Abstract
PI3K/AKT/mTOR pathway plays a key role in the tumorigenesis of many human cancers including prostate cancer. However, inhibitors of this pathway such as Rad001 have not shown therapeutic efficacy as a single agent. Through a high throughput screen of 5,000 widely used small molecules, we identified compounds that can synergize with Rad001 to inhibit prostate cancer cells. One of the compounds, Propachlor, synergizes with Rad001 to induce apoptosis of castration-resistant prostate cancer cells via enhanced autophagy. This enhanced autophagic cell death is accompanied by increased Beclin1 expression as well as upregulation of ATG5-ATG12 conjugate and LC3-2. Rad001 and Propachlor can also synergistically inhibit tumors in a xenograft animal model of prostate cancer. These findings provide a novel direction to develop combination therapies for advanced and metastatic prostate cancer that has failed the currently available therapies.
Keywords: Rad001, Propachlor, synergism, autophagy, apoptosis
Introduction
Prostate cancer (PC) is the most common malignancy and the second most common cause of cancer-related death among men in western countries (1-2). Although PC in early stages can be cured by local therapies, there is no cure for advanced and metastatic PC. Therefore, there is an urgent need to develop novel and effective systemic therapies. As has been observed in many human cancers, growth factor signaling pathways, particularly PI3K/AKT/mTORC1 pathway, are critical for the development of PC. A genomic survey of PC identified mutations in this pathway leading to its hyper activity (3). Inactivation of PTEN, a negative regulator of this pathway, has been found in a significant portion of human PC (3), and tissue-specific deletion of PTEN is sufficient to initiate PC in a mouse model (4). Thus, inhibition of the PI3K/AKT/mTORC1 pathway will likely suppress PC and provide therapeutic benefits. Rapamycin or its derivative, Rad001 (Everolimus) (Figure 1B), can specifically and potently inhibit mTOR1. Indeed, rapamycin (we will refer to Rad001 which is a more stable derivative thereafter) has been approved to treat advanced kidney cancer as well as neuroendocrine pancreatic cancer. However, efforts to expand its use in more prevalent cancers including PC have been unsuccessful. It is postulated that inhibition of mTOR by Rad001 leads to activation of compensatory signaling pathways thus countering the growth-inhibitory effects (5). Novel compounds that simultaneously inhibit several signaling pathways including mTORC1 have been identified, promising to more effectively suppress tumor growth (6). Alternatively, identification of compounds that synergize with Rad001 may enhance the potency of Rad001 to treat human cancers. We performed a high throughput screen of 5,000 compounds including over 1,000 FDA-approved drugs as well as purified natural products and other compounds with known safety profiles. We identified Propachlor (Figure 1B) as a compound that synergizes with Rad001 to induce cell death in PC cells.
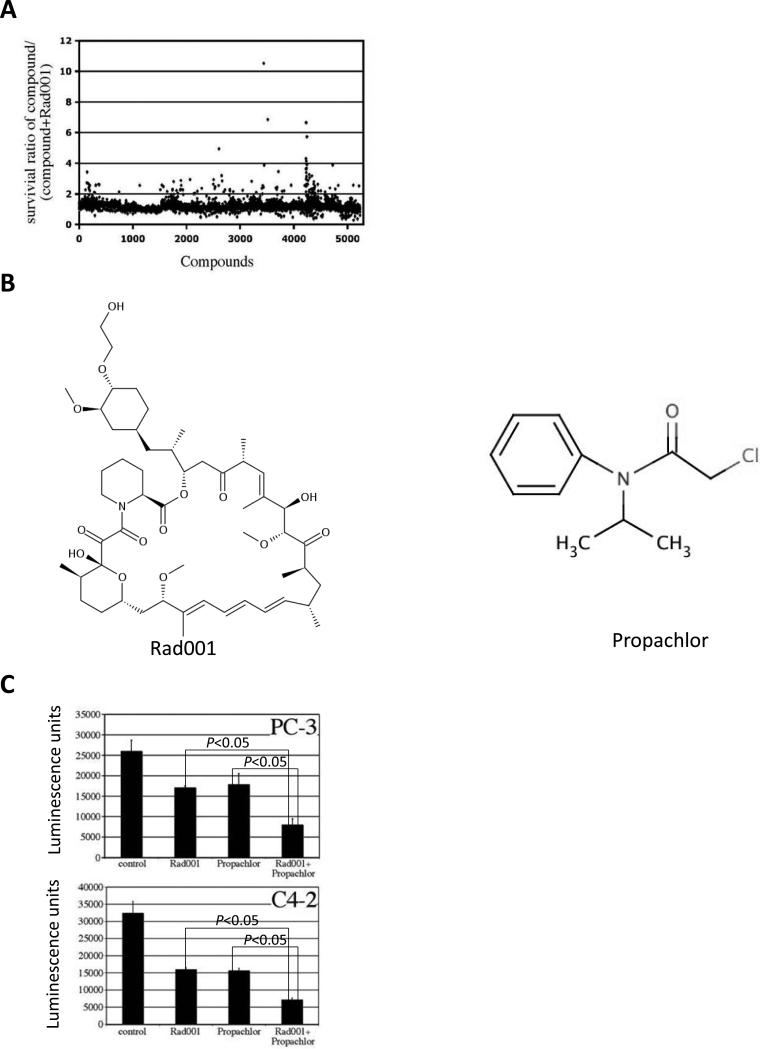
Figure 1.
Identification of Propachlor as a collaborating compound with Rad001. (A) 5000 compounds were added alone or together with 20nM Rad001 in C4-2 cells, the survival ratio gauged by ATP measurement between cells that are treated with single compound or combination with Rad001 is plotted. (B) The chemical structures of Rad001 and Propachlor. (C) Propachlor can collaborate with Rad001 to inhibit PC3 and C4-2 cells. Single compounds or combination with Rad001 were used to treat PC3 or C4-2 cells for 72 hrs followed by Celltiter Glo measurement to measure cell number. The two-way T-test showed P<0.05 between the two groups.
Materials and methods
Materials
PC3 cells were obtained from the American Type Culture Collection (Manassas, VA), and the C4-2 cells were kindly provided by Dr. Lily Wu at UCLA. Both cell lines were passaged for fewer than 6 months after resuscitation. The PC3 cells were tested and authenticated by the ATCC, and no authentication for either cell line was done by the authors. FBS, RPMI medium 1640, Dulbecco's modified Eagle's medium (DMEM), sodium pyruvate, L-glutamine, penicillin, and streptomycin were purchased from Hyclone; Propachlor and Rad001 were from Sigma; CellTiter assay was from Promega (Madison, USA); Caspase-3/7 Assay Kit was from Anaspec; Annexin V apoptosis detection Kit was from eBioscience; Dharmafect transfection reagent was from Thermo Scientific Life Science; Lipofectamine 2000 transfection reagent was from Invitrogen; Beclin1-siRNA (5’-rGrGrArArUrGrGrArArUrGrArrGrArUrUrArA, 5’-rArGrCrArGrCrArUrUrArArUrCrUrCrArUrU) and control-siRNA (5’-rGrArArArArArCrUrCrArUrArUrArArArUrCr, 5’-rGrUrGrGrGrGrCrGrArUrUrUrArUrArUrGrA) were from IDT.; RT-PCR primers for Beclin1, 5’-GGCCAATAAGATGGGTCTGA-3’ and 5’-CTGCACACAGTCCAGGAAAG-3’; for GAPDH 5’-CATGGGTGTGAACCATGAGA-3’ and 5’-CAGTGATGGCATGGACTGTG-3’, were from Valuegene. Rabbit anti-LC-3 polyclonal antibody was from GenScript; rabbit anti-Beclin1, rabbit anti-cleaved Caspase-3 and rabbit anti-PARP1 polyclonal antibodies were from Cell Signaling; rabbit anti- Caspase-3 polyclonal antibody was from Abgent; rabbit anti-Atg5 monoclonal antibody was from Epitomics; mouse anti-β-Actin antibody was from Sigma.
High throughput screen
C4-2 cells were seeded in 384-well plate at 1000 cells/well. After overnight incubation, compounds were delivered to the plates using SAGIAN core system with a Biomek FX equipped with 500 nl pin tool and Rad001 was added to a final concentration of 20nM using a Multidrop 384. The concentration of the compounds from the compound libraries was 10 μM final while the total volume was 50 μL and the DMSO concentration was 1% or less. 96 hours later, cell number was determined using Celltiter Glo (Promega) on a Victor3V (Perkin Elmer) according to the manufacturers’ instructions. The potential hits were identified as those that led to 50% or more reduction of cell number with the combination of Rad001 and the compound than with the compound alone. The hits were further tested in conventional tissue culture settings to verify their synergistic effects with Rad001 in decreasing cell viability.
Cell culture
The PC3 cells were maintained in the Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBXS), Penicillin-Streptomycin, L-glutamine, Na+-pyruvate, and the C4-2 cells were maintained in RPMI 1640 supplemented with 10% FBS and Penicillin-Streptomycin. Cells were grown at 37°C with 5% CO2.
Cell viability, combination index and growth curves
The cells were seeded into the 96-well plate at 3×103 cells per well. After 48 hrs, PC3 cells were treated with DMSO, 0.055 to 0.88 μM Rad001 for 24 hrs, 0.89 to 14.25 μM Propachlor or their combination for 24 hrs. The C4-2 cells were treated with DMSO, 0.041 to 0.65 μM Rad001 for 24 hrs, 0.25 to 4.08 μM Propachlor or their combination for 24 hrs. Three replicates were used for each treatment group. The cell viability/relative cell number was measured using the Promega CellTiter Glo (Madison, USA) assay according to manufacturer's instructions. The compound-interactions were analyzed with Calcusyn software (version2.1, Biosoft) to determine the combination index (CI) for the Rad001 and Propachlor.
Equal numbers of cells were seeded into 96-well plate and maintained with normal medium. After 48 hrs, the PC3 cells were treated with DMSO (control), Rad001 (0.70μM), Propachlor (6.15μM), or their combination, respectively. The C4-2 cells were treated with DMSO (control), Rad001 (0.56μM), Propachlor (7.05μM), or their combination, respectively. The cell viability/relative cell number was measured on day 1, 2, 3, 4 after compound addition using Celltiter Glo. All the experiments were performed in triplicates. The PC3 and C4-2 cells were also treated with compounds for 1 day and analyzed with Trypan Blue staining.
GFP-LC3 analysis
Cells were transfected with GFP-LC3 plasmid using Lipofectamine™ 2000 transfection reagent. After 24hrs, the medium was changed, and the PC3 cells were treated with DMSO (control), Rad001 (0.7μM), Propachlor (6.15μM), or their combination respectively, for one day. The C4-2 cells were treated with DMSO (control), Rad001 (0.56μM), Propachlor (7.05μM), or their combination respectively, for one day. The cells were fixed in 4% paraformaldehyde for 30min, washed twice with PBS and stained with DAPI, and observed under a fluorescence microscope (Eclipse 90i slide scope) with 40x lens.
Protein analysis
The cultured cells were washed with cold PBS and lysed with lysis buffer (20mM KCl, 150mM NaCl, 1% NP-40, 50mM NaF, 50mM TrisHCl, pH7.5, 1mM DTT, 1mM EGTA, 1 X Protease Inhibitor, 10% Glycerol) for 10 min on ice. The cells were centrifuged for 15min at 4°C. The protein concentration in the supernatant was determined with the Bradford assay (Bio-Rad). Equal amount of protein was loaded on 8% or 15% SDS-polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with nonfat dry milk for 1h, incubated with primary antibody in nonfat dry milk overnight, washed with PBS for 30 min, incubated with secondary antibody for 30 min, washed with PBS/0.1%Tween20 for 2 hrs and detected with enhanced chemiluminescence (Pierce).
Caspase-3/7 activity analysis
Equal number of PC3 and C4-2 cells was seeded into the 96 wells plates. After 48 hrs cells were treated the DMSO, Rad001, Propachlor or their combination for 15 hrs. The caspase-3/7 activity was measured by the SensoLyte Homogeneous AMC Caspase-3/7 Assay kit after reaction for 8 hrs. All the experiments were carried out in triplicates.
Analysis of apoptosis
After 15hs treatment, the PC3 and C4-2 cells were collected. The apoptosis was quantified by FACS analysis (BD FACSDiva™ Software v6) with Annexin-V/7-AAD staining following the manufacture's guidelines. The percentage of Annexin V positive cells was analyzed by FlowJo (Version 7.6.4).
Quantitative RT-PCR
The PC3 and C4-2 were treated with compounds for one day and total RNA was purified from the cells with Fermentas Gene RNA purification Kit. Equal amounts of RNA were reverse transcribed by reverse transcriptase (Fermentas) according to the manufacturer's instruction. Quantitative real-time PCR was performed using SA Biosciences RT2 Real-time™ SYBR Kit with the following parameters: 15μl, 95°C for 8min for one cycle followed by 43 cycles of 95°C for 15”/60°C for 60”.
Small interfering RNA transfection
4×103 of cells were seeded into the 96 wells plates and transfected with siRNA (100nM) by the Dharmafect general transfection reagent. The combination of Rad001 and Propachlor was added to the cells for one day after transfection followed by cell viability/relative cell number measurement. To determine the knockdown efficiency, cells were seeded into the 12-wells plate followed by transfection with Beclin1-siRNA and Control-siRNA, respectively, and collected after another 24 hrs.
Beclin1 mRNA half-life analysis
To determine the metabolic stability of Beclin1 mRNA, the C4-2 cells were treated with DMSO, Rad001, Propachlor, and their combination. Actinomycin D was added at 10 μg/ml to inhibit transcription. After 0, 1h, 2h and 4h, cells were collected and washed with PBS, and total RNA was extracted. Relative levels of mRNAs were determined by real-time PCR and normalized to that of GAPDH. All the experiments were performed in triplicates.
Cell cycle analysis
After 24 hours treatment, PC3 and C4-2 cells were harvested, washed with PBS, and fixed in 70% ethanol. After one day fixation, the cells were washed with PBS twice, treated with RNase (50μg/ml) and stained with propidium iodide (PI, 50μg/ml) for 30 min at 37°C. The cell cycle phase distribution was determined by Flow Cytometry (BD FACSDiva™ Software v6). The percentage of cells in each phase was analyzed by FlowJo (Version 7.6.4).
Prostate cancer xenograft
5-6 weeks-old SCID (severe combined immunodeficiency) mice were obtained from the UCLA Division of Laboratory Animal Medicine. All the mice were inoculated with 100μl (50% Matrigel/PBS) of PC3 cells suspension (7×106) to each dorsal flank with 25-gauge syringe. When the tumor size reached between 90 and 100 mm3, the mice were divided into the control, Rad001, Propachlor and combination group, with 6 mice per group. The Rad001 and Propachlor was delivered intraperitonealy 1mg/kg and 5mg/kg daily. The tumor size and mouse weight were measured every four days. These tumor measurements were converted to tumor volume using the formula (V = 0.52×L×W2), where W and L are the smaller and larger diameters. At the 44th day, all mice were sacrificed; tumors were dissected and collected. All the animal experiments were conducted according to the protocol approved by the UCLA Animal Research Committee.
Statistical analysis
The normalized isobologram analysis and bars were performed with Calcusyn software (version 2.1, Biosoft) and Microsoft Excel 2003, respectively. With median effects model described by Chou (7), the multiple compound dose-effect calculations were performed. Combination index (CI) values of <0.9, >0.9 and <1.2, >1.2 was considered as being synergistic, additive, antagonistic, respectively. Statistical analysis was performed by two-sided t test. P value less than 0.05 was regarded to be statistically significant.
The statistical significance of different tumor sizes between each treatment and control group was determined by One-way ANOVA followed by the Dunnett's test. Statistical analyses on body weights were performed by One-way ANOVA followed by Tukey's test. The level of significance was set at P<0.05. Statistical calculations were performed using the SPSS version 13.0.
Results
Identification of compounds that synergizes with Rad001 to inhibit PC cells
Phosphoinositide 3 kinase/AKT/mTOR signaling pathway plays a central role in many human cancers including PC. However, inhibitors of this pathway such as Rad001 have failed to show efficacy for PC as a single agent likely due to activation of compensatory pathways (5). We reasoned that certain chemical compounds may synergize with Rad001 to inhibit multiple pathways thus restore the full therapeutic potential of Rad001, a clinically useful drug with known safety profiles. Therefore, we chose 5,000 compounds available through our screening facility, and conducted a screen in castration-resistant prostate cancer (CRPC) cells using individual compound alone or in combination with Rad001 (Figure 1A). The primary screen yielded a number of compounds that decreased cell numbers by more than 50% in the presence of Rad001 compared to when the compounds were used alone. Several compounds collaborated with Rad001 in reducing cell numbers in secondary and tertiary screens and Propachlor (Figure 1B) was chosen for detailed studies.
Synergy of Rad001 and Propachlor in inhibiting PC cell lines PC3 and C4-2
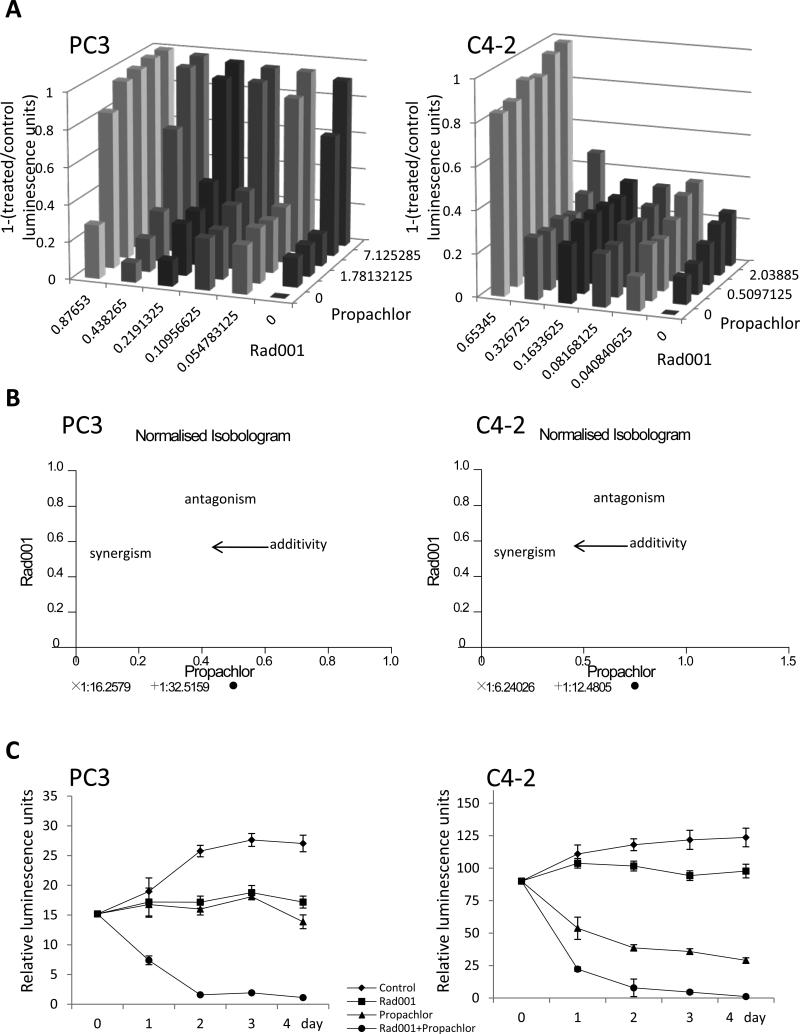
To confirm that Rad001 and Propachlor can synergistically inhibit PC cells, we added each of them at varying concentrations, and monitored the effects in PC3 and C4-2 cells, two PC cell lines that resist androgen withdrawal. As shown in Figure 2A, dose-dependent decrease of cell numbers were seen in both cell lines treated with either Rad001 or Propachlor. For PC3 cells, the ED50 for Propachlor was 6.55μM, and we did not reach ED50 for Rad001 under the experimental conditions. For C4-2 cells, we did not reach ED50 for Propachlor while the ED50 for Rad001 was 0.43μM. However, when the cells were treated with the combination of the two compounds, there was significantly more decrease in cell numbers in both cell lines (Z axis) compared to treatment with either Rad001 or Propachlor alone (Figure 2A). Combination Index (CI), a measure of synergistic activity, was determined with the CalcuSyn software program, which confirmed significant synergism of the two compounds (Figure 2B). For example, for PC3 cells, treatment with 0.44 μM Rad001 or 3.56 μM Propachlor alone resulted in 10.45% and 17.15% reduction in the cell number, respectively. However, when the two compounds were combined, the cell number was reduced by 67.21% (CI<0.9). Mixture-Algebraic estimate analysis, performed with CalcuSyn software, showed a strong synergism for most of the combinations of two compounds at various concentrations for the two cell lines (Supplementary Figures S1 and Supplementary Table S1), suggesting that the synergism is not limited to a particular threshold concentration of either compound. We also determined whether these compounds synergize at different time points post compound addition. As shown in Figure 2C, both PC3 and C4-2 cells exhibited pronounced reduction of cell numbers after combination treatment at multiple time points.
Figure 2.
The combination of Rad001 and Propachlor induces a synergistic reduction of cell number in PC cells. (A) Decreasing doses of Rad001 alone, Propachlor alone and their combination were used and cell numbers measured as in Figure 1 (X-axis: Rad001 μM, Y-axis: Propachlor μM, Z-axis: 1-(treatment luminescence unit/control luminescence). (B) Normalised isobologram analysis showed synergistic interactions in PC3 and C4-2 cells. The analysis was done with CalcuSyn software, which performs the drug does-effect calculation with the median effect method described by Chou TC (7). A large number of combination groups were below the line, indicating synergism. (C) Growth curves by measuring ATP level shows more reduction of cell number when cells were treated with the drug combination.
Combination of Rad001 and Propachlor synergistically induces apoptosis in PC3 and C4-2 cells
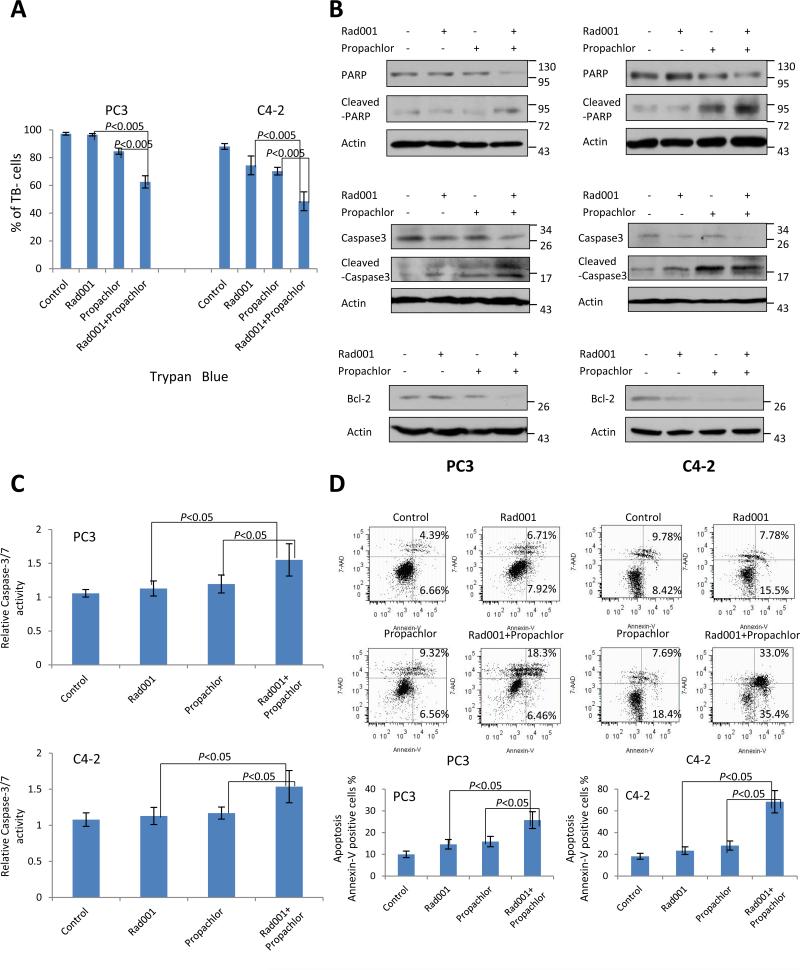
The synergistic reduction of cell number can be the result of a cell-cycle block or an induction of cell death or their combination. We performed a cell-cycle analysis of the cells treated with the compounds but did not detect a significant change in the distribution of cell cycle phases (Supplementary Figure S2). We performed trypan blue staining of the cells after the drug treatment. As shown in Figure 3A, there was a significant decrease in the percentage of cells negative for intracellular blue staining in both PC3 and C4-2 cells compared with single compound treatment (P<0.005), suggesting that the combination treatment resulted in decreased cell viability. This was further confirmed with the semi-quantitative analysis of the abundance of proteins involved in cell apoptosis. As shown in Figure 3B, when PC3 and C4-2 cells were treated with vehicle (DMSO), Rad001, Propachlor or their combination for 15 hrs, there was a significant increase of the cleaved form of apoptosis marker PARP1 (Figure 3B, top panel) (8). This was accompanied by the induction of cleaved Caspase-3 (Figure 3B, middle panel), reduced expression of Bcl-2 (Figure 3B, lower panel) and induced activity of caspase-3/7 (Figure 3C), suggesting that apoptotic pathway was activated in response to the combination treatment. To provide another level of confirmation, we performed flow cytometric analysis of cells stained with annexin V and 7-AAD to examines the population level of apoptotic response to the compounds. As shown in Figure 3D, combination of Rad001 and Propachlor increased the fraction of apoptotic cells significantly more than single treatment as quantified by the percentage of both early and late apoptotic cells in the two cell lines. These data strongly suggest that combination of Rad001 and Propachlor result in synergistic cytotoxicity through activation of the apoptotic pathway.
Figure 3.
Combination of Rad001 and Propachlor synergistically induces cell death in PC cells. PC3 cells were treated with DMSO (control), Rad001 (0.70μM), Propachlor (6.15μM), or their combination, and C4-2 cells were treated with DMSO (control), Rad001 (0.56μM), Propachlor (7.05μM), or their combination. (A) Trypan blue assay. Combination treatment resulted in lower cell viability than either compound alone. (Two-way T-test: P<0.005). (B) Increased levels of cleaved PARP1 and Caspase-3 and decreased Bcl-2 in PC3 and C4-2 cells treated for 15 hrs with the combination of Rad001 and Propachlor. Lysates from cells were separated by SDS-PAGE followed by immunoblot with respective antibodies. (C) Higher activities of Caspase-3/7 in PC3 and C4-2 cells treated for 15 hrs with the drug combination. (D) Flow cytometric analysis of apoptosis with Annexin-V and 7-AAD staining. There was a significant increase in the percentage of apoptotic cell after combination treatment vs single compound (P<0.05).
Combination of Rad001 and Propachlor synergistically induces autophagy in PC3 and C4-2 cells
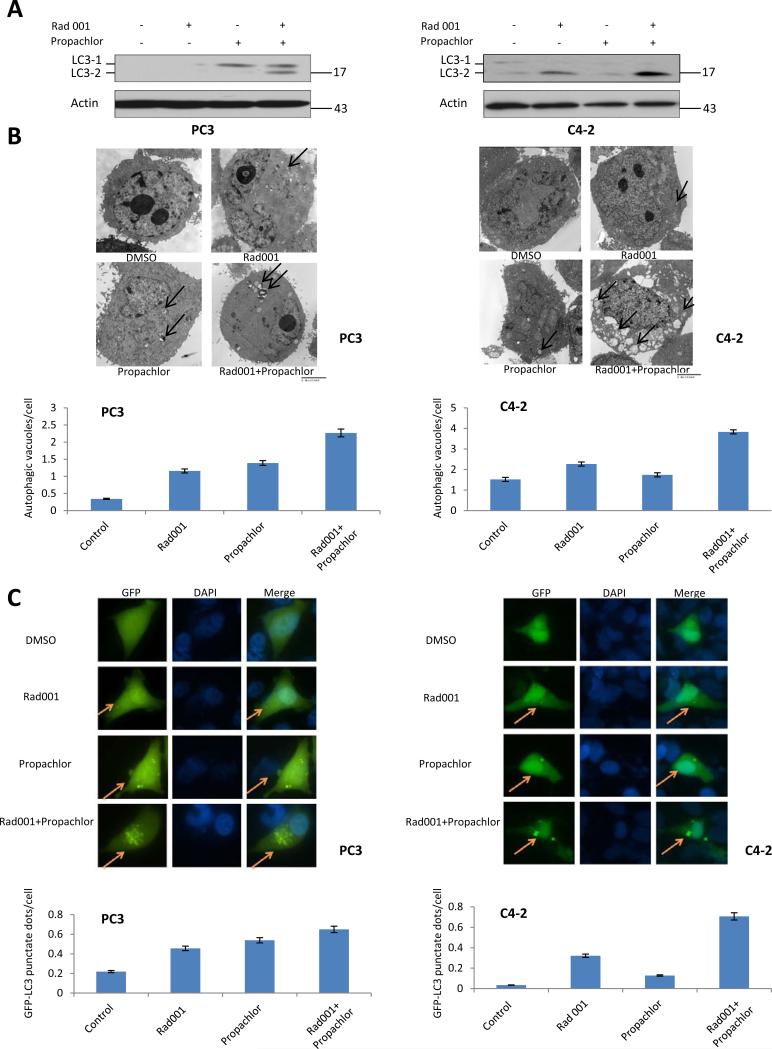
It is important to understand the mechanism by which the combination of the two compounds synergistically activates the apoptotic pathway. Since programmed cell death can be induced by autophagy, we determined if the two compounds synergistically induce autophagy. Enhanced conversion of microtubule-associated protein 1 light chain 3 (LC3-1) to its faster-migrating form LC3-2 is a hallmark of autophagy induction (9). As shown in Figure 4A, there was substantially more LC3-2 conversion after combination treatment compared with Rad001 or Propachlor treatment alone in both PC3 and C4-2 cell lines, suggesting that they can synergistically induce autophagy. We also examined the abundance of autophagosomes induced by the compounds. Autophagosomes are characterized by membranous structures with double or multiple membrane layers. Transmission electron microscopic analysis of the cells treated with various compounds demonstrated that the cells treated with the two compounds in combination had increased number of autophagosomes in comparison to single agent treatment (Figure 4B, upper panels), again confirming that there was a synergistic induction of autophagy. The quantification of this increase is shown in Figure 4B (lower panels).
Figure 4.
Rad001 and Propachlor combination synergistically induces autophagy in PC3 and C4-2 cells. Autophagy was analyzed after 1 day of treatment. PC3 cells were treated with DMSO (control), Rad001 (0.70μM), Propachlor (6.15μM), or their combination, and C4-2 cells were treated with DMSO (control), Rad001 (0.56μM), Propachlor (7.05μM), or their combination. (A) Upregulation of LC3-2 in PC3 and C4-2 cells treated with combination of the two compounds. Immunoblot was performed as in Figure 3. (B) Increased autophagosomes (arrows) were observed by electron microscopy in cells treated with Rad001/Propachlor than each compound alone. Quantified data from 30 cells are shown in the lower panel. (C) Autophagosome analysis through GFP-LC3 expression. C4-2 and PC3 cells expressing GFP-LC3 were treated with DMSO, Rad001, Propachlor, or their combination for 24 hrs. The cells were fixed with paraformaldehyde and visualized with epifluorescence. Yellow arrows indicate the punctate pattern of GFP-LC3, representative of autophagosome, and nuclei were visualized through DAPI staining. The number of GFP-LC3 punctuate dots/cells was quantitated in 50 GFP+ cells from each group (lower panel).
Autophagosomes can also be visualized through the expression of GFP-LC3 which upon autophagosome formation becomes clustered on the membrane vesicles and visible as a ring structure under a fluorescent microscope. Figure 4C (upper panels) demonstrates that there were more punctuate GFP-positive vesicles in response to the combination treatment in both PC3 and C4-2 cells, and the quantification of the results is shown in Figure 4C (lower panels). Consistent with the increase of autophagy in response to the combination treatment, we also found that Atg5-Atg12 conjugate which is involved in the first of the two ubiquitination-like reactions that control autophagy (10) was increased in the two cell lines treated with the two compounds in combination (Figure 5A). Taken together, we conclude that there is a synergistic induction of autophagy in response to the combinatorial treatment of Rad001 and Propachlor, resulting in autophagic cell death.
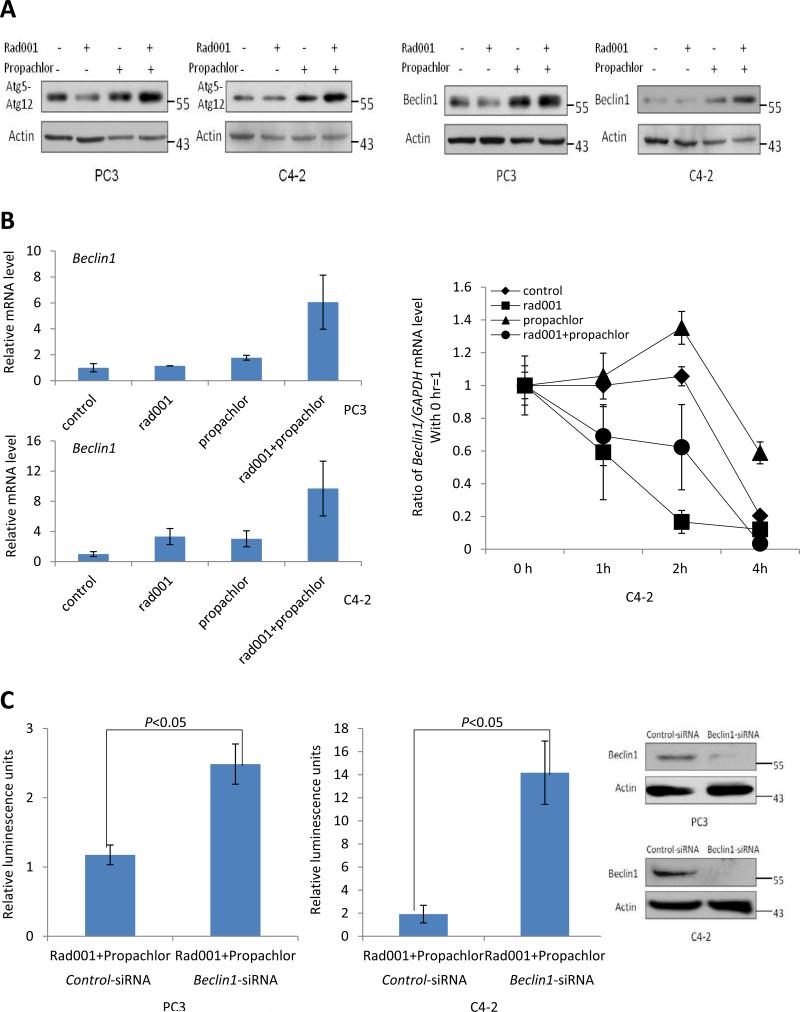
Figure 5.
Regulation of autophagy proteins in response to combination treatment of Rad001 and Propachlor. (A) Atg5-12 conjugates and Beclin1 were induced in response to combination treatment for 24 hrs. Immunoblot was performed as in Figure 3. (B) Beclin 1 mRNA was more significantly up-regulated by the combination treatment as shown by quantitative RT-PCR analyses (left panel). The right panel shows that Beclin1 mRNA stability does not increase in response to the combination treatment. (C) Beclin1 is critical for the autophagic death induced by the combination treatment. PC3 and C4-2 cells were transfected with control siRNA or siRNA for Beclin1 (right panel). Cells were treated with combination of Rad001 and Propachlor 24 hours after transfection. The cell number was determined with ATP measurement 24 hrs after the combination treatment.
Combination of Rad001 and Propachlor increases the expression of Beclin1 which is important for the induced autophagy and apoptosis
Beclin1 (Atg6) is critical for the initiation of autophagy pathway (11). Accordingly, we found that Beclin1 protein levels increased in response to the combination treatment (Figure 5A, right panel). The increase of Beclin1 protein is likely the result of increased mRNA levels of Beclin1 as shown by quantitative RT-PCR analysis (Figure 5B, left panel). To determine whether the increase of mRNA level is the result of enhanced transcription of Beclin1 gene or increased stability of Beclin1 mRNA, we examined the metabolic stability of Beclin1 mRNA by measuring its half-life. As shown in Figure 5B (right panel), Rad001 decreased Beclin1 mRNA stability while Propachlor slightly increased its stability and the combination of two did not increase Beclin1 mRNA stability as compared to the control group, suggesting that the increase of Beclin1 mRNA is likely the result of increased transcription of the gene.
To determine whether the induced autophagy is necessary for the increased cell death induced by the combination treatment, we used siRNA to reduce the expression of Beclin1, and examined whether its loss-of-function impacts on the cell death induced by the combination treatment. As shown in Figure 5C, the Beclin1 level was markedly reduced by siRNA treatment (right panel) and reduction of Beclin1 protein led to a significant rescue of cell death in response to the combination treatment (left and middle panels). These results suggest that synergistic induction of autophagy is likely the underlying mechanism for the increased cell death induced by the two compounds used in combination. Taken together these results suggest that Beclin1 is induced in response to the combination treatment, and the enhanced autophagy plays an essential role in the synergistic induction of cell death.
Rad001 and Propachlor combination inhibits PC xenograft tumor
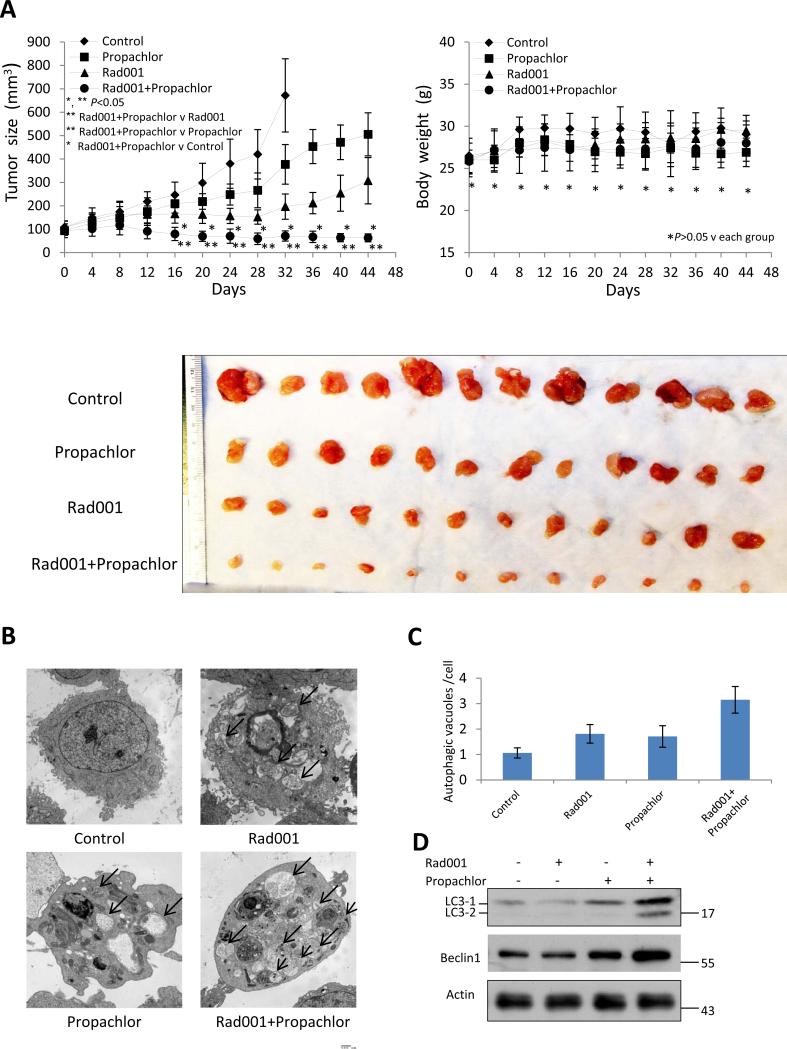
We next determined whether the synergism also exists in a preclinical PC xenograft mouse model. The toxicity of Propachlor is relatively low with LD50 at 550mg/kg (12), so we used it at 5 mg/kg·D-1 while using Rad001 at 1 mg/kg·D-1 which has been commonly reported (13). We initiated tumor with subcutaneous injection of PC3 cells on both flanks of SCID mice. When tumor size reached approximately 100mm3, we started daily intraperitoneal injection of the two compounds and measured tumor size and body weight on every fourth day. As shown in Figure 6A, the combination of Rad001 and Propachlor inhibited the growth of PC3 xenograft tumor significantly more than either compound alone (** P<0.05 compared with one compound administration, * P<0.05 compared with control). In addition, combination treatment showed no adverse effect on body weights (Figure 6A, right panel, P>0.05) and daily activities. No complications such as anaphylaxis and skin necrosis were observed throughout the course of the study. We also examined autophagosome formation in the xenograft tumors by transmission electron microscopy. Consistent with the in vitro results, combination of the two compounds increased autophagosome formation in the xenograft tumors (Figure 6 B-C). In addition, there was a significant increase of LC3-1, LC3-2 and Beclin1 levels in the combination treatment group compared with groups that received either compound alone (Figure 6 D). Therefore we have shown that Propachlor can synergize with Rad001 to induce apoptosis of PC cells through synergistic induction of autophagy in vitro and in vivo, establishing a foundation for potential clinical trials of similar combinations in PC patients.
Figure 6.
Combination of Rad001 and Propachlor significantly inhibits the PC3 xenograft tumors in mice. (A) Anti-tumor activity on PC3 xenografts by various compounds. Mice were administered daily i.p. with Rad001 (1 mg/kg), Propachlor (5 mg/kg) or both (6 mice/group). The tumor sizes (left) and body weights (right) were measured every four days. Tumors treated with two drugs were much smaller than single compound treatment or control. (B) Electron microscopic analysis of the tumors collected from various treatment groups. Arrows indicate autophagosomes. (C) Quantitation of the number of autophagosomes in 30 cells from the xenograft tumors. (D) Immunoblot analysis for LC3 and Beclin 1 from the xenograft tumors.
Discussion
Hormonal therapy has been the main treatment modality for advanced or metastatic PC for decades. However, hormonal therapy, including the newest drugs such as abiraterone and MDV3100, is palliative and nearly all patients will eventually experience tumor recurrence, for which there is no effective therapy. Thus, there is an urgent need to develop novel therapies targeting additional pathways for CRPC. It has been found that androgen enhances mTOR activity in an androgen receptor-dependent manner (14), and active mTOR signaling suppresses autophagy. Autophagosome formation is increased in the rat prostate epithelial cells upon castration (15-16). More recently, it was reported that PC cells can enhance autophagy to survive androgen deprivation treatment (17). Therefore it is likely that autophagy plays an important role in the development of PC, and targeting this pathway may be a novel therapeutic strategy.
Propachlor is a herbicide first marketed by Monsanto in 1965 (18) with well established safety profile. Although this particular compound may or may not be used in patients with PC, its synergy with Rad001 and the underlying mechanism provide us novel therapeutic targets and an opportunity to increase the therapeutic efficacy and reduce the side-effects of existing drugs. In this regard, the recent identification of combination treatment of rapamycin and thapsigargin for ras-driven cancer represents another excellent example (19). Identification of novel compounds that synergize with existing molecules will also expand our understanding of the molecular mechanisms of induced cancer cell death.
The main finding of our study is that combination of Rad001 and Propachlor induce apoptosis of CRPC cells in a synergistic manner, and enhanced autophagy is likely the underlying mechanism. Autophagy or macroautophagy is a major intracellular pathway for degrading and recycling cellular macromolecules, including proteins, ribosomes, and cytoplasmic organelles (20). In normal cells, autophagy functions to maintain cellular homeostasis by disposing of damaged or aged organelles. Autophagy is induced in response to stresses such as nutrient starvation, hormone treatment, chemotherapeutic agents, as well as in pathological conditions, including neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, Huntington's disease, hereditary myopathies, infectious diseases, and cancer (20-23). More recently, it was found that excessive autophagy can lead to cell death, especially in the absence of functional apoptotic molecules such as Bax/Bak (24). This type of cell death is termed the second type programmed cell death (20). Thus, it is conceivable that the induction of autophagy is cell context-dependent, and the extent of autophagy dictates the cellular outcome. At an appropriate level, autophagy protects cells by recycling and disposing of toxic and non-functional cellular proteins and organelles; while excessive autophagy or a particular kind of autophagy may result in damage of the cells, ultimately leading to cell death. As such, agents have been identified that can cause tumor cell death and reduce tumor burden by causing autophagic cell death, suggesting that modulation of autophagy is a new avenue for anti-cancer therapeutics (25-26). In gastric cancer cells, inhibition of Caspase-3 enhances the autophagic cell death, suggesting that inhibition of apoptosis resulted in induction of autophagy (27). For PC, induction of autophagy and caspase-independent apoptosis by arginine deiminase appears to be a novel therapeutic modality (28). It has also been reported that inhibition of autophagy can lead to induction of apoptosis (29). Simultaneous induction of autophagy and apoptosis seems to be responsible for cell death in response to the combination treatment of histone deacetylase inhibitor and the Bcl-2 homology domain-3 mimetic GX15-070 (30). Rad001 induces autophagy but is not potent (31). Since Propachlor can synergize with Rad001 to enhance autophagy and induce cell death in two CRPC cell lines, this may represent a new direction to develop therapies for advanced PC that has failed both traditional (e.g., LH-RH analog, casodex) and newer (e.g., abirateron, MDV3100) forms of hormonal therapy for which no effective treatment exists.
C4-2 was derived from LNCaP cells and has features of castration resistant adenocarcinoma expressing AR and PSA. PC3 cells are negative for AR and PSA but express neuroendocrine markers, similar to human prostate small cell carcinoma (32) which represents the most aggressive form of late stage PC. Although the two cell lines are similarly resistant to androgen deprivation, they appear to have different sensitivities to the agents used in this study which may have clinical implications.
In summary, we have demonstrated that Rad001 and Propachlor can synergize with each other to cause death of CRPC cells, thus providing a novel therapeutic possibility for this incurable disease. Our findings also establish autophagy induction as a novel direction of PC therapy.
Supplementary Material
Acknowledgements
We thank Dr. Hong Zhang for helpful discussions.
Financial Support: J. H. is supported by UCLA SPORE in Prostate Cancer (PI: Robert Reiter), Department of Defense Prostate Cancer Research Program (PC101008), a challenge award from the Prostate Cancer Foundation (PI: O. Witte), a creativity award from the Prostate Cancer Foundation (PI: Matthew Rettig), Cal-Tech-UCLA Joint Center for Translational Medicine Program, and National Cancer Institute (1R01CA158627-01; PI: Leonard Marks).
Grant Support: J. H. is supported by UCLA SPORE in Prostate Cancer (PI: Robert Reiter), Department of Defense Prostate Cancer Research Program (PC101008), a challenge award from the Prostate Cancer Foundation (PI: O. Witte), a creativity award from the Prostate Cancer Foundation (PI: Matthew Rettig), Cal-Tech-UCLA Joint Center for Translational Medicine Program, and National Cancer Institute (1R01CA158627-01; PI: Leonard Marks).
Abbreviations
- PC
Prostate cancer
- SCID
Severe combined immunodeficiency
- CRPC
castration-resistant prostate cancer
Footnotes
Potential conflict of interests: None
References
- 1.Morgan TM, Welty CJ, Vakar-Lopez F, Lin DW, Wright JL. Ductal adenocarcinoma of the prostate: increased mortality risk and decreased serum prostate specific antigen. J Urol. 2010;184:2303–7. doi: 10.1016/j.juro.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–20. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–21. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 5.Carracedo A, Baselga J, Pandolfi PP. Deconstructing feedback-signaling networks to improve anticancer therapy with mTORC1 inhibitors. Cell Cycle. 2008;7:3805–9. doi: 10.4161/cc.7.24.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santiskulvong C, Konecny GE, Fekete M, Chen KY, Karam A, Mulholland D, et al. Dual targeting of phosphoinositide 3-kinase and mammalian target of rapamycin using NVP-BEZ235 as a novel therapeutic approach in human ovarian carcinoma. Clin Cancer Res. 2011;17:2373–84. doi: 10.1158/1078-0432.CCR-10-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 8.Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, et al. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–9. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 9.Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods Mol Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 10.Ravikumar B, Futter M, Jahreiss L, Korolchuk VI, Lichtenberg M, Luo S, et al. Mammalian macroautophagy at a glance. J Cell Sci. 2009;122:1707–11. doi: 10.1242/jcs.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith DM, Patel S, Raffoul F, Haller E, Mills GB, Nanjundan M. Arsenic trioxide induces a beclin-1-independent autophagic pathway via modulation of SnoN/SkiL expression in ovarian carcinoma cells. Cell Death Differ. 2010;17:1867–81. doi: 10.1038/cdd.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. http://sitem.herts.ac.uk/aeru/footprint/en/Reports/543.htm.
- 13.Stelzer MK, Pitot HC, Liem A, Lee D, Kennedy GD, Lambert PF. Rapamycin inhibits anal carcinogenesis in two preclinical animal models. Cancer Prev Res (Phila) 2010;3:1542–51. doi: 10.1158/1940-6207.CAPR-10-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–92. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- 15.Kwong J, Choi HL, Huang Y, Chan FL. Ultrastructural and biochemical observations on the early changes in apoptotic epithelial cells of the rat prostate induced by castration. Cell Tissue Res. 1999;298:123–36. doi: 10.1007/s004419900057. [DOI] [PubMed] [Google Scholar]
- 16.Wilson MJ, Whitaker JN, Sinha AA. Immunocytochemical localization of cathepsin D in rat ventral prostate: evidence for castration-induced expression of cathepsin D in basal cells. Anat Rec. 1991;229:321–33. doi: 10.1002/ar.1092290306. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Jiang X, Liu D, Na Y, Gao GF, Xi Z. Autophagy protects LNCaP cells under androgen deprivation conditions. Autophagy. 2008;4:54–60. doi: 10.4161/auto.5209. [DOI] [PubMed] [Google Scholar]
- 18.Propachlor herbicide residue studies in cabbage using modified analytical procedure. Cornell University. Springerlink. 2009 doi: 10.1007/BF01605479. [DOI] [PubMed] [Google Scholar]
- 19.De Raedt T, Walton Z, Yecies JL, Li D, Chen Y, Malone CF, et al. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell. 2011;20:400–13. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine B. Cell biology: autophagy and cancer. Nature. 2007;446:745–7. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- 21.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–6. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 22.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–5. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–8. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 25.Zhang XQ, Huang XF, Hu XB, Zhan YH, An QX, Yang SM, et al. Apogossypolone, a novel inhibitor of antiapoptotic Bcl-2 family proteins, induces autophagy of PC-3 and LNCaP prostate cancer cells in vitro. Asian J Androl. 2010;12:697–708. doi: 10.1038/aja.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lian J, Wu X, He F, Karnak D, Tang W, Meng Y, et al. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell Death Differ. 2010;18:60–71. doi: 10.1038/cdd.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim MS, Jeong EG, Ahn CH, Kim SS, Lee SH, Yoo NJ. Frameshift mutation of UVRAG, an autophagy-related gene, in gastric carcinomas with microsatellite instability. Hum Pathol. 2008;39:1059–63. doi: 10.1016/j.humpath.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Kim RH, Coates JM, Bowles TL, McNerney GP, Sutcliffe J, Jung JU, et al. Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res. 2009;69:700–8. doi: 10.1158/0008-5472.CAN-08-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–36. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Y, Kadia T, Tong W, Zhang M, Jia Y, Yang H, et al. The combination of a histone deacetylase inhibitor with the BH3-mimetic GX15-070 has synergistic antileukemia activity by activating both apoptosis and autophagy. Autophagy. 2010;6:976–8. doi: 10.4161/auto.6.7.13117. [DOI] [PubMed] [Google Scholar]
- 31.Tsvetkov AS, Miller J, Arrasate M, Wong JS, Pleiss MA, Finkbeiner S. A small-molecule scaffold induces autophagy in primary neurons and protects against toxicity in a Huntington disease model. Proc Natl Acad Sci U S A. 2010;107:16982–7. doi: 10.1073/pnas.1004498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, et al. PC3 is a cell line characteristic of prostatic small cell carcinoma. The Prostate. 2011;71:1668–79. doi: 10.1002/pros.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.