Abstract
Clinical application of small interfering RNA (siRNA) requires safe and efficient delivery in vivo. Here, we report the design and synthesis of lipid nanoparticles (LNPs) for siRNA delivery based on cationic lipids with multiple tertiary amines and hydrophobic linoleyl chains. LNPs incorporating the lipid containing tris(2-aminoethyl)amine (TREN) and 3 linoleyl chain, termed TRENL3, were found to have exceptionally high siRNA transfection efficacy that was markedly superior to lipofectamine, a commercial transfection agent. In addition, inclusion of polyunsaturated fatty acids, such as linoleic acid and linolenic acids in the formulation further enhanced the siRNA delivery efficiency. TRENL3 LNPs were further shown to transported siRNA into the cytosol primarily via macropinocytosis rather than clathrin-mediated endocytosis. The new LNPs have demonstrated preferential uptake by the liver and hepatocellular carcinoma in mice, thereby leading to high siRNA gene silencing activity. These data suggest potential therapeutic applications of TRENL3 mediated delivery of siRNA for liver diseases.
Keywords: Cationic lipids, Lipid nanoparticles, Small interfering RNA, hepatocellular carcinoma
1. Introduction
The liver plays a pivotal role in metabolism and is affected by diseases such as hyperlipidemia, hepatitis, cirrhosis and primary liver cancer. Primary liver cancer is the fifth most common malignancy in the world. In addition, liver is often times the organ where tumor metastases occur. Current available treatment for hepatocellular carcinoma (HCC), the most common primary liver cancer, is rarely curative and therefore more effective therapy is needed [1]. Small interfering RNAs (siRNAs) can silence expressions of disease-causing genes in a sequence-specific manner. They hold great promise for therapeutic applications in a wide spectrum of diseases [2–4]. Liver represents an attractive target organ for siRNA therapeutics because of the numerous associated disease indications including cholesterol biosynthesis, hepatitis, and HCC [5–8]. However, inefficient cellular uptake and poor stability of free siRNAs have limited their clinical applications. Therefore, the greatest challenge associated with siRNA therapy is the development of safe and efficacious in vivo delivery systems [9–12].
Lipid nanoparticles (LNPs) have been increasingly recognized as one of the most promising delivery systems for siRNA due to their biocompatibility and the ease of large-scale production [10–13]. In fact, they are currently the most validated vehicles for systemic delivery of siRNA to the liver and HCC, and have been utilized in clinical trials [5, 6, 14–17]. Typically, a LNP formulation consists of a cationic lipid, a neutral lipid and/or cholesterol and a PEG-lipid. Major factors that determine the delivery efficiency of a particular cationic lipid are the structure of the head group, the linker, and the length and degree of unsaturation of the hydrocarbon chain. The widely used cationic lipid, 1,2-dioleyloxy-3-trimethylammonium propane (DOTAP), which has a quaternary ammonium head group attached to two oleyl chains via ester linkages, only provides modest transfection activity in liver [18–20].
A lipid-based formulation, “Stable nucleic acid lipid particles” (SNALP), containing conditionally ionizable cationic lipids such as 1,2-dilinoleyloxy-3-dimethylaminopropane (DLinDMA), has demonstrated effective knockdown of several targets in the liver in recent years [16, 17]. DLinDMA, with a tertiary amine head group, is able to efficiently interact with nucleic acids at low pH and achieve a neutral or low positive surface charge for the SNALP at pH 7.4 [21]. The two unsaturated bonds on the linoleic chains also account for the high transfection efficacy of DLinDMA [21]. Semple et al [7] further synthesized a series of cationic lipids from cyclic ketals between head groups and two hydrophobic lipid chains. Another cationic lipid material, known as lipidoids, which possess multiple tertiary amines in their head group, has also shown good efficacy for delivery of siRNA to the liver [5, 16].
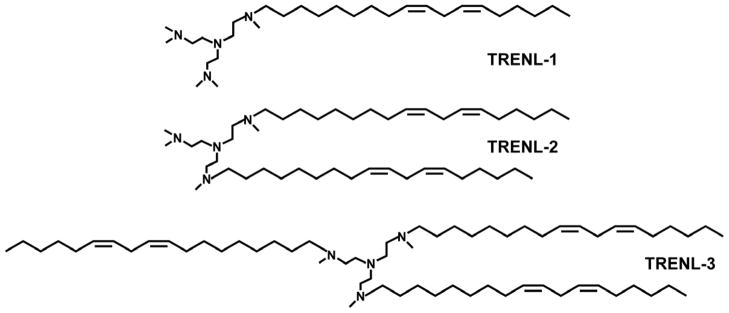
Since both the multiple tertiary amines in the head group in lipidoids and the high degree of unsaturation in hydrocarbon chains in DLinDMA appear to facilitate siRNA delivery, we designed a series of new cationic lipids by integrating a multi-tertiary amine head group and polyunsaturated hydrophobic linoleyl chain(s). Using tris(2-aminoethyl)amine (TREN) as a starting material, three new cationic lipids having four tertiary amines on the head group and one to three hydrophobic linoleyl chain(s) (termed as TRENL1, TRENL2 and TRENL3) were synthesized. To further enhance the delivery efficacy, we incorporated an unsaturated fatty acid, such as oleic acid, linoleic acid or linolenic acid, in LNPs incorporating these lipids. The physicochemical properties and intracellular trafficking behavior of these LNPs were investigated. In vitro and in vivo delivery efficacy of siRNA was examined as well.
2. Materials and Methods
2.1 Materials
Egg phosphatidylcholine (egg PC) was purchased from Lipoid (Newark, NJ). Cholesterol, oleic acid, linoleic acid and linolenic acid, tris(2-aminoethyl)amine (TREN) and 95% sodium hydride were purchased from Sigma-Aldrich (St. Louis, MO). (1,2-Dimyristoyl-sn-glycerol, methoxypolyethylene Glycol) (DMG-PEG) was from NOF America Corporation (White Plains, NY). Alkyl mesylates were purchased from Nu-Chek Prep, Inc. (Elysian, MN, USA). Deuterated chloroform (CDCl3) used as 1H NMR solvent was from Cambridge Isotope Laboratories (Andover, MA). All other organic solvents and reagents were obtained from Sigma-Aldrich (St. Louis, MO) and were used without further purification. RPMI-1640 media, fetal bovine serum (FBS), trypsin-EDTA and penicillin-streptomycin additives were purchased from Invitrogen (Grand Island, NY, USA). Luciferase (GL2 + GL3) siRNA (AM 4629), negative control siRNA (AM 4611), Cy3 labeled control siRNA (AM 4621) and FAM labeled control siRNA (AM 4620) were obtained from Applied Biosystems (Austin, TX, USA). Cy5 labeled oligonucleotide (ODN) (sequence: 5′-TCT CCC AGC GTG CGC CAT- 3′) was custom synthesized by Alpha DNA, Inc (Montreal, Canada).
2.2 Synthesis of TRENL series cationic lipids (TRENL1, TRENL2 and TRENL3)
TREN was first alkylated by linoleic mesylate (LM) at different TREN:LM ratios, and the remaining amines of TREN were methylated by a mixture of formic acid (HCOOH) and formaldehyde (HCHO) to obtain TRENL series lipids (Figure 1). The synthesis of TRENL3 is briefly described here as an example. Linoleyl mesylate (3.1g, 9 mmol) and 95% sodium hydride (0.075 g, 3 mmol) were added in a solution of TREN (0.44 g, 3 mmol) in 15 mL anhydrous chloroform. The mixture was stirred on a hot plate at 60°C for 12 h under nitrogen and the reaction mixture was then cooled in an ice bath followed by slow addition of ethanol. The reaction mixture was centrifuged at 2000 rpm for 5 min. The organic phase was transferred to a new flask and evaporated under vacuum. The crude product of TREN-LM (1:3) was dissolved in ethanol and filtered to remove precipitates. The raw product was collected for further methylation. Formic acid (2 g) was then added to a stirred aqueous solution of 1.5 g of TREN-LM (1:3) and 4 g of formaldehyde for 5 min in an ice bath. The mixture was refluxed under nitrogen for 24 h. After cooling, the reaction mixture was neutralized by NaOH (10 N) and washed by 30 ml of ethyl acetate twice. The aqueous layer was discarded and the organic phase was dried over magnesium sulfate at room temperature for 2 h and concentrated by rotary evaporation under vacuum. The oily residue was then dissolved in ethanol and evaporated again to get the final product TRENL3. As shown in Figure S2, the chemical composition of the starting materials and reaction products was determined by a 300 MHz 1H NMR spectroscope (Bruker 300 AM; Billerica, MA) in CDCl3.
Figure 1.
Chemical structures of three cationic lipid-like materials.
The precise linoleic chain/TREN ratio was calculated from the 1H NMR integral area of the protons from linoleic unit (0.81~0.84 ppm; terminal -CH3) and TREN unit (2.4~2.7 ppm;-N-CH2-CH2-NH2). The extent of methylation substitutions on TREN of the final TRENL analogs was analyzed by subtraction from the total number of –CH3 groups the number of –CH3 groups on linoleic chains. Data on the three TRENL based analogs are displayed in Table S1. Because of the high reactivity of mesylate with primary amine, TREN can be easily conjugated with LM and the position of carbon-carbon double bonds remains unchanged on the linoleic chain during the synthesis.
2.3 Preparation of LNP encapsulated siRNA
LNPs were prepared by ethanol dilution as described previously [22] with minor modification. Briefly, TRENL, Egg PC, Chol and DMG-PEG were dissolved in ethanol at a molar ratio of 45: 18: 35: 2. The lipid mixture was added to siRNA (20 mM citrate, pH 4) with a final ethanolic concentration of 30% (vol/vol), and incubated at room temperature for 10 min. The weight ratio of lipids/siRNA was kept at 10/1. Ethanol was removed by dialysis using a MWCO 100,000 Dalton Float-A-Lyser (Spectrum Laboratories Inc., Ranco Dominguez, CA) and against PBS (pH 7.4) for 2 h at room temperature. The resulting siRNA-LNPs were then sterilized using a 0.22 μm filter (Fisher Scientific, Pittsburgh, PA, USA). For tail vein injection to mice, the siRNA-LNPs were further concentrated to 200 μl using the Amicon® Ultra-4 Ultracel-50kDa centrifugal device (Millipore, Billerica, MA). For the preparation of siRNA-LNPs containing unsaturated fatty acid, the procedure was the same except that the Egg PC was replaced by fatty acids. The encapsulation efficiency of siRNA in LNPs was determined by RiboGreen assay (Invitrogen, Carlsbad, CA) [5, 7, 16].
2.4 Size and zeta potential measurements
The particle size of siRNA-LNPs was determined by dynamic light scattering using a particle sizer BI-200SM (Brookhaven Instruments Corp., Holtsville, NY, USA) in an intensity-weighted mode. Following dilution in water, the zeta potentials (ζ) of siRNA-LNPs was measured on a ZetaPALS zeta potential analyzer (Brookhaven Instrument Corp., Holtsville, NY).
2.5 Cell culture and transfection study
SK-HEP-1 cells, stably expressing the firefly luciferase gene, were plated at of 2 × 104 cells per well in 48-well plates and grown to 60–70% confluent prior to transfection. Luciferase specific siRNA (Luci-siRNA) and negative control (NC siRNA) were formulated into LNPs. Cells were treated with various siRNA-LNPs at indicated concentrations and incubated for another 24 h at 37°C and 5% CO2. The cells were then washed with PBS and lysed. The luciferase activity for each well was determined using Luciferase Reagent (Promega) on a Berthold MicroLumatPlus LB96V plate luminometer. The resulting luciferase activity was then normalized for the amount of protein using the Micro BCA assay kit (Pierce, Rockford, IL). Luciferase down-regulation relative to a control was then determined for each condition. Lipofectamine 2000 (Invitrogen, CA, USA) was used as a positive control. Untreated cells were used as a negative control.
2.6 Cytotoxicity study by MTS assay
The cytotoxicity of LNPs was evaluated in vitro using the MTS assay. SK-HEP-1 cells were seeded in 96-well plates at 1× 104 cells/well, allowed to adhere overnight and were transfected by LNPs containing NC siRNA. After 24 h, 20 μl of MTS reagent (Promega, Madison, WI) was added to each well and the incubation was continued for another 2 h. Subsequently, the optical density readings were performed using a microplate reader (GENios Pro, Tecan, USA) at 490 nm. Untreated SK-HEP-1 cells were used as a control and its viability was defined as 100%.
2.7. Cellular uptake of siRNA determined by flow cytometry
FAM-siRNA was used to study cellular uptake of LNPs. Briefly, 6 × 104 cells were seeded in 24-well plates prior to treatment. After 4 h exposure to the free FAM-siRNA or various FAM-siRNA encapsulated LNPs, the cells were rinsed three times with 500 μl PBS (pH 7.4) and fixed in 4% para-formaldehyde solution. The cell suspension was directly introduced into a Beckman Coulter EPICS XL (Beckman Coulter) to determine the fluorescence intensity of FAM (green). For each cell sample, a minimum of 10,000 events were collected under the LIST mode.
2.8 Cellular entry pathway study by confocal microscopy
To determine the endocytic pathways that may be involved in siRNA delivery by LNPs, co-localization experiments of Cy3 labeled siRNA with common pathway markers were performed by confocal microscopy. Briefly, 4 × 104 cells were seeded on a glass-bottom dish (Fisher Scientific, 12-545-82) in 24-well plates overnight, then incubated with various LNPs formulated Cy3-siRNA (100 nM) and common markers for macropinocytosis (70 kDa dextran-FITC, 2.5 mg/ml, Sigma), clathrin-mediated endocytosis (Transferrin-AlexaFluor 488, 100 mg/ml, Invitrogen), or caveolae mediated endocytosis (cholera toxin subunit B-AlexaFluor 488, 1 mg/ml, Invitrogen) [23, 24]. After incubation of 30 min at 37°C, the cells were washed twice with PBS followed by fixation with 4% para-formaldehyde for 20 min. Nuclei were stained with Hoechst 33342 (Invitrogen) for 5 min at room temperature. Red fluorescence of Cy3-siRNA and blue fluorescence of Hoechst were observed on an Olympus FV1000 Filter Confocal Microscope (Olympus Optical Co., Tokyo, Japan).
For confocal analysis of siRNA-LNPs trafficking, FAM-siRNA LNPs were used to perform transfection as described above. The seeded SK-HEP-1 cells were treated with various FAM-siRNA-LNPs (100 nM) for 2 h. To stain endosomes/lysosomes, the cells were incubated with culture medium containing 1 mg/ml of Lysotracker Red DND-99 (Invitrogen, CA, USA) for 30 min at 37°C, 5% CO2 and culture medium. The nuclei were stained with 5 mg/ml of Hoechst-33342 for 10 min at room temperature. After washing the cells twice with PBS, cells were fixed with 4% para-formaldehyde prior to confocal analysis.
2.9. Colloidal stability of siRNA encapsulated LNPs in serum
siRNA-LNPs were incubated at 37°C at a 1:1 volume ratio with fetal FBS diluted in PBS and the particle size of incubation mixtures were then determined by particle sizer BI-200SM (Brookhaven Instruments Corp., Holtsville, NY). The stability of Tf-LNPs was evaluated by monitoring changes in the mean particle diameter during storage at 4°C.
2.10 Cryogenic Transmission Electron Microscopy (Cryo-TEM)
Cryo-TEM imaging was performed at Technion-Israel Institute of Technology, Haifa, Israel, as previously described [22]. Briefly, samples were examined in a Philips CM120 microscope (Eindhoven, The Netherlands) at 120 kV, using an Oxford CT-3500 cooling holder and transfer station (Abingdon, England). Specimens were equilibrated in the microscope below −178°C, then examined in the low-dose imaging mode to minimize electron beam radiation damage, and recorded at a nominal under-focus of 1–2 μm to enhance phase contrast. Images were acquired digitally by a Gatan MultiScan 791 cooled charge-coupled device camera (Pleasanton, CA).
2.11 In vivo biodistribution study by confocal microscopy
LNPs containing 50 μg of Cy3-labeled siRNA were formulated as described above in a total volume of 0.2 ml and delivered into male ICR mice (20~25 g; Harlan Laboratories) by i.v. injection. Mice were sacrificed 4 h after injection. Then, the liver and tissue samples from lung, kidney, spleen and heart were harvested. To investigate the biodistribution in HCC, diethylnitrosamine (DEN) induced hepatocarcinoma model was developed according to the previously reported method [26]. Mice developed liver tumor upon DEN injection were treated with LNP containing Cy5-siRNA following the same protocol used for normal ICR mice. ODN was used because of its similar structure as but much lower cost than siRNA. Tissue samples from liver, lung, kidney, spleen, heart and tumor were harvested. Tissue samples were fixed in 4% paraformaldehyde/PBS for 6 h and then placed into a 30% sucrose/PBS solution overnight at 4°C. Fixed tissue samples were then placed into block holders containing OCT freezing medium (Fisher Scientific, Pittsburgh, PA) and snap-frozen in liquid nitrogen. Tissue sections were counterstained with Alexa-488 phalloidin (13 nM; Invitrogen, Carlsbad, CA) and Hoechst 33342 (1 μM; Invitrogen) in PBS for 20 min. The slides were mounted with the protection of anti-fade reagent (Invitrogen, Carlsbad, CA) and analyzed by using the Olympus FV1000 Filter confocal microscope (Olympus Optical Co., Tokyo, Japan).
2.12. Tissue distribution study by IVIS imaging
ICR mice and DEN induced tumor bearing mice were given i.v. injections of Cy5-labeled ODN containing LNPs. After 4 h, mice were euthanized and tissues were collected and fixed in 4% paraformaldehyde for 12 h. The tissues were then soaked in 30 wt% sucrose solution for another 12 h. The fluorescence signals of Cy5 emitted by the whole tissues were measured using a Xenogen IVIS-200 Optical In Vivo Imaging System (Caliper Life Sciences, Hopkinton, MA).
2.13 In vivo FVII siRNA down-regulation study
Five- to six-week-old ICR mice (20~25 g; Harlan Laboratories) received either saline or Factor VII siRNA (Ambion, Austin, TX) containing LNPs via tail vein injection at a dose of 1.0 mg/kg. Animals were anesthetized by isofluorane inhalation 48 h after administration, and blood was collected into serum separator tubes by retroorbital bleeding. Serum levels of Factor VII protein were determined in samples using a chromogenic assay (Biophen FVII, Aniara Corporation) according to the manufacturer’s protocols. Absorbance was measured at 405 nm and a calibration curve was generated using the serially diluted control serum to determine levels of Factor VII in serum from treated animals, relative to the saline-treated control animals.
2.14 Statistical analysis
The results were presented as the mean ± standard deviation (SD) of three repeat studies unless otherwise indicated. Statistical significance of the differences in fluorescent intensity data obtained by flow cytometry was examined using the Student’s t-Test.
3. Results
3.1 Synthesis of TRENL series lipids
The TRENL based analogs with structures shown in Figure 1 were synthesized by reacting TREN with LM and the remaining amines were methylated with the mixture of HCOOH and HCHO. Dependent on the initial ratio of LM to TREN, three TRENL based analogs (TRENL1, TRENL2 and TRENL3) were prepared. The precise chemical structures of resulting TRENL analogs were characterized by 1H-NMR (Figure S1) and the results of these new cationic lipids are summarized in Table S1.
3.2 Formation of siRNA LNPs containing TRENLs
Our cationic LNP formulations contain TRENLs, Chol and Egg PC, and DMG-PEG. Their siRNA encapsulation efficiency and physicochemical properties are given in Table 1. The siRNA-LNPs formulated by three TRENL cationic lipid-like materials resulted in approximately the same size (110 ~ 120 nm) and low polydispersity (< 0.2). siRNA encapsulation was more than 90% and the zeta potential was mildly positive. Overall, there is little difference among the three TRENLs containing siRNA-LNPs in terms of siRNA encapsulation efficiency and physicochemical properties.
Table 1.
Formulations of siRNA LNPs containing TRENLs
| Formulation | Particle size (nm) | PDI | Zeta potential (mV) (in water) | siRNA EE (%) |
|---|---|---|---|---|
| TRENL1 | 109 | 0.14 | 2.07 ± 0.85 | 98.4 |
| TRENL2 | 101 | 0.17 | 13.37 ± 1.01 | 98.7 |
| TRENL3 | 113 | 0.13 | 9.91 ± 1.11 | 99.4 |
PDI: polydispersity index. Values are mean +/− SD. EE: encapsulation efficiency
3.3 In vitro evaluation of siRNA-LNPs containing TRENL
The potential of these TRENL based LNPs for siRNA delivery was first evaluated in vitro in SK-HEP-1 cells stably expressing the luciferase gene. Luciferase specific and negative control siRNAs were delivered after complexation with a constant amount of TRENL lipids. The luciferase expression was determined 24 h after transfection. Lipofectamine 2000 was used as a positive control. All TRENL LNPs demonstrated effective luciferase silencing at 100 nM after 24 h transfection (see Figure 2A). Among them, TRENL2 and TRENL3 LNPs showed better luciferase silencing than TRENL1 LNPs. The transfection efficacy of TRENL3 LNPs was much better than that of lipofectamine 2000. The TRENL3 LNPs was very efficient even at a low siRNA concentration of 20 nM. These results clearly demonstrated the efficacy of TRENL lipids, which contain multi-tertiary amine head groups and hydrophobic linoleic chains.
Figure 2. In vitro evaluation of siRNA-LNPs containing TRENL.
(A) In vitro luciferase gene silencing by siRNA encapsulated in various TRENL based formulations. SK-HEP-1 cells, stably expressing the luciferase gene, were transfected with luciferase siRNA complexed with LNPs at concentrations of 100nM and 20nM for 24hr. As a positive control, lipofectamine 2000 was used in the experiment. (B) MTS study analysis of cell cytoxicity of various TRENL based LNPs. Values represent the mean ± S.D. (n = 4). (C) Down-regulation of Cdk4 by Cdk4 siRNA in TRENL3 based LNP. SK-HEP-1 cells were treated by Lipofectamine 2000/siRNA complex and TRENL3-LNP-siRNA at the concentration level of 100 nM for 48 h. Cdk4 protein level expression was determined by western blotting (1- untreated; 2-Lipofectamine-NC siRNA; 3-Lipofectamine-Cdk4 siRNA; 4- TRENL3-LNP-NC siRNA; 5-TRENL3-LNP-Cdk4 siRNA). The quantification of Cdk4 protein level was normalized by β-actin.
The cytotoxicity of negative control siRNA (NC siRNA) LNPs was evaluated in vitro. SK-HEP-1 cells were transfected with NC siRNA LNPs at various concentrations. At 24 h post transfection, the cell viability was measured using the MTS assay. As shown in Figure 2B, over 95% average cell viability was observed among cells transfected with LNPs vs. untreated cells, suggesting that the LNPs did not show significant cytotoxicity in SK-HEP-1 cells. However, the TRENL1 LNPs with single linolenic chain was cytotoxic at 200 nM.
To further assess the siRNA delivery efficiency of TRENL3, another therapeutic siRNA targeting Cdk4 was examined. SK-HEP-1 cells treated with Cdk4 siRNA in TRENL3 based LNPs exhibited a significant decrease in Cdk4 protein levels after 48 h as compared with cells without any treatment and cells treated with Lipofectamine complexed Cdk4 siRNA (Figure 2C). This result was in agreement with the luciferase siRNA silencing study in Figure 2A.
3.4 Enhancment of in vitro delivery efficiency by incorporating unsaturated fatty acid into LNPs
After identifying a promising cationic lipid component TRENL3, we tried to further improve the efficacy of the LNP formulation by incorporating unsaturated fatty acids. The monounsaturated oleic acid (OA) and polyunsaturated linoleic acid (LA) and linolenic acid (LNA) were placed in the formulation replacing Egg PC. Chemical structures of these materials are illustrated in Figure 3A. We hypothesize that the fatty acid would enhance pH-dependent destablization and enhance the endosomal escape of siRNA in the TRENL3 based LNPs. In addition, the TRENL3 based LNPs (TRENL3/Egg PC/Chol/DMG-PEG=45/18/35/2) showed a positive charge (Table 1). Neutralizing the charge of the LNPs with fatty acids may potentially improve the biodistribution following intravenously administration.
Figure 3. Enhanced in vitro delivery efficiency by including unsaturated fatty acid in LNPs.
(A) Chemical structures of three unsaturated fatty acids. (B) Cryo-TEM image of nanostrucure of siRNA-LNP containing TRENL3/LA. White arrows show the onion-like structure of LNPs. (C) In vitro luciferase gene silencing by TRENL3 based LNPs containing various fatty acids. SK-HEP-1 cells were transfected with luciferase siRNA complexed with LNPs at indicated concentrations for 24hr. The luciferase expression was analyzed by luminance. (D) MTS study analysis of cell cytoxicity of various TRENL3 based LNPs. Values represent the mean ± S.D. (n = 4).
The size and surface charge of the resultant particles are listed in Table 2. As expected, the addition of unsaturated fatty acids in the formulation greatly decreased the surface charge because of the neutralization of negative –COOH group by positive charged TRENL3. Comparing with the parent TRENL3 LNPs (~13 mV), TRENL3 LNPs with fatty acids (OA, LA and LNA) had a much lower surface charge even though they still contain 45% cationic TRENL3. Interestingly, the introduction of unsaturated fatty acids slightly reduced the mean diameter of particles and particle size distribution. The nanostructures of the siRNA-LNPs containing TRENL3/LA, were studied by cryo-TEM and are shown in Figure 3B. The white-arrow indicates “onion-like” nanostructures that are known as the classic siRNA/lipoplex structure [22, 25]. The onion-like nanostructures, where the siRNAs are sandwiched between two adjacent lipid bilayers, are formed by the so-called “zipper” effect [26]. There is little difference in morphology between TRENL3 and TREN3L/LA nanoparticles.
Table 2.
Formulations of siRNA LNPs containing TRENL3 and unsaturated fatty acids
| Formulation | Particle size (nm) | PDI | Zeta potential (mV) (in water) | siRNA EE (%) |
|---|---|---|---|---|
| TRENL3-OA | 123 | 0.09 | 3.43 ± 0.66 | 97.3 |
| TRENL3-LA | 125 | 0.08 | 4.02 ± 0.57 | 90.8 |
| TRENL3-LNA | 116 | 0.14 | −0.98 ± 1.15 | 93.2 |
PDI: polydispersity index. Values are mean +/− SD. EE: encapsulation efficiency
The luciferase silencing by siRNA was determined in SK-HEP-1 cells to assess the effect of unsaturated fatty acids on the delivery efficacy of LNPs. Figure 3C shows that the luciferase level, expressed relative to the level of untreated cells, was significantly reduced when siRNAs (100, 20 and 10 nM) were transfected using LA and LNA containing LNP formulations, compared to that of the parent TRENL3 formulation. The introduction of LA or LNA significantly promoted gene silencing at both 100 and 20 nM siRNA concentrations after 24 h transfection. The transfection efficiency of the formulation TRENL3-LA or TRENL3-LNA LNPs is approximately 5-fold of that of lipofectamine. At lower siRNA concentrations (1~5 nM), the LNP containing TRENL3/LA or TRENNL3/LNA still demonstrated potent down-regulation efficiency. Furthermore, as shown in Figure 3D, the introduction of unsaturated fatty acids (OA, LA and LNA) did not increase the cytotoxicity of the LNPs.
3.5 Mechanisms of siRNA delivery by TRENL based LNPs
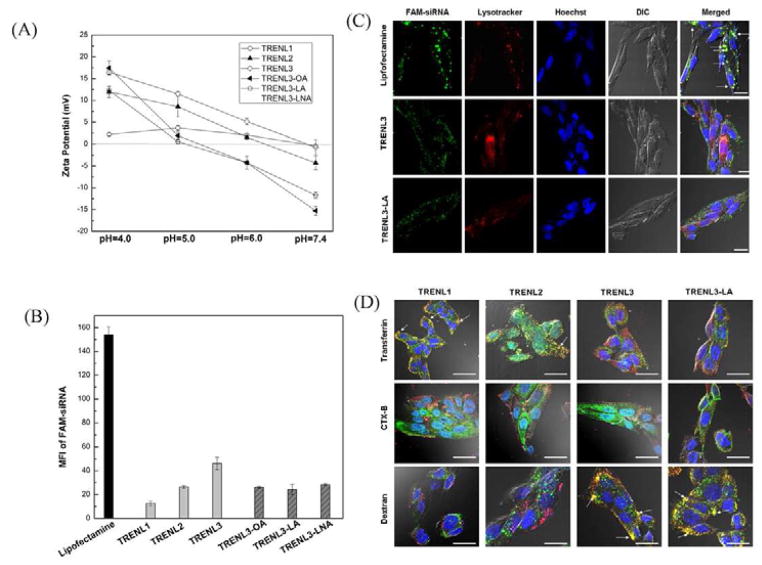
To confirm the ionizability of our TRENL based LNPs, we examined the zeta potential change of our LNPs as a function of the pH value. As shown in Figure 4A, TRENL3 showed much stronger ionization than TRENL1 and TRENL2, rendering it positively charged at acidic pH but close to charge-neutral at physiological pH. The incorporation of unsaturated fatty acids in TRNEL3 based LNPs further improved their pH-sensitive ionization. When pH was lower than 5.0, the nanoparticles showed positive surface charges, and the zeta potential was between 10 and 20 mV. When pH was higher than 6.0, the nanoparticles became negatively charged, and the zeta potential was between −5 and −20 mV (Figure 4A). Since the pH of lysosome was below 5.0, the surface charge reversal from negative to positive would occur when siRNA loaded LNPs were taken up and transported to endosome, providing a mechanism for the endosomal escape of siRNA. Furthermore, the cationic LNPs may interact with vesicular membranes leading to localized destabilization of the membrane and the escape of nanoparticles into cytoplasmic compartment. Thus, the LNPs containing TRENL3/LA or LNA could provide high potential for endosomal escape.
Figure 4. Mechanism study of siRNA delivery by TRENL based LNPs.
(A) Measurement of pH-dependent zeta potential of various TRENL based LNPs. (B) Ceullar uptake of LNPs carrying FAM- siRNA by SK-HEP-1 cells. Cells were treated various LNPs formulated FAM-siRNA (100 nM) at 4 h and the mean fluorescence intensity (MFI) was determined by flow cytometry. (C) Internalization mechanism of siRNA delivery by TRENL based LNPs. SK-HEP-1 cells were treated with 100 nM Cy3 labeled siRNA (red) in the presence of various labeled markers for 1 h. Markers for macropinosomes (70 kDa dextran), clathrin pits (transferrin), and caveosomes(CT-B). (D) Co-localization study of LNP-FAM-siRNA (green) with lysotracker (red). White arrows indicate the colocalization. Scale bar=20 μm.
Understanding the uptake mechanisms of LNPs is important. We therefore investigated the internalization and intra-cellular trafficking of siRNA-LNPs in SK-HEP-1 cells. For cellular uptake, nonspecific siRNA labeled by FAM was complexed with various TRENL based LNPs. Flow cytometry was used to study the cellular uptake of FAM-siRNA-LNPs. As shown in Figure 4B, the fluorescence signal of cells transfected with Cy3-siRNA-LNPs was much less than that of cells transfected with Cy3-siRNA/lipofectamine complexes at 4 h. In addition, the MFI of FAM-siRNA mediated by TRENL3 was higher than that mediated by TRENL1 or TRENL2. The inclusion of fatty acids in TRENL3 containing LNPs reduced the cellular uptake, which may be due to the reduced surface charge (Table 2).
To define the endocytotic route of TRENL mediated siRNA delivery, SK-HEP-1 cells were subjected to treatment with Cy3-siRNA-LNPs (red) and co-labeled with specific markers of endocytic pathways (green): clathrin-mediated endocytosis (transferrin), caveolae mediated endocytosis (cholera toxin B and macropinocytosis (70 kDa dextran). Figure 4C shows that siRNA delivered by TRENL3 based LNPs did not co-localize with transferrin or CT-B, a common pathway marker for the classical clathrin and caveolae mediated endocytosis, respectively [5, 23]. However, siRNA-LNPs formulated by TRENL3 co-localized well with the dextran marker, indicating macropinocytosis. In contrast, internalization of siRNA-LNPs containing TRENL1 or TRENL2 did not show macropinocytosis or caveolae mediated endocytosis, in view of the lack of co-localization of the Cy3-siRNA with either dextran or CTX-B (Figure 4C). Instead, LNPs containing TRENL1 or TRENL2 co-localized strongly with transferrin, suggesting that both TRENL1 and TRENL2 mediated siRNA delivery occurred by the classical clathrin associated endocytosis. Hence, better cellular uptake together with less entry into lysosomes might explain the observed high transfection efficiency of siRNA-LNPs containing TRENL3. Figure 4C shows that LNPs comprised of TRENL3/LNA also followed the macropinocytosis mechanism.
To further examine the intracellular trafficking of TRENL3 and TRENL3/LA LNPs, we performed co-localization study of LNPs with LysoTracker by confocal imaging to determine the endosomal escape rate of siNRA. As shown in Figure 4D, co-localization between the siRNA and the endosomal compartment was clearly observed at 4 h for lipofectamine, as revealed by the significant overlap of the fluorescence signals contributed by the FAM labeled siRNA (green) and the endosomal compartment labeled with NDN-189 (red) [27]. In contrast, the siRNA signals (green) of LNPs containing TRENL3 or TRENL3/LA were separated from the red endosomal compartment. This further explains why TRENL3 and TRENL3/LA LNPs mediated siRNA delivery could provide much greater gene silencing activity than Lipofectamine/siRNA complexes.
3.6 In vivo delivery of siRNA to liver and liver tumor by TRENL3 based LNPs
Prior to performing the in vivo study, we evaluated the storage and serum stability of two promising LNPs, TRENL3 and TRENL3-LA, by monitoring changes in the mean particle size. Storage stability assays for long-term colloidal stability were carried out over 1 month at 4°C. Particle sizing measurements demonstrated that TRENL3-LA based siRNA-LNPs were more stable than TRENL3 based siRNA-LNPs (Figure 5A). TRENL3 based siRNA-LNP showed gradual increase in particle size over the first two weeks and then remained essentially unchanged. To evaluate the serum stability, the siRNA encapsulated LNPs were mixed with FBS at a 1:1 (v/v) ratio and incubated at 37°C. According to Figure 5B, there was no significant change in the average size of the TRENL3/LA based siRNA-LNPs over 24 h, whereas the particle size of TRENL3 based siRNA-LNAs increased significantly over 6 h. This result suggests that the TRENL3/LA based LNPs has excellent stability.
Figure 5. Colloidal stability of siRNA encapsulated LNPs in serum.
(A) Colloidal stability of TRENL3-LA-based LNP versus TRENL based LNP. (B) Serum stability of TRENL3-LA-based LNP versus TRENL based LNP.
To assess siRNA delivery by LNPs in vivo, the liver distributions of mice injected with various LNPs were examined by confocal imaging. Confocal images of liver sections taken from mice 4 h after tail vein injection of two LNPs containing the Cy3-labeled siRNA (2.5 mg/kg) are shown in Figure 6A. Comparing to TRENL3 formulated LNPs, we observed preferential accumulation of Cy3-siRNA (red) in liver and nearly homogenous distribution throughout the different zones of the liver section by LNPs containing TRENL3/LA. This could be explained by the rapid increase in particle size in serum (Figure 5B) of the TRENL3 based LNPs, leading to poorer accumulation in liver. To examine the distribution of siRNA delivery to other organs, sections of kidney, spleen, heart and lung were also examined for fluorescence after single dose injection of Cy3-siRNA in TRENL3-LA based LNPs (Figure 6B). The results revealed slight accumulation in heart and lung, and minor accumulation in kidney. There was a small amount of uptake in spleen, but at a level that was significantly lower than that in liver (Figure 6A).
Figure 6. In vivo biodistribution study of LNP mediated systemic delivery of siRNA.
Confocal microscopic imaging of liver sections (A) and other organs (B). Mice were injected with TRENL3-LA based LNPs carrying Cy3-siRNA via tail vein. After 4 hours the liver was harvested and sections were counterstained with Alexa Fluor 488-Phalloidin (for actin) and Hoechst 33342 (for nuclei). Scale bar=20 μm. (C) Tissue distribution of Cy5-ODN containing LNP with TRENL3 and LA. 4 hr after intravenous administration, tissues were harvested and then Cy5 fluorescence signals were measured by IVIS imaging system. Left panel: a typical Cy5 fluorescence images of vital organs. Right panel: biodistribution based on the intensity of Cy5 signal (n=4). (D) A representative IVIS fluorescence image of tissues from a DEN induced mouse treated by Cy5-ODN (2.5 mg/kg) in LNP with TRENL3 and LA for 4 h. (E) Confocal images of liver and liver tumor sections from the DEN mouse treated by TRENL3-LA based LNP carrying Cy3-siRNA. After 4 hours the liver and liver tumor was harvested and sections were counterstained with Alexa Fluor 488-Phalloidin (for actin) and Hoechst 33342 (for nuclei). Scale bar=40 μm.
Besides the qualitative analysis of biodistribution by confocal imaging, we also evaluated the distribution of TRENL3-LA mediated siRNA delivery using the IVIS imaging system. We used Cy5 labeled ODN as a model material to screen the biodistribution of TRENL3-LA based LNPs in vivo. Two hundred μL of Cy5-ODN containing LNPs were given to each mouse at 2.5 mg/kg through tail vain injection. Major organs including liver, lung, kidney, spleen and heart were harvested 4 h later. The fluorescence signals of Cy5 were analyzed by the IVIS system and compared with untreated mice. Figure 6C clearly shows that the TRENL3-LA based LNPs facilitated preferential liver accumulation compared with other organs. Inspection of other organs revealed minor Cy5-fluorescence in spleen and kidney, with levels at least 6-fold lower than that in liver. These results further confirm our observation in the confocal imaging study (Figures 6A and 6B).
We next investigated the siRNA delivery by TRENL3-LA based LNPs in DEN induced tumor-bearing mouse model. DEN induced tumor model is frequently used as the animal model for HCC due to its similar tumor and vessel structure with that in human HCC. Therefore, we used this model to test if LNP can be systemically delivered to tumor localized in the liver. The biodistribution was evaluated by both IVIS imaging and confocal imaging. LNPs carrying Cy5-ODN (~100 nm in diameter and 0.48±1.28 mV in zeta potential) were injected through tail vein at the dose of 2.5 mg/kg. After 4 h harvest, the liver, liver tumor and other organs were subjected to the IVIS imaging system (Figure 6D). Then, the sections of liver and liver tumor were observed by confocal microscopy (Figure 6E). As shown in Figures 6D and 6E, the TRENL3-LA containing LNPs mediated high levels of accumulation of Cy5-ODN in normal liver as well as in liver tumor.
3.7 In vivo down-regulation of LNPs carrying siRNA
We further assessed the ability of TRENL-LA based siRNA-LNPs to knockdown targets in vivo. siRNA targeting the hepatocyte-specific blood-clotting protein Factor VII was used as a model. LNPs containing FVII siRNA were delivered to ICR mice by a single i.v. injection (1.0 mg/kg). At 48 h post-injection, mice were bled and assayed for FVII protein levels. Notably, LNPs carrying FVII siRNA was able to decrease the Factor VII level in serum by at least 30% when compared to a saline control and LNPs carrying NC-siRNA (Figure 7). No silencing of Factor VII was observed when a negative control siRNA was used. These data indicate that the observed gene silencing is a direct result of efficient siRNA delivery to liver by TRENL3-LA based LNPs.
Figure 7. In vivo down-regulation by siRNA encapsulated in the TRENL3-LA based LNP.
A single 1 mg/kg Factor VII siRNA dose was administered via tail vein injection to ICR mice. Livers were harvested at 48 hours postdose and assayed using a chromogenic assay. Data were normalized to PBS-treated mice and represented as group mean ± SD, n = 3/group. TRENL3-LA carrying Factor VII siRNA was that carrying NC siRNA(*P < 0.05).
4. Discussion
RNA interference is a powerful specific gene-silencing mechanism. Clinical application of RNAi requires the development of safe and effective delivery systems. Lipid and lipid-like materials are currently the most well-studied siRNA delivery systems for the liver, having been utilized in several animal models including non-human primates and in clinical trials [5, 6, 14–17]. Recent studies suggest that both the multiple tertiary amines in the head group in lipidoids and the high degree of unsaturation in hydrocarbon chains such as linoleic chains in DLinDMA are able to facilitate siRNA delivery [5, 21, 28]. In this study, designed and synthesized new cationic lipids possessing four protonatable tertiary amines and C18:2 linoleic chains. Our data demonstrated high efficacy by integrating a multi-tertiary amine head group and unsaturated hydrophobic linoleyl chains to create a potent nanocarrier for siRNA delivery.
There are a variety of factors that would influence the transfection efficiency of cationic lipid components and LNPs [7, 17, 21, 29]. Undergoing the efficient phase transition from a lamellar to an inverted hexagonal phase in the endosomal environment is one of the key design factors. The “lipid shape theory” is usually adopted to evaluate the phase transition, which assumes that amphiphilic molecules possessing broad “splayed” tails and small head groups are relatively easy to induce the transition from the lamellar phase to the hexagonal phase [7, 30]. This explains why TRENL3, which has triple hydrophobic linoleyl chains, possesses the strongest fusgenic property among the three TRENL based analogs due to its most broadly “splayed” tails. Cationic lipid based LNPs have positive surface charge, which enhances their interaction with cell membrane and subsequently facilitates endocytosis mediated cellular uptake. Endocytosis is the most common pathway for the cell entry of non-viral delivery vehicles, including LNPs [31, 32]. It has been well recognized that endosomal escape is a rate and efficiency limiting step for LNP based delivery systems [4, 31]. Our study revealed that the cell entry of TRENL3 mediated siRNA delivery is through macropinocytosis (Figure 4C), which could avoid the lysosomal degradation [5, 31]. Additionally, the cellular uptake of siRNA delivered by TRENL3 based LNPs was higher than that by TRENL1 or TRENL2 based LNPs (Figure 4B) possibly because of their different endocytosis pathway.
After identifying a lead TRENL3 molecule that showed potent in vitro efficacy, we further improved our LNP formulation by introducing an unsaturated fatty acid, such as LA and LNA, to replace the helper lipid egg PC to enhance the pH-triggered ionization and to shield the positive charge of TRENL3 based LNPs. Our results demonstrated that TRENL3 based LNPs containing LA or LNA have much stronger pH sensitivity in degree of ionization. Moreover, the introduction of LA did not influence the preference for macropinocytosis pathway of LNP cellular uptake (Figure 4). Although showing slightly lower cellular uptake compared to TRENL3 LNPs, the unsaturated fatty acids greatly enhanced the gene silencing ability of LNPs even at very low siRNA concentrations (1~5 nM) (Figure 3). The physical characterization (Table 2) and stability studies (Figure 5A) of LNPs revealed a smaller particle size, close to neutral charge, a higher siRNA entrapment efficiency and better stability of the TRENL-LA based LNPs.
Liver represents an ideal tissue target for LNP-based siRNA carriers. Due to the presence of sinusoidal endothelium, with fenestrae of 100~150 nm, hepatocytes are highly accessible to nanoparticles less than 150 nm in diameter [8, 10]. In contrast, endothelia of most tissues are impermeable to nanoparticles. In addition to the effect of particle size, surface charge also plays an important role to determine the uptake of nanoparticles by hepatocytes. ApoE is found to favorably adsorb onto neutral liposomes, enhancing uptake into hepatoma cells and primary hepatocytes [33]. Recent research suggested that ApoE could act as an endogenous targeting ligand to facilitate ionizable LNPs to hepatocytes in vivo [29]. Furthermore, the fatty acid metabolism has been mentioned as a way to enhance hepatocyte uptake of nanoparticles [8]. Since LA is an essential fatty acid involved in human fatty acid metabolism by hepatocytes via its putative plasma membrane transporter [8], thus, inclusion of LA in the formulation may facilitate preferential localization of LNPs to liver. For liver tumor such as spontaneous tumor model induced by DEN, LNPs can be more selectively accumulated there by passive targeting via the enhanced permeability and retention (EPR) effect [13, 34]. Although Kupffer cells (liver macrophages), which are part of the RES, are likely to take up a substantial fraction of nanoparticles due to their phagocytic activity and inherent affinity to nanoparticles, PEGylation of nanoparticles can reduce Kupffer cell uptake by reducing binding to plasma proteins.
To study the efficacy of LNP to liver tumor, we have generated the DEN induced mice model with the spontaneous tumor after 12 months of DEN treatment. This animal model is an ideal HCC model because the vasculature and morphological structure of HCC in this model better mimics human HCC compared to other commonly used animal model such as c-Myc transgenic, or orthotopic or subcutaneous HCC xenograft model. Clearly, the LNPs based on TRENL3/LA demonstrated preferential uptake by liver and liver tumor. However, this DEN induced HCC model may not be ideal for the theraputical study of siRNA mediated by LNPs because the mice require the siRNA treatment at young stage and it takes several months to evaluate the tumor growth. Furthermore, unlike xenograft mice model, it is difficult to monitor the tumor growth by the direct measurement of tumor size.
5. Conclusions
In summary, we have developed LNPs for the delivery of siRNA to liver and liver tumor. The most efficient cationic lipid-like material possesses four tertiary amines at head group and triple hydrophobic linoleyl chains. Adding unsaturated fatty acids such as LA into the LNP formulation further enhanced the potent siRNA delivery efficacy both in vitro and in vivo. The enhanced pH-sensitive, ionizability and the cellular uptake via macropinocytosis are believed to be the main reasons of the observed high efficacy of the TRENL3-LA based LNPs. The association with certain serum proteins such as ApoE could partially explain why the TRENL3-LA based LNPs showed the predominant uptake by liver and liver tumor. LNPs may serve as a valuable nanocarrier for in vivo targeting and siRNA therapeutic use in liver related diseases.
Supplementary Material
Acknowledgments
This work was supported by NSF Nanoscale Science and Engineering Center (NSEC) grant EEC-0425626 and NIH grants R01DK088076 and R21CA152969. We thank Brian Kemmenoe and Sara Cole at CMIF of OSU for advice and assistance in generating the confocal data. We are very grateful to Ms. Sharon Golan and Prof. Yeshayahu Talmon at Technion, Israel for their help in taking cryo-TEM images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arbuthnot P, Thompson LJ. Harnessing the RNA interference pathway to advance treatment and prevention of hepatocellular carcinoma. World J Gastroenterol. 2008;14:1670–81. doi: 10.3748/wjg.14.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59:75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–53. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juliano R, Alam MR, Dixit V, Kang H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008;36:4158–71. doi: 10.1093/nar/gkn342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A. 2010;107:1864–9. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–6. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 8.Li LJ, Wang HY, Ong ZY, Xu KJ, Ee PLR, Zheng SS, et al. Polymer- and lipid-based nanoparticle therapeutics for the treatment of liver diseases. Nano Today. 2010;5:296–312. [Google Scholar]
- 9.Rozema DB, Lewis DL, Wakefield DH, Wong SC, Klein JJ, Roesch PL, et al. Dynamic Poly Conjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Natl Acad Sci U S A. 2007;104:12982–7. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2010;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanton MG, Colletti SL. Medicinal chemistry of siRNA delivery. J Med Chem. 2010;53:7887–901. doi: 10.1021/jm1003914. [DOI] [PubMed] [Google Scholar]
- 12.Wu SY, McMillan NA. Lipidic systems for in vivo siRNA delivery. AAPS J. 2009;11:639–52. doi: 10.1208/s12248-009-9140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu B, Zhao X, Lee LJ, Lee RJ. Targeted delivery systems for oligonucleotide therapeutics. AAPS J. 2009;11:195–203. doi: 10.1208/s12248-009-9096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adami RC, Seth S, Harvie P, Johns R, Fam R, Fosnaugh K, et al. An amino acid-based amphoteric liposomal delivery system for systemic administration of siRNA. Mol Ther. 2011;19:1141–51. doi: 10.1038/mt.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin X, Li L, Wang R, Wilcox D, Zhao X, Song J, et al. A robust in vivo positive-readout system for monitoring siRNA delivery to xenograft tumors. RNA. 2011;17:603–12. doi: 10.1261/rna.2546011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561–9. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Judge AD, Robbins M, Tavakoli I, Levi J, Hu L, Fronda A, et al. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest. 2009;119:661–73. doi: 10.1172/JCI37515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podesta JE, Kostarelos K. Chapter 17 - Engineering cationic liposome siRNA complexes for in vitro and in vivo delivery. Methods Enzymol. 2009;464:343–54. doi: 10.1016/S0076-6879(09)64017-9. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Bathula SR, Yang Q, Huang L. Targeted nanoparticles deliver siRNA to melanoma. J Invest Dermatol. 2010;130:2790–8. doi: 10.1038/jid.2010.222. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Zhu X, Zhang X, Liu B, Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol Ther. 2010;18:1650–6. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyes J, Palmer L, Bremner K, MacLachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J Control Release. 2005;107:276–87. doi: 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Yang X, Koh CG, Liu S, Pan X, Santhanam R, Yu B, et al. Transferrin receptor-targeted lipid nanoparticles for delivery of an antisense oligodeoxyribonucleotide against Bcl-2. Mol Pharm. 2009;6:221–30. doi: 10.1021/mp800149s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaneda MM, Sasaki Y, Lanza GM, Milbrandt J, Wickline SA. Mechanisms of nucleotide trafficking during siRNA delivery to endothelial cells using perfluorocarbon nanoemulsions. Biomaterials. 2010;31:3079–86. doi: 10.1016/j.biomaterials.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Zhou C, Kwang KJ, Wang X, Yung B, Lee LJ, et al. Efficient siRNA delivery using a polyamidoamine dendrimer with a modified pentaerythritol core. Pharm Res. 2012;29:11–20. doi: 10.1007/s11095-012-0676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geusens B, Lambert J, De Smedt SC, Buyens K, Sanders NN, Van Gele M. Ultradeformable cationic liposomes for delivery of small interfering RNA (siRNA) into human primary melanocytes. J Control Release. 2009;133:214–20. doi: 10.1016/j.jconrel.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Weisman S, Hirsch-Lerner D, Barenholz Y, Talmon Y. Nanostructure of cationic lipid-oligonucleotide complexes. Biophys J. 2004;87:609–14. doi: 10.1529/biophysj.103.033480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C, Mao Y, Sugimoto Y, Zhang Y, Kanthamnen N, Yu B, et al. SPANosomes as delivery vehicles for small interfering RNA (siRNA) Mol Pharm. 2012;9:201–10. doi: 10.1021/mp200426h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akinc A, Goldberg M, Qin J, Dorkin JR, Gamba-Vitalo C, Maier M, et al. Development of lipidoid-siRNA formulations for systemic delivery to the liver. Mol Ther. 2009;17:872–9. doi: 10.1038/mt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18:1357–64. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lafleur M, Bloom M, Cullis PR. Lipid polymorphism and hydrocarbon order. Biochem Cell Biol. 1990;68:1–8. doi: 10.1139/o90-001. [DOI] [PubMed] [Google Scholar]
- 31.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145:182–95. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. Cell Mol Life Sci. 2009;66:2873–96. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng XH, Cao ZH, Xia JT, Carlson GW, Lewis MM, Wood WC, et al. Real-time detection of gene expression in cancer cells using molecular beacon imaging: new strategies for cancer research. Cancer Res. 2005;65:1909–17. doi: 10.1158/0008-5472.CAN-04-3196. [DOI] [PubMed] [Google Scholar]
- 34.Huang L, Liu Y. In vivo delivery of RNAi with lipid-based nanoparticles. Annu Rev Biomed Eng. 2011;13:507–30. doi: 10.1146/annurev-bioeng-071910-124709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.