Abstract
Efforts to enhance therapy for children and adults with sickle cell disease have proven more challenging than might have been predicted from the fact that an understanding of the underlying pathogenesis antedated that of many other diseases for which good treatments presently exist. The multi-organ injury that occurs with sickle cell disease certainly contributes to this clinical reality. Research over decades indicates that the primary defect in hemoglobin that results in polymerization of the protein under low oxygen conditions and resultant cellular deformity of the red blood cell initiates a complex downstream pathogenesis associated with vascular injury and organ ischemia. Deciphering this in a manner that informs successful therapies that improve all target organs continues to challenge hematologists. The National Heart, Lung and Blood Institute (NHLBI) is dedicated to support research across the basic science, translational a clinical spectrum to achieve these clinical outcomes. The following provides a brief summary of the research strategies which NHLBI is presently supporting and will support in the future to enhance care and ultimately, to effect cure of this hemoglobin disease that causes such suffering to those who inherit this monogenic disease.
Keywords: sickle cell disease, research, global health
Introduction
A year before sickle cell disease (SCD) was termed the first molecular disease by Linus Pauling in 1949, the National Heart Institute (now Heart, Lung, and Blood Institute, NHLBI) at the National Institutes of Health (NIH) had funded research on the basic pathogenesis of this multi-organ disease. The fundamental research focused on erythroid cells defined the chemistry of sickle hemoglobin polymerization, described the resultant red blood cell membrane dysfunction of irreversibly sickled red blood cells, and the increased vascular impedance created by these distorted erythrocytes.
More recent work has elucidated the resulting vascular inflammatory response to this sickling phenomenon. Population-based implementation of evidence obtained in research funded by NIH and other sponsors has resulted in enhanced survival in SCD and reduced the significant morbidities of selected complications such as stroke. In this discussion we will review some of these accomplishments, summarize briefly ongoing basic and clinical SCD research funded by NIH and, in particular the NHLBI, and broadly describe the proposed intermediate-term strategy for exploiting basic research findings in hemoglobinopathies for potential new therapies of this complex disease.
Historical Perspective and Successes
In 1972, Congress passed the Sickle Cell Treatment Act and subsequently the NHLBI assumed a leadership role in coordinating research and educational programs for persons with SCD. The Cooperative Study of Sickle Cell disease (CSSCD) was a national observational study, started in 1979, which observed over 3,000 American patients with sickle cell disease to better understand the natural history of this disorder and its complications. Much was learned from the CSSCD, which led to subsequent interventional studies.
NHLBI investigator-initiated studies focused on prevention of septic deaths, stroke, and a variety of complications using hydroxyurea to raise fetal hemoglobin. Survival of individuals with SCD has improved most dramatically in recent years, primarily because of early identification of neonates with HbSS through newborn screening programs in all 50 states. Early identification of affected infants, coupled with referral for education and initiation of penicillin prophylaxis, followed by vaccination with conjugated pneumococcal vaccine has dramatically reduced SCD infant deaths from sepsis throughout the developed world. SCD morbidity has been alleviated in many adults and children by treatment with hydroxyurea, which was approved in 1998 by the US Food and Drug Administration for treatment of adults with SCD. Regulatory agency approval followed publication of the NHLBI-funded Multicenter Study of Hydroxyurea in SCD, which has been followed by pediatric studies of hydroxyurea performed over the last three decades. Non-invasive early detection of patients at risk for stroke through the use of transcranial Doppler measurement of CNS arterial blood flow, followed by clinical use of regular RBC transfusions to prevent primary and recurrent strokes in children with SCD, has become the standard of care.
In parallel, basic science research funded by several NIH Institutes has dramatically extended the molecular understanding of such important biologic processes as globin gene expression (in particular, Hemoglobin F), has provided an explication of elements of inflammatory cell to cell cross-talk at sites of vaso-occlusion, and increased understanding of the differential perception of central versus peripheral pain in SCD, as well as provided a description of dysregulation of ion channels in irreversibly sickled cells (IRSC). To date, however, none of these has been fully exploited for therapies for patients with SCD. Providing essential resources to develop strategies to translate these and other fundamental discoveries into therapy are intrinsic to the NIH commitment to reducing the suffering of over 70,000 Americans and millions world-wide with SCD. The use of cellular therapies through hematopoietic stem cell transplantation and gene therapies to replace HbSS erythrocytes and/or HbSS gene replacement for cure are also fundamental to the long-term NIH research commitment.
The NIH and NHLBI Agenda in SCD Research
The NIH annual funding for SCD research is approximately $90 million from several NIH institutes including NHLBI, the National Institute for Digestive Diseases and Kidney (NIDDK), the National Institute of Neurologic Diseases and Stroke NINDS), the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD), and the National Institute for Neurological Diseases and Stroke (NINDS). NHLBI is the largest funder of such research and provides national leadership for basic, translational and clinical research as well as funding for professional education/training and dissemination of SCD scientific discoveries into the American healthcare system. NHLBI supports basic and translational research through both investigator-initiated research grants and Institute-initiated grants and contracts. Fundamental biologic grants from NIDDK complement this support. When targeted areas of need are identified, the NHLBI staff develops specific initiatives. For clinical research, both investigator-initiated research grants and targeted SCD initiatives provide the underpinnings of NHLBI support.
A series of Comprehensive Sickle Cell Centers were established under the Sickle Cell Act of 1972. These Centers conducted basic, translational and clinical research in SCD, and ran educational programs. Federal and state health care delivery changed over the decades, and the demographics of SCD was transformed by improved longevity. In 2008, the NHLBI, following the advice of its Advisory Council, reviewed the impact of existing programs and made adjustments to promote broader participation by the research community in SCD investigation, particularly in clinical trials research. Simultaneously, the NHLBI has led a broad program in the federal Department of Health and Human Services (HHS) to engage agencies in addressing major issues of epidemiology (Centers for Disease Control and Prevention, CDC) and health care delivery (Health Resources and Services Administration, HRSA and the Center for Medicaid and Medicare Services, CMS). Repurposing of research funds has allowed NHLBI to create a new strategic plan for SCD research congruent with the supervening NHLBI Strategic Research Plan, exploiting a wide variety of new research opportunities opened up by advances in genomics, molecular biology and cellular therapeutics, in spite of a flat budget with decreasing buying power. The broad elements of the Strategic Plan are delineated below with the relevant SCD research programs that address the NHLBI specific aim. Examples of ongoing programs, planned initiatives and intermediate-term plans are provided for each aim.
NHLBI Strategic Goal 1: Form to Function
To improve understanding of the molecular and physiological basis of health and disease and to use that understanding to develop improved approaches to disease diagnosis, treatment, and prevention
In SCD the challenges are to identify and understand the relationships of molecular events to pathophysiologic processes involved in vaso-occlusion and organ dysfunction and to identify biomarkers that differentiate clinically relevant phenotypes. Greater understanding of biologic processes essential to meeting these challenges include the biophysics of HbS, altered physical and membrane properties of IRSC, and the interactions among IRSC, the blood vessel wall, and inflammatory cells that appear to induce upregulation of endothelial adherence, activation of coagulation and resultant further enhanced impedance of blood flow.
Several NHLBI funded grants and initiatives are central to elucidating these biologic processes:
Thrombosis and Inflammation: In 2010 NHLBI issued a funding opportunity announcement entitled “Sickle Cell Disease: Inflammation, Thrombosis and Vascular Dysfunction” (R01; PA-11-013). This multi-principal investigator initiative is intended to identify key molecular and/or cellular targets for potential therapeutics.
Organ-specific Dysfunction: Investigations include pulmonary vascular injury (endothelial damage and fibroblast proliferation in SCD and modulation of vascular inflammatory damage by natural killer cells).
Genomics: Genome wide association studies (GWAS) are ongoing and have identified new loci in HbF expression/repression that are being exploited in animal models of SCD. Plans include utilizing whole genome sequencing to establish other potential modifiers of phenotype which may, in the future, allow for personalization of the therapeutic approach for patients with SCD.
Pain: Pain is the defining feature of SCD, but very little is known about either the neurobiology or optimal therapies which might take advantage of advances in our understanding of pain processes. This is particularly true in the setting of chronic pain and pain with a substantial inflammatory component. NHLBI is collaborating with NINDS to co-fund a request for application (RFA) entitled “ Exploratory Studies in the Neurobiology of Pain in Sickle Cell Disease” which is supporting the development of a preclinical (murine) model of pain and other central and peripheral studies of pain regulation and dysregulation.
Biomarkers: Better ways of identifying risks of complications, and of understanding the wide phenotypic variability of this single gene disorder will require markers of underlying processes. The spectrum of potential biomarkers that are being investigated include genetic risk factors, plasma markers (e.g., brain natriuretic peptide in SCD-associated pulmonary hypertension and secretory phospholipase A2 in acute chest syndrome), proteome analyses in animals and humans, and cellular and organ imaging utilizing existing clinical as well as rapidly developing research imaging tools.
Gene Therapy: NHLBI continues to support gene transfer strategies in preclinical models of SCD to ascertain optimal expression, safe transgene transfer using viral vectors and other strategies and exploration of gene targeting in hematopoietic cells. This complements ongoing support for the potential use of induced pluripotent stem cells (autologous with gene correction or allogeneic) to establish erythropoiesis with HbAA progenitors.
Future Directions
To develop the next generation of research funding opportunities for basic and translational science and build multidisciplinary teams of investigators: “Excellence in Hemoglobinopathy Research Awards (EHRA),” RFA-HL-13-005 will aim to create new scientific partnerships between scientists outside of the traditional areas of hematologic expertise with clinician-investigators to undertake hypothesis-directed basic and early translational studies targeting plausible future interventional strategies. These laboratories will serve as a foundation for the next generation of NHLBI SCD Bench to Bedside to Bench research strategy.
NHLBI Strategic Goal 2: Function to Causes
To improve understanding of the development of the clinical mechanism of sickle cell disease and thereby enable better prevention, diagnosis and treatment
The genotypic and biomarker data outlined in Goal 1 must be linked to defined phenotypes to identify new targets and permit personalization of therapies. Enhanced clinical trial design, subject recruitment and retention, and clinical site vetting (see below under discussion of the R34 program) to sponsor pivotal phase III clinical trials of new therapies for SCD are needed to advance research translation. Ongoing research projects helping to prepare the way for this developmental pipeline for new clinical trials in SCD include the following:
The Basic and Translational Research Program for Sickle Cell Disease (BTRP), created from the Comprehensive Sickle Cell Research Centers, will be succeeded by the planned EHRA discussed above. Approaches include induction of HbF production, use of sildenafil, the nitric oxide agonist, to treat priapism, and characterization of neurocognitive deficits in adults with SCD.
Studies designed to examine hydroxyurea use in children include the effect of hydroxyurea therapy when initiated in infancy on growth, development, and morbidity in children with SCD and stroke prevention in children with abnormally high transcranial Doppler (TCD) measurement, or to prevent future vascular flow abnormalities in children with normal TCD velocities. Publication of the NHLBI-funded BABY HUG Phase III infant hydroxyurea clinical trial [1] confirms the rationale for generation of these important clinical hypotheses-based trials.
Under the auspices of the NIH Therapeutics for Rare and Neglected Diseases (TRND) Program and in collaboration with the private pharmaceutical company AesRX, a new therapeutic anti-sickling agent (Aes-103) has been developed and regulatory early-phase human trials are planned to be undertaken at the NIH Clinical Center. NHLBI is a partner in this effort.
At the present, allogeneic hematopoietic stem cell transplantation represents the only potentially curative strategies for SCD. The NHLBI-funded Bone Marrow Transplantation Clinical Trials Network (BMTCTN) is collaborating with SCD investigators at several institutions to study new sources of hematopoietic stem cells in early stage clinical trials. The NHLBI Intramural Hematology Program is also continuing a non-myeloablative transplant program for adults with SCD.
Future Directions
An uninterrupted pipeline of clinical trials designed to assess possible beneficial therapies is essential to assure progress toward better health for people with SCD. The NHLBI is undertaking a new planning and feasibility funding program for Phase III clinical trials in SCD. This R34 program, the first phase of which began in late 2011, funds SCD investigators with proposals that have ranked well in peer review, to prepare the many components that are essential for a competitive clinical trials grant submission. Responding to experience with past studies, which have failed to enroll sufficient participants, the R34 will have a strong focus on recruitment and retention strategies, optimizing clinical trial site selection, facilitating logistical support for research subjects, and preparing for efficient trial initiation. The goal of this program is to ensure the timely completion of all studies that are initiated.
The NHLBI is cooperating with NINDS in investigating the biology of pain in SCD, and engaging the Clinical and Translational Sciences Awards (CTSA) Pain Researchers Interest Group (CPRIG) to design and implement new clinical strategies to ameliorate SCD-associated pain.
NHLBI Strategic Goal 3: Causes to Cures
Over the past half century, SCD has become a manageable condition for most affected children. Many opportunities remain to significantly the quality of life for people with SCD, especially adults. Bench discoveries and clinical trial results must be joined by behavioral and social science research that identifies cost-effective approaches for prevention, diagnosis and treatment.
In 2007, NHLBI issued a Request for Information to get input from the clinical, research and patient communities about top research priorities. Patients identified two major priorities: management of pain, and development of clear, evidence-based guidelines, which they and their physicians could use to ensure that they were receiving optimal care. The Division for the Application of Research Discoveries at NHLBI has been developing guidelines since 1978, and is in the process of developing guidelines for adults and children with SCD for use by primary care providers and patients. These will cover acute and chronic care, health care maintenance, and use of transfusions and hydroxyurea. These were made available for comment in late 2011, and will be issued in 2012 with a comprehensive approach to implementation, engaging federal and state agencies, professional groups, health care systems and others. These are intended to inform the practice of primary care providers, maximize appropriate referrals to hematologic specialists, and ensure that all members of the sickle cell community have access to proven, effective therapies to optimize their care. It is hoped that all eligible persons will be offered hydroxyurea, transfusion and stem cell transplantation; evidence suggests that this is not the case.
NIH is committed to working with our Federal partners to achieve these goals. Health and Human Services Secretary (HHS) Kathleen Sibelius recognized a more formalized structure under which HHS Agencies will collaborate to insure broader access of the SCD patient community to both care and research opportunities, each focusing on its own mission but coordinating with other agencies. The NHLBI has played a leadership role in establishing this collaboration. This joint effort will allow greater leveraging of resources dedicated to this population so that access to care and elective participation in clinical research is optimized.
NHLBI has long worked with other Federal agencies in SCD-related efforts. These efforts include an NHLBI-funded collaboration with CDC for SCD surveillance in seven states. The intent of this Registry and Surveillance System for Hemoglobinopathies (RuSH) is to identify unique individuals with SCD, thalassemia and other hemoglobinopathies in the six states and to use the data to model national prevalence. NHLBI then plans to build on this dataset to create a national registry for consented individuals with hemoglobinopathies. This protected data will be linked to individual blood specimens collected with informed consent to be housed in the NHLBI Biorepository and available for future research.
Healthy People 2020 is a partnership of Federal agencies and other organizations charged with developing measurable improvements in health outcomes by 2020. NHLBI has participated in a leadership role for developing such goals for SCD care.
Within NIH a trans-Institute effort to establish patient-reported outcomes measurements has resulted in the validated and reliability-tested PROMIS instruments. Using this template, scientists in NHLBI and extramural investigators are developing a SCD disease-specific quality of life patient outcome instrument (AScQ-Me), which will provide secondary clinical endpoints for future clinical and comparative effectiveness trials.
Sickle Cell Trait, although traditionally viewed as a genetic variant without clinical implications, has increasingly been shown to confer some morbidity risk in at least a few individuals. NHLBI hosted a workshop focused on this reality in June, 2010 and plans are underway for collaborative efforts with the Centers for Disease Control and Prevention (CDC), the Health Resources and Services Administration (HRSA), and the NIH-funded CTSA to conduct research in this important disparity population, representing approximately 8% of African Americans and 2–4% of Hispanic Americans.
Global Health
NHLBI funds two SCD clinical trials with international sites: the aforementioned trial assessing whether hydroxyurea is effective in forestalling abnormal TCDs in children with SCD is being conducted in Brazil and Jamaica. In addition, the Recipient Epidemiologic and Donor evaluation Study (REDS-III) has sites in China, South Africa and Brazil. The latter site is leading a first in country SCD-focused epidemiologic study.
NHLBI has recently created an Office of Global Health (OGH) that is coordinating international research activities. The OGH manages the NHLBI Centers of Excellence, a network of chronic disease research centers in 11 countries directed by local scientists working with developed world investigators, and supported jointly by UnitedHealth Group and is focused on cardiovascular and pulmonary diseases. The Division of Blood Diseases and Resources (DBDR) at NHLBI is working with the Office to expand hematologic research opportunities, with a strong interest in thalassemia in Southeast Asia and SCD in Africa. As with all the global health opportunities, the NHLBI will emphasize in-country investigators working in concert with US investigators to build capacity as well as advance research.
Summary
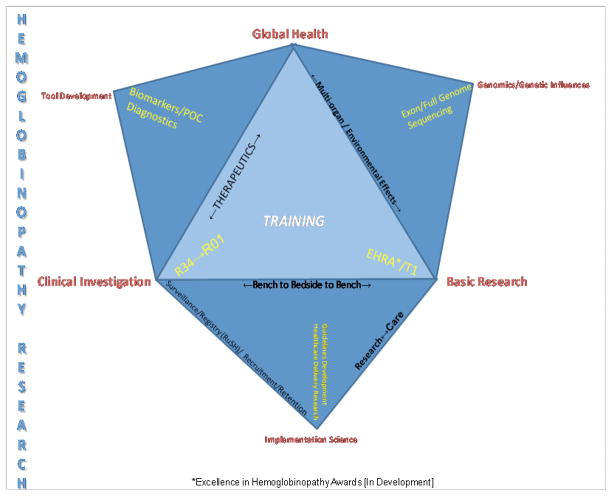
The NHLBI is committed to a research strategy that embodies a Bench-to-Bedside-to-Bench philosophy (Figure 1). The training of new investigators with interests in SCD along this entire continuum is intrinsic to this commitment. Complementary to funding basic, translational and clinical research are efforts to leverage other resources for implementation strategies for enhancing care of individuals with hemoglobinopathies in the U.S. and globally. By leveraging the resources of NHLBI with those of other NIH Institutes and Centers, sister U.S. HHS agencies, private research and care collaborators across the U.S. and established and potential global partners, our overarching goal will be to continue to advance understanding of fundamental biology while increasing both the duration and the quality of life of individuals with SCD.
Figure 1.
NHLBI bench-to-bedside-to-bench philosophy.
References
- 1.Wang WC, Ware RE, Miller ST, et al. Hydroxyucarbamide in very young children with sickle-cell anaemia: a multicenter, randomized, controlled trial (BABY HUG) Lancet. 2011;377:1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]