Abstract
Preclinical gene therapy studies both in-vitro and in-vivo require high purity preparations of adeno-associated virus (AAV). Current methods for purification of AAV entail the use of centrifugation over either a CsCl or iodixanol gradient, or the use of chromatography. These methods can be cumbersome and expensive, necessitating ultrahigh speed gradient centrifugation or, for chromatography the use of other expensive equipment. In addition, these methods are time consuming, and the viral yield is not high. Currently no commercial purification kits are available for other than AAV serotype 2. A simplified method was used for the purification of AAV, with a viral yield that is able to be used effectively in adult and embryo mice. The method does not require ultrahigh speed gradient centrifugation nor chromatography. Instead, polyethylene glycol (PEG) / aqueous two-phase partitioning is used to remove soluble proteins from the PEG8000 precipitated virus-protein mixture. The procedure obtained rapidly up to 95% recovery of high quality purified AAV. The entire purification process, including HEK-293 cell transfection, can be completed readily within one week, with purity seemingly higher than that obtained after one round of CsCl gradient purification.
Keywords: AAV8, Aqueous two-phase partition system (ATPs)
1. Introduction
Recombinant adeno-associated virus (AAV) is a non-pathogenic gene therapy method that has been used in clinical trials previously. AAV is a good candidate for clinical use as it provides long-term transgene expression in animal models, it is associated with little toxicity, and has good overall safety profiles in both pre-clinical animal studies and in clinical trials. Many investigators have published the need for a highly purified AAV in large quantity in order to perform pre-clinical and clinical trials (Ayuso et al.; Lock et al.).
Currently, the principal methods for purifying AAV include using CsCl ultrahigh speed density gradient centrifugation or chromatography, with the former being adopted by many investigators. This method, however, is time consuming (Ayuso et al.). Iodixanol (OptiPrep) ultrahigh speed density gradient centrifugation is a similar method developed for AAV purification, with a higher viral recovery compared to the CsCl method, but the Iodixanol reagent is expensive and the method still remains relatively time-consuming; the latter drawback prevents its use for assessing quickly gene expression effects of AAV in in-vivo studies (Klein et al., 2008; Zolotukhin et al., 1999).
The majority of chromatographic purification methods use either ion exchange or affinity based techniques to purify AAV. The latter method uses an antibody against AAV capsid, which therefore recognizes only assembled (serotype specific) particles, which has limited its popularity amongst investigators. Heparin-based affinity chromatography has also been used to purify AAV, a technique based on the property that AAV binds heparin sulphate proteoglycan with high efficiency during AAV infection; however, heparin affinity is known to be serotype specific (AAV 2). During heparin-based chromatography, other proteins present in the cell lysate that have affinity for heparin sulfate co-elute with AAV, and in the case of production of AAV in insect cells, baculovirus may also bind to heparin. This contamination would necessitate at least one additional step to purify further AAV from the eluate of heparin-based chromatography. Thus, both the CsCl or iodixanol ultrahigh speed density gradient centrifugation, as well as chromatography are time consuming and serotype-restricted techniques.
AAV has been shown to have more than ten serotypes that show tropism in-vivo in a tissue dependent manner (Wu et al., 2006). Commercial purification kits are available only for AAV serotype 2. Specifically, a serotype 2 AAV purification kit is the certain commercially available kit on the market. Current chromatography purification methods are not fit for a variety of AAV serotypes, and ultrahigh speed density gradient centrifugation is time consuming and needs expensive equipment (ultrahigh speed centrifuge) that are not always available.
The technique of chemical partitioning in aqueous two-phase systems (ATPs) has been shown to be a powerful method for separating and purifying mixtures of soluble proteins. ATPs can remove undesirable by-products in crude supernatants. These systems are composed of aqueous solutions of either two water-soluble polymers, usually polyethylene glycol (PEG) and dextrin, or else a polymer and a salt, usually PEG and a phosphate or sulfate. Compared with other commonly used separation and purification techniques, ATPs have a number of advantages, such as ease of scale-up, the ability to handle particulate materials, especially for the virus, and the ease of processing. No one has previously reported the use of ATPs to remove soluble bulk proteins from AAV-protein mixtures to further purify AAV.
AAV serotype 8 has been used widely in recent years to transduce multiple organs in animals, but a purification kit is not available commercially. A simple method described in this study can be used in a typical laboratory with only a standard desktop centrifuge, avoiding the need for ultrahigh speed centrifuge or chromatography, to purify AAV serotype 8 and also conventional AAV serotype 2. The method involves PEG8000 precipitation, chloroform treatment, PEG/(NH4)2SO4 aqueous two phase extraction and final dialysis. The whole purification process can be achieved in one week, starting with transfecting HEK293 cells in culture, through to harvesting cells to purify the virus for use in in-vivo studies. This rapid and simple method should be applicable for other serotype AAV purification and can be used to assess quickly the function of a gene or shRNA carried by the AAV vector. The purity of this method is high enough for use in in-vivo studies.
2. Material and Methods
2.1. Chemical Reagents and Equipment
Polyethylenimine (PEI) was purchased from Polysciences. (Warrington, PA). NaCt (sodium citrate), (NH4)2SO4, Na2CO3, 40% PEG1450, 40% PEG4000 and 40% PEG8000 (w/w) solution were purchased from Sigma-Aldrich (St. Louis, MO). Eppendorf 5810R and 5415R desktop centrifuges with cooling system, 37C water bath, sterile cell culture hood and cell culture incubator were used in this study.
2.2. Plasmids
Plasmids used to co-transfect in this study are (1) Vector, pAAV-CMV-ZsGreen plasmid, briefly pAAV-GFP carrying the expression cassette flanked by the viral ITRs (provided by Dr. Bing Wang); (2) a packaging plasmid carrying the serotype 8 or serotype 2 AAV rep and cap genes; and (3) a helper plasmid carrying the adenovirus helper functions was purchased from Applied Viromics, LLC. (Fremont, CA). The transgene used in the experiments was ZsGreen (Zoanthus sp. green fluorescence protein (GFP) under the control of a CMV promoter.
2.3. Cell culture and triple plasmid PEI transfection techniques
On day one HEK293 cell are maintained in DMEM (Cellgro, Mediatech. Manassas, VA) with 10% FBS (Cellgro, Mediatech. Manassas, VA). By day 2, at 80% confluent, the culture medium is changed to 2% FBS that morning before transfection in the evening. For PEI triple plasmid cotransfection of HEK293 cells (the plasmids are pAAV-GFP, pHELP and prep-Cap AAV8 or AAV2), plasmids in the ratio of 2:1:1 are PEI mixed by vortexing, incubated for 15 min at room temperature, and then added dropwise to plates. The PEI:DNA ratio is maintained at 2:1 (weight / weight ).
2.4. Virus harvest and primary treatment
The viral particles were harvested from the culture media of the transfected HEK293 cells. The culture media was centrifuged at 3,000x g for 10 min to pellet the cells and cell debris. Around 320 ml of culture medium from twenty 15cm dishes was taken, clarified and collected.
The cells in culture medium were pooled into one 50 ml conical tube with 5 ml of lysis buffer (50 mM TrisCl per 150 mM NaCl per 2 mM MgCl2, pH 8.0), and AAV particles were released from the cells with three freeze/thaw cycles between dry ice-ethanol and 37 0C water bath. 50 U/ml benzonase and 10 U/ml RNase I were added to the virus-released solution and incubated for 30 minutes at 370C water bath. The surfactant, 0.5% sodium deoxycholate, was then added and incubated for an additional 30 min for further treatment of virus released from the DNase and RNase-treated cells broth. To remove the cell debris, the cells were centrifuged at 2500x g for 10 minutes and the supernatant harvested. For the clarified cell lysate, a stock solution of 40% PEG 8000 (Sigma, St Louis, MO, USA), 2.5 N NaCl, was added to combined clarified cell lysate and culture supernatant to a final concentration of 8%, 0.5N respectively. The solution was then incubated on ice for 1 h, centrifuged at 2000x g for 30 min and the supernatant discarded. The pellet containing the virus was resuspended in a minimal volume of HEPES buffer(Grimm et al., 2003) (10 ml per 300 ml clarified cell lysate input from twenty 15cm dishes).
2.5. Downstream processing and aqueous two phases partitioning
The crude AAV solution was treated with an equal volume of chloroform at room temperature while vigorously vortexing for 2 minutes. The milk-like solution was centrifuged at 370x g using a desktop centrifuge for 5 min, and then the supernatant was allowed to stand for 30 min to evaporate the chloroform. Partitioning in the aqueous two-phase systems was carried out in different salt and PEG combinations. The phase system was prepared from stock solutions of PEG (50%, w/w), NaCt (20% w/w), (NH4)2SO4 (20%, w/w) and Na2CO3 (20%, w/w). The pH was measured after addition of the rough AAV. Stock solution was stored at 4°C. Before use, the temperature of the stock solution was equilibrated to room temperature. PEG/NaCt or PEG/sulfate or PEG/Na2CO3 two phase mixture was added to the supernatant and vortexed vigorously for two minutes at room temperature, and then left for 15 to 30 minutes at room temperature. For prompt partitioning of the soluble protein in the rough AAV, the two phase mixture was centrifuged at 3000x g for 15 min at room temperature. The phase volume ratios were determined in graduated centrifuge tubes. The proteins either salted out in precipitates at the bottom along with the unprecipitated virus, or else partitioned into the top PEG phase and inter-phase. The clear bottom phase containing virus was withdrawn carefully with a needle. Samples of the top and bottom phases were then assayed for concentration of virus and total protein. The bottom phase containing virus was transferred to a dialysis cassette (Slide-A-Lyser Dialysis Cassette)or dialysis spin tube (50K MWCO, Amicon) for removal of salt. Final concentrated AAV was stored in PBS or MEM medium with 0.001% pluronic F68. Usually, high concentration of stock virus should be kept in PBS or MEM medium with pluronic. Pluronic F68 can prevent AAV aggregation during storage in freezer.
For control, the first generation cesium chloride (CsCl) gradient-based protocol was used (Rose et al., 1966). Briefly, rough AAV solution was mixed with an equal volume of chloroform and centrifuged at 370x g for 5 minutes. The aqueous phase was then placed on top of CsCl gradient solution and centrifuged at 200,000x g using a SW-41 rotor in a Beckman ultracentrifuge for 40 hours (Rose et al., 1966).
2.6. SDS-PAGE and silver staining and Western blotting
For SDS-PAGE, wherever necessary, AAV was dialyzed to reduce the salt concentration in the sample. The samples were boiled for 5 minutes and electrophoresed on 12% SDS acrylamide gel for 2.5h at 100V under standard buffer conditions and visualized by Pierce silver staining kit followed the manufacture’s protocol (Pierce Biotechology, Rockford, IL).
The purified AAV8-GFP and AAV2-GFP as well as the top portion from the 10%PEG8000-13.2% (NH4)2SO4 (w/w) partitioning were used for Western blotting to identify the AAV capsids. Equal amount of proteins were loaded on a 12%SDS-PAGE and transferred to a nylon membrane. Blots were probed with mouse anti-AAV capsids antibody (American Research Products, Belmont, MA, USA) overnight at 4 °C. After extensive washing and incubation with rabbit anti-mouse secondary antibody conjugated to horseradish peroxidase, the membrane was developed using chemiluminescent reagents (Denville Scientific, South Plainfield, NY 07080) and exposed to an X-ray film.
2.7. Virus titration and gene transfer assay in vitro
The titer of dialyzed samples containing AAV was determined by dot blot. The samples were diluted in 100μl sterile ddH2O and boiled for 10 min, then chilled quickly on ice. An equal volume of freshly made 1 M NaOH was added to the samples. The solution was incubated at room temperature for 20 minutes, and then applied to the dot blot apparatus according to the manufacturer’s instructions. The virus particles were incubated with the membrane at room temperature for 10 min and then a vacuum applied to the dot blot apparatus to draw the solution through the membrane. Hybridization was performed using ExpressHyb hybridization solution (Clontech) according to the manufacturer’s protocol. The blot was developed using ECL blotting determination system (Pierce Biotechology, Rockford, IL). The dot blot signal intensity was quantified using the software Gel Pro Analyzer 4 (Media Cybernetics)
Virus gene transfer assay was done in-vitro in a 12-well culture plate with HEK293 cells plated at 0.5×10 6 cells/ml density . 1ul of the dialyzed AAV-GFP samples were added to each well. For counting cells infected with purified AAV-GFP, the first generation method purified AAVGFP were used as control. At 24h post-infection, GFP fluorescence of cells was visualized under Zeiss microscope with 525nm filter. The cells were then trypsinized, centrifuged and resuspended in 0.5ml Dulbecco’s PBS. The cells were then fixed in 2% paraformaldehyde and sorted by fluorescence activated cell sorting (FACS) technique using a flow cytometer (FACS, FACS Aria flow cytometer; BD, Franklin Lakes, NJ,USA). More than 10,000 cells gated on forward and side scattering were analyzed per sample. From the percentage of infected cells along with the cell count after 24h of infection and the dilution factor, the infective viral particles were calculated upon three repeated experiments.
2.8. AAV-GFP gene transfer assay in-vivo
All animal procedures were performed with prior approval of the University of Pittsburgh Institutional Animal Care and Use Committee (protocol number: 1103211). Viral gene transfer in-vivo into adult CD1 mice was performed at 6 to 10 weeks of age. Virus was inoculated at a dose of ~1.2×1012 genome-containing particles (gcp) of AAV-GFP in 200ul saline, a dose sufficient to transduce approximately 40-60% of the hepatocytes in the liver by a single tail vein injection with a 29-gauge stainless steel needle. A group of mice injected with same dose of CsCl purified AAV-GFP were used as controls. Five days post-injection the mice were killed and freshly harvested mouse livers were soaked in 4% PFA, thereafter the livers were examined by histofluorescence imaging. Livers were placed in the view platform of an Olympus SZX12 dissection microscope and imaged with GFP filter set by SPOT software (version 4.6, Diagnostic Instruments).
Viral gene transfer was also performed in-utero into embryonic mice on day 13 of gestation under ultrasound-guided embryonic transuterine intra cardiac microinjection. Four embryos in one mother were used as an experimental group. Briefly, intra cardiac microinjection was carried out in-utero after anesthetizing time-pregnant mice using inhaled isoflurane and performing midline laparotomy to access the uterine horn. A single uterine saccule was brought out through the midline incision and evaluated using high resolution ultrasound bio-microscopy (Vevo770, Visualsomics). The embryonic heart was visualized using ultrasound, and then an intra cardiac transuterine injection performed using the associated Vevo770 microinjector. The 13-day embryonic heart was injected with 2.5ul of AAV serotype 8 virus solution. The embryonic heart was then allowed to pump the AAV throughout the embryo for five minutes while observing for possible bleeding and contractile dysfunction, and then the embryo was re-implanted into mother’s abdomen, the abdomen was closed, and the animal recovered. The embryos were harvested on E17.5 and the GFP expression in liver was assessed and compared with same dosage of CsCl purified AAV-GFP used in controls(Tulachan et al., 2007).
2.9. GFP positive Cell counting
The AAV was purified by the PEG/sulfate partitioning or CsCl protocol and then injected into CD1 adult mouse through tail vein. Five days after the injection, the mouse liver was snap-frozen in liquid nitrogen, sectioned and stained. The number of GFP-positive cells in the adult CD1 mouse liver, (randomly chose five areas of GFP and DAPI positive cells) was counted at a high-power field (×200).
2.10. Statistical analysis
All results are given as the mean ± standard deviation. Means between PEG/Salt and Chloroform or PEG/Salt and CsCl purified virus were compared using Students’ t-test. Differences were considered statistically significant when the p-value was <0.05.
3. Results
AAV serotype 8-ZsGreen virus, which carries the green fluorescent protein (GFP) transgene (AAV8-GFP) was produced by triple plasmid co-transfection with the PEI chemical transfection reagent method (Lock et al.). The treatments were combined with benzonase, RNase A and 0.5% sodium deoxycholate in a 37°C water bath for 1 hr. Benzonase and RNase were used in low concentration (50U/ml and10ug/ml respectively) for nuclease treatment of AAV8-GFP virus released from cells. Sodium deoxycholate, which can be removed by dialysis, was used to enhance AAV release from the transfected HEK293 cells and gave the best yield.
Since the PEG8000/NaCl precipitated virus contained significant additional contaminated protein, including hydrophobic proteins released by the lysis of cells with sodium deoxycholate, the initial treatment of the precipitate included chloroform extraction, since AAV is resistant to chloroform. This extraction was followed by re-dissolving the PEG8000/NaCl precipitated virus in HEPES buffer. The rough virus mixture was mixed with an equal volume of chloroform and vigorously vortexed. The milky mixture separated quickly into aqua and chloroform phases. After centrifugation the white color was removed from the top aqueous phase.
Tris buffer had to be excluded from the buffer solution as even small traces of Tris can be highly toxic in in vivo experiments. HEPES buffer was used in our aqueous two phase partition (Table 1). HEPES and PBS buffers were compared for the aqueous two phase partition. The HEPES buffer is stronger in ion strength than PBS (data not show), dissolves PEG8000/NaCl precipitate virus more rapidly, and can keep buffered pH more stable (Table 1).
Table 1. effects of polymer/salt combinations and pH on two phase formation and virus recovery.
The aqueous two-phase partitioning of AAV was done at room temperature. Ten milliliter of HEPES buffer with dissolved crude AAV was extracted with equal volume of chloroform for two minutes, then separated by centrifugation and the aqueous phases carefully collected and pooled. This supernatant, referred to as “rough virus”, was used for the partitioning experiments. The amount of AAV present in 10 ml of this supernatant was 2.2 ×10 11 ±3.5×1010 gcp ml−1 by dot blot. Virus was not detectable in the top phase.
| Phase composition | Buffer | pH of Mixture | R(%) |
|---|---|---|---|
| 10%PEG1450-10% NaCt (pH5.2) | HEPES(pH=8.0) | 5.4 | 151.3±2.05* |
| 10%PEG4000-10% NaCt (pH5.2) | HEPES(pH=8.0) | 5.4 | 150.4±2.75* |
| 10%PEG8000-10% NaCt (pH5.2) | HEPES(pH=8.0) | 5.4 | 148.9±1.67* |
| 10%PEG1450-10%(NH4)2SO4) (pH6.5) | HEPES(pH=8.0) | 8.0 | 150.7±2.65* |
| 10%PEG 4000-10%(NH4)2SO4 (pH6.5) | HEPES(pH=8.0) | 8.0 | 145.7±2.35* |
| 20%PEG 4000-10%(NH4)2SO4 (pH6.5) | HEPES(pH=8.0) | 8.0 | 96.9±0.43 |
| 10%PEG 8000-10% (NH4)2SO4 (pH6.5) | HEPES(pH=8.0) | 8.0 | 97.8±0.55 |
| 10%PEG 8000-13.2% (NH4)2SO4 (pH6.5) | HEPES(pH=8.0) | 8.0 | 95.8±0.34 |
| 10%PEG1450-10%Na2CO3(pH12.0) | HEPES(pH=8.0) | 12.0 | 97.8±0.69 |
| 10%PEG 4000-10% Na2CO3 (pH12.0) | HEPES(pH=8.0) | 12.0 | 97.6±0.67 |
| 10%PEG 8000-10% Na2CO3 (pH12.0) | HEPES(pH=8.0) | 12.0 | 95.5±0.24 |
Bulk proteins were salted out and precipitated at the bottom, but two phases did not form. R: recovery ratio, measured as volume change before and after aqueous two-phase partitioning.
AAV Phase partitioning used an aqueous two phase system containing PEG (1450, 4000, or 8000) plus either NaCt, (NH4)2SO4 or Na2CO3 at 25°C, and at pH 5.4, 8.0 or 12. The rough or impure virus solutions treated with (NH4)2SO4 (10% w/w), Na2CO3 (10% w/w) or 10% PEG1450, 10%PEG4000 or 10%PEG8000 partitioning were analyzed for phase formation, protein concentration (Table 2) and recovery (Table 1). The 10%PEG1450-10%NaCt (w/w), 10%PEG4000-10%NaCt (w/w) and 10% PEG8000-10% NaCt (w/w), 10%PEG1450-10% (NH4)2SO4 (w/w) and 10%PEG4000-10% (NH4)2SO4 (w/w) in HEPES (pH=8.0) did not form two phases well, and had significant salted-out protein seen after centrifugation. The two phases formed well with re-adding the 10%PEG1450-10% (NH4)2SO4 (w/w) or 10%PEG4000-10% (NH4)2SO4 (w/w) solution. This secondary partitioning was performed in those situations where solubility decreased in the presence of the high concentration of PEG1450 or PEG4000 and (NH4)2SO4. This secondary step 10%PEG1450-10% (NH4)2SO4 (w/w) partitioning also caused the virus to enter the top PEG phase, which led to a decrease in viral recovery. In the latter step, protein concentration or removal was not improved (data not shown). The addition of 10%PEG8000-10% (NH4)2SO4 (w/w) in the impure virus formed two phases quickly, and moreover the bulk proteins were extracted and precipitated after centrifugation. Generally, protein solubility is increased by increasing pH. The partitioning of different molecular weight PEG with Na2CO3 (10% w/w) was assessed for the ability to remove bulk protein from rough virus. The rough virus was added to three molecular PEG combination solutions with Na2CO3 (pH=12.0). Once two phases formed and the bulk proteins were extracted into the PEG top phase and interface, no precipitates were observed after centrifugation. The bulk proteins removed from rough virus were assayed and compared between groups (Table 2). The purity of these partitioning purified viruses was assessed by SDS-PAGE gel silver staining (Figure 3). 10%PEG1450-10%Na2CO3 (w/w) provided more protein removal compared to 10%PEG4000-10%Na2CO3 (w/w) and 10%PEG8000-10%Na2CO3 (w/w), but less viral recovery ( Table 2). AAV2 is the first adeno-associated virus that was applied in clinical trial among a variety of the viral serotypes. The protocol in this study describes AAV8-GFP being successfully purified by PEG8000/(NH4)2SO4 aqueous partition. To determine whether AAV serotype 2 can be purified by this method, pAAV-GFP vector was co-transfected with AAV serotype 2 vector and helper vector plasmid DNA into HEK293 cells and purified with 10%PEG8000/13.2%(NH4)2SO4 partition.
Table 2. UV absorbance of denatured AAV-GFP vector.
All aqueous two-phase treatments were carried out in PEG/(NH4)2SO4 (pH=8.0) or PEG/ Na2CO3 (pH=12.0), and virus was dissolved in pH=8.0 HEPES buffer. Spectrophotometries of A260/A280 were taken for quantification of AAV particles. For spectrophotometry, an aliquot of AAV-GFP at dot blot-detected concentration was denatured in 0.1% SDS at 75°C for 10 minutes. The absorbance was measured in a spectrophotometer at 260 nm and 280 nm. The expected absorbance ratio for highly purified AAV particle is 1.44.
| Treatment | A260 | A280 | A260/A280 | gcp/ml(dot blot) |
|---|---|---|---|---|
| Chloroform | 0.431±0.0098 | 0.597±0.0037 | 0.721 | 2.2 ×10 11 ±3.5×1010 |
| 20%PEG4000- 10%(NH4)2SO4 |
0.367±0.0077 | 0.264±0.0048 | 1.391 | 5.2 ×10 12 ±2.6×1011 |
| 10%PEG8000- 10%(NH4)2SO4 |
0.391±0.0097 | 0.280±0.0065 | 1.398 | 1.1×10 12 ±5.5×1010 |
|
*10%PEG8000- 13.2%(NH4)2SO4 |
0.381±0.0087 | 0.268±0.0065 | 1.421 | 5.9×10 12 ±5.8×1011 |
| 10%PEG1450- 10%Na2CO3 |
0.277±0.0067 | 0.199±0.0057 | 1.394 | 1.8×10 12 ±4.7×1011 |
| 10%PEG4000- 10%Na2CO3 |
0.364±0.0034 | 0.265±0.0067 | 1.373 | 4.4×10 12 ±6.5×1011 |
| 10%PEG8000- 10%Na2CO3 |
0.287±0.0031 | 0.207±0.0069 | 1.385 | 4.6×10 12 ±4.6×1011 |
| CsCl Purified | 0.284±0.0089 | 0.214±0.0078 | 1.329 | 5.2×10 12 ±1.5×1011 |
P<0.005
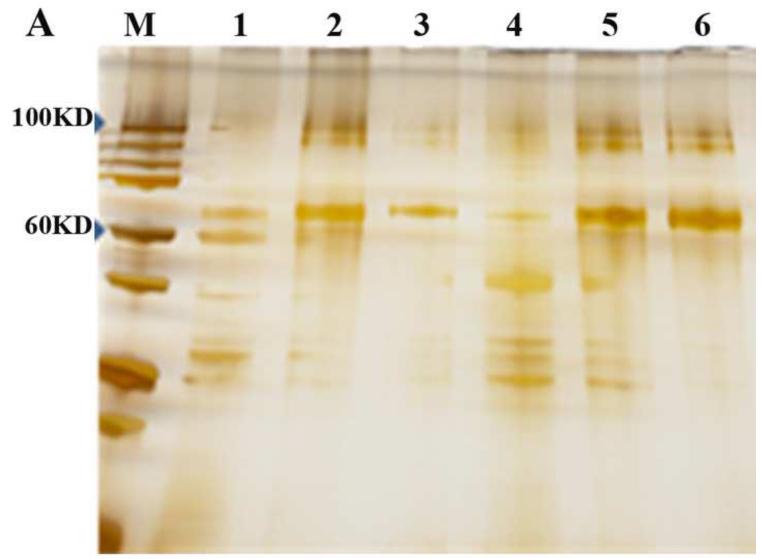
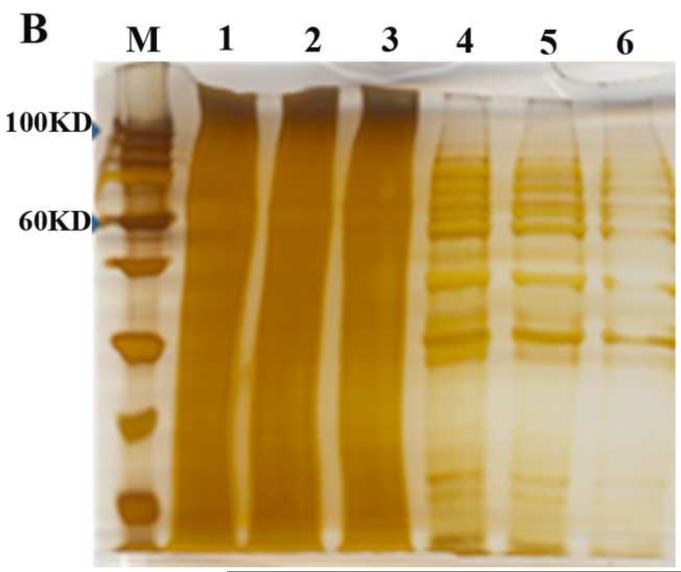
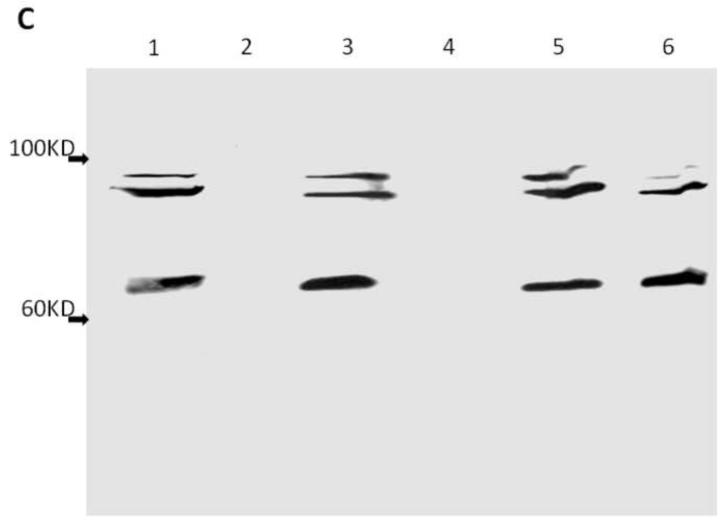
Figure 3. Silver staining of SDS-PAGE gel of purified AAV and Western Blotting identification.
Figure 3A. M, protein standard marker ( Stepview™ 10kD Prestained Protein MW Standard.); Lane1, 2 and 3 are PEG1450/Na2CO3, PEG4000/Na2CO3 and PEG8000/Na2CO3 APTs purified AAV, respectively; Lane 4,5 and 6 are 20%PEG4000-10% (NH4)2SO4, 10%PEG8000-10% (NH4)2SO4 and 10% PEG8000-13.2% (NH4)2SO4 purified AAV, respectively. Figure 3B. Lane 1,2 and 3 are triplicates of chloroform-treated rough AAV; Lane 4,5 and 6 are conventional CsCl gradient density centrifugation purified AAV. Figure 3C. Western blot of serotype 8 and serotype 2 AAV-GFP purified by 10%PEG8000-13.2 % (NH4)2SO4 partitioning. Lane1 and 3 are 10%PEG8000-13.2 % (NH4)2SO4 ATP partitioning purified serotype 8 and serotype 2 AAV-GFP; lane 2 and 4 are top portion of ATP partition, lane 5 and 6 are CsCl purified serotype 8 and serotype 2 AAV-GFP, respectively.
Purified virus on the Western blot showed the expected capsids bands (figure 3C). The SDS-PAGE and silver staining confirmed the purity of the virus purification using the protocol described earlier (figure 3A).
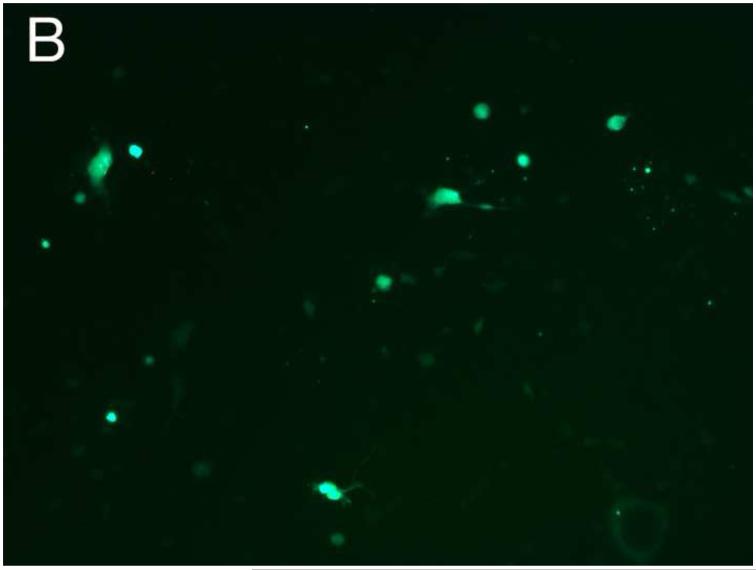
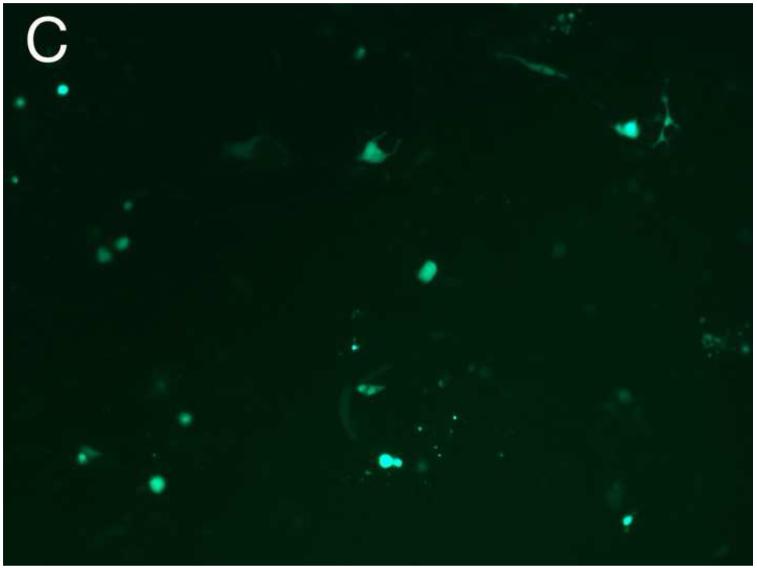
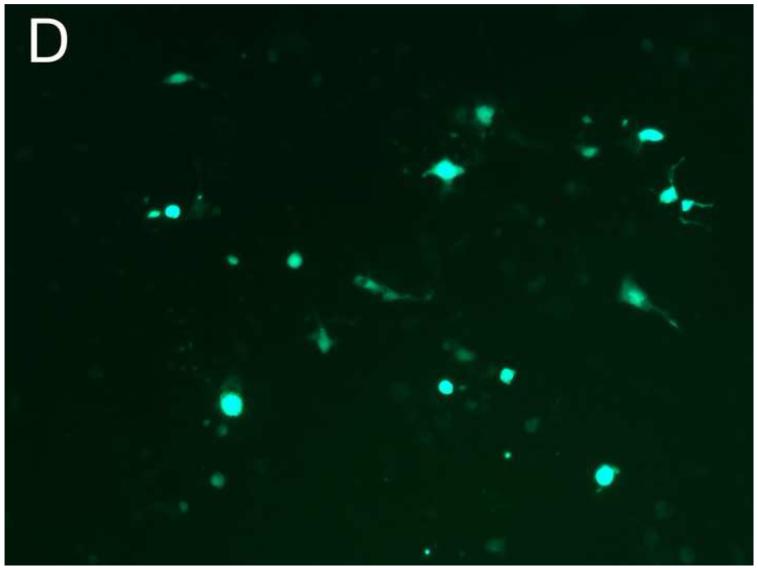
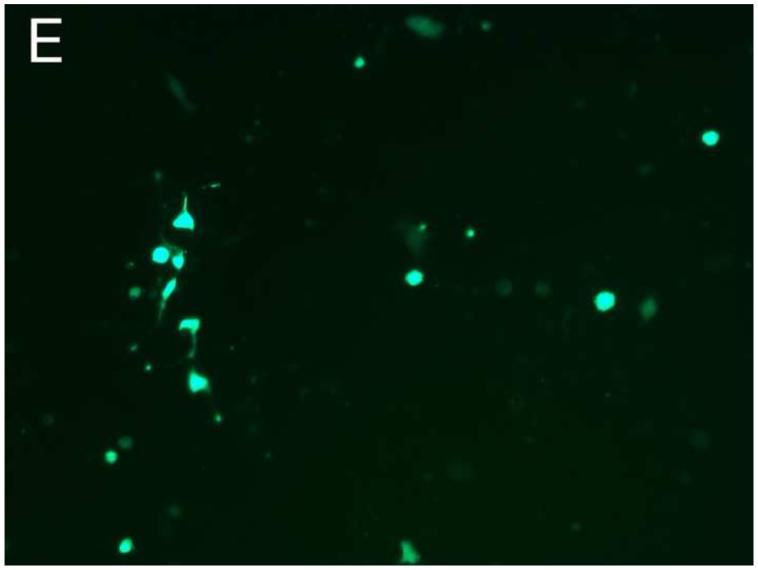
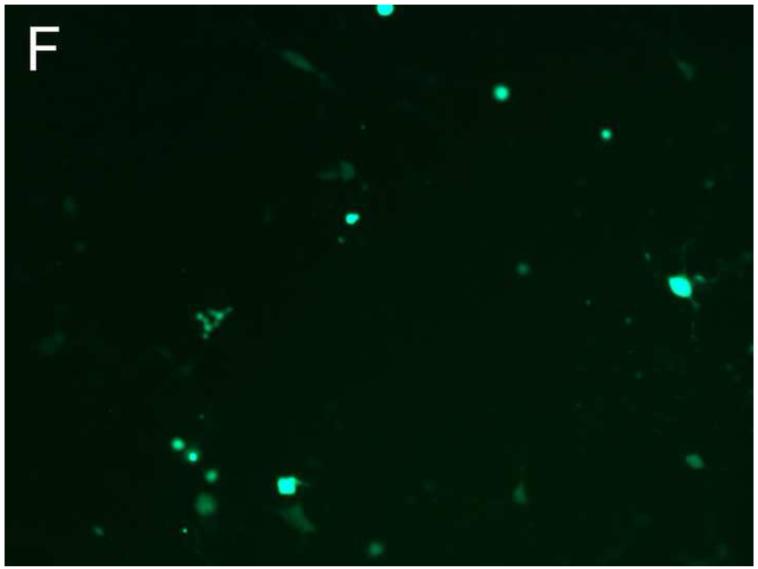
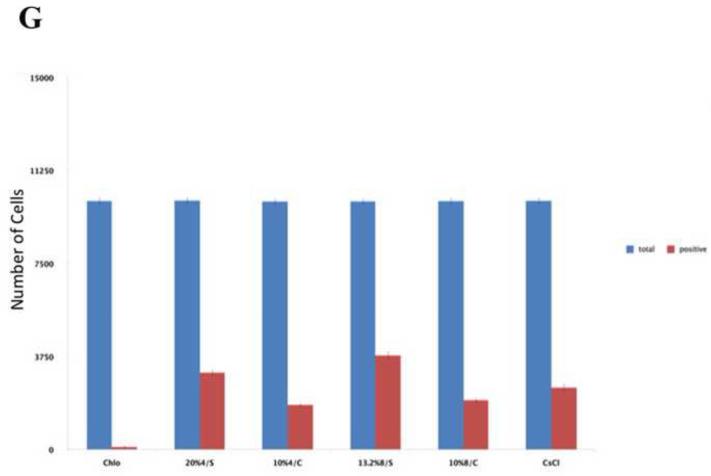
Different PEG/salt partitioning purified AAV8-GFP virus infection efficiencies were assayed using HEK293 cells (Figure 4). Chloroform-treated rough virus was used as a control to compare with the different PEG/salt partitioning purified AAV8-GFP virus solutions. The infective efficiency of 20%PEG4000-10% (NH4)2SO4 (w/w), 10%PEG8000- 10%Na2CO3 (w/w), 10%PEG4000-10%Na2CO3 (w/w) were similar. The 10% PEG8000-13.2% (NH4)2SO4 (w/w) partitioning purified virus showed significantly higher infectivity in the HEK293 cells (Figure 4D).
Figure 4. Transduction efficiency in vitro.
HEK 293 cells were infected for 24h with AAVGFP either as a rough virus (Chloroform treated, Chlo) (A), or purified with 20%PEG4000-10% (NH4)2SO4, (20%4/S) (B); 10%PEG4000-10%Na2CO3, (10%4/C) (C); 10%PEG8000-13.2% (NH4)2SO4, (13.2%8/S) (D), 10%PEG8000-10%Na2CO3, (10%8/C) (E); or CsCl purified (F). 24hr infection efficiency was assessed through green fluorescent sorting by FACS (G).
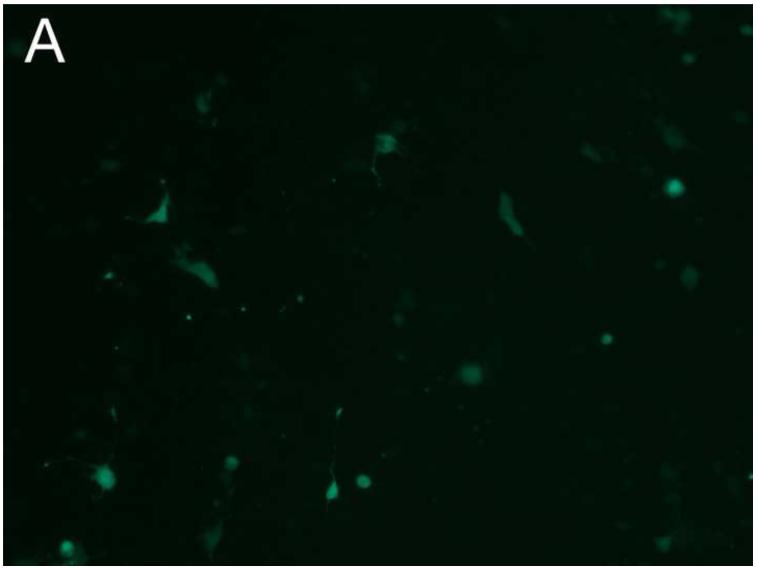
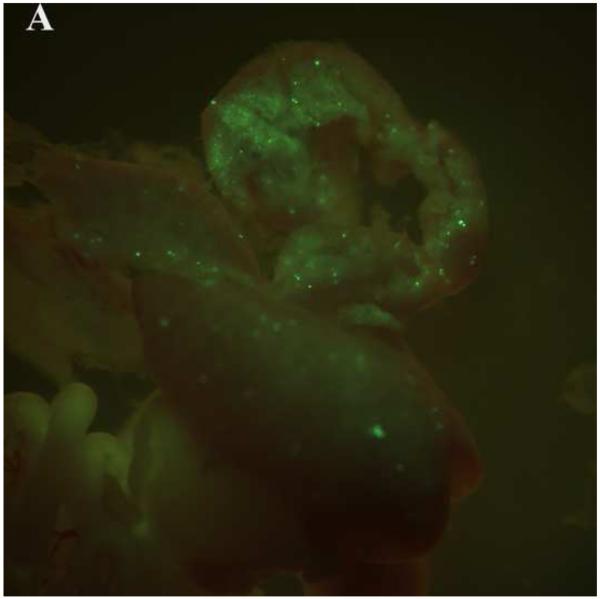
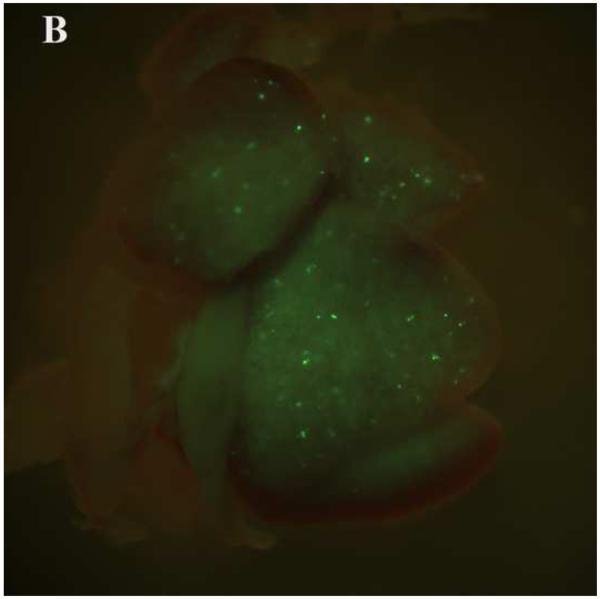
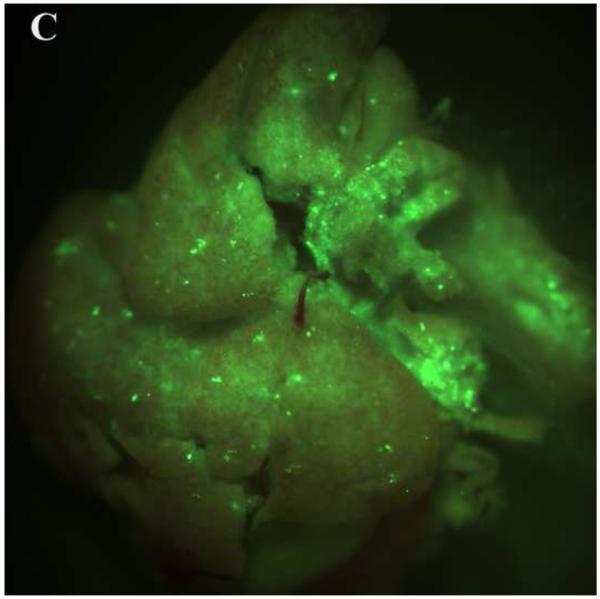
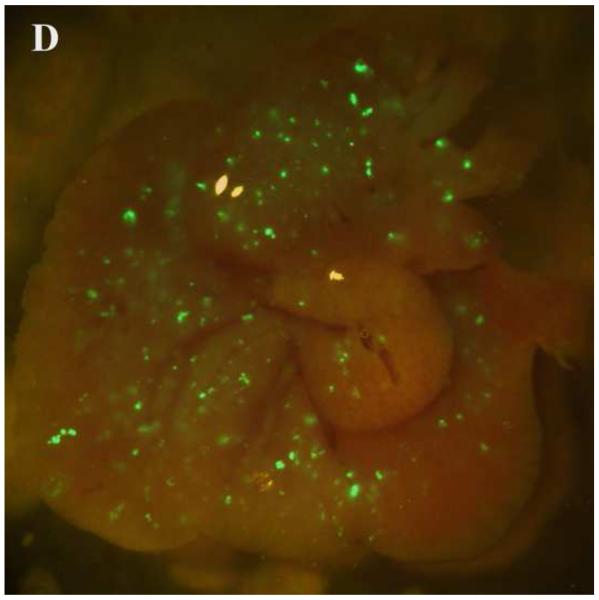
PEG/salt partitioning purified AAV8-GFP virus solutions were assayed for in vivo infectivity using 6 to 8 week old CD1 mice by a single tail vein injection at a dose of 1.2× 1011 −2.4× 1011particles, a dose potentially sufficient to transduce approximately 30-40% of hepatocytes in the adult mouse liver. For assessing infective efficiency in-utero in embryos, day 13 CD1 mouse embryos were injected (via transuterine intra cardiac injection, unpublished data, Figure 5) with 2.5ul of the different PEG/salt partitioning purified AAV8-GFP virus. The transduction of 10%PEG8000-13.2% (NH4)2SO4 (w/w) partitioning purified virus revealed better transduction rates, both for adult and embryonic mouse liver, when compared to transduction using CsCl purified virus.
Figure 5. Transduction of AAV-GFP in vivo.
Transduction of GFP in embryonic and adult mouse livers three days post injection with the aqueous two phase purified AAV-GFP by 10%PEG8000/13.2% (NH4)2SO4 (A) and CsCl purified control (B) in embryonic mouse livers; 10%PEG8000/13.2% (NH4)2SO4 (C) and CsCl purified control in adult mouse liver (D).
The purity was confirmed using the same protocol used to purify AAV2-GFP, Western blotting and silver staining. However, when injected into the mouse, the transduction of purified AAV2-GFP was weaker in the liver but stronger in the skeletal muscular tissue (Supplementary Figure1, 2).
4. Discussion
AAV serotype 8 has been reported to infect efficiently brain, liver, skeletal muscle, cardiac muscle and pancreas (Wang et al., 2006; Zincarelli et al., 2008). Our aqueous two-phase extraction method has the ability to extract AAV serotype 8 from lysed cells as a single process, delivering a near-purified AAV. Thus, this technology is potentially an attractive alternative to traditional AAV purification with CsCl density gradient ultrahigh speed centrifugation and chromatography. The first-generation CsCl-based protocol (Rose et al., 1966) was used as a control in this study. However, CsCl gradient centrifugation can be both time consuming and expensive. The modified three-step CsCl gradient centrifugation purification of AAV has shown highest purity compared to one round CsCl gradient centrifugation(Klein et al., 2008). The first-generation CsCl gradient centrifugation that required minimal time was used to compare with our method.
Thus, it was demonstrated that an aqueous two-phase system can be chosen to optimize for recovery of AAV away from both hydrophobic and hydrophilic bulk proteins that are released when AAV is released from lysed transfected HEK293 cells.
The presence of contaminating helper virus proteins in AAV vector preparations is particularly undesirable because the contaminating proteins may increase the immunogenicity of the AAV vector preparations when used in-vivo. Hence, the use of triple-plasmid transfection for AAV production was adopted to prevent helper viral proteins from being produced in the HEK293 cells (Matsushita et al., 1998). Generally, the empty viral capsids are formed in assembly procedure of vectors. However, the optimized ratio of plasmid was determined to be 1.2 :1:1 of pAAV-GFP, pHELP and prep-Cap AAV8 or AAV2 for transfection of HEK293 cells, after which there was little formation of empty viral capsid ( Supplementary Figure3).
Development of an aqueous two-phase partitioning system for AAV purification has been limited by an inability to characterize the effect of purification parameters on the viral yield and infectivity. In these sets of experiments, the first step, in which we utilized an 8%PEG8000/0.5N NaCl precipitation, which eliminates bulk proteins from the lysate of HEK293 cells (Grimm et al., 2003), the amount of co-precipitated protein was substantially reduced compared to traditional methods. This reduction in contaminated insoluble protein is critical for the next step of the aqueous two-phase partition. The second step, using chloroform extraction, was adopted to eliminate hydrophobic proteins prior to the aqueous two-phase partitioning. In this step the more hydrophobic proteins were readily removed by the chloroform.
Liquid-liquid partitioning with PEG/salt aqueous two-phase system was used to selectively extract AAV from PEG8000/NaCl precipitated rough virus in HEPES buffer. Several salt combinations were treated in order to find the suitable conditions for extraction. The data showed that many factors influenced the partitioning behavior of AAV in ATPs, making this system quite versatile.
In the aqueous two-phase partitioning, there are several factors which affect AAV partitioning such as the type of salt component, pH, PEG molecular weight, and amount of sample loading. AAV tolerates pH change well (pH 5.4 - 12 in our experiments), with no effect on its infectivity in HEK293 cells (Figure 4). The components of the salt solution in our experiments included NaCt, (NH4)2SO4 or Na2CO3, each of which affected the two-phase formation (Table 1). For example, with NaCt at pH5.4, the two-phase partition cannot form, and the proteins salted out. Or: 10% (NH4)2SO4 cannot form two phases with PEG1450 or PEG4000, respectively, in HEPES buffer(pH8.0).
PEG/salt aqueous phase formation can be influenced by many factors including polymer concentration, molecular weight and temperature. Generally, the partition phase volume ratio (volume of top phase/volume of bottom phase) changes with PEG molecular weight. The partitioning of total protein also depends on the PEG molecular weight. The ability of PEG to alter the solubility of proteins increased with increasing PEG molecular weight, so proteins in lower molecular weight PEG were partitioned to the bottom phase (salt phase). For example, 10%PEG4000-10%Na2CO3 (w/w) gave less protein removal compared to 10%PEG8000-10% (NH4)2SO4 or 10%PEG8000-10%Na2CO3 (w/w) ( Table 2). In some instances, unexplained results were observed, such as with PEG/Na2CO3, pH12. Thus, the behavior of PEG/Na2CO3 partitioning needs more experimental study. The protocol described in this study can be used for purification of AAV2-GFP. In-vivo experiments with the purified AAV2-GFP virus has shown GFP expression in the skeletal muscle and somewhat weaker expression in the liver.
CONCLUSION
The optimal condition for aqueous two-phase partitioning to purify the AAV8-GFP in these experiments, i.e. 10%PEG8000-13.2 (NH4)2SO4 at pH8.0 in HEPES buffer, yielded a purity even higher than conventional CsCl gradient density centrifugation methods (figure 3A), and a higher efficiency of infection in-vivo (Figure 5). In addition, the infection in embryonic mouse organs demonstrated that the PEG8000/(NH4)2SO4 aqueous two-phase partitioning purified AAV is not toxic to the embryos, and is pure enough for in-vivo studies in fragile mouse embryos.
Supplementary Material
Supplementary Figure 1. Transduction of serotype 2 AAV-GFP in vivo. Transduction of GFP in adult mouse skeletal muscle five days post injection with the aqueous two phase purified AAV2-GFP by 10%PEG8000/13.2% (NH4)2SO4.
Supplementary Figure 2. Comparison of AAV8-GFP and AAV2-GFP infection in vivo. Transduction of GFP in adult mouse liver five days post injection with AAV8-GFP(A,B) and AAV2-GFP(C,D) by CsCl purified control (A),10%PEG8000/13.2% (NH4)2SO4 (B) and CsCl purified control(C), 10%PEG8000/13.2% (NH4)2SO4 (D) in adult mouse liver. five days infection efficiency was assessed by counting of GFP-positive cells (E).
Supplementary Figure 3. Determination of empty viral particles by TEM The purified AAV8-GFP samples were negatively stained with uranyl acetate. Viral particles were viewed at 200000X magnification with a transmission electron microscope (TEM). Empty particles in five fields of the sample grids were counted. 19.0 ± 5.2 SEM percent empty particles were found in the ATPs purified virus.
Highlights.
The optimal condition for aqueous two-phase partitioning to purify AAV, i.e. 10%PEG8000-13.2 (NH4)2SO4 at pH8.0 in HEPES buffer, yielded a purity even higher than conventional CsCl gradient density centrifugation methods, and a higher efficiency of infection in vivo. In addition, the infection in embryonic mouse organs demonstrated that the PEG8000/(NH4)2SO4 aqueous two-phase partitioning purified AAV is not toxic to the embryos, and is sufficiently pure for in vivo studies in mouse embryos. The method does not require ultrahigh speed gradient centrifugation nor chromatography.
Figure 1. Flow chart of purification protocol.
The diagram illustrates the steps in AAV purification that were investigated in this study.
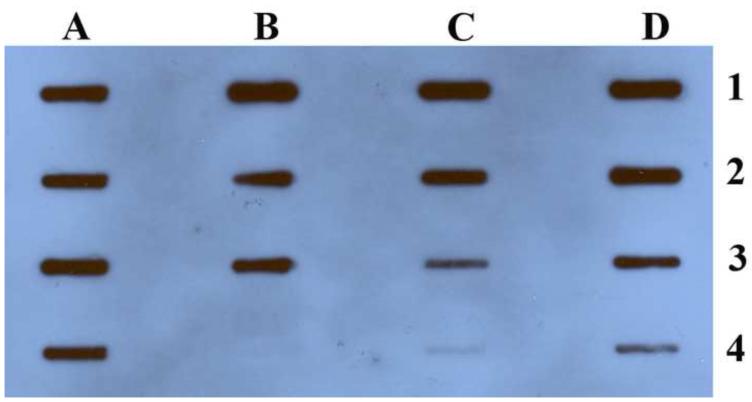
Figure 2. Titer determination of two-phase partitioning purified AAV-GFP by dot blot.
A1 to A3 stet repeated 10%PEG8000-13.2%(NH4)2SO4 (pH8.0) ATPs purified virus; A4 is chloroform treated rough virus; B1 to 3 are stet repeated 10% PEG8000-10%(NH4)2SO4 (pH8.0) ATPs purified virus; C1 to 3 are three-times repeated 20%PEG4000-13.2(NH4)2SO4 (pH8.0) ATPs purified virus; B4 and C4 are virus in the top PEG fraction; D1,2,3 and 4 are serial dilutions (10 times diluted in PBS) of CsCl purified AAV, starting at 5.2×10 12 ±1.5×1011.
Acknowledgements
We would like to thank Sean-Paul Williams and Jessica Thomas for their assistant with animal work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions P.G. designed and performed the experiments, analyzed data and wrote the manuscript. G.G. viewed the manuscript and finalized the manuscript. J.P. performed embryonic intra cardiac microinjection. K.P. and Y.G. viewed and corrected the manuscript.
Competing financial interests The authors declare no competing financial interests.
References
- Ayuso E, Mingozzi F, Montane J, Leon X, Anguela XM, Haurigot V, Edmonson SA, Africa L, Zhou S, High KA, Bosch F, Wright JF. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 17:503–10. doi: 10.1038/gt.2009.157. [DOI] [PubMed] [Google Scholar]
- Grimm D, Zhou S, Nakai H, Thomas CE, Storm TA, Fuess S, Matsushita T, Allen J, Surosky R, Lochrie M, Meuse L, McClelland A, Colosi P, Kay MA. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003;102:2412–9. doi: 10.1182/blood-2003-02-0495. [DOI] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Tatom JB, Henderson KM, Henning PP. AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol Ther. 2008;16:89–96. doi: 10.1038/sj.mt.6300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock M, Alvira M, Vandenberghe LH, Samanta A, Toelen J, Debyser Z, Wilson JM. Rapid, Simple and Versatile Manufacturing of Recombinant Adeno-Associated Virus Vectors at Scale. Hum Gene Ther. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock M, Alvira M, Vandenberghe LH, Samanta A, Toelen J, Debyser Z, Wilson JM. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther. 21:1259–71. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, Iwaki Y, Colosi P. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–45. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- Rose JA, Hoggan MD, Shatkin AJ. Nucleic acid from an adeno-associated virus: chemical and physical studies. Proc Natl Acad Sci U S A. 1966;56:86–92. doi: 10.1073/pnas.56.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulachan SS, Tei E, Hembree M, Crisera C, Prasadan K, Koizumi M, Shah S, Guo P, Bottinger E, Gittes GK. TGF-beta isoform signaling regulates secondary transition and mesenchymal-induced endocrine development in the embryonic mouse pancreas. Dev Biol. 2007;305:508–21. doi: 10.1016/j.ydbio.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Rehman KK, Bertera S, Zhang J, Chen C, Papworth G, Watkins S, Trucco M, Robbins PD, Li J, Xiao X. Widespread and stable pancreatic gene transfer by adeno-associated virus vectors via different routes. Diabetes. 2006;55:875–84. doi: 10.2337/diabetes.55.04.06.db05-0927. [DOI] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–27. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–80. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–85. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Transduction of serotype 2 AAV-GFP in vivo. Transduction of GFP in adult mouse skeletal muscle five days post injection with the aqueous two phase purified AAV2-GFP by 10%PEG8000/13.2% (NH4)2SO4.
Supplementary Figure 2. Comparison of AAV8-GFP and AAV2-GFP infection in vivo. Transduction of GFP in adult mouse liver five days post injection with AAV8-GFP(A,B) and AAV2-GFP(C,D) by CsCl purified control (A),10%PEG8000/13.2% (NH4)2SO4 (B) and CsCl purified control(C), 10%PEG8000/13.2% (NH4)2SO4 (D) in adult mouse liver. five days infection efficiency was assessed by counting of GFP-positive cells (E).
Supplementary Figure 3. Determination of empty viral particles by TEM The purified AAV8-GFP samples were negatively stained with uranyl acetate. Viral particles were viewed at 200000X magnification with a transmission electron microscope (TEM). Empty particles in five fields of the sample grids were counted. 19.0 ± 5.2 SEM percent empty particles were found in the ATPs purified virus.