Abstract
Adenomatous polyposis coli (APC) is a multifunctional protein commonly mutated in colon cancer. APC contains binding sites for multiple proteins with diverse roles in signaling and the structural and functional organization of cells. Recent evidence suggests roles for APC and some of its binding partners in regulating microtubules in mitosis. APC localizes to three key locations in mitosis: kinetochores, the cortex and centrosomes. Here, we discuss possible mechanisms for APC function at these sites and suggest new pathways by which APC mutations promote tumorigenesis.
Introduction
Mutations in adenomatous polyposis coli (APC) are associated with most colon cancers1,2 although how these mutations affect the development of cancer is not fully understood. In part, this is because APC is a multifunctional protein involved in a wide variety of cellular processes, as indicated by the number of APC interacting proteins (Fig. 1A). A subset of these interacting proteins, β-catenin and Axin, form a complex that regulates Wnt signalmg.3–7 Other interacting proteins, such as Kinesin-Associated Protein 3 (Kap3), Mitotic Centromere Associated Kinesin (MCAK), mDia, microtubules (MT) and End-Binding 1 (EB1) appear to play a role in microtubule dynamics.8–12 Studies have shown involvement of APC in cellular functions related to microtubule dynamics such as migration of epithelial cells and neuronal growth cones.13,14 Here we focus on the role of APC and its binding partners in regulating microtubules in mitosis. APC has been reported to act at three key locations for normal progression through mitosis: the kinetochore, cortex and centrosome.15–19 Potential roles for APC in mitosis suggest new pathways by which APC mutations can contribute to cancer progression.
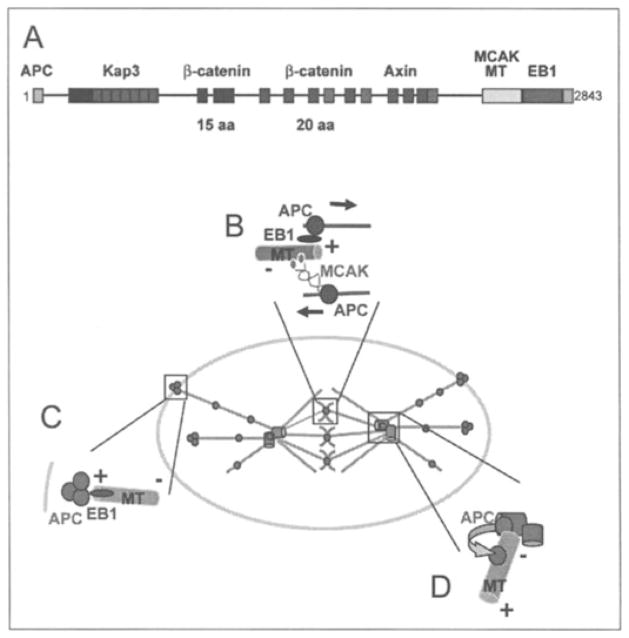
Figure 1.
APC regulates microtubules at multiple mitotic locations. a) Domains of vertebrate APC that are involved in microtubule regulation or Wnt signaling are indicated. APC can bind microtubules indirectly through its interaction with Kap3 (Kinesin associated protein 3) via the N-terminal Armadillo repeats (red), or MCAK (Mitotic Centromere Associated Kinesin) and EB1 (End-Binding 1) at the C-terminus. APC can also bind microtubules directly through its microtubule (MT) binding domain. APC has multiple binding sites for β-catenin (green and blue) and Axin (purple), which are located in the central domain. b) APC localizes to kinetochores where it may have key functions in regulating local microtubule plus-end dynamics. APC may coordinate stabilization and destabilization of microtubule plus-ends at kinetochores through its interactions with EB1 and MCAK, respectively. c) In spindle positioning, a proposed role for APC may be to stabilize and attach astral microtubules to the cortex. EB1 may target microtubule plus-ends to cortical APC clusters. Further work is required to determine whether APC localizes in clusters in mitosis. d) APC localizes to the mother centriole where it is proposed to load onto a subset of microtubules with specific functions, such as cortical attachment, spindle orientation and chromosome congression. A color version of this image is available at www.landesbioscience.com/curie.
APC at the Kinetochore: Regulation of Microtubule Dynamics
APC localizes to kinetochores and forms a complex with kinetochore-bound proteins.15,16 Microtubule-plus end proteins at kinetochores are thought to attach microtubules to chromosomes and/or regulate the local polymerization and depolymerization of microrubules.20 The opposing stabilizing and destabilizing activities of kinetochore-attached microtubules facilitate chromosome congression at the metaphase plate,21 which is required for equal and opposite segregation of chromosomes in anaphase.
Roles for APC in either kinetochore-microtubule capture and attachment, or regulation of kinetochore-microtubule plus-end dynamics have been proposed.22 In support of a role for APC in microtubule capture and attachment at kinerochores, colon cancer cell lines with mutant APC have fewer kinetochores with juxtaposed microtubule ends and an overall decrease in midzone microtubules.22 However, in contrast to depletion of other microtubule plus-end binding proteins (+Tips) that function in microtubule capture at kinetochores, such as CLIP170, cells depleted of APC have normal kinetochore-attached (cold-stable) microtubules.23 and decreased levels of Mad2 at kinetochores, which normally accumulates to unattached kinetochores.23,24 Thus, similar to the role of APC in regulating microtubule plus-end dynamics in migrating cells and mitotic Xenopus extracts,25–27 an alternative, but not mutually exclusive function for APC at kinetochores could be to regulate local microtubule dynamics. A role for APC in regulating microtubule dynamics at kinetochores is supported by the finding that the distances between kinetochores of sister chromatids is decreased in APC depleted cells.23,24 This is similar to what happens in response to taxol, which inhibits microtubule dynamics but not attachment at kinetochores.23,24 Thus, APC depletion results in reduced tension at kinetochores, an indicator of abnormal kinetochore-microtubule dynamics.28 Interestingly, reduced tension at kinetochores in APC depleted cells correlates with abnormal chromosome congression.28 Since localization of APC to kinetochores is microtubule-dependent,16 APC may regulate kinetochore microtubule plus-end dynamics of unattached microtubules and thereby promote microtubule capture at kinetochores. Once the rnicrotubules are attached, APC may regulate local kinetochore microtubule plus-end dynamics to generate tension, which is required for chromosome alignment at the metaphase plate.
APC may stimulate microtubule growth by directly interacting with microtubules through the basic domain or through its interaction with known microtubule stabilizing proteins such as EB1.9,29 Consistent with the latter idea, out of a panel of +Tips analyzed, EB1 was most related to APC in that its localization to kinetochores was microtubule dependent and depletion of EB1 caused reduced tension but not loss of microtubule attachment at kinetochores.23 The only noted difference was that EB1 depletion does not engage a spindle checkpoint arrest, whereas APC depletion results in a transient delay in metaphase.23 Nonetheless, depleting either EB1 or APC causes similar chromosome defects that correlate with and are likely a consequence of, abnormal microtubule dynamics at kinetochores.23 APC also binds to MCAK, which destabilizes microtubules.10,30 Thus, APC may coordinate microtubule polymerization and depolymerization at kinetochores allowing for proper chromosome movement and congression at the metaphase plate (Fig. 1B). Future work analyzing how kinetochore proteins that form a complex with APC, such as MCAK and EB1, are affected in APC depleted cells could test this possibility.
APC at the Cortex: Role in Spindle Positioning
A potential role for APC in orienting the mitotic spindle seems to be conserved in several organisms.31–33 The mechanism of spindle orientation is not well understood, but the importance for microtubule attachment at specialized cortical sites has been established.31,32,34 In Saccharomyces cerevisiae, the functional analogue for APC, Kar9, specifies the cortical site for astral microtubule attachment.35,36 In Drosophila, EAPC/dAPC2 at cell-cell adhesion sites is thought to mediate attachment of astral microtubules at the membrane for proper spindle positioning.17,18,37,38 Less is known about the role of APC in spindle positioning in mammalian cells although there is evidence to suggest that APC may regulate astral microtubule stability39 and/or provide a cortical site for astral microtubule attachment (see below).
In the current model in S. cerevisiae, Kar9 binds asymmetrically to the daughter-bound spindle pole body (SPB) where it interacts with Bim 1 (the yeast homologue of EB1) and is loaded onto the plus-end of a subset of microtubules polymerizing from the SPB (Fig. 2A).40,41 Kar9-Bim 1 bound rnicrotubules are guided along actin cables via Myo2 to the bud tip.33,42,43 Once attached to the bud tip, microtubule movement can be generated by microtubule plus-end dynamics against the cell cortex and cortically bound microtubule motors,44 which enables spindle positioning relative to the mother-bud axis. In budding yeast, the bud neck and later the bud tip direct spindle positioning by providing a predetermined axis of cell division (Fig. 2A). In multicellular organisms, cells often use cues from surrounding cells, specifically from cell-cell adhesion sites, to orient the spindle and thus the plane of division (Fig. 2B).38
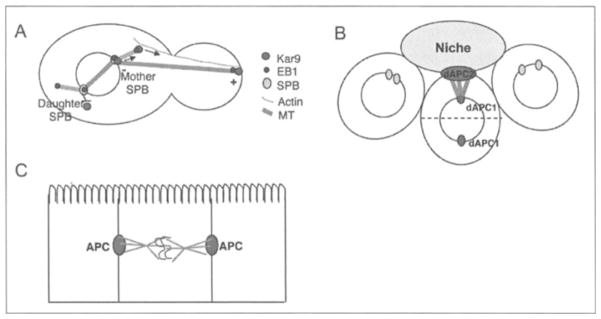
Figure 2.
Potential roles for APC in spindle positioning in budding yeast and Drosophila. A) Model for Kar9-Bim1 mediated spindle positioning in budding yeast. Kar9 binds asymmetrically to the daughter-bound spindle pole body (Mother SPB) where it interacts with Bim1 (the yeast homologue of EB1) and is loaded onto the plus-end of a subset of microtubules polymerizing from the SPB.40,41 Kar9-Bim1 bound microtubules are guided along actin cables via Myo2 (not shown) to the bud tip. Once attached to the bud tip, microtubule movement can be generated by microtubule plus-end dynamics against the cell cortex and cortically bound microtubule motors, which enables spindle positioning relative to the mother-bud axis. B) Model for dAPC2 and dAPC1 mediated spindle positioning in Drosophila male germline stem cells (GSC). dAPC2 localizes to the cell-cell junction between the stem cell and the hub cells where it may facilitate microtubule plus-end attachment.38 The unattached centrosome migrates to the opposite pole to orient the spindle relative to the hub cells (dotted line marks plane of division). dAPC1 is also involved in spindle positioning, however dAPC1 localizes to the centrosomes, not the cell cortex.38 C) In mammalian cells, tethering of astral microtubule plus-ends by APC at cell adhesion sites may orient the spindle in relation to the plane of the epithelium.
In Drosophila and vertebrates there are two APC genes: Drosophila E-APC/dAPC2 and dAPC1 and vertebrate APC and APCL.17,45–48 EAPC/dAPC2 has been reported to localize to cell-cell junctions throughout the embryo, in the neuroepithelium and germ cells of the ovary and testis,17 and this localization is actin dependent and coincides with Armadillo and DE-cadherin.49 Thus, dAPC2 is in the correct location to play a role in spindle attachment at cell junctions and thereby orient the spindle and plane of division. In the syncytial blastoderm, dAPC2 mutants lose nuclei from the cortex into the internal cytoplasm consistent with a loss of spindle attachment to the cortex.18 Similarly, RNAi of dAPC2 in the embryonic epidermis interferes with symmetric division along the planar axis of the embryo and instead these cells divide asymmetrically.37 This suggests that dAPC2 is required for spindle orientation. Finally, in male germline stem cells (GSC), centrosomes are mispositioned in dAPC2 mutants resulting in misoriented spindles (Fig. 2B).38 dAPC1 mutants also have defects in centrosome positioning in male GSCs, however only dAPC2 localizes to cortical attachment sites at cell junctions, whereas dAPC1 localizes to centrosomes in these cells.38 (see below and Fig. 2B). Thus, dAPC2 may play a key function in spindle orientation by providing cortical attachment sites at adherens junctions for microtubules (Fig. 2B), although the mechanism of attachment to adherens junctions by dAPC2 is not understood. One possibility is that dAPC2 functions in maintaining junctions and affects spindle positioning indirectly. However, fly embryos completely null for dAPC1 and dAPC2 do not display obvious defects in adhesion,47 suggesting that dAPC2 may have a direct role in spindle positioning at cell junctions.
In vertebrates, APC depletion in mitotic cells results in loss of astral microtubules and mispositioning of the spindle relative to the geometric center of the cell.39 APC localization to adherens junctions is weak and whether APC functions at adherens junctions in mammalian cells remains to be shown.50–52 In mitotic MDCK cells, small clusters of APC can be found at the cell cortex, some of which align with astral rnicrotubules (S.B., unpublished data). Thus, rather than tethering microtubules at adherens junctions, APC may provide cortical sites at the plasma membrane that stabilize and anchor the plus-ends of astral microtubules (Fig. 1C). Whether APC arrives at these sites independent of microtubules, or by traveling along microtubules and/or with microtubule plus-ends25,53 remains to be shown. Furthermore, it will be important to determine whether these cortical sites also provide cues to orient the axis of the spindle in relation to the plane of the epithelium.
Disruption of APC’s binding partner EB1 results in spindle mispositioining in Drosophila37,54 and mammalian cells,39 similar to the effects of disruption of APC. However, Drosophila dAPC2 lacks the vertebrate C-terminal EB1 and microtubule binding sites17 and may not bind EB1 directly.37 Therefore, it remains unclear whether dAPC2 and EB1 interact in a common pathway to regulate spindle orientation in Drosophila. In mammalian cells, depletion of both APC and EB1 does not have an additive affect, supporting the idea that APC and EB1 function in similar pathways governing spindle orientation.39 One possibility is that, similar to yeast, APC capture of astral microtubule plus ends through EB1 assists spindle orientation in mammalian cells (Fig. 1C). Further experiments are needed to confirm that interactions between APC and EB1 in mammalian cells are required for spindle orientation.
Our knowledge about the function of APC in orienting the mammalian mitotic spindle is still at the early stages. Nevertheless, it is tempting to speculate that APC’s role in positioning mitotic spindles is similar to its cytoskeletal roles at the cortex of interphase cells. In migrating cells, APC puncta localize to specialized cortical regions that promote microtubule growth.13,25,53,55 At the basal cortex, APC puncta localize along the path of microtubules and provide points at which microtubules pause and rescue.56,57 Thus, APC on the plasma membrane in mitotic cells may function similarly in guiding and stabilizing astral microtubules. Live cell imaging of mitotic cells to analyze the interaction of astral microtubule plus-ends with APC puncta is required to support this idea.
APC at Centrosomes: Potential Roles
APC localizes to mammalian centrosomes and this localization is conserved in the yeast analogue, Kar9 and the Drosophila homologue, dAPC1 (Fig. 2).19,38,58,87 Centrosomes nucleate and anchor microtubules and are essential for establishment of a bipolar spindle.59,60 APC mutant mouse embryonic stem cells (ES cells) have multipolar spindles and other centrosome abnormalities in mitosis indicating a role for APC at mitotic centrosomes.15 Very little is known about the function of APC at mammalian centrosomes. However, data from lower eukaryotes suggest several interesting functions for APC at centrosomes including recruiting interacting proteins to this site.
In S. cerevisiae, Kar9 localizes asymmetrically to the daughter-bound spindle pole body (SPB), the yeast equivalent of the centrosome (Fig. 2A).40,41,58 It is thought that the spindle pole body acts as a ‘loading dock’ for Kar9 assuring that only a subset of microtubules are loaded with the complex between Kar9 and Bim1.41 As noted in the previous section, Kar9-Bim1 bound microtubules are then directed to the bud tip where Kar9 mediates attachment of microtubule plus-ends to the cortex (Fig. 2A).34 In Drosophila testes, dAPC1 localizes to the centrosomes while dAPC2 localizes to the cortex (Fig. 2B).38 Although the relationship between dAPC1 at centrosomes and dAPC2 at the cortex in Drosophila testes is unknown, mutants in either gene cause centrosome and spindle misorientation.38 Vertebrate APC and EB1 localize specifically to the mother centriole, which is known to anchor a subset of microtubules.19,60 Furthermore, APC decorates a subset of microtubules in migrating cells.12 Thus, similar to yeast, a subset of microtubules anchored to the mother centriole may be loaded with APC and guided to their cortical destination (Fig. 1D).
In addition to APC, several other regulatory components of the Wnt signaling pathway localize to centrosomes. In the absence of a Wnt signal, a core destruction complex of APC, Axin and GSK3β control β-catenin levels by phosphorylating β-catenin7,61 (see Kennell and Cadigan, this volume). Recent reports show that β-catenin also localizes to centrosomes in interphase and mitosis,62–64 and regulation of its levels at centrosomes throughout the cell cycle may be important for proper centrosome function.62,63 Overexpression of β-catenin results in increased centrosome number in interphase (SB unpublished results),65 whereas β-catenin depletion inhibits centrosome separation in mitosis,62,63 suggesting that there may be a threshold level of β-catenin that is important in some aspect of centrosome organization and function. Consistent with a requirement for regulated levels of β-catenin at centrosomes, GSK3β is active at centrosomes during interphase and is inactive at centrosomes in rnitosis.66 However, it is not known whether GSK3β activity at centrosomes regulates β-catenin levels at this location.
Phosphorylated β-catenin is recognized by the SCF (Skp1-cullin-Fbox) ubiquitin ligase β-TrCP which ubiquitinates β-catenin marking it for degradation by the proteasome.2,7 Components of the SCF ubiquitin ligase, Skp1 and Cul1, localize to centrosomes and are required for centriole duplication and separation in Xenopus egg extracts.67 β-TrCP−/− mouse embryonic fibroblasts and Drosophila β-TrCP/Slimb mutants have overduplicated centrosomes.68 Furthermore, components of the proteasome machinery have been localized to the centrosome and requirements for proteolysis in centrosome duplication and separation has been suggested.69–71 Thus, most components of the core destruction complex and proteasome machinery are present and functional at centrosomes. A function for APC with the other components of the destruction complex at centrosomes may be in regulating β-catenin levels, which in turn could be important for normal centrosome duplication and separation.
APC localization to centrosomes together with several APC interacting proteins suggests several interesting possibilities for APC function at this site. APC, similar to Kar9 in yeast, may load onto a subset of microtubules in mitosis destined to perform specific functions such as cortical attachment, spindle orientation and chromosome congression. On the other hand, the centrosome may act as a signaling hub where Wnt signals coordinate with the cytoskeleton to perform specific tasks (see next section). Experiments that specifically inhibit APC at centrosomes will directly test for these potential functions.
Connecting APC Functions in Regulating Wnt Signaling and Microtubules
APC has divergent roles in cells: it is a regulator of Wnt signaling and also affects cytoskeletal function. In Wnt signaling, APC forms a complex that phosphorylates β-catenin leading to its degradation. As a cytoskeletal regulator, APC binds to proteins that either stabilize or destabilize microtubules at several locations in mitotic and interphase cells. Whether there is a functional link between Wnt signaling and regulation of microtubules through APC is unknown. One possibility is that APC can translate Wnt signals into cytoskeletal changes as it has been shown recently for a noncanonical Wnt signal during cell migration.72 In the absence of Wnt signal, the pool of APC in the destruction complex is distinct from the pool of APC that binds to microtubules,73 Moreover, GSK3β phosphorylation of APC inhibits the interaction of APC with microtubules,74 supporting the idea that the pool of APC in the destruction complex may be unavailable to regulate microtubules. In the presence of a Wnt signal, GSK3β in the destruction complex is inhibited,7 which may release APC from the destruction complex to interact with microtubules. Determining the influence of Wnt signaling on APC’s role in microtubule regulation will provide a link between the function of APC in gene expression and microtubule dynamics and will provide insight into the synergistic consequences of APC mutations that give cells the clonal advantage needed for tumor formation (see below).
Multiple Mechansims by which APC Mutations Contribute to Cancer
Mutations in APC result in unregulated expression of Wnt-responsive genes and mitotic spindle defects. The mechanism by which APC mutations lead to unregulated gene expression and the consequences of this deregulation on cancer progression has been thoroughly examined.7,75,76 Here, we will focus on potential consequences of APC mutations on centrosomes and kinetochores in mitosis, which could contribute to tumorigenesis by promoting chromosomal instability (see also Caldwell and Kaplan, this volume).
Most colorectal tumors exhibit chromosomal instability (CIN), which is caused by chromosome missegregation.77–78 Abnormal centrosome number84 and/or flawed attachment of microtubules to kinetochores82 can lead to chromosome segregation defects. APC mutant mouse ES cells have multipolar spindles and abnormal centrosomes indicating that APC mutations could lead to defects in centrosomes.15 Moreover, depletion of APC has been shown to cause lagging chromosomes, which correlates highly with reduced tension caused by lack of APC at kinetochores.23 Thus, abnormal APC function at centrosomes or kinetochores could cause defects in mitosis that may result in chromosomal instability. It will be necessary to determine whether mutations in APC correlate with centrosome or chromosome abnormalities in colon cancer tissues. Since centrosome abnormalities could arise through indirect effects of abnormal mitoses,85 it will be important to determine whether centrosome abnormalities are present in adenomas at the earliest stages of colon cancer.
A critical consequence of APC dysfunction in mitosis may be that in the absence of APC cells are able to bypass the mitotic checkpoint.23,24 Depletion of APC results in a less efficient mitotic checkpoint.23,24 which would allow cells with centrosome and spindle abnormalities to progress through mitosis with chromosome segregation defects.28 Checkpoint proteins sense lack of tension on kinetochores and respond by inhibiting transition into anaphase.28 After prolonged culture, colon cancer cells with APC mutations exhibit high levels of aneuploidy, indicating that problems in chromosome segregation bypass the checkpoint machinery.86 Furthermore, depletion of APC does not cause an arrest in mitosis and cells progress through mitosis with lagging chromosomes, potentially a direct cause of aneuploidy.23,24 In fact, APC depleted cells treated with low doses of nocodazole or taxol inappropriately exit from mitosis indicating a compromised mitotic checkpoint.24 Reduced accumulation of Bub1 and BubR1 at kinetochores in APC depleted cells24 might be the cause of premature mitotic exit allowing cells with kinetochore abnormalities to bypass the spindle checkpoint. Thus, APC function is required for two important kinetochore functions: proper microtubule attachment and regulation at kinetochores and activation of the mitotic checkpoint in response to chromosome missegregation. Mutations in APC may disrupt both these processes and promote CIN, thereby giving cells the clonal advantage needed for tumorigenesis.
In summary, disruption of the diverse functions of APC may synergistically contribute to cancer progression. Deregulated expression of Wnt-responsive genes promotes cell survival and proliferation, whereas defects in microtubule regulation at centrosomes and kinetochores could contribute to spindle abnormalities that are not properly recognized by the mitotic checkpoint and, therefore, cause CIN and, ultimately, cancer progression.
Acknowledgments
Work from the Nelson laboratory is supported by the NIH (GM35527) and a DOD predoctoral Fellowship to S.B. (BC050431).
References
- 1.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 2.Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta. 1997;1332(3):FI27–147. doi: 10.1016/s0304-419x(97)00008-5. [DOI] [PubMed] [Google Scholar]
- 3.Kishida S, Yamamoto H, Ikeda S, et al. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J Biol Chem. 1998;273(18):10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 4.Rubinfeld B, Tice DA, Polakis P. Axin-dependent phosphorylation of the adenomatous polyposis coli protein mediated by casein kinase lepsilon. J Biol Chem. 2001;276(42):39037–39045. doi: 10.1074/jbc.M105148200. [DOI] [PubMed] [Google Scholar]
- 5.Sakanaka C, Weiss JB, Williams LT. Bridging of beta-catenin and glycogen synthase kinase-3-beta by axin and inhibition of beta-catenin-mediated transcription. Proc Natl Acad Sci USA. 1998;95(6):3020–3023. doi: 10.1073/pnas.95.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda S, Kishida M, Matsuura Y, et al. GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene. 2000;19(4):537–545. doi: 10.1038/sj.onc.1203359. [DOI] [PubMed] [Google Scholar]
- 7.Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9(1):15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 8.Munemitsu S, Souza B, Muller O, et al. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54(14):3676–3681. [PubMed] [Google Scholar]
- 9.Su LK, Burrell M, Hill DE, et al. APC binds to the novel protein EB1. Cancer Res. 1995;55(14):2972–2977. [PubMed] [Google Scholar]
- 10.Banks JD, Heald R. Adenomatous polyposis coli associates with the microtubule-destabilizing protein XMCAK. Curr Biol. 2004;14(22):2033–2038. doi: 10.1016/j.cub.2004.10.049. [DOI] [PubMed] [Google Scholar]
- 11.Jimbo T, Kawasaki Y, Koyama R, et al. Identification of a link between the tumour suppressor APC and the kinesin superfamily. Nat Cell Biol. 2002;4(4):323–327. doi: 10.1038/ncb779. [DOI] [PubMed] [Google Scholar]
- 12.Wen Y, Eng CH, Schmoranzer J, et al. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6(9):820–830. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- 13.Nathke IS, Adams CL, Polakis P, et al. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J Cell Biol. 1996;134(1):165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou FQ, Zhou J, Dedhar S, et al. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42(6):897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Fodde R, Kuipers J, Rosenberg C, et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3(4):433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan KB, Burds AA, Swedlow JR, et al. A role for the adenomatous polyposis coli protein in chromosome segregation. Nat Cell Biol. 2001;3(4):429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- 17.McCartney BM, Dierick HA, Kirkpatrick C, et al. Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J Cell Biol. 1999;146(6):1303–1318. doi: 10.1083/jcb.146.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCartney BM, McEwen DG, Grevengoed E, et al. Drosophila APC2 and armadillo participate in tethering mitotic spindles to cortical actin. Nat Cell Biol. 2001;3(10):933–938. doi: 10.1038/ncb1001-933. [DOI] [PubMed] [Google Scholar]
- 19.Louie RK, Bahmanyar S, Siemers KA, et al. Adenomatous polyposis coli and EB1 localize in close proximity of the mother centriole and EB1 is a functional component of centrosomes. J Cell Sci. 2004;117(Pt 7):1117–1128. doi: 10.1242/jcs.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biggins S, Walczak CE. Captivating capture: how microtubules attach to kinetochores. Curr Biol. 2003;13(11):R449–460. doi: 10.1016/s0960-9822(03)00369-5. [DOI] [PubMed] [Google Scholar]
- 21.Rieder CL, Salmon ED. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 1998;8(8):310–318. doi: 10.1016/s0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green RA, Kaplan KB. Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J Cell Biol. 2003;163(5):949–961. doi: 10.1083/jcb.200307070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Draviam VM, Shapiro I, Aldridge B, et al. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. EMBO J. 2006;25(12):2814–2827. doi: 10.1038/sj.emboj.7601168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dikovskaya D, Schiffmann D, Newton IP, et al. Loss of APC induces polyploidy due to a combination of defects in mitosis and apoprosis. J Cell Biol. 2006 doi: 10.1083/jcb.200610099. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kita K, Wittmann T, Nathke IS, et al. Adenomatous polyposis coli on microtubule plus ends in cell extensions can promote microtubule net growth with or without EB1. Mol Biol Cell. 2006;17(5):2331–2345. doi: 10.1091/mbc.E05-06-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dikovskaya D, Newton IP, Nathke IS. The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts. Mol Biol Cell. 2004;15(6):2978–2991. doi: 10.1091/mbc.E03-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroboth K, Newton IP, Kita K, et al. Lack of adenomatous polyposis coli protein correlates with a decrease in cell migration and overall changes in microtubule stability. Mol Biol Cell. 2007;18(3):910–918. doi: 10.1091/mbc.E06-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinsky BA, Biggins S. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 2005;15(9):486–493. doi: 10.1016/j.tcb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Dikovskaya D, Zumbrunn J, Penman GA, et al. The adenomatous polyposis coli protein: in the limelight out at the edge. Trends Cell Biol. 2001;11(9):378–384. doi: 10.1016/s0962-8924(01)02069-4. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita K, Noetzel TL, Arnal I, et al. Global and local control of microtubule destabilization promoted by a catastrophe kinesin MCAK/XKCM1. J Muscle Res Cell Motil. 2006;27(2):107–114. doi: 10.1007/s10974-005-9045-2. [DOI] [PubMed] [Google Scholar]
- 31.Ahringer J. Control of cell polarity and mitotic spindle positioning in animal cells. Curr Opin Cell Biol. 2003;15(1):73–81. doi: 10.1016/s0955-0674(02)00018-2. [DOI] [PubMed] [Google Scholar]
- 32.Manneville JB, Etienne-Manneville S. Positioning centrosomes and spindle poles: looking at the periphery to find the centre. Biol Cell. 2006;98(9):557–565. doi: 10.1042/BC20060017. [DOI] [PubMed] [Google Scholar]
- 33.Beach DL, Thibodeaux J, Maddox P, et al. The role of the proteins Kar9 and Myo2 in orienting the mitotic spindle of budding yeast. Curr Biol. 2000;10(23):1497–1506. doi: 10.1016/s0960-9822(00)00837-x. [DOI] [PubMed] [Google Scholar]
- 34.Bloom K. It’s a kar9ochore to capture microcubules. Nat Cell Biol. 2000;2(6):E96–98. doi: 10.1038/35014089. [DOI] [PubMed] [Google Scholar]
- 35.Lee L, Tirnauer JS, Li J, et al. Positioning of the mitotic spindle by a cortical-microtubule capture mechanism. Science. 2000;287(5461):2260–2262. doi: 10.1126/science.287.5461.2260. [DOI] [PubMed] [Google Scholar]
- 36.Korinek WS, Copeland MJ, Chaudhuri A, et al. Molecular linkage underlying microtubule orientation toward cortical sites in yeast. Science. 2000 Mar 24;287(5461):2257–2259. doi: 10.1126/science.287.5461.2257. [DOI] [PubMed] [Google Scholar]
- 37.Lu B, Roegiers F, Jan LY, et al. Adherens junctions inhibit asymmetric division in the drosophila epithelium. Nature. 2001;409(6819):522–525. doi: 10.1038/35054077. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301(5639):1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 39.Green RA, Wollman R, Kaplan KB. APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment. Mol Biol Cell. 2005;16(10):4609–4622. doi: 10.1091/mbc.E05-03-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paoletti A, Bornens M. Kar9 asymmetrical loading on spindle poles mediates proper spindle alignment in budding yeast. Dev Cell. 2003;4(3):289–290. doi: 10.1016/s1534-5807(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 41.Liakopoulos D, Kusch J, Grava S, et al. Asymmetric loading of Kar9 onto spindle poles and microtubules ensures proper spindle alignment. Cell. 2003;112(4):561–574. doi: 10.1016/s0092-8674(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 42.Theesfeld CL, Irazoqui JE, Bloom K, et al. The role of actin in spindle orientation changes during the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1999;146(5):1019–1032. doi: 10.1083/jcb.146.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang E, Kusch J, Barral Y, et al. Spindle orientation in saccharomyces cerevisiae depends on the transport of microtubule ends along polarized actin cables. J Cell Biol. 2003;161(3):483–488. doi: 10.1083/jcb.200302030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearson CG, Bloom K. Dynamic microtubules lead the way for spindle positioning. Nat Rev Mol Cell Biol. 2004;5(6):481–492. doi: 10.1038/nrm1402. [DOI] [PubMed] [Google Scholar]
- 45.Yu X, Waltzer L, Bienz M. A new drosophila APC homologue associated with adhesive zones of epithelial cells. Nat Cell Biol. 1999;1(3):144–151. doi: 10.1038/11064. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa H, Murata Y, Koyama K, et al. Identification of a brain-specific APC homologue, APCL and its interaction with beta-catenin. Cancer Res. 1998;58(22):5176–5181. [PubMed] [Google Scholar]
- 47.McCartney BM, Price MH, Webb RL, et al. Testing hypotheses for the functions of APC family proteins using null and truncation alleles in drosophila. Development. 2006;133(12):2407–2418. doi: 10.1242/dev.02398. [DOI] [PubMed] [Google Scholar]
- 48.van Es JH, Kirkpatrick C, van de Wetering M, et al. Identification of APC2, a homologue of the adenomatous polyposis coli tumour suppressor. Curr Biol. 1999;9(2):105–108. doi: 10.1016/s0960-9822(99)80024-4. [DOI] [PubMed] [Google Scholar]
- 49.Mimori-Kiyosue Y, Tsukita S. Where is APC going? J Cell Biol. 2001;154(6):1105–1109. doi: 10.1083/jcb.200106113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barth AI, Nathke IS, Nelson WJ. Cadherins, catenins and APC protein: Interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9(5):68–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- 51.Bienz M, Hamada F. Adenomatous polyposis coli proteins and cell adhesion. Curr Opin Cell Biol. 2004;16(5):528–535. doi: 10.1016/j.ceb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Barth AI, Nelson WJ. What can humans learn from flies about adenomatous polyposis coli? Bioessays. 2002;24(9):771–774. doi: 10.1002/bies.10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mimori-Kiyosue Y, Shiina N, Tsukita S. Adenomatous polyposis coli (APC) protein moves along micro-tubules and concentrates at their growing ends in epithelial cells. J Cell Biol. 2000;148(3):505–518. doi: 10.1083/jcb.148.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogers SL, Rogers GC, Sharp DJ, et al. Drosophila EB1 is important for proper assembly, dynamics and positioning of the mitotic spindle. J Cell Biol. 2002;158(5):873–884. doi: 10.1083/jcb.200202032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mimori-Kiyosue Y, Shiina N, Tsukira S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol. 2000;10(14):865–868. doi: 10.1016/s0960-9822(00)00600-x. [DOI] [PubMed] [Google Scholar]
- 56.Reilein A, Nelson WJ. APC is a component of an organizing template for cortical microtubule networks. Nat Cell Biol. 2005;7(5):463–473. doi: 10.1038/ncb1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reilein A, Yamada S, Nelson WJ. Self-organization of an acentrosomal microtubule network at the basal Cortex of polarized epithelial cells. J Cell Biol. 2005;171(5):845–855. doi: 10.1083/jcb.200505071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller RK, Rose MD. Kar9p is a novel cortical protein required for cytoplasmic microtubule orientation in yeast. J Cell Biol. 1998;140(2):377–390. doi: 10.1083/jcb.140.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy SM, Stearns T. Cytoskeleton: Microtubule nucleation takes shape. Curr Biol. 1996;6(6):642–644. doi: 10.1016/s0960-9822(09)00437-0. [DOI] [PubMed] [Google Scholar]
- 60.Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol. 2002;14(1):25–34. doi: 10.1016/s0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- 61.Peifer M, Polakis P. Wnt Signaling in oncogenesis and embryogenesis—A look outside the nucleus. Science. 2000;287(5458):1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 62.Kaplan DD, Meigs TE, Kelly P, et al. Identification of a role for beta-catenin in the establishment of a bipolar mitotic spindle. J Biol Chem. 2004;279(12):10829–10832. doi: 10.1074/jbc.C400035200. [DOI] [PubMed] [Google Scholar]
- 63.Bahmanyar S, Kaplan DD, DeLuca JG, et al. B-catenin is a Nek2 substrate involved in centrosome separation. Genes Dev. 2006;22(1):91–105. doi: 10.1101/gad.1596308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang P, Senga T, Hamaguchi M. A novel role of phospho-beta-catenin in microtubule regrowth at centrosome. Oncogene. 2007;26(30):4357–4371. doi: 10.1038/sj.onc.1210217. [DOI] [PubMed] [Google Scholar]
- 65.Ligon LA, Karki S, Tokiro M, et al. Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol. 2001;3(10):913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- 66.Wakefield JG, Stephens DJ, Tavare JM. A role for glycogen synthase kinase-3 in mitotic spindle dynamics and chromosome alignment. J Cell Sci. 2003;116(Pt 4):637–646. doi: 10.1242/jcs.00273. [DOI] [PubMed] [Google Scholar]
- 67.Freed E, Lacey KR, Huie P, et al. Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 1999;13(17):2242–2257. doi: 10.1101/gad.13.17.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wojcik EJ, Glover DM, Hays TS. The SCF ubiquitin ligase protein slimb regulates centrosome duplication in Drosophila. Curr Biol. 2000;10(18):1131–1134. doi: 10.1016/s0960-9822(00)00703-x. [DOI] [PubMed] [Google Scholar]
- 69.Fabunmi RP, Wigley WC, Thomas PJ, et al. Activity and regulation of the centrosome-associated proteasome. J Biol Chem. 2000;275(1):409–413. doi: 10.1074/jbc.275.1.409. [DOI] [PubMed] [Google Scholar]
- 70.McDonald HB, Byers B. A proteasome cap subunit required for spindle pole body duplication in yeast. J Cell Biol. 1997;137(3):539–553. doi: 10.1083/jcb.137.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winey M, Baum P, Goetsch L, et al. Genetic determinants of spindle pole body duplication in budding yeast. Cold Spring Harb Symp Quant Biol. 1991;56:705–708. doi: 10.1101/sqb.1991.056.01.079. [DOI] [PubMed] [Google Scholar]
- 72.Schlessinger KME, Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol. 2007;178(3):355–361. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Penman GA, Leung L, Nathke IS. The adenomatous polyposis coli protein (APC) exists in two distinct soluble complexes with different functions. J Cell Sci. 2005;118(Pt 20):4741–4750. doi: 10.1242/jcs.02589. [DOI] [PubMed] [Google Scholar]
- 74.Zumbrunn J, Kinoshita K, Hyman AA, et al. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr Biol. 2001;11(1):44–49. doi: 10.1016/s0960-9822(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 75.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103(2):311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 76.Michor F, Iwasa Y, Lengauer C, et al. Dynamics of colorectal cancer. Semin Cancer Biol. 2005;15(6):484–493. doi: 10.1016/j.semcancer.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 77.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386(6625):623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 78.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396(6712):643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 79.Nowak MA, Komarova NL, Sengupta A, et al. The role of chromosomal instability in tumor initiation. Proc Natl Acad Sci USA. 2002;99(25):16226–16231. doi: 10.1073/pnas.202617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rajagopalan H, Nowak MA, Vogelstein B, et al. The significance of unstable chromosomes in colorectal cancer. Nat Rev Cancer. 2003;3(9):695–701. doi: 10.1038/nrc1165. [DOI] [PubMed] [Google Scholar]
- 81.Giaretti W, Venesio T, Prevosto C, et al. Chromosomal instability and APC gene mutations in human sporadic colorectal adenomas. J Pathol. 2004;204(2):193–199. doi: 10.1002/path.1623. [DOI] [PubMed] [Google Scholar]
- 82.Draviam VM, Xie S, Sorger PK. Chromosome segregation and genomic stability. Curr Opin Genet Dev. 2004;14(2):120–125. doi: 10.1016/j.gde.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 83.Michor F, Iwasa Y, Vogelstein B, et al. Can chromosomal instability initiate tumorigenesis? Semin Cancer Biol. 2005;15(1):43–49. doi: 10.1016/j.semcancer.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 84.Brinkley BR, Goepfert TM. Supernumerary centrosomes and cancer: Boveri’s hypothesis resurrected. Cell Motil Cytoskeleton. 1998;41(4):281–288. doi: 10.1002/(SICI)1097-0169(1998)41:4<281::AID-CM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 85.Nigg EA. Centrosome aberrations: Cause or consequence of cancer progression? Nat Rev Cancer. 2002;2(11):815–825. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
- 86.Tighe A, Johnson VL, Albertella M, et al. Aneuploid colon cancer cells have a robust spindle checkpoint. EMBO Rep. 2001;2(7):609–614. doi: 10.1093/embo-reports/kve127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akong K, Grevengoed EE, Price MH, et al. Drosophila APC2 and APC1 play overlapping roles in wingless signaling in the embryo and imaginal discs. Development. 2002;250:91–100. doi: 10.1006/dbio.2002.0776. [DOI] [PubMed] [Google Scholar]