Abstract
Background
The purpose of this paper is to present a guideline for beginning video-assisted thoracic surgery (VATS) lobectomy to junior surgeons, and to review the first year experience of a new surgeon performing VATS lobectomies who had not performed a VATS lobectomy unassisted during his training period.
Materials and Methods
A young surgeon opened a division of general thoracic surgery at a medical institution. The surgeon had performed about 100 lobectomies via conventional thoracotomy during his training period, but had never performed a VATS lobectomy unassisted while under the supervision of an expert. After opening the division of general thoracic surgery, the surgeon performed a total of 38 pulmonary lobectomies for various pulmonary diseases from March 2009 to February 2010. All data were collected retrospectively.
Results
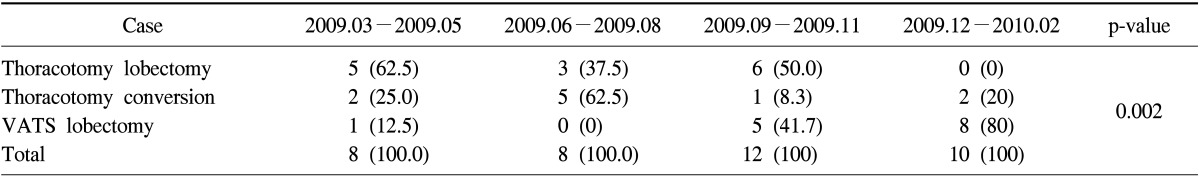
There were 14 lobectomies via thoracotomy, 14 VATS lobectomies, and 10 cases of attempted VATS lobectomies that were converted to open thoracotomies. The number of VATS lobectomies increased from the second quarter (n=0) to the third quarter (n=5). The lobectomies that were converted from VATS into thoracotomies decreased from the second quarter (n=5) to the third quarter (n=1) (p=0.002).
Conclusion
It can take 6 months for young surgeons without experience in VATS lobectomy in their training period to be able to reliably perform a VATS lobectomy.
Keywords: Thoracoscopy, Lobectomy, Learning curve
INTRODUCTION
Pulmonary lobectomy is the elementary surgical technique for lung diseases such as lung cancer and inflammatory lung disease. A pulmonary lobectomy can be performed using traditional thoracotomy or by video-assisted thoracoscopic surgery (VATS). The first report of a VATS lobectomy was in 1992 [1], and since then it has been replacing conventional lobectomy via thoracotomy because there is less pain, faster recovery, and other advantages over thoracotomy [2,3]. However, it is difficult for a trainee such as a thoracic surgical resident or fellow to perform a VATS lobectomy because of technical challenges such as the management of surgical instruments and disastrous complications such as pulmonary artery injury. Therefore, it is very difficult for a thoracic trainee or thoracic surgeon who has never singlehandedly performed a VATS lobectomy to perform one. This paper illustrates the progress in the performance of VATS lobectomy of a young surgeon who had never performed one in his training period and how he developed his skill in independently performing VATS lobectomy.
MATERIALS AND METHODS
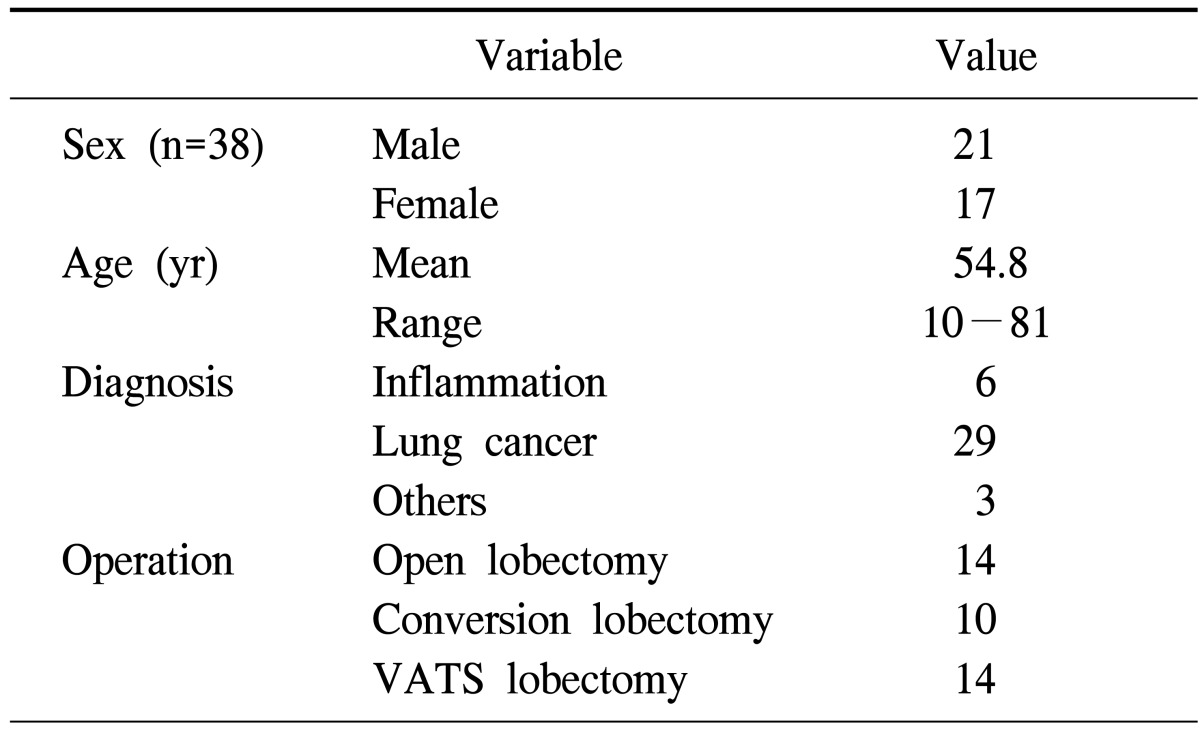
This study examines the procedures used for 38 pulmonary lobectomy cases performed by a single surgeon from March 2009 to February 2010 (Table 1). All data were reviewed retrospectively from the medical records. The data were grouped into the three groups: conventional lobectomy through thoracotomy, VATS lobectomy, and lobectomy via converted thoracotomy after a VATS attempt. We compared the increase or decrease in the frequency of use of each of the three approaches to lobectomy over time. We also compared parameters such as length of hospital stay, chest tube duration, and surgical time. The protocol for this retrospective study was approved by our institutional review board.
Table 1.
Characteristics of the patients
VATS, video-assisted thoracic surgery.
1) Our center
Our institute has been open since November 2008, but the general thoracic surgery service was not available until a thoracic surgeon joined the center in March of 2009 and opened the Division of the General Thoracic Surgery. This thoracic surgeon had performed more than 100 lobectomies via thoracotomy and was competent in minor VATS procedures, such as wedge resection, through the experience of his fellowship period. He had also assisted in approximately 100 VATS lobectomy procedures and observed many VATS lobectomies; however, he had never performed a VATS lobectomy by himself during his training period.
2) Surgical technique of video-assisted thoracoscopic surgery lobectomy
The patients were placed in the lateral decubitus position. After inserting a thoracoscopy at the junction of the 8th intercostal space and posterior axillary line, we then made three 5 cm working ports: one at the crossing point of the 5th intercostal space, another at the anterior axillary line, and a third port at the subscapular point of the 6th or 7th intercostal space. We divided the connective tissue, specifically the adhesion bands and the inferior pulmonary ligament with a Bovie electrocautery unit, lung parenchyme such as fissure were seprated with an Endo-GIA green stapler (Covidien, Norwalk, CT, USA). The large were ligated using an Endo-GIA white stapler, and small branches of the pulmonary artery were ligated using a Weck clip (Teleflex, Research Triangle Park, NC, USA). All the surgical procedures were performed by viewing the surgical field through the monitor. When the patients were diagnosed with lung cancer, mediastinal lymph nodes were dissected completely. To treat the cancer on the right side, the right inferior pulmonary ligament, subcarinal lymph nodes, right lower paratracheal nodes, and upper paratracheal nodes were dissected. To treat cancer on the left side, the left inferior pulmonary ligament, subcarinal lymph nodes, left lower paratracheal subaortic nodes, and para-aortic nodes. A 28 Fr sized chest tube was inserted and then the operation was finished. This process was established on the basis of the visual observation of the senior surgeon during the junior surgeon's fellowship.
3) Statistical analysis
The data were processed by SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) and the chi-squared test. Analysis of variance and logistic regression analysis were used for interpreting the data. A p-value less than 0.05 was considered statistically significant.
RESULTS
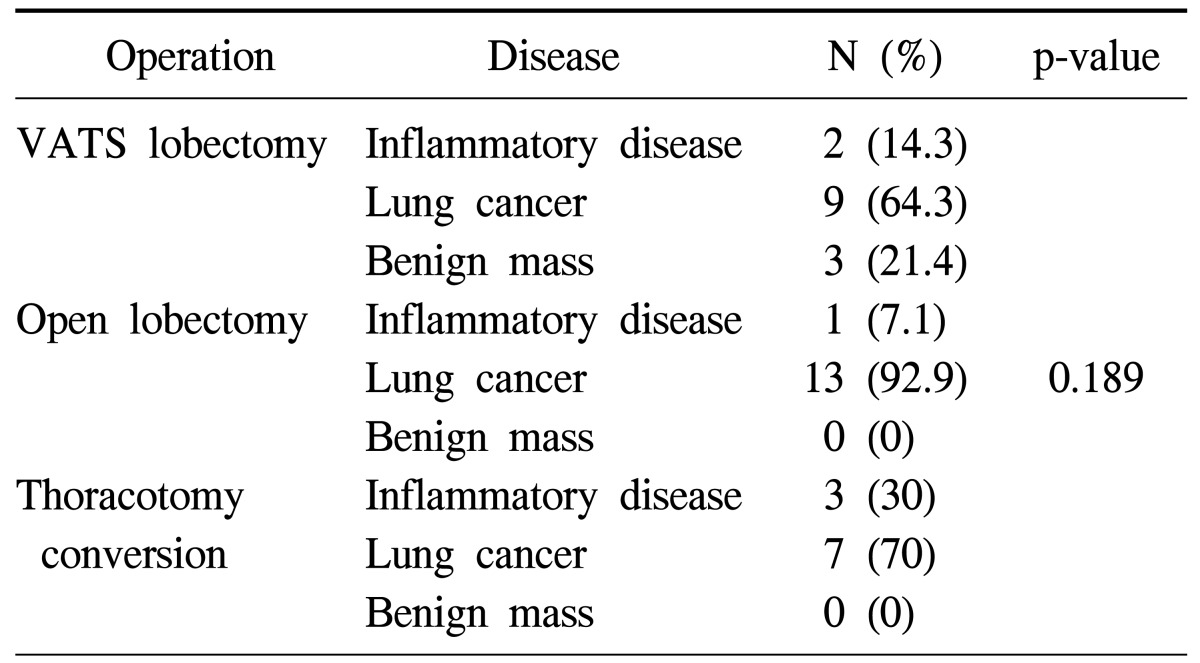
In our institute, the thoracic surgical team performed a total of 38 cases of lobectomy during the first year. Detailed characteristics of the patients are shown in Table 1. Of those patients, 14 lobectomies were performed using open thoracotomy, and the other 14 lobectomies were performed using VATS. In 10 patients, VATS lobectomy was initially attempted but the surgical procedure was converted into an open thoracotomy (Table 2).
Table 2.
Disease category for surgical procedures
VATS, video-assisted thoracic surgery.
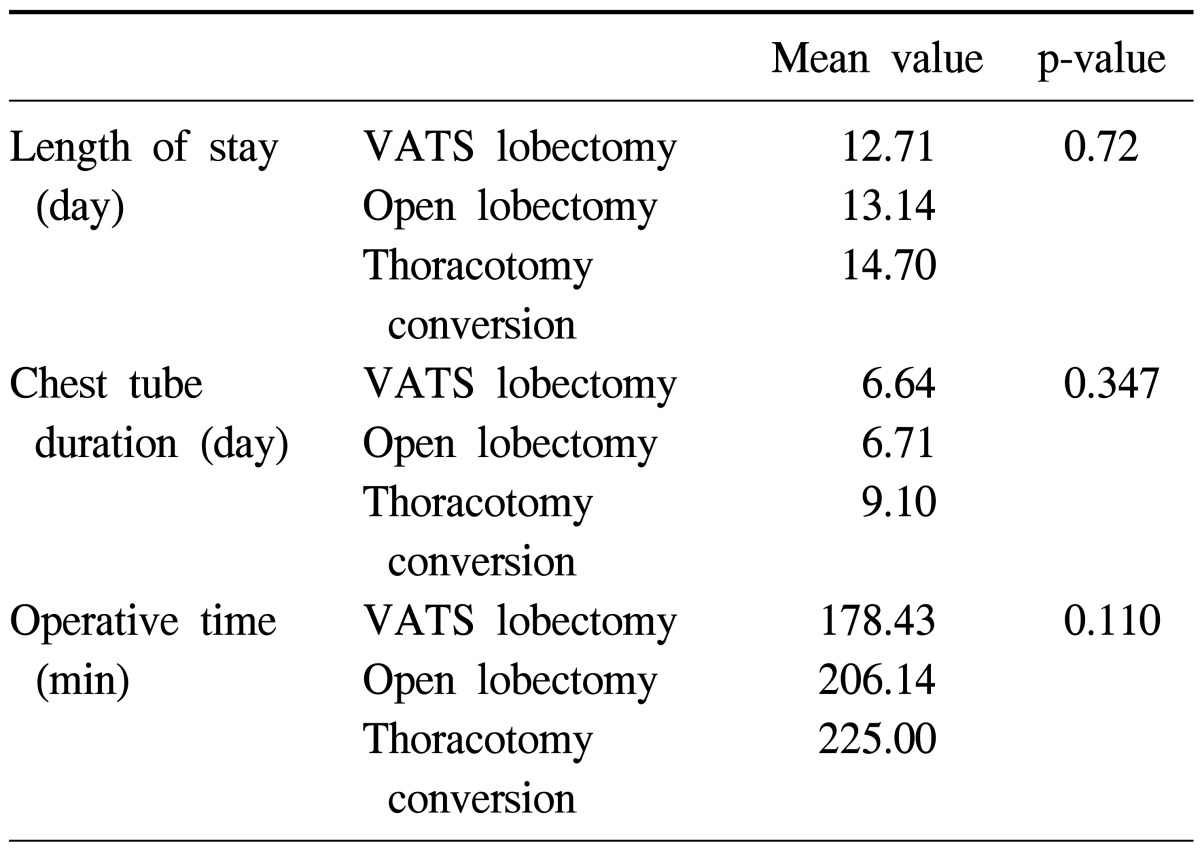
There was no operative mortality. The number of VATS lobectomy procedures increased from the second quarter of the year to the third quarter, while conventional lobectomy via thoracotomy decreased from the third to the forth quarter. The number of conversions of VATS to thoracotomy decreased from the second to the third quarter. These results show statistical significance (p=0.002). We concluded, therefore, that the third quarter was the turning point, in which VATS lobectomies increased and conversions from VATS to thoracotomy decreased (Table 3). Although there was no statistical difference in the operative time, days of hospitalization, and chest tube removal time were generally the shortest in VATS lobectomy group (Table 4). In the VATS to thoracotomy conversions, the most common cause of the conversion was fibrous adhesion, which occurred in 5 cases, followed by bleeding in 3 cases, and oncology considerations in 2 cases.
Table 3.
Surgical pattern according to the time period
Values are presented as number (%).
VATS, video-assisted thoracic surgery.
Table 4.
The differences between the various parameters of the procedures
VATS, video-assisted thoracic surgery.
DISCUSSION
Since the first series of lobectomies using VATS were reported in 1992 [1], several authors have reported on the feasibility and safety of this procedure [2,3]. Many reports have shown that the long term result of VATS major pulmonary resection and mediastinal lymph node dissection are equivalent to conventional thoracotomy in non-small cell lung cancer [2,4-6]. Therefore, VATS lobectomy has been substituted for conventional thoracotomy with the improvement of video equipment and auto-stapler devices for treating diverse pulmonary diseases. VATS lobectomy is also superior in the management of post-operative pain and decompression of respiratory function, and is outstanding in the reduction of health care costs.
However, even with these advantages, VATS lobectomy is a challenging procedure for beginner thoracic surgeons due to their inexperience in handling the instruments, obstacles created by the instruments that are caused by inappropriate port incisions, and most of all, catastrophic events resulting from pulmonary artery injury. When a general thoracic surgical division is opened in a center without a history of performing VATS lobectomy, it is especially challenging to perform VATS lobectomies due to inexperienced assistants and an unestablished protocol for pulmonary artery injury.
VATS lobectomy procedures can definitely be taught to thoracic trainees during their training program under supervision of a thoracic expert [7,8]. However, it is unclear whether it is advisable for thoracic surgeons who have opened a new division of thoracic surgery and have received training in an institute in which they could not perform VATS lobectomy unassisted while under the supervision of an expert, to start performing VATS lobectomies. This question could be answered with the data that few centers perform this type of surgery, and 45% of VATS lobectomies worldwide are performed in the UK [7].
This paper contributes to answering the question of whether junior surgeons who spent their training period in a center where they could not perform a VATS lobectomy unassisted while under the supervision of an expert could go on to perform VATS lobectomies independently. Even though a center may not provide such training, the competency of assistants for handling thoracoscopy and having a well-prepared protocol for complications such as pulmonary artery injury are definitely important. However, a surgeon must have a precise understanding of the anatomy of lung because this is the most important part of performing a VATS lobectomy. Therefore, if junior thoracic surgeons had a precise understanding of the anatomy of the pulmonary artery and lung parenchyme, learning to perform a VATS lobectomy would be a normal process. Acquiring this understanding, as well as training in the operating room, can be established at a VATS conference.
When the new division of thoracic surgery opened, I performed all the lobectomies only by open thoracotomy during the initial 2 months. This plan was intended to make the lobectomy procedure familiar to the surgical nurses and residents. After this period, VATS lobectomy was performed for benign diseases such as lobar bronchiectasis and was performed successfully. The surgical team then tried to perform a VATS lobectomy in a case of early stage lung cancer. The team became proficient in VATS lobectomy after a few conversions to open thoracotomy. Among the reasons for the conversions, except in inevitable case such as pulmonary artery injury, the most common cause for converting to thoracotomy was minor bleeding from the lung parenchyme. The minor bleeding made it difficult to distinguish the precise anatomy of the pulmonary artery and lung parenchyme, which necessitated a conversion to open thoracotomy. However, this problem can be solved after 6 months of experience (Table 3).
Although there was no statistical significance due to the small number of cases, the duration of hospital stay, tube removal time, and operation time were the shortest in the VATS lobectomy group, and the longest in the conversion group (Table 4). These results coincide with the results of previous reports [9]. Obviously, operating time is prolonged in conversion cases because of the additional time needed to control bleeding and repairing lung parenchymal or arterial injuries (Table 4). Over time, the time to perform VATS lobectomies was clearly reduced, but the wedge resection time and waiting time for frozen examination were included in operation time; we regret that we could not measure the precise operation time without those procedures.
Although there was no statistical significance due to the small number of cases, it became apparent that effort should be directed toward decreasing the conversion rate from VATS lobectomy to open thoracotomy for patients with inflammatory lung disease complicated by a distorted anatomy of the lung with severe fibrous adhesion (Table 2). Long-term follow up is mandatory for patients with lung cancer who have undergone VATS lobectomy.
This study supports the concept that junior thoracic surgeons, even if they have not performed a VATS lobectomy during their training period, can become competent in performing a VATS lobectomy after six months of experience in performing VATS lobectomies while concentrating entirely on open thoracotomy during the first two months as preparation for VATS lobectomy.
CONCLUSION
It took 6 months of the prescribed training for a young surgeon without experience in VATS lobectomy in his training period to be able to reliably perform VATS lobectomy.
References
- 1.Roviaro G, Rebuffat C, Varoli F, Vergani C, Mariani C, Maciocco M. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc. 1992;2:244–247. [PubMed] [Google Scholar]
- 2.McKenna RJ, Jr, Wolf RK, Brenner M, Fischel RJ, Wurnig P. Is lobectomy by video-assisted thoracic surgery an adequate cancer operation? Ann Thorac Surg. 1998;66:1903–1908. doi: 10.1016/s0003-4975(98)01166-7. [DOI] [PubMed] [Google Scholar]
- 3.Kim HR, Cho JS, Jang HJ, et al. Video-assisted thoracic surgery lobectomy for non-small cell lung cancer: experience of 133 cases. Korean J Thorac Cardiovasc Surg. 2009;42:615–623. [Google Scholar]
- 4.Iwasaki A, Shirakusa T, Shiraishi T, Yamamoto S. Results of video-assisted thoracic surgery for stage I/II non-small cell lung cancer. Eur J Cardiothorac Surg. 2004;26:158–164. doi: 10.1016/j.ejcts.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 5.Roviaro G, Varoli F, Vergani C, Nucca O, Maciocco M, Grignani F. Long-term survival after videothoracoscopic lobectomy for stage I lung cancer. Chest. 2004;126:725–732. doi: 10.1378/chest.126.3.725. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto K, Ohsumi A, Kojima F, et al. Long-term survival after video-assisted thoracic surgery lobectomy for primary lung cancer. Ann Thorac Surg. 2010;89:353–359. doi: 10.1016/j.athoracsur.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson J, Walker W. Developing a VATS lobectomy programme: can VATS lobectomy be taught? Eur J Cardiothorac Surg. 2006;29:806–809. doi: 10.1016/j.ejcts.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RH, Hansen HJ. Learning thoracoscopic lobectomy. Eur J Cardiothorac Surg. 2010;37:516–520. doi: 10.1016/j.ejcts.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Casali G, Walker WS. Video-assisted thoracic surgery lobectomy: can we afford it? Eur J Cardiothorac Surg. 2009;35:423–428. doi: 10.1016/j.ejcts.2008.11.008. [DOI] [PubMed] [Google Scholar]