Abstract
The epithelial apical–junctional complex is a key regulator of cellular functions. In addition, it is an important target for microbial pathogens that manipulate the cell to survive, proliferate and sometimes persist within a host. Out of a myriad of potential molecular targets, some bacterial and viral pathogens have selected a subset of protein targets at the apical–junctional complex of epithelial cells. Studying how microbes use these targets also teaches us about the inherent physiological properties of host molecules in the context of normal junctional structure and function. Thus, we have learned that three recently uncovered components of the apical–junctional complex of the Ig superfamily — junctional adhesion molecule, Nectin and the coxsackievirus and adenovirus receptor — are important regulators of junction structure and function and represent critical targets of microbial virulence gene products.
Introduction
The apical–junctional complex (AJC) is a highly specialized structure at the apical tip of the lateral membrane of polarized epithelial cells. In addition to its multi-faceted participation in regulating cell–cell adhesion between neighboring cells, integrity of the epithelial barrier, and contractile forces during morphogenesis and wound healing, the AJC is equally important as a hub for signaling pathways controlling cell proliferation, cell differentiation and cell polarity [1]. This signaling hub consists of complex networks of interconnected proteins that dynamically interact with each other to adjust and coordinate different cellular functions. Defects in one component of the junction often lead to changes in the entire AJC [2–4].
Pathogens have exploited the AJC as a strategy for overcoming the epithelial barrier and as a site for host colonization. They have evolved mechanisms to directly break down tight junction barriers to allow entry into, or exit from, a host organism, and to co-opt nutrients from the interstitium. Remarkably, they have also been able to access receptors hidden on the basal–lateral epithelial membrane to enter cells where they may replicate or seek protection from the immune system. Pathogenic microbes have even found ways to benefit from inflammation, increased cell turnover and prevention of wound healing, all of which are controlled to some extent by the AJC [5] (Figure 1).
Figure 1.
Bacteria and viruses interact with, and disrupt, apical junctions of polarized epithelia. From left to right, examples of different AJC targets of pathogens. Clostridia (yellow) and Vibrio cholerae (blue-green) secrete enzymes that cleave tight junction membrane proteins. Pathogenic E. coli (blue) deliver bacterial toxins to the host cell, and affect tight junction function by disrupting the peri-junctional actin cytoskeleton. Listeria monocytogenes (brown) exemplifies a bacterium that disrupts junctions to enter epithelial cells and uses AJC components as receptors. Helicobacter pylori (purple) adheres to gastric epithelial cells near apical junctions and injects the bacterial protein CagA into the host cell. CagA interacts with scaffolding components, growth factor receptors and transmembrane molecules of the AJC leading to defects in tight-junction barrier functions and changes in cell polarity. Various unrelated viruses (green) such as adenovirus, coxsackievirus, reovirus, and herpesvirus interact with transmembrane AJC components of the immunoglobulin superfamily. This is important for both viral entry into cells and exit from the basal-lateral surface to reach the apical surface.
The AJC contains several distinct protein sub-complexes. In general, each protein sub-complex consists of a transmembrane protein bound to scaffolding proteins, each of which has multiple protein–protein binding motifs that potentially link together different membrane sub-complexes. Scaffold proteins generally bind to the actin cytoskeleton, although links to microtubules may also be present [1,6]. The cadherin/catenin and claudin/occludin/ZO (zonula occludens) protein sub-complexes form the adherens and tight junctions, respectively, and together with the cytoskeleton provide the basic structural components of the AJC [7,8]. Of course, there are many regulatory elements that control the dynamic organization of the AJC and co-ordinate its many functions (Figure 2).
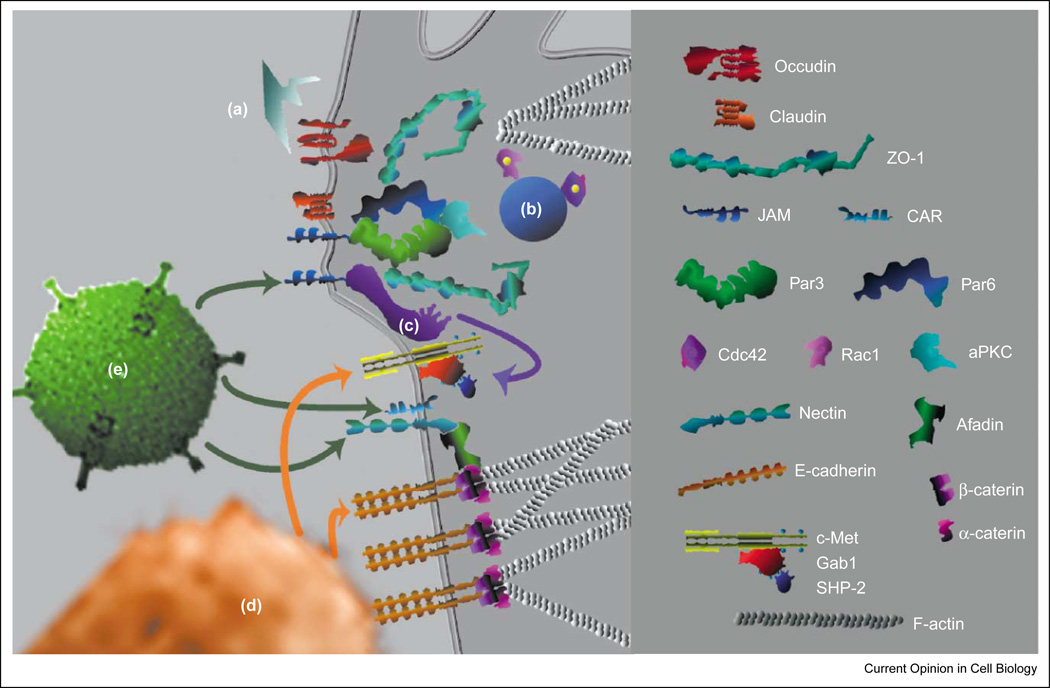
Figure 2.
The apical–junctional complex is organized into structural and regulatory domains by protein sub-complexes. Junctions are held together by transmembrane molecules of the occludin, claudin, cadherin and immunoglobulin superfamilies (red shading denotes proteins with a transmembrane role). Each transmembrane protein is linked via its cytoplasmic tail to scaffolding proteins of the ZO, afadin or catenin families (blue shading denotes proteins with a scaffolding role). Scaffolding proteins then form complexes with multiple signaling and adaptor proteins. For example, the Par3/Par6/aPKC/Rho-GTPase protein complex (purple shading), which is involved in regulating epithelial polarity, is linked to JAM. Scaffolding proteins also link transmembrane proteins to the peri-junctional actin cytoskeleton and its associated proteins and connect different sub-complexes with each other. The apical junctions are traditionally divided into tight and adherens junctions. The tight junctions form the epithelial barrier via their transmembrane proteins claudins and occludin. These are linked to the actin cytoskeleton through scaffolding proteins of the ZO family. At the adherens junction, E-cadherin is linked to the peri-junctional actin via β and α-catenins. These structural domains of the junctions (yellow shading) interact with regulatory protein sub-complexes that are involved in the control of cell polarity, cell division, cell movement and junction assembly. Several regulatory sub-complexes use transmembrane proteins of the immunoglobulin superfamily, such as JAM, CAR and nectin. Other regulatory systems include receptor tyrosine kinases such as c-met. (The key shows examples of the types of molecules at the AJC and is not meant to be exhaustive.)
Here, we review some of the strategies pathogens use to interact with the structural and regulatory components of the AJC. In particular, microbial virulence gene products point to an emerging role for a family of receptors belonging to the Ig superfamily as important regulators of the AJC. Focusing on these targets, we review what is known about the molecular regulation of the AJC by Ig superfamily receptors during junction assembly. The results of this work give us as much information about AJC structure and function as about microbial pathogenesis.
Breaking into the epithelial apical–junctional complex
Cleaving structural components
Bacterial pathogens have developed strategies to interfere with structural components of the AJC at the level of transmembrane proteins, scaffolding proteins and the cytoskeleton. Perhaps the simplest strategy used by pathogens is to secrete one or more enzymes that target the extracellular domain of AJC transmembrane proteins. This usually leads to barrier defects and disruption of the epithelial monolayer [9]. For example, Bacteroides fragilis secretes the toxin Fragilysin that cleaves the extracellular domain of E-cadherin [10]; Vibrio cholerae secretes a protease that can degrade occludin [11]; and adherent Staphylococcus aureus synthesizes several exfoliative toxins that cause disassembly of cell–cell contacts through cleavage of the more spatially distant transmembrane desmosome adhesion protein desmoglein 1 [12•] (Figure 3a). Proteolytic degradation by pathogens confirms the importance of their target transmembrane proteins for maintaining the structural and functional integrity at the AJC and that their disruption can have profound consequences for the host while benefiting the microbe by enabling it to establish itself, gain nutrients and replicate.
Figure 3.
Structural and regulatory sub-complexes of the AJC are targets for pathogens. (a) Bacterial enzymes can cleave structural transmembrane molecules of tight junctions; for example, the V. cholerae HA-protease cleaves the extracellular loops of occludin. (b) Several bacterial toxins and effector molecules target the peri-junctional actin cytoskeleton; for example, E. coli’s CNF-1 activates Rac1 and Cdc42 small GTPases at junctions. (c) Bacteria that translocate effector molecules into the host cell may target the AJC from the inside; for example, H. pylori’s CagA targets JAM and ZO-1 at the junctions as well as activating the c-met growth factor pathway. (d) Some bacteria target AJC components from the outside; for example, Listeria monocytogenes uses E-cadherin as a receptor for cell invasion, and activates the c-met receptor growth factor pathway. (e) Multiple viruses target the immunoglobulin superfamily at the AJC; for example, reovirus uses JAM as a receptor, adenovirus and coxsackievirus use CAR, and α-herpesviruses and poliovirus use nectins.
Targeting regulatory elements from inside the cell
Bacterial proteolytic toxins may be ineffective when applied to the intact apical compartment of an epithelial monolayer. Some bacterial pathogens have evolved alternative strategies that manipulate the AJC from the apical surface. One such strategy is to target regulatory elements of the actin cytoskeleton (Figure 3b). For example, cytotoxic necrotizing factor 1 (CNF-1), a toxin synthesized by certain pathogenic Escherichia coli, targets Rho family small GTPases to disrupt the tight-junctional seal of intact epithelial monolayer [13•]. CNF-1 applied to polarized epithelia activates RhoA, Rac1 and Cdc42 and leads to rearrangement of the actin cytoskeleton. This is followed by massive internalization of tight junctional proteins occludin, junctional adhesion molecule (JAM) and ZO-1 and concomitant disruption of the tight junction barrier. Interestingly, in CNF-1-treated epithelial cells the adherens junctions remained grossly intact. Toxin A and B from the bacterium Clostridium also intervene in small GTPase functions and affect tight junctions in a similar way to CNF-1 [14]. The regulation of junctions through small GTPases is highly complex and requires a balanced interaction between members of the Rho family (reviewed in detail [15,16]).
Bacterial pathogens often combine different strategies to change host cell functions, and have found ways to deliver their effectors to different sites of action. Several bacterial pathogens use molecular microinjection devices derived from flagellar or conjugation apparatuses (known as type III or type IV secretion systems) to introduce bacterial proteins into the host cell and target the AJC from inside. Enteropathogenic Escherichia coli (EPEC), for example, colonizes the apical surface of intestinal epithelial cells and becomes tightly adherent modifying the actin cytoskeleton beneath attached bacteria. This is mediated by bacterial effector proteins translocated into the host-cell membrane and cytosol through a type III secretion system. Independently of factors involved in bacterial attachment, some translocated effectors induce phosphorylation of the myosin light chain by myosin light chain kinase and concomitant contraction of the peri-junctional actomyosin ring [17]. In addition, EPEC infection leads to occludin dephosphorylation, redistribution of occludin and ZO-1, and activation of ezrin [18–20]. It was shown recently that ezrin regulates the assembly of adherens junctions in a Rac1-dependent way [21••]. These changes lead to the breakdown of tight junction barriers and thus to increased paracellular permeability in infected epithelia.
Helicobacter pylori also translocates at least one bacterial effector protein, CagA, into the host cell to unlock the AJC. CagA disrupts the epithelial AJC and forms a new protein complex with the junctional proteins JAM and ZO-1 at the bacterium–host-cell attachment site [22••] (Figure 3c). A functional consequence is that the epithelial barrier is perturbed (although gross cell–cell adhesion and integrity of the epithelial monolayer are maintained), resulting in leakage of ions and solutes from the interstitium into the apical compartment where bacteria reside [22••,23,24]. Furthermore, CagA activates the hepatocyte growth factor (HGF)/c-met pathway from within epithelial cells, which leads to changes in cell morphology and migration [25,26,27••]. HGF and other growth factors are known to be important regulators of the AJC, but the mechanism by which they interact with assembled junctions is poorly understood [28,29]. Similar to H. pylori, Listeria monocytogenes targets proteins of the AJC and growth factor pathways in order for the bacterium to enter host cells [30]. The Listeria surface protein In1A interacts with E-cadherin [31], and In1B activates the Met receptor tyrosine kinase for cell entry [32] (Figure 3d). Future studies on H. pylori CagA and L. monocytogenes In1A and In1B may provide new information about cellular regulation of the AJC through growth factor pathways.
Targeting regulatory elements from outside the cell
Viruses use receptors of the Ig superfamily to interact with host-cell junctions. Adenovirus interacts with the coxsackievirus and adenovirus receptor (CAR) to enter epithelial airway cells [33] (Figure 3e). CAR is an immunoglobulin-like cell–cell adhesion molecule that shares sequence similarities to JAM and is localized at the AJC in a variety of epithelial cells [34]. Disruption of CAR–CAR interactions in contacting cells causes a decrease in cell–cell adhesion and disruption of the barrier function of the epithelial monolayer [34,35••]. After adenoviruses enter epithelial cells and replicate, infected cells release complete viruses, viral particles and fiber protein. Fiber protein interacts with high affinity with CAR, which leads to disruption of cell–cell adhesion allowing viruses to exit from the space between opposing lateral membranes into the apical compartment [35••,36]. Adenovirus pathogenesis demonstrates that CAR receptors are very effective external regulators of AJC structure and function, although we know very little about the precise biochemical mechanisms involved.
Viruses such as reovirus and α-herpesviruses target JAM and nectin receptors, respectively, and use them for cell entry [37,38], but their effects on AJC structure and function have not been described [38–40]. It is not yet clear how JAM and nectin receptors regulate junctions once they are fully assembled. Glycoprotein D, a surface protein of α-herpesviruses, interacts with nectin receptors during cell entry, disrupts cell–cell adhesion in cells expressing nectin-1 and lacking E-cadherin, and interacts with nectin receptors in polarized epithelia [39]. However, in that study and others, effects on the integrity of mature junctions were not studied [41•,42].
Ig superfamily members as regulatory elements of the apical–junctional complex
Why have so many unrelated viral and bacterial factors such as CagA evolved to target the Ig superfamily at the AJC? Under physiological conditions, Ig superfamily receptors are implicated in controlling the disassembly and re-assembly of adherens and tight junctions in the AJC. For example, migration of leukocytes across cell monolayers requires the local opening of intercellular junctions. Live cell imaging of neutrophils migrating through an endothelial monolayer showed that GFP-labeled VE-cadherin, a component of the endothelial adherens junction, disappears from the plasma membrane of endothelial cells at the site of neutrophil penetration. This is followed by resealing of the local opening immediately after the neutrophil passes through the gap [43]. Presumably, tight junctions need to be opened in synchrony with the adherens junctions for this process to occur, and similar openings are required for the transmigration of leukocytes through epithelia. JAM, a member of the Ig superfamily, seems to be involved in the regulation of the AJC [44,45]. Inhibition of JAM receptor activity using antibodies directed against the JAM extracellular domain prevents transmigration of leukocytes through endothelial cell monolayers [46,47]. These results pinpoint the importance of JAM in regulating dynamic openings in the monolayer at the level of the AJC [48,49], but this effect is cell-type- and tissue-specific; for example, inhibition of JAM does not prevent leukocyte transmigration in other endothelial monolayer systems [50,51].
Studies on cell–cell junction assembly reveal how members of the Ig superfamily of cell–cell adhesion proteins regulate the AJC. Nectins, which are also members of the Ig superfamily, are linked to the actin cytoskeleton through afadin, and are closely associated with the cadherin/catenin system in adherens junctions [41•]. JAM also associates with afadin/AF-6 (AF-6 is the ALL-1 fusion partner from chromosome 6) and ZO-1 and is localized with tight-junctional proteins in polarized epithelia [45,52,53]. These protein–protein interactions highlight the interconnection between nectins, JAM and tight-junction proteins in different protein complexes.
The functions of nectin and JAM during assembly of the AJC in epithelial cells have been studied in calcium-switch experiments, in which calcium initiates cell–cell contact via the calcium-dependent cell–cell adhesion molecule E-cadherin. The nectin/afadin system seems to be closely associated with formation of adherens junctions. Nectin interacts with the cadherin/catenin system via afadin and α-catenin [41•], but the exact interaction is not completely understood. It is also thought that the nectin/afadin system is important for recruitment of JAM to sites of cell–cell adhesion. One study suggests that the recruitment of JAM occurs via afadin and ZO-1 and not through afadin alone [54••]. Although it has been shown that afadin can bind directly to ZO-1, this has not been confirmed in other studies and an indirect interaction between the two proteins is possible [55].
JAM seems to be important for the recruitment of the tight-junction transmembrane proteins occludin and claudin to sites of cell–cell adhesion. Inhibition of JAM with a functional blocking antibody prevents recruitment of JAM and occludin to cell–cell contacts and causes loss of barrier function during AJC assembly [56]. JAM may control tight junction assembly via the partitioning-defective protein (PAR) complex. JAM cytoplasmic domain binds PAR-3, thereby localizing the PAR-3/PAR-6/aPKC complex to the AJC [53,57]. In turn, the PAR complex seems to be important for targeting junctional proteins to tight junctions (reviewed in detail in [3,4,58]). JAM may also regulate tight-junction formation via recruitment of members of the ZO protein family, ZO-1, ZO-2 and ZO-3, which bind to the tight junctional proteins occludin and claudins. Overexpression of a ZO-3 mutant in epithelial cells causes a delay in tight junction formation indicating that the protein plays an important role during assembly [59].
As discussed above, CAR receptors are regulators of the AJC in epithelial cells. One mechanism of regulation may be through their interaction with components of the adherens and tight junctions. In one study CAR was found to be in a complex with ZO-1 and localized to tight junctions [34]. Another study found CAR to be localized underneath the tight junction belt in a co-immunoprecipitated complex with β-catenin, a component of the adherens junctions [35••]. Differences in these studies could be due to the studied cell type or to the assembly stage of the junctional complex. In analogy to JAM receptors and nectin receptors regulating junctional assembly, the physical link between CAR and these adaptor protein sub-complexes implicates them in regulation of the AJC.
As epithelial and endothelial cells express different receptors of the Ig superfamily at different levels and locations relative to the AJC [42,44], CAR, JAM and nectin receptors appear to give cells the regulatory diversity to reversibly control the structural integrity and function of the AJC. Is it possible that some microorganisms unlock the AJC using the same mechanisms utilized by transmigrating leucocytes? Are the cell-type- and tissue-specific features that are characteristic of bacterial and viral entry a reflection of the nature of the AJC and its molecular components in particular tissues? Future studies in these areas should provide us with some answers.
Conclusions
The AJC is a signaling hub regulating many important cellular functions. It comprises basic structural components, including the cadherin/catenin and claudin/occludin complexes that form the adherens and tight junctions, respectively, and the cytoskeleton, which connects these junctions into an integrated network. Regulatory sub-complexes comprising transmembrane receptors of the Ig superfamily resemble a software operating system, and may control the organization of the structural components during assembly and maintenance of the AJC. Pathogens have evolved mechanisms to target both the structural and regulatory components of the AJC. In some cases, pathogens, like hackers, have developed ways to finely manipulate the software that subtly adjusts AJC structure and function. Future studies based upon what work on pathogens has identified as key components of the AJC, should provide new insights into how the software controls the hardware to adapt the AJC signaling hub for different functions. Moreover, devising a ‘firewall’ to prevent disruptive hacking of the AJC hardware and software may provide us with novel preventive measures against microbial incursion.
Acknowledgements
Because of space constraints we have included examples of a limited number of pathogens and their targets rather than attempting to be complete. We apologize to the many investigators whose work we were unable to cite. Work from the Nelson and Falkow laboratory is supported by a Walter V and Idun Y Berry Fellowship (RV); Deutsche Forschungsgemeinschaft Fellowship VO 864/1-1 (RV); a Pediatric Infections Disease Society of America/St. Jude Fellowship in Pediatric Infectious Diseases (MRA); and NIH grants DDC DK56339 (RV, WJN, and SF), RO1GM35227 (WJN), and AI38459 (SF).
Abbreviations
- AJC
apical–junctional complex
- CAR
coxsackievirus and adenovirus receptor
- CNF-1
cytotoxic necrotizing factor 1
- EPEC
enteropathogenic Escherichia coli
- HGF
hepatocyte growth factor
- JAM
junctional adhesion molecule
- PAR
partitioning-defective protein
- ZO
zonula occludens
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- 2.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 3.Humbert P, Russell S, Richardson H. Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. Bioessays. 2003;25:542–553. doi: 10.1002/bies.10286. [DOI] [PubMed] [Google Scholar]
- 4.Etienne-Manneville S, Hall A. Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr Opin Cell Biol. 2003;15:67–72. doi: 10.1016/s0955-0674(02)00005-4. [DOI] [PubMed] [Google Scholar]
- 5.Falkow S. Cellular microbiology is launched. Cell Microbiol. 1999;1:3–6. doi: 10.1046/j.1462-5822.1999.00005.x. [DOI] [PubMed] [Google Scholar]
- 6.Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 8.Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–E108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 9.Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport and inflammation. Gut. 2003;52:439–451. doi: 10.1136/gut.52.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu S, Lim KC, Huang J, Saidi RF, Sears CL. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci U S A. 1998;95:14979–14984. doi: 10.1073/pnas.95.25.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, Nybom P, Magnusson KE. Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell Microbiol. 2000;2:11–17. doi: 10.1046/j.1462-5822.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- 12. Hanakawa Y, Schechter NM, Lin C, Garza L, Li H, Yamaguchi T, Fudaba Y, Nishifuji K, Sugai M, Amagai M, et al. Molecular mechanisms of blister formation in bullous impetigo and staphylococcal scalded skin syndrome. J Clin Invest. 2002;110:53–60. doi: 10.1172/JCI15766. Staphylococcus aureus exfoliative toxins bind specifically to desmoglein 1 in keratinocytes of the superficial epidermis. The toxins cleave mouse and human desmoglein 1 (Dsg1) after glutamic acid residue 381 between extracellular domains 3 and 4. The study demonstrates that some bacterial effectors are very specific for junctional elements.
- 13. Hopkins AM, Walsh SV, Verkade P, Boquet P, Nusrat A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci. 2003;116:725–742. doi: 10.1242/jcs.00300. CNF-1, which activates RhoA, Rac1 and Cdc42, affects the integrity of mature junctions and junction assembly. Following CNF-1 exposure, tight junctional proteins occludin, ZO-1 and JAM are displaced from the membrane, but not the adherens junctional proteins E-cadherin and β-catenin. CNF-1 produces an increase in paracellular permeability.
- 14.Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69:1329–1336. doi: 10.1128/IAI.69.3.1329-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Aelst L, Symons M. Role of Rho family GTPases in epithelial morphogenesis. Genes Dev. 2002;16:1032–1054. doi: 10.1101/gad.978802. [DOI] [PubMed] [Google Scholar]
- 16.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 17.Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- 18.Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305–315. doi: 10.1046/j.1462-5822.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- 19.Philpott DJ, McKay DM, Sherman PM, Perdue MH. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 20.Simonovic I, Arpin M, Koutsouris A, Falk-Krzesinski HJ, Hecht G. Enteropathogenic Escherichia coli activates ezrin, which participates in disruption of tight junction barrier function. Infect Immun. 2001;69:5679–5688. doi: 10.1128/IAI.69.9.5679-5688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pujuguet P, Del Maestro L, Gautreau A, Louvard D, Arpin M. Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol Biol Cell. 2003;14:2181–2191. doi: 10.1091/mbc.E02-07-0410. The study shows a new role for ezrin in E-cadherin-dependent cell–cell junction assembly. A constitutively active mutant of ezrin activates Rac1 in MDCK cells, but not RhoA and Cdc42, and causes a Rac1-dependent delay of E-cadherin delivery to the plasma membrane during junction assembly. Activated Ezrin perturbs E-cadherin internalization in mature junctions.
- 22. Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical–junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. CagA disrupts tight junction barrier function and AJC organization after translocation into epithelial cells. It specifically targets the tight junctional proteins ZO-1 and JAM to the attachment site of the bacterium and forms new protein complexes at the AJC. These effects are independent of the previously described phosphorylation-dependent effects of CagA on cell elongation.
- 23.Suzuki K, Kokai Y, Sawada N, Takakuwa R, Kuwahara K, Isogai E, Isogai H, Mori M. SS1 Helicobacter pylori disrupts the paracellular barrier of the gastric mucosa and leads to neutrophilic gastritis in mice. Virchows Arch. 2002;440:318–324. doi: 10.1007/s004280100430. [DOI] [PubMed] [Google Scholar]
- 24.Noach LA, Rolf TM, Tytgat GN. Electron microscopic study of association between Helicobacter pylori and gastric and duodenal mucosa. J Clin Pathol. 1994;47:699–704. doi: 10.1136/jcp.47.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- 26.Mimuro H, Suzuki T, Tanaka J, Asahi M, Haas R, Sasakawa C. Grb2 Is a key mediator of Helicobacter pylori CagA protein activities. Mol Cell. 2002;10:745–755. doi: 10.1016/s1097-2765(02)00681-0. [DOI] [PubMed] [Google Scholar]
- 27. Churin Y, Al-Ghoul L, Kepp O, Meyer TF, Birchmeier W, Naumann M. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J Cell Biol. 2003;161:249–255. doi: 10.1083/jcb.200208039. CagA interacts with and activates c-met receptor, and leads to cell morphology changes in epithelial cells. The study is in line with previous studies showing that the activation of proteins SHP-2 and Grb2 of the c-met pathway by H. pylori CagA is important for cell scattering of sub-confluent epithelial cells [25,26].
- 28.Grisendi S, Arpin M, Crepaldi T. Effect of hepatocyte growth factor on assembly of zonula occludens-1 protein at the plasma membrane. J Cell Physiol. 1998;176:465–471. doi: 10.1002/(SICI)1097-4652(199809)176:3<465::AID-JCP3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 29.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cossart P, Pizarro-Cerda J, Lecuit M. Invasion of mammalian cells by Listeria monocytogenes: functional mimicry to subvert cellular functions. Trends Cell Biol. 2003;13:23–31. doi: 10.1016/s0962-8924(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 31.Shen Y, Naujokas M, Park M, Ireton K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell. 2000;103:501–510. doi: 10.1016/s0092-8674(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 32.Mengaud J, Ohayon H, Gounon P, Mege RM, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 33.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 34.Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci U S A. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell. 2002;110:789–799. doi: 10.1016/s0092-8674(02)00912-1. Adenovirus fiber protein, which has a high affinity for CAR receptors, is released from infected bronchial epithelial cells into the lateral space of neighboring cells. The study demonstrates that fiber protein is sufficient to decrease the tight junction barrier when applied laterally to epithelial monolayers. Experiments with functional CAR-blocking antibodies also indicate that CAR is required for establishing and maintaining the airway epithelial barrier.
- 36.Freimuth P, Springer K, Berard C, Hainfeld J, Bewley M, Flanagan J. Coxsackievirus and adenovirus receptor amino-terminal immunoglobulin V-related domain binds adenovirus type 2 and fiber knob from adenovirus type 12. J Virol. 1999;73:1392–1398. doi: 10.1128/jvi.73.2.1392-1398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spear PG, Eisenberg RJ, Cohen GH. Three classes of cell surface receptors for αherpesvirus entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 38.Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, Nusrat A, Parkos CA, Dermody TS. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104:441–451. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 39.Krummenacher C, Baribaud I, Eisenberg RJ, Cohen GH. Cellular localization of nectin-1 and glycoprotein D during herpes simplex virus infection. J Virol. 2003;77:8985–8999. doi: 10.1128/JVI.77.16.8985-8999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon M, Spear PG. Disruption of adherens junctions liberates nectin-1 to serve as receptor for herpes simplex virus and pseudorabies virus entry. J Virol. 2002;76:7203–7208. doi: 10.1128/JVI.76.14.7203-7208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Honda T, Shimizu K, Kawakatsu T, Yasumi M, Shingai T, Fukuhara A, Ozaki-Kuroda K, Irie K, Nakanishi H, Takai Y. Antagonistic and agonistic effects of an extracellular fragment of nectin on formation of E-cadherin-based cell–cell adhesion. Genes Cells. 2003;8:51–63. doi: 10.1046/j.1365-2443.2003.00616.x. Recombinant extracellular fragments of nectin-3, which bind to nectin-1 and block nectin-1 homo-trans-dimer formation between neighboring cells, prevent the formation of E-cadherin-based adherens junctions during junction assembly in MDCK cells. Furthermore, beads coated with the recombinant extracellular fragment nectin-3 recruit the nectin/afadin and cadherin/catenin systems to sites of attachment in MDCK cells. E-cadherin-coated beads recruit both systems in the same way, demonstrating the close association between the two systems.
- 42.Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116:17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- 43.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 44.Luscinskas FW, Ma S, Nusrat A, Parkos CA, Shaw SK. The role of endothelial cell lateral junctions during leukocyte trafficking. Immunol Rev. 2002;186:57–67. doi: 10.1034/j.1600-065x.2002.18606.x. [DOI] [PubMed] [Google Scholar]
- 45.Liang TW, DeMarco RA, Mrsny RJ, Gurney A, Gray A, Hooley J, Aaron HL, Huang A, Klassen T, Tumas DB, et al. Characterization of huJAM: evidence for involvement in cell–cell contact and tight junction regulation. Am J Physiol Cell Physiol. 2000;279:C1733–C1743. doi: 10.1152/ajpcell.2000.279.6.C1733. [DOI] [PubMed] [Google Scholar]
- 46.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Maschio A, De Luigi A, Martin-Padura I, Brockhaus M, Bartfai T, Fruscella P, Adorini L, Martino G, Furlan R, De Simoni MG, et al. Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (JAM) J Exp Med. 1999;190:1351–1356. doi: 10.1084/jem.190.9.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunningham SA, Rodriguez JM, Arrate MP, Tran TM, Brock TA. JAM2 interacts with α4β1. Facilitation by JAM3. J Biol Chem. 2002;277:27589–27592. doi: 10.1074/jbc.C200331200. [DOI] [PubMed] [Google Scholar]
- 49.Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 50.Lechner F, Sahrbacher U, Suter T, Frei K, Brockhaus M, Koedel U, Fontana A. Antibodies to the junctional adhesion molecule cause disruption of endothelial cells and do not prevent leukocyte influx into the meninges after viral or bacterial infection. J Infect Dis. 2000;182:978–982. doi: 10.1086/315765. [DOI] [PubMed] [Google Scholar]
- 51.Shaw SK, Perkins BN, Lim YC, Liu Y, Nusrat A, Schnell FJ, Parkos CA, Luscinskas FW. Reduced expression of junctional adhesion molecule and platelet/endothelial cell adhesion molecule-1 (CD31) at human vascular endothelial junctions by cytokines tumor necrosis factor-α plus interferon-γ does not reduce leukocyte transmigration under flow. Am J Pathol. 2001;159:2281–2291. doi: 10.1016/s0002-9440(10)63078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem. 2000;275:27979–27988. doi: 10.1074/jbc.M002363200. [DOI] [PubMed] [Google Scholar]
- 53.Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154:491–497. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fukuhara A, Irie K, Nakanishi H, Takekuni K, Kawakatsu T, Ikeda W, Yamada A, Katata T, Honda T, Sato T, et al. Involvement of nectin in the localization of junctional adhesion molecule at tight junctions. Oncogene. 2002;21:7642–7655. doi: 10.1038/sj.onc.1205875. Recombinant extracellular fragments of nectin-3, which bind to nectin-1 and block nectin-1 homo-trans-dimer formation between neighboring cells, prevent the recruitment of JAM to sites of cell–cell adhesion during junction assembly in nectin-1α-overexpressing MDCK cells. Beads coated with recombinant extracellular fragments of nectin-3 recruit JAM to sites of attachment. JAM homo-trans-dimerization between neighboring cells is not required for JAM recruitment to sites of cell–cell adhesion. Although nectin-1 can be co-immunoprecipitated with afadin and ZO-1, and JAM with ZO-1 and afadin, nectin and JAM cannot be co-immunoprecipitated in nectin-1α-overexpressing MDCK cells. In this study, recombinant afadin can bind to recombinant nectin, but not recombinant JAM protein in vitro. Therefore, the authors suggest that nectin and JAM are associated via afadin and ZO-1.
- 55.Yokoyama S, Tachibana K, Nakanishi H, Yamamoto Y, Irie K, Mandai K, Nagafuchi A, Monden M, Takai Y. α-Catenin-independent recruitment of ZO-1 to nectin-based cell–cell adhesion sites through afadin. Mol Biol Cell. 2001;12:1595–1609. doi: 10.1091/mbc.12.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113:2363–2374. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 57.Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, Vestweber D. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM) EMBO J. 2001;20:3738–3748. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohno S. Intercellular junctions and cellular polarity: the PAR–aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol. 2001;13:641–648. doi: 10.1016/s0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- 59.Wittchen ES, Haskins J, Stevenson BR. Exogenous expression of the amino-terminal half of the tight junction protein ZO-3 perturbs junctional complex assembly. J Cell Biol. 2000;151:825–836. doi: 10.1083/jcb.151.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]