Abstract
Objective
Herpes simplex virus type 2 (HSV-2) suppression has been shown to reduce HIV-1 disease progression in non-pregnant women and men, but effects on pregnant and postpartum women have not been described.
Methods
We analyzed data from a cohort of Kenyan women participating in a randomized clinical trial of HSV-2 suppression. Pregnant HIV-1-seropositive, HSV-2-seropositive women who were not eligible for antiretroviral therapy (WHO stage 1–2, CD4>250 cells/µl) were randomized to either 500 mg valacyclovir or placebo twice daily from 34 weeks gestation through 12 months postpartum. Women received zidovudine and single-dose nevirapine for prevention of mother-to-child HIV-1 transmission. HIV-1 progression markers, including CD4 count and plasma HIV-1 RNA levels, were measured serially. Multivariate linear regression was used to compare progression markers between study arms.
Results
Of 148 women randomized, 136 (92%) completed 12 months of postpartum follow-up. While adjusted mean CD4 count at 12 months (565 cells/µl placebo arm, 638 cells/µl valacyclovir arm) increased from antenatal levels in both arms, the mean CD4 count increase was 73 cells/µl higher in the valacyclovir arm than placebo arm (p = 0.03). Mean increase in CD4 count was 154 cells/µl in the valacyclovir arm, almost double the increase of 78 cells/µl in the placebo arm. At 12 months, adjusted HIV-1 RNA levels in the placebo arm increased by 0.66 log10 copies/ml from baseline, and increased by only 0.21 log10 copies/ml in the valacyclovir arm (0.40 log10 copies/ml difference, p = 0.001).
Conclusion
Women randomized to valacyclovir suppressive therapy during pregnancy and postpartum had greater increases in CD4 counts and smaller increases in plasma HIV-1 RNA levels than women in the placebo arm. Valacyclovir suppression during pregnancy and breastfeeding may improve outcomes and delay antiretroviral therapy for HIV-1/HSV-2 co-infected women.
Introduction
Addressing herpes simplex virus type 2 (HSV-2) co-infection may be a pathway to improving health of HIV-1 infected mothers. More than 80% of HIV-1-seropositive women in sub-Saharan Africa are co-infected with HSV-2 [1]. HSV-2 co-infection has been associated with higher plasma HIV-1 RNA loads, and higher HIV-1 viral set points if HSV-2 infection precedes HIV-1 seroconversion [2], [3]. Trials of herpes virus suppression in HIV-1/HSV-2 co-infected persons have consistently shown reductions in HIV-1 plasma RNA levels, but these trials have excluded pregnant women [4], [5], [6], [7], [8], [9], [10], [11]. Recent randomized clinical trials of acyclovir for herpes suppression in co-infected persons have demonstrated 16–25% reductions in HIV-1 disease progression endpoints over 2 years of follow-up [12], [13]. Progression endpoints for both studies included CD4 thresholds and evidence of clinical disease progression including non-traumatic death and/or ART initiation.
Valacyclovir is now available in generic form, providing higher levels of acyclovir with a simpler regimen, improving adherence and potentially increasing effects on HIV-1 progression. We analyzed data from a randomized, double-blind, placebo-controlled trial to determine whether valacyclovir reduces maternal disease progression, using CD4 count as the primary marker, when taken in late pregnancy and for 12 months postpartum.
Methods
Study Design
Participants were part of a randomized, double-blind, placebo-controlled trial of valacyclovir suppressive therapy of 500 mg twice daily in pregnant HIV-1/HSV-2 co-infected women from 34 weeks gestation to 12 months postpartum, as previously described [14]. The original study was powered to detect at least a 0.50 log10 copies/ml difference in plasma HIV-1 RNA levels. The study was approved by Kenyatta National Hospital Ethical Review Committee and the University of Washington Institutional Review Board, and registered at ClinicalTrials.gov under Identifier NCT 00530777. All participants provided written informed consent.
Participants
Women presenting to public clinics in Nairobi, Kenya for antenatal care between April 2008 and June 2009 were recruited if they resided in Nairobi and were HIV-1-seropositive by rapid tests according to local protocols. Pregnant women with HIV-1 who were ≥18 years of age had HIV-1 confirmed by enzyme-linked immunosorbant assay (ELISA), HSV-2 antibodies, and CD4 count. HIV-1/HSV-2-seropositive women with CD4 counts >250 cells/µl and World Health Organization (WHO) clinical stage 1–2 were eligible.
Study participants received routine antenatal care and prevention of mother-to-child HIV-1 transmission (PMTCT) according to Kenya national guidelines: oral zidovudine (ZDV) 300 mg twice daily from 28 weeks gestation, oral ZDV at onset of labor and every 3 hours until delivery, single-dose nevirapine 200 mg at onset of labor, and ZDV or ZDV/lamivudine “tail” for 7 days postpartum. Baseline CD4 counts were measured prior to initiation of PMTCT regimens.
Enrollment took place at 34 weeks to allow completion of laboratory tests to determine eligibility. All women in the study received the following free of charge: antenatal and delivery care, co-trimoxazole prophylaxis, multivitamins in pregnancy, family planning, and prescribed outpatient medications.
Study Procedures
WHO stage was determined at enrollment. Women were seen at 38 weeks gestation, within 48 hours of delivery, at 2, 6, 10, and 14 weeks postpartum, and at 6, 9, and 12 months postpartum. Antenatal study visits focused on obstetric care; postpartum visits included a physical exam and assessment of WHO stage. If women became eligible for antiretroviral therapy (ART) after enrollment, they were referred for treatment but continued to participate in the study. Counseling to promote adherence to study drug was done regularly. Women returned all unused pills to the study each month and at study termination. Adherence was measured by self-report of number of pills missed and by pill count at follow-up visits.
Laboratory Methods
HIV-1 serostatus was confirmed by ELISA (Vironostika HIV Uni-Form II, bioMérieux, France) and HSV-2 serostatus was determined using ELISA (HerpeSelect, Focus Technologies, Cypress, CA) with a cutoff optical density (OD) ≥3.5 [15]. Tests were repeated if OD was between 1.1 and 3.4; women were eligible if the repeat assay was positive. CD4 counts were performed in Nairobi using flow cytometry (FACSCalibur or FACSCount, BectonDickinson, Franklin Lakes, NJ). HIV-1 RNA assays were conducted using a transcription-mediated amplification method validated for HIV-1 subtypes prevalent in Kenya (Gen-Probe Inc, San Diego, CA) [16]. HIV-1 RNA levels below the lower limit of detection (150 copies/ml) were recoded as half the value of the lower limit of detection.
Study Outcomes
The primary outcomes of this nested study were CD4 counts and log10 plasma HIV-1 RNA levels compared between participants in the two study arms. Secondary outcomes included maternal morbidity, initiation of ART, mortality events and changes in WHO stage. Other study results, including effects of valacyclovir on breast milk HIV-1, genital HIV-1, and genital HSV-2 have been reported elsewhere [14]. A data safety monitoring board regularly evaluated adverse events; there were no criteria for stopping the study early other than safety.
Statistical Methods
Analyses were conducted using Stata Version 10.0 (College Station, TX). All participants with a postpartum visit were included in this intention-to-treat analysis. Adequacy of randomization was assessed by chi-square and Fisher’s exact tests for categorical variables and Wilcoxon rank-sum tests for continuous variables comparing baseline values in both arms. Primary endpoints of CD4 counts and log10 HIV-1 plasma RNA levels at 6 months and 12 months were assessed using 2-sided t-tests and multivariate linear regression, controlling for baseline values. Cox proportional hazards regression was used to compare progression events in both arms. Adherence to study drug was measured by monthly pill counts and monthly self-report. Pill counts were totaled over the duration of the study and adherence was calculated as follows: (total pills dispensed – total pills missed)/total pills dispensed.
Results
Study Population
Women in both study arms had comparable baseline health status (Table 1). Most women lived in poverty in the urban slum neighborhoods surrounding the clinic. At enrollment, 85% of women in the placebo arm and 93% of women in the valacyclovir arm were WHO stage 1. Tuberculosis treatment was reported by 4% of women and 6% had prior herpes zoster. Participants in both study arms had similar baseline median CD4 counts and log10 HIV-1 plasma RNA levels. Almost all women (93% placebo, 100% valacyclovir) had started ZDV for PMTCT before study enrollment.
Table 1. Baseline Maternal Health Characteristics, by Treatment Arm.
| Baseline Health Characteristic | Placebo n = 73 | Valacyclovir n = 73 | |
| Median (IQR)or n (%) | Median (IQR)or n (%) | P value* | |
| Age (years) | 25 (22–29) | 25 (22–30) | 0.82 |
| Prior STI | 18 (25%) | 20 (27%) | 0.71 |
| Prior syphilis | 4 (5%) | 4 (5%) | 1.00 |
| Prior GUD | 13 (18%) | 10 (13%) | 0.5 |
| Prior TB | 3 (4%) | 3 (4%) | 1.00 |
| Prior herpes zoster | 5 (7%) | 4 (6%) | 0.73 |
| On ARVs for PMTCT | 68 (93%) | 73 (100%) | 0.02 |
| WHO Stage 1 | 62 (85%) | 68 (93%) | 0.11 |
| CD4 count (cells/µL) | 481 (340–598) | 452 (351–560) | 0.78 |
| Log10 HIV-1 plasma RNA level (copies/ml) | 3.84 (3.43–.45) | 3.99 (3.30–4.41) | 0.86 |
| No symptoms this pregnancy | 44 (60%) | 47 (64%) | 0.61 |
| Symptoms this pregnancy | |||
| Fever | 16 (22%) | 18 (25%) | 0.70 |
| Malaria | 12 (16%) | 12 (16%) | 1.00 |
| Diarrhea | 11 (15%) | 15 (21%) | 0.39 |
| Cough | 22 (30%) | 27 (37%) | 0.38 |
| Weight loss | 3 (4%) | 9 (12%) | 0.13 |
| Itchy rash | 4 (5%) | 4 (5%) | 1.00 |
| Thrush | 4 (5%) | 4 (5%) | 1.00 |
IQR = Interquartile range. USD = US Dollars. STI = sexually transmitted infection. GUD = genital ulcer disease. ARVs = antiretrovirals. TB = tuberculosis. PMTCT = prevention of mother-to-child transmission of HIV-1. *P value calculated using the Wilcoxon rank-sum test for continuous variables and chi-squared and Fisher’s exact test for categorical variables.
Retention and Adherence
Retention was high: 146 (99%) participants had at least one postpartum visit, and 136 women (92%) were followed for 12 months. Adherence to study drug was high among study participants, and was sustained over 12 months postpartum. By pill count, overall adherence averaged 85% (84% placebo, 85% valacyclovir).
CD4 Counts
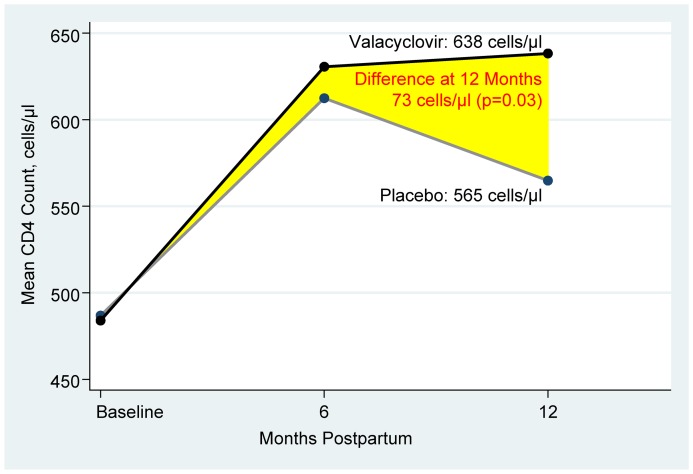
Mean CD4 counts improved from baseline in both randomization arms (Table 2). Of the 116 participants with CD4 count results available at 6 months postpartum, mean CD4 increased from 484 cells/µl to 631 cells/µl in the valacyclovir arm and 487 cells/µl to 612 cells/µl in the placebo arm (P = 0.72). At 12 months, with 136 (93%) participants measured, we observed a higher mean CD4 in the valacyclovir arm than in the placebo arm (638 cells/µl vs. 565 cells/µl, respectively; P = 0.09). After adjusting for baseline CD4, the 12 month mean increase in CD4 counts was 73 cells/µl higher in the valacyclovir arm (P = 0.03). Mean increase in CD4 count was 154 cells/µl in the valacyclovir arm, almost double the increase of 78 cells/µl in the placebo arm (Figure 1).
Table 2. Effect of Valacyclovir on Mean CD4 Count and Log10 Plasma HIV-1 RNA Level.
| Placebo | Valacyclovir | Difference, adjusted* | ||||
| n | Mean (SD) | Mean (SD) | Mean | 95% CI | P | |
| CD4 count (cells/µl) | ||||||
| Enrollment | 146 | 487 (174) | 484 (179) | −3 | −61, 55 | 0.92 |
| 6 months postpartum | 116 | 612 (232) | 631 (309) | 17 | −58, 93 | 0.72 |
| 12 months postpartum | 136 | 565 (206) | 638 (292) | 73 | 6, 140 | 0.03 |
| Log10 plasma HIV-1 RNA (copies/ml) | ||||||
| Enrollment | 146 | 3.87 (0.83) | 3.89 (0.95) | −0.01 | −0.18, 0.17 | 0.95 |
| 6 months postpartum | 136 | 4.40 (0.81) | 3.98 (1.05) | −0.42 | −0.65, −0.2 | <0.001 |
| 12 months postpartum | 136 | 4.53 (0.81) | 4.10 (1.07) | −0.40 | −0.63, −0.17 | 0.001 |
SD = standard deviation.
method used: Multivariate linear regression models control for baseline plasma HIV-1 RNA levels for plasma HIV-1 RNA models, and baseline CD4 for CD4 models.
Figure 1. Change in CD4 count over time in participants on valacyclovir compared to placebo.
Mean CD4 counts were compared at each timepoint by multivariate linear regression, adjusting for baseline values. Baseline CD4 counts were measured during pregnancy, prior to initiation of PMTCT antiretroviral short-course therapy.
HIV-1 RNA Levels
Mean plasma HIV-1 RNA levels were lowest in pregnancy (valacyclovir arm: 3.89 log10 copies/ml, placebo arm: 3.87 log10 copies/ml), when women were taking ZDV for PMTCT. Previously published data from this trial modeled plasma HIV-1 RNA from 6 weeks to 12 months postpartum and described an average 0.51 log copies/ml reduction in HIV-1 plasma RNA levels between study arms during that period [14]. In the present analysis, we report all antenatal, 6 month and 12 month plasma HIV-1 RNA data. During the postpartum period, HIV-1 RNA levels rebounded in both study arms after completion of maternal antiretrovirals (ARVs), but increases in HIV-1 RNA levels were smaller in the valacyclovir arm. At 6 months postpartum, after adjusting for baseline levels, plasma HIV-1 RNA levels were 0.42 log10 copies/ml lower in the valacyclovir arm than in the placebo arm (Table 2). At 12 months postpartum, adjusted plasma HIV-1 RNA levels were 0.40 log10 copies/ml lower in the valacyclovir arm than in the placebo arm; mean HIV-1 RNA increased by 0.21 log10 copies/ml in the valacyclovir arm, and increased by three times more, 0.66 log10 copies/ml, in the placebo arm (P = 0.001).
Maternal Morbidity, Mortality, and Obstetric Outcomes
Despite provision of free care to study participants, there were multiple poor maternal outcomes. Overall, 3 women (2%) died during the study (2 placebo, 1 valacyclovir) and 7 (5%) were hospitalized (5 placebo, 2 valacyclovir). Poor birth outcome with fetal demise occurred in 3 women (2 placebo, 1 valacyclovir) who had third-trimester stillbirths. Other morbidities included tuberculosis (6 women), diarrhea/colitis (26 women), malaria (20 women) and pneumonia (7 women). Overall, 6 women progressed to WHO Stage 3 or 4 during the study, 3 in each arm. Sixteen women (7 on placebo, 9 on valacyclovir) had a progression event (death, CD4<250 cells/µl, WHO stage 3–4; or opportunistic infection). Cox proportional hazards regression models assessing time to disease progression events revealed no significant differences in risk of any progression events, but the study was not powered for these outcomes and events were few. There were no serious adverse events related to use of valacyclovir.
Discussion
Our study of daily valacyclovir during late pregnancy and postpartum showed a significant increase in CD4 counts among women in the valacyclovir arm at 12 months postpartum. CD4 is the most reliable marker of HIV-1 disease progression and morbidity in pregnant and postpartum African women [17]. Although all women in this study received short-course maternal ARVs for PMTCT, the rise in maternal CD4 count in the valacyclovir arm was sustained months after maternal ARVs for PMTCT had been discontinued. This improvement in CD4 is plausible since we concurrently observed less increase in plasma HIV-1 RNA among women in the valacyclovir arm, modeled over the entire study period. Given that asymptomatic African patients with HIV-1 lose an estimated 30–60 CD4 cells/µl per year [18], [19], [20], improved CD4 counts of the magnitude observed in this study could translate into improved outcomes and delayed need for ART for postpartum women.
This is the first randomized trial reporting results of valacyclovir on HIV-1 disease progression specifically among pregnant women in Africa. Other studies in African non-pregnant HIV-1 infected populations have noted benefits of acyclovir, including the landmark trial by Lingappa et al, noting a 16% reduction in HIV-1 disease progression with acyclovir therapy [12], and a randomized controlled trial in Uganda of daily acyclovir among HIV-1/HSV-2 seropositive adults with CD4 counts 300–400 cells/µl that showed a 25% reduction in disease progression after 24 months [13]. Mugwanya et al published data showing that higher doses of valacyclovir may have more potential to impact HIV-1 disease [21]. Modeling studies have predicted similar declines in HIV-1 disease progression with herpes suppressive therapy [22], [23]. A recent cost-effectiveness analysis indicates that HSV-2 suppression to delay ART could be cost-effective in South Africa; HSV-2 suppression could potentially be more cost-effective in other African countries where labor costs are lower [24]. Further, long-term studies of acyclovir in HIV-1/HSV-2 co-infected adults showed no evidence of significant HIV-1 resistance mutations [25], and no evolution of HSV-2 resistance to acyclovir [26], even when adherence was sub-optimal, indicating that prolonged use of anti-herpes medication has few downsides.
Consistent with prior studies of daily acyclovir and valacyclovir, we found high adherence (86%) during the peri- and postpartum periods. Women successfully adhered to twice daily valacyclovir despite extreme poverty, motherhood, and even when concealing both HIV status and study participation. We believe that community health workers were instrumental in supporting adherence in our study, and conclude that widespread use of this therapy should be possible in most African settings, especially if accompanied by support for adherence.
The randomized design of this study proved helpful in comparing CD4 counts in pregnant and postpartum women, which fluctuate due to hemodilution of pregnancy and use of ARVs for PMTCT. Other strengths of this study include its extended duration of follow-up, excellent retention, and implementation within existing PMTCT programs. The small number of women in our study and the relatively brief follow-up time limited our ability to comment on disease progression events. Although valacyclovir is now a generic medication, it is not widely available in resource-limited settings, and continues to be relatively expensive.
What would be the role of valacyclovir, given the current push for elimination of PMTCT? Efforts to scale-up HIV treatment include increasing recognition of the benefits, both reduced infant HIV transmission and improved maternal outcomes, when pregnant women are started on ART or prophylaxis as early as possible [27], especially mothers who need ART for their own health. WHO guidelines recommend that, in addition to providing ART to all pregnant women with CD4≤350 cells/µl, countries choose either short-course maternal ZDV during pregnancy and infant prophylaxis during breastfeeding (“Option A”) or maternal ART during pregnancy and breastfeeding (“Option B”) as preferred interventions to eliminate MTCT [28]. Some countries, such as Malawi, are adopting “Option B-Plus” and placing women on lifelong ART [29], but there are ongoing concerns about retaining women in care who initiate ART solely for PMTCT [30], [31]. Thousands of women will continue to receive only ZDV prophylaxis under “Option A;” this trial shows that valacyclovir could benefit these women, especially since short-course regimens in pregnancy will continue to have a place in PMTCT programming for the foreseeable future.
In conclusion, pregnant and postpartum women randomized to 500 mg of valacyclovir twice daily had greater improvements in CD4 counts and lower postpartum maternal HIV-1 RNA levels when compared to women randomized to placebo. HIV-1 infected women and their children in Africa continue to face serious health consequences and excess mortality due to a lack of comprehensive and timely HIV-1 care and prevention. There is an urgent need to improve maternal CD4 counts and reduce maternal HIV-1 disease progression to promote maternal and child survival. The promising CD4 data from this trial indicate that valacyclovir may be a tool to reduce progression events in women. Further studies are needed to determine whether peripartum valacyclovir therapy may be useful to improve maternal outcomes in settings where access to ART is limited and HSV-2 co-infection is prevalent.
Acknowledgments
We appreciate the efforts and support of the dedicated staff of the Mathare North Health Centre. The Valacyclovir in Pregnancy research staff were instrumental in daily study operations: Sarah Githuku, Jane Waithira, Winnie Nekesa, Wambui Karuoya, Benetah Kendo, Jane Munuhe, and Samuel Kirichu. We remember and honor our colleague Robert Wainaina, who died in 2009. We thank our Data Safety and Monitoring Committee for their assistance: Dalton Wamalwa, Brandon Guthrie, Irene Inwani, and John Ong’ech. Finally, we thank the women who participated and made this research possible.
Presentation. An oral abstract of this work was presented at the International AIDS Society 2011 meeting in Rome, Italy, on July 18, 2011 (Abstract number TUAB0202).
Footnotes
Competing Interests: Dr. Wald has the following conflicts: she has received grant support from GlaxoSmithKline and Antigenics and has consulted for AiCuris and Agenus. All other authors have declared that no competing interests exist. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This work was supported by US National Institutes of Health (NIH) research grants [R03 HD 057773, R03 HD 057773-02S1, R01 AI076105; K24 AI087399 to C.F., K24 AI071113 and PO1 AI30731 to A.W.]; the University of Washington Center for AIDS Research (CFAR) (P30 AI027757), a Puget Sound Partners for Global Health Research and Technology Grant, and a University of Washington Royalty Research Fund Grant. GlaxoSmithKline donated study drug and matched placebo, but had no other role in the study. A.L.D. was supported by NIH-funded University of Washington CFAR Training Grant (T32AI07140-32), A.C.R. and D.M. were scholars in the International AIDS Research and Training Program, funded by the Fogarty International Center, NIH (D43-TW000007); A.C.R. and F.O.O. were Fogarty International Clinical Research Fellows funded by the Fogarty International Center, NIH (R24 TW007988). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Drake AL, John-Stewart GC, Wald A, Mbori-Ngacha DA, Bosire R, et al. Herpes simplex virus type 2 and risk of intrapartum human immunodeficiency virus transmission. Obstet Gynecol. 2007;109:403–409. doi: 10.1097/01.AOG.0000251511.27725.5c. [DOI] [PubMed] [Google Scholar]
- 2.Serwadda D, Gray RH, Sewankambo NK, Wabwire-Mangen F, Chen MZ, et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis. 2003;188:1492–1497. doi: 10.1086/379333. [DOI] [PubMed] [Google Scholar]
- 3.Duffus WA, Mermin J, Bunnell R, Byers RH, Odongo G, et al. Chronic herpes simplex virus type-2 infection and HIV viral load. Int J STD AIDS. 2005;16:733–735. doi: 10.1258/095646205774763298. [DOI] [PubMed] [Google Scholar]
- 4.Ouedraogo A, Nagot N, Vergne L, Konate I, Weiss HA, et al. Impact of suppressive herpes therapy on genital HIV-1 RNA among women taking antiretroviral therapy: a randomized controlled trial. AIDS. 2006;20:2305–2313. doi: 10.1097/QAD.0b013e328010238d. [DOI] [PubMed] [Google Scholar]
- 5.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeten JM, Strick LB, Lucchetti A, Whittington WL, Sanchez J, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198:1804–1808. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delany S, Mlaba N, Clayton T, Akpomiemie G, Capovilla A, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 co-infected women: a randomized placebo-controlled trial in South Africa. AIDS. 2009;23:461–469. doi: 10.1097/QAD.0b013e32831db217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuckerman RA, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 9.Tanton C, Weiss HA, Rusizoka M, Legoff J, Changalucha J, et al. Long-term impact of acyclovir suppressive therapy on genital and plasma HIV RNA in Tanzanian women: a randomized controlled trial. J Infect Dis. 2010;201:1285–1297. doi: 10.1086/651696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 11.Dunne EF, Whitehead S, Sternberg M, Thepamnuay S, Leelawiwat W, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV- and HSV-2-infected women, Chiang Rai, Thailand. J Acquir Immune Defic Syndr. 2008;49:77–83. doi: 10.1097/QAI.0b013e3181831832. [DOI] [PubMed] [Google Scholar]
- 12.Lingappa JR, Baeten JM, Wald A, Hughes JP, Thomas KK, et al. Daily aciclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375:824–833. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds SJ, Makumbi F, Newell K, Kiwanuka N, Ssebbowa P, et al. Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis. 2012. [DOI] [PMC free article] [PubMed]
- 14.Drake AL, Roxby AC, Ongecha-Owuor F, Kiarie J, John-Stewart G, et al. Valacyclovir suppressive therapy reduces plasma and breast milk HIV-1 RNA levels during pregnancy and postpartum: a randomized trial. J Infect Dis. 2011;205:366–375. doi: 10.1093/infdis/jir766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashley Morrow R, Krantz E, Friedrich D, Wald A. Clinical correlates of index values in the focus HerpeSelect ELISA for antibodies to herpes simplex virus type 2 (HSV-2). J Clin Virol. 2006;36:141–145. doi: 10.1016/j.jcv.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Emery S, Bodrug S, Richardson BA, Giachetti C, Bott MA, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38:2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown ER, Otieno P, Mbori-Ngacha DA, Farquhar C, Obimbo EM, et al. Comparison of CD4 cell count, viral load, and other markers for the prediction of mortality among HIV-1-infected Kenyan pregnant women. J Infect Dis. 2009;199:1292–1300. doi: 10.1086/597617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolbers M, Babiker A, Sabin C, Young J, Dorrucci M, et al. Pretreatment CD4 cell slope and progression to AIDS or death in HIV-infected patients initiating antiretroviral therapy–the CASCADE collaboration: a collaboration of 23 cohort studies. PLoS Med. 2010;7:e1000239. doi: 10.1371/journal.pmed.1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urassa W, Bakari M, Sandstrom E, Swai A, Pallangyo K, et al. Rate of decline of absolute number and percentage of CD4 T lymphocytes among HIV-1-infected adults in Dar es Salaam, Tanzania. AIDS. 2004;18:433–438. doi: 10.1097/00002030-200402200-00009. [DOI] [PubMed] [Google Scholar]
- 20.Katubulushi M, Zulu I, Yavwa F, Kelly P. Slow decline in CD4 cell count in a cohort of HIV-infected adults living in Lusaka, Zambia. AIDS. 2005;19:102–103. doi: 10.1097/00002030-200501030-00016. [DOI] [PubMed] [Google Scholar]
- 21.Mugwanya K, Baeten JM, Mugo NR, Irungu E, Ngure K, et al. High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared with standard dose acyclovir among HIV-1/HSV-2 coinfected persons: a randomized, crossover trial. J Infect Dis. 2011;204:1912–1917. doi: 10.1093/infdis/jir649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baggaley RF, Griffin JT, Chapman R, Hollingsworth TD, Nagot N, et al. Estimating the public health impact of the effect of herpes simplex virus suppressive therapy on plasma HIV-1 viral load. AIDS. 2009;23:1005–1013. doi: 10.1097/QAD.0b013e32832aadf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008;22:2179–2185. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vickerman P, Devine A, Foss AM, Delany-Moretlwe S, Mayaud P, et al. The cost-effectiveness of herpes simplex virus-2 suppressive therapy with daily aciclovir for delaying HIV disease progression among HIV-1-infected women in South Africa. Sex Transm Dis. 2011;38:401–409. doi: 10.1097/OLQ.0b013e31820b8bc8. [DOI] [PubMed] [Google Scholar]
- 25.Baeten JM, Lingappa J, Beck I, Frenkel LM, Pepper G, et al. Herpes simplex virus type 2 suppressive therapy with acyclovir or valacyclovir does not select for specific HIV-1 resistance in HIV-1/HSV-2 dually infected persons. J Infect Dis. 2010;203:117–121. doi: 10.1093/infdis/jiq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson-Jones D, Wald A, Celum C, Lingappa J, Weiss HA, et al. Use of acyclovir for suppression of human immunodeficiency virus infection is not associated with genotypic evidence of herpes simplex virus type 2 resistance to acyclovir: analysis of specimens from three phase III trials. J Clin Microbiol. 2010;48:3496–3503. doi: 10.1128/JCM.01263-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chibwesha CJ, Giganti MJ, Putta N, Chintu N, Mulindwa J, et al. Optimal time on HAART for prevention of mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011;58:224–228. doi: 10.1097/QAI.0b013e318229147e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach. 2010 Version. Geneva. 2010. [PubMed]
- 29.Schouten EJ, Jahn A, Midiani D, Makombe SD, Mnthambala A, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378:282–284. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- 30.Boyles TH, Wilkinson LS, Leisegang R, Maartens G. Factors influencing retention in care after starting antiretroviral therapy in a rural South african programme. PLoS One. 2011;6:e19201. doi: 10.1371/journal.pone.0019201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomson KA, Cheti EO, Reid T. Implementation and outcomes of an active defaulter tracing system for HIV, prevention of mother to child transmission of HIV (PMTCT), and TB patients in Kibera, Nairobi, Kenya. Trans R Soc Trop Med Hyg. 2011;105:320–326. doi: 10.1016/j.trstmh.2011.02.011. [DOI] [PubMed] [Google Scholar]