can be divided into at least two subsets: one that uses an invariant TCR receptor (invariant NKT cells) and a second with a diverse TCR (NKT cells). Invariant NKT cells constitute the low frequency of CD1d-restricted NKT cells that express NK cell surface receptors and highly restricted TCR repertoire encoded by Vα24 and Jα18 genes in humans are homologous to that encoded by Vα14 and Jα18 genes in mice. The majority are heterogeneous and coexpress other TCRs and various NK receptors, including CD56, CD94 and CD161.
†For clarity, only NK receptors are indicated.
NK: Natural killer; NKR+: Natural killer receptor-positive; TCR: T-cell receptor.
Table 4.
Alterations on natural killer T-cell receptors in multiple sclerosis.
| Receptor | Function | Alteration in MS [202] |
|---|---|---|
| CD56 | Marker correlates with cytotoxic function of CD8+ T cells [203] CD56+ T cells display both NK-like and T-cell-restricted cytotoxicity; capable of secreting cytokines IFN-γ, TNF-α and IL-4, which promote Th1 and Th2 adaptive immune responses [128,204] |
No differences in CD56+ T cells compared with HS and OND controls. No change between MS subtypes |
| CD161 | Costimulatory regulation of activity of CD1d-dependent T cells [205]; regulation of lymphocyte transmigration [206] CD161+ T cells are mainly memory cells Potential regulatory roles of CD161+ T cells, includes regulatory; in MS inferred from capability secrete IFN-γ upon stimulation [200] |
Reduced number of CD161+ T cells compared with HS; significant reductions in PPMS subgroup. CD161+ NK cells reduced compared with HS |
| CD94 | Expression on T cells protects against viral infection and autoimmunity [207] Recognizes/binds HLA-E; can be stimulatory or inhibitory to T cells and NK cells depending upon dimerization status [208,209] |
No change in CD94+ T cells compared with noninflammatory and inflammatory nature of OND controls. Reduced cell numbers compared with HS; significant reductions in SPMS cohort. No difference in cell numbers in neurological disease group compared with HS |
HS: Healthy subject; MS: Multiple sclerosis; NK: Natural killer, OND: Other neurological disease; PPMS: Primary progressive multiple sclerosis; SPMS: Secondary progressive multiple sclerosis.
Natural killer T cells are selected and restricted by CD1d – a nonpolymorphic MHC class I-like molecule that binds lipid antigens. The structural determination of CD1–ligand complex structures, such as CD1a-sGalCer [132], CD1a–lipopolypeptide [133], CD1b–phosphatidylinositol and CD1b–GM2 [35], CD1b–glucose monomycolate [36], mouse CD1d–α-GalCer (short chain, C8) [134], human CD1d–α-GalCer (long chain, C26) [135] and CD1d–phosphatidylcholine [136], has provided insights into general modes of ligand binding and elucidated the basis for CD1 isotype-specific variation. Each lipid ligand inserts its aliphatic moieties (two alkyl chains for glycolipids and usually two for lipopeptides) into the hydrophobic cavities of CD1 that position the diverse T-cell epitope carbohydrate moieties for glycolipids and peptidic moieties for lipopeptides above the hydrophobic binding groove on the CD1 surface. This renders them able to inspect TCRs specific for either ligand and/or CD1 isoform [137]. Promptly upon antigen stimulation, and consistent with preactivation status, NKT cells release large amounts of cytokines including IL-4 and IFN-γ, which affect the functions of neighboring cell populations of T cells, B cells, NK cells and dendritic cells.
Initially identified as the immune activator present in the extract of the marine sponge Agelas mauritianus, the development of a synthetic form of α-GalCer, known as KRN7000 ([2S,3S,4S]-1-O-[α-D-galactopyranosyl]-2-[N-hexacosanoylamino]-1,3,4-octadecanetriol), provided for the first time a well-characterized ligand that could be used to identify and activate virtually the entire population of iNKT cells. Although most mammalian ceramide glycolipids contain proximal sugars in the β-anomeric form (Figure 4B), KRN7000 is unusual in that the sugar moiety is linked in the α-anomeric configuration (Figure 4A). This glycolipid generates a high-affinity complex for the TCRs of most iNKT cells when presented by CD1d. However, because KRN7000 induces a mixed Th1- and Th2-cytokine response, the KRN7000 analog may be disadvantageous in many settings in which pure polarization of adaptive immune response is sought.
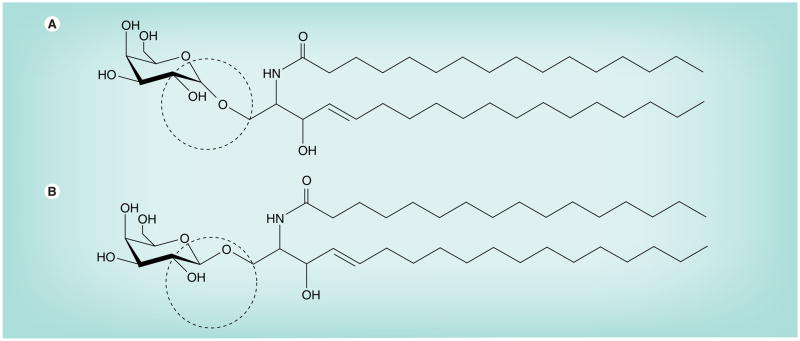
Figure 4. N-palmitoyl-galactosylceramide with galactose linked in either.
α (A) or β (B) configuration to ceramide backbone. β-linked forms of galactosylceramides (and glucosylceamides) are normal constituents of mammals, but α-linked forms are not. The α-linked form of galactosylceramides is found in Agelas mauritianus, a marine sponge. This glycolipid and its synthetic analog, KRN7000, have been used as specific activators of natural killer cells that are involved in the regulation of certain autoimmune diseases [210].
Subsequent studies have identified two other relevant analogs of α-GalCer; OCH [138] and the C-glycoside analog of α-GalCer, α-C-GalCer. The first analog, OCH, in which the sphingoid base is truncated to C9 and the fatty acid chain slightly shortened to C24:0, has distinct cytokine polarizing properties. The second one, α-C-GalCer, with the glycosidic oxygen, a polar hydrogen bond acceptor replaced by a nonpolar CH2 group, has generated great interest among NKT-cell biologists. The α-C linkage increases the stability of the lipid as this substitution renders it resistant to degradation by α-galactosidase in vivo. Contrary to the general impression that β-anomeric GalCer does not activate iNKT cells, investigation of the activity of four β-anomers that differed in the length of their acyl chains showed that two of these, β-GCa12 and β-GCa26, activated NKT cells in vivo associated with induction of IFN-γ and IL-4. Although these analogs were less potent than α-GalCer, they did induce transactivation of NK cells [139]. However, another study that showed activation of NKT cells by β-anomeric GalCer did not find evidence for NK cell transactivation [140].
Variability of efferent effectors is readily apparent from studies of the different patterns of cytokines that can be generated by different α-GalCer analogs, ranging from mixtures of Th1- and Th2-type cytokines (e.g., KRN7000) to a more pure Th2-like response (e.g., with OCH or α-GalCer-C20:2) or pure Th1-like response (e.g., α-C-GalCer). One would expect that if the strength of the TCR–glycolipid– CD1d interaction determines the Th1–Th2 balance, then changes in the carbohydrate moiety are likely to affect this balance.
Although it is thought that the stability of the CD1d–glycolipid complex is a factor contributing to the observed shift in cytokine-release profile, other factors might also contribute. To this end, characterization of lipid-reactive NKT cells and clarification of their functions will enable further understanding of the role of this cell subset in the immune response. NKT cells have regulatory effects in both experimental models and human auto-immune diseases including MS [141,142], and altered numbers and functions of NKT cells have been observed in MS [143–145]. Self-glycolipids may be activating ligands for NKT cells, an example of which is the self-reactivity of NKT cells [146,147]. The lack of CD1d polymorphism and the ligand-sharing cross-reactive responses of mouse and human NKT cells identify ligand-targeted NKT cells as attractive candidates for intervention in human autoimmune disease, such as MS [148]. This is entirely in keeping with NKT-cell regulation of autoimmune reactivity in EAE [149–151]. Since NKT cells produce both IFN-γ and IL-4 upon α-GalCer stimulation, α-GalCer was not expected to prevent EAE because NKT-cell-derived IFN-γ would mask the IL-4 protective effect that would be produced simultaneously [138,152]. Nevertheless, there have been reports of α-GalCer protection of mice against EAE [151,153]. Singh et al. proposed that the reason for protection was the repeated injections of α-GalCer and Th2 stimulation [151]. However, Jahng et al. protected C57BL/6 mice from EAE using only a single injection of α-GalCer [153], and Pal et al. observed no effect upon EAE in C57BL/6 mice of repeated α-GalCer injections [152]. More recently, Furlan et al. showed that EAE was suppressed only when α-GalCer with complete Freund’s adjuvant was administered subcutaneously, but not intraperitoneally, at the time of immunization [154]. Overall, the differences in these studies are confusing in regard to the role of NKT cells in the pathogenesis or prevention of autoimmunity in the CNS, and it may depend upon NKT subtype, disease stage, the timing or route of administration or the associated cytokine milieu; all being critical parameters in disease modulation. Overall, the consensus that NKT cells can be specifically targeted and manipulated in murine autoimmune disease using α-GalCer raises the possibility of influencing the NKT cell as a viable approach to therapeutic intervention in human autoimmune disease.
Besides α-GalCer and its analog-bearing shorter sphingosine, OCH, other exogenous lipids have been discovered that can serve as a ligand for NKT cells, including GSL-1 (Sphingomonas-derived glycosphingolipid [155]), BbGL-IIc (Borrelia burgdorferi-derived diacylglycerol glycolipid [156]), and iGb3 (isoglobotrihexosyl-ceramide [157]).
Little is known about self-glycolipid-reactive NKT although several self-glycolipid ligands for both invariant and variant CD1d-restricted NKT cells have been identified [157–159]. GSL that bind CD1d can stimulate specific T cells; and in MS an increased number of circulating T cells [160] recognizing different self-GSL and the acidic gangliosides GM1, GT1b and GQ1b, as well sGalCer and neutral GSL presented by CD1d molecules, have been reported [38,160,161]. Interestingly, T cells reacting to ganglioside GM1 also recognize intact GT1b, GQ1b and GD1a [161] indicating that the latter GSL may form complexes with CD1d in which the TCR recognizes a common minimal epitope constituting a five-sugar GM1 oligosaccharide. Moreover, GM1-containing sialic acid that is negatively charged does not induce proliferative cytokine responses, and GM1–CD1d tetramers do not stain NKT cells making it unlikely that the negative charge alone is responsible for specific recognition by NKT cells [162]. Other endogenous lipids that may stimulate CD1d-restricted T cells are phosphoglycerolipids such as phosphatidylinositol, phosphatidylethanolamine and phosphatidylglycerol [163].
For MS pathogenesis, brain-derived lipid stimulation of iNKT cells is now being examined. Although endogenous lipid ligands for iNKT cells have not yet been identified, sulfatide derived from myelin appears to be a ligand for non-iNKT cells [126] that have a diverse TCR repertoire but are restricted by CD1d [158,162].
It appears that recognition of sulfatide by CD1d-restricted NKT cells has important implications for CNS autoimmunity. Sulfatides are major glycolipids of myelin and in MS there is a T-cell-guided immune response that is either initiated from antigen presentation in CNS or induced after peripheral activation by a systemic molecular mimicry response [164,165]. Sulfatide stimulates a distinct population of CD1d-restricted NKT cells, and notably cis-tetracosenoyl sulfatide, an immunodominant myelin species in myelin, can mediate proliferation and cytokine secretion, and it can also be identified using CD1d tetramer staining. The 3′-sulfated galactose headgroup is exposed to the TCR as it projects from the binding pocket because of the β-linkage in contrast to the lower profile α-galactosyl headgroup. Sulfatide structure and binding to CD1d illustrates important implications for therapeutic design for target T cells reactive with myelin glycolipids. During the usual course of EAE, NKT cells reactive against sulfatide–CD1d tetramers, but not α-GalCer–CD1d tetramers, are increased several-fold within the CNS [158]. In addition, sulfatide treatment of mice prevents antigen-induced EAE employing the myelin oligodendrocyte glycoprotein 35–55 peptide. EAE was induced in CD1d+/+ mice, but not in CD1d−/− C57BL/6 mice, indicating that a sulfatide-reactive subset of NKT cells can be targeted for manipulation of autoimmune responses in experimental autoimmunity [158].
Sulfatide binds promiscuously to all of the CD1a, CD1b, CD1c, CD1d isoforms [38] and CD1 molecules are upregulated on macrophages in areas of demyelination in chronic-active MS lesions [166]. It appears then that myelin self-glycolipids could be presented to T cells in the course of local inflammatory events and microglia and infiltrating macrophages could engulf or internalize myelin by Fc receptors or by complement receptor-mediated phagocytosis binding to myelin-specific antibodies. Activated APCs in the CNS could present not only peptides (MHC) but also glycolipids (CD1) to T cells, and myelin glycolipid-reactive T cells could affect inflammation and demyelination of the CNS. It is also noteworthy that peripheral activation of sulfatide-reactive T cells after adjuvant-free administration of sulfatide ameliorates EAE [158].
Better delineation of the function of lipid-specific T cells in health and MS should provide useful insights into lipid use in the formulation of vaccines against microbial diseases and whether manipulation of CD1-restricted T cells is useful for treatment of autoimmune diseases including MS. Lipid-specific T cells are no longer an immunological curiosity and now rival the peptide-specific immune response for a role in the MS disease mechanism [167].
NKT-cell anergy in MS: role of FMCs in autoimmune responsiveness
Little is known of the physiology of self-glycolipid-reactive NKT cells compared with the α-GalCer-reactive iNKT cells. Glycolipid-reactive NKT cells regulate immunity in autoimmune diseases and, for MS pathogenesis, an intriguing question is whether brain-derived GSLs stimulate NKT cells. In our study, the responses to exogenous ligands, such as α-GalCer, as well as naturally occurring myelin glycolipids components (Figure 1B), including cerebroside, sulfatide, acetyl-cerebroside, FMCs, and the participation of iNKT cells in local regulation of MS were addressed. FMCs induced a two- to four-fold expansion of NKT cells and α-GalCer, an increase by sixfold of NKT-cell numbers. While half of healthy subject controls produced IFN-γ upon stimulation with α-GalCer, FMCs induced IFN-γ in all healthy subjects. Myelin-acetylated cerebrosides also induced a proliferative response similar to that with α-GalCer. We have observed that peripheral blood mononuclear cells of MS patients are hyporesponsive to GSL stimulation and thus manifest anergy to glycolipid stimulation, as shown by IFN-γ production or cytokine proliferation using the potent unnatural ligand α-GalCer [127]. We have recently found that FMC-7, an endogenous lipid antigen, has similar potent stimulation in healthy subjects and there is anergy in MS [O’Keeffe J, Hogan EL et al., Unpublished Data]. This indicates an anergy in peripheral blood mononuclear cell of MS immune response to certain GSLs.
The observation that NKT cells in MS peripheral blood failed to respond to stimulation with α-GalCer [127] or FMC-7 [O’Keeffe J et al., Manuscript In Preparation] in contrast to the robust increase in proliferation and IL-4 secretion observed in the healthy control subjects may stem from prior exposure to antigens including lipids and glycolipids that elicit innate immunity [127] and indicate continuing receptor occupancy by the ligands. Since α-GalCer benefits murine EAE, and might therefore have therapeutic value in MS [148,153], the finding of anergy in circulating leukocytes in MS to the glycolipid innate response has implications for drug development in MS immunotherapy [148].
Conclusion
Fast-migrating cerebrosides are acetyl derivatives of myelin mono-hexosyl glycolipid molecules that are enriched in CNS and PNS myelin and concentrated in spinal cord and white matter that are largely composed of myelinated nerve fibers. The appearance of the FMCs in the developing brain parallels that of GalCer and their enrichment in purified myelin from both the CNS and PNS indicate that the FMC (or 3-SAG series) GSLs affect myelin structure, and have significant biological meaning. Their hydrophobic surface structures are well suited to regulate the final phases of myelin compaction and achieve metabolic stability, thereby enabling function as a nerve fiber insulator that facilitates rapid saltatory impulse conduction. Owing to the greater FMC hydrophobicity (i.e., greater than cerebroside, sulfatide and GM4 ganglioside) that fosters hydrophobic inter-lipid binding, acetylated cerebrosides may play key roles during the critical stages of myelinogenesis or myelin maintenance. It is tempting to speculate that GSL–GSL interactions may occur during myelination - perhaps at the stage of the membrane wrapping the axon - to facilitate formation of a multilayered myelin sheath. Both FMC-1 and -2 contain an acetylated hydroxyl group on sphingosine at C3, are less susceptible to galactosidase digestion than GalCer, and are not recognized by O1 antibody, which is GalCer specific. The more complex FMCs that contain acetyl groups in addition to those of their 3-SAG backbone are more hydrophobic than GalCer. In addition, FMCs coappear with GalCer in the developing brain and disappear along with GalCer in demyelinating and dysmyelinating disorders.
The complex FMCs (FMC-5, -6 and -7) with penta- and hexa-acetylation of GalCer hydroxyls are unique, highly hydrophobic myelin lipids with potential beyond that of strengthening myelin membrane lipid interactions to act as potent antigens and provoke immune reactions both cellular and antibody/immunoglobulin mediated. Our study suggests that there may be a link between infection, immune response to novel myelin O-acetyl-cerebrosides, and demyelinating neurological disease in humans.
Future perspective
Knowledge of the physical and biochemical features of myelin lipids has now been expanded by the discovery of the acetylated cerebrosides that may be critical for the pathobiology of inflammatory demyelination in MS. The current needs are to determine their precise role in this process, define their synthetic pathway and the immune responses that they elicit. Taking into account that glycosphingolipids play important roles in neurological diseases, finding an MS-specific and likely lipid antigen(s) will be useful for differentiating the four currently postulated pathogenic mechanisms of demyelination. This could prove crucial for further understanding of MS pathogenesis, as well as for diagnosis, classification, evaluation of disease activity and therapeutic applications. Antilipid antibodies are candidate biomarkers for subclassification of MS phases and subtypes on the way to defining therapeutics and outcomes of individual therapies. Another important therapeutic objective could be the effective induction of remyelination within the demyelinated MS CNS lesions and the first indications of the possibility of the success of this approach have already appeared.
It is tempting to speculate that manipulation of the cellular and humoral facets of the immune system could promote endogenous CNS repair. The structure of CD1d lipid complexed with lipophilic invariant TCR may be critical to understanding MS pathoimmunology. Our understanding of the mechanisms whereby different glycolipid structures are able to stimulate qualitatively different iNKT-cell responses is still imperfect and continued development of this area of research is likely to enable the design of lipid antigen-specific therapeutics that will allow a more precise control of many potential activities of these lymphocytes for a broad range of applications. Although research in the field of pharmacological CD1d-based therapy is booming, the development of readily available therapeutics to modulate responses initiated by armed lipid-loaded CD1a, CD1b, CD1c and CD1e proteins remains largely unexplored. Analogs of α-GalCer with modified lipid structure may induce a bias in type of iNKT-cell effector functions, therefore preferentially reducing the release of proinflammatory Th1 cytokines and facilitating preferential production of the anti-inflammatory Th2 cytokines [138,168]. The rediscovery of lipids acting as antigenic molecules also advances the possibility of novel applications. CD1-presented lipids may become important targets of novel immunotherapy for the control of autoimmune diseases and for the induction of anti-inflammatory immune responses. Finally, there might be translation to clinical benefit, exemplified by lipids as novel components in vaccines, based on how specific lipids affect immunization and protection against autoimmune attack. Overall, there is reason for optimism with regard to therapeutic prospects in the foreseeable future.
Executive summary.
Myelin acetyl-cerebrosides: new molecular components of the myelin sheath
The myelin sheath is comprised of lipids (80%) and proteins (20%).
The three main classes of lipids that constitute CNS myelin are cholesterol, glycosphingolipids (derivatives of galactosylceramides [GalCer]) and phospholipids.
Myelin acetyl-cerebrosides, designated as fast-migrating cerebrosides (FMCs) by their TLC retention factor, are recently discovered glycosphingolipids (besides cerebrosides, sulfatides and GM1 and GM4 gangliosides) in the myelin sheath.
Myelin acetyl-cerebrosides: a glycolipid family with unique sphingosine 3-O-acetylation
FMC-1 and FMC-2 are 3-O-acetyl sphingosine-GalCers incorporating either nonhydroxy or 2-hydroxy fatty acyl, respectively.
FMC-3 and FMC-4 are analogs of FMC-1 and FMC-2 with an additional acetyl modification at the galactose C6 hydroxyl.
FMC-5, -6 and -7 have been characterized as more complex penta- and hexa-acetyl derivatives, all containing 3-O-acetyl-sphingosine- 2,3,4,6-tetra-O-acetyl-GalCer.
Occurrence of myelin acetyl-cerebrosides
FMCs are enriched in purified myelin fractions from vertebrate CNS and peripheral nervous system.
The pattern of appearance of the FMCs in the developing rat brain suggests that these nonpolar cerebrosides are regulated in concert with GalCer.
The pathway of FMC synthesis is not known and FMC function in oligodendrocyte-mediated myelinogenesis has not yet been examined.
FMCs are reduced quantitatively along with GalCer in de- and dys-myelinating disorders, including multiple sclerosis (MS), jimpy and quaking mouse mutants and GalCer-knockout mice.
Myelin acetyl-cerebrosides may trigger autoimmune demyelination in multiple sclerosis via a molecular mimicry mechanism involving lipids
The myelin acetyl-cerebrosides resemble the glycoconjugates of lipopolysaccharide.
FMCs are candidate lipid antigen mimics targeted by immune responses that are initially directed at bacterial acyl- or acetyl-conjugated glycosides and subsequently induce autoimmune inflammation in CNS.
Cross-reactivity of anti-FMC-7 antibody with microbial lipid (MfGL-II from Mycoplasma fermentans) has been observed.
Elevated titers for binding of FMC myelin lipid antigens has been observed in cerebrospinal fluid in relapsing–remitting MS in remission and primary progressive MS.
Hyporesponsiveness of myelin acetyl-cerebrosides in multiple sclerosis
Natural killer T-cells in MS peripheral blood failed to respond to stimulation with FMC-7 comparable with a similar response to α-GalCer stimulation.
The finding of anergy in circulating leukocytes in MS to a glycolipid innate response has implications for drug development in MS immunotherapy.
Acknowledgments
The authors thank Katarzyna Izydorczyk for graphic skills in rendering the myelin diagrams.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors gratefully acknowledge financial support from the National Multiple Sclerosis Society (NY, USA; Award RG3473), and NINDS/NIH (NS 115666). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Rosetti CM, Maggio B, Oliveira RG. The self-organization of lipids and proteins of myelin at the membrane interface. Molecular factors underlying the microheterogeneity of domain segregation. Biochim Biophys Acta. 2008;1778(7–8):1665–1675. doi: 10.1016/j.bbamem.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Norton WT, Autilio LA. The lipid composition of purified bovine brain myelin. J Neurochem. 1966;13(4):213–222. doi: 10.1111/j.1471-4159.1966.tb06794.x. [DOI] [PubMed] [Google Scholar]
- 3.Fewster ME, Hirono H, Mead JF. Lipid composition of myelin in multiple sclerosis. J Neurol. 1976;213(2):119–131. doi: 10.1007/BF00313273. [DOI] [PubMed] [Google Scholar]
- 4▪.Dasgupta S, Levery SB, Hogan EL. 3-O-acetyl-sphingosine-series myelin glycolipids: characterization of novel 3-O-acetyl-sphingosine galactosylceramide. J Lipid Res. 2002;43(5):751–761. First description of the acetylated derivatives of galactosylceramide. [PubMed] [Google Scholar]
- 5.Bennion B, Dasgupta S, Hogan EL, Levery SB. Characterization of novel myelin components 3-O-acetyl-sphingosine galactosylceramides by electrospray ionization Q-TOF MS and MS/CID-MS of Li+ adducts. J Mass Spectrom. 2007;42(5):598–620. doi: 10.1002/jms.1190. [DOI] [PubMed] [Google Scholar]
- 6▪▪.Podbielska M, Dasgupta S, Levery SB, et al. Novel myelin penta- and hexa-acetylgalactosyl- ceramides: structural characterization and immunoreactivity in cerebrospinal fluid. J Lipid Res. 2010;51(6):1394–1406. doi: 10.1194/jlr.M001396. Description of the penta- and hexa-acetyl derivatives of galactosylceramide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu L, Anchordoquy TJ. Cholesterol domains in cationic lipid/DNA complexes improve transfection. Biochim Biophys Acta. 2008;1778(10):2177–2181. doi: 10.1016/j.bbamem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Ramu Y, Lu Z. Removal of phosphohead groups of membrane lipids immobilizes voltage sensors of K+ channels. Nature. 2008;451(7180):826–829. doi: 10.1038/nature06618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu RK, Ledeen RW. Gangliosides of human, bovine, and rabbit plasma. J Lipid Res. 1972;13(5):680–686. [PubMed] [Google Scholar]
- 10.Wheeler D, Bandaru VV, Calabresi PA, Nath A, Haughey NJ. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 2008;131(Pt 11):3092–3102. doi: 10.1093/brain/awn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podbielska M, Hogan EL. Molecular and immunogenic features of myelin lipids: incitants or modulators of multiple sclerosis? Mult Scler. 2009;15(9):1011–1029. doi: 10.1177/1352458509106708. [DOI] [PubMed] [Google Scholar]
- 12.Kochetkov NK, Zhukova IG, Glukhoded IS. Sphingoplasmalogens. A new type of sphingolipids. Biochim Biophys Acta. 1963;70:716–719. doi: 10.1016/0006-3002(63)90823-0. [DOI] [PubMed] [Google Scholar]
- 13.Norton WT, Brotz M. New galactolipids of brain: a monoalkyl-monoacyl-glyceryl galactoside and cerebroside fatty acid esters. Biochem Biophys Res Commun. 1963;12:198–203. doi: 10.1016/0006-291x(63)90189-x. [DOI] [PubMed] [Google Scholar]
- 14.Kubota M, Taketomi T. Minor glycolipids being less polar than cerebroside in porcine spinal cord. Jpn J Exp Med. 1974;44(2):145–150. [PubMed] [Google Scholar]
- 15.Nudelman ED, Levery SB, Igarashi Y, Hakomori S. Plasmalopsychosine, a novel plasmal (fatty aldehyde) conjugate of psychosine with cyclic acetal linkage. Isolation and characterization from human brain white matter. J Biol Chem. 1992;267(16):11007–11016. [PubMed] [Google Scholar]
- 16.Levery SB, Nudelman ED, Hakomori S. Novel modification of glycosphingolipids by long-chain cyclic acetals: isolation and characterization of plasmalocerebroside from human brain. Biochemistry. 1992;31(23):5335–5340. doi: 10.1021/bi00138a013. [DOI] [PubMed] [Google Scholar]
- 17.Yachida Y, Kashiwagi M, Mikami T, et al. Stereochemical structures of synthesized and natural plasmalogalactosylceramides from equine brain. J Lipid Res. 1998;39(5):1039–1045. [PubMed] [Google Scholar]
- 18.Klenk E, Lohr JP. On the ester cerebrosides of brain. Hoppe Seylers Z Physiol Chem. 1967;348(12):1712–1714. [PubMed] [Google Scholar]
- 19.Tamai Y. Further study on the faster running glycolipid in brain. Jpn J Exp Med. 1968;38(1):65–73. [PubMed] [Google Scholar]
- 20.Kishimoto Y, Wajda M, Radin NS. 6-acyl galactosyl ceramides of pig brain: structure and fatty acid composition. J Lipid Res. 1968;9(1):27–33. [PubMed] [Google Scholar]
- 21.Dasgupta S, Everhart MB, Bhat NR, Hogan EL. Neutral monoglycosylceramides in rat brain: occurrence, molecular expression and developmental variation. Dev Neurosci. 1997;19(2):152–161. doi: 10.1159/000111201. [DOI] [PubMed] [Google Scholar]
- 22.Dasgupta S, Bhat NR, Spicer SS, Hogan EL, Furuya S, Hirabayashi Y. Cell-specific expression of neutral glycosphingolipids in vertebrate brain: immunochemical localization of 3-O-acetyl-sphingosine-series glycolipid(s) in myelin and oligodendrocytes. J Neurosci Res. 2007;85(13):2856–2862. doi: 10.1002/jnr.21419. [DOI] [PubMed] [Google Scholar]
- 23.Dupree JL, Girault JA, Popko B. Axo–glial interactions regulate the localization of axonal paranodal proteins. J Cell Biol. 1999;147(6):1145–1152. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki K, Suzuki Y. Globoid cell leucodystrophy (Krabbe’s disease): deficiency of galactocerebroside β-galactosidase. Proc Natl Acad Sci USA. 1970;66(2):302–309. doi: 10.1073/pnas.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki K, Suzuki Y. Galactosylceramide lipidosis: globoid cell leukodystrophy (Krabbe’s diesease) In: Stabury JC, Wyngaarden JB, Fredrikson DS, Goldstein JL, Brown MS, editors. The Metabolic Basis of Inherited Diesease. McGraw–Hill; NY, USA: 1983. pp. 857–880. [Google Scholar]
- 26.Svennerholm L, Vanier MT, Mansson JE. Krabbe disease: a galactosylsphingosine (psychosine) lipidosis. J Lipid Res. 1980;21(1):53–64. [PubMed] [Google Scholar]
- 27.Kobayashi T, Shinoda H, Goto I, Yamanaka T, Suzuki Y. Globoid cell leukodystrophy is a generalized galactosylsphingosine (psychosine) storage disease. Biochem Biophys Res Commun. 1987;144(1):41–46. doi: 10.1016/s0006-291x(87)80472-2. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Suzuki K. The twitcher mouse. A model of human globoid cell leukodystrophy (Krabbe’s disease) Am J Pathol. 1983;111(3):394–397. [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi H, Igisu H, Suzuki K, Suzuki K. Murine globoid cell leukodystrophy (the twitcher mouse). The presence of characteristic inclusions in the kidney and lymph nodes. Am J Pathol. 1983;112(2):147–154. [PMC free article] [PubMed] [Google Scholar]
- 30.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 31.Becher B, Bechmann I, Greter M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med. 2006;84(7):532–543. doi: 10.1007/s00109-006-0065-1. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee S, Soe TT, Maxfield FR. Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J Cell Biol. 1999;144(6):1271–1284. doi: 10.1083/jcb.144.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee S, Maxfield FR. Role of membrane organization and membrane domains in endocytic lipid trafficking. Traffic (Copenhagen, Denmark) 2000;1(3):203–211. doi: 10.1034/j.1600-0854.2000.010302.x. [DOI] [PubMed] [Google Scholar]
- 34.Sugita M, Porcelli SA, Brenner MB. Assembly and retention of CD1b heavy chains in the endoplasmic reticulum. J Immunol. 1997;159(5):2358–2365. [PubMed] [Google Scholar]
- 35.Gadola SD, Zaccai NR, Harlos K, et al. Structure of human CD1b with bound ligands at 2.3 A, a maze for alkyl chains. Nat Immunol. 2002;3(8):721–726. doi: 10.1038/ni821. [DOI] [PubMed] [Google Scholar]
- 36.Batuwangala T, Shepherd D, Gadola SD, et al. The crystal structure of human CD1b with a bound bacterial glycolipid. J Immunol. 2004;172(4):2382–2388. doi: 10.4049/jimmunol.172.4.2382. [DOI] [PubMed] [Google Scholar]
- 37.Moody DB, Ulrichs T, Muhlecker W, et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000;404(6780):884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- 38.Shamshiev A, Gober HJ, Donda A, Mazorra Z, Mori L, De Libero G. Presentation of the same glycolipid by different CD1 molecules. J Exp Med. 2002;195(8):1013–1021. doi: 10.1084/jem.20011963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Elzen P, Garg S, Leon L, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437(7060):906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- 40.Winau F, Schwierzeck V, Hurwitz R, et al. Saposin C is required for lipid presentation by human CD1b. Nat Immunol. 2004;5(2):169–174. doi: 10.1038/ni1035. [DOI] [PubMed] [Google Scholar]
- 41.Kang SJ, Cresswell P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat Immunol. 2004;5(2):175–181. doi: 10.1038/ni1034. [DOI] [PubMed] [Google Scholar]
- 42.Yuan W, Qi X, Tsang P, et al. Saposin B is the dominant saposin that facilitates lipid binding to human CD1d molecules. Proc Natl Acad Sci USA. 2007;104(13):5551–5556. doi: 10.1073/pnas.0700617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou D, Cantu C, 3rd, Sagiv Y, et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004;303(5657):523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai L, Sagiv Y, Liu Y, et al. Lysosomal recycling terminates CD1d-mediated presentation of short and polyunsaturated variants of the NKT cell lipid antigen αGalCer. Proc Natl Acad Sci USA. 2009;106(25):10254–10259. doi: 10.1073/pnas.0901228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85(3):299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 46.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26(9):485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Alt C, Laschinger M, Engelhardt B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood–brain barrier suggests their involvement in G-proteindependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur J Immunol. 2002;32(8):2133–2144. doi: 10.1002/1521-4141(200208)32:8<2133::AID-IMMU2133>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 48.Columba-Cabezas S, Serafini B, Ambrosini E, Aloisi F. Lymphoid chemokines CCL19 and CCL21 are expressed in the central nervous system during experimental autoimmune encephalomyelitis: implications for the maintenance of chronic neuroinflammation. Brain Pathol. 2003;13(1):38–51. doi: 10.1111/j.1750-3639.2003.tb00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greter M, Heppner FL, Lemos MP, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11(3):328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 50.Racke MK, Scott DE, Quigley L, et al. Distinct roles for B7–1 (CD-80) and B7–2 (CD-86) in the initiation of experimental allergic encephalomyelitis. J Clin Invest. 1995;96(5):2195–2203. doi: 10.1172/JCI118274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinberg AD, Wegmann KW, Funatake C, Whitham RH. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol. 1999;162(3):1818–1826. [PubMed] [Google Scholar]
- 52.Frohman EM, Filippi M, Stuve O, et al. Characterizing the mechanisms of progression in multiple sclerosis: evidence and new hypotheses for future directions. Arch Neurol. 2005;62(9):1345–1356. doi: 10.1001/archneur.62.9.1345. [DOI] [PubMed] [Google Scholar]
- 53.Chekhonin VP, Semenova AV, Gurina OI, Dmitrieva TB. Myelin oligodendrogliocyte glycoprotein: the structure, functions, role in pathogenesis of demyelinating disorders. Biomed Khim. 2003;49(5):411–423. [PubMed] [Google Scholar]
- 54.Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem. 2007;100(6):1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- 55.Jaskiewicz E. Epitopes on myelin proteins recognized by autoantibodies present in multiple sclerosis patients. Postepy Hig Med Dosw (Online) 2004;58:472–482. [PubMed] [Google Scholar]
- 56.Johns TG, Bernard CC. The structure and function of myelin oligodendrocyte glycoprotein. J Neurochem. 1999;72(1):1–9. doi: 10.1046/j.1471-4159.1999.0720001.x. [DOI] [PubMed] [Google Scholar]
- 57.Meinl E, Hohlfeld R. Immunopathogenesis of multiple sclerosis: MBP and beyond. Clin Exp Immunol. 2002;128(3):395–397. doi: 10.1046/j.1365-2249.2002.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tzakos AG, Troganis A, Theodorou V, et al. Structure and function of the myelin proteins: current status and perspectives in relation to multiple sclerosis. Curr Med Chem. 2005;12(13):1569–1587. doi: 10.2174/0929867054039026. [DOI] [PubMed] [Google Scholar]
- 59.Sobel RA, Greer JM, Kuchroo VK. Minireview: autoimmune responses to myelin proteolipid protein. Neurochem Res. 1994;19(8):915–921. doi: 10.1007/BF00968701. [DOI] [PubMed] [Google Scholar]
- 60.Musse AA, Harauz G. Molecular ‘negativity’ may underlie multiple sclerosis: role of the myelin basic protein family in the pathogenesis of MS. Int Rev Neurobiol. 2007;79:149–172. doi: 10.1016/S0074-7742(07)79007-4. [DOI] [PubMed] [Google Scholar]
- 61.Quarles RH. Myelin sheaths: glycoproteins involved in their formation, maintenance and degeneration. Cell Mol Life Sci. 2002;59(11):1851–1871. doi: 10.1007/PL00012510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menge T, Lalive PH, von Budingen HC, Cree B, Hauser SL, Genain CP. Antibody responses against galactocerebroside are potential stage-specific biomarkers in multiple sclerosis. J Allergy Clin Immunol. 2005;116(2):453–459. doi: 10.1016/j.jaci.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 63.Mata S, Lolli F, Soderstrom M, Pinto F, Link H. Multiple sclerosis is associated with enhanced B cell responses to the ganglioside GD1a. Mult Scler. 1999;5(6):379–388. doi: 10.1177/135245859900500i603. [DOI] [PubMed] [Google Scholar]
- 64.Sadatipour BT, Greer JM, Pender MP. Increased circulating antiganglioside antibodies in primary and secondary progressive multiple sclerosis. Ann Neurol. 1998;44(6):980–983. doi: 10.1002/ana.410440621. [DOI] [PubMed] [Google Scholar]
- 65.Acarin N, Rio J, Fernandez AL, et al. Different antiganglioside antibody pattern between relapsing-remitting and progressive multiple sclerosis. Acta Neurol Scand. 1996;93(2–3):99–103. doi: 10.1111/j.1600-0404.1996.tb00182.x. [DOI] [PubMed] [Google Scholar]
- 66.Ilyas AA, Chen ZW, Cook SD. Antibodies to sulfatide in cerebrospinal fluid of patients with multiple sclerosis. J Neuroimmunol. 2003;139(1–2):76–80. doi: 10.1016/s0165-5728(03)00131-0. [DOI] [PubMed] [Google Scholar]
- 67.Ryberg B. Multiple specificities of antibrain antibodies in multiple sclerosis and chronic myelopathy. J Neurol Sci. 1978;38(3):357–382. doi: 10.1016/0022-510x(78)90142-9. [DOI] [PubMed] [Google Scholar]
- 68.Sugiyama Y, Yamamoto T. Characterization of serum anti-phospholipid antibodies in patients with multiple sclerosis. Tohoku J Exp Med. 1996;178(3):203–215. doi: 10.1620/tjem.178.203. [DOI] [PubMed] [Google Scholar]
- 69▪▪.Kanter JL, Narayana S, Ho PP, et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med. 2006;12(1):138–143. doi: 10.1038/nm1344. Identification of lipid and oxidized lipid antigens reactive with IgG in multiple sclerosis cerebrospinal fluid. [DOI] [PubMed] [Google Scholar]
- 70.Zheng W, Kollmeyer J, Symolon H, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758(12):1864–1884. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 71.Taha TA, Mullen TD, Obeid LM. A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim Biophys Acta. 2006;1758(12):2027–2036. doi: 10.1016/j.bbamem.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stoffel W, Bosio A. Myelin glycolipids and their functions. Curr Opin Neurobiol. 1997;7(5):654–661. doi: 10.1016/s0959-4388(97)80085-2. [DOI] [PubMed] [Google Scholar]
- 73.Schenck M, Carpinteiro A, Grassme H, Lang F, Gulbins E. Ceramide: physiological and pathophysiological aspects. Arch Biochem Biophys. 2007;462(2):171–175. doi: 10.1016/j.abb.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 74.Obeid LM, Hannun YA. Ceramide: a stress signal and mediator of growth suppression and apoptosis. J Cell Biochem. 1995;58(2):191–198. doi: 10.1002/jcb.240580208. [DOI] [PubMed] [Google Scholar]
- 75.Pettus BJ, Chalfant CE, Hannun YA. Sphingolipids in inflammation: roles and implications. Curr Mol Med. 2004;4(4):405–418. doi: 10.2174/1566524043360573. [DOI] [PubMed] [Google Scholar]
- 76.Merrill AH, Jr, Schmelz EM, Dillehay DL, et al. Sphingolipids – the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol Appl Pharmacol. 1997;142(1):208–225. doi: 10.1006/taap.1996.8029. [DOI] [PubMed] [Google Scholar]
- 77.Kronke M. Involvement of sphingomyelinases in TNF signaling pathways. Chem Phys Lipids. 1999;102(1–2):157–166. doi: 10.1016/s0009-3084(99)00084-5. [DOI] [PubMed] [Google Scholar]
- 78.Kolesnick RN. Sphingomyelin and derivatives as cellular signals. Prog Lipid Res. 1991;30(1):1–38. doi: 10.1016/0163-7827(91)90005-p. [DOI] [PubMed] [Google Scholar]
- 79.Hannun YA, Obeid LM, Wolff RA. The novel second messenger ceramide: identification, mechanism of action, and cellular activity. Adv Lipid Res. 1993;25:43–64. [PubMed] [Google Scholar]
- 80.Wirguin I, Brenner T, Steinitz M, Abramsky O. In vitro synthesis of antibodies to myelin antigens by Epstein–Barr virus-transformed B lymphocytes from patients with neurologic disorders. J Neurol Sci. 1991;104(1):92–96. doi: 10.1016/0022-510x(91)90221-r. [DOI] [PubMed] [Google Scholar]
- 81.Richert JR, Robinson ED, Reuben-Burnside CA, et al. Measles virus-specific human T cell clones: studies of alloreactivity and antigenic cross-reactivity. J Neuroimmunol. 1988;19(1–2):59–68. doi: 10.1016/0165-5728(88)90035-5. [DOI] [PubMed] [Google Scholar]
- 82.Mameli G, Astone V, Arru G, et al. Brains and peripheral blood mononuclear cells of multiple sclerosis (MS) patients hyperexpress MS-associated retrovirus/HERV-W endogenous retrovirus, but not human herpesvirus 6. J Gen Virol. 2007;88(Pt 1):264–274. doi: 10.1099/vir.0.81890-0. [DOI] [PubMed] [Google Scholar]
- 83.Lunemann JD, Edwards N, Muraro PA, et al. Increased frequency and broadened specificity of latent EBV nuclear antigen-1-specific T cells in multiple sclerosis. Brain. 2006;129(Pt 6):1493–1506. doi: 10.1093/brain/awl067. [DOI] [PubMed] [Google Scholar]
- 84.Serafini B, Rosicarelli B, Franciotta D, et al. Dysregulated Epstein–Barr virus infection in the multiple sclerosis brain. J Exp Med. 2007;204(12):2899–2912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vartdal F, Vandvik B, Norrby E. Viral and bacterial antibody responses in multiple sclerosis. Ann Neurol. 1980;8(3):248–255. doi: 10.1002/ana.410080305. [DOI] [PubMed] [Google Scholar]
- 86.Vartdal F, Vandvik B. Multiple sclerosis: subclasses of intrathecally synthesized IgG and measles and varicella zoster virus IgG antibodies. Clin Exp Immunol. 1983;54(3):641–647. [PMC free article] [PubMed] [Google Scholar]
- 87.Salmi A, Reunanen M, Ilonen J, Panelius M. Intrathecal antibody synthesis to virus antigens in multiple sclerosis. Clin Exp Immunol. 1983;52(2):241–249. [PMC free article] [PubMed] [Google Scholar]
- 88.Burgoon MP, Williamson RA, Owens GP, et al. Cloning the antibody response in humans with inflammatory CNS disease: isolation of measles virus-specific antibodies from phage display libraries of a subacute sclerosing panencephalitis brain. J Neuroimmunol. 1999;94(1–2):204–211. doi: 10.1016/s0165-5728(98)00243-4. [DOI] [PubMed] [Google Scholar]
- 89.Cremer NE, Johnson KP, Fein G, Likosky WH. Comprehensive viral immunology of multiple sclerosis. II Analysis of serum and CSF antibodies by standard serologic methods. Arch Neurol. 1980;37(10):610–615. doi: 10.1001/archneur.1980.00500590034003. [DOI] [PubMed] [Google Scholar]
- 90.von Herrath MG, Fujinami RS, Whitton JL. Microorganisms and autoimmunity: making the barren field fertile? Nat Rev Microbiol. 2003;1(2):151–157. doi: 10.1038/nrmicro754. [DOI] [PubMed] [Google Scholar]
- 91.Wucherpfennig KW. Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest. 2001;108(8):1097–1104. doi: 10.1172/JCI14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92▪.Oldstone MB. Molecular mimicry, microbial infection, and autoimmune disease: evolution of the concept. Curr Top Microbiol Immunol. 2005;296:1–17. doi: 10.1007/3-540-30791-5_1. Early exposition of the possible role of molecular mimicry in the relationship of infection with autoimmune disease in man. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Noort JM, Bajramovic JJ, Plomp AC, van Stipdonk MJ. Mistaken self, a novel model that links microbial infections with myelindirected autoimmunity in multiple sclerosis. J Neuroimmunol. 2000;105(1):46–57. doi: 10.1016/s0165-5728(00)00181-8. [DOI] [PubMed] [Google Scholar]
- 94.Tejada-Simon MV, Zang YC, Hong J, Rivera VM, Zhang JZ. Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosis. Ann Neurol. 2003;53(2):189–197. doi: 10.1002/ana.10425. [DOI] [PubMed] [Google Scholar]
- 95.Sospedra M, Martin R. Molecular mimicry in multiple sclerosis. Autoimmunity. 2006;39(1):3–8. doi: 10.1080/08916930500484922. [DOI] [PubMed] [Google Scholar]
- 96.McCoy L, Tsunoda I, Fujinami RS. Multiple sclerosis and virus induced immune responses: autoimmunity can be primed by molecular mimicry and augmented by bystander activation. Autoimmunity. 2006;39(1):9–19. doi: 10.1080/08916930500484799. [DOI] [PubMed] [Google Scholar]
- 97.Barnett LA, Fujinami RS. Molecular mimicry: a mechanism for autoimmune injury. FASEB J. 1992;6(3):840–844. doi: 10.1096/fasebj.6.3.1740233. [DOI] [PubMed] [Google Scholar]
- 98.Oldstone MB. Molecular mimicry and immune-mediated diseases. FASEB J. 1998;12(13):1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McLean BN, Luxton RW, Thompson EJ. A study of immunoglobulin G in the cerebrospinal fluid of 1007 patients with suspected neurological disease using isoelectric focusing and the Log IgG-Index. A comparison and diagnostic applications. Brain. 1990;113(Pt 5):1269–1289. doi: 10.1093/brain/113.5.1269. [DOI] [PubMed] [Google Scholar]
- 100.Correale J, de los Milagros Bassani Molinas M. Oligoclonal bands and antibody responses in multiple sclerosis. J Neurol. 2002;249(4):375–389. doi: 10.1007/s004150200026. [DOI] [PubMed] [Google Scholar]
- 101▪.Antel J, Bar-Or A. Roles of immunoglobulins and B cells in multiple sclerosis: from pathogenesis to treatment. J Neuroimmunol. 2006;180(1–2):3–8. doi: 10.1016/j.jneuroim.2006.06.032. Recent review of the possible roles of B cells and immunoglobulins in multiple sclerosis. [DOI] [PubMed] [Google Scholar]
- 102.Melendez-Vasquez C, Redford J, Choudhary PP, et al. Immunological investigation of chronic inflammatory demyelinating polyradiculoneuropathy. J Neuroimmunol. 1997;73(1–2):124–134. doi: 10.1016/s0165-5728(96)00189-0. [DOI] [PubMed] [Google Scholar]
- 103.Goodyear CS, O’Hanlon GM, Plomp JJ, et al. Monoclonal antibodies raised against Guillain–Barre syndrome-associated Campylobacter jejuni lipopolysaccharides react with neuronal gangliosides and paralyze muscle-nerve preparations. J Clin Invest. 1999;104(6):697–708. doi: 10.1172/JCI6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yuki N. Molecular mimicry between gangliosides and lipopolysaccharides of Campylobacter jejuni isolated from patients with Guillain–Barre syndrome and Miller Fisher syndrome. J Infect Dis. 1997;176(Suppl 2):S150–S153. doi: 10.1086/513800. [DOI] [PubMed] [Google Scholar]
- 105.Yuki N, Miyatake T. Guillain–Barre syndrome and Fisher’s syndrome following Campylobacter jejuni infection. Ann NY Acad Sci. 1998;845:330–340. doi: 10.1111/j.1749-6632.1998.tb09685.x. [DOI] [PubMed] [Google Scholar]
- 106▪.Prendergast MM, Willison HJ, Moran AP. Human monoclonal immunoglobulin M antibodies to ganglioside GM1 show diverse cross-reactivities with lipopolysaccharides of Campylobacter jejuni strains associated with Guillain–Barre syndrome. Infect Immun. 1999;67(7):3698–3701. doi: 10.1128/iai.67.7.3698-3701.1999. Good example of the support for glycolipid-based molecular mimicry to Campylobacter jejuni bacterial infection and autoimmune disease, the Guillain–Barré syndrome, in the human peripheral nervous system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sack DA, Lastovica AJ, Chang SH, Pazzaglia G. Microtiter assay for detecting Campylobacter spp. and Helicobacter pylori with surface gangliosides which bind cholera toxin. J Clin Microbiol. 1998;36(7):2043–2045. doi: 10.1128/jcm.36.7.2043-2045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mori M, Kuwabara S, Miyake M, et al. Haemophilus influenzae has a GM1 ganglioside-like structure and elicits Guillain–Barre syndrome. Neurology. 1999;52(6):1282–1284. doi: 10.1212/wnl.52.6.1282. [DOI] [PubMed] [Google Scholar]
- 109.Yu RK, Ariga T. The role of glycosphingolipids in neurological disorders. Mechanisms of immune action. Ann NY Acad Sci. 1998;845:285–306. doi: 10.1111/j.1749-6632.1998.tb09682.x. [DOI] [PubMed] [Google Scholar]
- 110.Katzenellenbogen E, Kocharova NA, Korzeniowska-Kowal A, et al. Immunochemical studies of the lipopolysaccharides of Hafnia alvei PCM 1219 and other strains with the O-antigens containing D-glucose 1-phosphate and 2-deoxy-2-[(R)-3- hydroxybutyramido]-D-glucose. Arch Immunol Ther Exp (Warsz) 2008;56(5):347–352. doi: 10.1007/s00005-008-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parolis H, Parolis LA, Olivieri G. Structural studies on the Shigella-like Escherichia coli O121 O-specific polysaccharide. Carbohydr Res. 1997;303(3):319–325. doi: 10.1016/s0008-6215(97)00178-x. [DOI] [PubMed] [Google Scholar]
- 112.Ali T, Weintraub A, Widmalm G. Structural determination of the O-antigenic polysaccharide from the Shiga toxinproducing Escherichia coli O171. Carbohydr Res. 2006;341(11):1878–1883. doi: 10.1016/j.carres.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 113.Sidorczyk Z, Toukach FV, Zych K, et al. Structural and serological relatedness of the O-antigens of Proteus penneri 1 and 4 from a novel Proteus serogroup O72. Eur J Biochem. 2002;269(1):358–363. doi: 10.1046/j.0014-2956.2001.02660.x. [DOI] [PubMed] [Google Scholar]
- 114.Kubler-Kielb J, Vinogradov E, Chu C, Schneerson R. O-Acetylation in the O-specific polysaccharide isolated from Shigella flexneri serotype 2a. Carbohydr Res. 2007;342(3–4):643–647. doi: 10.1016/j.carres.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schroder NW, Schombel U, Heine H, Gobel UB, Zahringer U, Schumann RR. Acylated cholesteryl galactoside as a novel immunogenic motif in Borrelia burgdorferi sensu stricto. J Biol Chem. 2003;278(36):33645–33653. doi: 10.1074/jbc.M305799200. [DOI] [PubMed] [Google Scholar]
- 116.Yildirim HH, Li J, Richards JC, Hood DW, Moxon ER, Schweda EK. Complex O-acetylation in non-typeable Haemophilus influenzae lipopolysaccharide: evidence for a novel site of O-acetylation. Carbohydr Res. 2005;340(17):2598–2611. doi: 10.1016/j.carres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 117.MacLean LL, Webb AC, Perry MB. Structural elucidation of the O-antigenic polysaccharide from enterohemorrhagic (EHEC) Escherichia coli O48:H21. Carbohydr Res. 2006;341(15):2543–2549. doi: 10.1016/j.carres.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 118.Compston A. Complexity and heterogeneity in demyelinating disease. Brain. 2007;130(Pt 5):1178–1180. doi: 10.1093/brain/awm092. [DOI] [PubMed] [Google Scholar]
- 119.Lassmann H, Bruck W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med. 2001;7(3):115–121. doi: 10.1016/s1471-4914(00)01909-2. [DOI] [PubMed] [Google Scholar]
- 120.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2(1):11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 121.Ben-Nun A, Wekerle H, Cohen IR. Vaccination against autoimmune encephalomyelitis with T-lymphocyte line cells reactive against myelin basic protein. Nature. 1981;292(5818):60–61. doi: 10.1038/292060a0. [DOI] [PubMed] [Google Scholar]
- 122.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184(6):2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186(10):1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Van Kaer L. Natural killer T cells as targets for immunotherapy of autoimmune diseases. Immunol Cell Biol. 2004;82(3):315–322. doi: 10.1111/j.0818-9641.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- 125.Linsen L, Somers V, Stinissen P. Immunoregulation of autoimmunity by natural killer T cells. Hum Immunol. 2005;66(12):1193–1202. doi: 10.1016/j.humimm.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 126.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4(3):231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 127.O’Keeffe J, Gately CM, Counihan T, et al. T-cells expressing natural killer (NK) receptors are altered in multiple sclerosis and responses to α-galactosylceramide are impaired. J Neurol Sci. 2008;275(1–2):22–28. doi: 10.1016/j.jns.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Doherty DG, Norris S, Madrigal-Estebas L, et al. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163(4):2314–2321. [PubMed] [Google Scholar]
- 129.Snyder MR, Nakajima T, Leibson PJ, Weyand CM, Goronzy JJ. Stimulatory killer Ig-like receptors modulate T cell activation through DAP12-dependent and DAP12- independent mechanisms. J Immunol. 2004;173(6):3725–3731. doi: 10.4049/jimmunol.173.6.3725. [DOI] [PubMed] [Google Scholar]
- 130.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 131.Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2(5):336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 132.Zajonc DM, Elsliger MA, Teyton L, Wilson IA. Crystal structure of CD1a in complex with a sulfatide self antigen at a resolution of 2.15 A. Nat Immunol. 2003;4(8):808–815. doi: 10.1038/ni948. [DOI] [PubMed] [Google Scholar]
- 133.Zajonc DM, Crispin MD, Bowden TA, et al. Molecular mechanism of lipopeptide presentation by CD1a. Immunity. 2005;22(2):209–219. doi: 10.1016/j.immuni.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 134.Zajonc DM, Cantu C, 3rd, Mattner J, et al. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6(8):810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koch M, Stronge VS, Shepherd D, et al. The crystal structure of human CD1d with and without α-galactosylceramide. Nat Immunol. 2005;6(8):819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 136.Giabbai B, Sidobre S, Crispin MD, et al. Crystal structure of mouse CD1d bound to the self ligand phosphatidylcholine: a molecular basis for NKT cell activation. J Immunol. 2005;175(2):977–984. doi: 10.4049/jimmunol.175.2.977. [DOI] [PubMed] [Google Scholar]
- 137.Moody DB, Zajonc DM, Wilson IA. Anatomy of CD1-lipid antigen complexes. Nat Rev Immunol. 2005;5(5):387–399. doi: 10.1038/nri1605. [DOI] [PubMed] [Google Scholar]
- 138.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413(6855):531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 139.Parekh VV, Singh AK, Wilson MT, et al. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct α- and β-anomeric glycolipids. J Immunol. 2004;173(6):3693–3706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- 140.Ortaldo JR, Young HA, Winkler-Pickett RT, Bere EW, Jr, Murphy WJ, Wiltrout RH. Dissociation of NKT stimulation, cytokine induction, and NK activation in vivo by the use of distinct TCR-binding ceramides. J Immunol. 2004;172(2):943–953. doi: 10.4049/jimmunol.172.2.943. [DOI] [PubMed] [Google Scholar]
- 141▪.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1ddependent NKT cells. J Clin Invest. 2004;114(10):1379–1388. doi: 10.1172/JCI23594. Recent review of the role of glycolipidreactive invariant natural killer T cells in the regulation of immune responsiveness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol. 2003;3(3):211–222. doi: 10.1038/nri1028. [DOI] [PubMed] [Google Scholar]
- 143.Illes Z, Kondo T, Newcombe J, Oka N, Tabira T, Yamamura T. Differential expression of NK T cell V α 24J α Q invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J Immunol. 2000;164(8):4375–4381. doi: 10.4049/jimmunol.164.8.4375. [DOI] [PubMed] [Google Scholar]
- 144.Demoulins T, Gachelin G, Bequet D, Dormont D. A biased Vα24+ T-cell repertoire leads to circulating NKT-cell defects in a multiple sclerosis patient at the onset of his disease. Immunol Lett. 2003;90(2–3):223–228. doi: 10.1016/j.imlet.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 145.Araki M, Kondo T, Gumperz JE, Brenner MB, Miyake S, Yamamura T. Th2 bias of CD4+ NKT cells derived from multiple sclerosis in remission. Int Immunol. 2003;15(2):279–288. doi: 10.1093/intimm/dxg029. [DOI] [PubMed] [Google Scholar]
- 146.D’Andrea A, Goux D, De Lalla C, et al. Neonatal invariant Vα24+ NKT lymphocytes are activated memory cells. Eur J Immunol. 2000;30(6):1544–1550. doi: 10.1002/1521-4141(200006)30:6<1544::AID-IMMU1544>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 147.van Der Vliet HJ, Nishi N, de Gruijl TD, et al. Human natural killer T cells acquire a memory-activated phenotype before birth. Blood. 2000;95(7):2440–2442. [PubMed] [Google Scholar]
- 148.Miyake S, Yamamura T. Therapeutic potential of glycolipid ligands for natural killer (NK) T cells in the suppression of autoimmune diseases. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(3):315–322. doi: 10.2174/1568008054863772. [DOI] [PubMed] [Google Scholar]
- 149.Mars LT, Gautron AS, Novak J, et al. Invariant NKT cells regulate experimental autoimmune encephalomyelitis and infiltrate the central nervous system in a CD1d-independent manner. J Immunol. 2008;181(4):2321–2329. doi: 10.4049/jimmunol.181.4.2321. [DOI] [PubMed] [Google Scholar]
- 150.Winkler-Pickett R, Young HA, Cherry JM, et al. In vivo regulation of experimental autoimmune encephalomyelitis by NK cells: alteration of primary adaptive responses. J Immunol. 2008;180(7):4495–4506. doi: 10.4049/jimmunol.180.7.4495. [DOI] [PubMed] [Google Scholar]
- 151.Singh AK, Wilson MT, Hong S, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194(12):1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pal E, Tabira T, Kawano T, Taniguchi M, Miyake S, Yamamura T. Costimulationdependent modulation of experimental autoimmune encephalomyelitis by ligand stimulation of V α 14 NK T cells. J Immunol. 2001;166(1):662–668. doi: 10.4049/jimmunol.166.1.662. [DOI] [PubMed] [Google Scholar]
- 153.Jahng AW, Maricic I, Pedersen B, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194(12):1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Furlan R, Bergami A, Cantarella D, et al. Activation of invariant NKT cells by αGalCer administration protects mice from MOG35– 55-induced EAE: critical roles for administration route and IFN-γ. Eur J Immunol. 2003;33(7):1830–1838. doi: 10.1002/eji.200323885. [DOI] [PubMed] [Google Scholar]
- 155.Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434(7032):520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 156.Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7(9):978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 157.Zhou D, Mattner J, Cantu C, 3rd, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306(5702):1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 158.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199(7):947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198(1):173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shamshiev A, Donda A, Carena I, Mori L, Kappos L, De Libero G. Self glycolipids as T-cell autoantigens. Eur J Immunol. 1999;29(5):1667–1675. doi: 10.1002/(SICI)1521-4141(199905)29:05<1667::AID-IMMU1667>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 161.Shamshiev A, Donda A, Prigozy TI, et al. The αβ T cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity. 2000;13(2):255–264. doi: 10.1016/s1074-7613(00)00025-x. [DOI] [PubMed] [Google Scholar]
- 162.Zajonc DM, Maricic I, Wu D, et al. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J Exp Med. 2005;202(11):1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gumperz JE, Roy C, Makowska A, et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12(2):211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 164.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 165.Prat A, Antel J. Pathogenesis of multiple sclerosis. Curr Opin Neurol. 2005;18(3):225–230. doi: 10.1097/01.wco.0000169737.99040.31. [DOI] [PubMed] [Google Scholar]
- 166.Battistini L, Fischer FR, Raine CS, Brosnan CF. CD1b is expressed in multiple sclerosis lesions. J Neuroimmunol. 1996;67(2):145–151. doi: 10.1016/0165-5728(96)00045-8. [DOI] [PubMed] [Google Scholar]
- 167.De Libero G, Macdonald HR, Dellabona P. T cell recognition of lipids: quo vadis? Nat Immunol. 2007;8(3):223–227. doi: 10.1038/ni0307-223. [DOI] [PubMed] [Google Scholar]
- 168.Yu KO, Im JS, Molano A, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc Natl Acad Sci USA. 2005;102(9):3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Halder RC, Jahng A, Maricic I, Kumar V. Mini review: immune response to myelin-derived sulfatide and CNS-demyelination. Neurochem Res. 2007;32(2):257–262. doi: 10.1007/s11064-006-9145-4. [DOI] [PubMed] [Google Scholar]
- 170.Kolodny E, De Gasper R, GamaSosa MA, Weinreb HJ, Herbert J. Antisulfatide immunoglobulin G is elevated in the serum of multiple sclerosis patients. Ann Neurol. 1995;38:340. [Google Scholar]
- 171.Kirschning E, Rutter G, Uhlig H, Dernick R. A sulfatide-reactive human monoclonal antibody obtained from a multiple sclerosis patient selectively binds to the surface of oligodendrocytes. J Neuroimmunol. 1995;56(2):191–200. doi: 10.1016/0165-5728(94)00150-m. [DOI] [PubMed] [Google Scholar]
- 172.Dyer CA, Benjamins JA. Galactocerebroside and sulfatide independently mediate Ca2+ responses in oligodendrocytes. J Neurosci Res. 1991;30(4):699–711. doi: 10.1002/jnr.490300414. [DOI] [PubMed] [Google Scholar]
- 173.Rosenbluth J, Moon D. Dysmyelination induced in vitro by IgM antisulfatide and antigalactocerebroside monoclonal antibodies. J Neurosci Res. 2003;71(1):104–109. doi: 10.1002/jnr.10448. [DOI] [PubMed] [Google Scholar]
- 174.Bansal R, Pfeiffer SE. Reversible inhibition of oligodendrocyte progenitor differentiation by a monoclonal antibody against surface galactolipids. Proc Natl Acad Sci USA. 1989;86(16):6181–6185. doi: 10.1073/pnas.86.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dupree JL, Coetzee T, Suzuki K, Popko B. Myelin abnormalities in mice deficient in galactocerebroside and sulfatide. J Neurocytol. 1998;27(9):649–659. doi: 10.1023/a:1006908013972. [DOI] [PubMed] [Google Scholar]
- 176.Marcus J, Popko B. Galactolipids are molecular determinants of myelin development and axo-glial organization. Biochim Biophys Acta. 2002;1573(3):406–413. doi: 10.1016/s0304-4165(02)00410-5. [DOI] [PubMed] [Google Scholar]
- 177.Ishibashi T, Dupree JL, Ikenaka K, et al. A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation. J Neurosci. 2002;22(15):6507–6514. doi: 10.1523/JNEUROSCI.22-15-06507.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia. 2006;53(4):372–381. doi: 10.1002/glia.20292. [DOI] [PubMed] [Google Scholar]
- 179.Jeon SB, Yoon HJ, Park SH, Kim IH, Park EJ. Sulfatide, a major lipid component of myelin sheath, activates inflammatory responses as an endogenous stimulator in brain-resident immune cells. J Immunol. 2008;181(11):8077–8087. doi: 10.4049/jimmunol.181.11.8077. [DOI] [PubMed] [Google Scholar]
- 180.Stevens A, Weller M, Wietholter H. CSF and serum ganglioside antibody patterns in MS. Acta Neurol Scand. 1992;86(5):485–489. doi: 10.1111/j.1600-0404.1992.tb05129.x. [DOI] [PubMed] [Google Scholar]
- 181.Pender MP, Csurhes PA, Wolfe NP, et al. Increased circulating T cell reactivity to GM3 and GQ1b gangliosides in primary progressive multiple sclerosis. J Clin Neurosci. 2003;10(1):63–66. doi: 10.1016/s0967-5868(02)00270-9. [DOI] [PubMed] [Google Scholar]
- 182.Valentino P, Labate A, Nistico R, et al. Anti-GM1 antibodies are not associated with cerebral atrophy in patients with multiple sclerosis. Mult Scler. 2009;15(1):114–115. doi: 10.1177/1352458508096685. [DOI] [PubMed] [Google Scholar]
- 183.Giovannoni G, Morris PR, Keir G. Circulating antiganglioside antibodies are not associated with the development of progressive disease or cerebral atrophy in patients with multiple sclerosis. Ann Neurol. 2000;47(5):684–685. [PubMed] [Google Scholar]
- 184.Ruf P, Jager M, Ellwart J, Wosch S, Kusterer E, Lindhofer H. Two new trifunctional antibodies for the therapy of human malignant melanoma. Int J Cancer. 2004;108(5):725–732. doi: 10.1002/ijc.11630. [DOI] [PubMed] [Google Scholar]
- 185.Bjartmar C, Kinkel RP, Kidd G, Rudick RA, Trapp BD. Axonal loss in normal-appearing white matter in a patient with acute MS. Neurology. 2001;57(7):1248–1252. doi: 10.1212/wnl.57.7.1248. [DOI] [PubMed] [Google Scholar]
- 186.Bjartmar C, Trapp BD. Axonal degeneration and progressive neurologic disability in multiple sclerosis. Neurotox Res. 2003;5(1–2):157–164. doi: 10.1007/BF03033380. [DOI] [PubMed] [Google Scholar]
- 187.Zaprianova E, Deleva D, Ilinov P, et al. Serum ganglioside patterns in multiple sclerosis. Neurochem Res. 2001;26(2):95–100. doi: 10.1023/a:1011027125744. [DOI] [PubMed] [Google Scholar]
- 188.Roussel V, Yi F, Jauberteau MO, et al. Prevalence and clinical significance of anti-phospholipid antibodies in multiple sclerosis: a study of 89 patients. J Autoimmun. 2000;14(3):259–265. doi: 10.1006/jaut.2000.0367. [DOI] [PubMed] [Google Scholar]
- 189.Cordoliani MA, Michon-Pasturel U, Rerat K, et al. Multiple sclerosis and antiphospholipid antibodies: study of 62 consecutive patients. Rev Med Interne. 1998;19(9):635–639. doi: 10.1016/s0248-8663(99)80042-3. [DOI] [PubMed] [Google Scholar]
- 190.D’Olhaberriague L, Levine SR, Salowich-Palm L, et al. Specificity, isotype, and titer distribution of anticardiolipin antibodies in CNS diseases. Neurology. 1998;51(5):1376–1380. doi: 10.1212/wnl.51.5.1376. [DOI] [PubMed] [Google Scholar]
- 191.Tourbah A, Clapin A, Gout O, et al. Systemic autoimmune features and multiple sclerosis: a 5-year follow-up study. Arch Neurol. 1998;55(4):517–521. doi: 10.1001/archneur.55.4.517. [DOI] [PubMed] [Google Scholar]
- 192.Lolli F, Mata S, Baruffi MC, Amaducci L. Cerebrospinal fluid anti-cardiolipin antibodies in neurological diseases. Clin Immunol Immunopathol. 1991;59(2):314–321. doi: 10.1016/0090-1229(91)90027-8. [DOI] [PubMed] [Google Scholar]
- 193.Chaleomchan W, Hemachudha T, Sakulramrung R, Deesomchok U. Anticardiolipin antibodies in patients with rabies vaccination induced neurological complications and other neurological diseases. J Neurol Sci. 1990;96(2–3):143–151. doi: 10.1016/0022-510x(90)90127-9. [DOI] [PubMed] [Google Scholar]
- 194.Marchiori PE, Dos Reis M, Quevedo ME, et al. Cerebrospinal fluid and serum antiphospholipid antibodies in multiple sclerosis, Guillain–Barre syndrome and systemic lupus erythematosus. Arq Neuropsiquiatr. 1990;48(4):465–468. doi: 10.1590/s0004-282x1990000400010. [DOI] [PubMed] [Google Scholar]
- 195.Karussis D, Leker RR, Ashkenazi A, Abramsky O. A subgroup of multiple sclerosis patients with anticardiolipin antibodies and unusual clinical manifestations: do they represent a new nosological entity? Ann Neurol. 1998;44(4):629–634. doi: 10.1002/ana.410440408. [DOI] [PubMed] [Google Scholar]
- 196.Tartaglia MC, Narayanan S, De Stefano N, et al. Choline is increased in pre-lesional normal appearing white matter in multiple sclerosis. J Neurol. 2002;249(10):1382–1390. doi: 10.1007/s00415-002-0846-6. [DOI] [PubMed] [Google Scholar]
- 197.Qin J, Goswami R, Balabanov R, Dawson G. Oxidized phosphatidylcholine is a marker for neuroinflammation in multiple sclerosis brain. J Neurosci Res. 2007;85(5):977–984. doi: 10.1002/jnr.21206. [DOI] [PubMed] [Google Scholar]
- 198.Woodruff RH, Franklin RJ. Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide, and complement/anti-galactocerebroside: a comparative study. Glia. 1999;25(3):216–228. doi: 10.1002/(sici)1098-1136(19990201)25:3<216::aid-glia2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 199.Villar LM, Sadaba MC, Roldan E, et al. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J Clin Invest. 2005;115(1):187–194. doi: 10.1172/JCI22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.O’Keeffe J, Doherty DG, Kenna T, et al. Diverse populations of T cells with NK cell receptors accumulate in the human intestine in health and in colorectal cancer. Eur J Immunol. 2004;34(8):2110–2119. doi: 10.1002/eji.200424958. [DOI] [PubMed] [Google Scholar]
- 201.Kenna T, Golden-Mason L, Porcelli SA, et al. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J Immunol. 2003;171(4):1775–1779. doi: 10.4049/jimmunol.171.4.1775. [DOI] [PubMed] [Google Scholar]
- 202.O’Keeffe J, Gately CM, Counihan T, et al. T-cells expressing natural killer (NK) receptors are altered in multiple sclerosis and responses to α-galactosylceramide are impaired. J Neurol Sci. 2008;275(1–2):22–28. doi: 10.1016/j.jns.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. Cutting edge: cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J Immunol. 2000;164(3):1148–1152. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]
- 204.Loza MJ, Metelitsa LS, Perussia B. NKT and T cells: coordinate regulation of NK-like phenotype and cytokine production. Eur J Immunol. 2002;32(12):3453–3462. doi: 10.1002/1521-4141(200212)32:12<3453::AID-IMMU3453>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 205.Exley M, Porcelli S, Furman M, Garcia J, Balk S. CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T cells expressing invariant V α 24 J α Q T cell receptor α chains. J Exp Med. 1998;188(5):867–876. doi: 10.1084/jem.188.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Poggi A, Costa P, Zocchi MR, Moretta L. Phenotypic and functional analysis of CD4+ NKRP1A+ human T lymphocytes. Direct evidence that the NKRP1A molecule is involved in transendothelial migration. Eur J Immunol. 1997;27(9):2345–2350. doi: 10.1002/eji.1830270932. [DOI] [PubMed] [Google Scholar]
- 207.Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94–NKG2A receptors regulate antiviral CD8+ T cell responses. Nat Immunol. 2002;3(2):189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- 208.Lee N, Llano M, Carretero M, et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci USA. 1998;95(9):5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 210.Van Kaer L. α-galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5(1):31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]