Abstract
Epoxyeicosatrienoic acids (EETs) are cytochrome P450 metabolites of arachidonic acid that are produced by the vascular endothelium in response to agonists such as bradykinin and acetylcholine or physical stimuli such as shear stress or cyclic stretch. In the vasculature, the EETs have biological actions that are involved in the regulation of vascular tone, hemostasis and inflammation. In pre-constricted arteries in vitro, EETs activate calcium-activated potassium channels on vascular smooth muscle and the endothelium causing membrane hyperpolarization and relaxation. These effects are observed in a variety of arteries from experimental animals and humans; however, this is not a universal finding in all arteries. The mechanism of EET action may vary. In some arteries, EETs are released from the endothelium and are transferred to the smooth muscle where they cause potassium channel activation, hyperpolarization and relaxation through a G-protein coupled mechanism or transient receptor potential (TRP) channel activation. In other arteries, EETs activate TRP channels on the endothelium to causes endothelial hyperpolarization that is transferred to the smooth muscle by gap junctions or potassium ion. Some arteries use a combination of mechanisms. Acetylcholine and bradykinin increase blood flow in dogs and humans that is inhibited by potassium channel blockers and cytochrome P450 inhibitors. Thus, the EETs are endothelium-derived hyperpolarizing factors mediating a portion of the relaxations to acetylcholine, bradykinin, shear stress and cyclic stretch and regulate vascular tone in vitro and in vivo.
Keywords: Cytochrome P450, arachidonic acid, potassium channels, endothelial cells, smooth muscle cells
The endothelium regulates vascular tone through the release of soluble mediators that relax vascular smooth muscle [1-5]. Physical and chemical stimuli such as acetylcholine, bradykinin, thrombin, cyclic stretch and shear stress cause endothelium-mediated dilation by stimulating the generation of mediators including prostaglandin I2 (PGI2), nitric oxide (NO) and the endothelium-derived hyperpolarizing factor (EDHF). While PGI2 and NO are single, distinct chemical entities, the term EDHF refers to a chemically diverse group of compounds that share a common mechanism of action. They activate calcium (Ca)-activated potassium (KCa) channels on smooth muscle cells (SMC) and endothelial cell (EC) membranes to elicit membrane hyperpolarization and relaxation. Metabolites of arachidonic acid are among the EDHFs that have been identified.
Vascular Arachidonic Acid Metabolism
Endothelial cells metabolize arachidonic acid by the cyclooxygenase (COX), lipoxygenase (LO) and cytochrome P450 (CYP) pathways [6, 7]. The LO and CYP pathways produce oxygenated metabolites that cause hyperpolarization, vasodilation and contribute to the regulation of vascular tone [4, 8, 9]. Arteries differ in the contributions of LO and CYP metabolites to these biological effects. For example, LO metabolites mediate endothelium-dependent responses in mesenteric and cerebral arteries of some species while CYP metabolites function as endothelial mediators in coronary and renal arteries of most species including humans. In other arteries, arachidonic acid metabolites are not involved in endothelium-dependent responses but other mediators are implicated [10-15]. CYP epoxygenases add oxygen across the double bonds of arachidonic acid to produce four regioisomeric cis-epoxides, 14,15-, 11,12-, 8,9- and 5,6-epoxyeicosatrienoic acids (EETs)(Figure 1), and each of these epoxides may exist as two stereoisomers, S,R and R,S [16, 17]. CYP epoxygenases differ in the spectrum of EET regioisomers and stereoisomers that they produce. While a number of CYP isozymes have been detected in arteries, only two CYP epoxygenase have been cloned from the endothelium [18, 19]. In human ECs, these epoxygenases are CYP2C8/2C9 and CYP2J2. Both CYP2C and CYP2J isozymes produce mainly 14,15-EET with lesser amounts of 11,12-EET [17, 20, 21]. The latter are also the major EETs released from ECs, and their release is stimulated by acetylcholine, bradykinin, cyclic stretch and shear stress [22-26]. While the identity of the endothelial epoxygenase has not been established biochemically, functional studies indicate that CYP2C is the endothelial epoxygenase in coronary arteries [27, 28].
Fig 1.
Chemical structures of epoxyeicosatrienoic acid (EET) agonists and EET antagonists. (epoxyeicosa-5Z-enoic acid=EE5ZE; epoxyeicosa-8Z-enoic acid=EE8ZE; mSI=methylsulfonylimide)
In contrast, SMCs metabolize arachidonic acid by COX to prostaglandins and by LO to hydroxyeicosatetraenoic acids (HETEs)[29]. 20-HETE is the only CYP metabolite produced by SMCs. Thus, in the vascular wall, ECs are the sole source of EETs.
EETs are metabolized by ECs and SMCs by esterification into phospholipids or hydration to dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolase (sEH) [30, 31]. These pathways are rapid, occurring in minutes, but the rates differ for the various regioisomers and stereoisomers. EETs are also metabolized by beta-oxidation; however, this process is slow in vascular cells [30] and perhaps more apparent in cultured cells in which sEH expression tends to decrease after cell isolation. Generally, metabolism by all of these pathways result in a reduction in EET concentrations and thus activity.
While in the systemic circulation the sEH is more abundant in ECs than SMC’s [30, 32], the opposite situation occurs in the pulmonary circulation [33]. The physiological significance of this biochemical finding remains unexplored. It is important to point out that EETs and sEH inhibitors elicit vasodilatation of the systemic circulation but induce vasoconstriction in the lung [33-35]. There are a number of situations in which the altered expression of the sEH may contribute to pathophysiology. For example, the expression of the enzyme is increased in animals treated with angiotensin II [36], a finding that can contribute to the blood pressure lowering by some sEH inhibitors [37, 38]. Marked changes in sEH expression and EET concentrations are also observed in some models of inflammation (e.g. bacterial lipopolysaccharide) [39]. Soluble EH is rapidly downregulated by hypoxia which is linked with the development of pulmonary hypertension [34]. The activity of the sEH is also affected by posttranslational modification. Tyrosine nitration of the enzyme in two models of diabetes is associated with a decrease in enzyme activity [40].
EETs as EDHFs
Evidence from number of laboratories using arteries from a variety of species including humans indicates that EETs act as EDHFs [22, 25, 26, 41-44]. EDHF activity is defined in two ways: endothelium-dependent relaxations in arteries treated with COX and NO synthase inhibitors and endothelium-dependent SMC hyperpolarization. Bradykinin, acetylcholine, cyclic stretch and shear stress cause endothelium-dependent hyperpolarizations and relaxations that occur in the presence of COX and NO synthase inhibition [22, 25, 26, 41, 42, 45]. The relaxations and hyperpolarizations are inhibited by the K channel blockers charybdotoxin, iberiotoxin and tetraethylammonium.
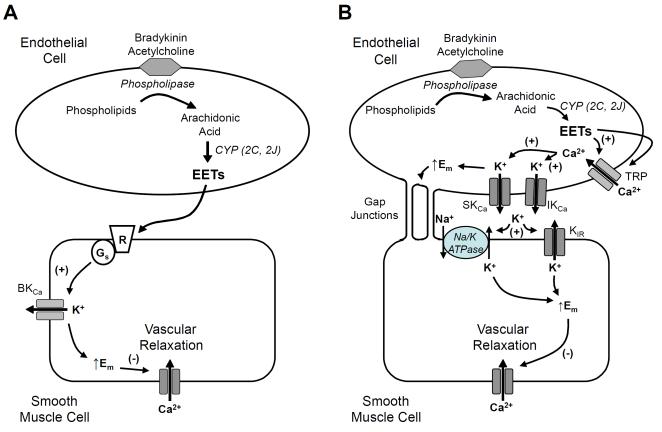
There are two mechanistic interpretations of the data indicating that EETs act as EDHFs. The traditional interpretation as proposed for endothelial relaxing factors by Furchgott and Zawadzki [2] indicates that EETs are released from the endothelium and act as transferrable factors that hyperpolarize and relax SMCs [8, 26] (Figure 2A). An equally plausible interpretation indicates that EETs act in an autocrine manner on ECs to increase intracellular Ca and activate apamin-sensitive, small conductance (SKCa) and charybdotoxin-sensitive, intermediate conductance (IKCa) KCa channels resulting in hyperpolarization of the ECs [46] (Figure 2B). This results in hyperpolarization and relaxation of the SMCs by spread of the hyperpolarizing current from ECs to SMCs through gap junctions and/or the endothelial release of K ions or another factor [12]. Arteries may use either of both of these mechanisms. Arteries such as the bovine coronary artery utilize predominately the traditional “transfer of a factor” pathway described in Figure 2A [26] while the rat hepatic artery utilizes predominately the “transfer of hyperpolarization” pathway outlined in Figure 2B [12]. In the porcine coronary, rat cremaster and mouse mesenteric arteries, both pathways seem to participate in NO and PGI2-independent relaxations [47-49].
Fig 2.
Pathway of EET synthesis and action in the vascular wall. EETs may function as an EDHF by two possible mechanisms: (A) EETs act as transferrable factors released from the endothelium and acting on smooth muscle cells to cause activation of the large conductance, calcium (Ca)-sensitive potassium (KCa) channels. This leads to K efflux, an increase in the membrane potential (Em) or hyperpolarization and relaxation. (B) EETs act in an autocrine manner on endothelial cells to promote Ca influx through TRPV4 or TRPC3 and TRPC6 channels. Calcium activates small conductance (SK) and intermediate conductance (IK) KCa channels to cause hyperpolarization and release of K ions into the sub-endothelial space. Potassium ions stimulate the sodium-potassium ATPase or inward rectifying (Kir) K channel. The endothelial hyperpolarization and K ion mediate hyperpolarization and relaxation of vascular smooth muscle. Gap junctions provide electrical coupling between endothelial cells and smooth muscle cells
EETs and the “Transfer of a Factor” Mechanism
There is evidence that EETs mediate the EDHF response in some arteries by the traditional “transfer of a factor” mechanism. The description of this mechanism is most complete in coronary arteries from several species, mouse skeletal muscle arteries and human internal mammary arteries [22, 25, 26, 41-44]. These findings are summarized as follows (see Figure 2A):
1. Endothelial EET synthesis and release
EETs are synthesized by the vascular endothelium but not SMCs [7, 29]. Bradykinin, acetylcholine, cyclic stretch and cyclic flow increase EET release from ECs or arteries with an intact endothelium [22-26]. Thus, EETs are released from the endothelium in response to chemical and physical stimuli that are known to release EDHF. The synthesis of the EETs is inhibited by CYP inhibitors such as miconazole, SKF525a, N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH), clotrimazole and ketoconazole. Treatment of coronary arteries with CYP2C antisense oligonucleotides decreases endothelial expression of CYP2C, but not CYP2J, and reduces the synthesis of EETs [27]. Scrambled and sense oligonucleotides are without effect. These tools provide a means to determine the physiological consequences of inhibiting EET synthesis.
2. EET-mediated relaxations
The relaxations to bradykinin, acetylcholine and shear stress are endothelium-dependent and are attenuated by inhibitors of COX and NO synthase [22, 25, 27, 42, 45, 50]. The relaxations to these agonist that remain after COX and NO synthase inhibition are blocked by CYP inhibitors. The interpretation of these findings are complex since some CYP inhibitors (clotrimazole and ketoconazole), but not others (miconazole, SKF525a and MSPPOH), inhibit KCa channels [51]. Pretreatment of the coronary artery with CYP2C antisense oligonucleotides, but not by scrambled or sense oligonucleotides, inhibited the relaxations to bradykinin [27, 28]. Additionally, the EET analog 14,15-epoxyeicosa-5Z-enoic acid (14,15-EE5ZE) acts as a selective EET antagonist blocking the relaxations to the four EET regioisomers [50](Figure 1). The non-NO, non-PG-mediated relaxations to acetylcholine and bradykinin were also blocked by 14,15-EE5ZE. These data implicate EETs as mediators of endothelium-dependent relaxations. The relaxations are also inhibited by K channel blockers such as tetraethylammonium, iberiotoxin and charybdotoxin but not glybenclamide indicating a role for KCa channels in the relaxations. In arteries using this mechanism, a single KCa channel blocker such as the large conductance KCa (BKCa) channel inhibitor iberiotoxin or non-selective KCa channel inhibitors such as charybdotoxin or tetraethylammonium inhibit the relaxations.
3. EET-mediated hyperpolarization
Bradykinin, acetylcholine and shear stress cause endothelium-dependent hyperpolarizations that are not blocked by NO synthase or COX inhibitors [22, 25, 27, 42, 45, 50]. The hyperpolarizations are blocked by CYP inhibitors, K channel blockers and 14,15-EE5ZE. The hyperpolarizations to bradykinin are also inhibited by treatment of the arteries with CYP2C antisense oligonucleotides [27]. Thus, an endothelium-derived CYP metabolite and K channels mediate the hyperpolarizations.
4. EET activity on SMCs
EETs have EDHF-like activity. EETs (10−9-10−5M) relax arteries in the presence and absence of the endothelium [6, 22, 45, 50, 52]. EETs also hyperpolarize SMCs. The relaxations and hyperpolarizations are inhibited by K channel blockers and 14,15-EE5ZE. Using patch clamp methods, EETs (10−9-10−6M) activate BKCa channels that are inhibited by iberiotoxin [22, 53, 54].
5. Transferrable Factor
Using bioassay methods, donor arteries with endothelium release a transferrable substance in response to bradykinin or shear stress that relaxes or hyperpolarizes a detector vessel without endothelium [25, 26, 55, 56]. This factor is not inhibited by COX or NO synthase inhibitors but is inhibited by CYP inhibitors and 14,15-EEZE. These studies indicate that EETs may act as an EDHF on SMCs.
EETs and the “Transfer of Hyperpolarization” Mechanism
There is evidence that EETs mediate the EDHF response in some arteries by the “transfer of hyperpolarization” mechanism. The description of this mechanism has been studied in porcine coronary arteries, rat hepatic arteries and mouse mesenteric arteries [12, 46, 48, 49]. The data supporting the “transfer of hyperpolarization” mechanism is summarized follows (Figure 2B):
1. Endothelial EET synthesis
EETs are synthesized by the endothelium [7]. The endothelial effects of the EETs are initiated intracellularly in the cells in which they are synthesized or extracellularly in an autocrine manner on the same or adjacent endothelial cell.
2. Endothelial K channels
ECs contain IKCa and SKCa channels which are inhibited by charybdotoxin and apamin, respectively [57, 58].
3. Gap junctions
Gap junctions between ECs, SMCs and ECs and SMCs have been described and allow dye transfer between ECs and SMCs [59, 60]. The gap junctions allow electrical continuity between cells of the vascular wall and are important in propagation of hyperpolarization along arterioles. EETs also affect gap junctional communication between ECs [61].
4. EET-mediated actions
In ECs, agonists such as acetylcholine and bradykinin promote an increase in intracellular Ca and cause membrane hyperpolarization [62]. Inhibitors of CYP and 14,15-EEZE inhibit the increase in Ca and hyperpolarization. Charybdotoxin and apamin also inhibit the hyperpolarization. These findings implicate EETs.
5. Endothelial action of EETs
In some arteries, removal of the endothelium attenuates the relaxations to EETs suggesting EETs have both an EC and SMC sites of action [48, 49]. On the endothelium, EETs increase intracellular Ca in ECs, activate the KCa channels and hyperpolarize the membrane [62, 63]. The hyperpolarization in ECs by EETs is inhibited by the combination of apamin and charybdotoxin.
6. Endothelial K release
Through activation of endothelial KCa channels, acetylcholine increases the concentration of K ion in the subendothelial space by approximately 5.9 mM, enough to stimulate hyperpolarization and relaxation of SMCs [12].
7. Role of gap junctions and K channels
Acetylcholine and bradykinin hyperpolarize and relax SMCs in an endothelium-dependent manner [12]. These responses are attenuated by inhibitors of gap junctions as well as the combination of apamin and charybdotoxin. These findings suggest that gap junctions and K ion mediate the hyperpolarization and relaxation of SMCs [12]. In arteries that use this mechanism, the hyperpolarization and relaxation of SMCs is inhibited by the combination of apamin and charybdotoxin or apamin and TRAM-34 but not either inhibitor alone.
Taken together, these findings support the view that endothelium-derived EETs act as EDHFs in some arteries. However, in certain arteries from humans and experimental animals, either EETs are not synthesized or are without activity, and mediators other than EETs are implicated in the EDHF response [9-15, 64, 65]. These other mediators include K ion, hydrogen peroxide, C-type natriuretic peptide and LO metabolites of arachidonic acid such as 11,12,15-trihydroxyeicosatrienoic acid and 12-HETE. Thus, EETs are not universal mediators of the EDHF response in all vascular beds. This conclusion fits with the relatively hetereogenous expression of the CYP2C and CYP2J enzymes in the vascular endothelium.
Mechanism of EET Action
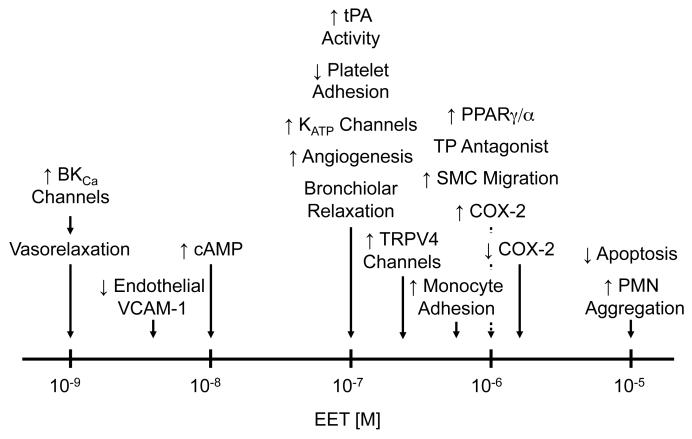
EETs exert a number of effects on vascular tone, hemostasis and inflammation [31, 66, 67]. With a few exceptions, the actions attributed to the EETs are based on studies using the racemic, RS and SR, mixture. Figure 3 indicates a number of actions of an EET regioisomer(s) that are placed on a concentration line. It indicates the threshold concentration of an exogenously applied EET regioisomer that causes the particular action. Some of the actions occur at low physiological concentrations while others require much higher concentrations. One of the most studied actions that occurs at low concentrations is the hyperpolarization and relaxation of SMCs.
Fig 3.
Biological effects of exogenous EETs. Effects of EETs are listed on a concentration-response line. Threshold concentrations for the activity are indicated
Effects of EETs on Vascular SMCs--G proteins and possible receptors
EETs relax the bovine coronary artery and mediate a portion of the endothelium-dependent relaxations to arachidonic acid [6, 22, 68]. Arachidonic acid causes a concentration-dependent relaxation that is partially inhibited by the COX inhibitor indomethacin, partially inhibited by the CYP inhibitor SKF525a and blocked by the combination of COX and CYP inhibition. All four EET regioisomers relax the bovine, canine and human coronary arteries in a concentration-dependent manner, and the regioisomers are of similar potency [6, 22, 68, 69]. These findings should not be interpreted as an indication of non-specificity as coronary artery relaxation is, in fact, stereospecific with 14(S),15(R)-EET being more potent than 14(R),15(S)-EET [70]. The relaxations of other arteries differ with regioisomer. For example, 11,12-EET, but not 14,15-EET, relaxes the rat renal artery, and only 5,6-EET relaxes the rat tail artery [71-73]. Interestingly, the 11(R),12(S)-EET relaxes the rat renal artery while the 11(S),12(R)-EET isomer is inactive. The reasons for the differential responses of arteries to EET regioisomers are not known but are related directly to the mechanisms of action of the EETs rather than the means preconstriction. This is important to mention as relaxations to EETs only occur in preconstricted arteries. However, as similar responses to EETs have been recorded in arteries preconstricted by the thromboxane mimetic U-46619, endothelin-1, phenylephrine or norepinephrine and myogenic tone, it seems safe to state that the means of vasoconstriction is not a decisive factor.
A development that has proven helpful is the synthesis of a series of 14,15-EET analogs by Falck et al which allows a comparison of the biological activities and determination of the key structural features in the molecule that are necessary for vasorelaxation [70]. Using bovine coronary arteries, these studies indicate that a 20 carbon backbone, a carbon-1 carboxyl group, a cis-8,9 double bond and a 14(S),15(R)-cis-epoxide are required for full agonist activity. Thus, the basic structure for full agonist activity is 14(S),15(R)-cis-epoxyeicosa-8Z-enoic acid (14,15-EE8ZE)(Figure 1). Thus, there are strict structural and stereoisomeric requirements for relaxations suggesting a specific binding site or receptor for 14,15-EET action. Unfortunately, there have been no studies for the other EET regioisomers documenting the structural requirements for activity.
Changes in the EET structure through metabolism by sEH or beta-oxidation generally results in a loss of biological activity [30, 31]. In the rat renal arterioles, 11,12-EET causes relaxation while the sEH metabolite, 11,12-DHET, is without activity [73]. In other tissues, the differences in activity are not as clear. For example, 14,15-EET is five-fold more potent than 14,15-DHET in causing relaxation and 10-fold more potent in activating the BKCa channels in the bovine coronary artery [32, 70]. However, the EETs and DHETs are equipotent in relaxing canine coronary arteries [74]. Dilations also differs with the regioisomers in human coronary arteries [69]. 14,15- and 8,9-EETs are more potent than their respective DHETs whereas 11,12-EET and 11,12-DHET are of similar potency. Thus, it is important to compare the activities of the EETs and DHETs in various arteries and tissues to understand the contribution of metabolism by sEH. This has become particularly important with the development of potent inhibitors of sEH and their testing in physiological and pathological settings [37].
Using whole cell and cell-attached patch clamp configurations nanomolar concentrations of 14,15-, 11,12- and 8,9- EET all activate BKCa channels [22, 53, 54, 72, 75] in an iberiotoxin- and tetraethylammonium- (both blockers of the BKCa channel) sensitive manner. As with relaxation, 14(S),15(R)-EET, but not 14(R),15(S)-EET, increases BKCa channel activity in coronary SMCs [29] while in rat renal SMCs, 11(R),12(S)-EET is the active BKCa channel-opening isomer [72]. EET activation of BKCa channels is not simply by direct binding to an extracellular domain as the activation of the SMC BKCa channel by EETs and EDHF requires a guanine nucleotide binding protein (G protein) [54, 75, 76]. Moreover, while 11,12-EET activates the BKCa channel in cell-attached patches of bovine coronary arterial SMCs, it is without effect in inside-out patches. Such observations imply that some cytosolic component or cellular signaling pathway that is absent in inside-out patches is required for EET activity. Currently, the missing component is thought to be GTP as addition of GTP to the cytoplasmic surface of inside-out patches restores the ability of 11,12-EET to open the BKCa channel. Indeed, the potency of 11,12-EET and extent of BKCa channel activation by the EET is the same in cell-attached patches and inside-out patches in the presence of GTP. Underlining a role for GTP is the observation that BKCa channel activation by EETs in GTP-treated inside-out patches is inhibited by the G protein inhibitor GDPβS and by an anti-Gαs antibody. Antibodies against Gαi or Gβγ are without effect. Thus, 11,12-EET acts in a membrane-delimited manner to activate BKCa channels by a Gαs-mediated mechanism.
Biochemical studies measuring GTP binding to G proteins of ECs confirm the importance of a G protein and reveal that 11,12-EET increases GTPγ35S binding to Gαs, but not Gαi, in a concentration-dependent manner [77]. Stimulation of GTPγ35S binding to Gαs occurs with the other EET regioisomers; however, they are all less active than 11,12-EET. The logical next question is how can 11,12-EET affect GTP binding to Gαs? Studies in bovine coronary arteries indicate that 11,12-EET stimulates ADP-ribosylation of the G protein or rather a 52 kDa protein with the properties of Gαs [78]. Cholera toxin, like 11,12-EET, stimulates the ADP-ribosylation of a 52 kDa protein and activates Gαs, and both 11,12-EET and cholera toxin stimulate BKCa channel activity in SMCs. Added to these findings, inhibitors of ADP-ribosylation block the ability of 11,12-EET to activate the BKCa channel and cause relaxation. Currently, the relationship between the membrane-delimited and ADP-ribosylation mechanisms of BKCa channel activation by EETs is not clear. However, the EET activation of BKCa channels in inside-out patches is rapidly reversible upon removal of the EET while the reversibility of the EET is slow in cell-attached patches [54]. Thus, it is possible that ADP-ribosylation of the G protein in intact cells slows K channel inactivation upon EET removal and prolongs the duration of EET action. In SMCs and ECs, EETs increase cyclic AMP accumulation that is consistent with the involvement of Gαs in EET action [77, 79, 80]. It is also possible that cyclic AMP contributes to prolonging the action of the EET. Overall, data from a number of laboratories indicate that EET activation of BKCa channels allows K ion to leave the SMC along its electrochemical gradient resulting in membrane hyperpolarization and relaxation [22, 45, 69] (Figure 2).
Differential responsiveness to EET regioisomers and the apparent involvement of a G protein, probably Gαs, for activity suggest that a specific binding site is a prerequisite for the initiation of an EET-stimulated response. On the basis of the data available to date, it is certainly reasonable to suggest that a G protein coupled receptor(s) for the EETs exists. There is accumulating evidence suggesting a cell surface binding site or receptor for EETs on SMCs. For example, 11,12- and 14,15-EET inhibit dibutyryl cAMP-induced aromatase activity in SMCs [81]. Although tethering 14,15-EET to silica beads restricts its entry into SMCs, the biological effects remain the same i.e., silica bead teathered 14,15-EET inhibits aromatase activity just like 14,15-EET. Thus, EETs may initiate their action by interacting with a cell surface protein. Additional evidence in support of this possibility is obtained using [3H]-14,15-EET to detect a high affinity binding site in monocytes, U937 cells and guinea pig mononuclear cell membrane fractions [82-84]. In the membranes, [3H]-14,15-EET binding is specific, reversible and saturable with a Kd of 5.7 nM. The ligand is not displaced by antagonists of the thromboxane, platelet activating factor or leukotriene receptors. Moreover, 20-Iodo-14,15-EE8ZE (20-I-14,15-EE8ZE), like 14,15-EE8ZE, is a full agonist causing relaxation of bovine coronary arteries and increasing cyclic AMP content in U937 cells [70, 85]. 20-125I-14,15-EE8ZE binds to U937 cell membranes with high affinity in a specific, saturable and reversible manner. Binding is inhibited by 14,15- and 11,12-EETs but not by inactive analogs of 14,15-EET or 15-HETE. Importantly, ligand binding is inhibited by GTPγS indicating that the binding site or receptor is coupled to a G protein. Such findings are in agreement with other reports indicating the involvement of a G protein in the actions of the EETs [54, 77]. Although a group of 47 known receptors has been screened for the ability of EET regioisomers to displace high affinity radioligands [86], to date no EET receptor has been identified.
Effects of EETs on Vascular SMC-EET antagonists
In determining the structural requirements for agonist activity, 14,15-EE5ZE was identified as an analog with low agonist activity that inhibits the relaxations to the four EET regioisomers [50] (Figure 1). This analog proves most effective at inhibiting the actions of 14,15-EET and has no effect on contraction to 20-HETE or the synthesis of either the EETs or 20-HETE [50]. 14,15-EE5ZE does not affect relaxations to the NO donor sodium nitroprusside, the ATP-sensitive K (KATP) channel opener bimakalim, the BKCa channel opener NS1619 or the PGI2 analog iloprost [50]. Thus, this is the first compound described to inhibit the actions of the EETs but not other endothelial mediators or K channels. Clearly, the characteristics of 14,15-EE5ZE are similar to what is expected of a receptor antagonist. As further evidence in support of this possibility, 14,15-EE5ZE displaces 20-125I-14,15-EE8ZE from its binding site on U937 cell membranes with an affinity similar to that of the EETs [85]. Like 14,15-EE5ZE, 20-I-14,15-EE5ZE is also inhibits the relaxations to 14,15-EET [87]. 20-125I-14,15-EE5ZE binds to U937 cell membranes in a specific, saturable and reversible manner with high affinity. Importantly, the binding is not altered by GTPγS confirming that the ligand binds to an antagonist’s binding site. Thus, 14,15-EE5ZE binds to the same binding site or receptor as the EETs and acts as a selective inhibitor of EETs.
Modification of the carboxyl group of 14,15-EE5ZE with a methylsulfonylimide changes its pharmacological properties [88] (Figure 1). Methylsulfonylimides of the EETs do not enter cells and are not subject to esterification or beta-oxidation [81]. 14,15-EE5ZE-methylsulfonylimide inhibits the relaxations to 14,15- and 5,6-EET but does not alter the relaxations to 11,12- or 8,9-EET [88]. These findings raise the possibility for the development of regioisomer-specific antagonists and imply that regioisomers may have distinct binding sites/receptors.
A series of CYP and sEH inhibitors were tested for their ability to displace 20-125I-14,15-EE5ZE from its binding site on U937 cell membranes [87]. The CYP inhibitors miconazole and MS-PPOH inhibit binding of the EET ligand and inhibit the relaxation to 14,15-EET in bovine coronary arteries (Figure 1). They do not inhibit the relaxations to the BKCa channel opener, NS1619. Thus, some CYP inhibitors also act as EET antagonists. These findings may lead to new classes of drugs that inhibit EET actions.
Actions of EETs on Vascular SMCs and ECs--TRP Channels
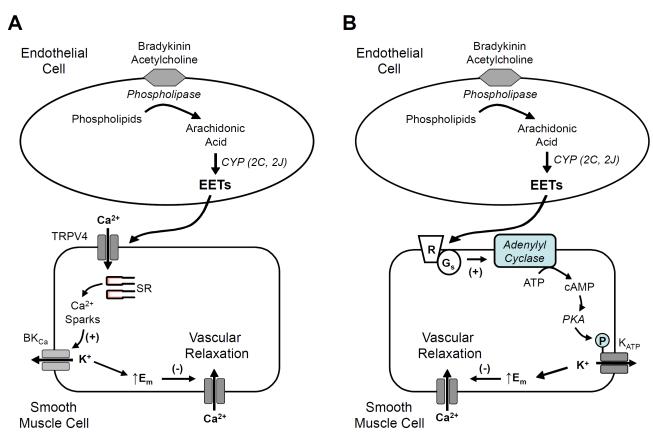
ECs and SMCs contain a number of types of transient receptor potential (TRP) channels that function as non-selective cation channels and can mediate Ca influx [89]. Two different classes of TRP channel are of importance when considering the mechanism of action of the EETs. The first is the vanilloid-type 4, TRPV4, which can be activated by 5,6-EET and to a lesser extent by 8,9-EET [90]. Interestingly neither 11,12- nor 14,15-EET affect Ca influx into murine endothelial cells through the TRPV4 channel. In contrast to these findings, 11,12-EET and the TRPV4 agonist 4α-phorbol-12,13-didecanoate (4-PDD) increase TRPV4 currents in SMCs from rat cerebral arteries [91]. In addition, 11,12-EET stimulates rapid Ca release events termed Ca sparks that activate KCa channels promoting transient outward K currents and membrane hyperpolarization. These ionic changes to 11,12-EET are associated with cerebral artery relaxation. Suppression of TRPV4 expression with an antisense oligonucleotide inhibits 11,12-EET-induced Ca sparks, outward K currents, hyperpolarization and relaxation. On the basis of these findings, it was concluded that the TRPV4 channels form a complex with BKCa channels so that activation by 11,12-EET results in membrane hyperpolarization and relaxation (Figure 4A). As the effects were EET-specific, it was proposed that the TRPV4 channel may represent the EET extracellular receptor.
Fig 4.
Pathway of EET synthesis and action in the vascular wall. EETs may hyperpolarize and relax vascular smooth muscle by two additional mechanisms: (A) EETs are released by the endothelium and activate TRPV4 channels on smooth muscle cells. The calcium (Ca) influx through TRPV4 channels promotes Ca sparks from the endoplasmic reticulum activating large conductance, Ca-activated K (BKCa) channels. This promotes K efflux, an increase in the membrane potential (Em) or hyperpolarization and relaxation. (B) EETs are released by the endothelium and stimulate cyclic AMP production through activation of adenylyl cyclase by the guanine nucleotide binding protein Gαs. Protein kinase (PKA) is activated by cyclic AMP leading to phosphorylation and activation of ATP-sensitive potassium channels. This promotes hyperpolarization and relaxation
Like SMCs, mouse and human ECs express TRPV4 channels, and these channels are activated by arachidonic acid as well as 5,6- and 8,9-EETs resulting in an increase in intracellular Ca [62, 63, 90]. Inhibition of CYP2C9 with sulfaphenazole blocks the arachidonic acid-induced increase in intracellular Ca. Increasing the expression of CYP2C9 with nifedipine enhances the effect of arachidonic acid. These findings implicate a CYP2C9 metabolite of arachidonic acid, an EET, in TRPV4 activation and increases in intracellular Ca in ECs. In murine mesenteric arteries, acetylcholine normally induces hyperpolarization and dilation; however, in arteries from TRPV4-deficient mice, both responses are reduced by 75% [49]. Fluid shear stress and flow-induced dilatation is also linked to Ca sparks. In mice, the NO- and PG-independent flow-induced dilatation of carotid arteries is dependent on the expression of a CYP epoxygenase and on an EET, probably 5,6-EET. This CYP-dependent component to dilation is missing in arteries from TRPV4-deficient mice [92]. The activation of TRPV4 by EETs is not only related to a direct effect on the channel but also to the rapid translocation of the channel to the plasma membrane. Altogether, TRPV4 seems to be an important regulator of agonist and flow-induced dilation. This is reflected by the fact that blood pressure is higher in mice lacking TRPV4 channels [49].
There are distinct differences in TRP channel sensitivity to the different EETs. While flow-induced, EDHF-mediated dilatation is linked to 5,6-EET and the TRPV4 channel, the situation is very different in vessels stimulated with bradykinin. Bradykinin increases CYP activity as well as Ca influx in human ECs [62]. The bradykinin-induced Ca influx is inhibited by CYP inhibitors and by the EET antagonist, 14,15-EE5ZE and is enhanced by blocking EET hydration with a sEH inhibitor. All in all, these data support the suggestion that EETs may act as “calcium influx factors” [93, 94]. As Ca is required for the activation of KCa channels, an effect of EETs on Ca influx may account for the EET-dependent activation of SKCa and IKCa channels in ECs (Figure 2B). Certainly ruthenium red, a non-selective TRP channel inhibitor as well as sulfaphenazole, 14,15-EE5ZE and the KCa channel blockers, charybdotoxin plus apamin, inhibit the bradykinin-induced hyperpolarization [62]. The endothelial TRP channels implicated in the responses to bradykinin are not TRPV4, but rather TRPC3 and TRPC6, channels [62]. These channels are activated by bradykinin and translocate very rapidly to caveolae to modulate Ca influx in response to 11,12-EET. TRP channel translocation is dependent on the activation of protein kinase (PK) A [62]. 11,12-EET that is generated in response to acute hypoxia stimulates pulmonary vasoconstriction by a mechanism involving SMC TRPC6 channels [33]. Specifically, pulmonary vasoconstriction to either hypoxia or 11,12-EET occurs in lungs from normal mice but not in lungs from TRPC6-deficient mice.
Since EC hyperpolarization and KCa channel activation can mediate SMC hyperpolarization and relaxation as described above, both SMC and EC TRP channels may play important roles. Indeed, 11,12-EET elicits hyperpolarization and dilation of mesenteric arteries from wild type mice but is without effect in arteries from TRPV4-deficient mice [49]. The hyperpolarization and dilation responses to 11,12-EET are reduced by approximately 50% by removal of the endothelium in arteries from wild type mice indicating that a component of the responses can be attributed to K channels in the SMC and a component to K channels on ECs. Similarly, a portion of both responses is inhibited by charybdotoxin and apamin implicating SKCa and IKCa channels on ECs, and a portion is inhibited by iberiotoxin implicating BKCa in SMCs [49, 91] (Figure 4A). The combination of the three KCa channel inhibitors blocks both responses.
How is it possible to reconcile the link between the effects of EETs on TRP channels and the need for EET binding to a G protein-coupled receptor? Little is actually known about the mechanisms by which EETs activate TRP channels. The assumption that EETs bind directly to a cytoplasmic domain of the TRP protein such as a putative arachidonate recognition sequence [91, 95] is indeed difficult to reconcile with the theory that EET responses are EET receptor-dependent [81-85, 87]. However, as mentioned above, EETs elicit the rapid intracellular translocation of TRP channels from a perinuclear site into caveolae [33, 62]. This translocation process depends on the activation of PKA by cyclic AMP, which is consistent with the activation of a Gαs-coupled receptor. Indeed, blocking the actions of cyclic AMP inhibits TRP channel translocation and inhibits the bradykinin-induced, CYP-dependent increase in intracellular Ca and hyperpolarization in ECs [62]. Further investigation of the regulation of TRP channel activity and translocation by EETs and by G-protein coupled mechanisms is warranted.
Actions of EETs Vascular SMCs-Thromboxane Prostanoid (TP) Receptor Antagonist
In murine aorta and mesenteric arteries preconstricted with the TP agonist U46619, 14,15-EET induces relaxation with an IC50 of 5.4 and 0.9 μM, respectively [96]. Intriguingly, the effects are similar in arteries from wild type and TRPV4-deficient as well as BKCa-deficient mice and are not affected by iberiotoxin. Thus, in these studies, the EET-induced relaxation is atypical as neither TRPV4 nor BKCa channels are involved. Moreover, 14,15-EET fails to relax the same vessels preconstricted with either phenylephrine, endothelin-1 or potassium chloride. Such observations gave rise to the suggestion that the EETs may selectively antagonize contractions induced by thromboxane agonists. Supporting this suggestion, 14,15-EET inhibits binding to the TP, FP and DP, but not EP or leukotriene, receptors with a Ki of 6.1 μM. This conclusion and these findings may not be extended to all arteries and species and in fact, contradict previously published studies. For example, 11,12-EET relaxes the phenylephrine-preconstricted mouse mesenteric artery from wild type mice but not TRPV4-deficient mice [49]. Additionally, the relaxations to 11,12-EET in wild type mice are inhibited by approximately 50% by iberiotoxin and by 50% by the combination of charybdotoxin and apamin. While it is possible that the mechanism of relaxation differs with the various regioisomers, e.g., 14,15-EET antagonizing TP receptors and 11,12-EET acting through TRPV4 and KCa channels, it seems safe to state that inhibition of TP receptors by the EETs cannot be considered a universal mechanism for EET-mediated relaxation. Certainly, there are a wealth of studies (too many to list herein) reporting the ability of EETs to relax vessels precontracted with serotonin, phenylephrine or endothelin via activation of BKCa channels [53, 68, 69, 72, 74, 97-99]. Thus, while EETs may be TP receptor antagonists in some arteries, it is important to determine the effect of dilators on arteries preconstricted with several different agonists to determine the signaling pathways that mediate contraction and relaxation.
EET Action on Vascular SMCs-ATP-sensitive K (KATP) channels
SMCs from rat mesenteric arteries express BKCa and KATP channels, and both types of K channel contribute to EET-induced relaxation and hyperpolarization [97, 98]. For example, 11,12-EET activates KATP channels in isolated SMCs treated with iberiotoxin with an EC50 of 87 nM [97]. The activation of the KATP channel depends on PKA, but not PKC, activity, and 11,12-EET-stimulated relaxations in the same arteries are inhibited, but not blocked, by the KATP channel inhibitor glibelclamide or by PKA inhibition. 14,15-EET also activates KATP channels in SMCs [98]. This activation by the EET is inhibited by the EET antagonist 14,15-EE5ZE as well as an antibody against Gαs and inhibitors of ADP-ribosylation [98]. Thus, 14,15-EET activates KATP channels by a mechanism similar to that described for the activation of BKCa channels [78]. Since EETs stimulate cyclic AMP accumulation in rat mesenteric SMCs [80], it is proposed that EETs activate Gαs by ADP-ribosylation resulting in stimulation of adenylyl cyclase and elevation of cyclic AMP [97, 98](Figure 3B). Activation of PKA by cyclic AMP then phosphorylates and activates the KATP channel contributing to membrane hyperpolarization and relaxation.
EET Action on Vascular SMCs-Activation of Heme Oxygenase and Carbon Monoxide (CO)
The 11,12-EET-stimulated dilation of phenylephrine-constricted rat mesenteric arteries is prevented by the heme oxygenase inhibitor chromium mesoporphyrin (CrMP) as well as by iberiotoxin [99]. Not all EETs are equal in this respect. Relaxations to 8,9- and 14,15-EETs are also inhibited by CrMP; however, relaxations to 5,6-EET are unaffected. Consistent with the pharmacological data, 11,12-EET increases the release of CO from mesenteric arteries which is inhibited by CrMP. It was not determined whether ECs or SMCs are the source of the CO. CO causes slight dilation in CrMP-treated arteries and binds to heme to activate the BKCa channel [100]. These observations suggest that 11,12-EET does not stimulate BKCa directly but rather that the EETs stimulate heme oxygenase to generate CO which mediates EET-induced dilation, presumably by activating BKCa channels and membrane hyperpolarization.
From the above, it is clear that EETs induce relaxation potentially through a variety of mechanisms, most of which involve K channel activation and membrane hyperpolarization. Depending on the artery, EETs may act on ECs and/or SMCs to cause K channel activation and relaxation. Further study is needed to clarify these mechanisms and their relative importance to regulating vascular tone.
Role of EETs in the regulation of vascular tone in vivo
While most of the aforementioned studies were performed in vitro in isolated vascular cells or arteries, there is also evidence that EETs participate in the regulation of vascular tone in vivo in experimental animals and humans. In anesthetized, open chest dogs, changes in the diameter of small coronary arteries are measured following the application of endothelium-dependent dilators. Intracoronary infusion or topical application of acetylcholine causes a concentration-dependent dilation of small coronary arteries [101, 102]. Inhibition of COX with indomethacin or NO synthase with N nitro-L-arginine (L-NA) or N -monomethyl-L-arginine (L-NMMA) or CYPs with clotrimazole has little effect on the dilations to acetylcholine. However, in indomethacin and L-NA-treated dogs, the dilations to acetylcholine are blocked after inhibition of K channels with iberiotoxin or superfusion of a high concentration of potassium chloride in the field of measurement [102]. These results suggest the involvement of an EDHF in the dilations elicited by acetylcholine. Additionally, these responses are blocked by CYP inhibition with either clotrimazole, 17-octadecynoic acid or miconazole indicating the involvement of a CYP metabolite in mediating dilations to acetylcholine [101, 102]. There is also evidence linking bradykinin to CYP activation and EET production. In indomethacin- and L-NMMA-treated dogs, bradykinin dilates coronary arteries, and these dilations are blocked by high concentrations potassium chloride or the CYP inhibitor miconazole [103]. Topical application of arachidonic acid also causes a concentration-dependent dilation of the small coronary arteries [104]. These in vivo studies suggest that a CYP metabolite of arachidonic acid is involved in the dilations to acetylcholine and bradykinin in the canine coronary circulation.
Measurements of changes in forearm blood flow induced by endothelium-dependent dilators led to similar conclusions in human subjects. Bradykinin causes a concentration-dependent increase in forearm blood flow [105-108]. A portion of the increase in flow to bradykinin is inhibited by infusing potassium chloride into the brachial artery or treatment with tetraethylammonium chloride implicating K channel activation and an EDHF in the vasodilatation. Treatment with the CYP inhibitors sulfaphenazole or miconazole has no effect [105, 107, 108]. However, in subjects treated with NO synthase and COX inhibitors, bradykinin increases forearm blood flow, and the increases are inhibited by miconazole [105]. The effects of sulfaphenazole vary. In one study, sulfaphenazole inhibits bradykinin-induced increases in forearm blood flow [108] whereas it is without effect in another study [107]. Similarly, when acetylcholine is used to increase forearm blood flow in subjects treated with COX and NO synthase inhibitors, sulfaphenzole inhibits the increases in flow in one study [108] but has no effect in another [109]. Flow-induced vasodilation in the radial artery is inhibited by sulfaphenzole, and the combination of sulfaphenzole and L-NMMA has additive effects on inhibition of vasodilation [110]. The reasons for these differences with sulfaphenazole are not apparent but may be related to the experimental design, the vascular beds or the stimulus applied.
The increases in forearm blood flow to acetylcholine and bradykinin are reduced in hypertensive patients when compared to normal subjects [108]. The mediator of this endothelial dysfunction has been studied pharmacologically. L-NMMA reduces the blood flow increase to both acetylcholine and bradykinin in normal subjects but is without effect in hypertensive patients [108]. These findings suggest that the diminished endothelium-dependent dilation in hypertensive patients is due to an absence of the NO-mediated component. Intriguingly, sulfaphenazole inhibits the increase in blood flow to both agonists in hypertensive patients but not in normal subjects. As in in vivo studies in dogs and in vitro studies in coronary arterial rings [27, 103], inhibition of NO production is required to observe the CYP-mediated component of bradykinin-induced dilation in humans. Thus, the decrease in NO synthesis that occurs during endothelial dysfunction may alleviate the inhibition of CYP enzymes by NO, increase EET production and sustain endothelium-dependent dilation.
As discussed above, sulfaphenazole has been used in many of the human studies to evaluate the contribution of the CYP epoxygenase pathway to changes in blood flow under basal conditions and with endothelium-dependent dilators in normal subjects and patients. Since sulfaphenazole is a CYP2C9 inhibitor [111], its use assumes that CYP2C9 is the endothelial epoxygenase contributing to dilation in human arteries. Both CYP 2C8/9 and CYP 2J2 have been cloned from human ECs so either may participate in CYP/EET-mediated responses [18, 19]. Sulfaphenazole will only inhibit the CYP 2C9 contribution, which may explain the variable results with the drug. None of the human studies measured urinary or plasma EETs or DHETs as an index of CYP inhibition. Interpretation of data with sulfaphenazole is further complicated by the ability of CYP2C9 to produce reactive oxygen species such as superoxide under some pathological circumstances [112]. Thus, inhibiting CYP2C9 with sulfaphenazole may reduce the synthesis of EETs and/or reactive oxygen species depending on the conditions. In fact, in patients with coronary artery disease, sulfaphenazole enhances acetylcholine-induced increases in forearm blood flow, and this enhanced response is attributed to an increase in the bioavailability of NO due to a reduction in reactive oxygen species [113].
Summary and Perspective
Studies from a number of laboratories indicate that EETs mediate a portion of the endothelium-dependent relaxations to acetylcholine, bradykinin, shear stress and cyclic stretch in some, but not all, arteries. In this regard, they cause hyperpolarization and relaxation of SMCs and function as EDHFs. Their mechanism of action and role as EDHFs varies with the vascular bed and species. Endothelial dysfunction, a loss of endothelium-dependent relaxation, is a sentinel of disturbances in the cardiovascular system and is associated with the disease process. It is often due to the loss of the NO component of dilation. When NO is reduced, the CYP and EET-mediated pathway sustains endothelium-dependent dilation. Reversal of endothelial dysfunction may represent an important approach for treatment of cardiovascular diseases. Enhancing the production of vascular EETs or inhibiting the degradation of the EETs may represent a new therapeutic approach to correct endothelial dysfunction and cardiovascular diseases.
Acknowledgements
The authors thank Dr. Kathryn Gauthier for her comments and help with the figures and Ms. Gretchen Barg for her secretarial assistance. Support was provided by a grant from the National Heart, Lung and Blood Institute (HL-51055) and by the Deutsche Forschungsgemeinschaft (Exzellenzcluster 147 “Cardio-Pulmonary Systems”).
References
- 1.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- 2.Furchgott RF, Zawadzki JW. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 3.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization: Beyond nitric oxide and cyclic GMP. Circulation. 1995;92:3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- 4.Fleming I, Busse R. Endothelium-derived epoxyeicosatrienoic acids and vascular function. Hypertension. 2006;47:629–633. doi: 10.1161/01.HYP.0000208597.87957.89. [DOI] [PubMed] [Google Scholar]
- 5.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolaizing factor. Where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 6.Rosolowsky M, Campbell WB. Role of PGI2 and EETs in the relaxation of bovine coronary arteries to arachidonic acid. Am J Physiol. 1993;264:H327–H335. doi: 10.1152/ajpheart.1993.264.2.H327. [DOI] [PubMed] [Google Scholar]
- 7.Rosolowsky M, Campbell WB. Synthesis of hydroxyeicosatetraenoic acids (HETEs) and epoxyeicosatrienoic acids (EETs) by cultured bovine coronary artery endothelial cells. Biochim Biophys Acta. 1996;1299:267–277. doi: 10.1016/0005-2760(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 8.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 9.Chawengsub Y, Gauthier KM, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone. Am J Physiol. 2009;297:H495–H507. doi: 10.1152/ajpheart.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zygmunt PM, Edwards G, Weston AH, Davis SC, Hogestatt ED. Effects of cytochrome P450 inhibitors on EDHF-mediated relaxation in the rat hepatic artery. Brit J Pharmacol. 1996;118:1147–1152. doi: 10.1111/j.1476-5381.1996.tb15517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Evidence against a role of cytochrome P450-derived arachidonic acid metabolites in endothelium-dependent hyperpolarization by acetylcholine in rat isolated mesenteric artery. Brit J Pharmacol. 1997;120:439–446. doi: 10.1038/sj.bjp.0700932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 13.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kandaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci USA. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batenburg WW, Garrelds IM, van Kats JP, Saxena PR, Danser AHJ. Mediators of bradykinin-induced vasorelaxation in human coronary microarteries. Hypertension. 2004;43:488–492. doi: 10.1161/01.HYP.0000110904.95771.26. [DOI] [PubMed] [Google Scholar]
- 16.Capdevila J, Chacos N, Werringloer J, Prough RA, Estabrook RW. Liver microsomal cytochrome P450 and the oxidative metabolism of archidonic acid. Proc Natl. Acad Sci USA. 1981;78:5362–5366. doi: 10.1073/pnas.78.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 18.Lin JH-C, Kobari Y, Stemerman MB, Pritchard KA. Human umbilical vein endothelial cells express P450 2C8 mRNA: Cloning of endothelial P450 epoxygenase. Endothelium. 1996;4:219–229. [Google Scholar]
- 19.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin D, Liao J. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeldin DC, Dubois RN, Falck JR, Capdevila JH. Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch Biochem Biophys. 1995;322:7686. doi: 10.1006/abbi.1995.1438. [DOI] [PubMed] [Google Scholar]
- 21.Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem. 1996;271:3460–3468. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
- 22.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 23.Nithipatikom K, Pratt PF, Campbell WB. Determination of EETs using microbore liquid chromatography with fluorescence detection. Am J Physiol. 2000;279:H857–H862. doi: 10.1152/ajpheart.2000.279.2.H857. [DOI] [PubMed] [Google Scholar]
- 24.Fissllthaler B, Popp R, Michaelis UR, Kiss L, Fleming I, Busse R. Cyclic stretch enhances the expression and activity of coronary endothelium-derived hyperpolarizing factor synthase. Hypertension. 2001;38:1427–1432. doi: 10.1161/hy1201.096532. [DOI] [PubMed] [Google Scholar]
- 25.Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res. 2005;96:376–383. doi: 10.1161/01.RES.0000155332.17783.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauthier KM, Edwards EM, Falck JR, Reddy DS, Campbell WB. 14,15-Epoxyeicosatrienoic acid represents a transferable endothelium-dependent relaxing factor in bovine coronary arteries. Hypertension. 2005;45:666–671. doi: 10.1161/01.HYP.0000153462.06604.5d. [DOI] [PubMed] [Google Scholar]
- 27.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 28.Bolz S-S, Fissllthaler B, Pieperhoff S, De Wit C, Fleming I, Busse R, Pohl U. Antisense oligonucleotides against cytochrome P450 2C8 attenuates EDHF-mediated Ca2+ changes and dilation in isolated resistance arteries. FASEB J. 2000;14:255–260. doi: 10.1096/fasebj.14.2.255. [DOI] [PubMed] [Google Scholar]
- 29.Campbell WB, Holmes BB, Falck JR, Capdevila JH, Gauthier KM. Adenoviral expression of cytochrome P450 epoxygenase in coronary smooth muscle cells: Regulation of potassium channels by endogenous 14(S),15(R)-EET. Am J Physiol. 2006;290:H64–H71. doi: 10.1152/ajpheart.00516.2005. [DOI] [PubMed] [Google Scholar]
- 30.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): Metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 31.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 32.Campbell WB, Deeter C, Gauthier KM, Ingraham RH, Falck JR, Li P-L. 14,15-Dihydroxyeicosatrienoic acid relaxes bovine coronary arteries by activation of KCa channels. Am J Physiol. 2002;282:H1656–H1664. doi: 10.1152/ajpheart.00597.2001. [DOI] [PubMed] [Google Scholar]
- 33.Kerseru B, Barbosa-Sicard E, Popp R, Fissllthaler B, Dietrich A, Gudermann T, Hammock BD, Falck JR, Weissmannn N, Busse R, Fleming I. Epoxyeicosatrienoic acids and the soluble epoxide hydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstriction response. FASEB J. 2008;22:4306–4315. doi: 10.1096/fj.08-112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerseru B, Barbosa-Sicard E, Schermuly RT, Tanaka H, Hammock BD, Weissman M, Fissllthaler B, Fleming I. Hypoxia-induced pulmonary hypertension: Comparison of soluble epoxide hydrolase deletion versus inhibiton. Cardiovas Res. 2010;85:232–240. doi: 10.1093/cvr/cvp281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pokreisz P, Fleming I, Kiss L, Barbosa-Sicard E, Fissllthaler B, Falck JR, Hammock BD, Kim I-H, Szelid Z, Vermeersch P, Gillijns H, Pellens M, Grimminger F, van Zonneveld AJ, Collen D, Busse R, Janssens S. Cytochrome P450 epoxygenase gene function in hypoxic pulmonary vasoconstriction and pulmonary vascular remodeling. Hypertension. 2006;47:762–770. doi: 10.1161/01.HYP.0000208299.62535.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ai D, Guo D, Tanake H, Wang N, Tang C, Hammock BD, Shyy JY-J, Zhu Y. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Nat Acad Sci USA. 2007;104:9018–9023. doi: 10.1073/pnas.0703229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nature Rev. Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung O, Brandes RP, Kim IH, Schweda F, Schmidt R, Hammock BD, Fleming I. Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension. 2005;45:759–765. doi: 10.1161/01.HYP.0000153792.29478.1d. [DOI] [PubMed] [Google Scholar]
- 39.Fife KL, Liu Y, Schmelzer KR, Tasai HJ, I.M. K, Morisseau C, Hammock BD, Kroetz DL. Inhibition of soluble epoxie hydrolase does not protect against endotoxin-mediated hepatic inflammation. J Pharmacol Exp Ther. 2008;327:707–715. doi: 10.1124/jpet.108.142398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbosa-Sicard E, Fromel T, Kerseru B, Brandes RP, Morisseau C, Hammock BD, Braun T, Kruger M, Fleming I. Inhibition of the soluble epoxide hydrolase by tyrosine nitration. J Biol Chem. 2009;284:28156–28163. doi: 10.1074/jbc.M109.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popp R, Fleming I, Busse R. Pulsatile stretch in coronary arteries elicits release of endothelium-derived hyperpolarizing factor: A modulator of arterial compliance. Circ Res. 1998;82:696–703. doi: 10.1161/01.res.82.6.696. [DOI] [PubMed] [Google Scholar]
- 42.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hasimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BKca channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- 43.Coats P, Johnston F, MacDonald J, McMurray JJV, Hillier C. Endothelium-derived hyperpolarizing factor: Identification and mechanism of action in human subcutaenous resistance arteries. Circulation. 2001;103:1702–1708. doi: 10.1161/01.cir.103.12.1702. [DOI] [PubMed] [Google Scholar]
- 44.Miura H, Wachtel RE, Liu Y, Loberiza J, F. R, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: Important role of Ca2+-activated K+ channels. Circulation. 2001;103:1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- 45.Hecker M, Bara AT, Bauersachs J, Busse R. Characterization of endothelium-derived hyperpolarizing factor as a cytochrome P450-derived arachidonic acid metabolite in mammals. J Physiol. 1994;481:407–414. doi: 10.1113/jphysiol.1994.sp020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: Bringing the concepts together. TiPS. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 47.McSherry IN, Sandow SL, Campbell WB, Falck JR, Hill MA, Dora KA. A role for heterocellular coupling and EETs in dilation of rat cremaster arteries. Microcirculation. 2006;13:119–130. doi: 10.1080/10739680500466400. [DOI] [PubMed] [Google Scholar]
- 48.Weston AH, Feletou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: Involvement of potassium channel activation and epoxyeicosatrienoic acids. Brit J Pharmacol. 2005;145:775–784. doi: 10.1038/sj.bjp.0706256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol. 2009;297:H1096–H1102. doi: 10.1152/ajpheart.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: A selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 51.Campbell WB, Harder DR. Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circ Res. 1999;84:484–488. doi: 10.1161/01.res.84.4.484. [DOI] [PubMed] [Google Scholar]
- 52.Pratt PF, Li P, Hillard CJ, Kurian J, Campbell WB. Endothelium-independent, ouabain-sensitive relaxation of bovine coronary arteries by EETs. Am J Physiol. 2001;280:H1113–H1121. doi: 10.1152/ajpheart.2001.280.3.H1113. [DOI] [PubMed] [Google Scholar]
- 53.Gebremedhin D, Ma Y-H, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol. 1992;263:H519–H525. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- 54.Li P-L, Campbell WB. Epoxyeicosatrienoic acids activate potassium channels in coronary smooth muscle through guanine nucleotide binding protein. Circ Res. 1997;80:877–884. doi: 10.1161/01.res.80.6.877. [DOI] [PubMed] [Google Scholar]
- 55.Popp R, Bauersachs J, Hecker M, Fleming I, Busse R. A transferable, beta-naphthoflavone-inducible, hyperpolarizing factor is synthesized by native and cultured porcine coronary endothelial cells. J Physiol. 1996;497:699–709. doi: 10.1113/jphysiol.1996.sp021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gebremedhin D, Harder DR, Pratt PF, Campbell WB. Bioassay of an endothelium-derived hyperpolarizing factor from bovine coronary arteries: role of a cytochrome P450 metabolite. J Vasc Res. 1998;35:274–284. doi: 10.1159/000025594. [DOI] [PubMed] [Google Scholar]
- 57.Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, Edwards G. Characterization of an apamn-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: Relevance to EDHF. Brit J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Feletou M, Vanhoutte PM. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: Relevance to EDHF. Brit J Pharmacol. 2002;137:1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaytor AT, Martin PEM, Edwards DH, Griffith TM. Gap junctional communication underpins EDHF-type relaxations evoked by acetylcholine in the rat hepatic artery. Am J Physiol. 2001;280:H2441–H2450. doi: 10.1152/ajpheart.2001.280.6.H2441. [DOI] [PubMed] [Google Scholar]
- 60.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 61.Popp R, Brandes RP, Ott G, Busse R, Fleming I. Dynamic modulation of interendothelial gap junctional communication by 11,12-epoxyeicosatrienoic acid. Circ Res. 2002;90:800–806. doi: 10.1161/01.res.0000015328.20581.d6. [DOI] [PubMed] [Google Scholar]
- 62.Fleming I, Rueben A, Popp R, Fissllthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R. Epoxyeicosatrienoic acids regulate Trp-channel-dependent Ca signaling and hyperpolariztion in endothelial cells. Arterio Thromb Vasc Biol. 2007;27:2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- 63.Vriens J, Owsianik G, Fissllthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 64.Petersson J, Zygmunt PM, Jonsson P, Hogestatt ED. Characterization of endothelium-dependent relaxations in guinea pig basilar ratery-effect of hypoxia and role of cytochrome P450 monooxygenase. J Vas Res. 1998;35:285–294. doi: 10.1159/000025595. [DOI] [PubMed] [Google Scholar]
- 65.Chataigneau T, Feletou M, Duhault J, Vanhoutte PM. Epoxyeicosatrienoic acids, potassium channel blockers and endothelium-dependent hyperpolarizations in the guinea pig carotid artery. Brit J Pharmacol. 1998;123:574–580. doi: 10.1038/sj.bjp.0701629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Larsen BT, Campbell WB, Gutterman DD. Beyond vasodilation: Non-vasomotor roles of epoxyeicosatrienoic acids in the cardiovascular system. TIPS. 2007;28:32–38. doi: 10.1016/j.tips.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Fleming I. Vascular cytochrome P450 enzymes: Physiology and pathophysiology. TCM. 2008;18:20–25. doi: 10.1016/j.tcm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Rosolowsky M, Falck JR, Willerson JT, Campbell WB. Synthesis of lipoxygenase and epoxygenase products of arachidonic acid by normal and stenosed canine coronary arteries. Circ Res. 1990;66:608–621. doi: 10.1161/01.res.66.3.608. [DOI] [PubMed] [Google Scholar]
- 69.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BKca channels: Implications for soluble epoxide hydrolase inhibition. Am J Physiol. 2005;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Falck JR, Krishna UM, Reddy YK, Kumar PS, Reddy KM, Hittner SB, Deeter C, Sharma KK, Gauthier KM, Campbell WB. Comparison of the vasodilatory properties of 14,15-EET analogs: Structural requirements for dilation. Am J Physiol. 2003;284:H337–H349. doi: 10.1152/ajpheart.00831.2001. [DOI] [PubMed] [Google Scholar]
- 71.Carroll MA, Schwartzman M, Capdevila J, Falck JR, McGiff JC. Vasoactivity of arachidonic acid epoxides. Europ J Pharmacol. 1987;138:281–283. doi: 10.1016/0014-2999(87)90445-6. [DOI] [PubMed] [Google Scholar]
- 72.Zou A, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K+-channel activity. Am J Physiol. 1996;270:F822–F832. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]
- 73.Imig JD, Navar LG, Roman RJ, Reddy KK, Falck JR. Actions of epoxygenase metabolites on the preglomerular vasculature. J Am Soc Nephrol. 1996;7:2364–2370. doi: 10.1681/ASN.V7112364. [DOI] [PubMed] [Google Scholar]
- 74.Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res. 1998;83:932–939. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- 75.Hayabuchi Y, Nakaya Y, Matsuoka S, Kuroda Y. Endothelium-derived hyperpolarizing factor activates Ca2+-activated K+ channels in porcine coronary artery smooth muscle cells. J Cardiovasc Pharmacol. 1998;32:642–649. doi: 10.1097/00005344-199810000-00018. [DOI] [PubMed] [Google Scholar]
- 76.Fukao M, Mason HS, Kenyon JL, Horowitz B, Keef KD. Regulation of BKCa channels expressed in human embryonic kidney 293 cells by epoxyeicosatrienoic acid. Molec Pharmacol. 2001;59:16–23. doi: 10.1124/mol.59.1.16. [DOI] [PubMed] [Google Scholar]
- 77.Node K, Ruan X-L, Dai J, Yang S-X, Graham L, Zeldin DC, Liao JK. Activation of Gas mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem. 2001;276:15983–15989. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- 78.Li P-L, Chen C-L, Bortell R, Campbell WB. 11,12-Epoxyeicosatrienoic acid stimulates endogenous mono-ADP-ribosylation in bovine coronary arterial smooth muscle. Circ Res. 1999;85:349–356. doi: 10.1161/01.res.85.4.349. [DOI] [PubMed] [Google Scholar]
- 79.Sun J, Sui X-X, Bradbury A, Zeldin DC, Conte MS, Liao JK. Inhibition of vascular smooth muscle cell migration by cytochrome P450 epoxygenase-derived eicosanoids. Circ Res. 2002;90:1020–1027. doi: 10.1161/01.res.0000017727.35930.33. [DOI] [PubMed] [Google Scholar]
- 80.Dimitropoulou C, West L, Field MB, White RE, Reddy LM, Falck JR, Imig JD. Protein phosphatase 2A and Ca-activated K channels contribute to 11,12-epoxyeicosatrienoic acid analog mediated mesenteric arterial dilation. Prostag and Other Lipid Mediat. 2007;83:50–61. doi: 10.1016/j.prostaglandins.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 81.Snyder GD, Krishna UM, Falck JR, Spector AA. Evidence for a membrane site of action for 14,15-EET on expression of aromatase in vascular smooth muscle. Am J Physiol. 2002;283:H1936–H1942. doi: 10.1152/ajpheart.00321.2002. [DOI] [PubMed] [Google Scholar]
- 82.Wong PY, Lin KT, Yan YT, Ahern D, Iles J, S.Y. S, Bhatt RK, Falck JR. 14(R), 15(S)-Epoxyeicosatrienoic acid receptor in guinea pig mononuclear cell membranes. J Lipid Mediat Cell Signal. 1993;6:199–208. [PubMed] [Google Scholar]
- 83.Wong PY-K, Lai P-S, Falck JR. Mechanism and signal transduction of 14(R), 15 (S)-epoxyeicosatrienoic acid (14,15-EET binding in guinea pig monocytes. Prostag Other Lipid Med. 2000;62:321–333. doi: 10.1016/s0090-6980(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 84.Wong PY-K, Lai P-S, Shen S-Y, Belosludtsev YY, Falck JR. Post-receptor signal transduction and regulation of 14(R), 15(S)-epoxyeicosatrienoic acid (14,15-EET) binding in U-937 cells. J Lipid Med Cell Signal. 1997;16:155–169. doi: 10.1016/s0929-7855(97)00005-9. [DOI] [PubMed] [Google Scholar]
- 85.Yang W, Tuniki VR, Anjaiah S, Falck JR, Hillard CJ, Campbell WB. Characterization of epoxyeicosatrienoic acid binding site in U937 membranes using a novel radiolabeled agonist, 20-125I-14,15-epoxyeicosa-8(Z)-enoic acid. J Pharmacol Exp Ther. 2008;324:1019–1027. doi: 10.1124/jpet.107.129577. [DOI] [PubMed] [Google Scholar]
- 86.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostag and Other Lipid Mediat. 2007;82:42–49. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Y, Falck JR, Tuniki VR, Campbell WB. 20-125Iodo-14,15-epoxyeicosa-5Z-enoic acid: a high affinity radioligand used to characterize the epoxyeicosatrienoic acid antagonist binding site. J Pharmacol Exp Ther. 2009;331:1137–1145. doi: 10.1124/jpet.109.157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gauthier KM, Jagadeesh SG, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic-mSI: A 14,15- and 5,6-EET antagonist in bovine coronary arteries. Hypertension. 2003;42:555–561. doi: 10.1161/01.HYP.0000091265.94045.C7. [DOI] [PubMed] [Google Scholar]
- 89.Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97:853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe H, Viriens J, Prenin J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 91.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BK Ca channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 92.Loot AE, Popp R, Fissllthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilation. Cardiovas Res. 2008;80:445–452. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- 93.Alonso MT, Alvarez J, Montero M, Sanchez A, Garcia-Sancho J. Agonist-induced Ca influx into human platelets is secondary to emptying of intracellular Ca stores. Biochem J. 1991;280:783–789. doi: 10.1042/bj2800783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alvarez J, Montero M, Garcia-Sancho J. Cytochrome P450 may link intercellular lCa stores with plasma membrane Ca influx. Biochem J. 1991;274:193–197. doi: 10.1042/bj2740193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol. 2004;286:C195–C205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 96.Behm DJ, Ogbonna A, Wu C, Burns-Kurtis CL, Douglas SA. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: Identification of a novel mechanism of vasodilation. J Pharmacol Exp Ther. 2009;328:231–239. doi: 10.1124/jpet.108.145102. [DOI] [PubMed] [Google Scholar]
- 97.Ye D, Zhou W, Lee H-C. Activation of rat mesenteric arterial Katp channels by 11,12-epoxyeicosatrienoic acid. Am J Physiol. 2005;288:H358–H364. doi: 10.1152/ajpheart.00423.2004. [DOI] [PubMed] [Google Scholar]
- 98.Ye D, Zhou W, Lu T, Jagadeesh SG, Falck JR, Lee H-C. Mechanism of rat mesenteric arterial KATP channel activation by 14,15-epoxyeicosatrienoic acid. Am J Physiol. 2006;290:J1326–H1336. doi: 10.1152/ajpheart.00318.2005. [DOI] [PubMed] [Google Scholar]
- 99.Sacerdoti D, Bolognesi M, DiPascoli M, Gatta A, McGiff JC, Schwartzman ML, Abraham NG. Rat mesenteric arterial dilator response to 11,12-epoxyeicosatrienoic acid is mediated by activating heme oxygenase. Am J Physiol. 2006;291:H1999–H2002. doi: 10.1152/ajpheart.00082.2006. [DOI] [PubMed] [Google Scholar]
- 100.Jaggar JH, Li A, Parfenova H, Liu J, E.S. U, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conducatance C-activated K channels. Circ Res. 2005;97:805–812. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Widmann MD, Weintraub NL, Fudge JL, Brooks LA, Dellsperger KC. Cytochrome P-450 pathway in acetylcholine-induced canine coronary microvascular vasodilation in vivo. Am J Physiol. 1998;274:H283–H289. doi: 10.1152/ajpheart.1998.274.1.H283. [DOI] [PubMed] [Google Scholar]
- 102.Nishikawa Y, Stepp DW, Chilian WM. In vivo location and mechanism of EDHF-mediated vasodilation in canine coronary microcirculation. Am J Physiol. 1999;277:H1252–H1259. doi: 10.1152/ajpheart.1999.277.3.H1252. [DOI] [PubMed] [Google Scholar]
- 103.Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol. 2000;279:H459–H465. doi: 10.1152/ajpheart.2000.279.2.H459. [DOI] [PubMed] [Google Scholar]
- 104.Oltman CL, Kane NL, Fudge JL, Weintraub NL, Dellsperger KC. Endothelium-derived hyperpolarizing factor in coronary microcirculation: Responses to arachidonic acid. Am J Physiol. 2001;281:H1553–H1560. doi: 10.1152/ajpheart.2001.281.4.H1553. [DOI] [PubMed] [Google Scholar]
- 105.Halcox JPJ, Narayanan S, Crames-Joyce L, Mincemoyer R, Quyyumi AA. Characterization of endothelium-derived hyperpolarizing factor in the human forearm microcirculation. Am J Physiol. 2001;280:H2470–H2477. doi: 10.1152/ajpheart.2001.280.6.H2470. [DOI] [PubMed] [Google Scholar]
- 106.Honing MLH, Smits P, Morrison PJ, Rabelink TJ. Bradykinin-induced vasodilation of human forearm resistance vessels is primarily mediated by endothelium-dependent hyperpolarization. Hypertension. 2000;35:1314–1318. doi: 10.1161/01.hyp.35.6.1314. [DOI] [PubMed] [Google Scholar]
- 107.Passauer J, Bussemaker E, Lassing G, Pistrosch F, Fauler J, Gross P, Fleming I. Baseline blood flow and bradykinn-induced vasodilator responses in human forearm are insensitive to the cytochrome P450 2C9 (CYP2C9) inhibitor sulfaphenazole. Clin Sci. 2003;105:513–518. doi: 10.1042/CS20030118. [DOI] [PubMed] [Google Scholar]
- 108.Taddei S, Varsari D, Cipriano A, Ghiadoni L, Glaetta F, Franzoni F, Magagna A, Virdis A, Salvetti A. Identification of a cytochrome P450 2C9-derived endothelium-derived hyperpolarizing factor in human essential hypertensive patients. J Am Coll Cardiol. 2006;48:508–15. doi: 10.1016/j.jacc.2006.04.074. [DOI] [PubMed] [Google Scholar]
- 109.Donato AJ, Eskurza I, Jablonski KL, Gano LB, Pierce GL, Seals DR. Cytochrome P450 2C9 signaling does not contribute to age-associated vascular endothelial dysfunction in humans. J Appl Physiol. 2008;105:1359–1363. doi: 10.1152/japplphysiol.90629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fisher D, Landmesser U, Spiekermann S, Hilfiker-Kleiner D, Hospely M, Muller M, Busse R, Fleming I, Drexler H. Cytochrome P450 2C9 is involved in flow-dependent vasodilation of peripheral conduit arteries in healthy subjects and patients with chronic heart failure. Europ J Heart Failure. 2007;9:770–775. doi: 10.1016/j.ejheart.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 111.Marques-Soares C, Dijols S, Macherey A-C, Wester MR, Johnson EF, Dansette PM, Mansuy D. Sulfaphenazole derivatives as tools for comparing cytochrome P450 2C5 and human cytochrome P450 2Cs. Biochemistry. 2003;42:6363–6369. doi: 10.1021/bi027391+. [DOI] [PubMed] [Google Scholar]
- 112.Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- 113.Fichtlscherer S, Dimmeler S, Breuer S, Busse R, Zeiher AM, Fleming I. Inhibition of cytochrome P450 2C9 improves endothelium-dependent nitric oxide-mediated vasodilation in patients with coronary artery disease. Circulation. 2004;109:178–183. doi: 10.1161/01.CIR.0000105763.51286.7F. [DOI] [PubMed] [Google Scholar]