Abstract
Purpose
The aim of this study was to evaluate the relationship between the detection of circulating tumor cell molecular markers from localized colorectal cancer and the time-course of a surgical manipulation or surgical modality.
Methods
From January 2010 to June 2010, samples from the peripheral blood and the inferior mesenteric vein were collected from 42 patients with cancer of the sigmoid colon or rectum. Pre-operative, intra-operative (both pre-mobilization and post-mobilization), and post-operative samples were collected. We examined carcinoembryonic antigen (CEA) mRNA and cytokeratin-20 (CK20) mRNA by real-time reverse-transcriptase polymerase chain reaction. Changes in mRNA detection rates were analyzed according to the time of blood sample collection, the surgical modality, and patient clinicopathological features.
Results
mRNA expression rates before surgical resection did not differ between blood samples from the peripheral and inferior mesenteric veins. The detection rate for CEA and CK20 mRNA showed a tendency to increase after operative mobilization of the cancer-bearing bowel segment. Furthermore, the cumulative detection rates for CEA and CK20 mRNA increased significantly over the course of surgery (pre-mobilization vs. post-mobilization). The cumulative detection rate decreased significantly after surgical resection compared with the pre-operative rates. However, no significant difference was observed in the detection rates between different surgical modalities (laparoscopy vs. open surgery).
Conclusion
The results of this study suggest that surgical manipulation has a negative influence on the dissemination of circulating tumor cells during operations on localized colorectal cancer. However, the type of surgical technique did not affect circulating tumor cells.
Keywords: Colorectal cancer, Circulating tumor cells, mRNA
INTRODUCTION
Although advances in treatment modalities have improved, the survival rate of patients with colorectal cancer (CRC) after surgical removal, 30 to 50% of patients with CRC develop a recurrence following complete surgical resection of a primary tumor [1,2]. Distant metastasis through the hematogenous and lymphatic pathways is a major cause of disease recurrence, which has a substantial impact on patient prognosis. Micrometastasis is assumed to be the cause of metastasis in patients who have undergone curable surgical resection. Hematogenous micrometastasis has been studied in circulating tumor cells (CTCs), which shed from the primary tumor, spread through the blood stream, invade distant organs, and result in distant metastasis [3]. CTCs in patients with CRC were first detected in 1955, and many studies thereafter have focused on detecting CTCs and their clinical implications for patients with CRC [4].
Animal studies have shown that malignant cells are shed into the blood stream during surgical manipulation of a primary tumor [5,6]. Minimal manipulation of the malignant lesion is a generally accepted concept to reduce these micrometastases during surgery. Fisher and Turnbull [7] suggested that tumor cells are scattered by surgical manipulation. "No-touch isolation", a surgical technique involving early lymphovascular ligation before tumor manipulation, has been proposed to minimize micrometastasis during an operation [8,9]. However, few reports have examined the presence of free cancer cells in blood samples in relation to the whole time-course of surgery or analyzed the relationship between surgical manipulation and the detection of CTCs. Furthermore, it remains unclear whether the surgical modality (laparoscopy vs. open surgery) differently affects CTC detection.
Development of a reliable detection method is essential to understand the mechanisms and implication of CTCs. The reverse-transcriptase polymerase chain reaction (RT-PCR) technique was developed to enable the detection of a small number of cancer cells, which is not possible with cytology or immunological techniques. Moreover, real-time RT-PCR can be used to monitor the low-level expression of marker mRNAs and establish cut-off values. This technique has been used to detect disseminated tumor cells in the peripheral blood, bone marrow, and peritoneal lavage of patients with CRC by detecting epithelial marker mRNAs [10]. The most reliable RT-PCR targets in CRC are cytokeratins (CKs), and carcinoembryonic antigen (CEA).
In the present study, we used real-time RT-PCR to detect CEA and CK20 mRNA expression in the peripheral and inferior mesenteric vein (IMV) in relation to the time-course of CRC surgery. The purpose of the study was to assess the influence of surgical manipulation and two different surgical modalities on the presence of CTC markers in patients with curable CRC.
METHODS
Study patients
This study involved 53 consecutive patients with CRC who underwent potentially curative surgical resection in a single CRC center between January 2010 and June 2010. Enrolled patients had been diagnosed with primary CRC, which was confirmed by colonoscopic biopsy. Cancer location was limited to the sigmoid colon and rectum. Patients underwent either laparoscopic or open surgery. None of the patients received chemotherapy or radiation therapy before surgery. Patients with palliative resection, prior endoscopic mucosal resection, distant metastasis, need for an emergency operation, age >80 years, and American Society of Anesthesiology score >3 points were also excluded. This study was conducted prospectively after gaining approval from the local Institutional Review Boards. All patients provided written informed consent.
Blood sample collection
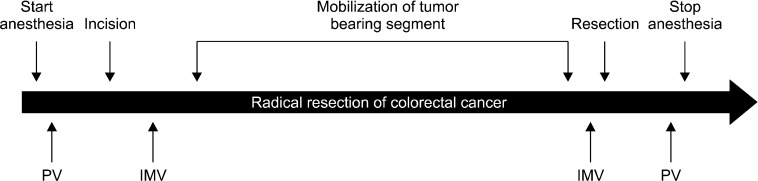
Samples from the peripheral blood and the IMV were collected from patients at four different time-points during the peri-operative period (Fig. 1). Blood from the peripheral vein was extracted just before skin incision (pre-operation) and immediately after closure of skin incision (post-operation). During the operation, blood samples from the IMV were obtained (pre-mobilization) before beginning the manipulation of the cancer-bearing bowel segment. Blood samples from the IMV were obtained by direct puncture of the vein with a 23-gauge needle, and the vein was then ligated (Fig. 2). After mobilizing the cancer-bearing bowel and dividing the distal margin, post-mobilization samples were collected from the IMV distal from the ligation prior to cancer removal. To prevent any contamination of epithelial cells, the initial 10 mL of blood was discarded from all blood samples and the following 10 mL of blood, drawn using a new syringe, was used for RNA extraction.
Fig. 1.
Time-frame for collecting blood sample. PV, peripheral vein; IMV, inferior mesenteric vein.
Fig. 2.

Blood samples from the inferior mesenteric vein were obtained by direct puncture with a 23-gauge needle.
Real-time RT-PCR
Five mL blood samples were collected in ethylenediaminetetraacetic acid-containing tubes. Three mL of whole blood was transferred to a 15 mL conical centrifuge tube with the same volume of Histopaque-10771 separation medium (Sigma-Aldrich Co., St. Louis, MO, USA) and centrifuged at 400 × g at room temperature for 30 minutes. Cells at the interface were transferred to a clean conical centrifuge tube and washed with 10 mL of phosphate-buffered saline (PBS) or appropriate cell culture medium. After the cells were centrifuged with washing buffer at 250 × g for 10 minutes, the supernatant was aspirated and discarded. Pelleted cells were resuspended in 5 mL of isotonic PBS solution and mixed by gently drawing the solution with a Pasteur pipette.
Total RNA was extracted by using Trizol reagent (15596-18; Invitrogen, Carlsbad, CA, USA), according to the manufacturer's protocol. The volume of total RNA obtained was checked spectrophotometrically at 260 nm using a Nanodrop spectrophotometer (Thermo Fisher Scientific Inc., Rockford, IL, USA). Reverse transcription was conducted in a reaction mixture consisting of 10 × RT buffer (2 µL), 25 × dNTP mix (0.8 µL), 10 × RT random primers (2 µL), RNase inhibitor (1 µL), distilled water (3.2 µL), and 10 µL RNA (100 ng/µL). The reaction mixture was incubated for 10 minutes at 25℃, 120 minutes at 37℃, heated to 85℃ for 5 minutes, and then stored at -20℃ until analysis. The integrity of the isolated RNA was established by real time-PCR analysis of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Real time-PCR reactions of CEA, CK20, and GAPDH were performed on an ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). Primers and probes were synthesized by Applied Biosystems. Real-time PCR reactions were prepared using TaqMan probe, a primer set for CEA (Hs00944025_m1), CK20 (Hs00300643_m1), and GAPDH (Hs99999905_m1), TaqMan Gene Expression Master Mix (ABI. 4369016), a MicroAmp optical 96-well reaction plate (ABI. N8010560), and MicroAmp optical adhesive film (ABI. 4311971). The thermocycling conditions were as follows: 50℃ for 2 minutes, 95℃ for 10 minutes, followed by 43 cycles at 95℃ for 15 seconds, and 60℃ for 1 minute. Data were analyzed with SDS relative quantification software ver. 2.2.3 (Applied Biosystems), using the automatic cycle threshold setting for assigning the baseline and threshold for positive results. The threshold for the epithelial tumor markers of CRC CTC has been reported as 35 to 50 cycles according to an individualized assay [11]. The patients were considered to have CTCs if mRNA was detected after 36 to 40 PCR cycles (threshold cycle value, Ct), which was determined through our individualized pilot study. Samples from 10 healthy volunteers and dextrose solution from which no mRNA was detected even after more than 45 to 48 cycles served as negative controls. The PCR products were confirmed by 2% agarose gel electrophoresis. Real time-PCR was evaluated by independent investigators unaware of patient status.
Statistical analysis
Changes in these mRNA levels were analyzed according to collection time, surgical modality, and patient clinicopathological features. Statistical calculations were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). The McNemar test was used to examine differences between the detection rate in peripheral and IMV blood. Statistical differences between pre- and post-operative positive rates for mRNA markers were calculated with the chi-square test. The chi-square and Fisher's exact tests were used to compare clinicopathological parameters between mRNA marker-positive patients and mRNA-negative patients at the post-mobilization time-point. The Mann-Whitney U-test was used to compare continuous variables. Statistical significance was set at P < 0.05.
RESULTS
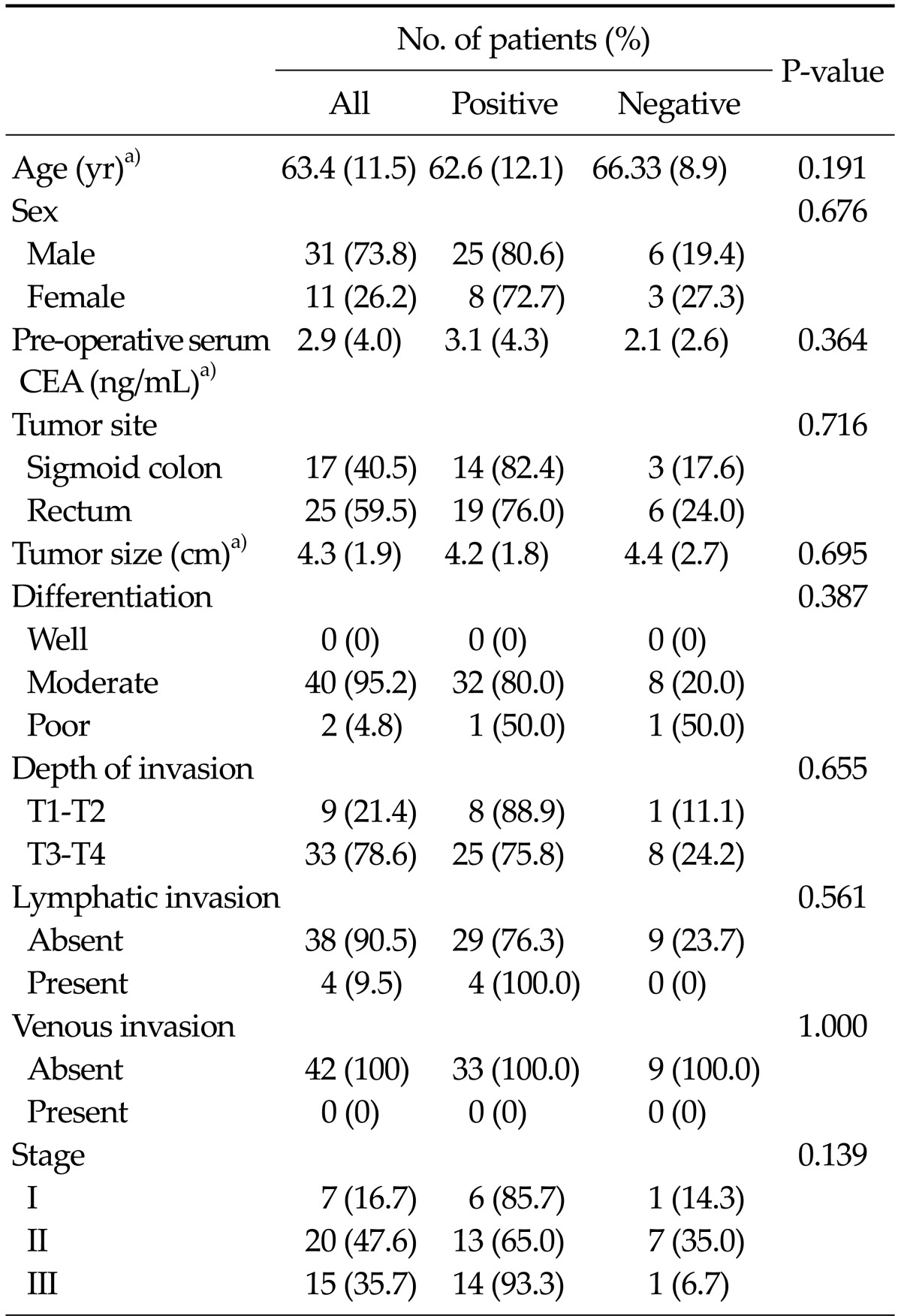
Fifty-three patients were included in the study. Sampling from the IMV before or after bowel mobilization failed in 11 patients. The remaining 42 patients were included in the analysis. The clinicopathological characteristics of all patients, including age, gender, tumor size, location, stage, and lymph node metastasis, are summarized in Table 1. The mean age of the patients was 63.4 years. Of all patients, 17 had sigmoid colon cancer and 25 had rectal cancer. Forty patients had moderately differentiated and two had poorly differentiated carcinomas. Based on the tumor-node-metastasis classification of resected specimens, seven patients had stage I, 20 had stage II, and 15 had stage III cancer. Comparison between mRNA-positive patients and -negative patients at the post-mobilization stage showed no significant differences in the clinicopathological characteristics between the two groups. The mRNA expression at the post-mobilization time-point was not significantly different with regard to the overall operation time and intra-operative blood loss. A comparison between the laparoscopic and the open surgery groups showed no differences in the clinicopathological characteristics, except tumor size, which was greater in the open surgery group.
Table 1.
Clinicopathologic characteristics of all patients and comparison between mRNA (CEA or CK20) expression-positive and -negative patients, based on samples collected at the post-mobilization time-point
CEA, carcinoembryonic antigen; CK20, cytokeratin-20.
a)Values are presented as mean (SD).
CEA and CK20 mRNA expression before surgery was similar in blood samples from the periphery and the IMV. CEA mRNA was identified in 28.6% of peripheral samples and 19.0% of IMV samples before surgery. The expression rates showed 66.6% consistency between blood from the periphery and IMVs. CK20 mRNA was identified in 50.0% of peripheral samples and 52.3% of IMV samples. The CK20 mRNA expression rates showed a 64.3% consistency between blood from the periphery and that from IMVs.
Comparison of samples from the IMV between before and after mobilization showed that the detection rate of CEA mRNA increased after mobilization (P = 0.07) (Fig. 3). The CK20 mRNA detection rate did not differ between pre- and post-mobilization samples. Cumulative detection rates were calculated to assess overall detection rates, resulting in a score ranging from 0 (mRNA-negative for both markers) to 1 (positive for at least one marker). The cumulative detection rates increased significantly after surgical manipulation (P = 0.032).
Fig. 3.
Comparison of circulating tumor markers (sampled from the inferior mesenteric vein) between the pre-mobilization and post-mobilization time-points. CEA, carcinoembryonic antigen; CK20, cytokeratin-20. a)P < 0.05.
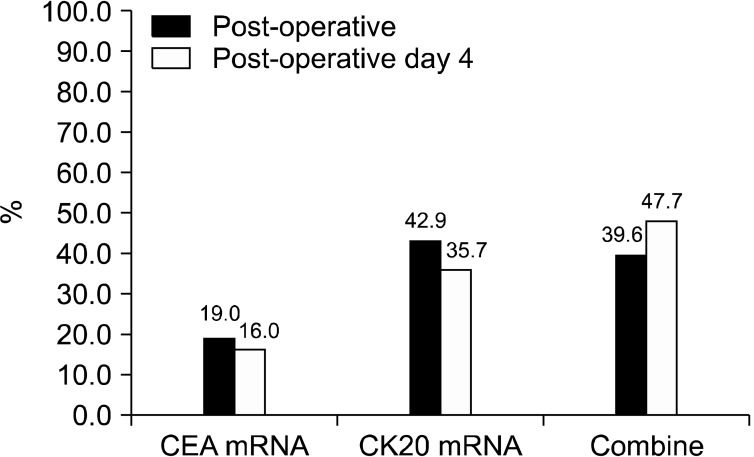
Comparing the samples from peripheral blood between the pre-operative and post-operative time-points revealed that the cumulative detection rates decreased significantly after surgical resection (P = 0.027). The detection rate for each individual mRNA also tended to decrease after operation compared with that before surgery, but without statistical significance (CEA mRNA, P = 0.306; CK20 mRNA, P = 0.512) (Fig. 4). Comparing peripheral blood samples between the post-operative time-point and post-operative day 4 showed that the detection rate for mRNA markers did not decrease significantly (Fig. 5).
Fig. 4.
Comparison of circulating tumor markers (sampled from the peripheral vein) between the pre-operative and post-operative time-points. CEA, carcinoembryonic antigen; CK20, cytokeratin-20. a)P < 0.05.
Fig. 5.
Comparison of circulating tumor markers (sampled from the peripheral vein) between the pre-operative and post-operative day 4 time-points. CEA, carcinoembryonic antigen; CK20, cytokeratin-20.
To assess the effect of surgical modality, detection rates for both mRNAs were compared between the laparoscopic and open surgery groups. No differences in the detection rate were observed for either of the two markers in relation to the surgical modality (laparoscopy vs. open surgery) at any time-point (Table 2). The cumulative detection rate also showed no significant difference between the two surgery groups.
Table 2.
Comparison of circulating tumor markers between laparoscopy and open surgery
CEA, carcinoembryonic antigen; CK20, cytokeratin-20; p, blood sampled from the peripheral blood; i, blood sampled from the inferior mesenteric vein.
DISCUSSION
The present study showed that the presence of CEA and CK20 mRNA was similar in both peripheral blood and mesenteric blood, and that mRNA detection rate increased after surgical manipulation of the cancer-bearing bowel segment. Expression of both mRNAs decreased after completion of the surgery. The detection rate of the CTC markers was not affected by surgical modality (laparoscopy vs. open surgery).
In the past, detection of CTCs in the blood of patients with cancer was achieved by cytology, immunocytochemistry, and flow cytometry techniques. As the number of cells in the blood is as low as one CTC in 105 to 107 leukocytes, these techniques have a high specificity but limited sensitivity [11]. PCR techniques can detect trace amounts (1/106) of cells in peripheral blood, lymph nodes, cerebrospinal fluid, and bone marrow [12]. The advantage of identifying RNA is that this technique implies that the cell is viable, because extracellular RNA is rapidly degraded, and only viable cells produce mRNA [13]. Therefore, current studies usually use RT-PCR techniques. CKs (such as CK20 and CK19), and CEA are the most commonly used RT-PCR markers for CRC. In the present study, we applied the RT-PCR assay to examine CEA and CK20 mRNA as surrogate markers to detect CRCs in the blood of patients with CRC. Great variability in CTC detection rates has been observed across studies, ranging from 4 to 57% in stage I to III CRC patients [14,15]. These variable detection rates are caused by technical errors, different sampling time-points, and using different veins for sampling. We used two different mRNA markers and samples taken at four time-points based on the surgical procedure to minimize variability. We observed that CEA mRNA was detected in 28.6% of samples, whereas the CK20 mRNA detection rate was 50.0% from peripheral blood during pre-operative state.
Surgical manipulation has been suggested to be an aggravating factor for tumor dissemination. The impact of surgical manipulation on oncological outcome can be approached based on long-term oncological results (survival and recurrence), but the direct effect of surgery can be assessed by detecting free cancer cells in the blood. In animal studies, detached cancer cells resulting from surgical manipulation are associated with blood-borne metastases [6]. Several human studies have also shown an association between surgical manipulation and CTC dissemination. Intra-operative dissemination of free cancer cells during resection of colorectal liver metastases was a significant predictive factor for intrahepatic or extrahepatic tumor recurrence [16]. The number of CTCs detected in post-operative or post-dissection blood samples was significantly higher than that in pre-operative or pre-dissection blood samples in patients with curable CRC [17]. These studies suggested that surgery causes cancer cells to be shed into circulation, which consequently results in worse outcomes. However, most of these studies obtained blood samples at one or two time-points, such as before and after surgery or before and after tumor dissection. We hypothesized that detecting mRNA markers in the blood at four different time-points (pre-operative, pre-mobilization, post-mobilization, and post-operative) would be more useful to identify a relationship between surgical manipulation and CTC dissemination. We found that the detection rate of mRNAs increased after surgical manipulation compared with before mobilization, but decreased postoperatively. Our results highlight the unfavorable impact of surgical manipulation on the manifestation of CTC mRNA.
During the early development of laparoscopy, the oncological safety of laparoscopic surgery was a main concern regarding expansion of the laparoscopic area, although laparoscopic surgery showed the same long-term oncological outcome (survival and recurrence) compared with that of open surgery. The effect of this technique on CTCs has been evaluated in some animal and human studies to assess the safety of laparoscopic surgery. Pneumoperitoneum-induced tumor growth or dissemination of tumor cells has been described in experimental animal models [18-20]. But, Chen et al. [21] found no elevation in CTCs during laparoscopic resection, and Wind et al. [22] found that fewer CTCs were observed in laparoscopically operated patients compared with those who underwent open surgery. The authors of the latter study hypothesized that the "no-touch isolation" technique, rather than surgical modality, was the cause for the lower CTC detection rate in the laparoscopy group, because they used the technique only in this group, and not in patients who underwent open surgery. The prior study showed no deleterious effect of laparoscopic surgery on CTC, but that study did not compare between open and laparoscopic surgery. Therefore, we compared the detection of mRNA markers in blood between laparoscopic and open surgery in this study. Both groups were similar in clinicopathological characteristics (data not shown). Both surgical approaches basically used the "no-touch isolation" technique. We hypothesized that the laparoscopic group would show less dissemination of CTCs because of the smaller instruments used and less hand-touch of the tumor-bearing bowel segment, which should reduce the release of cancer cells. However, surgical modality did not affect mRNA detection rates, and both markers were detected at a similar rate during both surgical modalities at the four time-points. This result is consistent with the results of Bessa et al. [23], who also found no difference in CTC detection rates with respect to the surgical approach. This result may be partly evidence of the observation that surgical modalities (open surgery and laparoscopy) have not shown difference in overall and 5-year survival rates [24, 25].
Several studies have shown that the detection rate of cancer cells in the portal or mesenteric veins is higher than that in peripheral blood [16,22,26]. The higher detection rate of cancer cells in drainage veins is explained by the fact that tumor cells are filtered by the action of the liver, through which tumor cells must pass before entering the systemic venous circulation. Furthermore, the large blood volume of the peripheral blood can dilute the concentration of tumor cells [9]. Unlike previous studies, we were unable to detect any difference in CTC expression rates between peripheral and IMV samples. Furthermore, we are unable to clearly explain why 8% and 7% of patients showed conversion from positive mRNA expression in peripheral blood before operation to negative expression in mesenteric blood before mobilization and vice versa. It is possible that differing concentrations of cancer cells at the time of blood aspiration resulted in statistical sampling errors, because the sampled blood may not exactly represent the cancer burden from the primary cancer, and tumor cells released into the bloodstream may not be a continuous process [27]. Moreover, the heterogeneity of cancer cells can also affects mRNA expression. These problems should be resolved in future studies with advancement of laboratory techniques.
We found that the clinicopathological characteristics did not differ significantly between mRNA marker-positive patients and -negative patients at the post-mobilization time-point. The mRNA expression rate at the post-mobilization time-point tended to increase with advancing cancer stage, but without statistical significance, which may be explained by the low number of patients included in the mRNA marker-negative group. An interesting finding of this study was that although the mRNA marker detection rate decreased after surgical resection, some patients showed continued expression following the operation and on the fourth post-operative day. The relationship between clinicopathologic characteristics and the prognostic value of CTCs in CRC has been discussed in previous reports. Many of these studies found that perioperative mRNA expression is correlated with aggravated cancer stage, but not deteriorated long-term prognosis, which has only been demonstrated in a few studies [11,15,28]. Significant correlations have been found between poor disease-free survival or recurrence and the detection of CTCs or markers in samples obtained 2 or more days after surgical resection. Chen et al. [21] found that the late post-operative CTC level (14 days after resection), but not the peri-operative level, was highly related to disease-free survival rate. Sadahiro et al. [29] also found that detecting CTCs in blood samples taken 7 days after curative resection is an independent factor associated with recurrence. However, many studies differ in their recommendation of the time-point and method of CTC detection to predict patient prognosis. Therefore, the prognostic implications and most suitable methodology for CTC detection remain to be established. Because of our study period, we were unable to assess the association between post-operative detection of CTC markers and prognosis. We have been continuously following up our patients and will assess the prognostic results after a sufficient follow-up period. Clearly, an international consensus on optimal CTC detection methodology and supporting large-scale studies are warranted to assess the prognostic implication of CTCs.
In conclusion, we found that surgical manipulation influenced CTCs and their dissemination during surgical resection of primary CRC. This result suggests that surgical manipulation plays an important role in the processes involved in detachment of primary tumor cells and their entry into systemic circulation. We did not find that the type of surgical technique affected CTCs. CTCs were detected in some patients after surgical resection, which may be indicative of a poor outcome. Longer follow-up and larger-scale studies are warranted to understand the long-term effects of peri-operative changes in CTCs.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Shibata D, Paty PB, Guillem JG, Wong WD, Cohen AM. Surgical management of isolated retroperitoneal recurrences of colorectal carcinoma. Dis Colon Rectum. 2002;45:795–801. doi: 10.1007/s10350-004-6300-3. [DOI] [PubMed] [Google Scholar]
- 2.Manfredi S, Bouvier AM, Lepage C, Hatem C, Dancourt V, Faivre J. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg. 2006;93:1115–1122. doi: 10.1002/bjs.5349. [DOI] [PubMed] [Google Scholar]
- 3.Fehm T, Sagalowsky A, Clifford E, Beitsch P, Saboorian H, Euhus D, et al. Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin Cancer Res. 2002;8:2073–2084. [PubMed] [Google Scholar]
- 4.Engell HC. Cancer cells in the circulating blood; a clinical study on the occurrence of cancer cells in the peripheral blood and in venous blood draining the tumour area at operation. Acta Chir Scand Suppl. 1955;201:1–70. [PubMed] [Google Scholar]
- 5.Romsdahl MM, McGrath RG, Hoppe E, McGrew EA. Experimental model for the study of tumor cells in the blood. Acta Cytol. 1965;9:141–145. [PubMed] [Google Scholar]
- 6.Nishizaki T, Matsumata T, Kanematsu T, Yasunaga C, Sugimachi K. Surgical manipulation of VX2 carcinoma in the rabbit liver evokes enhancement of metastasis. J Surg Res. 1990;49:92–97. doi: 10.1016/0022-4804(90)90116-j. [DOI] [PubMed] [Google Scholar]
- 7.Fisher ER, Turnbull RB., Jr The cytologic demonstration and significance of tumor cells in the mesenteric venous blood in patients with colorectal carcinoma. Surg Gynecol Obstet. 1955;100:102–108. [PubMed] [Google Scholar]
- 8.Turnbull RB, Jr, Kyle K, Watson FR, Spratt J. Cancer of the colon: the influence of the no-touch isolation technic on survival rates. Ann Surg. 1967;166:420–427. doi: 10.1097/00000658-196709000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkin G, Chopada A, Mitchell I. Colorectal cancer metastasis: in the surgeon's hands? Int Semin Surg Oncol. 2005;2:5. doi: 10.1186/1477-7800-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khair G, Monson JR, Greenman J. Epithelial molecular markers in the peripheral blood of patients with colorectal cancer. Dis Colon Rectum. 2007;50:1188–1203. doi: 10.1007/s10350-006-0875-9. [DOI] [PubMed] [Google Scholar]
- 11.Sergeant G, Penninckx F, Topal B. Quantitative RT-PCR detection of colorectal tumor cells in peripheral blood--a systematic review. J Surg Res. 2008;150:144–152. doi: 10.1016/j.jss.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Sakakura C, Takemura M, Hagiwara A, Shimomura K, Miyagawa K, Nakashima S, et al. Overexpression of dopa decarboxylase in peritoneal dissemination of gastric cancer and its potential as a novel marker for the detection of peritoneal micrometastases with real-time RT-PCR. Br J Cancer. 2004;90:665–671. doi: 10.1038/sj.bjc.6601544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zehentner BK. Detection of disseminated tumor cells: strategies and diagnostic implications. Expert Rev Mol Diagn. 2002;2:41–48. doi: 10.1586/14737159.2.1.41. [DOI] [PubMed] [Google Scholar]
- 14.Thorsteinsson M, Jess P. The clinical significance of circulating tumor cells in non-metastatic colorectal cancer: a review. Eur J Surg Oncol. 2011;37:459–465. doi: 10.1016/j.ejso.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Peach G, Kim C, Zacharakis E, Purkayastha S, Ziprin P. Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: a systematic review. Br J Cancer. 2010;102:1327–1334. doi: 10.1038/sj.bjc.6605651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch M, Kienle P, Hinz U, Antolovic D, Schmidt J, Herfarth C, et al. Detection of hematogenous tumor cell dissemination predicts tumor relapse in patients undergoing surgical resection of colorectal liver metastases. Ann Surg. 2005;241:199–205. doi: 10.1097/01.sla.0000151795.15068.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito S, Nakanishi H, Hirai T, Kato T, Kodera Y, Feng Z, et al. Quantitative detection of CEA expressing free tumor cells in the peripheral blood of colorectal cancer patients during surgery with real-time RT-PCR on a LightCycler. Cancer Lett. 2002;183:195–203. doi: 10.1016/s0304-3835(02)00157-x. [DOI] [PubMed] [Google Scholar]
- 18.Han SA, Lee WY, Park CM, Yun SH, Chun HK. Comparison of immunologic outcomes of laparoscopic vs open approaches in clinical stage III colorectal cancer. Int J Colorectal Dis. 2010;25:631–638. doi: 10.1007/s00384-010-0882-0. [DOI] [PubMed] [Google Scholar]
- 19.Tomita H, Marcello PW, Milsom JW, Gramlich TL, Fazio VW. CO2 pneumoperitoneum does not enhance tumor growth and metastasis: study of a rat cecal wall inoculation model. Dis Colon Rectum. 2001;44:1297–1301. doi: 10.1007/BF02234787. [DOI] [PubMed] [Google Scholar]
- 20.Ishida H, Hashimoto D, Nakada H, Takeuchi I, Hoshino T, Murata N, et al. Increased insufflation pressure enhances the development of liver metastasis in a mouse laparoscopy model: possible mechanisms. Surg Endosc. 2002;16:331–335. doi: 10.1007/s00464-001-8318-0. [DOI] [PubMed] [Google Scholar]
- 21.Chen WS, Chung MY, Liu JH, Liu JM, Lin JK. Impact of circulating free tumor cells in the peripheral blood of colorectal cancer patients during laparoscopic surgery. World J Surg. 2004;28:552–557. doi: 10.1007/s00268-004-7276-9. [DOI] [PubMed] [Google Scholar]
- 22.Wind J, Tuynman JB, Tibbe AG, Swennenhuis JF, Richel DJ, van Berge Henegouwen MI, et al. Circulating tumour cells during laparoscopic and open surgery for primary colonic cancer in portal and peripheral blood. Eur J Surg Oncol. 2009;35:942–950. doi: 10.1016/j.ejso.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Bessa X, Castells A, Lacy AM, Elizalde JI, Delgado S, Boix L, et al. Laparoscopic-assisted vs. open colectomy for colorectal cancer: influence on neoplastic cell mobilization. J Gastrointest Surg. 2001;5:66–73. doi: 10.1016/s1091-255x(01)80015-9. [DOI] [PubMed] [Google Scholar]
- 24.Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ, et al. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–1645. doi: 10.1002/bjs.7160. [DOI] [PubMed] [Google Scholar]
- 25.Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW, Jr, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655–662. doi: 10.1097/SLA.0b013e318155a762. [DOI] [PubMed] [Google Scholar]
- 26.Iinuma H, Okinaga K, Egami H, Mimori K, Hayashi N, Nishida K, et al. Usefulness and clinical significance of quantitative real-time RT-PCR to detect isolated tumor cells in the peripheral blood and tumor drainage blood of patients with colorectal cancer. Int J Oncol. 2006;28:297–306. [PubMed] [Google Scholar]
- 27.Weitz J, Koch M, Kienle P, Schrodel A, Willeke F, Benner A, et al. Detection of hematogenic tumor cell dissemination in patients undergoing resection of liver metastases of colorectal cancer. Ann Surg. 2000;232:66–72. doi: 10.1097/00000658-200007000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SY, Min KS, Chung JK, Jung IM, Ahn YJ, Hwang KT, et al. Carcinoembryonic antigen level of draining venous blood as a predictor of recurrence in colorectal cancer patient. J Korean Surg Soc. 2011;81:387–393. doi: 10.4174/jkss.2011.81.6.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadahiro S, Suzuki T, Maeda Y, Yurimoto S, Yasuda S, Makuuchi H, et al. Detection of carcinoembryonic antigen messenger RNA-expressing cells in peripheral blood 7 days after curative surgery is a novel prognostic factor in colorectal cancer. Ann Surg Oncol. 2007;14:1092–1098. doi: 10.1245/s10434-006-9289-0. [DOI] [PubMed] [Google Scholar]