Abstract
Recent studies suggest that HDL levels are inversely related to colon cancer risk. HDL mimetics constructed from a number of peptides and proteins with varying structures possess anti-inflammatory and antioxidant properties reminiscent of HDL. In this report, we examined whether HDL mimetics, L-4F (an apolipoprotein A-I mimetic peptide) and G* (an apolipoprotein J mimetic peptide) affect tumor growth and development, in mouse models of colon cancer. HDL mimetics reduced viability and proliferation of CT26 cells, a mouse colon adenocarcinoma cell line and decreased CT26 cell-mediated tumor burden in BALB/c mice when administered subcutaneously or orally. Plasma levels of lysophosphatidic acid (LPA), a serum biomarker for colon cancer, were significantly reduced in mice that received HDL mimetics, suggesting that binding and removal of pro-inflammatory lipids is a potential mechanism for the inhibition of tumor development by HDL mimetics. Furthermore, L-4F significantly reduced size and number of polyps in APCmin/+ mice, a mouse model for human familial adenomatous polyposis, suggesting that HDL mimetics are effective in inhibiting the development of both induced and spontaneous cancers of the colon. Our results, for the first time, identify HDL mimetics as a novel therapeutic strategy for the treatment of colon cancer.
Keywords: HDL, Mimetic Peptides, Colon Cancer, LPA, Cancer Therapeutics
Introduction
Colon cancer is the third most common cancer worldwide and the third leading cause of cancer death in both men and women in the U.S., with approximately 150,000 new cases diagnosed and 50,000 disease related deaths every year (1). Like most cancers, early diagnosis and surgery significantly improve the chances of cure for colon cancer (2). Therefore the development of serum-based biomarkers and novel therapeutic targets for treating colorectal cancer are greatly needed.
High density lipoprotein (HDL) is an important mediator of lipid homeostasis. HDL and HDL associated molecules (proteins and lipids) provide a number of protective functions including anti-inflammatory, antioxidant, anti-microbial, and innate immunity (3). HDL cholesterol (HDL-C) is an accepted marker for cardiovascular risk assessment (4) and several clinical strategies for cardiovascular therapy are designed to elevate HDL-C (5–6). Although there is significant correlation between lipid metabolism and cancer, until recently very little is known about the potential role for lipoproteins in cancer biology. There is a significant inverse association between HDL-C and the risk of incident cancer, which is independent of low density lipoprotein cholesterol (LDL-C), age, BMI and smoking status (7). The concentrations of HDL and apolipoprotein A-I (apoA-I, the major protein component of HDL) were found to be inversely associated with the risk of colon cancer (8–9).
Recent studies suggest that increasing the amount of circulating HDL-cholesterol alone does not reduce the risk of coronary heart disease (CHD) events, CHD deaths, or total deaths (10). One method that has been reported to modify the lipid and protein cargo of HDL involves treatment with HDL mimetic peptides (11). Our previous studies showed that apoA-I is a biomarker for detection of early stage ovarian cancer and a promising therapeutic target for the treatment of ovarian cancer (12–17).
In the present study, we demonstrate that two HDL mimetics, the apoA-I mimetic peptide L-4F (Ac-D-W-F-K-A-F-Y-D-K-V-A-E-K-F-K-E-A-F-NH2 synthesized from all L -amino acids) and the apoJ peptide termed G* (Ac-L-V-G-R-Q-L-E-E-F-L-NH2 corresponding to aminoacids 113 to 122 in apoJ (L- [113–122]), decrease tumor burden in mice injected with CT26 cells. We further demonstrate that L-4F and G* peptides reduce plasma LPA levels in mice. Our results demonstrate that HDL mimetics L-4F and G*, may serve as therapeutic agents for the treatment of colon cancer.
Materials and Methods
Mice
The Animal Research Committee at the University of California at Los Angeles approved all mouse protocols. 6-week-old BALB/c female mice and 6-week-old C57BL/6J-APCMin/+ male mice were purchased from The Jackson Laboratory.
Peptides
HDL mimetics, the apoA-I peptide L-4F (Ac-D-W-F-K-A-F-Y-D-K-V-A-E-K-F-K-E-A-F-NH2) and a scrambled peptide (sc-4F) containing the same amino acids as in the 4F peptides but arranged in a sequence (Ac-D-W-F-A-K-D-Y-F-K-K-A-F-V-E-E-F-A-K-NH2) that prevents the formation of a class A amphipathic helix, and the apoJ mimetic, named G* peptide {Ac-L-V-G-R-Q-L-E-E-F-L-NH2 corresponding to aminoacids 113 to 122 in apoJ (L- [113–122] apoJ)} were synthesized from all L-amino acids. The peptides were dissolved in H2O for administration by injection. For administration of peptides in the diet, the peptides were mixed into standard mouse chow (Ralston Purina) using techniques essentially as described previously for a Western diet (18). However, the Western diet was not administered in any of the experiments reported here; the mice only received standard mouse chow with or without the peptides.
Cell-Culture Experiments
CT26 cell line derived from N-nitroso-N-methyl urethane-induced mouse colon carcinoma of BALB/c origin was purchased from the American Type Culture Collection (ATCC). CT26 cells (2,000 cells per well) were first cultured in complete medium in 96-well culture plates, and 24 hours later the medium was replaced with serum free medium. Following an overnight incubation, the cells were either treated with vehicle (control), or treated with 10 μg/mL of either L-4F or G* peptide. The peptides were dissolved in H2O. Cells were incubated for an additional 48 hours and assayed for viability using the MTS assays kit (Promega) according to the manufacturer’s protocol. For proliferation assay, cells were labeled with BrdU for the last 4 hours of the 48 hours incubation. Cells were subsequently washed, fixed, and incubated with mouse anti-BrdU antibody for 1 hour at room temperature and detected by a peroxidase-coupled goat anti-mouse secondary antibody (Calbiochem). Absorbance was measured using dual wavelengths 450 and 540 nm.
Tumor-Load Study
6-week-old BALB/c female mice were given a 100μL subcutaneous injection of 1 × 106 CT26 cells prepared as a single cell suspension in PBS, and the mice were treated with sc-4F or L-4F at 10 mg/kg administered subcutaneously (SQ) daily for 15 days. The mice were sacrificed and tumor weights were measured.
Pulmonary Metastasis in Vivo
BALB/c mice were intravenously injected with 2 × 104 CT26 cells in 100μL of PBS via tail vein injection and the mice were treated with L-4F or sc-4F at 10 mg/kg/day administered SQ for 3 weeks; or treated with sc-4F or L-4F or G* peptide at 100mg/kg/day administered in a chow diet for 3 weeks. After 3 weeks treatment, the mice were sacrificed; lungs were harvested, weighed and fixed with Bouin’s solution (Sigma). Tumor nodules on the lung surface were counted.
APC Min/+ Mice Study
6-week-old APC Min/+ malemice on a C57BL/6J background were treated with L-4F or sc-4F at 100mg/kg/day administered in a chow diet. After 8 weeks treatment, mice were sacrificed. The entire intestine was immediately removed, fixed in formalin and 70% ethanol. The intestine was opened and examined under a dissecting microscope to count and measure the tumors.
Immunohistochemistry (IHC) Staining
Tumor tissues from the lung surface were fixed and embedded with paraffin, sectioned at 5μm thickness. Sections were deparaffinized with xylene, rehydrated with 100%, 90%, 70%, and 50% ethanol, treated with proteinase K at 20μg/mL for 30 min, and treated with 3% H2O2 for 30 min at room temperature to inhibit endogenous peroxidase, blocked with 10% normal goat serum and 4% BSA prepared in PBS for 3 h, and then incubated with 1:50 rat anti-mouse monoclonal CD31 antibody overnight at 4°C. The sections were incubated with corresponding biotinylated secondary antibody for 1 hour, followed by incubation with Vectastain ABC Elite reagents.
Cell Cycle Analysis
CT26 cells were cultured in 6-well plates overnight and then serum starved for 48 hours. Cells were either treated with vehicle (control), or treated with 10 μg/mL of L-4F or G* peptide, and incubated for an additional 48 hours. Cells were collected, washed with PBS, and fixed with 70% ice-cold methanol overnight at 4°C. The fixed cells were collected by centrifugation, washed with PBS, and resuspended in 0.3 ml of PBS containing 40 μg/mL RNaseA and 100 μg/mL Propidium Iodide, and subjected to flow cytometric cell-cycle analysis by FACScan from BD Biosciences.
Western Blot Analysis
Total cell proteins were collected after treatment in cell lysis buffer containing 0.1M NaCl, 5 mM EDTA, 50 mM sodium orthovanadate, 1% Triton X-100, and protease inhibitor tablet in 50 mM Tris buffer (pH 7.5). 20μg of total proteins were separated by SDS-PAGE and transferred onto nitrocellulose membrane, and followed by incubation with primary antibody at 4°C in 5% skim milk and 0.1% Tween-20. Anti-Cyclin D1 and anti-Cyclin A rabbit polyclonal antibodies were used at 1:1000 dilution, and anti-β-actin molyclonal antibody was used at 1:2000 dilution.
ELISA Analysis
Il-6 concentrations were measured in plasma by a competition ELISA according to the manufacture’s protocol (Invitrogen).
LPA Binding Affinity and Serum LPA Levels
LPA (20:4) was purchased from Avanti Polar Lipids. LPA levels were determined as described previously (19).
Statistical Analyses
The data are shown as means ± SD for each group. We performed statistical analyses by unpaired t test. All results were considered statistically significant at P < 0.05.
Results
HDL mimetic L-4F inhibits tumor development following CT26 cell injection in BALB/c mice
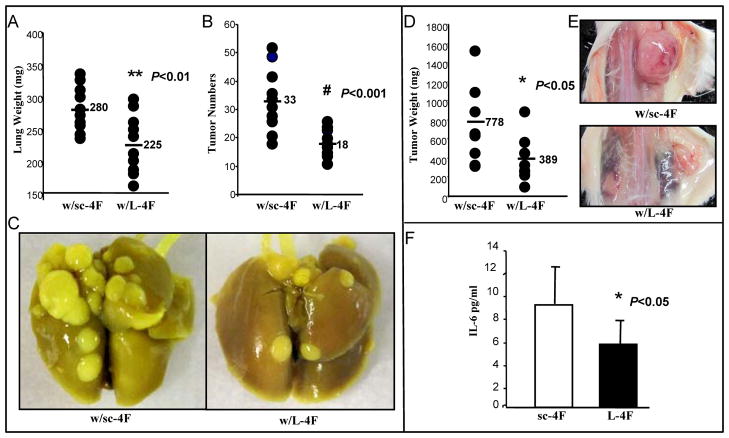
CT26 is a colon adenocarcinoma cell line which develops metastatic pulmonary tumors when introduced intravenously into immunocompetent BALB/c mice (20–22). CT26 cell line has been widely used as a syngeneic tumor model to study therapeutic applications for cancer in mouse models and therefore we chose CT26 cells for the colon cancer study in our HDL mimetic studies. We first examined the effect of L-4F and sc-4F (a scrambled peptide containing the same amino acids as in the 4F peptide but arranged in a sequence that prevents the formation of a class A amphipathic helix) administered SQ at 10 mg/kg/day for 3 weeks on lung tumor formation in BALB/c mice injected with 2 X104 CT26 cells via tail vein. The lung weights (Fig 1A) and the tumor numbers counted on the lung surface (Fig 1B) in BALB/c mice treated with L-4F (n=11 per group) were significantly reduced compared with mice treated with sc-4F (280 mg vs. 225 mg, P < 0.01; 33 vs.18, P < 0.001). Representative photographs of lung tumors from the two groups are shown in Figure 1C. We next examined whether L-4F treatment effects the development of tumors in the flanks of BALB/c mice. 6-week-old BALB/c female mice were injected with 1×106 CT26 cells SQ in the flank. The mice were treated with either sc-4F (n=9) or L-4F (n=8) at 10 mg/kg administered SQ daily for 15 days at a site distant from the site where the CT26 cells were injected. The flank tumor weights were significantly larger in BALB/c mice treated with sc-4F compared with mice treated with L-4F (778 mg vs. 389 mg, P < 0.05) (Fig 1D). Representative photographs of lung tumors from the two groups are shown in Figure 1E. We also measured IL-6 levels in plasma from the experiment shown in Fig 1A. IL-6 was significantly decreased in mice with L-4F treatment compared to control group (Fig 1F).
Figure 1. CT26 cell-mediated lung tumors and flank tumors are significantly decreased in BALB/c mice treated with HDL mimetic, L-4F by SQ.
Lung tumors were established in BALB/c mice (n=11 per group) as described in Materials and Methods. Mice were sacrificed 3 weeks after CT26 cells were administered by tail vein injection. Lungs were harvested and weighed. Lung tumors were counted. (A) The data shown are lung weights for mice receiving sc-4F or L-4F administered subcutaneously (SQ) daily at 10 mg/kg. P< 0.01. (B) The data shown are the number of tumors counted on the lung surface from the two groups of mice. P< 0.001. (C) Representative tumors from the two groups of mice showing tumor nodules on the lung surface. (D & E) Flank tumors were established in BALB/c mice as described in Materials and Methods. Mice were sacrificed 15 days after CT26 cells were administered SQ and tumor weight was measured. (D)The data shown are tumor weights for mice receiving sc-4F or L-4F at 10mg/kg SQ daily. P < 0.05. (E) Representative tumors are shown from two groups of mice. w/sc-4F, mice treated with sc-4F; w/L-4F, mice treated with L-4F. (F) Plasma IL-6 levels from the experiment shown Fig 1A. P < 0.05.
Tumor development following CT26 cell injection is significantly decreased in mice that were treated with L-4F administered in mouse chow
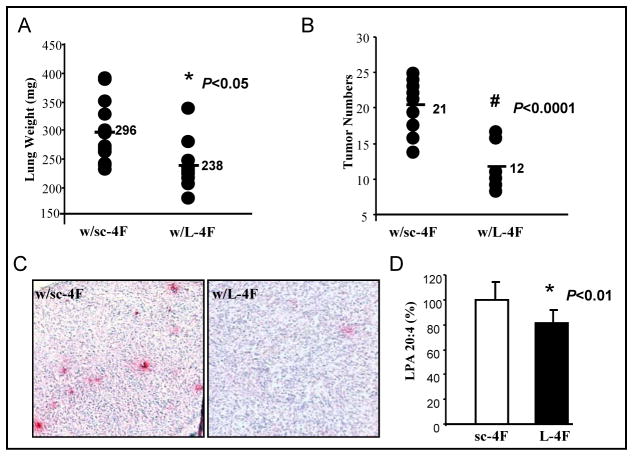
We recently reported that 4F is effective in animal models of atherosclerosis whether administered SQ or orally (18). To determine whether L-4F could reduce tumor development when administered orally, BALB/c mice were injected with 2 × 104 CT26 cells via tail vein, and treated with L-4F (n=9) or sc-4F (n=12) at 100 mg/kg/day administered in the chow diet for 3 weeks. The lung weights (Fig 2A) and the tumor numbers (Fig 2B) in BALB/c mice treated with sc-4F were significantly larger compared with mice treated with L-4F (296 mg vs. 238 mg, P < 0.05; 21 vs. 12, P < 0.0001). We previously reported that L-4F inhibits angiogenesis in vivo (23). IHC staining of tumor sections from this experiment showed a significant decrease in CD31 expression in tumors derived from mice treated with L-4F compared to control mice (Fig 2C). Furthermore, plasma LPA levels were significantly reduced in mice receiving L-4F peptide compared with their corresponding control mice, P < 0.01 (Fig 2D).
Figure 2. CT26 cell-mediated lung tumors are significantly decreased in BALB/c mice treated with L-4F administered in mouse chow.
Lung tumors were established in BALB/c mice as described in Materials and Methods. Mice were sacrificed 3 weeks after CT26 cells were administered by tail vein injection. Lungs were harvested and weighed. Lung tumors were counted. (A) The data shown are lung weights for mice receiving sc-4F (n=12) or L-4F (n=9) mixed into the chow diet at 100 mg/kg/day (2 mg/mouse/day). P < 0.05. (B) The data shown are the tumor numbers counted on the lung surface from the two groups of mice. P < 0.0001. (C) Tumor tissues from the lung surface were sectioned and CD31 immunostaining was performed with anti-CD31 antibody for detection of endothelial cells in microvessels. The red stain represents CD31 staining. w/sc-4F, mice treated with sc-4F; w/L-4F, mice treated with L-4F. (D) Plasma LPA levels were measured as desribed under materials and methods. P < 0.01.
Tumor numbers and sizes in the intestinal tract are significantly decreased in C57BL/6J-ApcMin/+ mice treated with L-4F administered in mouse chow
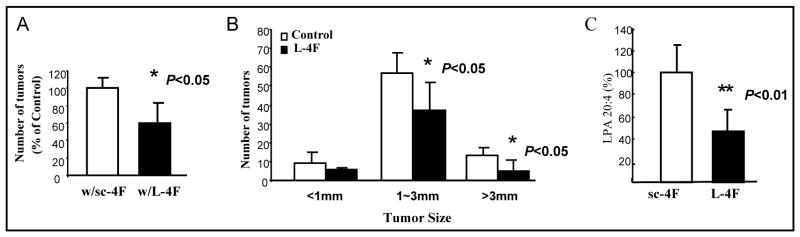
We next examined whether HDL mimetics could effect the development of colon tumors in a spontaneous model of colon cancer. APCMin/+ mouse is an established mouse model for colon cancer and mirrors the development of familial adenomatous polyposis in humans (24–25). 6-week-old C57BL/6J-ApcMin/+ male mice were treated with L-4F (n=5) or sc-4F (n=6) at 100 mg/kg/day administered in mouse chow for 8 weeks. The tumor numbers and sizes in the intestinal tract from mice treated with L-4F were significantly reduced compared with mice treated with sc-4F (100% vs. 60%, P < 0.05; 1–3 mm: 56.5 vs. 36.8, P < 0.05; >3 mm: 12.8 vs. 5, P < 0.05) (Fig 3A and 3B). Plasma LPA levels from this experiment were significantly reduced in mice receiving L-4F peptide compared with to control mice, P < 0.01 (Fig 3C).
Figure 3. Effect of L-4F treatment in chow diet on tumor number and size in the intestinal tract of C57BL/6J-ApcMin/+ mice.
ApcMin/+ mice were sacrificed after 8 weeks treatment with sc-4F or L-4F administered in mouse chow as described under Materials and Methods. (A) Total tumor numbers in the intestinal tract after treatment with L-4F administered in mouse chow for 8 weeks represented as a percent of the control (i.e. mice treated with sc-4F), P < 0.05. (B) Numbers of tumors in different size categories defined by the diameter of the tumor in mm. w/sc-4F, mice treated with sc-4F; w/L-4F, mice treated with L-4F. (C) Plasma LPA levels are significantly decreased (> 50%) in C57BL/6J-ApcMin/+ mice treated with L-4F compared to control mice. P < 0.01.
L-4F alters CT26 cell viability, proliferation, cell cycle, and expression of cell cycle related proteins in vitro
To examine the mechanisms by which HDL mimetic, L-4F inhibits CT26 cell-mediated tumor development in mice, the effect of L-4F on CT26 cell viability was determined in vitro. Cell viability was reduced by more than 25% (P < 0.001) in CT26 cells that were treated with L-4F (10 μg/mL) when compared with control (Fig 4A). Moreover, L-4F significantly inhibited proliferation of CT26 cells (P < 0.001) as measured by BrdU incorporation (Fig 4B). To investigate whether L-4F inhibited cell proliferation through changes in cell cycle progression, the effect of L-4F on the cell cycle profile was assessed in CT26 cells. Cell cycle analysis demonstrated that L-4F treatment for 48 hours induced an increase in G0/G1 phase and arrest in S phase (Fig 4C). Moreover, Western blot analysis demonstrated that expression of the cell cycle proteins Cyclin D1 and Cyclin A were significantly lower in cells treated with L-4F (Fig 4D).
Figure 4. HDL mimetic, L-4F reduces viability, inhibits proliferation and affects cell cycle and cyclin proteins in CT26 cells.
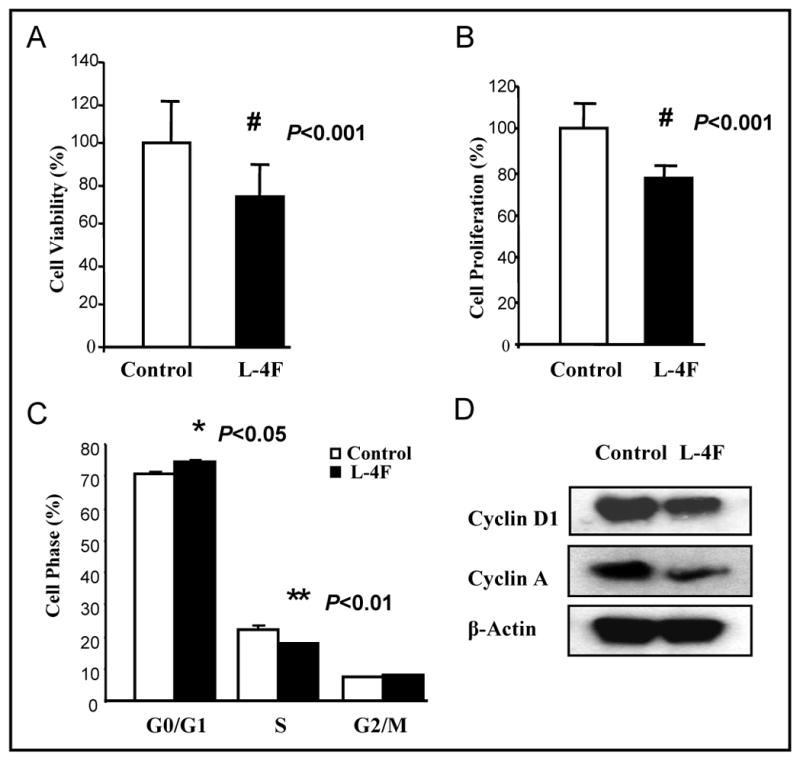
CT26 cells were cultured as described in Materials and Methods, and incubated with either vehicle (control) or L-4F at a concentration of 10μg/mL. (A) Cells were assayed for viability using the MTS assay kit. P < 0.001. (B) BrdU incorporation was analyzed as described under Materials and Methods. P < 0.001. (C) Quantitative analysis of cells in different phases in cell cycle. Data are represented as the mean ± SD of the percent of control cells. (D) The expression of Cyclin D1 and Cyclin A. All experiments were performed in triplicate and each assay was carried out in quadruplicates.
HDL mimetic L-4F inhibits LPA-induced proliferation of CT26 cells
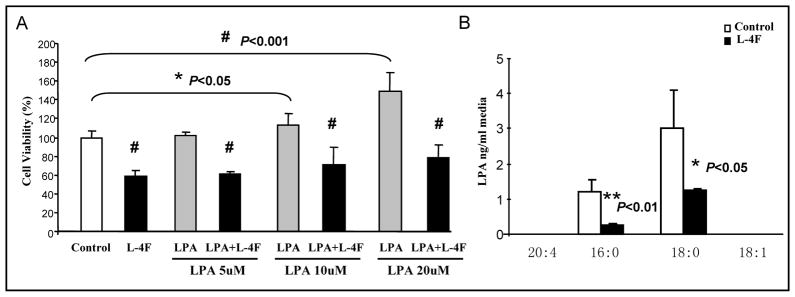
LPA has been identified as an important mediator of tumor development, progression, and metastases in humans (26–27). We have previously demonstrated that apoA-I mimetic peptides inhibit LPA-induced proliferation of ID8 cells and reduce serum LPA levels in mice injected with ID8 cells (17). L-4F binds LPA (17), as expected, LPA (10–20 μM) significantly improved CT26 cell growth, and L-4F significantly reduced LPA-induced viability at all doses tested, P < 0.001 (Fig 5A). We measured LPA levels in cell culture medium by LC/MS and found that LPA 16:0 and 18:0 were significantly decreased with L-4F treatment compared with the control medium. LPA 20:4 and 18:1 were not detectable in cell culture medium (Fig 5B).
Figure 5. HDL mimetic, L-4F inhibits LPA induced proliferation of CT26 cells and reduces serum LPA levels in mice injected with CT26 cells.
(A) CT26 cells were cultured as described in Materials and Methods, and incubated with either L-4F at 10 μg/mL or LPA at a concentration 5, 10, 20μM, or cells were treated with both L-4F and LPA for 48 hours. All experiments were performed in triplicate and each assay was carried out in quadruplicates. Data are represented as the mean ± SD of the percent of control cells. (B) LPA levels were measured in the cell culture medium after 48 hour of treatment.
HDL mimetic, G* peptide (L-[113–122]apoJ) inhibits CT26 cell growth and CT26-mediated tumor development
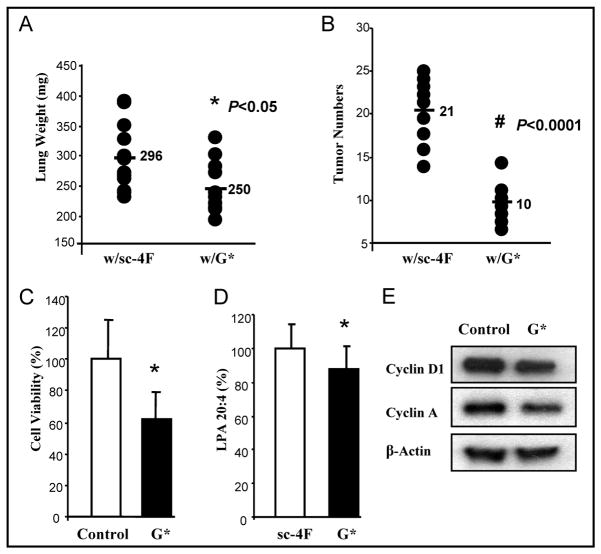
G* (L-[113–122]apoJ) peptide was used to repeat the studies in vivo and in vitro. Pulmonary tumor development following CT26 cell injection was significantly decreased in mice treated with G* peptide at 100 mg/kg/day administered in mouse chow for 3 weeks (Lung weights were 296 mg vs. 250mg, P < 0.05; tumor numbers were 21 vs.10, P < 0.0001) (Fig 6A, 6B). Cell viability was ~ 40% lower in CT26 cells treated with G* peptide (10 μg/mL) when compared with no treatment (Fig 6C). In the mouse experiment shown in Fig 6A and 6B, plasma LPA levels were significantly reduced in mice receiving G* peptide compared with their corresponding control mice P < 0.05 (Fig 6D). Western Blot showed the expression of Cyclin D1 and Cyclin A was lower with G* peptide treatment compared with no treatment (Fig 6E).
Figure 6. G* (L-[113–122]apoJ) peptide has effects similar to L-4F in vivo and in vitro.
Lung tumors were established in BALB/c as described in Materials and Methods. Mice were sacrificed 3 weeks after CT26 cells were injected into the tail vein. Lungs were harvested and weighed. Lung tumors were counted. (A) The data shown are lung weights for mice receiving sc-4F (n=12), G* peptide (n=12) at 100 mg/kg/day (2mg/mouse/day) administered in mouse chow. P < 0.05. (B) The data shown are the tumor numbers on the lung surface from two group mice of (A). P < 0.0001. (C) Cells were assayed for viability using the MTS assay. P < 0.05. (D) Serum LPA levels from the mice described in Fig 6A and 6B were determined as described in Materials and Methods. (E) The expression of Cyclin D1 and Cyclin A by Western Blot. w/sc-4F, mice treated with sc-4F; w/G*, mice treated with G* peptide.
Discussion
There is a significant correlation between lipid metabolism and cancer, and inflammatory oxidative stress has long been thought to be associated with the pathophysiology of cancer (28–30). Lipid oxidation and resulting oxidized lipid mediated inflammation appear to be common to the etiology of a number of inflammatory diseases (31–32) implicating a role for lipoproteins in the development and progression of several diseases, including cancer.
HDL is recognized as an integral part of the innate immune system. HDL is a complex macromolecule whose functional repertoire includes anti-oxidant, anti-inflammatory, and anti-microbial activities. Unlike LDL, HDL is a heterogeneous mixture of proteins and lipids, which determine HDL’s structural and functional integrity. Several protein/enzyme constituents of HDL including phospholipid transfer protein (PLTP), cholesterol ester transfer protein (CETP), and lecithin cholesterol acyl transferase (LCAT) are important for its formation and maturation, while other protein/enzyme constituents such as apolipoprotein A-I (apoA-I), apoJ, and paraoxonase-1 (PON1) confer functional properties on HDL (33). Over the last decade, HDL mimetics have shown extraordinary therapeutic promise in preclinical studies in a number of inflammatory diseases (34–40).
We have recently shown that L-4F and L-5F, two apoA-I mimetic peptides, reduced viability and proliferation of mouse ovarian cancer cells (ID-8 cells) and cis-platinum-resistant human ovarian cancer cells, and decreased ID-8 cell-mediated tumor burden in C57BL/6J mice when administered subcutaneously or orally (17). We further demonstrated that apoA-I mimetic peptides inhibit tumorigenesis by i) inhibiting angiogenesis (23) and ii) inducing expression and activity of MnSOD (41). Since angiogenesis and redox pathways are common features of many cancers, we examined the effect of two HDL mimetics, apoA-I mimetic peptide L-4F and an apoJ mimetic peptide G* (42), in the development and progression of colon cancer. Consistent with our hypothesis our results demonstrated that HDL mimetics inhibit the development of colon cancer generated by injecting CT26 cells into immunocompetent BALB/c mice. Furthermore, we show here for the first time using the mouse model of FAP (APCMin/+) that oral administration of HDL mimetics is able to suppress the spontaneous development of colon cancer in a mouse model.
There have been two sets of clinical trials using the 4F peptides. Bloedon et al (43) found that administration of doses of 4F orally of 4.3 and 7.14 mg/kg significantly improved HDL ant-inflammatory properties despite very low plasma levels (8 – 16 ng/mL). Bloedon et al. (43) also found that administering doses of peptide of 0.43 and 1.43 mg/kg were not effective. Watson et al. (44) targeted plasma levels and L-4F was administered daily by either intravenous (IV) infusion for 7 days or SQ for 28 days in patients with coronary heart disease. Using a dose of 0.43 mg/kg, Watson et al (44) achieved very high plasma levels but did not achieve any improvement in HDL anti-inflammatory properties. It was concluded that the doses needed for improving HDL function in humans maybe much higher than those used by Watson et al (44) and at least as high as those used by Bloedon et al (43). Recently, Navab et al. (45) reported that the dose of the HDL mimetic peptide 4F that was administered and not the plasma level achieved determines efficacy and the intestine may be a major site of action for the peptide regardless of the route of administration. Our results show that the HDL mimetics are effective whether given orally or SQ in mouse models at doses greater than those used by Bloedon et al (43). Given our results with HDL mimetics in mouse colon cancer models and the results of Navab et al. (45) indicating that dose determines efficacy and not plasma levels, it will be important to test the high doses used here in any future clinical trials.
One of the downstream targets for the general mechanism of anti-tumorigenic activity of HDL mimetics appears to be angiogenesis as seen by the reduction in CD31 staining in treated tumors. LPA plays an important role in inflammation, angiogenesis, and cancer, and has become a promising target for therapy (46). Moreover, consistent with our previous findings (17, 23) and current findings, the binding and removal of pro-inflammatory/pro-angiogenic lipids such as LPA may be a major part of the mechanism of action for the HDL mimetics.
In conclusion, our results show that HDL mimetics inhibit both induced and spontaneous colon cancer development in mice. The binding and removal of pro-tumorigenic lipids by HDL mimetic peptides likely alters the proliferation capacity of the tumor cells as well as angiogenesis associated with the tumors. Identifying the target lipid(s) is an important next step in delineating the specific mechanism of action for these HDL mimetics. Future studies to determine the clinical efficacy of HDL mimetics seem warranted to evaluate these new anti-tumorigenic agents.
Acknowledgments
We thank Feng Gao for technical support. This work was supported by funds from the Women’s Endowment, the Carl and Roberta Deutsch Family Foundation, the Joan English Fund for Women’s Cancer Research, the VA Merit I Award (to R.F.-E.), the Ovarian Cancer Coalition, the Helen Beller Foundation, Wendy Stark Foundation, Sue and Mel Geleibter Family Foundation, US Public Health Service Grants HL-30568 (to A.M.F., S.T.R., M.N.) and HL-082823 (to S.T.R.), and the Laubsich and M. K. Grey funds at the University of California, Los Angeles.
Abbreviation List
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- apoA-I
apolipoprotein A-I
- apoJ
apolipoprotein J
- LPA
lysophosphatidic acid
Footnotes
Disclosures: A.M.F, S.T.R, and M.N. are principals in Bruin Pharma and A.M.F. is an officer in Bruin Pharma.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Vámosi-Nagy I, Köves I. Endoscopic diagnostics of colorectal cancers. Acta Chir Hung. 1992–1993;33:149–56. [PubMed] [Google Scholar]
- 3.Navab M, Reddy ST, Van Lenten BJ, Anantharamaiah GM, Fogelman AM. The role of dysfunctional HDL in atherosclerosis. J Lipid Res. 2009;50:S145–9. doi: 10.1194/jlr.R800036-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Després Jean-Pierre, Lemieux Isabelle, Dagenais Gilles-R, Cantin Bernard, Lamarche Benoît. HDL-cholesterol as a marker of coronary heart disease risk: the Québec cardiovascular study. Atherosclerosis. 2000;153:263–272. doi: 10.1016/s0021-9150(00)00603-1. [DOI] [PubMed] [Google Scholar]
- 5.Kapur Navin K, Ashen Dominique, Blumenthal Roger S. High density lipoprotein cholesterol: an evolving target of therapy in the management of cardiovascular disease. Vasc Health Risk Manag. 2008;4:39–57. doi: 10.2147/vhrm.2008.04.01.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah PK. Evolving concepts on benefits and risks associated with therapeutic strategies to raise HDL. Curr Opin Cardiol. 2010;25:603–8. doi: 10.1097/HCO.0b013e32833f0382. [DOI] [PubMed] [Google Scholar]
- 7.Jafri Haseeb, Alsheikh-Ali Alawi A, Karas Richard H. High-Density Lipoprotein Cholesterol Levels Are Inversely Associated With Cancer Risk. Circulation. 2009;120:S406. [Google Scholar]
- 8.Cust Anne E, kaaks Rudolf, Friedenreich Christine, Bonnet Fabrice, Laville Martine, Tjønneland Anne, et al. Metabolic syndrome, plasma lipid, lipoprotein and glucose levels, and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2007;14:755–67. doi: 10.1677/ERC-07-0132. [DOI] [PubMed] [Google Scholar]
- 9.van Duijnhoven FJ, Bueno-De-Mesquita HB, Calliqaro M, Jenab M, Pischon T, Jansen EH, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut. 2001;60:1094–1102. doi: 10.1136/gut.2010.225011. [DOI] [PubMed] [Google Scholar]
- 10.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navab M, Shechter I, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Fogelman AM. Structure and function of HDL mimetics. Arterioscler Thromb Vasc Biol. 2010;30:164–8. doi: 10.1161/ATVBAHA.109.187518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozak KR, Amneus MW, Pusey SM, Su F, Luong MN, Luong SA, et al. Identification of biomarkers for ovarian cancer using strong anion-exchange ProteinChips: potential use in diagnosis and prognosis. Proc Natl Acad Sci USA. 2003;14(100):12343–8. doi: 10.1073/pnas.2033602100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozak KR, Su F, Whitelegge JP, Faull K, Reddy S, Farias-Eisner R. Characterization of serum biomarkers for detection of early stage ovarian cancer. Proteomics. 2005;5:4589–96. doi: 10.1002/pmic.200500093. [DOI] [PubMed] [Google Scholar]
- 14.Su F, Lang J, Kumar A, Ng C, Heieh B, Suchard MA, et al. Validation of candidate serum ovarian cancer biomarkers for early detection. Biomark Insights. 2007;16:369–75. [PMC free article] [PubMed] [Google Scholar]
- 15.Nossov V, Amneus M, Su F, Lang J, Janco JM, Reddy ST, et al. The early detection of ovarian cancer: from traditional methods to proteomics. Can we really do better than serum CA-125? Am J Obstet Gynecol. 2008;199:215–23. doi: 10.1016/j.ajog.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Nossov V, Su F, Amneus M, Birrer M, Robins T, Kotlerman J, et al. Validation of serum biomarkers for detection of early-stage ovarian cancer. Am J Obstet Gynecol. 2009;200:639.e1–5. doi: 10.1016/j.ajog.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 17.Su F, Kozak KR, Imaizumi S, Gao F, Amneus MW, Grijalva V, et al. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc Natl Acad Sci USA. 2010;16; 107:19997–20002. doi: 10.1073/pnas.1009010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navab M, Reddy ST, Anantharamaiah GM, Imaizumi S, Hough G, Hama S, et al. Intestine may be a major site of action for the apoA-I mimetic peptide 4F whether administered subcutaneously or orally. J Lipid Res. 2011;52:1200–10. doi: 10.1194/jlr.M013144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murph M, Tanaka T, Pang J, Felix E, Liu S, Trost R, et al. Liquid chromatography mass spectrometry for quantifying plasma lysophospholipids: potential biomarkers for cancer diagnosis. Methods Enzymol. 2007;433:1–25. doi: 10.1016/S0076-6879(07)33001-2. [DOI] [PubMed] [Google Scholar]
- 20.Wang M, Chen PW, Bronte V, Rosenberg SA, Restifo NP. Anti-tumor activity of cytotoxic T lymphocytes elicited with recombinant and synthetic forms of a model tumor-associated antigen. J Immunother Emphasis Tumor Immunol. 1995;18:139–46. doi: 10.1097/00002371-199510000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao HF, Chen YY, Liu JJ, Hsu ML, Shieh HJ, Liao HJ, et al. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J Agric Food Chem. 2003;51:7907–12. doi: 10.1021/jf034729d. [DOI] [PubMed] [Google Scholar]
- 22.Chang KH, Liao HF, Chang HH, Chen YY, Yu MC, Chou CJ, et al. Inhibitory effect of tetrandrine on pulmonary metastases in CT26 colorectal adenocarcinoma-bearing BALB/c mice. Am J Clin Med. 2004;32:863–72. doi: 10.1142/S0192415X04002478. [DOI] [PubMed] [Google Scholar]
- 23.Gao F, Vasqiez SX, Su F, Roberts S, Shah N, Grijalva V, et al. L-5F, an apolipoprotein A-I mimetic, inhibits tumor angiogenesis by suppressing VEGF/basic FGF signaling pathways. Inteqr Biol (Camb) 2011;3:479–89. doi: 10.1039/c0ib00147c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corpet DE, Pierre F. Point: From animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 25.Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, et al. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002;11:535–40. [PubMed] [Google Scholar]
- 26.Baker DL, Morrison P, Miller B, Riely CA, Tolley B, Westermann AM, et al. Plasma lysophosphatidic acid concentration and ovarian cancer. JAMA. 2002;19; 287:3081–2. doi: 10.1001/jama.287.23.3081. [DOI] [PubMed] [Google Scholar]
- 27.Sutphen R, Xu Y, Wilbanks GD, Fiorica J, Grendys EC, Jr, LaPolla JP, et al. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1185–91. [PubMed] [Google Scholar]
- 28.Nicotera TM, Privalle C, Wang TC, Oshimura M, Barrett JC. Differential proliferative responses of Syrian hamster embryo fibroblasts to paraquat-generated superoxide radicals depending on tumor suppressor gene function. Cancer Res. 1994;54:3884–3888. [PubMed] [Google Scholar]
- 29.Loo G. Redox-sensitive mechanisms of phytochemical-mediated inhibition of cancer cell proliferation (review) J Nutr Biochem. 2003;14:64–73. doi: 10.1016/s0955-2863(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y, Rosen DG, Zhou Y, Feng L, Yang G, Liu J, et al. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J Biol Chem. 2005;280:39485–39492. doi: 10.1074/jbc.M503296200. [DOI] [PubMed] [Google Scholar]
- 31.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stöckl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res. 2009;50:204–213. doi: 10.1194/jlr.M700505-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James RW, Deakin SP. The contribution of high density lipoprotein apolipoproteins and derivatives to serum paraoxonase-1 activity and function. Adv Exp Med Biol. 2010;660:173–81. doi: 10.1007/978-1-60761-350-3_16. [DOI] [PubMed] [Google Scholar]
- 34.Getz GS, Wool GD, Reardon CA. Biological properties of apolipoprotein a-1 mimetic peptides. Curr Atheroscler Rep. 2010;12:96–104. doi: 10.1007/s11883-010-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherman CB, Peterson SJ, Frishman WH. Apolipoprotein A-I mimetic peptides: a potential new therapy for the prevention of atherosclerosis. Cardiol Rev. 2010;18:141–7. doi: 10.1097/CRD.0b013e3181c4b508. [DOI] [PubMed] [Google Scholar]
- 36.Getz GS, Wool GD, Reardon CA. Apoprotein A-I mimetic peptides and their potential anti-atherogenic mechanisms of action. Curr Opin Lipidol. 2009;20:171–5. doi: 10.1097/MOL.0b013e32832ac051. [DOI] [PubMed] [Google Scholar]
- 37.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Navab M, Reddy ST, Buga GM, et al. Apolipoprotein A-I mimetic peptides. Curr Atheroscler Rep. 2009;11:52–7. doi: 10.1007/s11883-009-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navab M, Anantharamaiah GM, Fogelman AM. The effect of apolipoprotein mimetic peptides in inflammatory disorders other than atherosclerosis. Trends Cardiovasc Med. 2008;18:61–6. doi: 10.1016/j.tcm.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Datta G, Garber D, et al. Potential clinical utility of high-density lipoprotein-mimetic peptides. Curr Opin Lipidol. 2006;17:440–4. doi: 10.1097/01.mol.0000236371.27508.d4. [DOI] [PubMed] [Google Scholar]
- 40.Reddy ST, Anantharamaiah GM, Navab M, Hama S, Hough G, Grijalva V, et al. Oral amphipathic peptides as therapeutic agents. Expert Opin Investig Drugs. 2006;15:13–21. doi: 10.1517/13543784.15.1.13. [DOI] [PubMed] [Google Scholar]
- 41.Ganapathy E, Su F, Meriwether D, Devarajan A, Grijalva V, Gao F, et al. D-4F, an apoA-I mimetic peptide, inhibits proliferation and tumorigenicity of epithelial ovarian cancer cells by upregulating the antioxidant enzyme MnSOD. Int J Cancer. 2011;130:1071–81. doi: 10.1002/ijc.26079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Wagner AC, Hama S, Hough G, Bachini E, Garber DW, Mishra VK, Palgunachari MN, Fogelman AM. An oral apoJ peptide renders HDL anti-inflammatory in mice and monkeys and dramatically reduces atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2005;25:1932–1937. doi: 10.1161/01.ATV.0000174589.70190.e2. [DOI] [PubMed] [Google Scholar]
- 43.Bloedon LT, Dunbar R, Duff D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watson CE, Weissbach N, Kjems L, Ayalasomayajula S, Zhang Y, Chang I, et al. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J Lipid Res. 2011;52:361–73. doi: 10.1194/jlr.M011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navab M, Reddy T, Anantharamaiah GM, Imaizumi S, Hough G, Hama S, et al. Intestine may be a major site of action for the apoA-I mimetic peptide 4F whether administered subcutaneously or orally. J Lipid Res. 2011;52:1200–10. doi: 10.1194/jlr.M013144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tigyi G. Aiming drug discovery at lysophosphatidic acid targets. Br J Pharmacol. 2010;161:241–70. doi: 10.1111/j.1476-5381.2010.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]