Abstract
The exocyst complex is an evolutionarily conserved multisubunit protein complex implicated in tethering secretory vesicles to the plasma membrane. Originally identified two decades ago in budding yeast, investigations using several different eukaryotic systems have since made great progress toward determination of the overall structure and organization of the eight exocyst subunits. Studies point to a critical role for the complex as a spatiotemporal regulator through the numerous protein and lipid interactions of its subunits, although a molecular understanding of exocyst function has been challenging to elucidate. Recent progress demonstrates that the exocyst is also important for additional trafficking steps and cellular processes beyond exocytosis, with links to development and disease. In this review, we discuss current knowledge of exocyst architecture, assembly, regulation and its roles in a variety of cellular trafficking pathways.
Exocytosis, or secretion, is the process by which cargo-filled vesicles fuse with the plasma membrane to incorporate proteins and lipids into the plasma membrane and to release molecules into the extracellular space. Exocytic events are often restricted to a distinct region of the plasma membrane, resulting in polarized growth and secretion. The budding yeast Saccharomyces cerevisiae has been a critical model system to study the mechanisms of polarized exocytosis and these findings have guided and complemented experiments in multicellular eukaryotes, due to the high conservation of trafficking mechanisms. These studies resulted in the identification of a multitude of proteins and lipids critical for establishing cell polarity and the trafficking of vesicles between cellular membranes.
Exocytic vesicles are generated at the Golgi apparatus and those that function in polarized exocytosis are transported using cytoskeletal tracks and motor proteins to the plasma membrane (1). Vesicle fusion at the target membrane is facilitated by SNARE proteins present on the vesicle and target membranes. Yeast genetic studies identified a number of proteins that are required for a step after vesicle delivery but preceding SNARE-mediated vesicle fusion. Temperature-sensitive mutations in these genes result in an accumulation of vesicles that fail to fuse with the plasma membrane, leading to growth and secretion defects (2, 3). Many of these proteins were later identified as components of an evolutionarily conserved complex and named the exocyst (4), which is the focus of this review. The exocyst is a member of the Complex Associated with Tethering Containing Helical Rods (CATCHR) family (5), of which two other family members are reviewed in this issue (Ungar, COG review; Spang, Dsl review) and the other, GARP, was recently reviewed (6). Several functions have been proposed for these complexes, including tethering vesicles to their target membranes, as well as spatial and temporal regulation of SNARE complex assembly (7–9) (Figure 1).
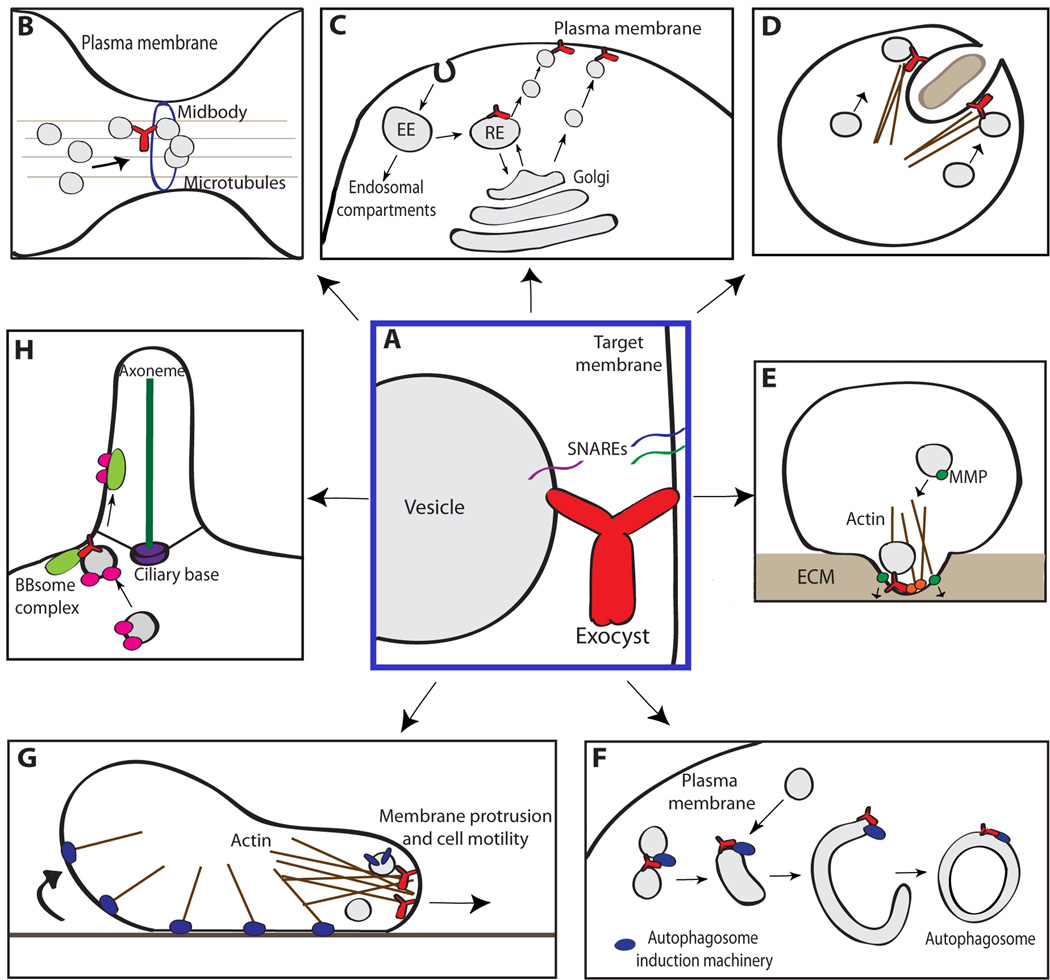
Figure 1. Exocyst functions in a variety of processes in single- and multicellular eukaryotes.
A) The exocyst is proposed to form an initial connection between vesicle and target membrane through interactions with proteins and lipids on both surfaces. The interactions may bring the vesicle close enough to promote SNARE complex formation and vesicle fusion and/or the exocyst may play an active role in regulating SNARE assembly. B) The exocyst localizes to the site of cytokinesis to direct and tether vesicles at these sites, leading to formation of a new membrane and facilitating abscission. C) During polarized secretion, the exocyst tethers both exocytic vesicles generated at the Golgi apparatus and vesicles that are being recycled to the plasma membrane from the recycling endosome (RE=Recycling Endosome; EE=Early Endosome). D) An invading pathogen mediates its entry into the cell by hijacking host cell processes including the exocyst complex, to polarize the cytoskeleton and vesicle delivery for membrane ruffling and macropinocytosis. E) The exocyst colocalizes with IQGAP1 (orange) in invadopodia, directing the growth of invasive processes and the delivery of matrix metalloproteinases (MMPs) that degrade the extracellular matrix (ECM). F) Exo84 and a possible subcomplex of exocyst subunits interact with autophagosome induction machinery (blue), promoting the formation of the autophagosome. The exocyst may function to tether vesicles or tubules to each other leading to the production of this compartment. G) The exocyst interacts with lipids and proteins to localize to the leading edge of migrating cells, promoting the outgrowth of the leading edge and delivering focal adhesion (blue) components recycled from the rear. H) The exocyst directs membrane and protein delivery to the ciliary base to promote ciliogenesis and the BBsome complex shuttles proteins into the cilium beyond the diffusion barrier. For references, see the text and Table 1.
For nearly two decades the exocyst has been a major focus of research in a variety of eukaryotic model systems, including the identification of numerous protein-protein interactions, and determination of crystal structures of several subunits (10–12). However, our understanding of the molecular mechanisms of its function is still limited. Additionally, the more we learn about the exocyst complex, the more it becomes clear that the complex is important in multiple stages of membrane trafficking and likely plays more active roles in exocytosis than simply tethering two lipid bilayers (Figure 1). Interactions with SNAREs, SNARE regulators and many key cell signaling molecules, as well as the unique roles of several of its subunits, suggest that the exocyst is a key integrator of many signals and is a spatiotemporal regulator of multiple membrane trafficking processes.
Exocyst architecture
The composition of the exocyst is highly conserved in eukaryotic systems, with eight single-copy subunits: Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84 (4, 13, 14). Six of the eight subunits were identified in the original S. cerevisiae screen for secretory mutants and all but SEC3 are essential genes in yeast (2, 15). Since the identification of the complex, budding yeast has proved to be a powerful tool for elucidating functional and structural information about the exocyst complex. Moreover, homologues of all the subunits exist in multicellular eukaryotes and the essential role for the complex in growth and development is conserved as well. Null mutants in a number of exocyst subunits result in early lethality in both mice and Drosophila indicating a critical role in development (16–18).
The exocyst belongs to the CATCHR family of multisubunit protein complexes, which have low sequence identity but conserved helical bundle structures (see (5, 6, 19) for review). The crystal structures of exocyst subunits display a common motif of tandem helical bundles that form extended rod-like structures. Electron microscopy (EM) studies of the mammalian brain exocyst (20) and biochemical studies (21) predict that the subunits pack together in a side-by-side manner in the assembled holocomplex. The EM images show the glutaraldehyde-fixed exocyst complex in a “Y-shaped” structure, suggesting that the two arms may connect apposing members to mediate its putative tethering function (10, 20). Supporting this idea, electron tomography studies of cell plate formation in Arabidopsis thaliana showed “Y-shaped” structures linking vesicles (22).
Although the exocyst subunits share structural homology, their surfaces are characterized by unique hydrophobic and electrostatic patterns (23). This diversity of surface properties indicates unique binding interfaces and functions of the individual subunits, either within the complex or individually. Structural studies have been important in characterizing these unique binding sites and interactions (reviewed in (1, 10). In addition to the conserved helical bundles, several exocyst subunits contain additional functional domains. The yeast Sec3 N-terminal region contains a novel Pleckstrin Homology domain in a region demonstrated to interact with PI(4,5)P2 and a number of small GTPases (11, 12). Moreover, mammalian Sec5 and Exo84 were each crystallized in complex with the RalA GTPase, structures that were invaluable in defining the specificity of binding to the GTP-bound form of RalA (24, 25).
Exocyst localization and activation
As expected for a complex involved in polarized vesicle exocytosis, the exocyst is localized to limited regions of the plasma membrane, where it mediates the delivery of lipids and proteins necessary for polarized membrane growth. In yeast, these sites are the tip of the growing bud and the mother-bud neck during cytokinesis (26). Similarly, the Schizosaccharomyces pombe exocyst is localized at the division septum during membrane scission (27). Studies in Drosophila and mammalian neurons indicate that the exocyst is found at the ends of neuronal growth cones during neurite branching, as well as at sites of synaptogenesis (28–30). Cell-cell contact sites in polarized epithelial cells and the leading edge of cell motility processes are also sites of exocyst concentration (31, 32). Data is rapidly emerging about the role of the exocyst in plants, where the complex localizes to the growing ends of pollen tubes, root hair tips and the cell plate for division (33, 34). How the exocyst is recruited and maintained at polarized sites is a critical question, and one that has been the focus of much effort since the complex was identified.
Early studies in budding yeast implicated Sec3 as a spatial landmark for exocytosis, as the localization of Sec3-GFP appeared unaffected by disruptions of the secretory pathway, actin, and cell cycle proteins (35). Immunofluorescence of endogenous Sec3 called this result into question, however, and later reports demonstrated that Sec3 is not sufficient to target and/or retain exocyst complexes at sites of secretion (36–38). Consistent with exocyst localization being dependent on secretion and polarized actin, live imaging and fluorescence recovery after photobleaching (FRAP) analyses suggested that six of the eight exocyst subunits arrive at polarized sites on vesicles via transport on actin cables (39). Sec3 and Exo70 were the exceptions in that Sec3-GFP seemed to localize independently of these mechanisms and Exo70-GFP appeared to use both vesicle-independent and -dependent routes to polarized sites. The model proposed that exocyst subunits arriving on vesicles assembled with Exo70 and Sec3 at the plasma membrane, although it is not clear whether the rest of the subunits arrive individually or already assembled together. It remains to be determined if assembly and disassembly of the complex are important for tethering and targeting vesicles; it seems likely that disassembly of the complex must follow to initiate another round of vesicle fusion, but there is no direct evidence yet to suggest whether this occurs in vivo.
Exo70 and Sec3 are effectors for Rho GTPases, which are master cell polarity regulators that are localized to the plasma membrane and are critical in polarizing the actin cytoskeleton for vesicle delivery. For a more thorough review of the role of small GTPases in exocytosis, see Wu et al., 2008 (40). The yeast Sec3 N-terminal domain interacts with Rho1, Cdc42 and PI(4,5)P2, while Exo70 binds Rho3, Cdc42 and PI(4,5)P2 (41–46). The GTPase interactions of Exo70 are conserved, as mammalian Exo70 interacts with the Rho protein TC10 (47).
To understand the functional significance of these interactions, specific temperature-sensitive mutants in yeast were studied. cdc42-6 and rho3-V51 alleles display severe growth and secretion defects without disrupting exocyst localization or actin polarization (42, 43). The latter Rho3 effector domain mutation abrogates binding to both Exo70 and the myosin motor Myo2, and recently identified loss-of-function exo70 mutants mimic the phenotypes of these specifically exocytosis-deficient Rho mutants (43, 48). However, deletion of the N-terminal domain of Sec3 resulted in the mislocalization of only Sec3 with no growth or secretion defects (44). It was possible that this lack of phenotype resulted from a redundant or parallel pathway involving Exo70 and synthetic genetic interactions have been tested extensively. Interestingly, the double mutant, rho3-V51 sec3ΔN, shows no synthetic effects on growth, polarity, or localization of any exocytic machinery (36). However, sec3ΔN was synthetically lethal in combination with cdc42-6 arguing that there may be functional overlap between Exo70 and Sec3 through Cdc42, but because exo70 loss-of-function mutants alone demonstrate similar phenotypes to cdc42-6 it seems plausible that Exo70 is the primary effector for this GTPase (36, 48).
Interestingly, these mutants can be rescued by GTP-hydrolysis deficient versions of the Rho GTPases, suggesting that the GTPase cycle is not required for their exocytosis-specific functions (36). Small GTPases, such as Cdc42, function both through GTP hydrolysis and hydrolysis-independent mechanisms. Commonly, molecular recognition and timing events that require binding and release of effectors require hydrolysis of GTP; for example, the Sec4 interaction with the exocyst requires this function (see below). Hydrolysis-independent mechanisms are proposed to be allosteric regulatory events, where binding of the GTPase activates the binding partner through a conformational change (40). Since exocyst interactions with Rho appear to fit this allosteric model, it is possible that these interactions function primarily to activate the exocyst at polarized sites, potentially to accelerate SNARE complex assembly for vesicle fusion.
Because Rho GTPase interactions are not critical for polarized exocyst localization, phospholipid interactions may provide this function. sec3ΔN mutants crossed to exo70 mutants defective in binding to PI(4,5)P2 are severely growth defective or synthetically lethal in yeast, indicating possible redundant functions for these subunits in stabilizing exocyst localization through lipid binding (49, 50). Furthermore, mutations in yeast MSS4 the kinase that produces PI(4,5)P2, cause diffuse exocyst localization (51). Mammalian Exo70 is also dependent on PI(4,5)P2 binding for its localization and the residues involved in this interaction constitute the most conserved domain on the protein (52). The lipid binding residues in the N-terminal region of Sec3 are also highly conserved among Sec3 homologues (11). Finally, in addition to phospholipid interactions, it is likely that additional factors critical for exocyst localization remain to be identified. The yeast sec6 mutant alleles, sec6-49 and sec6-54 result in mislocalization of all eight exocyst subunits, which remain fully assembled (38). The mutations are in regions suggestive of protein-protein, rather than protein-lipid, interactions. Therefore, these mutants are proposed to be defective in binding to a protein factor that anchors the assembled complex at the plasma membrane.
Exocyst assembly
A key aspect to understanding exocyst function is to determine the mechanism(s) of its assembly and disassembly. Many questions remain unanswered, such as when, where and how many of the subunits assemble together, and if they always stay assembled. Whether the exocyst requires disassembly is unknown, although considering the ~750 kDa size of the complex, disassembly may remove the exocyst as an obstacle for vesicle fusion and/or facilitate recycling of the complex. Hints of functional subcomplexes and monomeric pools of subunits have been observed, but biochemical isolation and characterization of these pools remains elusive, as does the fully assembled complex.
Early biochemical experiments discovered that the eight unique polypeptides of the exocyst form a high molecular weight complex (4, 14). Differential centrifugation, cell fractionation, and immunofluorescence experiments in both yeast and higher eukaryotes indicated that the exocyst subunits are found primarily as a single complex, with both cytosolic and plasma membrane pools (4, 33, 53–57). This is consistent with the localization of all of the exocyst subunits to sites of polarized secretion at the bud tip and mother-bud neck in yeast, and polarized sites of membrane expansion in plant and animal cells. Moreover, in unpolarized epithelial cells, the exocyst subunits Sec6 and Sec8 are primarily cytosolic, but the majority shifts to polarized sites on the plasma membrane upon cell-cell contact (55).
Individual temperature-sensitive mutations in each of the budding yeast exocyst subunits result in the loss of specific combinations of subunits from the complex (54), suggesting that many individual interactions are necessary for maintaining the architecture of the exocyst. Additional work using yeast two-hybrid analyses and in vitro binding studies identified weak pairwise binding interactions among the subunits of the exocyst (reviewed in (10, 32)). Structural and biochemical studies of the holocomplex remain an outstanding challenge for the field, to achieve an understanding of how the subunits are pieced together, and details of assembly and disassembly of the complex. Moreover, it will be interesting to see if these mechanisms are conserved across all eukaryotes.
Exocyst subcomplexes
The existence of subcomplexes or monomeric free pools would lead to a greater array of functional possibilities and mechanisms for exocyst regulation. Assembly of the subunits into the full octameric complex at the proper place and time would ensure the spatiotemporal specificity of a vesicle tethering event. Indeed, it has been proposed that a subset of subunits arrives on vesicles and assembles with the remaining subunits waiting at the plasma membrane, thus mediating a connection between membranes (39). Although the exocyst subunits predominantly co-migrate when examined by centrifugation and gel filtration studies, the broad distributions and trailing peaks for some exocyst subunits suggest that some of the subunits may exist in free pools outside of the complex (53, 57). Additionally, localization studies in Drosophila melanogaster indicate that specific exocyst subunits exhibit unique localization patterns during oogenesis, development and adulthood, suggesting that the subunits might not always function as a single entity (18).
Cell fractionation studies in mammalian cells also provide strong evidence for subcomplexes. Ral GTPases function in trafficking, but are unique to metazoan systems. Activated RalA and RalB are associated with secretory vesicles (58) and each binds to two exocyst subunits: Sec5 and Exo84, which are predicted to be in separate subcomplexes by cell fractionation (59, 60). Recent studies also showed that Ral GTPases interact with Exo84 and Sec5 in distinct subcellular locations, with Sec5 at the plasma membrane and Exo84 associated with vesicles (61, 62). It seems likely that there would be a greater need for functional subcomplexes in mammalian systems, where different combinations of subunits could respond to a complex array of signals.
Despite a number of studies suggesting that subcomplexes of exocyst subunits exist, the isolation or reconstitution of these has proven challenging, likely due to the weak pairwise interactions between the subunits (21, 23). Weak interactions are likely to be functionally important for cooperative assembly and disassembly of the complex. More sensitive quantitative techniques for detection of these subcomplexes, as well as robust activity assays, will be important for determining their physiological relevance. Furthermore, identification of specific mutants that disrupt intra-exocyst interactions is crucial to tease apart the functions of individual subunits, the complex as a whole, and to understand which subunits are critical for stabilization of exocyst structure.
Vesicle recognition and regulation of assembly by GTPases
Rab GTPases are the largest group of the Ras superfamily of small GTPases, with 11 Rab proteins in yeast and over 60 in humans. They are important regulators at all stages of trafficking, particularly through interactions with vesicle motility machinery (see below), tethering factors, and other regulatory molecules (63). The yeast exocyst subunit Sec15 interacts with the GTP-bound Rab protein Sec4 on vesicles, presumably for specific secretory vesicle recognition; furthermore, functional Sec4 is required for proper exocyst localization and stable assembly (53, 54, 64). It is not yet known whether the Sec4-GTP-Sec15 interaction only facilitates exocyst subunit delivery on vesicles, or if it plays a more active role in assembly/disassembly of the complex.
Exocyst interactions with Rab GTPases are conserved in higher eukaryotes as well. In both mammals and Drosophila Sec15 interacts with the Rab GTPase Rab10; this interaction appears to be important for endocytic recycling (65–67). Additionally, interactions with Rab8 and Rab11 function in trafficking from the Golgi and recycling endosome to the plasma membrane, as well as to the base of the primary cilium during ciliogenesis (68). It will be interesting to determine if these Rab GTPases function similarly to Sec4 in regulation of the exocyst complex.
The interaction of RalA with two different exocyst subcomplexes in metazoans may also be functionally important for exocyst assembly. The reduction of RalA expression results in decreased association of Sec10 with Sec6, each being a component of separate Exo84 and Sec5 subcomplexes (59). Additionally, release of the Ral-exocyst interactions may be triggered by phosphorylation events (69), possibly leading to dissociation of the exocyst from vesicles or disassembly of the complex.
Exocyst Functions
Tethering
Tethering is defined as the initial, long-distance connection between the vesicle and the target membrane (7, 70).The act of tethering would capture and stabilize vesicles before fusion, thus indirectly facilitating SNARE-docking and fusion (Figure 1). Tethering factors take the form of either long coiled-coil proteins or multisubunit protein complexes, which interact with proteins and/or lipids on both the vesicle and target membranes (9).
Despite their classification as tethers, many of these proteins and complexes, such as the exocyst, have not been experimentally shown to perform this function, with the exception of the long coiled coil tethers, TRAPP (71), and (72). One landmark experiment demonstrated that Uso1, a coiled coil tether implicated in ER to Golgi trafficking of COPII vesicles, and the GTPase Ypt1, were sufficient to anchor vesicles at the Golgi and this function was physically separable from SNARE-mediated fusion (70). Tethering has also been shown for other coiled coils and a few of the multisubunit tethers, but most are challenging to test experimentally (5). Demonstration of tethering by the exocyst complex awaits in vitro reconstitution experiments; the large sizes and low solubilities of the eight subunits are a significant challenge for purification of the holocomplex, or for reconstituting an assembled and functional complex in vitro. Not only would an in vitro assay be valuable for demonstrating tethering, but would also be able to distinguish between tethering and a direct effect on SNARE complex assembly and fusion. Other possible approaches include the use of super high resolution imaging for observing tethering of vesicles in vivo and powerful electron microscopy techniques such as that previously used to observe homotypic vesicle fusion during cytokinesis in Arabidopsis (22).
SNARE regulation
In addition (or alternatively) to tethering, the recognition of exocytic vesicles by the exocyst may directly ensure the fidelity of secretion by activating specific SNARE complex assembly. For example, the yeast exocyst subunit Sec6 binds to the exocytic plasma membrane SNARE Sec9 both in vitro and in vivo and this interaction inhibits the in vitro assembly of the plasma membrane SNARE complex (57, 73). Sec9 binding to Sec6 is incompatible with Sec6-exocyst interactions, suggesting that assembly of the exocyst would lead to release of Sec9 for SNARE complex assembly (57). Additionally, an interaction between Sec6 and the SNARE regulatory protein Sec1 (74) was recently identified, and was suggested to recruit and/or stabilize Sec1 at sites of secretion (57); together, the exocyst and Sec1 may function to spatially and temporally control SNARE assembly. In vitro reconstitution of SNAREs with purified exocyst complexes, and other regulators, such as Rab and Rho GTPases, Sec1 and Sro7/77 (75), will be necessary to determine the effect of the exocyst on SNARE assembly and membrane fusion.
SNARE regulation may be a general feature of many tethering complexes. HOPS, the vacuolar tethering complex, binds to SNARE complexes and proofreads vacuolar SNARE pairing (76, 77). Similarly, COG binds to SNAREs and increases the stability of intra-Golgi SNARE complexes, possibly preventing disassembly and promoting fusion. It is unclear whether COG may have an effect on the rate of SNARE complex assembly; Dsl1has a slight stimulatory effect on Golgi to endoplasmic reticulum SNARE complex assembly in vitro and GARP promotes the assembly of trans-Golgi network SNARE complexes (6, 78, 79). As the mechanistic details for these functions are explored further, it will be interesting to discover whether all the tethering complexes function similarly in SNARE complex regulation, or if there are interesting organelle-specific (or species-specific) differences.
Diverse cellular functions
In contrast to the traditional view of the exocyst as a simple tether of secretory vesicles to the plasma membrane, the complex has been implicated in a great variety of cellular processes (Figure 1). The common theme seems to involve exocyst-mediated localization of membrane-bound vesicles or compartments to specific target sites at the appropriate time. For example, at least three yeast exocyst subunits (Sec3, Sec5, and Sec8) have been implicated in ER inheritance, potentially by anchoring the cortical ER at the bud tip where the exocyst is localized (80). A later study also identified an interaction between yeast Sec6 and Rtn1, a protein important for ER reticulation, with Rtn1 potentially serving as an exocyst receptor on the ER (81). Several studies implicate the exocyst in prospore membrane formation during meiosis in budding yeast (82, 83).
In higher eukaryotes, the exocyst subunits are expressed in all tissue types analyzed thus far (14). Similar to the phenotype in yeast, exocyst mutants or knock-downs in more complex organisms are associated with cell growth and developmental defects, as has been shown in mouse, plant, and Drosophila model systems (16–18, 84). The function of the exocyst in growth and secretion in many cell types reflects its critical role in tethering and SNARE-mediated fusion of exocytic vesicles. Furthermore, as suggested by its bud neck localization in budding yeast, the exocyst also appears to direct vesicles to the midbody during cytokinesis in mammalian cells (85). In addition, the exocyst has been shown to be important for endocytic recycling in animal cells (26, 86). Highly specialized secretory pathways, such as the insulin-stimulated delivery of the glucose transporter Glut4 in adipocytes, also require functional exocyst complexes (47, 87).
The exocyst is required for many other types of membrane expansion, including ciliogenesis, tubulogenesis and cell migration in mammalian systems (31, 32, 68) (Figure 1). Due to its promotion of cell growth, cell migration, and its interactions with Ral GTPases, the exocyst has been linked with cancer progression and metastasis (31, 88). In one example, the secretion of matrix metalloproteinases (MMPs) in tumor cell invadopodia requires exocyst-mediated exocytosis (89, 90). Furthermore, the exocyst-mediated exocytic pathway has also been shown to play a role in bacterial pathogenesis; the exocyst is co-opted by the bacteria Salmonella to promote its invasion of intestinal epithelial cells (91). The exocyst also has roles in host survival responses—several studies have linked exocyst function to various aspects of the innate immune response (92, 93).
The newest facet to exocyst function was discovered through the study of the involvement of the GTPase RalB in autophagosome biogenesis (62). RalB triggers its exocyst binding partner Exo84 to serve as a platform for the assembly of the autophagy induction complex and vesicle formation machinery. It will be interesting to see whether the exocyst’s role in autophagy is yet another aspect of its tethering/membrane fusion activities, or a novel function for the complex or its subunits.
In contrast to these various roles for the exocyst, several secretory processes appear not to be dependent on wild-type levels of exocyst function. In Schizosaccharomyces pombe for example, severely reduced levels of Sec8 protein blocked septum cleavage with only a modest effect on cargo secretion and no significant effect on polarized growth (27). It is possible that exocyst function is rate-limiting during cytokinesis and not growth, but recent results suggested the presence of parallel actin-dependent and exocyst-dependent secretory pathways in S. pombe (94). Additionally, Drosophila Sec5 mutants suggested a requirement for the exocyst during neuronal development, but not for synaptic vesicle fusion (17). This specialized system may have evolved additional mechanisms to mediate the fine-tuned release of synaptic vesicles. However, Sec8 was found on purified mammalian synaptic vesicles, so it is possible that the exocyst could be required for synaptic transmission in other animals (95).
Cytoskeleton interactions: role in vesicle transport?
Yeast post-Golgi vesicles are transported from the trans-Golgi network to the plasma membrane along actin filaments using the type V myosin motor Myo2. The Rab GTPases Ypt31/32 and Sec4 both associate with post-Golgi vesicles and bind to Myo2, but not simultaneously, as they exchange during the progression of vesicle transport (96). Due to this GTPase shuffling, it seems unlikely that Rabs would be the sole interactors maintaining the cytoskeletal connection of the vesicle. Indeed, it was recently shown that the cargo-binding domain of Myo2 is structurally homologous to the exocyst subunits (97) and this domain of Myo2 directly binds to Sec15; abrogation of the Myo2-Sec15 interaction leads to growth and secretion defects in yeast (96). Immunoprecipitation of Myo2 pulls down all of the exocyst subunits, suggesting association with the full complex, although it is unclear whether this occurs during vesicle transport or upon arrival at sites of secretion (96). The function of this interaction is unclear, however, as disruption of exocyst assembly and function by a variety of mutants does not lead to defects in vesicle delivery to polarized sites (2). More specific mutant alleles of Sec15 and Myo2 may be required to tease apart this molecular mechanism.
In mammalian systems, vesicles are transported from the Golgi by microtubules and their associated kinesin motors to cortical actin networks at the plasma membrane. Numerous approaches have demonstrated an interaction between the exocyst complex and microtubules; furthermore, Exo70 was shown to inhibit the polymerization of tubulin in vitro (98). The exocyst or one or more of its subunits may play a role as adaptors in the connection of vesicles to microtubules, analogous to its proposed role in actin-based transport in yeast. Moreover, it was proposed that the exocyst may be needed to release vesicles from microtubules to the actin networks (98). There is no mechanistic understanding yet for the role of the exocyst in these processes but the interactions provide important clues that the exocyst is involved in multiple stages of trafficking including vesicle transport up through SNARE complex assembly.
Conclusions
The recent years have shown an explosion in studies of exocyst function, in yeast and in many Drosophila plant, and mammalian cell types. These advances argue that the exocyst is more than just a tethering complex, and that it has many roles in multiple stages of vesicle trafficking. A common theme arising from all model systems is the role of the exocyst at many sites in the cell as a spatiotemporal regulator of membrane trafficking. It is well-suited to these functions, having eight subunits characterized by unique sets of interacting partners, with multiple layers of regulation available (Table 1).
Table 1.
Exocyst protein interactions reveal roles for the complex in a variety of basic and complex eukaryotic cellular processes.
| Cellular Process | Interactor | Exocyst Partner |
Kingdom | References |
|---|---|---|---|---|
| Bud site selection | Iqg1 | Sec3 | Fungi | (99) |
| Endoplasmic reticulum structure | Rtn1 | Sec6 | Fungi | (81) |
| SNARE complex regulation | Sec9 and Sec1 | Sec6 | Fungi | (57, 73) |
| Post-Golgi vesicle transport | Myo2 | Sec15 | Fungi | (96) |
| Cell polarity | Sro7/Sro77 | Exo84 | Fungi | (75) |
| Cell polarity | ICR1 | Sec3 | Plant | (100) |
| Exocyst disengagement from Ral GTPase | Protein Kinase C | Sec5 | Animal | (69) |
| Cytokinesis | Centriolin | Sec15 | Animal | (85) |
| GLUT4 trafficking | TC10 | Exo70 | Animal | (47) |
| Ciliogenesis | Rab8 and Rab11 | Sec15 | Animal | (68) |
| Invadopodia function, MMP secretion | IQGAP1 | Sec3 and Sec8 | Animal | (89) |
| Cancer, cell migration | RalA/RalB | Sec5 and Exo84 | Animal | (31, 59, 60) |
| Cell migration | aPKCs | unknown | Animal | (101) |
| Actin polymerization and cell migration | ARPC1 of Arp2/3 complex | Exo70 | Animal | (102) |
| Directional cell migration | PIPKIγi2 | Exo70 and Sec6 | Animal | (103) |
| Neuronal and epithelial cell polarity | RalA/RalB | Sec5 and Exo84 | Animal | (30, 55) |
| Tight junction establishment and function | RalA/RalB | Sec5 and Exo84 | Animal | (61) |
| Innate immunity signaling | TBK1 | Sec5 | Animal | (92, 93) |
| Salmonella invasion | SipC | Exo70 | Animal | (91) |
| Autophagy induction | Beclin/ULK1/VPS34 | Exo84 | Animal | (62) |
Recent work links the exocyst with disease and bacterial pathogenesis in mammalian systems. The key to understanding the role of the exocyst in these processes will be deeper mechanistic examination of exocyst function using structural and quantitative biochemical studies of the octameric complex, putative subcomplexes and their binding partners. Further insight is provided by mutational analyses, especially in yeast, to isolate the functions specific to particular subunits within and outside of the complex. These studies will complement localization and in vivo functional assays in multicellular organisms, where genetic techniques are more challenging. Finally, although classified as a tethering complex, no direct evidence for tethering has been experimentally demonstrated; development of tethering and other in vitro activity assays presents the greatest challenge to advance our understanding of the molecular details of exocyst complex function.
Supplementary Material
Acknowledgments
We thank Chavela Carr, Heidi Hehnly and members of the Munson laboratory for critical reading of this manuscript. Research in the Munson lab is supported by the National Institutes of Health Grant GM068803.
References
- 1.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12(5):671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- 3.Novick P, Ferro S, Schekman R. Order of events in the yeast secretory pathway. Cell. 1981;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- 4.TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. Embo J. 1996;15(23):6483–6494. [PMC free article] [PubMed] [Google Scholar]
- 5.Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- 6.Bonifacino JS, Hierro A. Transport according to GARP: receiving retrograde cargo at the trans-Golgi network. Trends Cell Biol. 2011;21(3):159–167. doi: 10.1016/j.tcb.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeffer SR. Transport vesicle docking: SNAREs and associates. Annu Rev Cell Dev Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- 8.Grote E, Carr CM, Novick PJ. Ordering the final events in yeast exocytosis. J Cell Biol. 2000;151(2):439–452. doi: 10.1083/jcb.151.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whyte JR, Munro S. Vesicle tethering complexes in membrane traffic. J Cell Sci. 2002;115(Pt 13):2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- 10.Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol. 2006;13(7):577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- 11.Baek K, Knodler A, Lee SH, Zhang X, Orlando K, Zhang J, Foskett TJ, Guo W, Dominguez R. Structure-function study of the N-terminal domain of exocyst subunit Sec3. J Biol Chem. 2010;285(14):10424–10433. doi: 10.1074/jbc.M109.096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita M, Kurokawa K, Sato Y, Yamagata A, Mimura H, Yoshikawa A, Sato K, Nakano A, Fukai S. Structural basis for the Rho- and phosphoinositide-dependent localization of the exocyst subunit Sec3. Nat Struct Mol Biol. 2010;17(2):180–186. doi: 10.1038/nsmb.1722. [DOI] [PubMed] [Google Scholar]
- 13.Guo W, Grant A, Novick P. Exo84p is an exocyst protein essential for secretion. J Biol Chem. 1999;274(33):23558–23564. doi: 10.1074/jbc.274.33.23558. [DOI] [PubMed] [Google Scholar]
- 14.Hsu SC, Ting AE, Hazuka CD, Davanger S, Kenny JW, Kee Y, Scheller RH. The mammalian brain rsec6/8 complex. Neuron. 1996;17(6):1209–1219. doi: 10.1016/s0896-6273(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 15.Finger FP, Novick P. Sec3p is involved in secretion and morphogenesis in Saccharomyces cerevisiae. Mol Biol Cell. 1997;8(4):647–662. doi: 10.1091/mbc.8.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedrich GA, Hildebrand JD, Soriano P. The secretory protein Sec8 is required for paraxial mesoderm formation in the mouse. Dev Biol. 1997;192(2):364–374. doi: 10.1006/dbio.1997.8727. [DOI] [PubMed] [Google Scholar]
- 17.Murthy M, Garza D, Scheller RH, Schwarz TL. Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron. 2003;37(3):433–447. doi: 10.1016/s0896-6273(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 18.Murthy M, Ranjan R, Denef N, Higashi ME, Schupbach T, Schwarz TL. Sec6 mutations and the Drosophila exocyst complex. J Cell Sci. 2005;118(Pt 6):1139–1150. doi: 10.1242/jcs.01644. [DOI] [PubMed] [Google Scholar]
- 19.Munson M. Tip20p reaches out to Dsl1p to tether membranes. Nat Struct Mol Biol. 2009;16(2):100–102. doi: 10.1038/nsmb0209-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu SC, Hazuka CD, Roth R, Foletti DL, Heuser J, Scheller RH. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 1998;20(6):1111–1122. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 21.Dong G, Hutagalung AH, Fu C, Novick P, Reinisch KM. The structures of exocyst subunit Exo70p and the Exo84p C-terminal domains reveal a common motif. Nat Struct Mol Biol. 2005;12:1094–1100. doi: 10.1038/nsmb1017. [DOI] [PubMed] [Google Scholar]
- 22.Segui-Simarro JM, Austin JR, 2nd, White EA, Staehelin LA. Electron tomographic analysis of somatic cell plate formation in meristematic cells of Arabidopsis preserved by high-pressure freezing. Plant Cell. 2004;16(4):836–856. doi: 10.1105/tpc.017749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sivaram MV, Furgason MLM, Brewer DN, Munson M. The structure of the exocyst subunit Sec6p defines a conserved architecture with diverse roles. Nat Struct Mol Biol. 2006;13(6):555–556. doi: 10.1038/nsmb1096. [DOI] [PubMed] [Google Scholar]
- 24.Fukai S, Matern HT, Jagath JR, Scheller RH, Brunger AT. Structural basis of the interaction between RalA and Sec5, a subunit of the sec6/8 complex. Embo J. 2003;22(13):3267–3278. doi: 10.1093/emboj/cdg329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin R, Junutula JR, Matern HT, Ervin KE, Scheller RH, Brunger AT. Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. Embo J. 2005;24(12):2064–2074. doi: 10.1038/sj.emboj.7600699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009;21(4):537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Tang X, Liu J, Trautmann S, Balasundaram D, McCollum D, Balasubramanian MK. The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol Biol Cell. 2002;13(2):515–529. doi: 10.1091/mbc.01-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazuka CD, Foletti DL, Hsu SC, Kee Y, Hopf FW, Scheller RH. The sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J Neurosci. 1999;19(4):1324–1334. doi: 10.1523/JNEUROSCI.19-04-01324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta SQ, Hiesinger PR, Beronja S, Zhai RG, Schulze KL, Verstreken P, Cao Y, Zhou Y, Tepass U, Crair MC, Bellen HJ. Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron. 2005;46(2):219–232. doi: 10.1016/j.neuron.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Lalli G, Hall A. Ral GTPases regulate neurite branching through GAP-43 and the exocyst complex. J Cell Biol. 2005;171(5):857–869. doi: 10.1083/jcb.200507061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hertzog M, Chavrier P. Cell polarity during motile processes: keeping on track with the exocyst complex. Biochem J. 2011;433(3):403–409. doi: 10.1042/BJ20101214. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Guo W. The exocyst complex in exocytosis and cell migration. Protoplasma. 2011 doi: 10.1007/s00709-011-0330-1. [DOI] [PubMed] [Google Scholar]
- 33.Hala M, Cole R, Synek L, Drdova E, Pecenkova T, Nordheim A, Lamkemeyer T, Madlung J, Hochholdinger F, Fowler JE, Zarsky V. An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell. 2008;20(5):1330–1345. doi: 10.1105/tpc.108.059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fendrych M, Synek L, Pecenkova T, Toupalova H, Cole R, Drdova E, Nebesarova J, Sedinova M, Hala M, Fowler JE, Zarsky V. The Arabidopsis exocyst complex is involved in cytokinesis and cell plate maturation. Plant Cell. 2010;22(9):3053–3065. doi: 10.1105/tpc.110.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finger FP, Hughes TE, Novick P. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 1998;92(4):559–571. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- 36.Roumanie O, Wu H, Molk JN, Rossi G, Bloom K, Brennwald P. Rho GTPase regulation of exocytosis in yeast is independent of GTP hydrolysis and polarization of the exocyst complex. J Cell Biol. 2005;170(4):583–594. doi: 10.1083/jcb.200504108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Zajac A, Zhang J, Wang P, Li M, Murray J, TerBush D, Guo W. The critical role of Exo84p in the organization and polarized localization of the exocyst complex. J Biol Chem. 2005;280(21):20356–20364. doi: 10.1074/jbc.M500511200. [DOI] [PubMed] [Google Scholar]
- 38.Songer JA, Munson M. Sec6p anchors the assembled exocyst complex at sites of secretion. Mol Biol Cell. 2009;20(3):973–982. doi: 10.1091/mbc.E08-09-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyd C, Hughes T, Pypaert M, Novick P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J Cell Biol. 2004;167(5):889–901. doi: 10.1083/jcb.200408124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H, Rossi G, Brennwald P. The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 2008;18(9):397–404. doi: 10.1016/j.tcb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He B, Xi F, Zhang J, TerBush D, Zhang X, Guo W. Exo70p mediates the secretion of specific exocytic vesicles at early stages of the cell cycle for polarized cell growth. J Cell Biol. 2007;176(6):771–777. doi: 10.1083/jcb.200606134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adamo JE, Moskow JJ, Gladfelter AS, Viterbo D, Lew DJ, Brennwald PJ. Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J Cell Biol. 2001;155(4):581–592. doi: 10.1083/jcb.200106065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adamo JE, Rossi G, Brennwald P. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol Biol Cell. 1999;10(12):4121–4133. doi: 10.1091/mbc.10.12.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo W, Tamanoi F, Novick P. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat Cell Biol. 2001;3(4):353–360. doi: 10.1038/35070029. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Bi E, Novick P, Du L, Kozminski KG, Lipschutz JH, Guo W. Cdc42 interacts with the exocyst and regulates polarized secretion. J Biol Chem. 2001;276(50):46745–46750. doi: 10.1074/jbc.M107464200. [DOI] [PubMed] [Google Scholar]
- 46.Robinson NG, Guo L, Imai J, Toh EA, Matsui Y, Tamanoi F. Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol Cell Biol. 1999;19(5):3580–3587. doi: 10.1128/mcb.19.5.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoue M, Chang L, Hwang J, Chiang SH, Saltiel AR. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422(6932):629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- 48.Wu H, Turner C, Gardner J, Temple B, Brennwald P. The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol Biol Cell. 2010;21(3):430–442. doi: 10.1091/mbc.E09-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol. 2008;180(1):145–158. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutagalung AH, Coleman J, Pypaert M, Novick PJ. An internal domain of Exo70p is required for actin-independent localization and mediates assembly of specific exocyst components. Mol Biol Cell. 2009;20(1):153–163. doi: 10.1091/mbc.E08-02-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He B, Xi F, Zhang X, Zhang J, Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. Embo J. 2007;26(18):4053–4065. doi: 10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Zuo X, Yue P, Guo W. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol Biol Cell. 2007;18(11):4483–4492. doi: 10.1091/mbc.E07-05-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. Embo J. 1999;18(4):1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.TerBush DR, Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J Cell Biol. 1995;130(2):299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93(5):731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- 56.Bowser R, Muller H, Govindan B, Novick P. Sec8p and Sec15p are components of a plasma membrane-associated 19.5S particle that may function downstream of Sec4p to control exocytosis. J Cell Biol. 1992;118(5):1041–1056. doi: 10.1083/jcb.118.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgera F, Sallah MR, Dubuke ML, Gandhi P, Brewer DN, Carr CM, Munson M. Regulation of exocytosis by the exocyst subunit Sec6 and the SM protein Sec1. Mol Biol Cell. 2012;23(2):337–346. doi: 10.1091/mbc.E11-08-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bielinski DF, Pyun HY, Linko-Stentz K, Macara IG, Fine RE. Ral and Rab3a are major GTP-binding proteins of axonal rapid transport and synaptic vesicles and do not redistribute following depolarization stimulated synaptosomal exocytosis. Biochim Biophys Acta. 1993;1151(2):246–256. doi: 10.1016/0005-2736(93)90109-d. [DOI] [PubMed] [Google Scholar]
- 59.Moskalenko S, Tong C, Rosse C, Mirey G, Formstecher E, Daviet L, Camonis J, White MA. Ral GTPases regulate exocyst assembly through dual subunit interactions. J Biol Chem. 2003;278(51):51743–51748. doi: 10.1074/jbc.M308702200. [DOI] [PubMed] [Google Scholar]
- 60.Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA. The exocyst is a Ral effector complex. Nat Cell Biol. 2002;4(1):66–72. doi: 10.1038/ncb728. [DOI] [PubMed] [Google Scholar]
- 61.Hazelett CC, Sheff D, Yeaman C. RalA and RalB differentially regulate development of epithelial tight junctions. Mol Biol Cell. 2011;22(24):4787–4800. doi: 10.1091/mbc.E11-07-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bodemann BO, Orvedahl A, Cheng T, Ram RR, Ou YH, Formstecher E, Maiti M, Hazelett CC, Wauson EM, Balakireva M, Camonis JH, Yeaman C, Levine B, White MA. RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell. 2011;144(2):253–267. doi: 10.1016/j.cell.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103(32):11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goud B, Salminen A, Walworth NC, Novick PJ. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- 65.Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem. 2004 doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
- 66.Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol. 2005;12:879–885. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- 67.Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ. Sec15, a Component of the Exocyst, Promotes Notch Signaling during the Asymmetric Division of Drosophila Sensory Organ Precursors. Dev Cell. 2005 doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 68.Das A, Guo W. Rabs and the exocyst in ciliogenesis, tubulogenesis and beyond. Trends Cell Biol. 2011;21(7):383–386. doi: 10.1016/j.tcb.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen XW, Leto D, Xiao J, Goss J, Wang Q, Shavit JA, Xiong T, Yu G, Ginsburg D, Toomre D, Xu Z, Saltiel AR. Exocyst function is regulated by effector phosphorylation. Nat Cell Biol. 2011;13(5):580–588. doi: 10.1038/ncb2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao X, Ballew N, Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. Embo J. 1998;17(8):2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, Reinisch K, Hay JC, Ferro-Novick S. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445(7130):941–944. doi: 10.1038/nature05527. [DOI] [PubMed] [Google Scholar]
- 72.Stroupe C, Hickey CM, Mima J, Burfeind AS, Wickner W. Minimal membrane docking requirements revealed by reconstitution of Rab GTPase-dependent membrane fusion from purified components. Proc Natl Acad Sci U S A. 2009;106(42):17626–17633. doi: 10.1073/pnas.0903801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sivaram MV, Saporita JA, Furgason MLM, Boettcher AJ, Munson M. Dimerization of the exocyst protein Sec6p and its interaction with the t-SNARE Sec9p. Biochemistry. 2005;44(16):6302–6311. doi: 10.1021/bi048008z. [DOI] [PubMed] [Google Scholar]
- 74.Carr CM, Grote E, Munson M, Hughson FM, Novick PJ. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J Cell Biol. 1999;146(2):333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X, Wang P, Gangar A, Zhang J, Brennwald P, TerBush D, Guo W. Lethal giant larvae proteins interact with the exocyst complex and are involved in polarized exocytosis. J Cell Biol. 2005;170(2):273–283. doi: 10.1083/jcb.200502055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Starai VJ, Hickey CM, Wickner W. HOPS Proofreads the trans-SNARE Complex for Yeast Vacuole Fusion. Mol Biol Cell. 2008;19(6):2500–2508. doi: 10.1091/mbc.E08-01-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kramer L, Ungermann C. HOPS drives vacuole fusion by binding the vacuolar SNARE complex and the Vam7 PX domain via two distinct sites. Mol Biol Cell. 2011 doi: 10.1091/mbc.E11-02-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shestakova A, Suvorova E, Pavliv O, Khaidakova G, Lupashin V. Interaction of the conserved oligomeric Golgi complex with t-SNARE Syntaxin5a/Sed5 enhances intra-Golgi SNARE complex stability. J Cell Biol. 2007;179(6):1179–1192. doi: 10.1083/jcb.200705145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ren Y, Yip CK, Tripathi A, Huie D, Jeffrey PD, Walz T, Hughson FM. A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1. Cell. 2009;139(6):1119–1129. doi: 10.1016/j.cell.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wiederkehr A, Du Y, Pypaert M, Ferro-Novick S, Novick P. Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol Biol Cell. 2003;14(12):4770–4782. doi: 10.1091/mbc.E03-04-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Craene JO, Coleman J, Estrada de Martin P, Pypaert M, Anderson S, Yates JR, 3rd, Ferro-Novick S, Novick P. Rtn1p is involved in structuring the cortical endoplasmic reticulum. Mol Biol Cell. 2006;17(7):3009–3020. doi: 10.1091/mbc.E06-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mathieson EM, Suda Y, Nickas M, Snydsman B, Davis TN, Muller EG, Neiman AM. Vesicle docking to the spindle pole body is necessary to recruit the exocyst during membrane formation in Saccharomyces cerevisiae. Mol Biol Cell. 2010;21(21):3693–3707. doi: 10.1091/mbc.E10-07-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neiman AM. Prospore membrane formation defines a developmentally regulated branch of the secretory pathway in yeast. J Cell Biol. 1998;140(1):29–37. doi: 10.1083/jcb.140.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y, Liu CM, Emons AM, Ketelaar T. The plant exocyst. J Integr Plant Biol. 2010;52(2):138–146. doi: 10.1111/j.1744-7909.2010.00929.x. [DOI] [PubMed] [Google Scholar]
- 85.Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123(1):75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 86.Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, Frey AM, Peden AA, Gould GW, Prekeris R. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell. 2005;16(2):849–860. doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inoue M, Chiang SH, Chang L, Chen XW, Saltiel AR. Compartmentalization of the exocyst complex in lipid rafts controls Glut4 vesicle tethering. Mol Biol Cell. 2006;17(5):2303–2311. doi: 10.1091/mbc.E06-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Camonis JH, White MA. Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol. 2005;15(6):327–332. doi: 10.1016/j.tcb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 89.Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D'Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181(6):985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu J, Yue P, Artym VV, Mueller SC, Guo W. The Role of the Exocyst in MMP Secretion and Actin Dynamics during Tumor Cell Invadopodia Formation. Mol Biol Cell. 2009 doi: 10.1091/mbc.E08-09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nichols CD, Casanova JE. Salmonella-directed recruitment of new membrane to invasion foci via the host exocyst complex. Curr Biol. 2010;20(14):1316–1320. doi: 10.1016/j.cub.2010.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chien Y, Kim S, Bumeister R, Loo YM, Kwon SW, Johnson CL, Balakireva MG, Romeo Y, Kopelovich L, Gale M, Jr, Yeaman C, Camonis JH, Zhao Y, White MA. RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127(1):157–170. doi: 10.1016/j.cell.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 93.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461(7265):788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bendezu FO, Martin SG. Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast. Mol Biol Cell. 2011;22(1):44–53. doi: 10.1091/mbc.E10-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127(4):831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 96.Jin Y, Sultana A, Gandhi P, Franklin E, Hamamoto S, Khan AR, Munson M, Schekman R, Weisman LS. Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev Cell. 2011;21(6):1156–1170. doi: 10.1016/j.devcel.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pashkova N, Jin Y, Ramaswamy S, Weisman LS. Structural basis for myosin V discrimination between distinct cargoes. Embo J. 2006;25(4):693–700. doi: 10.1038/sj.emboj.7600965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang S, Hsu SC. The molecular mechanisms of the mammalian exocyst complex in exocytosis. Biochem Soc Trans. 2006;34(Pt 5):687–690. doi: 10.1042/BST0340687. [DOI] [PubMed] [Google Scholar]
- 99.Osman MA, Konopka JB, Cerione RA. Iqg1p links spatial and secretion landmarks to polarity and cytokinesis. J Cell Biol. 2002;159(4):601–611. doi: 10.1083/jcb.200205084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, Sternberg H, Yalovsky S. A Novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol. 2007;17(11):947–952. doi: 10.1016/j.cub.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 101.Rosse C, Formstecher E, Boeckeler K, Zhao Y, Kremerskothen J, White MD, Camonis JH, Parker PJ. An aPKC-exocyst complex controls paxillin phosphorylation and migration through localised JNK1 activation. PLoS Biol. 2009;7(11) doi: 10.1371/journal.pbio.1000235. e1000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zuo X, Zhang J, Zhang Y, Hsu SC, Zhou D, Guo W. Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat Cell Biol. 2006;8(12):1383–1388. doi: 10.1038/ncb1505. [DOI] [PubMed] [Google Scholar]
- 103.Thapa N, Sun Y, Schramp M, Choi S, Ling K, Anderson RA. Phosphoinositide signaling regulates the exocyst complex and polarized integrin trafficking in directionally migrating cells. Dev Cell. 2012;22(1):116–130. doi: 10.1016/j.devcel.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.