Abstract
Microglia and astrocytes are the primary immune cells within the central nervous system. Microglia influence processes including neural development, synaptic plasticity and cognition; while their activation and production of immune molecules can induce stereotyped sickness behaviors or pathologies including cognitive dysfunction. Given their role in health and disease, we propose that glia may be also be a critical link in understanding the etiology of many neuropsychiatric disorders that present with a strong sex-bias in their symptoms or prevalence. Specifically, males are more likely to be diagnosed with disorders that have distinct developmental origins such as autism or schizophrenia. In contrast, females are more likely to be diagnosed with disorders that present later in life, after the onset of adolescence, such as depression and anxiety disorders. In this review we will summarize the evidence suggesting that sex differences in the colonization and function of glia within the normal developing brain may contribute to distinct windows of vulnerability between males and females. We will also highlight the current gaps in our knowledge as well as the future directions and considerations of research aimed at understanding the link between neuroimmune function and sex differences in mental health disorders.
INTRODUCTION
Microglia and astrocytes combined comprise 80–90% of the cell population within the brain. Taken together, glia perform a dynamic range of functions essential for maintaining homeostasis within the nervous system and re-establishing homeostasis following insult, infection, or injury. Microglia are the primary immune cells of the central nervous system (CNS). They produce multiple immune factors during neuroinflammatory or infectious events and scavenge dead or dying cells, a characteristic that is consistent with their monocyte/macrophage lineage. Microglia also exert crucial physiological functions in the healthy CNS. Astrocytes, the largest glial cell population within the brain, are also capable of synthesizing pro-inflammatory immune molecules at rest and during an insult or challenge, and thus are similarly considered immunocompetent CNS cells.
In addition to these well-known functions of glia within the brain, recent evidence indicates that astrocytes, microglia, and their expressed or secreted immune molecules can significantly affect the development of the nervous system. As the brain develops into adulthood, glial cells change in morphology and function and this process continues into old age. This developmental approach to studying glial function has allowed researchers to identify a novel role for glial cells in brain development, as well as a role for glia in the early-life programming of later-life processes such as learning, memory, anxiety, and other behavioral outputs. This approach to studying glial function has been important for identifying potential mechanisms underlying long-term glial and immune dysfunction, a process that has been linked to the etiology of many neuropsychiatric disorders.
It is well-known that the sex of an individual, being male or female, can have profound influences on the function of the nervous and immune system, the physiology and behavior of an individual, and an individual’s mental health or disease outcomes. Despite this, very few studies have directly examined sex differerences in the number, activation state, or function of glia during distinct periods of development either within the healthy brain or during pathology. Based on the lack of information in the field of sex differences and neuroimmune function, we propose that this particular field of study is long overdue for investigation. We anticipate that investigating sex differences in glial number or function during early brain development and into adulthood will lend valuable insight into the mechanisms underlying the expression of basic sex differences within the brain, which are often established during critical periods of development and maintained into adulthood. We also posit that investigating sex differences in glial number and function (e.g., the synthesis of immune molecules) from a developmental perspective might lend critical insight into the sex disparities of many mental health disorders, which exhibit a strong dysregulation of the immune system and often a distinct etiology in development.
Thus, the purpose of this review is to synthesize the small but growing literature on sex differences in glia and their production of immune molecules within the healthy or pathological brain (e.g., following infection or injury), throughout early neurodevelopment, with the secondary goal of identifying the gaps in our knowledge and highlighting the importance of future research focusing on neural-endocrine-immune interactions from the perspective of sex differences.
Sex Differences in Peripheral Immune Function
Sex differences in the physiology and function of the vertebrate peripheral immune system have been documented for decades. Females of many species generally exhibit enhanced immune responses and increased resistance to disease and infection than males (Gaillard and Spinedi, 1998; Klein, 2000; McClelland and Smith, 2011; Schuurs and Verheul, 1990). For example, female mice have higher titers of immunoglobulins (Ig) (e.g. IgG, IgM, IgA) (Grossman, 1989; Klein, 2000), and display higher splenocyte blastogenic responses to T and B cell mitogens than males (Krzych et al., 1981; Schneider et al., 2006). Sex differences in the rates and severity of many infections are often greater in human males than in females (McMillen, 1979; Washburn et al., 1965), and this sex difference is true in many other species as well (Klein, 2000). For example, in humans, parasite infections are generally more severe in male than in female hosts (Poulin, 1996), and males of wild species such as deer and birds often have higher parasite loads than females (Eens et al., 2000; Klein, 2004; Zuk and McKean, 1996).
These sex differences have been attributed, in large part, to the direct and indirect immuno-modulatory actions of sex steroid hormones (Bouman et al., 2005; Gaillard and Spinedi, 1998; Klein, 2000; Olsen and Kovacs, 1996). In general, exogenous estradiol has immuno-enhancing effects on humoral immunity (Cutolo et al., 2004; d'Elia and Carlsten, 2008; Seaman and Gindhart, 1979; Song et al., 2008), but may either enhance or suppress cell-mediated immunity depending on low or high doses, respectively (Kovacs et al., 2002). Exogenous testosterone generally depresses both humoral and cell-mediated immunity, and increases susceptibility to bacterial and viral infections (Muller et al., 2005; Roberts and Peters, 2009; Roberts et al., 2007; Viselli et al., 1995).
In addition, recent evidence indicates that the X chromosome is partly responsible for sex differences in peripheral immune responses (Libert et al., 2010; Pessach, 2010; Selmi, 2008). X-inactivation, a developmental process which eliminates one copy of a gene along the X chromosome, creates a “cellular mosaic” of gene expression in female cells. It is thought that potentially disadvantageous gene expression in certain cells can be compensated for by different gene expression in other cells. In fact, there are several immunodeficiencies that are present in males that are not usually present or with lesser severity in females [see (Libert et al., 2010) for review]. For example, CD40 ligand is a protein expressed on T cells that is important for activating B cells within the periphery. CD40 ligand is on the X chromosome, and mutation of this gene can cause X-linked immunodeficiency with hyper–immunoglobulin M. Males are more at risk for this disorder while the presence of two X chromosomes, and thus two copies of this particular gene, counteracts the potential mutation of this gene that may be present on one of the X chromosomes. Other genes escape X-inactivation altogether [see (Berletch et al., 2011) for review], and as such this only increases the cellular mosaic of genes expressed by individual immune cells in the female. Thus, the expression pattern of immune genes from the X-chromosome in males and females can greatly affect the function of the peripheral, and likely the central, immune system.
The current hypothesis among researchers in the field of sex differences and immunology suggests that the more robust nature of the female peripheral immune response significantly increases the risk of developing autoimmune diseases when compared to males (Kivity and Ehrenfeld, 2010; McCombe et al., 2009). For example, more than 80% of the patients diagnosed with diseases such as Grave’s disease, Addison’s disease, and systemic lupus erythematosus (SLE) are female (Cooper and Stroehla, 2003); while between 65–70% of patients diagnosed with rheumatoid arthritis, multiple sclerosis, and myasthenia gravis are females (Selmi, 2008). The mechanism underlying this sex difference in the prevalence of autoimmune disorders likely involves a combination of chromosomal differences and hormonal differences. In fact, the symptoms of many of these disorders can change significantly with hormonal or pregnancy status in females (Lleo et al., 2008). Given the robust sex difference in the prevalence of autoimmune diseases, research has been extensive in trying to understand the interactions of sex chromosomes and hormones and the underlying mechanisms of these devastating disorders, listed extensively in (Cooper and Stroehla, 2003; Libert et al., 2010; Selmi, 2008). In contrast, very little research has been done to understand the effects of sex, sex chromosomes, or hormones on the central immune system, or the resident immune cells of the CNS.
Glia are the immune cells of the brain
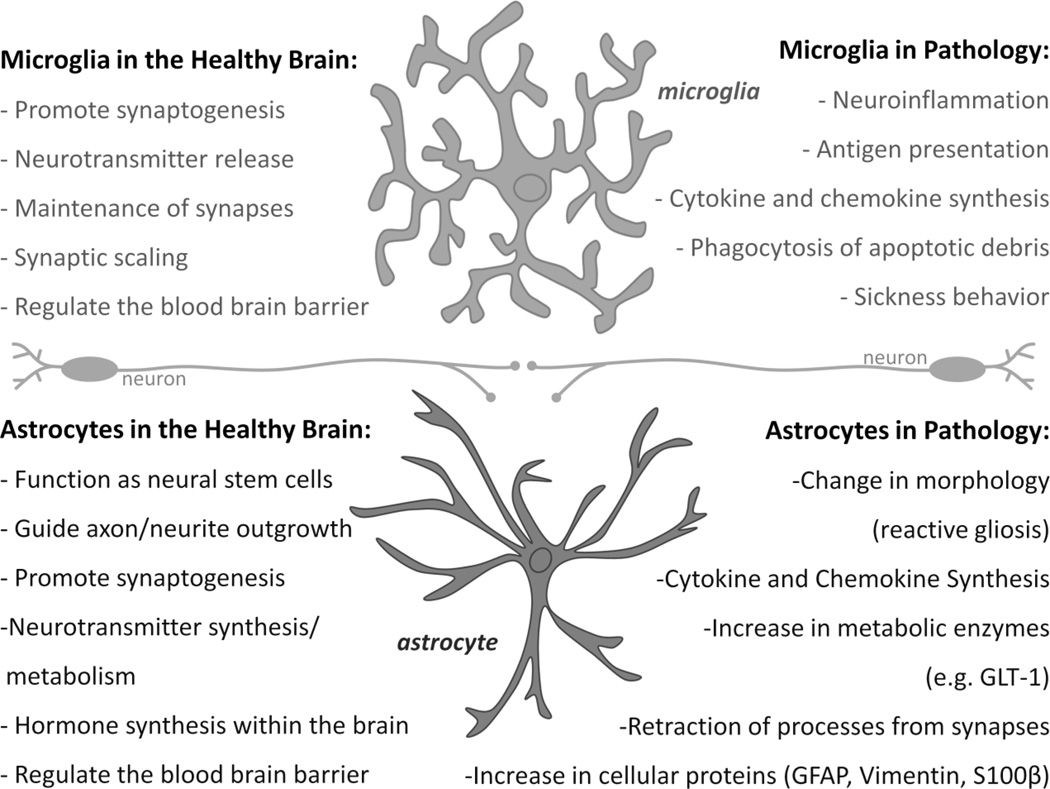
Astrocytes are the largest glial cell population within the brain, and their role in synaptic plasticity mechanisms is now well accepted (Araque and Navarrete, 2010; Pfrieger, 2010; Sharif and Prevot, 2010; Stipursky et al., 2011). In the adult brain, astrocytes are physically and functionally appositioned with most glutamatergic synapses, now known as a “tripartite synapse” (Halassa et al., 2009; Perea et al., 2009). Astrocytes express many neurotransmitter receptors, allowing them to rapidly perceive and respond to synaptic activity (Stipursky et al., 2011). Microglia are the primary immunocompetent cells of the brain, but evidence also suggests a role for microglia in synaptic plasticity mechanisms during early brain development, via interactions with the extracellular matrix composition, dendritic spine remodeling, and synapse elimination (Tremblay et al., 2010; Tremblay and Majewska, 2011; Tremblay et al., 2011). Microglia continually survey their microenvironments by extending and contracting processes into nearby synapses, with a frequency that is activity-dependent (Tremblay et al., 2010), and they have receptors for multiple neurotransmitters and neuromodulators (e.g., ATP, norepinephrine, glutamate, DA) (Kettenmann et al., 2011), suggesting a rapid and direct role for these cells in normal plasticity, similar to astrocytes. Under non-pathological conditions, microglia and astrocytes have a highly ramified/stellate morphology with low expression of surface antigen or activation markers (e.g., CD11b [complement 3 receptor/CR3/Mac-1] and glial fibrillary acidic protein [GFAP], respectively). In response to injury or immune stimulation, microglia in particular become considerably more reactive, by up-regulating a number of surface antigen receptors, including CD11b, and those for cytokines (e.g., interleukin (IL)-1, interferon (IFN)) and chemokines (chemotactic cytokines) (e.g., CCL4, CXCL1) (FIGURE 1). Astrocytes increase GFAP expression and exhibit hypertrophy and proliferation, a process known as reactive gliosis or astrocytosis, and astrocytes also produce their own cohort of proinflammatory mediators. Both types of glia thereby adopt full immune effector functions (e.g., antigen presentation, and cytokine production), a process known generally as “activation”, which occurs in response to any threat to body or brain homeostasis (Czlonkowska and Kurkowska-Jastrzebska, 2011; Robel et al., 2011) (FIGURE 1).
Figure 1. Microglia and astrocytes in health and pathology.
This figure outlines the well-known actions and phenotype of microglia and astrocytes within the healthy brain (left side) and the pathological brain, following injury, disease, or trauma (right side).
Mental Health, Immune Function, Development and Sex
Given their critical role in normal synaptic plasticity and brain homeostasis, we hypothesize that glia are perfectly positioned to play a crucial role in pathology, when homeostasis is disrupted or threatened. Moreover, the existence of sex differences in glial function could provide a mechanistic basis for the well-known sex differences in the prevalence or severity of many mental health disorders [see (Swaab, 2004) for review]. In support of this hypothesis, scientists and medical professionals have identified many similarities between sickness behaviors caused by acute illness or infection and the behaviors expressed by individuals with certain neuropsychiatric disorders (Dantzer and Kelley, 2007). For example, the behavioral and physiological symptoms of depression are strikingly similar to sickness behaviors, which include decreased food intake, decreased activity, increased sleep disturbances, and decreased social/sexual interactions. This similarity suggests that the expression of many psychiatric disorders may involve a dysregulation of either the peripheral or central immune system, even in the absence of an overt immune challenge. In 2006, Dantzer proposed that there are at least three potential causes for such abnormal changes in immune function: (1) pro-inflammatory cytokine production is exaggerated or prolonged; (2) the regulatory molecules (i.e. anti-inflammatory molecules) that would normally down-regulate the actions of pro-inflammatory cytokines and sickness behaviors are faulty; or (3) the neuronal circuits or targets of inflammatory cytokines that organize sickness behavior have become sensitized (Dantzer, 2006). This link between neuropsychiatric disorders and neuroimmune dysfunction has been explored and described for many diseases including depression, schizophrenia, post-traumatic stress disorder (PTSD), generalized anxiety disorder, autism, and Rett syndrome (Abazyan et al., 2010; Ashwood et al., 2010; Ashwood et al., 2011; Careaga et al., 2010; Garay and McAllister, 2010; Muller and Ackenheil, 1998; Pace and Heim, 2011; Watanabe et al., 2010). In addition, exaggerated glial activation has been implicated in the cognitive decline associated with aging and Alzheimer’s disease (Bilbo, 2010; Bilbo et al., 2011; Capuron and Miller, 2011; Caserta et al., 2009; Gate et al., 2010; Lee and Landreth, 2010; Lynch, 2009).
Equally as important as the link between immune dysfunction and neuropsychiatric disorders is the strong link between neuropsychiatric disorders and their origins in development. Many of these studies have been performed in humans, linking the early-life environment of an individual with the later-life risk of cognitive disorders (depression, PTSD, autism and schizophrenia) (Amstadter et al., 2011; Caspi and Moffitt, 2006; Robinson et al., 2011; Rutter et al., 2006; Toyokawa et al., 2011). Similar studies have been done in rodents, which have linked early-life immune challenges to the later-life risk of cognitive disorder with direct implications for glial function in these long-term consequences (Bilbo and Schwarz, 2009; Bilbo and Tsang, 2010; Bilbo, 2010; Bilbo et al., 2011; Williamson et al., 2011).
Thus investigating neuropsychiatric disorders from the perspective of the neuroimmune system and development will likely yield important and informative insights into the etiology of many neuropsychiatric disorders, and most important for this review, the well-known sex difference in the expression of many of these disorders. We propose that glia may be particularly vulnerable to the early-life environment because of the critical role these cells have in the development of the brain, thus we will explore the important role of these cells in brain development and in establishing sex differences within the brain.
A Brief Overview of Microglia and Astrocytes within the Developing Brain
Though they are often lumped together as “glia”, microglia and astrocytes each have a distinct ontogeny within the developing brain. Microglia originate relatively early in the life of the fetus and may be very long-lived, meaning that microglia have the capacity to reside in the brain for most of the life of the animal. Microglial progenitor cells begin colonizing the rodent brain around embryonic day (E) 9–10 via the infiltration of primitive monocyte precursors from the yolk sac of the embryo (Chan et al., 2007; Ginhoux et al., 2010). Microglia themselves are identifiable within the developing brain around E13 or 14, initially localized within the embryonic brain around subcortical regions such as the hippocampus and around the corpus callosum via the blood stream and ventricles (Cuadros and Navascués, 1998; Wang et al., 2002; Xu et al., 1993). From that point, microglia migrate to their final destination within the brain where they continue to proliferate and differentiate.
In contrast, astrocytes are derived from specific populations of progenitor cells within the rodent brain [see (Zhang and Barres, 2010) for review] towards the end of embryonic development. Astrocytes continue to proliferate in select niches of the adult rodent brain, though the proliferation potential of astrocytes is dependent upon their function and location within the brain (Kriegstein and Alvarez-Buylla, 2009). During mammalian nervous system development, neural progenitor cells (NPCs) generate neurons first and astrocytes second, though the switch that guides this determination is complex and brain-region dependent [for review see (Freeman, 2010)]. The differentiation of neural progenitor cells into neurons first and astrocytes second requires a discrete turning on and turning off of particular genes, such as gfap or s100b, using epigenetic mechanisms including DNA methylation (Takizawa et al., 2001). In fact, a recent experiment determined that co-culture of neural progenitor cells with microglia can promote the differentiation of neural progenitors into astrocytes (Gu et al., 2011), which is not surprising given that microglia are present within the developing rodent brain prior to the presence of astrocytes within the developing brain. These data also indicate that 1) factors released by microglia can influence the differentiation of neural progenitor cells, and 2) the role of glial cells within the brain is constantly evolving.
Both microglia and astrocytes have a distinct morphology within the developing brain. Protoplasmic astrocytes begin to sprout processes within the final weeks of embryonic development and the first weeks of postnatal development, though they look nothing like the intricate “bushy” astrocytes described in the adult brain (Bushong et al., 2004). One technical difficulty with characterizing the morphology of astrocytes regards the markers used to identify them. Many markers, including the widely used glial fibrillary acidic protein (GFAP), stain only the primary stellate cytoskeleton of protoplasmic astrocytes, leaving many of the thin astrocytic processes undetected (Bushong et al., 2002). However, using extensive filling methods with Lucifer yellow researchers have been able to determine the developmental pattern of protoplasmic astrocytes within the hippocampus during the first postnatal weeks. In particular, astrocytes develop long processes relatively rapidly. By postnatal day (P) 7, astrocytes within the hippocampus display thin processes similar to filopodia that often terminate with small bulbous structures, with usually one primary but sometimes more primary, thicker processes (Bushong et al., 2004). One week later, astrocytes within the hippocampus display much greater ramification of their processes, though many of these processes are still thin and filopodial in nature; and by P21, astrocytes display multiple primary processes, thinner processes are more ramified/mature, and astrocytes have established distinct boundaries from neighboring astrocytes.
In contrast to astrocytes, microglia within the embryonic rodent brain have a larger, round, amoeboid morphology, similar to the morphology of microglia seen in the adult brain following injury or immune activation (Male and Rezaie, 2001; Rezaie et al., 1999). This morphology is consistent with their role in the phagocytosis of new cells. From birth to P4, microglia change their morphology rapidly as they develop from a round amoeboid shape to a shape characterized by a smaller cell body with thinner, longer processes (Schwarz et al., 2011). However, even in the juvenile and adolescent rodent brain, microglia within certain brain regions continue to show a more activated morphology, with thick variegated branches, suggesting that certain brain regions and the microglia within them are continuing to undergo maturational changes long after the postnatal period (Schwarz et al., 2011).
As microglia and astrocytes mature, they function in distinct ways to influence the on-going development of the neonatal brain. Neural development can be broken down into specific processes, which overlap in timing throughout brain development. These include cell genesis (including neurogenesis), migration, axon guidance, synaptogenesis, synaptic pruning, and programmed cell death. To date, it is not well-known what factors drive the infiltration and migration of immature microglia throughout the brain; however, many researchers have noted that the invasion of microglia within the developing brain coincides with programmed cell death that occurs during early brain development (Ashwell, 1990; Ashwell, 1991; Perry et al., 1985). However, little is known about the exact relationship between developmental cell death and microglial colonization of neural tissues. During early brain development, microglia produce elevated levels of diffusible immune factors, such as cytokines and chemokines, which have a critical role in many neurodevelopmental processes. For example, Interleukin (IL)-1β is produced at detectable levels within the cortex from approximately E14 to P7 (Giulian et al., 1988). In contrast, the cerebellum, which develops significantly later just prior to birth in rodents, produces a peak in IL-1β levels that occurs from P2 to P14 (Giulian et al., 1988). Microglia and their releasable factors have a demonstrated role in cell proliferation, differentiation, axon guidance, synaptogenesis and synaptic pruning, either via the production of certain immune molecules or via the identification of other immune molecules produced by neurons [see (Boulanger, 2009; Deverman and Patterson, 2009) for review]. Astrocytes have an important function in synaptogenesis and synaptic scaling, either through the release of diffusible factors or the production of extracellular matrix proteins [see (Eroglu and Barres, 2010) for review}. Most synaptogenesis occurs after birth and depends significantly on astrocyte function. Despite the fact that a single astrocyte can associate with nearly 2 million synapses, relatively little is known regarding the chemical and mechanical signaling processes of astrocytes that can influence synaptic function [see (Freeman, 2010) for review].
Sex and the Developing Brain
Very few studies have investigated potential sex differences in the morphology, number, or function of microglia and astrocytes within the developing brain, though these cells have a critical role in many on-going processes of neural development as described above. Sex differences exist in many neurodevelopmental processes, and these sex differences are critical for establishing the well-known differences in physiology and behavior between males and females. In male rats, testosterone production begins around embryonic day (E) 18 with a significant peak shortly thereafter and a second larger peak at birth (P0). Testosterone is converted to estradiol in the rat brain via the enzyme aromatase and it is estradiol that primarily influences the developmental patterning of the male brain. Testosterone itself or its other primary conversion product, dihydrotestosterone (DHT), can also affect the development of the male rat brain; and sex differences in the genes expressed by the sex chromosomes themselves (e.g. genes expressed on the Y chromosome of the male) can also influence the development of the male brain. However, estradiol is the primary mechanism by which the male rodent brain is shaped and determined for sex-specific behaviors in adulthood.
Estradiol affects the number of neurons in particular brain nuclei (most famously the sexually dimorphic nucleus of the preoptic area), the number of synaptic connections along individual neurons in other brain regions, and the density of particular fiber tracts [see (McCarthy and Arnold, 2011) for review]. In the absence of testosterone secretion, the female brain develops and thus this difference in hormone exposure early in brain development is the primary mechanism by which sex differences between the male and female brain are established.
Astrocytes: sex differences and immune molecules in the developing brain
Given the multitude of sex differences within the developing brain, it is perhaps no surprise that sex differences have also been detected within the developing brain in the number and morphology of glia (see FIGURE 2 for an overview). Within the arcuate nucleus (ARC), a brain region critical for in the control of gonadotropin secretion, neonatal males have significantly fewer dendritic spines than females, and treatment of females with testosterone during the critical period of sexual differentiation decreases the dendritic spine density, while castration of males increases dendritic spines in this particular brain region. Concurrently, astrocytes within the male ARC have a more processes and branches, appearing more stellate in morphology; while astrocytes in the female ARC have significantly fewer and shorter astrocyte processes. This sex difference in astrocyte morphology can be reversed by either castration or treatment of females with testosterone (Mong et al., 1996; Mong et al., 1999) and suggests that there is, at least within the ARC, an inverse relationship between dendritic spine density and astrocyte complexity that is dependent upon neonatal testosterone exposure during the critical period of sexual differentiation of the brain. In a brain region next to the ARC, the ventromedial nucleus of the hypothalamus (VMN), a similar sex difference exists in the number of dendritic spines; however, astrocytes within this brain region are significantly underdeveloped and show no correlation to the sex difference in dendritic spines (Mong et al., 1999). These data indicate that the responsiveness of glia to hormones may be dependent upon the brain region being analyzed, the developmental stage of the cells within each brain region, and the underlying mechanism of action (Laping et al., 1994).
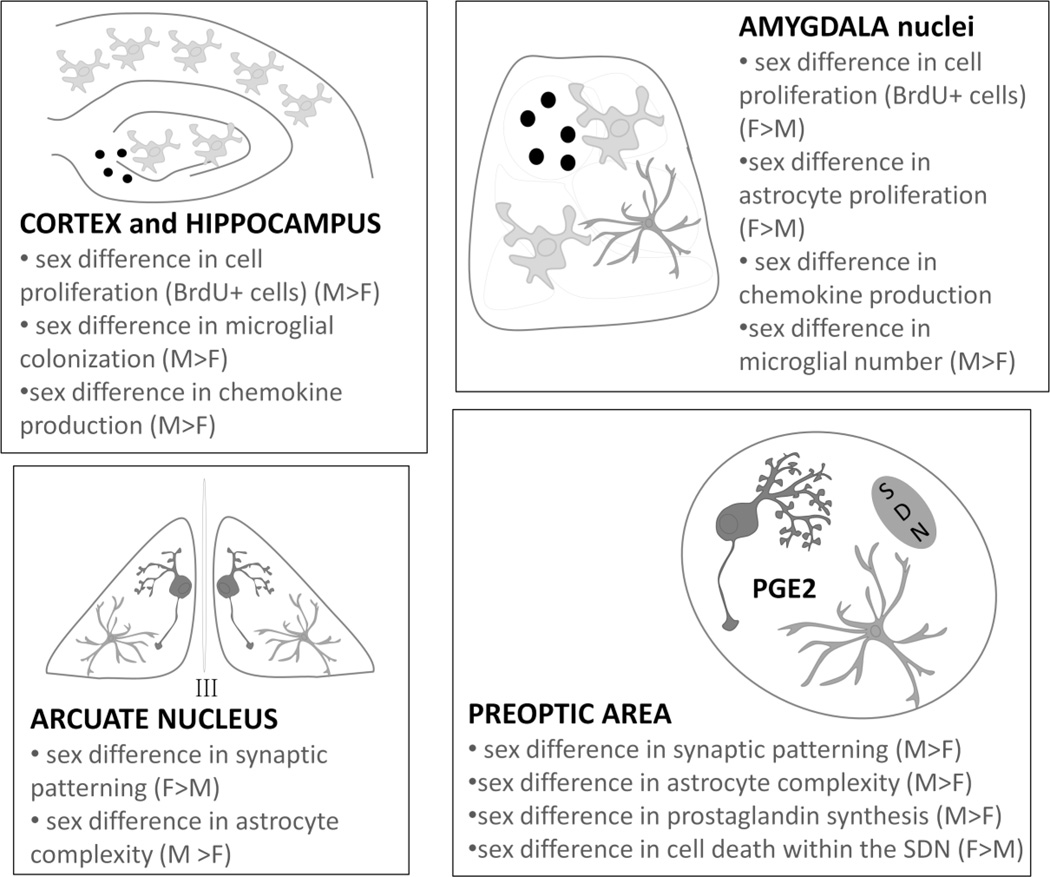
Figure 2. Sexual differentiation, glia and immune molecules in the developing brain.
Within the neonatal cortex and hippocampus, sex differences have been identified in the proliferation of new cells (BrdU+ cells), the colonization/number of microglia, and the expression level of certain chemokines (in particular CCL4 and CCL20). Within the neonatal amygdala, a sex difference has been identified in the proliferation of new cells (BrdU+ cells) which is representative of an increase in new astrocytes. A sex difference in the production of chemokines (CXCL9 is significantly increased in females compared to males) and the colonization/number of new microglia has also been identified within the developing amygdala. Within the neonatal arcuate nucleus, females have significantly more dendritic spines on neurons and a corresponding decrease in astrocyte complexity when compared to males, suggesting an inverse relationship between astrocyte complexity and synaptic patterning within the developing arcuate nucleus. In the neighboring preoptic area, males have significantly more dendritic spines on neurons; males have more complex astrocytes and increased levels of prostaglandin (PG) E2, a pro-inflammatory immune molecule. In contrast, females have increased levels of programmed cell death within the POA creating the robust size difference of the sexually dimorphic nucleus (SDN) between males and females.
Though the data from the ARC provided the first evidence of sex differences in astrocyte morphology and correlated sex differences in neuronal morphology, the mechanism underlying this sex difference remains relatively unknown. In an adjacent brain region, the preoptic area (POA), sex differences in neuronal morphology critical for male sex behavior in adulthood are dependent upon the morphology of astrocytes and their synthesis of the immune molecule, prostaglandin E2 (PGE2). PGE2 is up-regulated by the enzyme cyclooxygenase 1 or 2. COX-2 expression, in particular, is induced in response to peripheral inflammatory stimuli, and is a rate-limiting enzyme for prostaglandin (PG) synthesis in the brain. Prostaglandins, in general, are inflammatory molecules that are produced in cells along blood vessels within the blood brain barrier, and propagate the inflammatory signal into other brain regions. PG is involved in the activation of the HPA axis during infection, the release of CRH, and the onset of fever (Blatteis, 2007). Within the developing male POA, however, PGE2 is up-regulated by neonatal estradiol exposure in a “non-inflammatory” manner. Males have significantly more dendritic spines and this sex difference is established by estradiol during the critical period of sexual differentiation and is maintained into adulthood (Amateau and McCarthy, 2002) (FIGURE 2). The POA is critical for the expression of male sexual behavior, and thus it is no surprise that the wiring of these neurons is different within the male brain than in the female brain. PGE2 or estradiol treatment can significantly increase the density of dendritic spines during the critical period of sexual differentiation for the life of the rodent, and subsequently masculinize sexual behavior (Amateau and McCarthy, 2004). The proposed mechanism of this action involves the neuronal synthesis of PGE2, which is subsequently released and activates neighboring astrocytes that have prostaglandin (EP) receptors (Wright et al., 2008). Astrocytes in turn release glutamate and alter the synaptic connectivity of the neurons within the POA (McCarthy et al., 2003; Wright and McCarthy, 2009). This is an excellent demonstration that molecules, typically considered immune molecules, can significantly affect the developing brain in a sexually dimorphic manner.
Microglia: sex differences and immune molecules in the developing brain
Considering that microglia are the primary immune cells within the brain, we can learn a great deal regarding the role of immune molecules in the development of sex differences in the brain by examining microglia themselves. However, this cell type has been examined to a much greater extent in males than in females. Our lab has recently observed profound sex differences in the colonization of the developing rodent brain. At P4, male rats have significantly more microglia than females within many brain regions critical for cognition, learning and memory including the hippocampus, the parietal cortex, and the amygdala (FIGURE 2 and FIGURE 3). In general, these cells are large round amoeboid cells that produce elevated levels of cytokines and chemokines when compared to the adult brain (Schwarz et al., 2011). In addition to the robust sex difference in glial colonization that occurs during early brain development, males have nearly 200-fold greater expression of the chemokine ligand (CCL) 20 within the hippocampus/cortex than females and nearly 50-fold higher expression of the chemokine ligand, CCL4, than females (FIGURE 3). One might hypothesize that these two chemokines may be critical for driving the sex difference in colonization that arises just days later, though this remains to be determined.
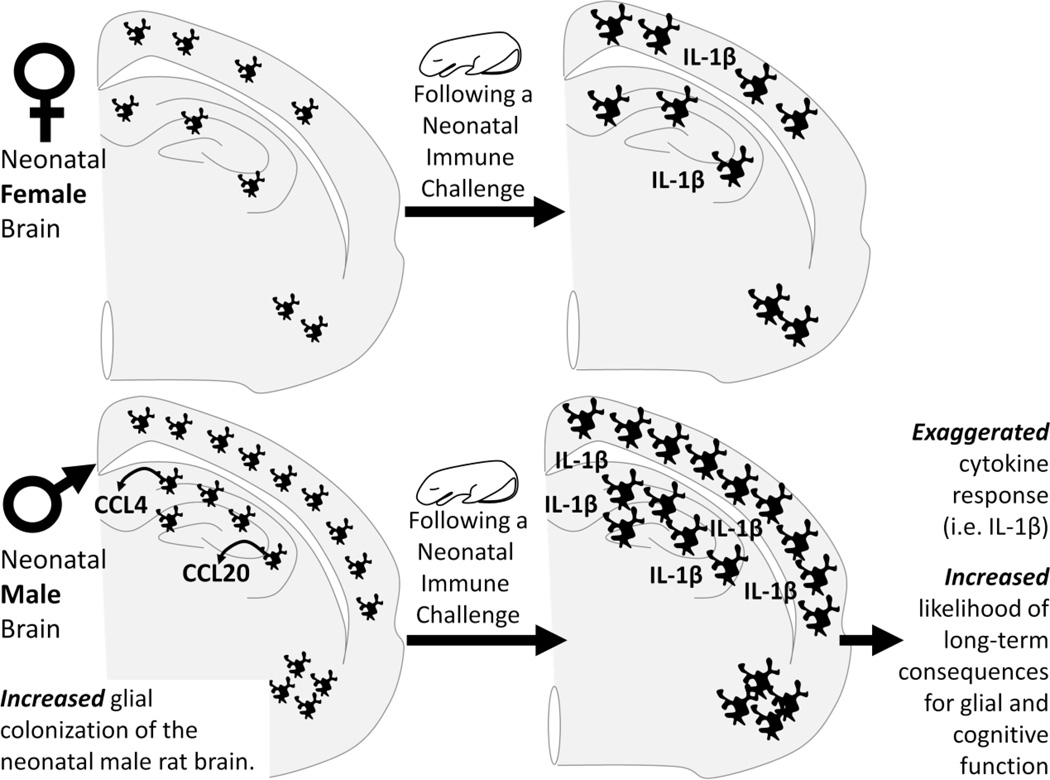
Figure 3. Sex differences in microglial colonization may make males more vulnerable to a neonatal immune challenge.
Within the developing hippocampus and cortex, males express nearly 200- and 50-fold higher levels of two specific chemokines, CCL20 and CCL4, respectively. Sex differences in chemokine (chemotactic cytokine) expression such as this may drive the sex difference in colonization of microglia into the neonatal brain from the periphery. Males have significantly more microglia within the cortex, hippocampus, and amygdala by postnatal day 4 than females. This sex difference in the number of microglia may make males more vulnerable in the event of a neonatal infection, causing exaggerated expression of cytokines. This increase in cytokine production during neural development causes long-term changes in glial function and increased risk of cognitive deficits in adulthood.
Interestingly, this sex difference in glial colonization does not exist within the paraventricular nucleus of the hypothalamus (PVN). Microglia within the adult PVN produce cytokines following an immune challenge (e.g. infection), driving the activation of the stress axis and the release of stress hormones, corticosterone, into the circulation; thus the PVN is critical for mediating the physiological response to an immune challenge (Berkenbosch et al., 1987; Bernardini et al., 1990; Navarra et al., 1991; Sapolsky et al., 1987). Despite this, it appears that the factors influencing sex differences in microglial colonization within cortical brain regions may not affect the colonization of microglia into the PVN and potentially other brain regions not yet assessed.
At this time it is not clear whether sex differences in glial colonization occur in response to the well-known sex differences in neurodevelopmental processes mentioned above, driven by sex differences in hormone exposure; or whether glial colonization may be differentially affected by sex and/or hormones and thus subsequently affect ongoing neurodevelopmental processes in a sex-dependent manner. The sex difference in glial colonization does not exist at E17, a time point prior to the onset of hormone production in the rat. In addition, microglia express sex hormone receptors (Milner et al., 2005), and steroid-converting enzymes (Gottfried-Blackmore et al., 2008; Gottfried-Blackmore et al., 2010), thus taken together, it is likely that sex differences in the colonization of microglia within the developing brain may be driven by differential exposure to sex hormones. Alternatively, it is not clear whether the sex differences in glial colonization occur in response to sex differences in the processes of neural development, which are caused by hormone exposure; or whether glial colonization may be differentially affected by sex or hormones and thereby subsequently affect the ongoing processes of neural development in a sex-dependent manner.
For example, within a brain region known to be sexually dimorphic and important for masculine vocalizations in the gerbil, the sexually dimorphic area pars compacta (SDApc), microglia were similarly sexually dimorphic in number corresponding to the sex difference in cell death observed at the same time point (Holman and Rice, 1996; Holman, 1998). These data suggest that microglia either drive the sex difference in cell death or microglia are present to scavenge the cells during the on-going sexually dimorphic process of cell death (Holman and Collado, 2001).
Are males more sensitive than females to early-life immune challenges?
Perhaps regardless of the underlying mechanisms or the potentially yet unknown role of sex differences in immune molecules within the developing rodent brain, one thing may be certain: the sex difference in the number of glial cells and levels of immune molecules is likely to have profound sex-specific effects on the function of the neuroimmune system during an early-life immune challenge. Our lab has proposed that because of the distinct function/plasticity of glia throughout early brain development, they are particularly sensitive to the effects of early-life immune challenges or injury. We and others have provided significant evidence that the developing brain is particularly sensitive to long-term changes in glial function via early-life immune activation. In particular, male rats infected with a low dose of E.coli on postnatal day (P) 4 have long-term changes in the function of microglia, which in turn has profound consequences for cognitive behaviors in adulthood, such as learning and memory (Bilbo et al., 2005; Bilbo et al., 2005; Bilbo et al., 2008; Williamson et al., 2011). The same immune challenge later in development, at P30, has no long-term effects on glial function or behavior into adulthood (Bilbo et al., 2006), indicating that the long-term programming of glial function in males is limited to the postnatal period.
Our lab has also recently determined that the treatment of females with the same dose of E.coli has no long-term effects on cognitive behaviors in adulthood (Bilbo et al., 2011). Similarly, male rat pups exposed to bacterial endotoxin, lipopolysaccharide (LPS), during the early post-natal period exhibit increased tumor colonization within the lung and depressed activity of peripheral immune cells in adulthood. This same effect is not seen in females (Hodgson and Knott, 2002). Data such as these suggest that males and females may have a fundamentally different response to neonatal activation of the immune system. Specifically, females may be resilient to the long-term consequences of neonatal alterations in the immune system (FIGURE 3). While there is recent cellular and molecular evidence to suggest this, sex differences in neuroimmune function during early brain development have been vastly under-studied.
We do know that treatment of male rat pups with E.coli at P4 produces a robust and relatively pathway-focused increase in immune molecules within the hippocampus that are related to the production of IL-1β. For example, neonatal infection results in a 6-fold increase in Caspase-1 by 2 hours after infection and a 10-fold increase in Caspase-1 by 24 hours post-infection (Schwarz and Bilbo, 2011). Caspase-1 is the enzyme that cleaves the pro-form of IL-1β into its active form. We have recently determined that Caspase-1 is elevated significantly more so in males than in females within the cortex at P4 following an E.coli infection. Specifically, Caspase-1 is elevated only 3-fold from baseline, 24 hours post-infection in females. In the hippocampus, we see a similar pattern for Caspase-1 expression following a neonatal infection; the up-regulation is attenuated in females when compared to males. These data suggest that IL-1β may be a critical molecule for producing long-term changes in neuroimmune function in males, and that the decreased response within the female brain may be protective against these long-term negative consequences of an early-life infection. In addition, the exaggerated response seen in males may be related to the significant increase in the number of microglia present in the hippocampus and cortex at this time. Thus, while this sex difference in cell number may be “normal” in the course of brain development, it may be responsible for creating the sex disparity in the negative long-term consequences to an early-life infection (FIGURE 3).
A similar finding was recently published using cultured astrocytes from neonatal males and females. Specifically, cortical astrocytes were cultured from P1 males or females, and from females masculinized with testosterone treatment 24 hours prior. Treatment of these astrocytes with LPS produced a significantly greater cytokine response in the astrocytes retrieved from males or the masculinized females than the cytokine response produced by astrocytes retrieved from females. This effect was consistent for IL-1β, IL-6, and TNFα (Santos-Galindo et al., 2011). Astrocytes can also express TLR4, the receptor which recognizes LPS (Gorina et al., 2011); however these authors found no significant difference in TLR4 expression between males and females on neonatal astrocytes (Santos-Galindo et al., 2011), similar to our own findings that there are no significant differences in TLR4 expression between males and females during development (Schwarz et al., 2011). These data suggest there may be an intrinsic factor underlying the sex difference in inflammatory responses generated by neonatal microglia and astrocytes that is driven by neonatal hormone exposure. Regardless, these data provide strong evidence that males may be more sensitive to neonatal infection because of sex differences in glial reactivity (FIGURE 3).
Males are also more sensitive to other early-life challenges. For example, males exhibit significantly more hippocampal neuronal death and astrocyte reactivity when compared to females following maternal separation (Llorente et al., 2009). Males also exhibit increased cell death in the hippocampus in models of neonatal ischemia, and this effect is dependent upon neonatal hormone exposure (Hilton et al., 2003; Nunez et al., 2003). In contrast, females are more sensitive to disruptions in later-life reproductive physiology and behavior, the result of a neonatal immune challenge (Walker et al., 2011); suggesting that in certain circumstances, differential sensitivity to early-life immune activation or injury may be dependent upon the age of the insult, the brain region of analysis, and the behavioral endpoint being assessed. Factors other than glial function may also influence the sensitivity of males and females to an early-life infection or injury, including sex differences in the responsiveness of the HPA axis (Kentner and Pittman, 2010). However, based on the data presented here, further investigation into the role of the immune system and specifically glial function may lend even greater insight into the mechanisms underlying this differential sensitivity to perinatal immune activation and injury.
Are females more sensitive than males to later-life immune challenges?
We have also recently determined that the sex difference in microglial colonization observed in the neonatal brain reverses just prior to adolescence in the juvenile brain, and this sex difference is maintained into adulthood (Schwarz et al., 2011). During this time, females have significantly more microglia than males within brain regions important for cognition including the hippocampus, the parietal cortex, and the amygdala. Microglia within the female brain also display a more activated morphology compared to the microglia within the male brain at the same age. Specifically, microglia within the adolescent and adult female brain have significantly thicker and more branched processes than microglia within the adolescent and adult male brain (FIGURE 4). A close relationship exists between the morphology of microglia and their function [see (Ransohoff and Perry, 2009) for review], suggesting that microglia within the juvenile/adolescent female brain may have a very different functional output from that of males at the same time point. This has also been examined in the mouse brain. Female mice have significantly more microglia and astrocytes within the hippocampus than males, and as aging progresses females are more likely to have a significant increase in both cell populations within the hippocampus (Mouton, 2002).
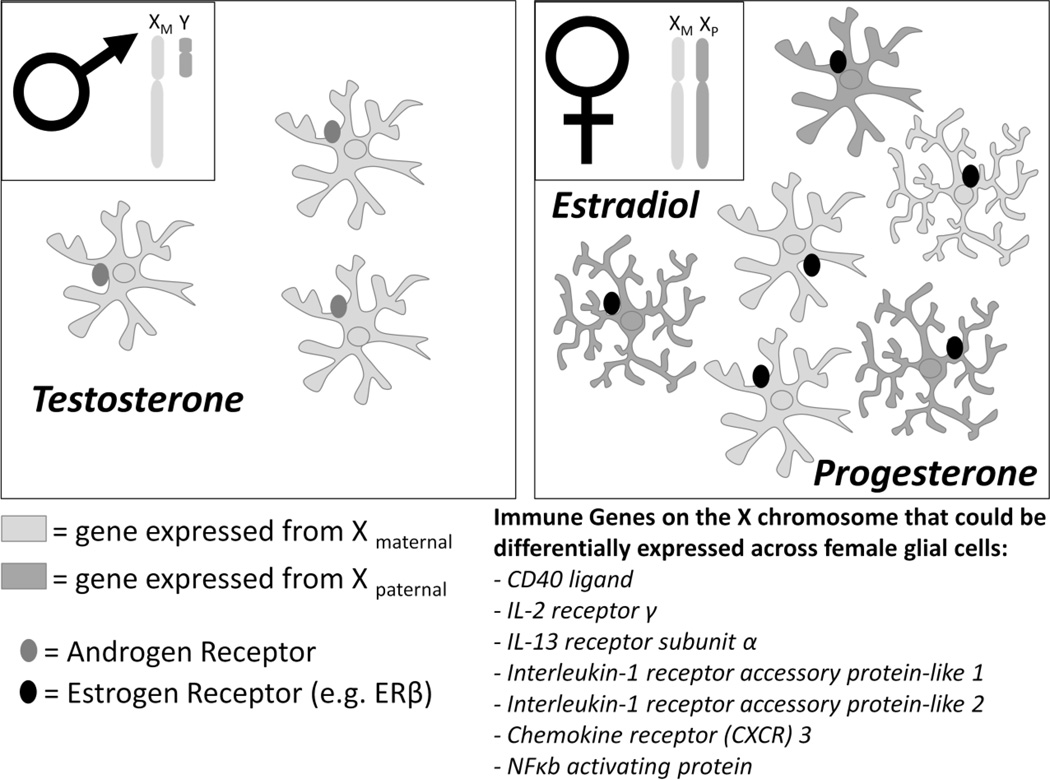
Figure 4. Factors that may influence glial function in adult males and females.
Males have two sex chromosomes, a maternal X chromosome and a paternal Y chromosome. As a result, all glia within the male brain express the same copy of X-linked genes. In contrast, females have two X chromosomes, one maternal X chromosome and one paternal X chromosome. During X-inactivation, a process that occurs exclusively in females, one copy of each gene is randomly silenced, creating a mixture of maternal or paternal gene expression in glial cells. A significant number of immune genes are located on the X chromosome, and thus differences in expression across different cell types may influence the function of glia at baseline or during an immune challenge. Adult females also have significantly more microglia with thicker, longer branches than males within the hippocampus, cortex, and amygdala; and this morphology has been linked to increased activation (Ransohoff and Perry, 2009). In addition, adult males and females have different levels of circulating hormones. Males have constant levels of testosterone that can act at androgen receptors, present on glial cells. In contrast, females have fluctuating levels of estradiol (the receptor for which, ER β, has been localized to glia) and progesterone which may influence glial function.
In support of the idea that glia within the adult female brain may be more “active” than microglia in the adult male brain, females have significantly higher levels of several genes within the hippocampus at P60 compared to males, including the proinflammatory cytokines IL-1α and IL-1β, a well-known marker of microglial activation called cluster of differentiation molecule 11 B (CD11b), and a signaling molecule necessary for the recognition of certain pathogens called Toll Interacting Protein (TOLLIP).
A significant amount of research has been done to examine the effects of female sex hormones (estradiol and progesterone) on glial function in adulthood because human females are more sensitive to multiple sclerosis, an autoimmune disease that attacks the CNS and is commonly diagnosed between the ages of 20 and 40. In general, female sex hormones such as estradiol are seen as neuroprotective (Veiga et al., 2004); and in support of this idea, experiments have determined that estradiol and/or progesterone can reduce LPS-induced expression of many pro-inflammatory cytokines (TNFα, IL-18, or nitric oxide for example) and chemokines (CCL2 and CCL5 for example) (Baker et al., 2004; Drew and Chavis, 2000; Drew et al., 2003; Kipp et al., 2007). However, many of these experiments are performed on astrocytes or microglia ex vivo, collected from neonates (age and sex unknown/mixed), or from microglial cell lines. Thus the results of these experiments can be difficult to interpret given the aforementioned differences in both microglia and astrocyte function/morphology within the neonatal brain versus the adult brain and the differences between the sexes.
Just as chromosomal sex has an important role in determining the function of individual immune cells within the peripheral immune system, we predict that genetic sex may play an important role in determining glial function in maintaining homeostasis or during an immune challenge. Female rats have significantly more microglia with a more activated phenotype than males by P30 in many brain regions including the amygdala, hippocampus, and cortex (Schwarz et al., 2011). This specific time point represents the end of the juvenile period of rodent development, yet is prior to the onset of circulating female hormones that begins during adolescence. These data indicate that other factors, potentially genetic factors, may drive the sex difference in glial morphology and number within the juvenile and adult female rodent brain (FIGURE 4). In sum, the age and sex of collected cells must remain a critical factor when considering the results of in vitro studies so that we might expand our knowledge of the effects of sex and hormones on glial cell function in general.
Females are often more likely to be diagnosed with disorders such as depression and anxiety that present later in life, around adolescence. Just as females have a more robust peripheral immune response to infection, females may also have a more robust central immune response to disease given the increase in cell number and gene expression that arises before adolescence. As we mentioned, this robust peripheral immune response can be a disadvantage, increasing a female’s risk of developing autoimmune disorders. In a similar manner, the increase in glial cell number and function may be a disadvantage, increasing a female’s risk of developing depression or anxiety related disorders. Thus, an exaggerated or sensitized neuroimmune response becomes abnormal when not in the context of an overt infection (Dantzer, 2006). More research focusing on the link between depression, anxiety and glial function will likely yield a great wealth of knowledge; however, the challenge will be to study this link in females, a task which has not yet been undertaken.
Conclusions and Future Directions
Many neuropsychiatric disorders exhibit a striking sex difference in their etiology and prevalence. Males are more likely to be diagnosed with disorders that present at birth or early in childhood. In contrast, females are more often diagnosed with disorders that arise around the onset of adolescence or puberty [see (Bao and Swaab, 2010) for review]. These same neuropsychiatric disorders, both early- and late-onset, are also associated with alterations in immune function. Given the critical role of both microglia and astrocytes in neural function and immune function, it is surprising that very few studies have addressed sex differences in the phenotype and function of these cells throughout different points in development. Taken together, we propose that this field warrants further research into the role that sex-dependent mechanisms may play in glial colonization/differentiation, number, and function, and their potential contribution to neural and cognitive dysfunction. In particular, great care needs to be taken in assuming that the glial cells within the neonatal brain are identical to the glia found in the adult brain. The morphology, function, and synthesis of immune molecules from glial cells is markedly different during early postnatal development as these cells continue, themselves, to develop and direct the development of the cells around them. In addition, the developmental time course for each brain region is likely distinct. Certain brain regions develop early (either embryonically or immediately postnatal) while other brain regions continue to undergo maturational changes into adolescence, which has been deemed a second “critical period for sexual differentiation” (Sisk and Zehr, 2005), and this can significantly influence the function of glial cells. We hypothesize that many of the sex differences observed here, first in males early in development and then in females later in life, may account for disparities in the onset, prevalence, or severity of symptoms associated with many neuropsychiatric disorders. However, future research aimed at investigating the effects of sex and glial function on behavior either via long-term consequences on postnatal brain development or in adulthood is certainly warranted.
Schwarz and Bilbo – Highlights.
Sex, Glia, and Development: potential interactions in health and disease
Glia, including astrocytes and microglia, are critical for brain development.
Astrocytes are important for establishing sex differences in synaptic patterning.
Neonatal male rats have significantly more microglia in brain regions than females.
Adult female rats have more microglia in the same brain regions than males.
Sex differences in glia may underlie vulnerabilities to particular disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, Pogorelov V, Ladenheim B, Yang C, Krasnova IN, Cadet JL, Pardo C, Mori S, Kamiya A, Vogel MW, Sawa A, Ross CA, Pletnikov MV. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol. Psychiatry. 2010;68:1172–1181. doi: 10.1016/j.biopsych.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat. Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Yang BZ, Miller A, Siburian R, Moorjani P, Haddad S, Basu A, Fagerness J, Saxe G, Smoller JW, Koenen KC. Corticotrophin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Dis. Markers. 2011;30:89–99. doi: 10.3233/DMA-2011-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Navarrete M. Glial cells in neuronal network function. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010;365:2375–2381. doi: 10.1098/rstb.2009.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell K. Microglia and cell death in the developing mouse cerebellum. Brain Res. Dev. Brain Res. 1990;55:219–230. doi: 10.1016/0165-3806(90)90203-b. [DOI] [PubMed] [Google Scholar]
- Ashwell K. The distribution of microglia and cell death in the fetal rat forebrain. Brain Res. Dev. Brain Res. 1991;58:1–12. doi: 10.1016/0165-3806(91)90231-7. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain Behav. Immun. 2010 doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J. Neuroimmunol. 2011;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145:5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- Bao AM, Swaab DF. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscientist. 2010;16:550–565. doi: 10.1177/1073858410377005. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Berletch JB, Yang F, Xu J, Carrel L, Disteche CM. Genes that escape from X inactivation. Hum. Genet. 2011;130:237–245. doi: 10.1007/s00439-011-1011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini R, Kamilaris TC, Calogero AE, Johnson EO, Gomez MT, Gold PW, Chrousos GP. Interactions between tumor necrosis factor-alpha, hypothalamic corticotropin-releasing hormone, and adrenocorticotropin secretion in the rat. Endocrinology. 1990;126:2876–2881. doi: 10.1210/endo-126-6-2876. [DOI] [PubMed] [Google Scholar]
- Bilbo SD. Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiol. Learn. Mem. 2010;94:57–64. doi: 10.1016/j.nlm.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav. Neurosci. 2005;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav. Brain Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front. Behav. Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Smith SH, Schwarz JM. A Lifespan Approach to Neuroinflammatory and Cognitive Disorders: A Critical Role for Glia. J. Neuroimmune Pharmacol. 2011 doi: 10.1007/s11481-011-9299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24:2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Barrientos RM, Eads AS, Northcutt A, Watkins LR, Rudy JW, Maier SF. Early-life infection leads to altered BDNF and IL-1β mRNA expression in rat hippocampus following learning in adulthood. Brain Behav. Immun. 2008;22:451–455. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Blatteis CM. The onset of fever: new insights into its mechanism. Prog. Brain Res. 2007;162:3–14. doi: 10.1016/S0079-6123(06)62001-3. [DOI] [PubMed] [Google Scholar]
- Boulanger LM. Immune Proteins in Brain Development and Synaptic Plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum. Reprod. Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. International Journal of Developmental Neuroscience. 2004;22:73–86. doi: 10.1016/j.ijdevneu.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol. Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Van de Water J, Ashwood P. Immune dysfunction in autism: a pathway to treatment. Neurotherapeutics. 2010;7:283–292. doi: 10.1016/j.nurt.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta MT, Bannon Y, Fernandez F, Giunta B, Schoenberg MR, Tan J. Normal brain aging clinical, immunological, neuropsychological, and neuroimaging features. Int. Rev. Neurobiol. 2009;84:1–19. doi: 10.1016/S0074-7742(09)00401-2. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat. Rev. Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Res. Rev. 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun. Rev. 2003;2:119–125. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- Cuadros MA, Navascués J. The origin and differentiation of microglial cells during development. Prog. Neurobiol. 1998;56:173–189. doi: 10.1016/s0301-0082(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Seriolo B, Straub RH. Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus. 2004;13:635–638. doi: 10.1191/0961203304lu1094oa. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Kurkowska-Jastrzebska I. Inflammation and gliosis in neurological diseases--clinical implications. J. Neuroimmunol. 2011;231:78–85. doi: 10.1016/j.jneuroim.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurol. Clin. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Elia HF, Carlsten H. The impact of hormone replacement therapy on humoral and cell-mediated immune responses in vivo in post-menopausal women with rheumatoid arthritis. Scand. J. Immunol. 2008;68:661–667. doi: 10.1111/j.1365-3083.2008.02186.x. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J. Neuroimmunol. 2000;111:77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- Drew PD, Chavis JA, Bhatt R. Sex steroid regulation of microglial cell activation: relevance to multiple sclerosis. Ann. N. Y. Acad. Sci. 2003;1007:329–334. doi: 10.1196/annals.1286.031. [DOI] [PubMed] [Google Scholar]
- Eens M, Van Duyse E, Berghman L, Pinxten R. Shield characteristics are testosterone-dependent in both male and female moorhens. Horm. Behav. 2000;37:126–134. doi: 10.1006/hbeh.1999.1569. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR. Specification and morphogenesis of astrocytes. Science. 2010;330:774–778. doi: 10.1126/science.1190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard RC, Spinedi E. Sex-and stress-steroids interactions and the immune system: evidence for a neuroendocrine-immunological sexual dimorphism. Domest. Anim. Endocrinol. 1998;15:345–352. doi: 10.1016/s0739-7240(98)00028-9. [DOI] [PubMed] [Google Scholar]
- Garay PA, McAllister AK. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Front. Synaptic Neurosci. 2010;2:136. doi: 10.3389/fnsyn.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gate D, Rezai-Zadeh K, Jodry D, Rentsendorj A, Town T. Macrophages in Alzheimer's disease: the blood-borne identity. J. Neural Transm. 2010;117:961–970. doi: 10.1007/s00702-010-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Young DG, Woodward J, Brown DC, Lachman LB. Interleukin-1 is an astroglial growth factor in the developing brain. J. Neurosci. 1988;8:709–714. doi: 10.1523/JNEUROSCI.08-02-00709.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorina R, Font-Nieves M, Marquez-Kisinousky L, Santalucia T, Planas AM. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFkappaB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59:242–255. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- Gottfried-Blackmore A, Sierra A, Jellinck PH, McEwen BS, Bulloch K. Brain microglia express steroid-converting enzymes in the mouse. J. Steroid Biochem. Mol. Biol. 2008;109:96–107. doi: 10.1016/j.jsbmb.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried-Blackmore A, Sierra A, McEwen BS, Ge R, Bulloch K. Microglia express functional 11 beta-hydroxysteroid dehydrogenase type 1. Glia. 2010;58:1257–1266. doi: 10.1002/glia.21007. [DOI] [PubMed] [Google Scholar]
- Grossman C. Possible underlying mechanisms of sexual dimorphism in the immune response, fact and hypothesis. J. Steroid Biochem. 1989;34:241–251. doi: 10.1016/0022-4731(89)90088-5. [DOI] [PubMed] [Google Scholar]
- Gu F, Wang J, Fu L, Ma YJ. Co-culture with microglia promotes neural stem cells differentiation into astrocytes. Chin. Med. J. (Engl) 2011;124:3394–3398. [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57:343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton GD, Nunez JL, McCarthy MM. Sex differences in response to kainic acid and estradiol in the hippocampus of newborn rats. Neuroscience. 2003;116:383–391. doi: 10.1016/s0306-4522(02)00716-9. [DOI] [PubMed] [Google Scholar]
- Hodgson DM, Knott B. Potentiation of tumor metastasis in adulthood by neonatal endotoxin exposure: sex differences. Psychoneuroendocrinology. 2002;27:791–804. doi: 10.1016/s0306-4530(01)00080-4. [DOI] [PubMed] [Google Scholar]
- Holman S, Collado P. Postnatal cell proliferation and death in a lateralized, gender-related, asymmetric nucleus. J. Neurobiol. 2001;47:150–158. doi: 10.1002/neu.1022. [DOI] [PubMed] [Google Scholar]
- Holman SD. Neuronal cell death during sexual differentiation and lateralisation of vocal communication. Neurosci. Biobehav. Rev. 1998;22:725–734. doi: 10.1016/s0149-7634(98)00001-3. [DOI] [PubMed] [Google Scholar]
- Holman SD, Rice A. Androgenic effects on hypothalamic asymmetry in a sexually differentiated nucleus related to vocal behavior in Mongolian gerbils. Horm. Behav. 1996;30:662–672. doi: 10.1006/hbeh.1996.0067. [DOI] [PubMed] [Google Scholar]
- Kentner AC, Pittman QJ. Minireview: early-life programming by inflammation of the neuroendocrine system. Endocrinology. 2010;151:4602–4606. doi: 10.1210/en.2010-0583. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kipp M, Karakaya S, Johann S, Kampmann E, Mey J, Beyer C. Oestrogen and progesterone reduce lipopolysaccharide-induced expression of tumour necrosis factor-alpha and interleukin-18 in midbrain astrocytes. J. Neuroendocrinol. 2007;19:819–822. doi: 10.1111/j.1365-2826.2007.01588.x. [DOI] [PubMed] [Google Scholar]
- Kivity S, Ehrenfeld M. Can we explain the higher prevalence of autoimmune disease in women? Expert Rev. Clin. Immunol. 2010;6:691–694. doi: 10.1586/eci.10.60. [DOI] [PubMed] [Google Scholar]
- Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci. Biobehav. Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- Klein SL. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26:247–264. doi: 10.1111/j.0141-9838.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- Kovacs EJ, Messingham KA, Gregory MS. Estrogen regulation of immune responses after injury. Mol. Cell. Endocrinol. 2002;193:129–135. doi: 10.1016/s0303-7207(02)00106-5. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzych U, Strausser HR, Bressler JP, Goldstein AL. Effects of sex hormones on some T and B cell functions, evidenced by differential immune expression between male and female mice and cyclic pattern of immune responsiveness during the estrous cycle in female mice. Am. J. Reprod. Immunol. 1981;1:73–77. doi: 10.1111/j.1600-0897.1981.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Laping NJ, Teter B, Nichols NR, Rozovsky I, Finch CE. Glial fibrillary acidic protein: regulation by hormones, cytokines, and growth factors. Brain Pathol. 1994;4:259–275. doi: 10.1111/j.1750-3639.1994.tb00841.x. [DOI] [PubMed] [Google Scholar]
- Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 2010;117:949–960. doi: 10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat. Rev. Immunol. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- Lleo A, Battezzati PM, Selmi C, Gershwin ME, Podda M. Is autoimmunity a matter of sex? Autoimmun. Rev. 2008;7:626–630. doi: 10.1016/j.autrev.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Llorente R, Gallardo ML, Berzal AL, Prada C, Garcia-Segura LM, Viveros MP. Early maternal deprivation in rats induces gender-dependent effects on developing hippocampal and cerebellar cells. Int. J. Dev. Neurosci. 2009;27:233–241. doi: 10.1016/j.ijdevneu.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Lynch MA. The multifaceted profile of activated microglia. Mol. Neurobiol. 2009;40:139–156. doi: 10.1007/s12035-009-8077-9. [DOI] [PubMed] [Google Scholar]
- Male D, Rezaie P. Colonisation of the human central nervous system by microglia: the roles of chemokines and vascular adhesion molecules. In: Castellano Lopez MNB, editor. Progress in Brain Research. Elsevier; 2001. pp. 81–93. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Todd BJ, Amateau SK. Estradiol modulation of astrocytes and the establishment of sex differences in the brain. Ann. N. Y. Acad. Sci. 2003;1007:283–297. doi: 10.1196/annals.1286.027. [DOI] [PubMed] [Google Scholar]
- McClelland EE, Smith JM. Gender specific differences in the immune response to infection. Arch. Immunol. Ther. Exp. (Warsz) 2011;59:203–213. doi: 10.1007/s00005-011-0124-3. [DOI] [PubMed] [Google Scholar]
- McCombe PA, Greer JM, Mackay IR. Sexual dimorphism in autoimmune disease. Curr. Mol. Med. 2009;9:1058–1079. doi: 10.2174/156652409789839116. [DOI] [PubMed] [Google Scholar]
- McMillen MM. Differential mortality by sex in fetal and neonatal deaths. Science. 1979;204:89–91. doi: 10.1126/science.571144. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Kurzweil RL, Davis AM, Rocca MS, McCarthy MM. Evidence for sexual differentiation of glia in rat brain. Horm. Behav. 1996;30:553–562. doi: 10.1006/hbeh.1996.0058. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists. 2002 [Google Scholar]
- Muller N, Ackenheil M. Psychoneuroimmunology and the cytokine action in the CNS: implications for psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1998;22:1–33. doi: 10.1016/s0278-5846(97)00179-6. [DOI] [PubMed] [Google Scholar]
- Muller W, Groothuis TG, Kasprzik A, Dijkstra C, Alatalo RV, Siitari H. Prenatal androgen exposure modulates cellular and humoral immune function of black-headed gull chicks. Proc. Biol. Sci. 2005;272:1971–1977. doi: 10.1098/rspb.2005.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra P, Tsagarakis S, Faria MS, Rees LH, Besser GM, Grossman AB. Interleukins-1 and -6 stimulate the release of corticotropin-releasing hormone-41 from rat hypothalamus in vitro via the eicosanoid cyclooxygenase pathway. Endocrinology. 1991;128:37–44. doi: 10.1210/endo-128-1-37. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Alt JJ, McCarthy MM. A new model for prenatal brain damage. I. GABAA receptor activation induces cell death in developing rat hippocampus. Exp. Neurol. 2003;181:258–269. doi: 10.3201/eid0906.030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr. Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav. Immun. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Pessach IM. The relationship of x-linked primary immune deficiencies and autoimmunity. Curr. Allergy Asthma Rep. 2010;10:311–319. doi: 10.1007/s11882-010-0127-x. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW. Role of glial cells in the formation and maintenance of synapses. Brain Res. Rev. 2010;63:39–46. doi: 10.1016/j.brainresrev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Poulin R. Helminth growth in vertebrate hosts: does host sex matter? Int. J. Parasitol. 1996;26:1311–1315. doi: 10.1016/s0020-7519(96)00108-7. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Patel K, Male DK. Microglia in the human fetal spinal cord--patterns of distribution, morphology and phenotype. Brain Res. Dev. Brain Res. 1999;115:71–81. doi: 10.1016/s0165-3806(99)00043-7. [DOI] [PubMed] [Google Scholar]
- Robel S, Berninger B, Gotz M. The stem cell potential of glia: lessons from reactive gliosis. Nat. Rev. Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- Roberts M, Peters A. Is testosterone immunosuppressive in a condition-dependent manner? An experimental test in blue tits. J. Exp. Biol. 2009;212:1811–1818. doi: 10.1242/jeb.031047. [DOI] [PubMed] [Google Scholar]
- Roberts ML, Buchanan KL, Hasselquist D, Evans MR. Effects of testosterone and corticosterone on immunocompetence in the zebra finch. Horm. Behav. 2007;51:126–134. doi: 10.1016/j.yhbeh.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V, Happe F, Plomin R, Ronald A. A Multivariate Twin Study of Autistic Traits in 12-Year-Olds: Testing the Fractionable Autism Triad Hypothesis. Behav. Genet. 2011 doi: 10.1007/s10519-011-9500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. J. Child Psychol. Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Santos-Galindo M, Acaz-Fonseca E, Bellini MJ, Garcia-Segura LM. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol. Sex. Differ. 2011;2:7. doi: 10.1186/2042-6410-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Schneider CP, Schwacha MG, Chaudry IH. Influence of gender and age on T-cell responses in a murine model of trauma-hemorrhage: differences between circulating and tissue-fixed cells. J. Appl. Physiol. 2006;100:826–833. doi: 10.1152/japplphysiol.00898.2005. [DOI] [PubMed] [Google Scholar]
- Schuurs AH, Verheul HA. Effects of gender and sex steroids on the immune response. J. Steroid Biochem. 1990;35:157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. LPS elicits a much larger and broader inflammatory response than Escherichia coli infection within the hippocampus of neonatal rats. Neurosci. Lett. 2011;497:110–115. doi: 10.1016/j.neulet.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman WE, Gindhart TD. Effect of estrogen on natural killer cells. Arthritis Rheum. 1979;22:1234–1240. doi: 10.1002/art.1780221110. [DOI] [PubMed] [Google Scholar]
- Selmi C. The X in sex: how autoimmune diseases revolve around sex chromosomes. Best Pract. Res. Clin. Rheumatol. 2008;22:913–922. doi: 10.1016/j.berh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Sharif A, Prevot V. ErbB receptor signaling in astrocytes: a mediator of neuron-glia communication in the mature central nervous system. Neurochem. Int. 2010;57:344–358. doi: 10.1016/j.neuint.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Song W, Condron S, Mocca BT, Veit SJ, Hill D, Abbas A, Jerse AE. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17beta-estradiol-treated mice. Vaccine. 2008;26:5741–5751. doi: 10.1016/j.vaccine.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipursky J, Romao L, Tortelli V, Neto VM, Gomes FC. Neuron-glia signaling: Implications for astrocyte differentiation and synapse formation. Life Sci. 2011;89:524–531. doi: 10.1016/j.lfs.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Swaab DF. Sexual differentiation of the human brain: relevance for gender identity, transsexualism and sexual orientation. Gynecol Endocrinol. 2004;19:301–312. doi: 10.1080/09513590400018231. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Nakashima K, Namihira M, Ochiai W, Uemura A, Yanagisawa M, Fujita N, Nakao M, Taga T. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev. Cell. 2001;1:749–758. doi: 10.1016/s1534-5807(01)00101-0. [DOI] [PubMed] [Google Scholar]
- Toyokawa S, Uddin M, Koenen KC, Galea S. How does the social environment 'get into the mind'? Epigenetics at the intersection of social and psychiatric epidemiology. Soc. Sci. Med. 2011 doi: 10.1016/j.socscimed.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000527. e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Commun. Integr. Biol. 2011;4:220–222. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The Role of Microglia in the Healthy Brain. J. Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga S, Garcia-Segura LM, Azcoitia I. The neuroprotective properties of sex steroids and neurosteroids. Rev. Neurol. 2004;39:1043–1051. [PubMed] [Google Scholar]
- Viselli SM, Stanziale S, Shults K, Kovacs WJ, Olsen NJ. Castration alters peripheral immune function in normal male mice. Immunology. 1995;84:337–342. [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Hiles SA, Sominsky L, McLaughlin EA, Hodgson DM. Neonatal lipopolysaccharide exposure impairs sexual development and reproductive success in the Wistar rat. Brain Behav. Immun. 2011;25:674–684. doi: 10.1016/j.bbi.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Wang C, Wu C, Shieh J, Wen C. Microglial distribution and apoptosis in fetal rat brain. Dev. Brain Res. 2002;139:337–342. doi: 10.1016/s0165-3806(02)00584-9. [DOI] [PubMed] [Google Scholar]
- Washburn TC, Medearis DN, Jr, Childs B. Sex Differences in Susceptibility to Infections. Pediatrics. 1965;35:57–64. [PubMed] [Google Scholar]
- Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin. Neurosci. 2010;64:217–230. doi: 10.1111/j.1440-1819.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- Williamson LL, Sholar PW, Mistry RM, Smith SH, Bilbo SD. Microglia and Memory: Modulation by Early-Life Infection. Journal of Neuroscience. 2011 doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, Burks SR, McCarthy MM. Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev. Neurobiol. 2008;68:1406–1419. doi: 10.1002/dneu.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, McCarthy MM. Prostaglandin E2-induced masculinization of brain and behavior requires protein kinase A, AMPA/kainate, and metabotropic glutamate receptor signaling. J. Neurosci. 2009;29:13274–13282. doi: 10.1523/JNEUROSCI.3603-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kaur C, Ling EA. Variation with age in the labelling of amoeboid microglial cells in rats following intraperitoneal or intravenous injection of a fluorescent dye. J. Anat. 1993;182(Pt 1):55–63. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Zuk M, McKean KA. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 1996;26:1009–1023. [PubMed] [Google Scholar]