Abstract
Neuroblastoma is a solid tumor that mostly occurs in children. Malignant neuroblastomas have poor prognosis because conventional chemotherapeutic agents are hardly effective. Survivin, which is highly expressed in some malignant neuroblastomas, plays a significant role in inhibiting differentiation and apoptosis and promoting cell proliferation, invasion, and angiogenesis. We examined consequences of survivin knockdown by survivin short hairpin RNA (shRNA) plasmid and then treatment with (−)-epigallocatechin-3-gallate (EGCG), a green tea flavonoid, in malignant neuroblastoma cells. Our Western blotting and laser scanning confocal immunofluorescence microscopy showed that survivin was highly expressed in malignant neuroblastoma SK-N-BE2 and SH-SY5Y cell lines and slightly in SK-N-DZ cell line. Expression of survivin was very faint in malignant neuroblastoma IMR32 cell line. We transfected SK-N-BE2 and SH-SY-5Y cells with survivin shRNA, treated with EGCG, and confirmed knockdown of survivin at mRNA and protein levels. Survivin knockdown induced morphological features of neuronal differentiation, as we observed following in situ methylene blue staining. Combination of survivin shRNA and EGCG promoted neuronal differentiation biochemically by increases in expression of NFP, NSE, and e-cadherin and also decreases in expression of Notch-1, ID2, hTERT, and PCNA. Our in situ Wright staining and Annexin V-FITC/PI staining showed that combination therapy was highly effective in inducing, respectively, morphological and biochemical features of apoptosis. Apoptosis occurred with activation of caspase-8 and cleavage of Bid to tBid, increase in Bax:Bcl-2 ratio, mitochondrial release of cytochrome c, and increases in expression and activity of calpain and caspase-3. Combination therapy decreased migration of cells through matrigel and inhibited proliferative (p-Akt and NF-κB), invasive (MMP-2 and MMP-9), and angiogenic (VEGF and b-FGF) factors. Also, in vitro network formation ability of cells was significantly inhibited by survivin silencing and completely by combination of survivin silencing and EGCG treatment. Collectively, survivin silencing potentiated anti-cancer effects of EGCG in human malignant neuroblastoma cells having survivin overexpression.
Keywords: Angiogenesis; Apoptosis; Differentiation; (−)-Epigallocatechin-3-Gallate; Neuroblastoma, Survivin shRNA
Introduction
Neuroblastoma is an extracranial, heterogeneous, and malignant solid tumor of the sympathetic nervous system in children and it accounts for approximately 15% of pediatric cancer deaths in the United States [1]. Despite multimodal therapeutic approaches including chemotherapy, radionuclide therapy, and immunotherapy, the survival rate for patients with malignant neuroblastoma remains poor. Newer therapeutic strategies are urgently warranted for successful treatment this devastating childhood malignancy.
Survivin is the smallest member of inhibitor-of-apoptosis (IAP) gene family in mammalian cells that has attracted attention of basic and translational research scientists. The survivin gene is alternatively spliced extensively to generate several protein isoforms [2]. The expression of survivin is transcriptionally controlled in a cell cycle-dependent manner and the expression of protein peaks at G2/M phase of cell cycle [3]. Survivin protein functions as a key regulator of mitosis and programmed cell death or apoptosis [4]. The role of survivin in the pathogenesis of cancer is not limited to the inhibition of apoptosis but also in the regulation of the mitotic spindle checkpoint and the promotion of angiogenesis and chemoresistance [4]. Survivin is highly expressed in most human tumors and fetal tissue, but it is completely absent in terminally differentiated cells [5]. Tumors that highly express survivin generally bear a poor prognosis and are associated with resistance to radiation and chemotherapy [6]. Survivin gene expression is transcriptionally repressed by wild-type p53 and can be deregulated in cancers by several mechanisms, including gene amplification, hypomethylation, increased promoter activity, and loss of p53 function [7, 8]. Therefore, survivin is an ideal target for killing tumor cells specifically and leaving the normal cells unaffected.
Flavonoids are polyphenolic compounds that are ubiquitous in plants. The role of dietary flavonoids in cancer prevention is widely recognized [9]. Epigallocatechin-3-gallate (EGCG), a major polyphenol found in green tea, is a widely studied chemopreventive agent that has shown potential anti-cancer actions [9]. The anti-tumor mechanism of EGCG in culture is due to modulation of the expression of key molecules in cell cycle progression, inhibition of the inflammatory molecule nuclear factor-kappaB (NF-κB), binding to Fas, and activation of mitogen-activated protein kinase cascade [10]. There are increasing evidence demonstrating that EGCG alone or in combination with another compound can be beneficial in curbing the growth of various cancers including prostate cancer [11], breast cancer [12], and pancreatic cancer [13]. We previously reported that EGCG induced cell death in neuroblastoma SH-SY5Y cells by activation of the receptor and mitochondria mediated pathways [9].
The purpose of this investigation was to induce apoptosis via the knockdown of survivin using shRNA plasmid and simultaneous EGCG treatment in two neuroblastoma cell lines, SK-N-BE2 and SH-SY-5Y, and to examine whether such a combination therapy could induce apoptosis and cell differentiation, and inhibit cell migration, angiogenesis, and network formation. The present study demonstrated that knockdown of survivin in combination of EGCG treatment controlled the growth of neuroblastoma cells (SK-N-BE2 and SH-SY-5Y) by modulating the expression of the molecules involved cell differentiation apoptosis, migration, invasion, and angiogenesis.
Experimental procedures
Cell culture conditions
The human malignant neuroblastoma SK-N-DZ (wild-type p53), SK-N-BE2 (mutant p53), SH-SY5Y (wild-type p53), and IMR32 (wild-type p53) cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA). SK-N-DZ and IMR32 cell lines were maintained in DMEM while SK-N-BE2 and SH-SY5Y cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Atlanta Biological, Atlanta, GA) and 1% antibiotics in a humidified incubator containing 5% CO2 at 37°C. Survivin shRNA plasmid and control shRNA plasmid (encoding scrambled shRNA sequence) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). EGCG (Sigma–Aldrich, St. Louis, MO) was dissolved in dimethyl sulfoxide (DMSO) to make stock solutions and aliquots were stored at −20°C until ready to use.
Immunofluorescence microscopy to examine expression of survivin in neuroblastoma cell lines
Laser scanning confocal immunofluorescence microscopy was performed according to a previously described method [14]. Briefly, cells were grown on poly-D-lysine camber slides (BD Biosciences, Bedford, MA) at 37°C in presence of 5% CO2 for 24 h. Cells in the slide were washed twice in PBS (without Ca2+and Mg2+) followed by fixation in 4% formaldehyde/phosphate-buffered saline (PBS) for 30 min at room temperature. After fixation cells were washed in PBS and made permeable with 0.25% Triton X-100/PBS for 10 min. Cells were washed and non-specific binding were blocked by 3% bovine serum albumin (BSA) in PBS for 10 min. Primary IgG antibody against survivin was diluted (1:200) in 1% BSA/PBS. After the incubation with primary antibody for 1 h at room temperature cells were washed twice with PBS and stained with fluorescein isothocyanate (FITC) conjugated anti-rabbit secondary IgG antibody (Jackson Immunoresearch, West Grove, PA) in 1% BSA/PBS for 1 h at room temperature and washed again with PBS. To counter stain the nucleus, cells were then incubated with 300 nM DAPI (Invitogen, Eugene, OR) for 5 min and washed four times before mounting cells with Dabco 33-LV (Sigma, St. Louis, MO). Fluorescence images were captured using Zeiss LSM 510 META confocal microscopy and analyzed by Zeiss LSM image browser software.
Flow cytometry to monitor the expression of survivin in neuroblastoma cell lines
Adherent cells were detached from culture flasks by incubating in 0.02% EDTA/PBS (Sigma–Aldrich, St. Louis, MO) and then dissociated into monodispersed cells. Cells (1×105 cells/sample) were collected and suspended in 100 μl 1× flow cytometry buffer (1% BSA and 0.1% NaN3 in PBS, filtered through 0.22 μm filter unit) containing an FcBlocker (eBioscience, San Diego, CA) and incubated at 4°C for 15 min. Then, primary IgG antibody againt survivin (1:200 dilution) was added to the cell suspension. Parallel staining was also performed with rabbit IgG antibody (1:200) (Jackson Immunoresearch, West Grove, PA), as isotype matched control for survivin. After incubation at 4°C for 30 min, cells were washed twice with the 1× flow cytometry buffer. Cells were then incubated in 100 μl 1× flow cytometry buffer containing FITC-conjugated goat anti-rabbit secondary IgG antibody (1:200) at 4°C for 30 min. Subsequently, cells were washed as above, fixed in 2% paraformaldehyde in PBS and then transferred into a 5-ml polystyrene round-bottom tube capped with a cell-strainer 183 cap (BD Biosciences, Franklin Lakes, NJ). Antibody-bound cells were then analyzed in a Calibur flow cytometer using CellQuest software (BD Biosciences, Bedford, MA), and FlowJo software (Tree Star, Ashland, OR).
Transfection with survivin shRNA plasmid and treatment with EGCG
SK-N-BE2 and SH-SY-5Y cells were grown at 37°C in 5% CO2 and full-humidity. Both cell lines were transfected with mammalian expression vector carrying the survivin shRNA plasmid or scrambled shRNA plasmid, treated with 50 μM EGCG, or in combination of survivin shRNA plasmid and 50 μM EGCG in medium containing 2% FBS. The concentration of EGCG was chosen according to our previous study [15]. The cells were transfected with 1 μg plasmid in 6-well dishes using Lipofectamine 2000 transfection reagent and incubated at 37°C in 5% CO2. After 6 h, transfection reagent was exchanged with fresh medium containing 2%FBS. After 18 h, the medium were replaced with fresh medium or medium containing 50 μM EGCG and incubated for another 24 h. The transfection efficiency was measured by counting the percentage of green-fluorescent cells.
Reverse transcription-polymerase chain reaction (RT-PCR) to examine survivin mRNA
The RT-PCR experiments were conducted to monitor the knockdown of expression of survivin mRNA after treatment with the survivin shRNA. SK-N-BE2 and SH-SY-5Y cells were grown at 37°C in 5% CO2. Both cell lines were transfected with mammalian expression vector carrying the survivin shRNA plasmid or scrambled shRNA plasmid, treated with 50 μM EGCG, or in combination of survivin shRNA plasmid and 50 μM EGCG in medium containing 2% FBS. Total RNA was isolated from the cells (1×106 cells/sample) using TRIZOL Reagent (Invitrogen, Carlsbad, CA). We used the following primer sequences for PCR amplifications of survivin (forward: 5'- CAT TCA AGA ACT GGC CCT TC -3' and reverse: 5'- CTA AGA CAT TGC TAA GGG GC -3') and GAPDH (forward: 5'-ATG GGG AAG GTG AAG GTC GG-3' and reverse: 5'-AGA CGC CAG TGG ACT CCA CGA CG-3') genes. The cDNA was synthesized using SuperScript one-step RT-PCR kit (Invitrogen, Carlsbad, CA) on a PCR cycler (Eppendorf, Westbury, NY) at 50°C for 30 min followed by 30 cycles of amplification (denaturation at 94°C for 15 s, annealing at 55°C for 30 s and extension at 72°C for 1 m) and final extension 72°C for 10 min. The RT-PCR products were resolved by electrophoresis on 1% agarose gels, stained with ethidium bromide (1 μg/ml), and visualized using a UV chamber (Alpha Innotech, San Leandro, CA). Expression of GAPDH was used as an internal standard.
In situ methylene blue staining to examine morphology of neuronal differentiation
After the transfection with mammalian expression vector carrying the survivin shRNA plasmid or scrambled shRNA plasmid, cells were cultured in 6-well dishes for 3 days. The transfected cells were washed twice with ice-cold PBS, in the culture plate followed by fixation of cells with 2 ml of ice-cold 95% (v/v) ethanol 5 min. After fixation, we aspirated ethanol, washed the cells twice with PBS, and then stained with 2 ml of ice-cold 0.2% (v/v) methylene blue solution (prepared in 50% ethanol) for 20 sec. After staining, the cells were washed with distilled water and air dried before taking photograph under the microscope. The dimensions of the cells (length and width) and length of neurite were measured (n = 20) using ImagePro Plus software version 4.5.1.29 (Media Cybernetics, Silver Spring, MD).
In situ Wright staining for the detection of morphological features of apoptosis
After the treatments, both adherent and non-adherent cells were spun down at 3,500 rpm for 10 min. The cells were washed with PBS and then fixed and stained HEMA 3 stain set according to the manufacturer's instruction (Fisher Scientific, Kalamazoo, MI). Cells were allowed to dry after the staining and the morphological features of the cells were observed under the light microscope and pictures were taken. All the experiments were conducted in triplicates and only representative pictures were presented.
Annexin V staining and flow cytometry for detection of a biochemical feature of apoptosis
After the treatments, both adherent and non-adherent cells were collected in 15 ml tubes and washed twice with 10 ml PBS. Cells (1×105 cells/sample) were stained with Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI), processed as per the manufacturer's instructions (BD Bioscineces, San Diego, CA), and then analyzed on Epics XL-MCL Flow Cytometer (Beckman Coulter). Both PI and Annexin V negative cells (quadrant B3) were considered as normal, PI negative and Annexin V positive cells were considered as early apoptotic (quadrant B4), cells that were both PI and Annexin V positive (quadrant B2) were considered as late necrotic, and cells that were PI positive and Annexin V negative were considered as mechanically injured (quadrant B1) during the experiment. All the experiments were conducted in triplicates and only representative pictures were shown.
Protein extraction
Cells were grown in 150 mm dishes and treated as above before protein extraction. Cells were scraped into growth medium, collected into 50 ml tube, and centrifuged to harvest the pellet. The cell pellets were washed twice in 20 ml ice-cold PBS. The cell pellets were suspended in 400 μl ice-cold homogenization solution (50 mM Tris-HCl, pH 7.4, 320 mM sucrose, 0.1 mM phenylmethylsulfonyl fluride, and 1 mM EDTA), transferred to eppendorf tube, and subjected to sonication gently in micro-ultrasonic cell disruptor (Kontes, Vineland, NJ). The cell lysates were centrifuged at 12000 rpm for 10 min at 4°C and the supernatants were collected. The protein concentrations in the supernatant were measured using Coomassie Plus protein assay reagents (Pierce Biotechnology, Rockford, IL). All the samples were divided into small aliquots and kept at −20°C until used.
Western blotting using specific antibodies
The protein samples (10 μg) were mixed with Laemmli buffer and boiled in boiling water for 5 min. The boiled protein samples were loaded onto precast 4–20% polyacrylamide gradient gels (Bio-Rad Laboratories, Hercules, CA) and electroblotted to the polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA). The non-specific binding sites in the membrane were blocked with 5% non-fat dry milk for 1 h at room temperature. The membranes were then incubated overnight at 4°C shaking on a rocker with appropriate dilution of primary IgG antibody followed by three times washing in washing buffer (20 mM Tris-HCl, pH 7.6, 137 mM NaCl, 0.1% Tween 20). After washing, the membranes were incubated with the appropriate alkaline horseradish peroxidase (HRP)-conjugated secondary IgG antibody for 1 h followed by three times washing in washing buffer. Specific protein bands were detected by incubation for 5 min at room temperature with Immun-Star™ HRP Lumino/Enhancer (Bio-Rad Laboratories, Hercules, CA) and exposing to BIOMAX XAR films (Kodak, Rochester, NY) for autoradiography.
The antibody against β-actin (clone AC-15) was purchased from Sigma (Sigma-Aldrich, St. Louis, MO), and antibodies against survivin, NFP, NSE, e-cadherin, Notch-1, ID2, PCNA, hTERT, caspase-8, Bid, Bcl-2, Bax, calpain, caspase-3, SBDP, ICAD, p-Akt (Thr 308), p65 NF-κB, VEGF, b-FGF, MMP-2, and MMP-9 were from Santa Cruz Biotechnology (Santa Cruz, CA). The alkaline HRP-conjugated anti-rabbit and anti-mouse secondary IgG antibodies were purchased from Biomeda (Foster City, CA) and anti-goat secondary IgG antibody was from Santa Cruz Biotechnology (Santa Cruz, CA).
In vitro cell migration assay
In vitro cell migration assay was performed to determine the effects of survivin shRNA or EGCG alone and in combination on the migratory property of neuroblastoma cells. The migration assay was carried out in 6-well transwell inserts of polycarbonate filter with 8.0 μm pore size (Corning, Lowell, MA). The transwell inserts were coated with Matrigel (BD Biosciences, San Jose, CA) of final concentration of 1.0 mg/ml in an ice-cold serum-free medium and allowed to dry at 37°C for 4 h. After the treatments, cells were trypsinized, washed twice with serum-free medium. Then, 500 μl of cell suspension (1×105 cells) from each sample was added to each Transwell insert in triplicate. Cells were incubated at 37°C in presence of 5% CO2 for 48 h, the membranes were collected and stained with DIFF quick stain kit stain (IMEB, San Marcos, CA). The cells that migrated to the undersurface of the membrane were observed by light microscope, photographed, and counted in 10 randomly selected microscopic fields.
In vitro network formation assay
In vitro angiogenic network formation assay was performed to evaluate the effect of survivin shRNA, EGCG alone, or in combination on network formation of human microvascular endothelial (HME) cells (Cascade Biologics, Portland, OR) in co-culture. Neuroblastoma cells (1×105 cells/sample) were seeded into 2-well chamber slides. After overnight incubation at 37°C in 5% CO2, the cells were transfected with survivin shRNA or scrambled shRNA plasmid. After 6 h, transfection reagent was exchanged with fresh medium containing 2% FBS. After 18 h, the medium were replaced with fresh medium or medium containing 50 μM EGCG and incubated for another 24 h. The cells were incubated for another 24 h and then co-cultured with 1×105 HME cells in a 50:50 mixture of serum-free medium and HME medium (Medium 131, Cascade Biologics, Portland, OR). The co-cultures were further incubated at 37°C in presence of 5% CO2 for 72 h and fixed in 95% cold ethanol and treated with Von Willebrand factor (VWF) antibody (Santa Cruz, CA). After washings, the slides were further treated with biotinylated secondary antibody for 30 min, washed twice in PBS, and then incubated with HRP-labeled streptavidin for 30 min. Cells were stained with the HRP substrate according to the instruction of ImmunoCruz™ staining System (Santa Cruz, CA). The cells were viewed under the light microscope and photographed. The numbers of network formation were quantified using Image-Pro Discovery software (Media Cybernetics, Silver Spring, MD).
Statistical analysis
Results from different experiments were analyzed using Minitab® 15 Statistical Software (Minitab, State College, PA). Data were expressed as mean ± standard deviation (SD) of separate experiments (n ≥ 3) and compared by one-way analysis of variance (ANOVA) followed by Fisher's post hoc test. Difference between control and a single or combination treatment was considered to be significant at p < 0.05.
Results
Levels of expression of survivin in neuroblastoma cell lines
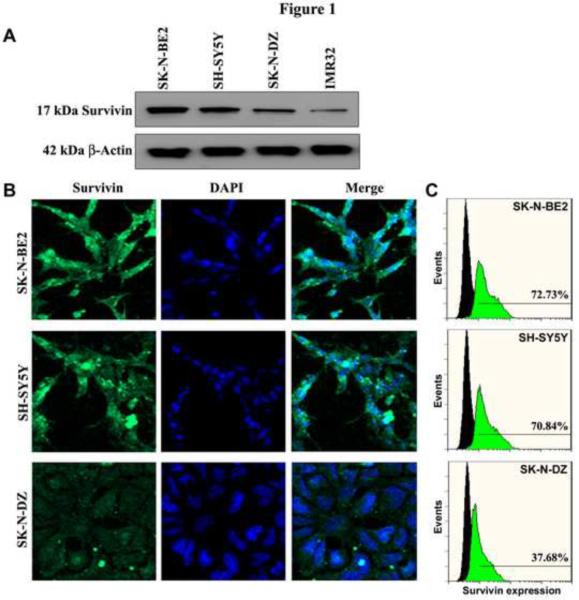
We examined the expression of survivin in four neuroblastama (SK-N-DZ, SK-N-BE2, IMR32, and SHSY5Y) cell lines (Fig. 1). Western blotting showed high expression of survivin protein in SK-N-BE2 and SH-SY5Y cells, low in SK-N-DZ cells, and very low in IMR32 cells (Fig. 1A). Further, in situ immunofluorescence analysis showed similar variations in survivin expression in SK-N-BE2, SH-SY5Y, and SK-N-DZ cells (Fig. 1B). We also quantitated the levels of expression of this protein by flow cytometric analysis (Fig. 1C). Flow cytometric data revealed that the levels of survivin expression were 72.73%, 70.84%, and 37.68% in SK-N-BE2, SH-SY5Y, and SK-N-DZ cells, respectively (Fig. 1C). Our immunofluorescence and flow cytometric analyses detected very low expression of survivin in IMR32 cells (data not shown). Based on these findings, we confirmed relatively overexpression of survivin in SK-N-BE2 and SH-SY5Y cell lines, which were used for further experiments.
Fig. 1.
Expression of survivin in neuroblastoma cell lines. (A) Representative Western blots to show the presence of survivin in neuroblastoma cell lines (SK-N-BE2, SH-SY5Y, SK-N-DZ, and IMR32). Expression of β-actin used as a loading control. (B) Representative (n = 3) immunofluorescence staining to show the expression of survivin (FITC, green). DAPI (blue) was used as a counter stain for the nucleus. (C) Flow cytometry to show survivin expression (FITC, green) in comparison with control (no stain, black).
Down regulation of survivin mRNA and protein levels in SK-N-BE2 and SH-SY-5Y cells
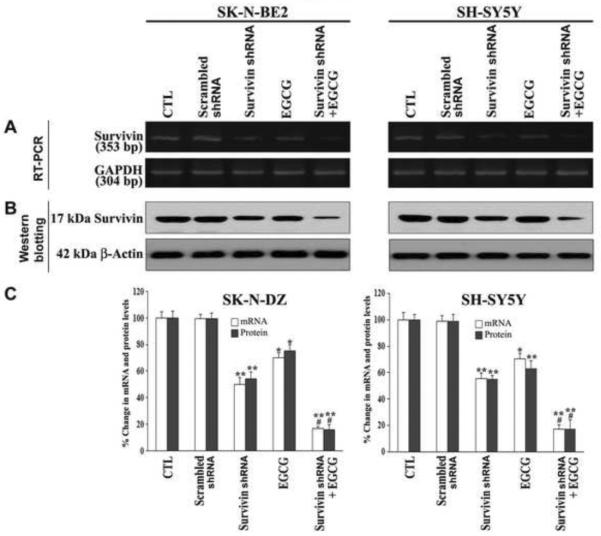
We examined the down regulation of survivin mRNA and protein levels in neuroblastama SK-N-BE2 and SH-SY5Y cells after scrambled shRNA plasmid transfection, survivin shRNA plasmid transfection, EGCG treatment, and combination of survivin shRNA transfection and EGCG treatment (Fig. 2). RT-PCR and Western blot analyses showed the extents of survivin down regulation at, respectively, mRNA (Fig. 2A) and protein (Fig. 2B) levels in both cell lines after these traetments. There was no change in mRNA and protein levels when cells were transfected with the scrambled shRNA plasmid. The levels of expression of survivin mRNA and protein were further down regulated if concurrently treated with EGCG. We used GAPDH mRNA expression and β-actin protein expression as the internal controls in the RT-PCR and Western blotting. Quantification of mRNA and protein levels by Gel-Pro analyzer software demonstrated more than 80% knockdown of survivin at mRNA and protein levels in both neuroblastoma cell lines after treatment with a combination of survivin shRNA and EGCG (Fig. 2C).
Fig. 2.
Alterations in survivin mRNA and protein levels in SK-N-BE2 and SH-SY5Y cells after transfection (48 h) with plasmid vector carrying survivin shRNA cDNA (0.5 μg/ml), treatment (24 h) with EGCG (50 μM) or both agents together (n ≥ 3). (A) Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis for survivin mRNA expression. Expression of GAPDH mRNA was used as an internal control. (B) Western blotting for examining expression of survivin protein. Western blots were reprobed for β-actin content to demonstrate that all lanes were loaded with equal amounts of protein. (C) Determination of survivin mRNA and protein levels after the treatments. *p < 0.05; **p < 0.01; #p < 0.05 (* or ** compared with control; # compared combination therapy with survivin shRNA or EGCG alone).
Survivin knockdown induced the morphological and biochemical features of neuronal differentiation in SK-N-BE2 and SH-SY-5Y cells
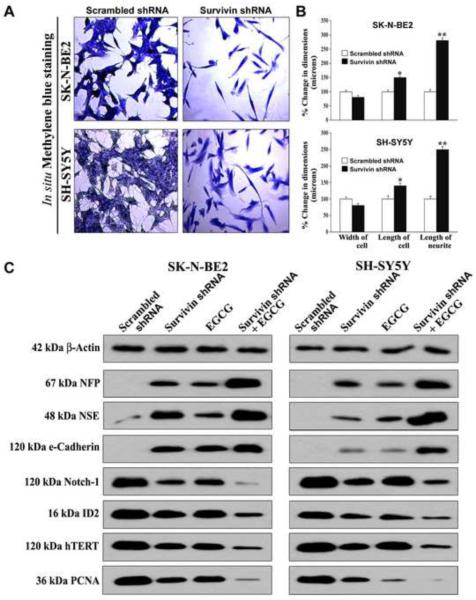
Transfection with survivin shRNA plasmid for 72 h induced neuronal differentiation in both SK-N-BE2 and SH-SY5Y cell lines (Fig. 3). The morphological features of neuronal differentiation were demonstrated following in situ methylene blue staining (Fig. 3A). Survivin shRNA trasfection caused induction of neuronal differentiation morphologically resulting in cells with small and retracted cell bodies having thin elongated and branched neurite extensions, while scrambled shRNA transfection in neuroblastoma cells did not change cell width, length, or neurite. We measured the width, length, and neurite extensions and observed that down regulation of survivin due to survivin shRNA transfection tended to significantly shrink the width but increased the length of the cells and neurite extensions, when compared with the cells transfected with scrambled shRNA (Fig. 3B). We performed Western blotting to examine the changes in biochemical features of neuronal differentiation after the treatments (Fig. 3C). Knockdown of survivin and concurrent EGCG treatment increased expression neurofilament protein (NFP), neuron specific enolase (NSE) and the tight junction protein (e-cadherin), confirming the expression of biochemical markers of neuronal differentiation. Differentiation of neuroblastoma both SK-N-BE2 and SH-SY5Y cells was also clearly evidenced from the down regulation of Notch-1, ID2 (a member of inhibitor of differentiation family), catalytic subunit of human telomerase (hTERT), and proliferation cell nuclear antigen (PCNA) following survivin knockdown and EGCG treatment.
Fig. 3.
Down regulation of survivin inhibited cell proliferation and induced morphological and biochemical features of neuronal differentiation. (A) In situ methylene blue staining showed inhibition of cell growth and increase in morphological features of neuronal differentiation. Photographs were taken after transfection (72 h) with plasmid vector carrying survivin shRNA cDNA (0.5 μg/ml) or scrambled shRNA cDNA (0.5 μg/ml). (B) Measurement of morphological features of neuronal differentiation (width of cell, length of cell, and length of neurite extension). Mean values of 3 independent experiments are shown (*p < 0.05; **p < 0.01). (C) Representative (n = 3) Western blots to show changes in biochemical markers of neuronal differentiation and cell proliferation. Treatments: transfection with plasmid vector carrying scrambled shRNA cDNA (0.5 μg/ml) for 48 h, transfection with plasmid vector carrying survivin shRNA cDNA (0.5 μg/ml) for 48 h, 50 μM EGCG for 24 h, and survivin shRNA (0.5 μg/ml) for 48 h + 50 μM EGCG for last 24 h. Expression of β-actin was used as a loading control.
Survivin knockdown and EGCG treatment induced the morphological and biochemical features of apoptosis in SK-N-BE2 and SH-SY-5Y cells
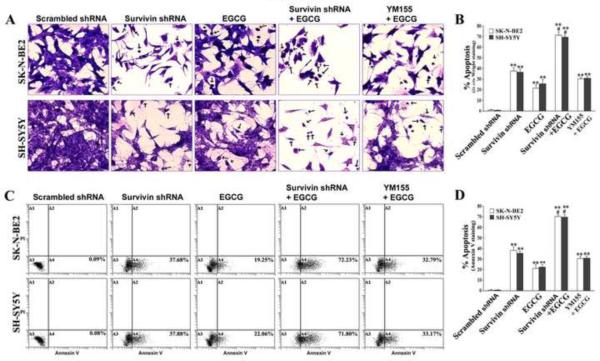
We examined the effect of survivin knockdown and EGCG treatment in the increases in morphological and biochemical features of apoptosis in both SK-N-BE2 and SH-SY5Y cells (Fig. 4). We performed in situ Wright staining and found characteristics morphological features of apoptosis including blebbing, loss of cell membrane assembly, shrinkage of cell volume, nuclear fragmentation, chromatin condensation and fragmentation, and membrane attached apoptotic bodies (Fig 4A). The characteristic morphological changes, which were evident after survivin knockdown and increased after addition of EGCG, were clear in Wright staining and shown by arrows (Fig. 4A). Determination of percentage of apoptotic cells showed that survivin knockdown caused apoptosis in both neuroblastoma cell lines and amounts of apoptosis were significantly increased by concurrent EGCG treatment (Fig. 4B). We confirmed the presence of apoptotic population by further analyzing the Annexin V staining of cells, an early biochemical feature of apoptosis, after the treatments (Fig. 4C). Annexin V-FITC/PI staining and flow cytometry in both SK-N-BE2 and SH-SY5Y cells showed that Annexin V positive cells were increased moderately in the A4 quadrant after survivin knockdown. Interestingly, when cells were subjected to survivin knockdown and concurrent EGCG treatment, we found that population of Annexin V positive apoptotic cells was increased dramatically in the A4 quadrant. The percentage of cells in A4 quadrant presented in the bar graphs clearly showed that survivin knockdown and concurrent EGCG treatment significantly increased the apoptotic cell death (Fig. 4D). We also tested a pharmacological inhibitor of survivin (YM155) in combination with EGCG and found that YM155 was not as effective as the genetic inhibitor (survivin shRNA) in promoting apoptosis.
Fig. 4.
Determination of morphological and biochemical features of apoptosis. Treatments: transfection with plasmid vector carrying scrambled shRNA cDNA (0.5 μg/ml) for 48 h, transfection with plasmid vector carrying survivin shRNA cDNA (0.5 μg/ml) for 48 h, 50 μM EGCG for 24 h, survivin shRNA (0.5 μg/ml) for 48 h + 50 μM EGCG for last 24 h, and 50 nM YM155 for 48 h + 50 μM EGCG for last 24 h. (A) In situ Wright staining for morphological features of apoptosis. (B) Bar diagrams showing percentage of apoptosis based on in situ Wright staining. Mean values of 3 independent experiments are shown. **p < 0.01; #p < 0.05 (**compared with scrambled shRNA; # compared combination therapy with survivin shRNA or EGCG alone). (C) Staining with Annexin V-FITC and flow cytometry for determination of percentage of apoptotic cells. Apoptotic cells (Annexin V-FITC positive and PI negative) are represented by dot plot in A4 quadrant (bottom right) on x-axis. (D) Bar diagrams to show percentage of apoptosis based on accumulation of Annexin V positive cells in A4 quadrant. Mean values of 3 independent experiments are shown. **, p < 0.01; #p, <0.05 (**compared with scrambled shRNA; # compared combination therapy with survivin shRNA or EGCG alone).
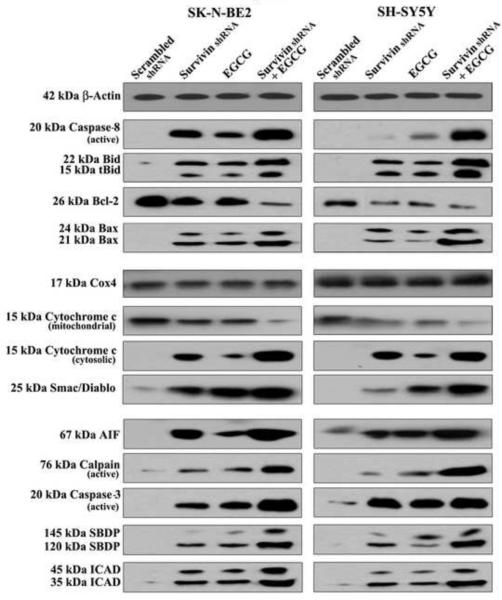
Survivin knockdown in combination with EGCG activated the classical extrinsic intrinsic caspase cascades to induce apoptosis in SK-N-BE2 and SH-SY-5Y cells
In order to determine the changes in expression or activation of apoptosis related proteins in both SK-N-BE2 and SH-SY5Y cell lines following treatments, we performed Western blotting (Fig. 5). Survivin knockdown stimulated the classical receptor-mediated extrinsic pathway leading to activation of caspase-8 for apoptosis in both SK-N-BE2 and SH-SY5Y cell lines. The increased activation of caspase-8 was further augmented with the combination of surviving knockdown and EGCG treatment. Increased activity of caspase-8 was recognized in the cleavage of Bid to tBid, which could be translocated to mitochondria for induction of cell death through intrinsic pathway.
Fig. 5.
Western blotting to examine molecules involved in induction of apoptosis. Treatments: transfection with plasmid vector carrying scrambled shRNA cDNA (0.5 μg/ml) for 48 h, transfection with plasmid vector carrying survivin shRNA cDNA (0.5 μg/ml) for 48 h, 50 μM EGCG for 24 h, survivin shRNA (0.5 μg/ml) for 48 h + 50 μM EGCG for last 24 h. Induction of extrinsic and intrinsic caspase pathways for apoptosis in neuroblastoma SK-N-BE2 and SH-SY5Y cells. Expression of β-actin was used as a loading control. All experiments were conducted in triplicates.
The Bcl-2 family of proteins plays a key role in regulation of mitochondrial permeability during apoptosis via intrinsic pathway. Survivin knockdown caused an increase in pro-apoptotic Bax expression and decrease in anti-apoptotic Bcl-2 expression in both cell lines (Fig. 5). Combination of survivin knockdown and EGCG treatment most dramatically increased Bax expression and decreased Bcl-2 expression, which could lead to increase in the Bax:Bcl-2 ratio in both cell lines. An increase in the Bax:Bcl-2 ratio could cause mitochondrial release of pro-apoptotic molecules such as cytochrome c, Smac/Diablo, and apoptosis-inducing factor (AIF) to trigger mitochondrial caspase-dependent and caspase-independent pathways for apoptosis.
We also examined the activation and activity of cysteine proteases, calpain and caspase-3, in SK-N-BE2 and SH-SY5Y cell lines after the treatments (Fig. 5). We found that both calpain and caspase-3 were activated simultaneously after survivin knockdown but activated most prominently after combination of survivin knockdown and EGCG treatment. We found the increases in calpain and caspase-3 activities in the formation of calpain-specific 145 kD spectrin breakdown products (SBDP) and caspase-3 specific 120 kD SBDP, respectively, in both SK-N-BE2 and SH-SY5Y cells following combination therapy (Fig. 5).
We further examined caspase-3 activity in the cleavage of inhibitor of caspase-3 activated DNase (ICAD) (Fig. 5). Our results showed that survivin knockdown in combination with EGCG treatment markedly increased the cleavage of ICAD in both SK-N-BE2 and SH-SY5Y cells so as to release of CAD from the CAD/ICAD complex and translocation to the nucleus for DNA fragmentation.
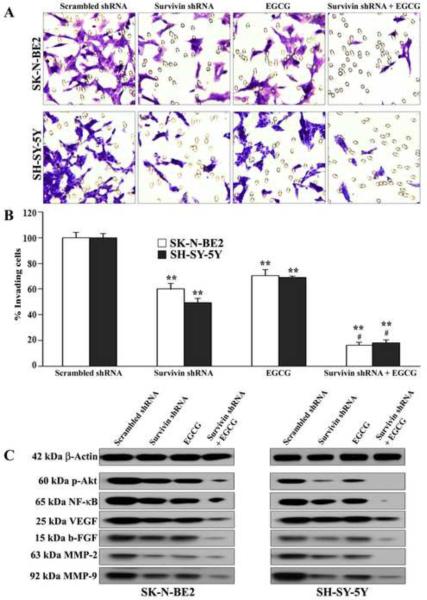
Combination therapy prevented cell migration and down regulated molecules involved in survival, angiogenesis, and invasion pathways in SK-N-BE2 and SH-SY-5Y cells
We examined the effects of survivin knockdown or/and EGCG treatment on the capability of migration of malignant neuroblastoma cells through the polycarbonate membrane and also examined the expression of molecules involved in survival, angiogenesis, and invasion pathways (Fig. 6). Cells underneath the membrane that migrated through matrigel on the polycarbonate membrane were stained and examined under the light microscope (Fig. 6A). We found that scrambled shRNA plasmid transfection did not prevent cells from migration through the matrigel coated membrane but urvivin knockdown or EGCG treatment inhibited the cell migration. Importantly, there was a dramatic reduction in the cell migration through matrigel coated membrane after combination of survivin knockdown and EGCG treatment (Fig. 6A). The percentage of cells migrated through the transwell membrane presented in the bar graphs clearly showed that combination of survivin knockdown and EGCG treatment most significantly reduced cell migration (Fig. 6B). Our Western blotting showed that survivin knockdown in combination with EGCG treatment drastically down regulated the molecules involved in pro-survival (p-Akt and NF-κB), angiogenesis (VEGF and b-FGF), and invasion (MMP-2 and MMP-9) pathways (Fig. 6C).
Fig. 6.
(A) Cell migration assay in neuroblastoma SK-N-BE2 and SH-SY5Y cells. Treatments: transfection with plasmid vector carrying scrambled shRNA cDNA (0.5 μg/ml) for 48 h, transfection with plasmid vector carrying survivin shRNA cDNA(0.5 μg/ml) for 48 h, 50 μM EGCG for 24 h, survivin shRNA (0.5 μg/ml) for 48 h + 50 μM EGCG for last 24 h. After treatments, cells were seeded for migration on the matrigel coated membrane of tranwell insert and incubated pores at 37°C in presence of 5% CO2. After incubation for 48 h, the membranes were collected, stained, and photographs were taken under the light microscope. (B) Quantitation of matrigel invaded cells underneath the membrane. Mean values of 3 independent experiments are shown. **p < 0.01; #p <0.05 (** compared with scrambled shRNA; # compared combination therapy with survivin shRNA or EGCG alone). (C) Western blotting to examine the molecules involved in cell survival and proliferation (p-Akt, NF-κB), angiogenesis (VEGF, b-FGF), and cell migration (MMP-2, MMP-9). Expression of β-actin was used as a loading control. All experiments were conducted in triplicates.
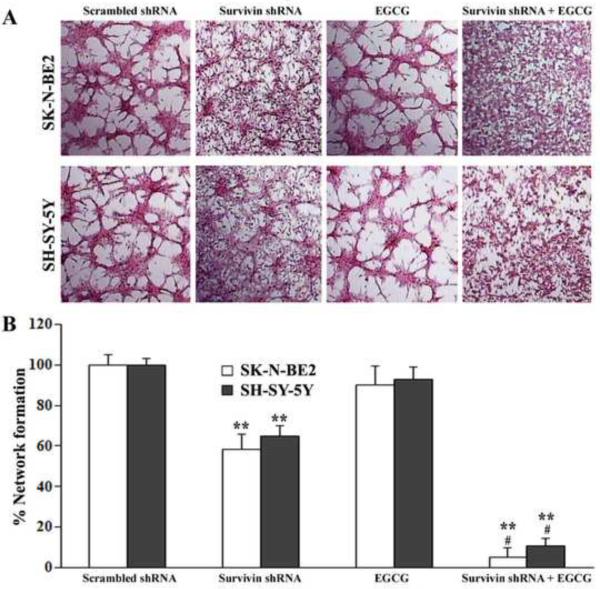
Survivin knockdown and EGCG treatment inhibited in vitro angiogenic network formation
We examined the in vitro angiogenic network formation in co-culture of HME cells and SK-N-BE2 or SH-SY-5Y cells after survivin knockdown and EGCG treatment alone or in combination (Fig. 7). Co-culturing of scrambled shRNA transfected SK-N-BE2 or SH-SY-5Y cells with HME cells did not prevent angiogenic network formation ability of HME cells (Fig. 7A). However, network formation was marginally reduced with the use of SK-N-BE2 or SH-SY-5Y cells treated with EGCG alone. There was a drastic reduction in network formation when HME cells were co-cultured with the SK-N-BE2 or SH-SY-5Y cells subjected to survivin knockdown and EGCG treatment (Fig. 7A). The percentage of network formation presented in the bar graphs clearly showed that co-culturing of combination therapy exposed SK-N-BE2 or SH-SY-5Y cells with HME cells most significantly reduced the angiogenic network formation ability of HME cells (Fig. 7B).
Fig. 7.
Changes in the in vitro network formation ability of HME cells in co-culture with neuroblastoma cells. Treatments of neuroblastoma cells before co-culturing: transfection with plasmid vector carrying scrambled shRNA cDNA (0.5 μg/ml) for 48 h, transfection with plasmid vector carrying survivin shRNA cDNA(0.5 μg/ml) for 48 h, 50 μM EGCG for 24 h, survivin shRNA (0.5 μg/ml) for 48 h + 50 μM EGCG for last 24 h. (A) Representative network formation in HME cells co-cultured with neuroblastoma (SK-N-BE2 or SH-SY5Y) cells. Cells were grown on chamber slides and transfected with survivin shRNA plasmid or treated with EGCG or both agents together. After 24 h, HME cells were co-cultured with neuroblastoma cells. The co-cultures were terminated at 72 h and immunohistochemically stained for Von Willebrand factor. (B) Quantitation of in vitro network formation ability of HME cells. Mean values of 3 independent experiments are shown. **p < 0.01; #p, <0.01 (** compared with control; # compared combination therapy with survivin shRNA or EGCG alone).
Discussion
Our study clearly demonstrated that the combination of survivin knockdown and EGCG treatment successfully induced differentiation and apoptosis and inhibited capabilities of cell migration and angiogenesis in human malignant neuroblastoma SK-N-BE2 and SH-SY-5Y cells harboring survivin overexpression.
Survivin is a bi-functional cancer-specific protein at the interface between cell proliferation and cell survival due to inhibition of apoptosis [16]. We observed that survivin was expressed constitutively in human malignant neuroblastoma SK-N-BE2, SH-SY-5Y, SK-N-DZ, and IMR-32 cell lines; however, highly in SK-N-BE2 and SH-SY-5Y cell lines, slightly in SK-N-DZ cells, and very slightly in IMR-32 cell line. Thus, survivin could be a potential therapeutic target for treatment of malignant neuroblastomas that overexpress survivin. EGCG is a widely known polyphenolic compound from green tea and it has been effective as pro-apoptotic agent in several cancers including human colon cancer [17,18] and pancreatic cancer [19]. Survivin expression can be down regulated in cancer cells by several mechanisms, including the prevention of amplification of the survivin locus on chromosome 17q25 [20], the use of survivin inhibitor such as YM155 [21], and knockdown of survivin by survivin shRNA plasmid trasfection. In the present study, we successfully employed survivin shRNA plasmid to knockdown survivin mRNA and protein levels in two human malignant neuroblastoma SK-N-BE2 and SH-SY-5Y cell lines. We found that shRNA directed against survivin resulted in more than 40% suppression of surviving expression and this transfection followed by EGCG treatment resulted in more than 80% suppression of survivin at the mRNA and protein levels in SK-N-BE2 and SH-SY5Y cells.
We also found that survivin shRNA plasmid transfection induced neuronal differentiation in SK-N-BE2 and SH-SY5Y cells resulting in reduced width of the cell along with significant increase in the length of the cell as well as in the neurite extensions. The increases in expression of the differentiation markers such as NFP and NSE indicate induction of neuronal differentiation [22]. We found that both NFP and NSE were highly increased indicating neuronal differentiation in SK-N-BE2 and SH-SY-5Y cells after the combination therapy. Transition to neuronal differentiation phenotype was accompanied by dramatic decreases in expression of Notch-1 and Id2, which were previously shown to promote dedifferentiation of neuroblastoma cells under hypoxic conditions [23]. Our data showed that cells transfected with shRNA plasmid and concurrently treated with EGCG showed clear inhibition of expression of Notch-1 and ID2. Expression of e-cadherin, known to promote cell differentiation [24], was highly induced due to survivin knockdown in combination with EGCG treatment. Induction of neuronal phenotype was correlated with repression of cell proliferation markers such as PCNA and hTERT [25].
In situ Wright staining and Annexin V staining demonstrated significant increases in, respectively, morphological and biochemical features of apoptosis in neuroblastoma cells after survivin shRNA plasmid transfection and EGCG treatment. Survivin blocks apoptosis by inhibiting several members of the caspases such as caspase-9, caspase-3, and caspase-7 [26,27]. It has previously been reported that green tea polyphenol induces death receptor mediated caspase-8 activation followed by caspase-3 activation [9,28]. We analyzed expression of various molecules involved in apoptosis after the treatments. We first analyzed the expression of caspase-8, which could confirm activation of the receptor mediated pathway of apoptosis. Caspase-8 cleaves of Bid to tBid and translocates tBid to mitochondrial membrane for promoting mitochondrial release of several pro-apoptotic factors including cytochrome c, Smac/Diablo, and apoptosis-inducing factor (AIF) into the cytosol to facilitate apoptosis [24,29]. Direct interaction between survivin and Smac/Diablo can prevent the release of Smac from mitochondria to block cell death [30]. We found that down regulation of survivin by surviving shRNA plasmid transfection and EGCG treatment highly upregulated caspase-8 for cleavage of Bid to tBid, followed by mitochondrial release of cytochrome c Smac/Diablo and AIF in SK-N-BE2 and SH-SY-5Y cells, indicating the induction mitochondria mediated intrinsic apoptotic pathway as well.
Caspase-8 mediated cleavage of Bid to tBid and translocation to mitochondria provides a link between death receptor and mitochondrial pathways of apoptosis [31]. Caspase-8 mediated cleaved Bid to tBid alters the expression of Bax and Bcl-2 [32]. Increase of Bax:Bcl-2 ratio is a key factor to induce the release of several pro-apoptotic molecules from mitochondria [33]. Upregulation of various anti-apoptotic molecules such as Bcl-2 protects the cancer cells from apoptosis [34]. In this study, survivin shRNA plasmid transfection in combination with EGCG treatment caused significant upregulation of Bax with a concomitant down regulation of Bcl-2 so as to cause an increase in the Bax:Bcl-2 ratio in SK-N-BE2 and SH-SY-5Ycells.
Mitochondria mediated activation of the effector caspases, including caspase-3, can cleave a number of cytoplasmic and nuclear substrates leading to apoptosis [33]. Incraeses in calpain and caspase-3 activities are simultaneously activated in human in glioblastoma cells [32] as well as in glioblastoma T98G xenograft [35] to facilitate the apoptosis. Cytochrome c is released from mitochondria into cytosol and it binds to apoptosis-activating-factor-1 (Apaf-1) to produce apoptosome leading to the activation caspase-9 and caspase-3 for caspase-dependent apoptosis [24, 36]. Caspases play significant roles in the apoptosis of mammalian cells. In the present study, we demonstrated that survivin shRNA plasmid transfection in combination with EGCG treatment played an important role in the dramatic upregulation of calpain and caspase-3. Increases in the activities of calpain and caspase-3 were confirmed in the formation of 145 kD SBDP and 120 kD SBDP, respectively. Also, caspase-3 activity caused ICAD cleavage in both SK-N-BE2 and SH-SY-5Y cells. Cleavage of ICAD could allow the neuroblastoma cells to translocate CAD to the nucleus for nuclear DNA fragmentation, the final step in apoptosis.
The distinct ability of tumor cells to infiltrate the extracellular matrix of normal tissue makes it impossible to treat the highly invasive neuroblastomas using surgery and radiation. The dislodgment of tumor cells from the primary site and their subsequent migration of normal adjacent tissues is a defining feature of malignant neuroblastoma. MMPs, especially MMP-9 and MMP-2, can play a significant role in tumor cell migration through proteolytic degradation of the extracellular matrix. Tumor cell migration through matrigel and subsequently through a polycarbonate membrane with a particular pore size is an excellent technique to measure any change in the migration potency of tumor cells after exposure to radiation or chemotherapy. Not all sorts of tumor cells have the ability to migrate through a polycarbonate membrane with a particular pore size. However, almost all neuroblastoma cells are highly migrating and possess this ability. In the present study, we used cell migration assay to measure the ability of two highly malignant neuroblastoma cell lines that migrate and then pass through the matrix on polycarbonate membrane (8 μm pore size) before and after survivin knockdown or/and EGCG treatment. The combination of survivin knockdown and EGCG treatment resulted in up to 80% inhibition in the ability of neuroblastoma cells to migrate through the matrix on polycarbonate membrane. We did not use any chemoattractant in the medium placed underneath the membrane. This indicated that the treated tumor cells lost the ability to secrete the required amount of proteolytic enzymes such as MMP-9 and MMP-2 to degrade the matrix and pass through the membrane.
Phosphorylation of Akt (p-Akt) promotes cell survival and proliferation via activation of NF-κB survival pathway [37]. NF-κB is a protein complex that controls the transcription of DNA in the nucleus. Abberant regulation of NF-κB is linked to cancers and many inflammatory and autoimmune diseases. The active form of Akt (p-Akt) has high potential to deregulate cell cycle, induce cell proliferation, avoid apoptosis, and stimulate cell survival through upregulation of NF-κB [38]. In this study, we demonstrated that survivin knockdown and EGCG treatment resulted in remarkable decreases in the expression of molecules such as p-Akt, and NF-κB in SK-N-BE2 and SH-SY-5Y cells that could otherwise promote tumor cell proliferation.
Angiogenesis, the formation of new blood vessels from pre-existing ones, plays an important role in tumor progression and metastasis. Angiogenesis is a key regulatory factor in the progression and growth of malignant tumors such as neuroblastomas. Therefore, development of an appropriate targeted molecular strategy to prevent angiogenesis is an important milestone in the therapeutic intervention against neuroblastomas. The mechanism of angiogenesis is primarily mediated by hypoxia through chronic activation of the hypoxia-inducible factor pathway leading to the production of VEGF and bFGF [39,40]. In the present study, we observed that cells after transfection with survivin shRNA and treatment with EGCG failed to produce potent angiogenic factors such as VEGF and bFGF. Thus, this combination therapy would inhibit the secretion of potent angiogenic stimulants and subsequently prevent tumor progression. In vitro network formation requires special angiogenic stimulants such VEGF and bFGF secreted by the tumor cells. In this study, we observed that neuroblastoma cells transfected with survivin shRNA and treated with EGCG and then co-cultured with HME cells markedly reduced the network formation ability of HME cells.
In conclusion, the present study demonstrated that survivin knockdown and EGCG treatment effectively induced differentiation and apoptosis and inhibited cell migration, angiogenesis, and growth of malignant neuroblastoma cells through down regulation of molecules involved in these processes. Therefore, the combination of survivin silencing and EGCG treatment can be a novel therapeutic strategy for controlling the growth of human malignant neuroblastomas.
Highlights.
Survivin shRNA + EGCG controlled growth of human malignant neuroblastoma cells.
Survivin knockdown of induced neuronal differentiation in neuroblastoma cells.
Survivin shRNA + EGCG induced morphological and biochemical features of apoptosis.
Combination therapy inhibited invasion, proliferation, and angiogenesis as well.
So, combination therapy showed multiple anti-cancer mechanisms in neuroblastoma.
Acknowledgements
This investigation was supported in part by a grant (R01 NS-57811) from the National Institutes of Health (Bethesda, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosures The authors have declared that no conflict of interest exists.
REFERENCES
- [1].Hiyama E, Iehara T, Sugimoto T, Fukuzawa M, Hayashi Y, Sasaki F, Sugiyama M, Kondo S, Yoneda A, Yamaoka H, Tajiri T, Akazawa K, Ohtaki M. Effectiveness of screening for neuroblastoma at 6 months of age: a retrospective population-based cohort study. Lancet. 2008;371:1173–1180. doi: 10.1016/S0140-6736(08)60523-1. [DOI] [PubMed] [Google Scholar]
- [2].Sampath J, Pelus LM. Alternative splice variants of survivin as potential targets in cancer. Curr. Drug. Discov. Technol. 2007;4:174–191. doi: 10.2174/157016307782109652. [DOI] [PubMed] [Google Scholar]
- [3].Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr. Opin. Cell. Biol. 2006;18:609–615. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- [4].Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin. Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- [5].Sah NK, Khan Z, Khan GJ, Bisen PS. Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006;244:164–171. doi: 10.1016/j.canlet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- [6].Yamamoto H, Ngan CY, Monden M. Cancer cells survive with urviving. Cancer Sci. 2008;99:1709–1714. doi: 10.1111/j.1349-7006.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic urviving gene by wild type p53. J. Biol. Chem. 2002;277:3247–3257. doi: 10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]
- [8].Pennati M, Folini M, Zaffaroni N. Targeting urviving in cancer therapy. Expert. Opin. Ther. Targets. 2008;12:463–476. doi: 10.1517/14728222.12.4.463. [DOI] [PubMed] [Google Scholar]
- [9].Das A, Banik NL, Ray SK. Mechanism of apoptosis with the involvement of calpain and caspase cascades in human malignant neuroblastoma SH-SY5Y cells exposed to flavonoids. Int. J. Cancer. 2006;119:2575–2585. doi: 10.1002/ijc.22228. [DOI] [PubMed] [Google Scholar]
- [10].Chen C, Shen G, Hebbar V, Hu R, Owuor ED, Kong AN. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis. 2003;24:1369–1378. doi: 10.1093/carcin/bgg091. [DOI] [PubMed] [Google Scholar]
- [11].Siddiqui IA, Malik A, Adhami VM, Asim M, Hafeez B, Sarfaraz S, Mukhtar H. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2008;27:2055–2063. doi: 10.1038/sj.onc.1210840. [DOI] [PubMed] [Google Scholar]
- [12].Mittal A, Pate MS, Wylie RC. EGCG down-regulates telomerase in human breast carcinoma MCF-7 cells, leading to suppression of cell viability and induction of apoptosis. Int. J. Oncol. 2004;24:703–710. [PubMed] [Google Scholar]
- [13].Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front. Biosci. 2008;13:440–445. doi: 10.2741/2691. [DOI] [PubMed] [Google Scholar]
- [14].Rasper M, Schafer A, Piontek G, Teufel J, Brockhoff G, Ringel F, Heindl S, Zimmer C, Schlegel J. Aldehyde dehydrogenase 1 positive glioblastoma cells show brain tumor stem cell capacity. Neuro-Oncol. 2010;12:1024–1033. doi: 10.1093/neuonc/noq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mohan N, Karmakar S, Banik NL, Ray SK. SU5416 and EGCG work synergistically and inhibit angiogenic and survival factors and induce cell cycle arrest to promote apoptosis in human malignant neuroblastoma SH-SY5Y and SK-N-BE2 cells. Neurochem. Res. 2011;36:1383–1396. doi: 10.1007/s11064-011-0463-9. [DOI] [PubMed] [Google Scholar]
- [16].Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat. Rev. Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- [17].Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, Bucana CD, Gallick GE, Ellis LM. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br. J. Cancer. 2001;84:844–850. doi: 10.1054/bjoc.2000.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brierley-Hobson S. Binding of (−)-epigallocatechin-3-gallate to the Hsp70 ATPase domain may promote apoptosis in colorectal cancer. Biosci. Horiz. 2001;1:9–18. [Google Scholar]
- [19].Qanungo S, Das M, Haldar S, Basu A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis. 2005;26:958–967. doi: 10.1093/carcin/bgi040. [DOI] [PubMed] [Google Scholar]
- [20].Islam A, Kageyama H, Takada N, Kawamoto T, Takayasu H, Isogai E, Ohira M, Hashizume K, Kobayashi H, Kaneko Y, Nakagawara A. High expression of survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene. 2000;19:617–623. doi: 10.1038/sj.onc.1203358. [DOI] [PubMed] [Google Scholar]
- [21].Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, Daud AI, Patnaik A, Papadopoulos K, Takimoto C, Bartels P, Keating A, Antonia S. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of surviving. J. Clin. Oncol. 2008;26:5198–5203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mohan N, Banik NL, Ray SK. Synergistic efficacy of a novel combination therapy controls growth of Bcl-x(L) bountiful neuroblastoma cells by increasing differentiation and apoptosis. Cancer Biol. Ther. 2011;12:846–854. doi: 10.4161/cbt.12.9.17715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jögi A, Øra I, Nilsson H, Lindeheim H, Makino Y, Poellinger L, Axelson H, Påhlman S. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc. Natl. Acad. Sci. USA. 2002;99:7021–7026. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim S, Schein AJ, Nadel JA. E-cadherin promotes EGFR-mediated cell differentiation and MUC5AC mucin expression in cultured human airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L1049–L1060. doi: 10.1152/ajplung.00388.2004. [DOI] [PubMed] [Google Scholar]
- [25].Janardhanan R, Banik NL, Ray SK. N-Myc down regulation induced differentiation, early cell cycle exit, and apoptosis in human malignant neuroblastoma cells having wild type or mutant p53. Biochem. Pharmacol. 2009;78:1105–1214. doi: 10.1016/j.bcp.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chandele A, Prasad V, Jagtap JC, Shukla R, Shastry PR. Upregulation of survivin in G2/M cells and inhibition of caspase 9 activity enhances resistance in staurosporine-induced apoptosis. Neoplasia. 2004;6:29–40. doi: 10.1016/s1476-5586(04)80051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117–1123. doi: 10.1021/bi001603q. [DOI] [PubMed] [Google Scholar]
- [28].Roy M, Chakrabarty S, Sinha D, Bhattacharya RK, Siddiqi M. Anti-clastogenic, anti-genotoxic and apoptotic activity of epigallocatechin gallate: a green tea polyphenol. Mutat. Res. 2003;523–524:33–41. doi: 10.1016/s0027-5107(02)00319-6. [DOI] [PubMed] [Google Scholar]
- [29].Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- [30].Song Z, Yao X, Wu M. Direct Interaction between Survivin and Smac/DIABLO Is Essential for the Anti-apoptotic Activity of Survivin during Taxol-induced Apoptosis. J. Biol. Chem. 2003;278:23130–23140. doi: 10.1074/jbc.M300957200. [DOI] [PubMed] [Google Scholar]
- [31].Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- [32].Choudhury SR, Karmakar S, Banik NL, Ray SK. Valproic acid induced differentiation and potentiated efficacy of taxol and nanotaxol for controlling growth of human glioblastoma LN18 and T98G cells. Neurochem. Res. 2011;26:2292–2305. doi: 10.1007/s11064-011-0554-7. [DOI] [PubMed] [Google Scholar]
- [33].Karmakar S, Banik NL, Patel SJ, Ray SK. Curcumin activated both receptor-mediated and mitochondria-mediated proteolytic pathways for apoptosis in human glioblastoma T98G cells. Neurosci. Lett. 2006;407:53–58. doi: 10.1016/j.neulet.2006.08.013. [DOI] [PubMed] [Google Scholar]
- [34].George J, Banik NL, Ray SK. Bcl-2 siRNA augments taxol mediated apoptotic death in human glioblastoma U138MG and U251MG cells. Neurochem. Res. 2009;34:66–78. doi: 10.1007/s11064-008-9659-z. [DOI] [PubMed] [Google Scholar]
- [35].Karmakar S, Choudhury SR, Banik NL, Ray SK. Activation of multiple molecular mechanisms for increasing apoptosis in human glioblastoma T98G xenograft. J. Cancer Sci. Ther. 2010;2:107–113. doi: 10.4172/1948-5956.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J. Biol. Chem. 1999;274:5053–5060. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- [37].Maddika S, Ande SR, Panigrahi S, Paranjothy T, Weglarczyk K, Zuse A, Eshraghi M, Manda KD, Wiechec E, Los M. Cell survival, cell death and cell cycle pathways are interconnected: Implications for cancer therapy. Drug Resist. Updates. 2007;10:13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- [38].Pelloski CE, Lin E, Zhang L, Yung WK, Colman H, Liu JL, Woo SY, Heimberger AB, Suki D, Prados M, Chang S, Barker FG, 3rd, Fuller GN, Aldape KD. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin. Cancer Res. 2006;12:3935–3941. doi: 10.1158/1078-0432.CCR-05-2202. [DOI] [PubMed] [Google Scholar]
- [39].Argyriou AA, Giannopoulou E, Kalofonos HP. Angiogenesis and anti-angiogenic molecularly targeted therapies in malignant gliomas. Oncology. 2009;77:1–11. doi: 10.1159/000218165. [DOI] [PubMed] [Google Scholar]
- [40].Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int. J. Cancer. 1994;59:520–529. doi: 10.1002/ijc.2910590415. [DOI] [PubMed] [Google Scholar]