Abstract
Micro/nano-fabrication techniques, such as soft lithography and electrospinning, have been well-developed and widely applied in many research fields in the past decade. Due to the low costs and simple procedures, these techniques have become important and popular for biological studies. In this review, we focus on the studies integrating micro/nano-fabrication work to elucidate the molecular mechanism of signaling transduction in cell biology. We first describe different micro/nano-fabrication technologies, including techniques generating three-dimensional scaffolds for tissue engineering. We then introduce the application of these technologies in manipulating the physical or chemical micro/nano-environment to regulate the cellular behavior and response, such as cell life and death, differentiation, proliferation, and cell migration. Recent advancement in integrating the micro/nano-technologies and live cell imaging are also discussed. Finally, potential schemes in cell biology involving micro/nano-fabrication technologies are proposed to provide perspectives on the future research activities.
Keywords: Micro/nano-fabrication, Surface pattern, Soft lithography, Live cell imaging
1 Introduction
Micro/nano-fabrication technologies have become more and more important for biology, especially for cell biology. As one of the central methods to manipulate the cell environment for the study of the signaling pathways and the underlying molecular mechanism of cellular responses, including cell life and death, stem cell differentiation, proliferation, cell migration and so on, micro/nano-fabrication technologies have several advantages. First, the fabrication techniques can create local cellular microenvironment to closely mimic the physiological and pathological environment. Second, the microenvironment can be generated in a precisely predictable manner to study the live cell real time response under different environment. Third, it is easy to modulate the topography and density of the extracellular matrix (ECM) proteins to control the local chemical environment for cell adhesion; Finally, these micro/nano-technologies can help the designation and generation of well-controlled three-dimensional structures, which can allow the culturing or co-culturing of cells in an environment similar to in vivo conditions for tissue engineering and regenerative medicine. As such, micro/nano-fabrication technologies become essential to modern biological studies.
In this review, we first introduce several well-developed micro/nano-fabrication technologies which can be applied to create different micro/nano-structured environment, both in 2D and 3D. We then discuss the applications of these technologies in studying cellular responses and behaviors, such as apoptosis, differentiation, proliferation, cell migration. Subsequently, the integration of micro/nano-technologies and molecular imaging in live cells is discussed. Finally, we briefly describe the potential future applications of micro/nano-fabrication technologies in the research field of cell biology.
2 Micro/nano-fabrication technologies
Many micro/nano-fabrication technologies have been invented and developed during the past decades. Indeed, some of them have already widely applied in the cell biology study. In this section, we introduce and emphasize on several prominent technologies, such as soft lithography, electrospinning, nano-structured patterning technologies (including dip pen, e-beam writing, nano-imprint lithography, nano-shaving, and so on), and three-dimensional fabrications. A table is also presented to highlight the pros and cons of different major technologies (Table 1).
Table 1. Summary of the pros and cons of different micro/nanotechnologies.
| Technologies | Pros | Cons | Resolution | Dimension(s) |
|---|---|---|---|---|
| Soft lithography |
|
Diffusion from the ink can lower the resolution | 30 nm–100 mm (Nano-imprint lithography can reach the resolution < 5 nm) | 2D or 3D |
| Electrospinning |
|
Low yield | 3 nm–5 μm | 3D |
| Dip-pen | Allow the creation of biocompatible nanosized patterns |
|
30 nm | 2D |
| Electron beam writing | High resolution |
|
5 nm | 2D |
2.1 Soft lithography
Soft lithography is the keystone of a set of techniques for non-photolithographic fabrication, which is mainly based on the elastometric stamp to mold desirable two- or three-dimensional micro/nano-scale patterns on different substrata [3, 4, 25, 65]. In general, it includes several major techniques: microcontact printing, replica molding, microtransfer molding micromolding, and solvent-assisted micromolding. Due to its low cost and easy fabrication characteristics, it has been widely applied in biology studies during the past decade.
Soft lithography process is usually divided into two parts: (1) create and fabricate the elastomeric elements; (2) apply these elements to release the desirable geometrical patterns onto substrata. Figure 1 is a representative scheme illustrating the major steps of soft lithography: the preparation of a poly(dimethylsiloxane) (PDMS) stamp using replica molding, and the transfer of patterns by micro-contact printing (lCP). The whole process starts with the exposure of a spinning-coated photoresist film to ultraviolet lights on a silicon support through a mask. After dissolving the unexposed photoresist, the remaining photoresist is developed to form the master with designed patterns. The size of the patterns is usually determined by the dimension of patterns on masks. In order to achieve the desirable dimensions and precision, it is also necessary to control the spinning coating speed, the periods of exposure time and development. Subsequently, the master needs to be exposed to vapors of CF3(CF2)6(CH2)2SiCl3 for 2 h in a desiccator with a low pressure to reduce its tendency of adhering to elastomer stamps, which is generated by pouring elastomer (typically PDMS; Sylgard 184) over the master. The elastomer will then be cured for 2 h at 80°C to form the PDMS stamp, which can be peeled off from the master afterwards. Before doing μCP, the uncured ingredients can be extracted by CH2Cl2 to clean the mold surface. The stamps are then ready to be inked with an alkanethiol solution with ethanol as the solvent. The ethanol can be removed by evaporation in a stream of nitrogen and then brought into contact with a thin film of gold on a solid surface for around 30 s. This patterned self-assembled monolayers (SAMs) is now ready for the coupling of functional molecules/proteins to create micro/nano-environments for cell culture.
Fig. 1.
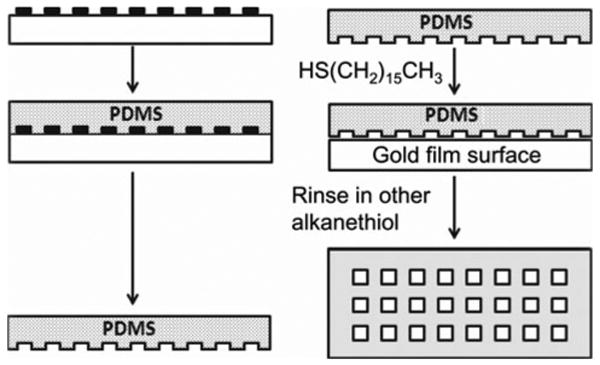
A scheme shows the procedure of developing micro-patterns by soft lithography. The process starts with fabricating photo resist patterns on silicon wafer by photolithography and remolding the patterns by PDMS. This is followed by micro-contact printing during which the PDMS stamp is rinsed with an ethanolic solution of a thiol to transfer thiol patterns on gold surfaces
Due to the advantages of soft lithography, e.g., it can be fabricated at low costs and cells constrained on a certain location with desirable shapes, there are a lot of applications of soft lithography in cell biology, such as creating patterned cell culture and co-culture systems, manipulating topography of ECM, and imposing cells with gradient chemical stimulation [25]. SAM is not the only way to obtain soft lithography patterning surface. Ma et al. created micro-patterns via micro-contact printing method [27, 37]. With this method, the patterns created by comb polymer were quite stable. While the micro-contract printing technology has been exceedingly useful and applied in cell biological studies, there is a practical limit in the resolution of the created patterns, typically at the scale of 100– 200 nm [53]. This is mainly due to the surface diffusion of the molecular inks and the molecular disorder at the pattern edges.
Microfluidics system is another major application of soft lithography technology. Microfluidics systems are typically applied for the screening purpose of protein crystallization, bio-analysis, synthesis, examination, and manipulation of samples consisting of a single or multiple cells, and drug development. Different kinds of stimuli (chemical molecules or mechanic stimulation) can also be delivered to cells by this system in microfluidic channels at subcellular levels. For example, Takayama et al. reported the application of multiple laminar streams in a microfluidic channel to deliver membrane permeable molecules to highlight subcellular microdomains [59]. This method can also be readily applied for the potential non-invasive visualizing, probing, and manipulating the cellular metabolic and structural machinery. Chiu et al. have created a three-dimensional microfluidic system which can deliver cells to an adhesive tissue culture surface patterned in concentric squares [57]. Different cell types, e.g., bovine capillary endothelial and human bladder cancer cells, can be deposited at different squares. This system can also allow the deposition of the same type of cells on defined areas where each area can be treated with different substances.
2.2 Electrospinning
Electrospinning is a fabrication technique utilizing electrical charges to draw fine fibers at the scale of submicron and nano meters. It was invented by Cooley and Morton in 1902, more than 100 years ago. The generation of fiber electrospinning was also achieved during 1930s [58]. In the past decade, largely due to the need in the field of tissue engineering, electrospinning has been significantly advanced to create different materials of scaffolds and hence gained a high popularity. This technique is able to generate fibers with diameters ranging from 3 nm to 5 μm, with small pore sizes and high ratio between the surface area and volume. As shown in Fig. 2 [50], a typical setup of electrospinning consists mainly three parts: a syringe pump containing the materials, a high voltage source which can generate high electric field for spinning, and a collector to collect the fibers. Potential applications for this technique include filtration membrane, catalytic nanofibers, fiber-based sensors, and tissue engineering [50].
Fig. 2.
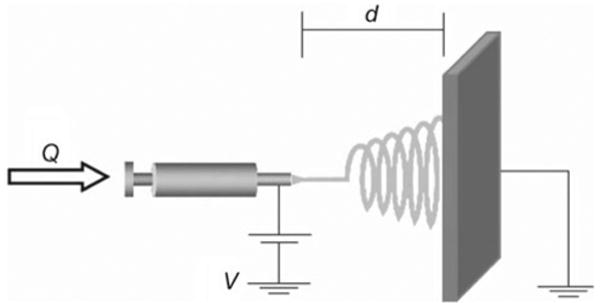
A typical electrospinning setup. Q represents the flow rate and d the distance between the needle tip and patterned plate. V is the applied voltage to control the spinning of the thread (reprinted with permission from [50])
Electrospinning technique has been widely applied in the area of tissue engineering due to its various advantages. (1) Electrospinning technique can create nano-sized fibers and scaffolds with high ratio between surface area and volume. Indeed, studies have shown that this kind of nano-scale property of the scaffolds is capable of supporting different types of cells better than the micron sized fibers. Kwon et al. [33] demonstrated that human umbilical endothelial vein cells (HUVECs) on fibers between 300 nm and 1.2 μm in size were well-elongated on fibrous meshes, whereas HUVECs on fibers with diameters at 7 μm were round with limited spreading area. Furthermore, the densities of HUVECs cultured on submicron-sized fibers increased continuously whereas HUVECs adhered on the 7 μm fibers remained at a very low density without any sign of proliferation even after 1-week culture. (2) Electrospinning technique can form non-woven fabrics based on a variety of materials, such as polysaccharides (cellulose, chitin, chitosan, and dextrose), proteins including collagen [39, 56] and gelatin [56], silk [7, 41, 45], DNA [56], as well as some biopolymer derivatives and composites. As such, researchers can have more freedom in choosing the materials to be compatible for desired cell types. For example, collagen is considered as one of the major component of ECM proteins. Matthews et al. [39] have chosen collagen as the material to create scaffolds to host aortic smooth muscle cells (ASMCs). The results revealed that the scaffolds were densely populated with ASMCs within 7 days. Indeed, ASMCs can be observed deep within the scaffold, fully enmeshed within the fibrils of the electrospun collagen. Huang et al. [56] also developed a method to gain elastin-mimetic protein fibers and networks by electrospinning an aqueous solution of a genetically engineered 81 kDa peptide polymer based upon the repeated sequence of (Val-Pro-Gly-Val-Gly)4(Val-Pro-Gly-Lys-Gly). Fibers based on DNA component and created by electrospinning have also been demonstrated [16]. (3) Composite scaffolds can also be created by electrospinning. Boland and his co-workers created multi-layer scaffold with both collagen type I and type III. Smooth muscle cells could be observed to infiltrate comfortably into the scaffold [5].
In summary, because the properties of the scaffolds created by electrospinning, such as the structure, chemical and mechanical stability, and functionality, can be easily modified to match desired applications, electrospinning is an ideal method to create scaffolds for tissue engineering, so that the micro-environment of cultured cells can mimic more closely the environment in vivo.
2.3 Nano-structured fabrication
Various nano-structured fabrication techniques other than electrospinning have achieved tremendous progress in applying to cell biology during last decade. Nano-structured materials have many unique properties: (i) large fraction of surface and high ratio of surface area to volume; (ii) high surface energy; (iii) precise spatial confinement; (iv) reduced imperfections. These properties do not exist in the corresponding bulk materials. In this section, we discuss some nano-fabrication methods which have already been applied in the studies of cell biology.
A “dip-pen” nanolithography (DPN) in a direct-write fashion was invented in 1999 [51]. In brief, an atomic force microscope tip was used to deliver collections of molecules in a positive printing mode. The results indicate that alkanethiols can be printed in lines on a gold thin film with 30 nm resolution, analogous to what a dip pen can do. During the past 10 years since this technique was invented, DPN has been developed as a general method for generating functional surface-patterns on the sub-100 nm scale, with the chemical composition and structure precisely tailored by an atomic force microscope. For example, Lee et al. [35] applied DPN to fabricate nanoarrays of ECM domains of fibronectin on a thin-film gold surface. 3T3 Swiss fibroblast cells were successfully seeded onto these fabricated patterns consisting of nanoarrays. Therefore, DPN can provide the opportunity to study a variety of surface-mediated biological processes at subcellular levels.
Electron beam fabrication is another method which can create nano-patterns with different materials. Indeed, a resolution of 10 nm was achieved in 1976 [6] and Yasin et al. [63] successfully fabricated <5 nm poly(methyl-methacrylate)-resist lines utilizing a developer assisted by an ultrasonic approach. Other nano-fabrication systems, like nano-imprint lithography [22], nanoshaving, and scanning probe microscopy [36, 66], have also been developed for studies in cell biology.
2.4 Three-dimensional techniques
Apparently, cells in vivo are subjected to environment in three dimensions. In addition, cells in vivo tightly interact with the surrounding environment which contains various physical and chemical cues. As such, the cell environment in vivo is more complicated than what can be generated in 2D tissue culture dishes. Indeed, the cellular functions and structure under 3D environment demonstrated features significantly different from 2D cultured cells [12, 15]. Therefore, three-dimensional techniques are becoming more and more important in cell biology to generate micro-environment mimicking the physiological condition closer than that of two-dimensional devices and patterns. These three-dimensional techniques can offer powerful tools to study the different mechanisms of cellular behaviors and functions under the physiological and pathological conditions.
While not the mainstream activities, three-dimensional devices can also be generated by soft lithography at micron scale and by electrospinning at nano-scale. In this part, we introduce other methods aimed to specifically create three-dimensional patterns or devices for the studies of cell biology. Giang et al. reported a method using a mold composed of deep reactive ion etched (DRIE) trenches to get concave cavities in three-dimensional structures [17]. In this case, partially degassed PDMS pre-polymer was applied to a hydrophobic mold with a high curing temperature to create the desired patterns. Ice-lithography was another method newly developed [10]. This method utilizes patterned water droplets created by microscale plasma-activated templates. The pre-cured PDMS is applied to encapsulate patterned water. The space occupied by water can then formulate the 3D cavities. Joong Park et al. further improved this technique by utilizing DMSO as the liquid to occupy the space within PDMS [48]. This approach allows easier control of the size and shape of the solidified template. With this method, multiple concave micro-wells can be conveniently generated to successfully entrap cells. Therefore, ice-lithography has potential biological and biomedical applications in areas such as the fabrication of cell docking devices. Electroplating is a method to obtain a durable metal replication master for cylindrical micro-channels [64]. The master dimension can be controlled by the electroplating time and swapping power. As such, PDMS micro-channels can be obtained without soft lithography. Chan-Park et al. have demonstrated that concave or convex micron patterns can be generated by gas expansion [9]. In this case, pre-polymer substances trapping argon gas in the microcavities can be polymerized by applying UV lights to develop 3D PDMS molds. These micro-channels can be, subsequently, applied to study cell biology.
3 The applications of micro/nano-technologies in biology
Cells are the basic structural and functional unit of living systems. It is hence important to understand the mechanism of how cells interact with the environment. Indeed, cells are sensitive to biochemical, mechanical, and topological cues. Our understanding of the mechanisms on how cells sense these cues and our ability to manipulate cellular behaviors accordingly will allow efficient and therapeutic intervention of related diseases and expedite the development of new methods for drug screening and discovery. Micro/nano-fabrication techniques are particularly useful in facilitating the research in this aspect by creating and mimicking the physiological and pathological micro/nano environment. In this section, we discuss the recent progress in applying micro/nano-fabrication techniques in cell biological studies, mainly in the field of apoptosis, differentiation, proliferation, and cell migration.
3.1 Apoptosis
Normal cells usually will undergo a self-termination or apoptosis, but for cancer cells, they become immortal. It is therefore important to understand the underlying mechanisms governing the cell fate, life, or death. This understanding would help to intervene and cure different diseases. Apoptosis is a programmed cell death, involving a series of biochemical events which eventually lead to the cell death, with morphological and molecular characteristics including blebbing, changes at the cell membrane, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation. Apoptosis can be triggered by various stimuli involving different molecular pathways. After many years of continuous effect, the molecular mechanism of apoptosis becomes relatively clear. Among the many techniques which have contributed to the understanding of apoptosis, surface micro/nano pattern technique plays an important role. Chen et al. found that cells could switch from growth to apoptosis when seeded onto small micro-patterned islands containing ECM proteins [11]. The results showed that cells attached to non-patterned surfaces would not undergo apoptosis, but about 20% of the cells seeded on 20 μm-diameter circle-shaped patterns would undergo apoptosis. By further decreasing the diameter size of these circle-patterns from 20 to 10 μm, the percentage of apoptotic cells adhered on these patterns progressively increased. Similar results were observed in a later study from Dike et al. [13]. These results demonstrate that the cell shape is one of the important factors to determine the fate of a cell. Chiu et al. fabricated cell culture dishes utilizing collagen gels with different stiffness [57]. The results indicate that soft substrates could induce apoptosis in polarized cells. It appears that the soft collagen gels can up-regulate the store-operated calcium influx across the cell membrane to increase the cytosolic calcium levels. This disturbed calcium homeostasis eventually resulted in the activation of μ-calpain, which cleaves μ-spectrin to induce actin disorganization and apoptosis.
3.2 Differentiation
Although the molecular mechanisms involved in the renewal and apoptosis of cells appear different, ample evidence has shown that those two processes are related in cells under different mechanical/physical/chemical environment. Indeed, geometric control of the substrate can switch the adhered cells between growth, apoptosis, and differentiation [13]. Differentiation is the process of cells becoming more specialized types. It starts right after the formation of a zygote, which differentiates into a complex system containing different tissue and cell types. This process also exists in the adult organism, usually occurring as a part of regenerative process for wound repair and tissue regeneration. For stem cells, differentiation is one of their most important features. External cues, including microenvironment, biomolecules, and the substrates, can regulate the gene expression of stem cells to switch and govern the cell fate. Here, we highlight several cases demonstrating that micro/nano-fabrication techniques were applied to manipulate the cellular microenvironment to regulate stem cell differentiation. McBeath et al. showed that different shapes of micropatterns can lead human mesenchymal stem cells to differentiate into adipocytes or osteoblasts [40]. The results showed that stem cells seeded on ECM with low density could differentiate into adipocyte-like cells, while those seeded on ECM with high density appeared more osteogenic. Further results indicated that these mesenchymal stem cells seeded on small or large-size patterns could differentiate into adipocytes or osteoblasts, respectively. A recent study from the same group further revealed the mechanism of stem cell differentiation on different shapes of patterns [54]. It appears that geometry determines spatial distribution of cytoskeletal tension, which regulates the differentiation of stem cells. Indeed, the cell tensions of different patterns at different locations are different, as shown in Fig. 3. Cells on the convex part of the patterns are subjected to high cellular tension, which guides MSCs to differentiate into osteoblasts. Cells at the concave part of the patterns have lower cellular tension which leads MSCs to differentiate into adipocytes. Similar results were obtained in three-dimensional cell patterns. These results are consistent with previous observations that stem cells can differentiate differently on substrates with different stiffness [14]. Consistently, direct mechanical loading can also affect the differentiation commitment of stem cells [42].
Fig. 3.
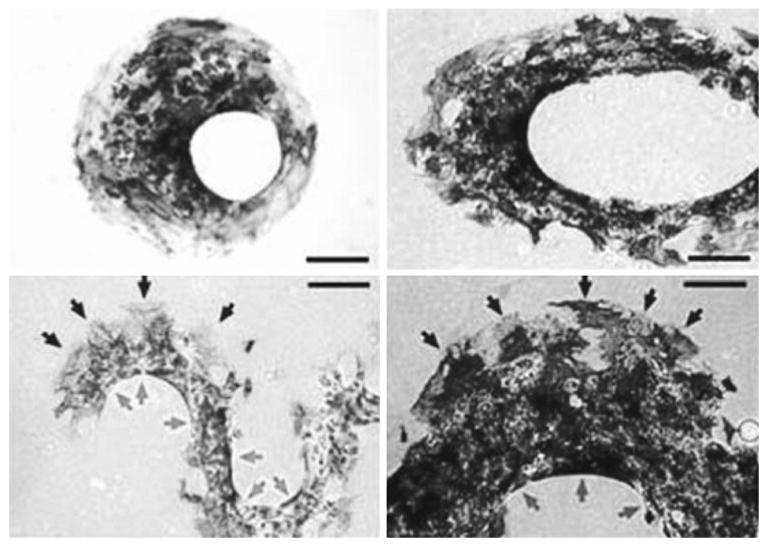
Geometry determines spatial patterning of differentiation Mesenchymal stem cell aggregates in the shape of an offset annulus (Upper left), an elliptical annulus (Upper right), and sinusoidal bands (Bottom: left and right: low and high magnifications, respectively) stained for oil droplets and alkaline phosphatase after 14 days in culture. Gray indicate adipogenesis at concave edges, and black indicate osteogenesis at convex edges. Scale bars 250 μm (reprinted with permission from [54])
3.3 Cell growth and proliferation
Cell growth and proliferation refer to the growth of cell population, which are dependent on the cell adherence to solid surfaces. The whole process of cell proliferation starts with cells adhering to the substrate surface, with parental cells dividing to generate daughter cells. More populated cells then migrate out to continuously colonize the surface until a crowded cell sheet is formed and proliferation stopped. O'Neill et al. seeded whole mouse embryo (WME) and NIH 3T3 cells on patterned areas with different size [44]. The results indicate that the DNA synthesis for WME cells is limited whereas a 6-fold increase could be observed in NIH 3T3 cells when the cells were constrained on a small growing area. Therefore, the alteration of shape or area where cells are seeded can significantly affect the synthesis of DNA and, subsequently, cell proliferation. Consistently, it has been shown that cells cultured on nano-structured PDMS surfaces have higher proliferation and lower apoptosis rate [60].
3.4 Cell migration
Cell migration plays a central role in regulating cellular functions, including embryonic development, immune response, and wound healing process. Any error occurring during the cell migration process can lead to severe path-ophysiological consequences, including tumor formation, vascular disfunction, or malformation during the development of organisms. Micro/nano-fabrication technologies have provided powerful tools to study the migration of single or multiple cells, by controlling the adhesion areas or the topography of the ECM where cells can migrate on. It becomes clear that cell migration is mainly determined by two systems: intracellular signaling mechanism and extracellular physical/biochemical cues. The intracellular signaling mechanism includes the signals controlling the cell protrusion in the leading edge, integrin-mediated membrane adhesion to the substrate, formation and stabilization of attachments, and contraction and detachment from the substrate in the rear edge. Physical/biochemical cues are mainly related to the cellular microenvironment which includes chemical stimulation and physical topography [34, 49, 52]. In this section, we will focus on the regulation of cell migration directed by microenvironment cues.
The lamellipodia-like protrusion of cells seeded on micro-patterned surface can be induced by EGF stimulation. Bailly et al. created vitronectin-patterned lines on the gold surface where MTLn3 cells were constrained to grow [3]. EGF can be clearly observed to stimulate lamellipodia extension out from the adhesive pattern toward the inert areas. Saadi et al. [55] developed a simple microfluidic device for generating stable concentration gradients of IL-8 in 2D and 3D environments. With this device, neutrophil chemotaxis and migration can be observed and monitored. Similar method was also developed by Jeon et al. [28]. The topography of ECM can also control cell migration through physical cues. Wood showed that mesenchymal fish cells cultured on different patterns on micro-fabricated quartz discs could predominantly align and migrate parallelly along the long axis of the adhesive patterns after a few hours of culture [62]. These polarization and alignment effects can also be found in other cell types, including oligodendrocytes [61] and hippocampal neurons [19] and fibroblast (Fig. 4) [23].
Fig. 4.
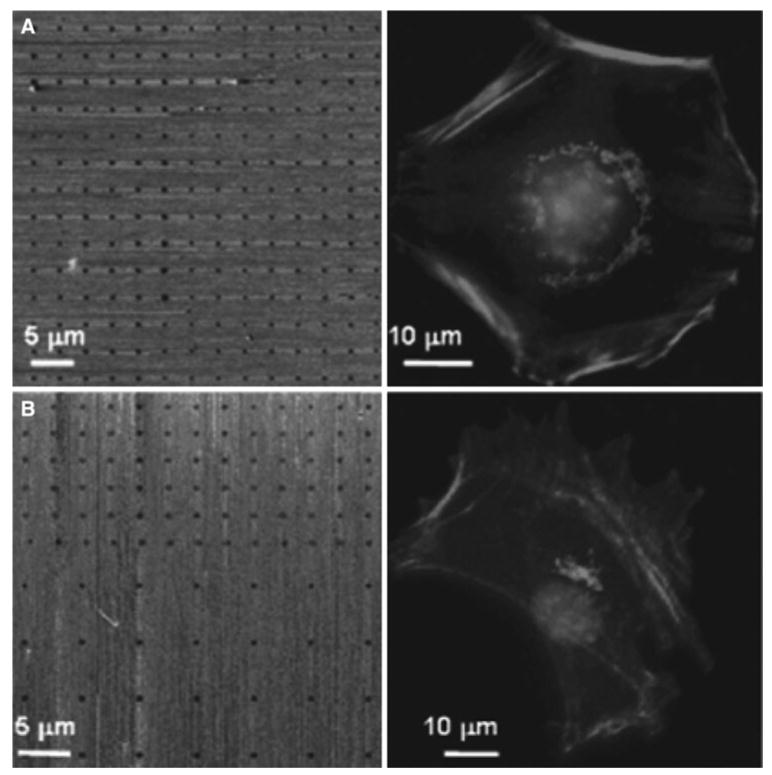
Effect of microenvironment on single cell polarization. a The panels show (left) the lateral force microscopy image of the control symmetric nanoarray of immobilized linear RGD peptide (500 nm spots with 3 μm distance apart) and (right) the representative fluorescence micrograph of a 3T3 Swiss Albino mouse fibroblast adhered on this symmetric nanoarray. The diffusive distribution of the Golgi complex surrounding the nucleus indicates that the cell is not polarized on the symmetric nanoarray. b The panels show (left) the lateral force microscopy image of an expanded area of an asymmetric nanoarray composed of two different patterns and (right) the representative fluorescence micrograph of a 3T3 Swiss Albino mouse fibroblast adhered on this asymmetric nanoarray. The position and orientation of the Golgi complex with respect to the nucleus indicates that the cell is polarized toward the higher density region of the array. The cells were stained for nuclei (bright circle in the middle), Golgi complex (bright clusters next to the nuclei), and actin (bright fiber bundles) (reprinted with permission from [23])
This platform of applying micro/nano-fabrication technologies to create special microenvironment has proven to be a versatile and powerful tool for the study of cell migration. It provides a well-controlled cell culture environment in which cells can be observed in real time. Furthermore, it allows us to integrate both biophysical and biochemical factors, which is necessary in mimicking biological conditions as cells constantly receive environmental signals in both soluble and insoluble forms. Indeed, micro/nano-fabrication technique has been successfully applied for the study of cellular functions related to migration, such as adhesion on quasi three-dimensional cell microenvironment [4, 26], and artificial mirco/nanostructures [38].
4 Live cell imaging of signaling transduction and micro/nano-technologies
Recent advancement in imaging technologies has allowed the visualization of molecular signaling cascades in live cells [46, 60]. In particular, the development of various fluorescent proteins (FPs) and genetically encoded molecular biosensors based on fluorescence resonance energy transfer (FRET) utilizing FPs have provided unprecedented capability for researchers to study molecular signals in live cells with high spatiotemporal resolutions. Together with micro/nano-fabrication technologies, these visualization tools can investigate the dynamic molecular signals at subcellular levels when cells are subjected to different micro/nano-environment. As such, the detailed molecular hierarchies and networks governing the cellular responses toward the environment can be elucidated. Here, we briefly introduce the recent work in this area. A synthetic oligopeptide with a FRET capability was successfully designed to monitor the cell traction force and the mechanical stiffness of substrate where cells are seeded [32]. FRET has also been applied to measure the RhoA activities at cell edges on top of micro-patterned inert surfaces [21]. When cells were constrained to grow and migrate on micro-patterned strips, a membrane-type matrix metalloproteinase (MT1-MMP) can be observed by a FRET biosensor to concentrate at the leading edge of migrating cells with a high activity [46]. Together with the FRET biosensors of Rac1 and Src, the same micro-patterned strips allowed the observations of a highly polarized Rac1 activity concentrated at the leading edge, but a relatively uniform distribution of Src activity [46]. Since Src and Rac1 are mutually regulating each other globally, the results suggest that molecules within the same signaling feedback loop can be differentially regulated at different subcellular locations. Further results revealed that when cells constrained on micro-patterned strips were exposed to epidermal growth factor (EGF), a decrease of RhoA and an increase of Src activities with biphasic time courses can be observed [31]. This is different from cells adhered on non-patterned areas where both RhoA and Src activities upon EGF stimulation appeared monophasic. Further experiments indicate that the microenvironment effect in causing the biphasic RhoA activation time course is mediated by Src and actomyosin machinery. Another study utilizing a calcium FRET biosensor indicates that mesenchymal stem cells cultured on engineered soft substrates have reduced calcium oscillation comparing to those on stiff surfaces [30]. Further results indicate that this substrate rigidity-regulated Ca2+ oscillation is mediated by RhoA and its downstream molecule ROCK. Therefore, the integration of molecular imaging technologies, particularly FRET biosensors, with micro/nano-fabrication tools can allow the revelation of molecular signals in live cells controlling the cell–environment interactions.
5 Conclusion and future directions
In summary, micro/nano-fabrication techniques have provided scientists with powerful tools to control cellular micro-environment, mimicking both the physiological and pathological environment. The control of cellular micro-environment, both physically and biochemically, can allow the study of fundamental molecular mechanisms regulating vital cellular functions. In particular, the regulation of stem cell differentiation by microenvironment can not only advance our understanding of the mechanism of stem cell differentiation, but also facilitate the development of new methods to grow biocompatible artificial organs/tissues to replace the dysfunctional counter parts in vivo. Indeed, Hartman et al. [20] have recently shown that cancer cells on three-dimensional environment were suitable models for the development and testing of anti-neoplastic drugs. Micro/nano-technologies have also been applied in the clinic studies [43, 47], medical assays [2, 8], tissue engineering [1, 18, 24, 26], and drug development [29]. While all these applications have had significant impact on different fields, they are not discussed in detail because this review article is mainly focused on the studies integrating micro/nano-fabrication technologies to elucidate the molecular mechanism of signaling transduction in cell biology.
For the future research activities, we expect more work on the integration of micro/nano-technology and molecular imaging in live cells to elucidate the detailed molecular mechanisms on how cells perceive the micro/nano-environmental cues and coordinate the signals cascades for appropriate responses. This will further our in-depth understanding of the cell–environment interaction and help the development of new technologies/reagents against related diseases.
Contributor Information
Tongcheng Qian, Department of Bioengineering and the Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA; Department of Biomedical Engineering, Peking University, Beijing 100871, People's Republic of China.
Yingxiao Wang, Email: yingxiao@uiuc.edu, Department of Bioengineering and the Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA, Department of Integrative and Molecular Physiology, Center for Biophysics and Computational Biology, Institute for Genomic, Biology, Neuroscience Program, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.
References
- 1.Abraham LC, Dice JF, Finn PF, Mesires NT, Lee K, Kaplan DL. Extracellular matrix remodeling methods to quantify cell matrix interactions. Biomaterials. 2007;28:151–161. doi: 10.1016/j.biomaterials.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Alyassin MA, Moon S, Keles HO, Manzur F, Lin RL, Haeggstrom E, Kuritzkes DR, Demirci U. Rapid automated cell quantification on HIV microfluidic devices. Lab on a Chip. 2009;9:3364–3369. doi: 10.1039/b911882a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailly M, Yan L, Whitesides GM, Condeelis JS, Segall JE. Regulation of protrusion shape and adhesion to the substratum during chemotactic responses of mammalian carcinoma cells. Exp Cell Res. 1998;241:285–299. doi: 10.1006/excr.1998.4031. [DOI] [PubMed] [Google Scholar]
- 4.Biggs MJP, Richards RG, Gadegaard N, McMurray RJ, Affrossman S, Wilkinson CDW, Oreffo ROC, Dalby MJ. Interactions with nanoscale topography: adhesion quantification and signal transduction in cells of osteogenic and multipotent lineage. J Biomed Mater Res A. 2009;91A:195–208. doi: 10.1002/jbm.a.32196. [DOI] [PubMed] [Google Scholar]
- 5.Boland ED, Matthews JA, Pawlowski KJ, Simpson DG, Wnek GE, Bowlin GL. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front Biosci. 2004;9:1422–1432. doi: 10.2741/1313. [DOI] [PubMed] [Google Scholar]
- 6.Broers AN, Molzen WW, Cuomo JJ, Wittels ND. Electron-beam fabrication of 80 Å metal structures. Appl Phys Lett. 1976;29:596–598. [Google Scholar]
- 7.Buchko CJ, Chen LC, Shen Y, Martin DC. Processing and microstructural characterization of porous biocompatible protein polymer thin films. Polymer. 1999;40:7397–7407. [Google Scholar]
- 8.Carraro A, Hsu WM, Kulig KM, Cheung WS, Miller ML, Weinberg EJ, Swart EF, Kaazempur-Mofrad M, Borenstein JT, Vacanti JP, Neville C. In vitro analysis of a hepatic device with intrinsic microvascular-based channels. Biomed Microdevices. 2008;10:795–805. doi: 10.1007/s10544-008-9194-3. [DOI] [PubMed] [Google Scholar]
- 9.Chan-Park MB, Yang C, Guo X, Chen LQ, Yoon SF, Chun JH. Fabrication of 3-D curved microstructures by constrained gas expansion and photopolymerization. Langmuir. 2008;24:5492–5499. doi: 10.1021/la703608p. [DOI] [PubMed] [Google Scholar]
- 10.Chao SH, Carlson R, Meldrum DR. Rapid fabrication of microchannels using microscale plasma activated templating (mu PLAT) generated water molds. Lab on a Chip. 2007;7:641–643. doi: 10.1039/b618269k. [DOI] [PubMed] [Google Scholar]
- 11.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 12.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 13.Dike LE, Chen CS, Mrksich M, Tien J, Whitesides GM, Ingber DE. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micro-patterned substrates. In Vitro Cell Dev Biol Anim. 1999;35:441–448. doi: 10.1007/s11626-999-0050-4. [DOI] [PubMed] [Google Scholar]
- 14.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 15.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17:524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Fang X, Reneker DH. DNA fibers by electrospinning. J Macromol Sci Phys. 1997;B36:169–173. [Google Scholar]
- 17.Giang UBT, Lee D, King MR, DeLouise LA. Microfabrication of cavities in polydimethylsiloxane using DRIE silicon molds. Lab on a Chip. 2007;7:1660–1662. doi: 10.1039/b714742b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillette BM, Jensen JA, Tang BX, Yang GJ, Bazargan-Lari A, Zhong M, Sia SK. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nat Mater. 2008;7:636–640. doi: 10.1038/nmat2203. [DOI] [PubMed] [Google Scholar]
- 19.Gomez N, Chen SC, Schmidt CE. Polarization of hippocampal neurons with competitive surface stimuli: contact guidance cues are preferred over chemical ligands. J R Soc Interface. 2007;4:223–233. doi: 10.1098/rsif.2006.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartman O, Zhang C, Adams EL, Farach-Carson MC, Petrelli NJ, Chase BD, Rabolt JF. Microfabricated electrospun collagen membranes for 3-D cancer models and drug screening applications. Biomacromolecules. 2009;10:2019–2032. doi: 10.1021/bm8012764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodgson L, Chan EWL, Hahn KM, Yousaf MN. Combining surface chemistry with a FRET-based biosensor to study the dynamics of RhoA GTPase activation in cells on patterned substrates. J Am Chem Soc. 2007;129:9264–9265. doi: 10.1021/ja072900m. [DOI] [PubMed] [Google Scholar]
- 22.Hoff JD, Cheng LJ, Meyhofer E, Guo LJ, Hunt AJ. Nanoscale protein patterning by imprint lithography. Nano Lett. 2004;4:853–857. [Google Scholar]
- 23.Hoover DK, Chan EWL, Yousaf MN. Asymmetric peptide nanoarray surfaces for studies of single cell polarization. J Am Chem Soc. 2008;130:3280–3281. doi: 10.1021/ja711016m. [DOI] [PubMed] [Google Scholar]
- 24.Hsiong SX, Boontheekul T, Huebsch N, Mooney DJ. Cyclic arginine-glycine-aspartate peptides enhance three-dimensional stem cell osteogenic differentiation. Tissue Eng A. 2009;15:263–272. doi: 10.1089/ten.tea.2007.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu SH, Eberhard L, Chen JX, Love JC, Butler JP, Fredberg JJ, Whitesides GM, Wang N. Mechanical anisotropy of adherent cells probed by a three-dimensional magnetic twisting device. Am J Physiol Cell Physiol. 2004;287:C1184–C1191. doi: 10.1152/ajpcell.00224.2004. [DOI] [PubMed] [Google Scholar]
- 26.Hwang YS, Chung BG, Ortmann D, Hattori N, Moeller HC, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci USA. 2009;106:16978–16983. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyun JH, Ma HW, Zhang ZP, Beebe TP, Chilkoti A. Universal route to cell micropatterning using an amphiphilic comb polymer. Adv Mater. 2003;15:576–579. [Google Scholar]
- 28.Jeon NL, Baskaran H, Dertinger SKW, Whitesides GM, Van de Water L, Toner M. Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat Biotechnol. 2002;20:826–830. doi: 10.1038/nbt712. [DOI] [PubMed] [Google Scholar]
- 29.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 30.Kim TJ, Seong JH, Ouyang MX, Sun J, Lu SY, Hong JP, Wang N, Wang YX. Substrate rigidity regulates Ca2+ oscillation via RhoA pathway in stem cells. J Cell Physiol. 2009;218:285–293. doi: 10.1002/jcp.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TJ, Xu J, Dong R, Lu SY, Nuzzo R, Wang YX. Visualizing the effect of microenvironment on the spatiotemporal RhoA and Src activities in living cells by FRET. Small. 2009;5:1453–1459. doi: 10.1002/smll.200801846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong HJ, Boontheekul T, Mooney DJ. Quantifying the relation between adhesion ligand-receptor bond formation and cell phenotype. Proc Natl Acad Sci USA. 2006;103:18534–18539. doi: 10.1073/pnas.0605960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon IK, Kidoaki S, Matsuda T. Electrospun nano- to microfiber fabrics made of biodegradable copolyesters: structural characteristics, mechanical properties and cell adhesion potential. Biomaterials. 2005;26:3929–3939. doi: 10.1016/j.biomaterials.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 35.Lee KB, Park SJ, Mirkin CA. Protein nanoarrays generated by dip-pen nanolithography. Abstr Pap Am Chem Soc. 2002;223:506-PHYS. doi: 10.1126/science.1067172. [DOI] [PubMed] [Google Scholar]
- 36.Liu GY, Xu S, Qian YL. Nanofabrication of self-assembled monolayers using scanning probe lithography. Acc Chem Res. 2000;33:457–466. doi: 10.1021/ar980081s. [DOI] [PubMed] [Google Scholar]
- 37.Ma HW, Hyun J, Zhang ZP, Beebe TP, Chilkoti A. Fabrication of biofunctionalized quasi-three-dimensional micro-structures of a nonfouling comb polymer using soft lithography. Adv Funct Mater. 2005;15:529–540. [Google Scholar]
- 38.Martinez E, Engel E, Planell JA, Samitier J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann Anat Anatomischer Anzeiger. 2009;191:126–135. doi: 10.1016/j.aanat.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 40.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 41.Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004;25:1289–1297. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 42.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci USA. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neeley WL, Redenti S, Klassen H, Tao S, Desai T, Young MJ, Langer R. A microfabricated scaffold for retinal progenitor cell grafting. Biomaterials. 2008;29:418–426. doi: 10.1016/j.biomaterials.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Neill C, Jordan P, Ireland G. Evidence for two distinct mechanisms of anchorage stimulation in freshly explanted and 3T3 Swiss mouse fibroblasts. Cell. 1986;44:489–496. doi: 10.1016/0092-8674(86)90470-8. [DOI] [PubMed] [Google Scholar]
- 45.Ohgo K, Zhao CH, Kobayashi M, Asakura T. Preparation of non-woven nanofibers of Bombyx mori silk, Samia cynthia ricini silk and recombinant hybrid silk with electrospinning method. Polymer. 2003;44:841–846. [Google Scholar]
- 46.Ouyang MX, Sun J, Chien S, Wang YX. Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors. Proc Natl Acad Sci USA. 2008;105:14353–14358. doi: 10.1073/pnas.0807537105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park J, Toner M, Yarmush ML, Tilles AW. Microchannel bioreactors for bioartificial liver support. Microfluid Nanofluid. 2006;2:525–535. [Google Scholar]
- 48.Park JY, Hwang CM, Lee SH. Ice-lithographic fabrication of concave microwells and a microfluidic network. Biomed Microdevices. 2009;11:129–133. doi: 10.1007/s10544-008-9216-1. [DOI] [PubMed] [Google Scholar]
- 49.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 51.Piner RD, Zhu J, Xu F, Hong SH, Mirkin CA. “Dip-pen” nanolithography. Science. 1999;283:661–663. doi: 10.1126/science.283.5402.661. [DOI] [PubMed] [Google Scholar]
- 52.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 53.Rogers JA, Nuzzo RG. Recent progress in soft lithography. Mater Today. 2005;8:50–56. [Google Scholar]
- 54.Ruiz SA, Chen CS. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells. 2008;26:2921–2927. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saadi W, Rhee SW, Lin F, Vahidi B, Chung BG, Jeon NL. Generation of stable concentration gradients in 2D and 3D environments using a microfluidic ladder chamber. Biomed Microdevices. 2007;9:627–635. doi: 10.1007/s10544-007-9051-9. [DOI] [PubMed] [Google Scholar]
- 56.Seong J, Lu S, Ouyang M, Huang H, Zhang J, Frame MC, Wang Y. Visualization of Src activity at different compartments of the plasma membrane by FRET imaging. Chem Biol. 2009;16:48–57. doi: 10.1016/j.chembiol.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shiu YT, Li S, Yuan S, Wang Y, Nguyen P, Chien S. Shear stress-induced c-fos activation is mediated by Rho in a calcium-dependent manner. Biochem Biophys Res Commun. 2003;303:548–555. doi: 10.1016/s0006-291x(03)00388-7. [DOI] [PubMed] [Google Scholar]
- 58.Subbiah T, Bhat GS, Tock RW, Pararneswaran S, Ramkumar SS. Electrospinning of nanofibers. J Appl Polym Sci. 2005;96:557–569. [Google Scholar]
- 59.Takayama S, Ostuni E, LeDuc P, Naruse K, Ingber DE, White-sides GM. Laminar flows: subcellular positioning of small molecules. Nature. 2001;411:1016. doi: 10.1038/35082637. [DOI] [PubMed] [Google Scholar]
- 60.Wang YX, Wang N. FRET and mechanobiology. Integr Biol. 2009;1:565–573. doi: 10.1039/b913093b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Webb A, Clark P, Skepper J, Compston A, Wood A. Guidance of oligodendrocytes and their progenitors by substratum topography. J Cell Sci. 1995;108:2747–2760. doi: 10.1242/jcs.108.8.2747. [DOI] [PubMed] [Google Scholar]
- 62.Wood A. Contact guidance on microfabricated substrata: the response of teleost fin mesenchyme cells to repeating topographical patterns. J Cell Sci. 1988;90:667–681. doi: 10.1242/jcs.90.4.667. [DOI] [PubMed] [Google Scholar]
- 63.Yasin S, Hasko DG, Ahmed H. Fabrication of <5 nm width lines in poly(methylmethacrylate) resist using a water:isopropyl alcohol developer and ultrasonically-assisted development. Appl Phys Lett. 2001;78:2760–2762. [Google Scholar]
- 64.Yi Y, Kang JH, Park JK. Moldless electroplating for cylindrical microchannel fabrication. Electrochem Commun. 2005;7:913–917. [Google Scholar]
- 65.Yu J, Xiao J, Ren XJ, Lao KQ, Xie XS. Probing gene expression in live cells, one protein molecule at a time. Science. 2006;311:1600–1603. doi: 10.1126/science.1119623. [DOI] [PubMed] [Google Scholar]
- 66.Zhou D, Bruckbauer A, Ying LM, Abell C, Klenerman D. Building three-dimensional surface biological assemblies on the nanometer scale. Nano Lett. 2003;3:1517–1520. [Google Scholar]


