Abstract
Objective
The objectives of this study were to 1) determine the correlation between osteoarthritis (OA) and Ihh expression, and 2) establish the effects of Ihh on expression of markers of chondrocyte hypertrophy and MMP-13 in human OA cartilage.
Design
OA cartilage and synovial fluid samples were obtained during total knee arthroplasty. Normal cartilage samples were obtained from intra-articular tumor resections, and normal synovial fluid samples were obtained from healthy volunteers and the contralateral uninjured knee of patients undergoing anterior cruciate ligament reconstruction. OA was graded using the Mankin score. Expression of Ihh in synovial fluid was determined by western blot. Ihh, type X collagen and MMP-13 mRNA were determined by real time PCR. Protein expression of type X collagen and MMP-13 in cartilage samples were analyzed with immunohistochemistry. Chondrocyte size was measured using image analysis.
Results
Ihh expression was increased 2.6 fold in OA cartilage and 37% in OA synovial fluid when compared to normal control samples. Increased expression of Ihh was associated with the severity of OA and expression of markers of chondrocyte hypertrophy: type X collagen and MMP-13, and chondocyte size. Chondrocytes were more spherical with increasing severity of OA. There was a significant correlation between Mankin score and cell size (r2= 0.80) and Ihh intensity (r2 = 0.89). Exogenous Ihh induced a 6.8 fold increase of type X collagen and 2.8 fold increase of MMP-13 mRNA expression in cultured chondrocytes. Conversely, knockdown of Ihh by siRNA and Hh inhibitor Cyclopamine had the opposite effect.
Conclusions
Ihh expression correlates with OA progression and changes in chondrocyte morphology and gene expression consistent with chondrocyte hypertrophy and cartilage degradation seen in OA cartilage. Thus, Ihh may be a potential therapeutic target to prevent OA progression.
Keywords: Ihh, cartilage, osteoarthritis, chondrocyte hypertrophy, MMP-13
Introduction
Osteoarthritis (OA), featured by destruction and loss of articular cartilage, causes chronic joint pain and disability (1). It has been shown that aging, trauma, excessive mechanical load, and genetics are risk factors associated with the development of OA (2-4). However, the pathogenesis of the disease remains unknown. Indian hedgehog (Ihh), a key signaling molecule, is primarily synthesized and expressed in prehypertrophic chondrocytes during growth plate development. Ihh regulates chondrocyte hypertrophy and endochondral ossification (5) (6) (7). Genetic studies using knockout mice have demonstrated that activation of Ihh downstream signaling pathways results in a decrease in articular cartilage thickness and proteoglycan (PG) content while inhibiting Ihh signaling results in an increase of articular cartilage thickness and PG (7) (8). Consistent with these observations, upregulation of hedgehog (Hh) signaling in postnatal cartilage promotes chondrocyte hypertrophy and cartilage degradation (9).
Studies have demonstrated that the OA chondrocytes recapitulate some of the differentiation processes that occur during embryogenesis (10-13) (14). During growth plate development, hypertrophic chondrocytes express type X collagen and matrix metalloproteases (MMPs), which subsequently induce cartilage degradation as part of the endochondral ossification process. Cartilage degradation in the growth plate is similar to cartilage degeneration in OA, which has also been shown to be mediated by MMPs (15-17). Furthermore, recent studies indicate that increased Hh signaling is involved in mouse OA development (9), and that increased type X collagen expression, a hypertrophic marker, has been observed in human knee joint cartilage with early focal OA-like lesions (12, 18). Mechanical overload is a proposed mechanism of OA development and the Ihh signaling pathway may mediate the response of chondrocytes to mechanical stress (19). These data suggest that Ihh may play a critical role during OA cartilage degradation.
Although increased downstream of Hh signaling has been associated with OA development (9), direct genetic evidence linking Ihh to OA development by promoting chondrocyte hypertrophy has not been reported. To test the hypothesis that Ihh may play a role in human OA development, we analyzed human normal and OA cartilage and synovial fluid samples for Ihh expression to determine if the Ihh expression correlates with OA cartilage damage. In order to establish a functional role for Ihh in OA development, expression of factors associated with chondrocyte hypertrophy, type X collagen and MMP-13, were quantified after Ihh treatment or knockdown in human OA chondrocytes in vitro. Our findings suggest that Ihh signaling may promote OA progression.
Methods
Specimens
The study was approved by the Institutional Review Board at Rhode Island Hospital, and informed consent was obtained from each donor. Articular cartilage samples were obtained from patients with OA at the time of total joint arthroplasty (N=17, 11 female, 6 male, age 68.6±8.6 (mean±SD), range 55-79). Normal control samples of articular cartilage were obtained from patients undergoing tumor resections (N=6, 6 male, age 23.8±13.6 (mean±SD), range 15-51). OA synovial fluid was also obtained during knee joint arthroplasty (N= 32, 20 female, 12 male, age 69.6±9.0 (mean±SD), range 42-86). Normal synovial fluid samples were also collected from the contralateral uninjured knee of patients undergoing unilateral ACL reconstruction (n=30) and one healthy volunteer (N=31, 17 male, 14 female age 25.1±10.5 (mean±SD), range 15-54). Inclusion criteria for using the contralateral knee of ACL reconstruction patients as a normal control were the absence of prior knee injury and normal standing radiographs.
Synovial Fluid Analysis
Synovial fluid samples were centrifuged at 2,000 g for 10 min to remove cells and debris. The synovial fluid samples were aliquoted and frozen at −80°C until analysis (20). Before performing the experiments, the synovial fluid samples were treated with 15 U/ml of bovine testicular hyaluronidase (HA) (Sigma-Aldrich, St Louis, MO) for 15 min at 37°C to reduce viscosity and diluted 1:10 with cell lysis buffer containing proteinase inhibitor (Roche, Basel, Switzerland) (21).
Cartilage Tissue Samples
Cartilage samples from the tibia obtained during total knee arthroplasty were divided into OA cartilage from the more affected compartment (usually medial) and “relatively normal” cartilage from the uninvolved compartment (usually lateral). Normal cartilage samples were also obtained from intra-articular tumor resections. Absence of cartilage degeneration was confirmed in the normal cartilage samples using Safranin-O staining.
Chondrocyte isolation and primary culture
Cartilage slices were removed from the tibial plateau and washed in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA). Chondrocytes were isolated as previously described (22). Briefly, pieces of cartilage were minced with a scalpel and digested with pronase (2 mg/ml) (Roche, Basel, Switzerland) in Hank’s balanced salt solution (HBSS) (Invitrogen, Carlsbad, CA) for 30 min at 37°C with shaking. After digestion and removal of the supernatant, the cartilage pieces were washed with DMEM, and digested with crude bacterial collagenase (Type IA, 1 mg/ml) (Sigma-Aldrich, St Louis, MO) for 6-8 h at 37°C with shaking. Enzymatic digestion was stopped by adding DMEM containing 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA). Residual multi-cellular aggregates were removed by filtering, and the cells were washed 3 times with DMEM. Chondrocytes were incubated in DMEM containing 10% FBS, L-glutamine (Invitrogen, Carlsbad, CA), and antibiotics (penicillin and streptomycin) (Sigma-Aldrich, St Louis, MO), and allowed to attach to the surface of the culture dishes. Cells were trypsinized, washed 3 times, and plated either in 8-well chamber (Nalge Nunc International Corp, Naperville, IL) at 1 × 105 cells/well or in 6-well culture plates (Becton Dickinson Labware, Franklin Lakes, NJ) 1 × 106 cells/plate. At 90% confluence, the second passage cells were transfected with Ihh siRNA or scrambled siRNA control or exposed to recombinant human Ihh protein (R&D, Minneapolis, MN) at different concentrations. Two Duplex small interfering RNAs (siRNA) that specifically targeted the mRNA encoding human Ihh and scrambled siRNA were used in the study (Santa Cruz, Santa Cruz, CA and Invitrogen, Carlsbad, CA). Duplex siRNAs (200 nM) were transfected into human chondrocytes that were cultured in 6-well tissue culture plates using the GeneMute siRNA transfection reagent (SignaGen Laboratories, USA). After 48 hours in culture without removing the transfection reagent, the total RNA was isolated from the chondroytes. Immunocytochemistry analyses of chondrocyte phenotype were performed as described previously (23) using anti-type-I and anti-type-II collagen monoclonal antibodies (mAb; Chemicon International, Temecula, CA). These cells are positive for type II collagen and negative for type I staining (Supplement Figure 4).
Histology
Full thickness 2×2 cm cartilage samples were taken from the OA cartilage and adjacent “relatively normal” cartilage samples from each knee joint with a scalpel. Approximately half of the section (0.4-0.5 g) was used for RNA isolation and the remainder was fixed in 10% formalin (Sigma-Aldrich, St Louis, MO) for 72 hr. The specimens were decalcified in Richman-Gelfand-Hill solution, processed in a Tissue-Tek VIP 1000 tissue processor (Miles, Elkhart, IN), and embedded in a single block of Paraplast X-tra (Thermo-Fisher, Hampton, NH). Blocks were trimmed to expose tissue using a rotary microtome (Reichert-Jung, Wien, Austria). The slices were then cut into 6-μm sections and mounted on slides. Safranin-O staining was performed and the severity of cartilage damage was assessed using the modified Mankin grading system (24). Grade I (Mankin score 0-2) represents minimal cartilage damage, while Grade II (Mankin score 3-10) and Grade III (Mankin score 11-18) represent more severe cartilage damage. Two independent and blinded observers scored each section, and the scores were averaged.
Immunohistochemistry
To detect the distribution of type X collagen and MMP-13 in cartilage, 6-μm sections were collected on positively charged glass slides (Thermo-Fisher, Hampton, NH). The sections were dried on a hot plate to increase adherence to the slides. Immunohistochemistry was carried out using the Histostain-SP Kits (Zymed-Invitrogen, Carlsbad, CA). Sections were de-paraffined and rehydrated using conventional methods. Endogenous peroxidase was blocked by treating the sections with 3% hydrogen peroxide (Sigma-Aldrich, St Louis, MO) in methanol (Sigma-Aldrich, St Louis, MO) for 30 min. The sections were digested by 5 mg/ml HA in PBS (Sigma-Aldrich, St Louis, MO) for 20 min. Nonspecific protein binding was blocked by incubation with a serum blocking solution (LICOR, Lincoln, NE). The sections were incubated with affinity-isolated IgG fractions of antibody against human type X collagen (2 μg/ml) (EMD, Gibbstown, NJ) and an antibody against MMP-13 (2 μg/ml) (Santa Cruz, Santa Cruz, CA) respectively at 4°C overnight. The negative control sections were incubated with IgG isotype control (2 μg/ml) (R&D, Minneapolis, MN) in PBS. Thereafter, the sections were treated sequentially with biotinylated secondary antibody and streptavidin-peroxidase conjugate (Zymed-Invitrogen, Carlsbad, CA), and then were developed in DAB chromogen (Zymed-Invitrogen, Carlsbad, CA). The sections were counterstained with hematoxylin (Zymed-Invitrogen, Carlsbad, CA). Photography was performed with a Nikon E800 microscope (Nikon, Melville, NY) (25).
To detect the distribution of Ihh in cartilage, 6-μm sections were analyzed by immunofluorescent staining with a polyclonal antibody against Ihh (sc-1196, Santa Cruz, Santa Cruz, CA). The negative control sections were incubated with isotype control (sc-1196-P, Santa Cruz, Santa Cruz, CA) in PBS. The specificity of Ihh antibodies used in this study has been validated by immunofluorescence staining (26) and western blotting (27). The sections were incubated with primary antibody at 4°C overnight. After washing with PBS, affinity-purified TRITC conjugated donkey anti-goat secondary antibody (1:500) (Jackson, West Grove, PA) was applied with Hoechst nuclear dye (0.5 mg/ml) (Pierce, Rockford, IL). The sections were washed and mounted in GEL/MOUNTTM (Biomeda, Foster city, CA). Single or multiple exposure photography was performed with a Nikon E800 microscope (28). The intensity of Ihh expression and the size of chondrocytes were visualized under fluorescence microscopy and quantified using Ivision software (BioVision, Exton, PA). The intensity of Ihh is a pixel unit of gray scale.
Western blot
Total protein of synovial fluid was quantified using the BAC Protein Assay Reagent Kit (Pierce, Rockford, IL). 14 μg of total protein was electrophoresed in 10% SDS PAGE under reducing conditions.
After electrophoresis, proteins were transferred onto Immobilon-PVDF membrane (Thermo-Fisher, Hampton, NH) and probed with a polyclonal antibody against Ihh (sc-1196, Santa Cruz, Santa Cruz, CA). The antibody was diluted 1:1,000 in PBS-T containing 1% bovine serum albumin (BSA) (Sigma-Aldrich, St Louis, MO). Horseradish peroxidase-conjugated second antibody IgG (H+L) (Bio-Rad, Hercules, CA) was diluted 1:3,000 in PBS-T and used as the secondary antibody. Visualization of immunoreactive proteins was achieved by using the ECL Western blotting detection reagents (Amersham, Arlington Heights, IL) and by subsequently exposing the membrane to Kodak X-Omat AR film (Kodak, Rochester, NY). Band densities were quantified using Image Acquisition and Analysis Software (UVP, Upland, CA). Parallel gels were prepared for Coomassie Blue staining to confirm equal loading of samples. Equal amounts of protein were electrophoresed in 10% SDS PAGE and the gel was prefixed in 50% MeOH, 10% HoAC, 40% H2O for 30 minutes and then stained with 0.25% Coomassie Brilliant blue R-250 (Bio-Red Laboratories, Hercules, CA) in the above solution for 4 hours. The gel was detained in 5% MeOH, 7.5% HoAC, 87.5% H2O until background was clear. The detained gel was stored in 7% HoAc and a photograph was taken using Canon camera (SD-1000, Canon Inc, Japan).
Real Time RT-PCR (qPCR)
Total RNA was isolated from OA cartilage (Mankin scores: 11 or 18) and the adjacent “relatively normal” cartilage from the opposite compartment (usually lateral) in the same specimen (Mankin scores: 0 or 1) or from chondrocytes incubated with recombinant human Ihh protein or transfected with Ihh siRNA 48 hours after the transfection, using RNeasy isolation kit (Qiagen, Valencia, CA). 1 μg of RNA was reverse-transcribed to obtain first-strand cDNA using the iScriptTM cDNA synthesis Kit (Bio-Rad, Hercules, CA). Total RNA was also isolated from OA cartilage and the normal cartilage from the intraarticular tumor resections incubated with Hh inhibitor cyclopamine (20μM) 48 hours after the treatment. The quantification of mRNA for Ihh, type X collagen, and MMP-13 were determined using the QuantiTect SYBR Green PCR kit (Qiagen, Valencia, CA) with the DNA Engine Opticon 2 Continuous Fluorescence Detection System (MJ Research, Waltham, MA). Each reaction was performed in triplicate. Ihh primers were as follows: forward, 5′-CAT TGA GAC TTG ACT GGG CAA C-3′, and reverse, 5′-AGA GCA GGC TGA GTT GGG AGT CGC-3′. Type X collagen primers were as follows: forward, 5′- TGC CTC TTG TCA GTG CTA ACC -3′, and reverse, 5′- GCG TGC CGT TCT TAT ACA GG-3′. MMP-13 primers were as follows: forward, 5′- TGC TGC ATT CTC CTT CAG GA-3′, and reverse, 5′- ATG CAT CCA GGG GTC CTG GC-3′. MMP-1 primers were as follows: forward, 5′-CTG TTC AGG GAC AGA ATG TGC T-3′, and reverse, 5′-TTG GAC TCA CAC CAT GTG TT-3′. MMP-3 primers were as follows: forward, 5′-CCC TCC AGA ACC TGG GAC-3′, and reverse, 5′-ATA AAA GAA CCC AAA TTC TTC AAA A-3′ (29, 30). Amplification conditions were as follows: 2 min of preincubation at 50°C, 10 min at 95°C for enzyme activation, and 40 cycles at 95°C denaturation for 10 sec, 55°C annealing for 30 sec, and 72 °C extension for 30 sec. The cycle threshold (Ct) values for 18s RNA and that of target genes were measured and calculated by computer software (MJ Research, Waltham, MA). Relative transcription levels were calculated as x=2−ΔΔCt, in which ΔΔCt = ΔE-ΔC, and ΔE =Ctexp-Ct18s; ΔC = Ctctl-Ct18s, as previously described (28).
Statistical analysis
Two-tailed paired t-tests were used to compare mRNA levels from OA cartilage to its adjacent “relatively normal” cartilage as well as from Ihh siRNA treated group or recombinant human Ihh protein treated group to their respective controls. T-test was also used to compare Ihh band densities from OA synovial fluid to normal synovial fluid at a rejection level of 5% unless otherwise noted. Random effects models (31) were used to examine the relationships between Mankin score and (a) Ihh intensity, (b) chondrocyte size, and (c) ratio of major: minor axis (major/minor). Within-person correlation due to repeated measurements was accounted for by random intercepts which were assumed to have a mean-zero normal distribution. Log-transformed major/minor ratio was used as a dependent variable to capture its nonlinear relationships with Mankin score. Reader difference was accounted for by including a dummy variable as a fixed effect in the model. All models were fit using restricted maximum likelihood (REML) (32) to reduce the bias of estimating the residual variance. Model goodness of fit was assessed using a likelihood ratio (LR) statistic that has an interpretation similar to R2 in a simple linear model (33). All statistical analyses were conducted using the statistical program R (version 2.13.1) (34).
Results
Increased Ihh expression in OA cartilage
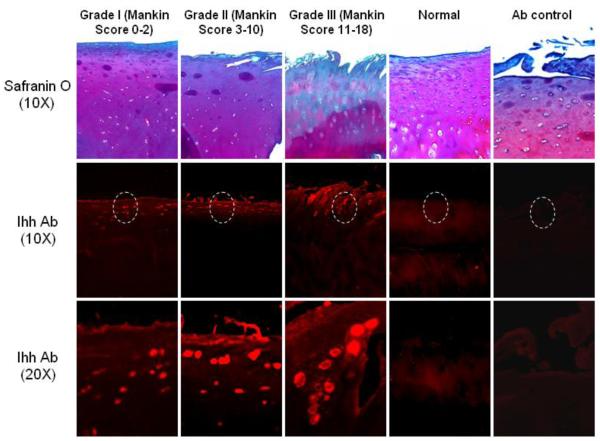
Knee joint cartilage samples from the involved compartment of OA patients (OA) were compared to normal cartilage from patients who had undergone intraarticular tumor resections (normal controls). The normal cartilage was collected from the central region of cartilage (thickest region), while the OA cartilage was collected from the corresponding area from OA patients. Immunofluorescent staining showed that the expression of Ihh protein was low in normal articular cartilage, but significantly greater in the OA cartilage samples (Fig. 1). The more intense staining was mainly found in the upper clusters of cells in OA cartilage, and the intensity of Ihh staining was associated with the severity of OA cartilage damage as determined by modified Mankin score (r2 = 0.89). When compared to OA cartilage, no obvious staining of Ihh was observed in the normal cartilage.
Figure 1. Increased Ihh expression in OA cartilage.
Ihh was analyzed with immunohistochemistry in knee joint cartilage from OA knees (n=17) and resection specimens with normal cartilage (n=6). The expression of Ihh is significantly increased in OA cartilage. Increased staining for Ihh protein is associated with increased severity of OA cartilage damage as demonstrated by Safranin O staining. In contrast, Ihh staining was minimal in normal cartilage.
Increased Ihh levels in OA synovial fluid
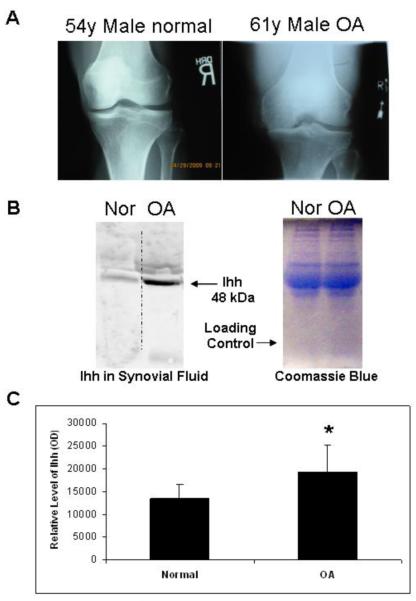
Synovial fluid samples collected from OA patients and normal controls (the contralateral side of patients undergoing unilateral ACL reconstruction and a normal volunteer) were processed for Ihh content by western blot. Ihh concentration was 137.2% higher in the synovial fluid of the OA patients when compared to that of the normal controls (Fig. 2). Within normal patients aged 15-54, there was no difference in Ihh in synovial fluid (supplement fig 1).
Figure 2. Increased Ihh expression in OA synovial fluid.
(A) Radiographs confirmed cartilage damage and joint space narrowing in the OA patients and no joint changes in the normal controls. (B) Representative Western blot demonstrates a high level of Ihh in human OA synovial fluid compared to normal control (54-year-old male healthy volunteer). Coomassie Blue staining was used to confirm equal loading. (C) Densitometry showed that the mean Ihh concentration was 137.2% higher in the OA group (n=32) relative to the control group (n=31) (p=0.002).
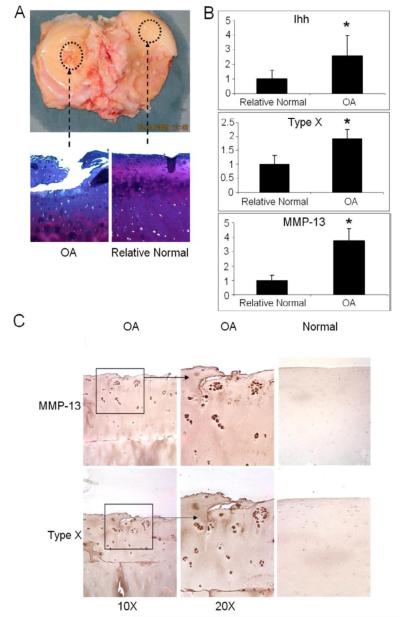
Increased Ihh, type X collagen, and MMP-13 expression in OA cartilage
Representative histologic sections are shown of OA cartilage and “relatively normal” cartilage from the same knees (Fig. 3A). Real time PCR indicates that the levels of Ihh, type X collagen, and MMP-13 expression were increased in OA cartilage samples compared to the adjacent “relatively normal” cartilage (Fig. 3B). Immunostaining showed increased type X collagen and MMP-13 in OA cartilage compared to normal cartilage obtained from intra-articular tumor resections (Fig. 3C).
Figure 3. Increased expression of Ihh, type X collagen, and MMP-13 in OA cartilage.
(A) Representative histologic sections from OA cartilage and adjacent relatively normal cartilage are shown. (B) Expression of Ihh, and typical molecular markers of chondrocyte hypertrophy: type X collagen and MMP-13, were determined by qPCR. Samples from the relatively normal are arbitrarily defined as 1. Bar graphs show the averages with SD, N=3, *: p=0.0019. (C) Representative immunohistochemistry for type X collagen and MMP-13 in OA cartilage and cartilage from a control patient without OA.
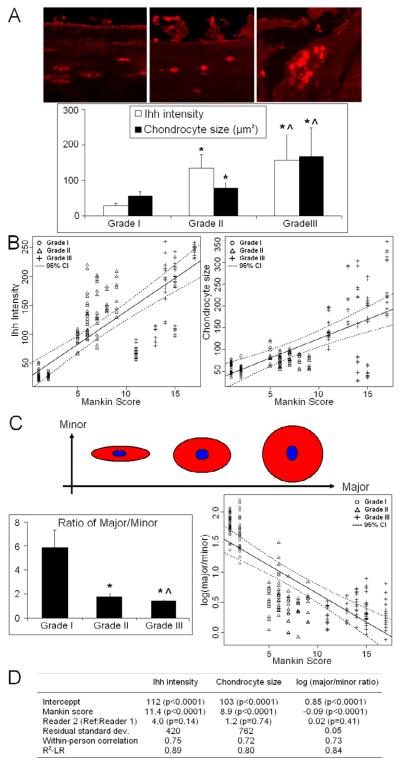
Increased Ihh levels in OA cartilage associated with altered cell morphology
There was a significant correlation between Ihh intensity and Mankin score (R2 = 0.89, p<0.0001) (Figs. 4A and 4B-left). Cell size is also correlated with Mankin score (R2= 0.80, p<0.0001) (Figs. 4A and 4B-right), which is analogous to the changes seen in chondrocyte morphology in the growth plate as chondrocytes become hypertrophic. In addition, chondrocytes became more spherical as measured by the ratio of major to minor axis with increasing severity of OA (Fig. 4C, R2 = 0.84, p<0.0001). Therefore, increased Ihh expression was associated with the larger size of chondrocytes observed in OA cartilage and with increased cartilage damage.
Figure 4. Ihh expression is associated with cartilage damage and chondrocyte morphological changes.
(A) An increase in Ihh staining in OA cartilage correlates with OA grade and a larger cell size. Bar graphs show the averages of quantified data of Ihh intensity and cell size from two hundred and twenty-eight cells from nine patients. (B) There was a significant correlation (p<0.0001) between Ihh intensity of the chondrocytes and Mankin score (R2=0.89) (left) and between cell size and Mankin score (R2=0.80) (right). (C) Chondrocytes change in shape from spindle to spherical (decrease ratio of major: minor axis, major/minor) with OA progression. Bar graphs show the averages of quantified data of major/minor ratio from two hundred and twenty-eight cells (left) from nine patients. Log-transformed major/minor ratio was used as dependent variable to capture its nonlinear relationships between Mankin score (R2=0.84) (right). (D) The detail information for plot 4B and 4C. *: compared to grade I p<0.0001; ^: compared to grade II OA, p<0.0001.
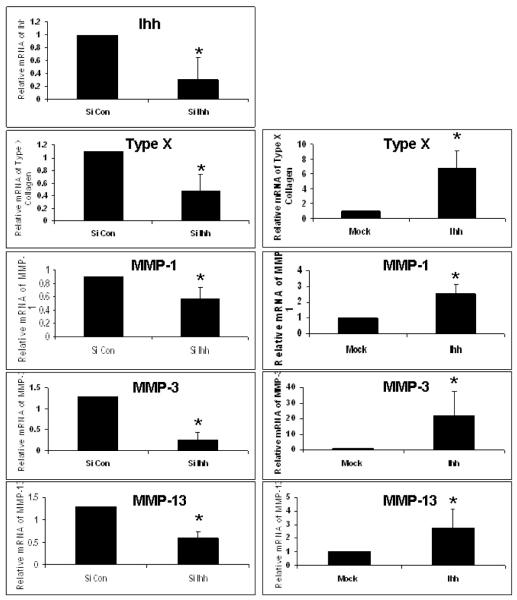
Ihh induces hypertrophy and regulates expression of type X collagen and MMPs in OA chondrocytes
Treatment of OA chondrocytes with recombinant Ihh protein increased the expression of type X collagen and MMP-13 mRNA. In contrast, knockdown of Ihh by transfecting chondrocytes with Ihh siRNAs inhibited the expression of Ihh, type X collagen, and MMPs (Fig. 5). A similar result was also observed in the normal or OA cartilage incubated with Hh inhibitor, cyclopamine (Supplement figure 6). The mRNA levels of type X collagen and MMP-13 were increased 340% and 302% respectively in OA cartilage compared to the normal cartilage. While the levels of type X collagen and MMP-13 were decreased by 85% and 57% respectively after inhibiting Hh pathway with cyclopamine in OA cartilage. Similar decreases of type X and MMP-13 were found after Hh inhibition with cyclopamine in normal cartilage by 52% and 48% respectively.
Figure 5. Ihh induces chondrocyte hypertrophy and regulates type X collagen and MMPs in OA chondrocytes.
Human OA chondrocytes were transfected with Ihh siRNA or treated with recombinant Ihh protein (5.0 μg/ml). The mRNA levels of Ihh, type X collagen, and MMPs were determined by qPCR. The endogenous Ihh was knocked down 70% by Ihh siRNA (p=0.0003). Knockdown of Ihh inhibited the expression of type X collagen and MMP-13. Ihh treatment increased the expression of type X collagen (p=0.0001), and MMP-1 (p=0.0089), MMP-3 (p=0.045), and MMP-13 (p=0.001). Samples treated with carrier or siRNA control (a scrambled sequence) are arbitrarily defined as “1”, and data from samples treated with either siRNA for Ihh or recombinant Ihh protein are given as a mean. Bar graphs show the means and SD of data from three independent experiments (chondrocytes from three different patients) using qPCR. *: compared to Mock for Ihh protein or to siRNA control for siRNA treatment.
Discussion
In this study, we determined that cartilage and synovial fluid samples from patients with OA have elevated levels of Ihh compared to normal control samples, and that the amount of Ihh was associated with severity of OA (Figs. 1, 2) and the hypertrophic chondrocyte phenotype (Fig. 4). OA chondrocytes have more of a spherical cell shape and larger cell size than chondrocytes from normal cartilage (Fig. 4). Our data show that upregulated Ihh promotes the hypertrophic phenotype and regulates typical hypertrophic markers such as type X collagen and MMP-13 (Fig. 5). Therefore upregulation of Ihh signaling may facilitate OA development by inducing chondrocyte hypertrophy and expression of genes known to cause cartilage degeneration. This result is consistent with the finding that type X collagen and MMP-13 are increased in OA cartilage as previously reported (35) (11) (36) (37). This result is in agreement with Lin et al who reported that human cartilage explants treated with Hh blocking agents exhibited decreased expression of type X collagen and MMP-13, while Hh ligand stimulation led to induction of these two genes in the cartilage explants (9). Thus, it is likely that induction of type X collagen and MMP-13 may be caused by increased Ihh signaling in OA cartilage in vivo.
In addition to increased Ihh in OA tissues, we also demonstrated that the increase of Ihh is correlated with the severity of OA cartilage damage (Fig. 1), suggesting that upregulation of Ihh signaling may play a role in OA progression. Our data show that high levels of Ihh are accompanied by increased cell size, altered cell morphology, and upregulated hypertrophic markers type X collagen and MMP-13 (Figs. 3, 4), suggesting that upregulated Ihh signaling might regulate the chondrocyte hypertrophic phenotype and cartilage matrix degradation. Increased cell size (Fig. 4A) and increased ratio of minor axis to major axis correlated with OA progression (Fig. 4B) suggesting that OA chondrocyte morphology changes with OA progression in a way that is analagous to the changes that occur with maturation of growth plate chondrocytes, which is known to be mediated by IHH. In healthy articular cartilage, chondrocytes in the superficial zone are usually spindle-shaped, while chondrocytes in the deeper zone are more spherical, which are similar to hypertrophic cells. Thus, our finding that OA chondrocytes have a more spherical cell morphology indicates the possibility that OA chondrocytes may enter the hypertrophic stage as a result of Ihh signaling. Another possibility is that the increasing cartilage damage in advanced OA leads to severe disruption or complete loss of the superficial zone, and thus may expose more spherical chondrocytes originally located in the deeper zones. In either case, these spherical chondrocytes undergo clustering in OA, a phenomenon not seen in normal articular cartilage.
Obtaining age matched controls without OA is challenging for studies of human OA. Therefore, cartilage analyses were performed using “relatively normal” cartilage and OA cartilage samples from the same patient. We found that the level of Ihh was increased in the OA samples when compared with the “relatively normal” samples from the same patient, despite the fact that this relatively normal cartilage was exposed to higher levels of Ihh found in OA synovial fluid. We recognize that the regions in which cartilage appears normal in the OA joint may not be entirely normal (38). However, it provides us with a reasonable benchmark for comparison since it is tissue with minimal damage. Another explanation for some of the differences we found could be related to the age of the patients from which the specimens were obtained. Normal cartilage and synovial fluid were from younger patients. However, there was a wide range of patient ages from which normal synovial fluid was obtained and our supplemental data indicates that the level of Ihh in synovial fluid is independent of age but is associated with the cartilage damage (Supplement Figures 1 and 2). Taken together with the effects of Ihh on expression of factors known to cause cartilage degradation, it is likely that Ihh is associated with the severity of cartilage damage. An animal model utilizing an inducible tissue specific Ihh knockout would add additional support of this concept.
In this study, we found that Ihh increased hypertrophic markers in cultured OA chondrocytes, including type X collagen and MMPs, while knockdown of Ihh inhibited the expression of these genes (Fig. 5). In murine OA cartilage, MMP-13 activity led to structural damage and knockdown of MMP-13, which may alleviate cartilage damage in OA (36). Thus, our finding that Ihh inhibition downregulated MMP-13 (Fig. 5) may be a means to provide chondroprotection in patients with early stage disease. Interestingly, Lin et al. observed that inhibition of Hh signaling attenuated OA severity in a mouse model in vivo and in human explants in vitro (9), which is consistent with our model.
In conclusion, this study provides evidence that Ihh expression correlates with OA progression and induces chondrocyte hypertrophy and expression of genes known to cause cartilage degradation in chondrocytes isolated from OA cartilage. Further analysis of Ihh inhibition as a therapeutic strategy is warranted.
Supplementary Material
Acknowledgments
This project was supported by grants R01AR059142, AR052479, AR047910, AG 017021, P20RR024484 from NIH, grants from the Aircast Foundation and Arthritis National Research Foundation, and NSFC 81071495, 81171676, and SXNSFC 2011011042. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center of Research Resources or the National Institutes of Health. The authors would like to thank Dr. Michael G. Ehrlich and Dr. Gregory Jay for their support and help with collection of synovial fluid samples.
Role of the funding source in the publication None
Footnotes
Author contributions Fangyuan Wei, conception and design of the study, acquisition of data, revising the article, final approval of the version to be submitted.
Jingming Zhou, acquisition of data, analysis and interpretation of data, drafting and revising the article, approval of the version to be submitted.
Xiaochun Wei and Juntao Zhang, acquisition of data, drafting and revising the article, approval of the version to be submitted.
Braden C. Fleming, provision of study materials, analysis and interpretation of data, revising the article, final approval of the version to be submitted.
Richard Terek, provision of study materials, revising the article, final approval of the version to be submitted.
Ming Pei, analysis and interpretation of data, revising the article, final approval of the version to be submitted.
Qian Chen, analysis and interpretation of data, revising the article, final approval of the version to be submitted.
Tao Liu, a statistician from Department of Biostatistics/Center for Statistical Sciences, Brown University School of Public Health
Lei Wei, conception and design of the study, analysis and interpretation of data, drafting and revising the article, final approval of the version to be submitted.
Competing interests None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clinical Orthopaedics & Related Research. 1986;(213):34–40. [PubMed] [Google Scholar]
- 2.Wei L, Svensson O, Hjerpe A. Correlation of morphologic and biochemical changes in the natural history of spontaneous osteoarthrosis in guinea pigs. Arthritis & Rheumatism. 1997;40(11):2075–83. doi: 10.1002/art.1780401121. [DOI] [PubMed] [Google Scholar]
- 3.Wei L, Hjerpe A, Brismar BH, Svensson O. Effect of load on articular cartilage matrix and the development of guinea-pig osteoarthritis. Osteoarthritis & Cartilage. 2001;9(5):447–53. doi: 10.1053/joca.2000.0411. [DOI] [PubMed] [Google Scholar]
- 4.Wei L, de Bri E, Lundberg A, Svensson O. Mechanical load and primary guinea pig osteoarthrosis. Acta Orthopaedica Scandinavica. 1998;69(4):351–7. doi: 10.3109/17453679808999046. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Lian N, Li L, Moss HE, Wang W, Perrien DS, et al. Atf4 regulates chondrocyte proliferation and differentiation during endochondral ossification by activating Ihh transcription. Development. 2009;136(24):4143–53. doi: 10.1242/dev.043281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda Y, Nakamura E, Nguyen M-T, Suva LJ, Swain FL, Razzaque MS, et al. Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(15):6382–7. doi: 10.1073/pnas.0608449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mak KK, Kronenberg HM, Chuang P-T, Mackem S, Yang Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development. 2008;135(11):1947–56. doi: 10.1242/dev.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaupre GS, Stevens SS, Carter DR. Mechanobiology in the development, maintenance, and degeneration of articular cartilage. Journal of Rehabilitation Research & Development. 2000;37(2):145–51. [PubMed] [Google Scholar]
- 9.Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nature Medicine. 2009;15(12):1421–5. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 10.Hoyland JA, Thomas JT, Donn R, Marriott A, Ayad S, Boot-Handford RP, et al. Distribution of type X collagen mRNA in normal and osteoarthritic human cartilage. Bone & Mineral. 1991;15(2):151–63. doi: 10.1016/0169-6009(91)90005-k. [DOI] [PubMed] [Google Scholar]
- 11.Aigner T, Reichenberger E, Bertling W, Kirsch T, Stoss H, von der Mark K. Type X collagen expression in osteoarthritic and rheumatoid articular cartilage. Virchows Archiv. B, Cell Pathology Including Molecular Pathology. 1993;63(4):205–11. doi: 10.1007/BF02899263. [DOI] [PubMed] [Google Scholar]
- 12.von der Mark K, Kirsch T, Nerlich A, Kuss A, Weseloh G, Gluckert K, et al. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis & Rheumatism. 1992;35(7):806–11. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]
- 13.Aigner T, Soder S, Gebhard PM, McAlinden A, Haag J. Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis-- structure, chaos and senescence. Nature Clinical Practice Rheumatology. 2007;3(7):391–9. doi: 10.1038/ncprheum0534. [DOI] [PubMed] [Google Scholar]
- 14.Slagboom E, Meulenbelt I. Genetics of osteoarthritis: early developmental clues to an old disease. Nature Clinical Practice Rheumatology. 2008;4(11):563. doi: 10.1038/ncprheum0935. [DOI] [PubMed] [Google Scholar]
- 15.Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, Lopez-Otin C, et al. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(49):17192–7. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005;13(7):632–41. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Kamekura S, Kawasaki Y, Hoshi K, Shimoaka T, Chikuda H, Maruyama Z, et al. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis & Rheumatism. 2006;54(8):2462–70. doi: 10.1002/art.22041. [DOI] [PubMed] [Google Scholar]
- 18.Tchetina EV, Squires G, Poole AR. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. Journal of Rheumatology. 2005;32(5):876–86. [PubMed] [Google Scholar]
- 19.Wu Q, Zhang Y, Chen Q. Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. The Journal of Biological Chemistry. 2001;276(38):35290–35296. doi: 10.1074/jbc.M101055200. [DOI] [PubMed] [Google Scholar]
- 20.Kanbe K, Takagishi K, Chen Q. Stimulation of matrix metalloprotease 3 release from human chondrocytes by the interaction of stromal cell-derived factor 1 and CXC chemokine receptor 4. Arthritis & Rheumatism. 2002;46(1):130–7. doi: 10.1002/1529-0131(200201)46:1<130::aid-art10020>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 21.Moreno MJ, Clayburne G, Schumacher HR., Jr Processing of noninflammatory synovial fluids with hyaluronidase for cytospin preparations improves the accuracy of differential counts. Diagnostic Cytopathology. 2000;22(4):256–8. doi: 10.1002/(sici)1097-0339(200004)22:4<256::aid-dc13>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Wei L, Sun X, Kanbe K, Wang Z, Sun C, Terek R, et al. Chondrocyte death induced by pathological concentration of chemokine stromal cell-derived factor-1. Journal of Rheumatology. 2006;33(9):1818–26. [PubMed] [Google Scholar]
- 23.Wei LSX, Terek R, Chen Q. CD95 induced osteoarthritic chondrocytes apoptosis and necrosis: dependency on p38 mitogen-activated protein kinase. Arthritis Research & Therapy. 2006;8(2):R37. doi: 10.1186/ar1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson CS, Loeser RF, Purser CB, Gardin JF, Jerome CP. Osteoarthritis in cynomolgus macaques. III: Effects of age, gender, and subchondral bone thickness on the severity of disease. Journal of Bone & Mineral Research. 1996;11(9):1209–17. doi: 10.1002/jbmr.5650110904. [DOI] [PubMed] [Google Scholar]
- 25.Wei L, Fleming BC, Sun X, Teeple E, Wu W, Jay GD, et al. Comparison of differential biomarkers of osteoarthritis with and without posttraumatic injury in the Hartley guinea pig model. Journal of Orthopaedic Research. 28(7):900–6. doi: 10.1002/jor.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adolphe C, Narang M, Ellis T, Wicking C, Kaur P, Wainwright B. An in vivo comparative study of sonic, desert and Indian hedgehog reveals that hedgehog pathway activity regulates epidermal stem cell homeostasis. Development. 2004;131(20):5009–19. doi: 10.1242/dev.01367. [DOI] [PubMed] [Google Scholar]
- 27.van den Brink GR, Bleuming SA, Hardwick JCH, Schepman BL, Offerhaus GJ, Keller JJ, et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nature Genetics. 2004;36(3):277–82. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 28.Wei LKK, Lee M, Wei X, Pei M, Sun X, Terek R, Chen Q. Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation. Dev Biol. 2010;341(2010):236–245. doi: 10.1016/j.ydbio.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins JR, Thomas B, Tan L, Choy B, Arbiser JL, Berenbaum F, et al. Immortalized human adult articular chondrocytes maintain cartilage-specific phenotype and responses to interleukin-1beta. Arthritis & Rheumatism. 2000;43(10):2189–201. doi: 10.1002/1529-0131(200010)43:10<2189::AID-ANR6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 30.Kayed H, Kleeff J, Keleg S, Guo J, Ketterer K, Berberat PO, et al. Indian hedgehog signaling pathway: expression and regulation in pancreatic cancer. International Journal of Cancer. 2004;110(5):668–76. doi: 10.1002/ijc.20194. [DOI] [PubMed] [Google Scholar]
- 31.McLean RAS, William L, Stroup Walter W. A Unified Approach to Mixed Linear Models. The American Statistician. 1991;45(1):54–64. [Google Scholar]
- 32.Harville DA. Maximum Likelihood Approaches to Variance Component Estimation and to Related Problems. Journal of the American Statistical Association. 1977;72(358):320–338. [Google Scholar]
- 33.L M. R2 measures based on Wald and likelihood ratio joint significance tests. The American Statistician. 1990;44:250–253. [Google Scholar]
- 34.Team RDC . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: ISBN 3-900051-07-0, URL http://www.R-project.org/2011. [Google Scholar]
- 35.Aigner T, Dietz U, Stoss H, von der Mark K. Differential expression of collagen types I, II, III, and X in human osteophytes. Laboratory Investigation. 1995;73(2):236–43. [PubMed] [Google Scholar]
- 36.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis & Rheumatism. 2009;60(12):3723–33. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clements DN, Carter SD, Innes JF, Ollier WER, Day PJR. Analysis of normal and osteoarthritic canine cartilage mRNA expression by quantitative polymerase chain reaction. Arthritis Research & Therapy. 2006;8(6):R158. doi: 10.1186/ar2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rout RMS, Hollander A, Davidson R, Clark I, Murray D, Gill H, Hulley P, Price A. Increased type I collagen in undamaged cartilage of anteromedial osteoarthritis of the knee. Journal of Bone and Joint Surgery - British. 2011;Vol 93-B(Issue SUPP_I, 30) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.