SUMMARY
Follicular lymphoma (FL) comprises nearly 25% of non-Hodgkin lymphoma cases and is clinically characterized by initial sensitivity to chemotherapy followed by relapse. FL stroma contains a special type of stromal cell found in germinal centre of lymph nodes—the follicular dendritic cell (FDC). We first isolated tumourigenic cells from the FL cell line FLK-1 by side population (SP) technique, and found that SP cells, which express ABCG2, were enriched by chemotherapy and radiation treatments. In vitro, SP cells were attracted by and adhered to FDCs through chemokine (C-X-C motif) ligand 12/chemokine (C-X-C motif) receptor 4 (CXCL12/CXCR4) signalling. In vivo, limiting dilution assays showed SP cells were highly enriched in cancer stem cell (CSC), but required FDC for tumour formation in non-obese diabetic/severe combined immunodeficiency mice. Treatment with AMD3100, a specific CXCL12/CXCR4 inhibitor, eliminated tumour growth. These findings were then verified with FL cells isolated from an FL patient’s ascitic fluid (FLA-1). Finally, we detected the ABCG2 expressing lymphoma cells in FL clinical specimens. Thus, we found that the highly tumourigenic FL cells having CSC-like activities (FL-SC) interact with FDCs in a CXCL12/CXCR4 dependent manner to resist chemotherapy. Our results indicate the importance of FL-SC and niche cell signalling in maintaining tumourigenicity. These signals represent novel targets for CSC eradication.
Keywords: Follicular lymphoma, Tumour/stromal cell interactions, Side population, Follicular dendritic cells, CXCL12 (SDF-1α)
INTRODUCTION
Follicular lymphoma (FL) is the second most common form of non-Hodgkin lymphoma (NHL) in the Western Hemisphere, representing nearly 25% of all NHL cases (Armitage and Weisenburger 1998). FL arises from B cells, and its clinical course is frequently indolent and responsive to initial chemotherapy. However, multiple relapses are common. More than half of relapsed patients become refractory to treatment and do not survive more than five years (van Oers, et al 2006). Hence, it is important to gain insight into the cellular and molecular mechanisms of relapse following a successful response to chemotherapy.
The potential mechanisms of relapse may involve the interaction of tumourigenic FL cells with the stromal cell counterpart present in the lymph node (LN) (Arai, et al 2000). One of the putative stromal cell types that may interact with FL cells is the follicular dendritic cell (FDC). FDC is present in the germinal centre (GC) of lymphoid follicles, the site of FL origin (Klein, et al 1998). FDCs were found to initiate and maintain a pro-tumourigenic microenvironment (Torlakovic, et al 2002) by producing appropriate cytokines (Li and Choi 2002) to promote lymphoma cell proliferation (Li, et al 2004, Li, et al 2000). Our preliminary observations suggested that the FDC in GC expresses chemokine (C-X-C motif) ligand 12 (CXCL12) (Supplementary Fig 1). This preliminary data, coupled with previous reports that the transformation of FL to therapy-resistant aggressive large B cell lymphoma (De Jong and de Boer 2009) is associated with the induction of stromal gene signatures including CXCL12 (Lenz, et al 2008), motivated us to investigate the potential interaction between tumourigenic FL cells and FDCs through the CXCL12/ chemokine (C-X-C motif) receptor 4 (CXCR4) signalling pathway.
Previous cell line-based studies showed that not all cancer cells are tumourigenic (Wang, et al 2007). It has also been reported that some cancer cells require co-injection with stromal cells to form tumour in immunodeficient mice (Li, et al 2004, Margolin, et al 2011), suggesting the importance of the interaction between tumourigenic cancer cells and stromal cells to form tumours in vivo.
In this study, we developed an experimental model to isolate the tumourigenic FL cells with cancer stem cell (CSC)-like activities (henceforth known as FL-SC) to study the potential interaction between the FL-SC and FDC. To isolate FL-SC, we have employed a commonly used CSC enrichment technique known as the side-population (SP) cells. Recently, we and others have identified a quiescent population of drug-resistant CSC in the SP fraction in various malignancies, including Hodgkin lymphoma (Das, et al 2008, Ho, et al 2007, Jones, et al 2009, Wu and Alman 2008, Zhang, et al 2009). The SP fraction expressed the drug-resistant gene ABCG2 and demonstrated higher tumourigenic capacity than the non-SP fraction (Das, et al 2008, Jakubikova, et al 2011, Kruger, et al 2006). Hence, we isolated the SP fraction from both FL cell line and primary tumours, and confirmed that FL-SC interact with FDC in a CXCL12/CXCR4-dependent manner both in vitro and in vivo to maintain tumourigenicity. Finally, we demonstrated the existence of FL-SC in FL patient specimens.
METHODS
Patient specimen, cell lines, antibodies and reagents
This research was approved by the institutional review board of Ochsner Clinic Foundation. Ascitic fluid from an untreated FL patient was collected after obtaining informed consent. B lymphoma cells (FLA-1) were isolated from the ascitic fluid and used in various experiments. The FLK-1 cell line (obtained from Dr. Kagami, Aichi Cancer Centre Hospital, Nagoya, Japan) was established by co-culturing lymphoma cells derived from the bone marrow (BM) of an FL patient with an FDC cell line, HK (Kagami, et al 2001, Kim, et al 1995). FLA-1 and FLK-1 cells express CD10, CD20, CD27, CD77, high CD38, but low CD44, and no surface IgD. These phenotypes are similar to GC-centroblasts but different from naïve B cells (Supplementary Table 1), suggesting B cell tumours of GC-origin. The HK cell line, established from human tonsil FDC, was maintained as described (Kim, et al 1995). FLK-1 cells were co-cultured with HK cells in Iscove’s media supplemented with 10% fetal calf serum (FCS; Life Technologies, Grand Island, NY), 2 mM glutamine, 100 u/ml penicillin G, and 100 mg/ml streptomycin (Irvine Scientific, Santa Ana, CA).
Antibodies used for this work were fluorescein isothiocyanate (FITC)-labelled mouse anti-human ABCG2 (clone 5D3) and goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA); anti-human CD20 (clone L-26, BioGenex, Cherlapally, Hyderabad, India); anti-human BCRP1 (clone BXP-21); biotinylated anti-human ABCG2, anti-human Oct 3/4, and normal goat IgG negative control (all from R&D Systems, Minneapolis. MN); alkaline phosphatase (AP)-conjugated rabbit anti-mouse IgG (Southern Biotechnology Associates, Birmingham, AL); and horseradish peroxidase (HRP)-conjugated streptavidin (Jackson Laboratory, Bar Harbor, ME). Reagents used were goat ABC Elite Kit; Vector Red Alkaline Phosphatase developing kit (both from Vector Laboratories, Burlingame, CA); and Sigma FAST 3,3-Diaminobenzidine tablets (Sigma Aldrich, St. Louis, MO).
Hoechst 33342 labelling and SP cell sorting
Hoechst 33342 dye (5 µg/ml, Molecular Probes, Carlsbad, CA) was added to lymphoma cells with or without 100 mM verapamil, and cells were incubated at 37°C for 90 min, mixed occasionally, and then washed. For fluorescence-activated call sorting (FACS) analysis, lymphoma cells were resuspended in phosphate-buffered saline (PBS) containing 1% FCS. Prior to analysis, propidium iodide (2 µg/ml) was added to exclude nonviable cells. Verapamil-sensitive SP cells were identified and electronically gated for cell sorting on an FACS Aria cell sorter using Becton Dickinson Diva software (BD Biosciences, San Jose, CA). Hoechst dye was excited with 150 mW of 350 nm ultra-violet light. SP fluorescence emissions were directed toward a 610-nm dichroic filter and captured simultaneously through a blue (440/24 nm) and a red (695/40 nm) filter on a linearly amplified fluorescence scale.
Cell cycle analysis
Lymphoma cells (1 × 106/0.5 ml in PBS) were mixed with 4.5 ml 70% ethanol and incubated for 2 h. Ethanol-suspended cells were washed once with PBS and resuspended in staining solution (1 × 106 cells/ml) containing 0.1% (v/v) Triton X-100 (Rohm & Haas Company, Philadelphia, PA) in PBS, 0.2 mg/ml of DNase-free RNase A (Sigma) and 20 µg/ml of propidium iodide. Cells were allowed to equilibrate for 30 min in the dark. The distribution of cells in various cell cycle compartments was analysed using FACS Caliber equipped with Cell Quest software (BD Biosciences). The percentages of cells in G0+G1, G2+M, or S phase were obtained from DNA histograms using ModFit software (Versity Software House, Topsham, ME). The proliferating index (PI) was calculated as PI = G2+M+S/G0+G1.
Tumourigenicity
Female non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice, 6 to 8 weeks old, were obtained from the NCI-Frederick Cancer Research Facility (Frederick, MD) and acclimated for 1 week. All animal studies were conducted under approved guidelines of the Animal Care and Use Committee of Ochsner Clinic Foundation. NOD/SCID mice were pre-treated with anti-Natural Killer cell antibody and sublethally irradiated (4 Gy) 24 h before cell injection. The Extreme Limiting Dilution Analysis (ELDA) was performed as described by Hu and Smyth (2009). Briefly, different numbers of lymphoma cells or FACS-sorted SP cells were mixed with HK cells (1 × 106) in 0.1 ml of RPMI medium and then injected subcutaneously into the posterior flank of NOD/SCID mice. Tumour formation was measured in three dimensions with calipers twice a week for up to 90 days. Tumour volume was calculated as length×width×height. The frequency of tumour initiating cells in FLK-1 cell line was determined using the extreme limiting dilution analysis (ELDA) webtool at http://bioinf.wehi.edu.au/software/elda.
Tumours were removed when tumour size reached 1 cm3. A portion of each tumour was placed in 10% formalin for paraffin embedding or placed in optimum cutting temperature (OCT) compound to be snap frozen in liquid nitrogen. In some experiments, to improve tumour detection sensitivity, green fluorescence protein (GFP)--tagged FLK-1 cells were used for tumour formation. To visualize GFP-labelled tumour cells, mice were imaged with the IVIS Lumina Imaging System; and images were analysed using Living Image Software (Caliper Life Sciences, Hopkinton, MA). Mice were anesthetized using isofluorane (2.5% in 100% oxygen, 1 l/min) during all imaging procedures.
FACS analysis
For cell surface antigen staining, lymphoma cells were stained with unconjugated or directly FITC-conjugated antibodies (Abs). Unconjugated monoclonal antibodies (mAbs) were detected by FITC-labelled goat anti-mouse Ig. In brief, cells were incubated with the appropriate concentration of Abs for 15 min at 4°C. After washing with PBS containing 0.2% bovine serum albumin and 0.1% sodium azide, cells were analysed by FACS Calibur and CellQuest software.
Immunohistochemistry
Formalin-fixed paraffin embedded tissue sections (5 µm) were deparaffinized, rehydrated, and blocked for endogenous peroxidase activity. Following high temperature antigen retrieval in Trilogy unmasking solution (Cell Marque, Rocklin, CA), slides were biotin-blocked, serum-blocked, and immunostained using a goat ABC Elite Kit. Biotin-conjugated Ab to Oct-3/4 was applied at 1:50 for 1 h at room temperature (RT). Positive staining was detected with 3,3V-diaminobenzidene (DAB). For double staining, anti-CD20 mAb was applied after DAB for 2 h at RT. AP-conjugated rabbit anti-mouse IgG secondary Ab was applied at 1:40 at RT for 1 h. The Vector Red alkaline phosphatase detection kit was used to detect CD20+ cells, followed by light green counter-stain. Frozen slides were fixed with cold 100% acetone for 10 min before specific mAbs staining.
Migration assay
Lymphoma cells (105 cells/100 µl) were added to the upper compartments of 24-well transwell chambers with 8 µm pore filters (Corning Life Sciences, Lowell, MA). Lower compartments contained medium, pre-seeded HK cells, or CXCL12 (100 ng/ml, R&D Systems). In some experiments, lymphoma cells were pre-incubated with 50 µM AMD3100 (Sigma) for 30 min at 37°C. Migrating cells were collected after 24 h and the absolute numbers of viable lymphoma cells were evaluated by flow cytometry using FlowCount beads (Beckman-Coulter, Fullerton, CA). Data were analysed as a migration index corresponding to the number of cells migrating in response to testing medium divided by the number of cells migrating in response to control medium.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). To assess statistical significance of differences, an unpaired t test (Prism Version 4.03, GraphPad Software, Inc., La Jolla, CA) was used. P values <0.001 and <0.05 were considered very significant (***) and significant (**) as indicated by asterisks, respectively.
RESULTS
FL tumour formation is dependent on FDC cell line HK
The FLK-1 cell line was established by co-culturing lymphoma cells derived from the BM of a FL patient with HK cells, an FDC cell line (Kagami, et al 2001, Kim, et al 1995). FLK-1 cells have been previously reported to be dependent on FDC to proliferate in vitro (Kagami, et al 2001). We used the FLK-1 cells and the HK cells to study the putative CSC and niche cell interaction. First, we determined whether FLK-1 tumour formation was dependent on stromal cell support by evaluating the tumour formation from subcutaneous injection of both cell types in NOD/SCID mice. We found that FLK-1 cell tumour formation was dependent on HK cells (Supplementary Fig 2A); No tumour was formed when FLK-1 cells were injected into mice without HK cells. FLK-1 cells maintained tumourigenic potential in the presence of HK cells, and these tumours could be serially transplanted in NOD/SCID mice with shortened tumour formation latency in each generation (Supplementary Fig 2B). Lymphoma cells isolated from a FL patient’s ascitic fluid (FLA-1) were used to confirm FLK-1 results. As shown in Supplementary Fig 2A and 2C, FLA-1 cells were similarly dependent on HK cells for proliferation and tumour formation in NOD/SCID mice. These results demonstrate that HK cells are required for FL tumourigenesis.
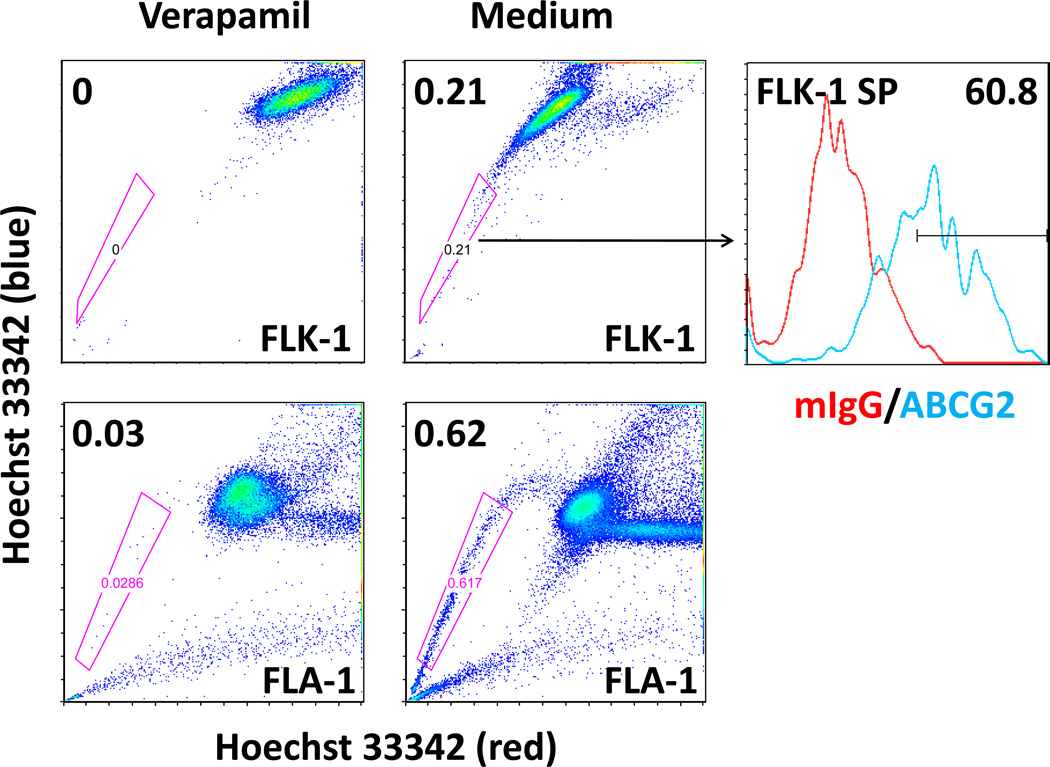
FL cells contain an SP fraction with CSC characteristics
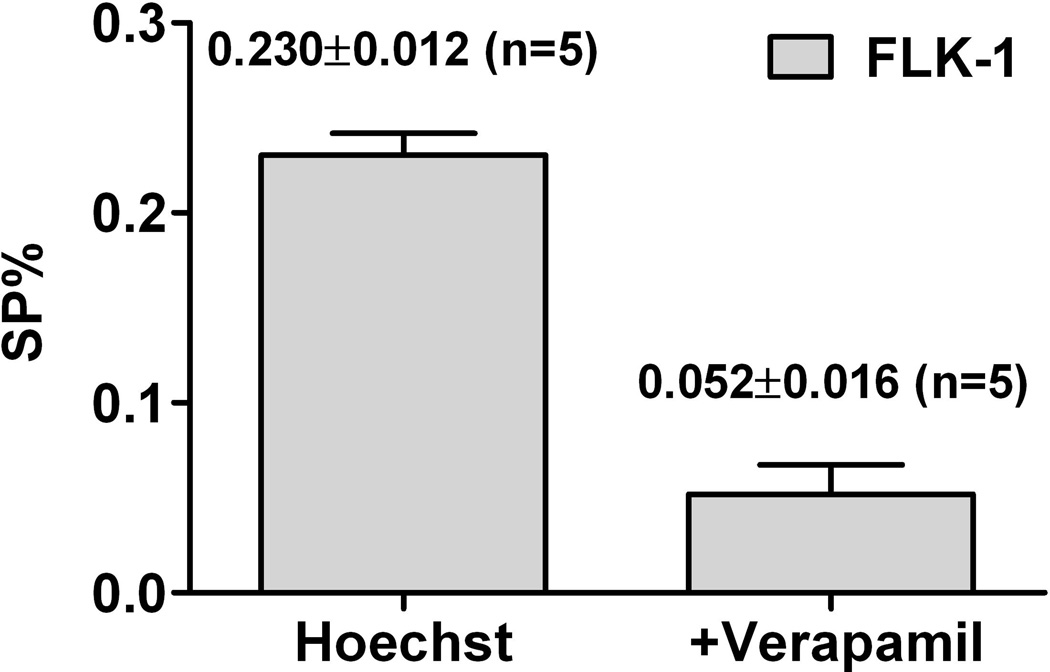
To isolate and further characterize the putative FL-SC population, we used the Hoechst 33342 dye efflux-based isolation of SP cells. To detect SP, FLK-1 cells were labelled with Hoechst 33342 with or without treatment of verapamil, an ABCG2 inhibitor (Fig 1A) (Seigel, et al 2005). In 5 separate experiments, we consistently found Hoechst dim SP from FLK-1 cells, 0.230±0.012% with Hoechst staining and 0.052±0.016% with Hoechst staining in the presence of verapamil (P=0.001). The presence of Hoechst 33342 dye efflux and verapamil-sensitive SP was confirmed with patient-derived lymphoma cells isolated from ascitic fluid (FLA-1 cells, Fig 1A).
Fig. 1. Identification and characterization of SP in the FL cell line, FLK-1, and FL patient lymphoma cells (FLA-1).
(A) Hoechst efflux and verapamil-sensitive SP cells are detected in FLK-1 cells (top, left and middle panels), and FLA-1 cells (bottom). Histograms (upper right) indicate FLK-1 SP cells stained with FITC-conjugated anti-ABCG2 antibody (blue line) for FACS analysis, and isotype control (red line) was used for background staining. Numbers indicate percentage of gated cells. (B) Hoechst efflux and verapamil-sensitive SP cells detected in FLK-1 cells in 5 separate experiments (P=0.001 by t test).
Moreover, the sorted FLK-1 SP cells were characterized for ABCG2 expression and cell cycle status by appropriate assays. FACS staining data showed that 60.8% of FLK-1 SP cells were positive for ABCG2 staining (Fig 1A, right panel), whereas 1.14% ABCG2 positive cells were found in FLK-1 parental cells (Supplementary Table 2). It has been reported that CSC phenotype, such as ABCG2 expression, is modulated by the microenvironment in malignant lymphomas (Singh, et al 2011). However, FLK-1 cells are maintained in co-culture with HK cells. It is not likely that HK cells induce SP cells from non-SP cells under these co-culture conditions.
CSC are known to be quiescent and proliferate slower than non-CSC. Indeed, identically treated FLK-1 parental and sorted SP cells are distinguishable by their cell cycle states. Cell cycle distribution showed that 41.87±2.94% of FLK-1 parental cells were in G0G1 phases, 51±1.96% was in S phase, and 8.22±0.32% was in G2M phases (PI=1.45), while a majority of sorted SP cells was in G0G1 state (68.39±4.39%). 30.27±0.97% and 1.35±0.04% of SP cells were in S and G2M phases, respectively (N=3, PI=0.46, P<0.0001). These results suggest that FLK-1 cell line contains an ABCG2 expressing and slow cycling SP fraction.
FL-SP cells are enriched in chemotherapy- and irradiation-treated cell populations
A hallmark of CSC is their resistance to conventional treatments, including chemotherapy and radiation (Visvader and Lindeman 2008). Therefore, we examined the effects of chemotherapy and radiation on FLK-1 SP cells. FLK-1 cells were co-cultured with HK cells, and then treated with media (Control, Table 1), chemotherapy drugs or irradiation. Following treatment, SP cells were detected by Hoechst dye staining and FACS analysis in each condition. The relative SP fraction of FLK-1 cells increased 128-fold after treatment with irradiation (3 Gy) compared with untreated cells (Control, Table 1). After treatment with two commonly used chemotherapy drugs in the treatment of lymphoma, cyclophosphamide and doxorubicin, the SP fraction of FLK-1 cells increased 114- and 156-fold, respectively when compared with untreated cells (Control, Table 1). When primary tumour FLA-1 cells were tested for methylcellulose colony formation, doxorubicin treatment enriched the number of colony-forming cells by at least 3-fold (Supplementary Fig 2D). Drug or irradiation treatment increased the proportion of SP cells whereas the non-SP fraction decreased. These results suggest that SP cells are enriched in the chemotherapy and radiation-treated population.
Table 1.
Detecting FL-SP cells
| Treatment | Average SP (%) | Average SP enrichment (fold)** |
|---|---|---|
| FLK-1 cell | ||
| Control* | 0.230 | 1 |
| Verapamil | 0.052 | - |
| Irradiation1 | 30.7 | 128 |
| Cyclophosphamide2 | 27.3 | 114 |
| Doxorubicin3 | 37.4 | 156 |
| Adhere cells (to HK cell) | 13.3 | 55 |
| Migrating cells (to HK cell) | 14.4 | 60 |
| Non-migrating cells (to HK cell) | 0.22 | 1 |
| FL patient lymph node suspension cells | ||
| Medium | 0.37 | N/A |
| Verapamil | 0.08 | N/A |
| FL patient ascitic suspension cells (FLA-1) | ||
| Medium | 0.62 | N/A |
| Verapamil | 0.03 | N/A |
Control indicates side population (SP) fractions in untreated FLK-1 cells. The SP (%) was an average of 5 separate experiments (see Fig 1B).
Average SP enrichment (fold) = Average SP (%) of Treatment/Average SP (%) of Control. Untreated (Control) or treated lymphoma cells were stained with Hoechst dye for SP analysis. Verapamil was used to validate the SP. FLK-1 cells were treated with irradiation1 (3 Gy) then cultured for 72 h; Cyclophosphamide2 (2000 µM) for 48 h; or Doxorubicin3 (10 nM) for 28 h. All lymphoma cell cultures were in the presence of HK cells. Relative SP cells were also enriched in the populations that are adherent to HK cells, or migrating towards HK cells.
Numbers show percentage of SP. Data shows one representative of at least 2 similar experiments.
N/A: not applicable.
FL-SP cell sorting enriched tumour-initiating FL-SC
To investigate whether the SP in FLK-1 is enriched with the CSC population, we first compared the clonogenic ability of SP versus parental cells in a methylcellulose based in vitro assay. When the primary FL tumour-derived cells (FLA-1 cells) were tested in a methylcellulose colony formation assay, a significantly higher percentage of cells formed colonies in the SP cell fraction compared with parental cells (Supplementary Fig 2D).
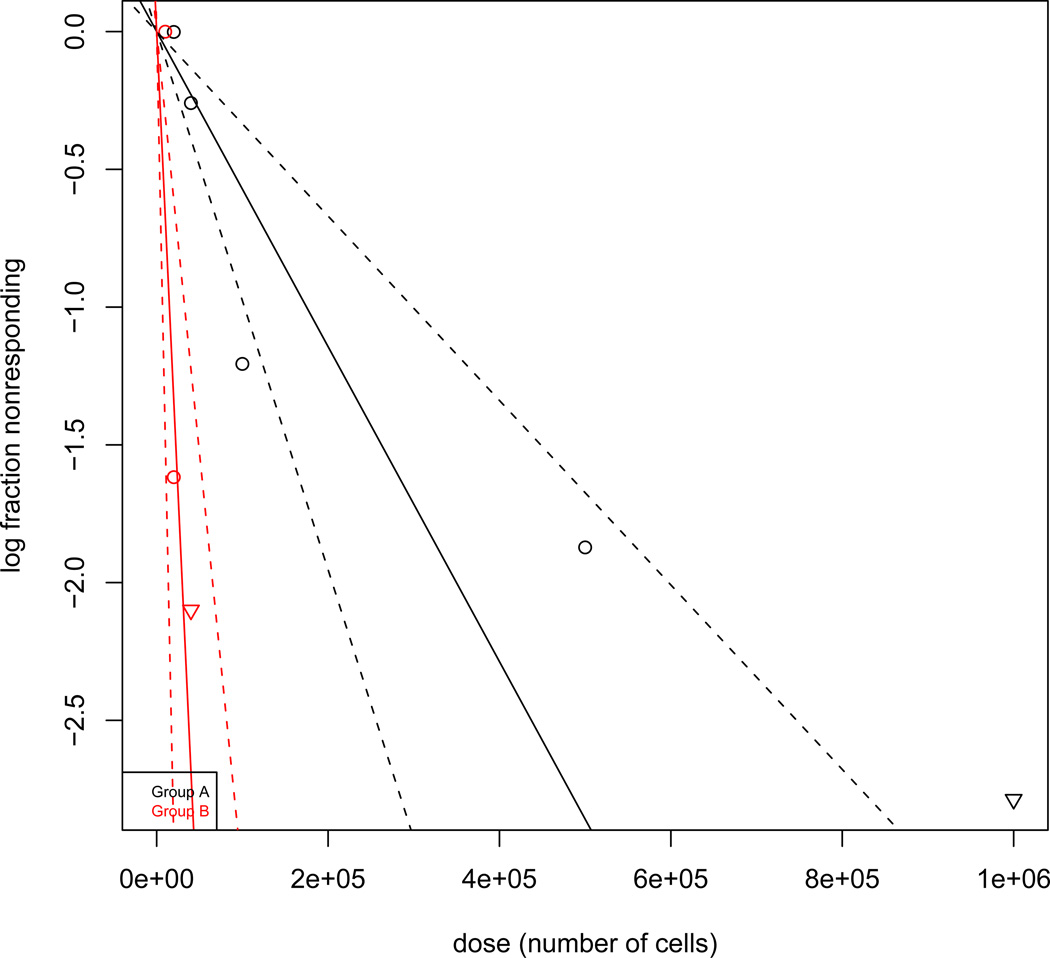
Next, we investigated the tumourigenicity of FLK-1 SP versus parental cells in NOD/SCID mice using a limiting dilution tumour transplantation assay. Lymphoma cells were mixed with HK cells and injected subcutaneously to NOD/SCID mice, and limiting dilution assays were performed. The data were analysed by an ELDA method (Hu and Smyth 2009). Tumour initiating cells were found to be one out of 1.75×105 for FLK-1 parental cells and 1.49×104 for FLK-1 SP cells. There was an 11.7-fold enrichment of tumour-initiating FLK-1 cells in SP compared with parental cells (Fig 2). A summary of tumour formation experiments with various numbers of parental or FACS-sorted SP cells from FLK-1 cells are shown in Table 2. These functional in vitro and in vivo assay results demonstrated that SP cells obtained from FL were highly enriched with CSC.
Fig. 2. SP cells are highly tumourigenic.
Limiting dilution assays of tumour formation were performed with various cell doses of FLK-1 parental and SP cells in the presence of HK cells. A log-fraction plot of the limiting dilution model fitted to the data in Table 2. The slope of the line is the tumour-initiating cell fraction for FLK-1 parental (black) and FLK-1 SP (red) cells. The dotted lines give the 95% confidence interval. Tumour-initiating cells comprise one out of 1.75×105 cells for FLK-1 parental cells and one out of 1.49×104 cells for FLK-1 SP cells (P=9.18×10−6).
Table 2.
Extreme limiting dilution analysis of tumour formation frequency of FLK-1 and FLK-1 SP cells
| cells/injection | FLK-1 parental cells | FLK-1 SP cells | ||
|---|---|---|---|---|
| Injections (n) | tumour formations (n) | Injections (n) | tumour formations (n) | |
| 1,000,000 500,000 100,000 40,000 20,000 10,000 |
8 13 10 8 2 |
8 11 7 2 0 |
4 5 2 |
4 4 0 |
| Tumour formation frequency (95% CI) |
1/174,918 (1/102,417-1/298,743) | 1/14,899 (1/6,798-1/32,652) | ||
| P value | 9.18e−6 | |||
CI, confidence interval
FL-SC interact with FDC in a CXCL12/CXCR4-dependent manner in vitro
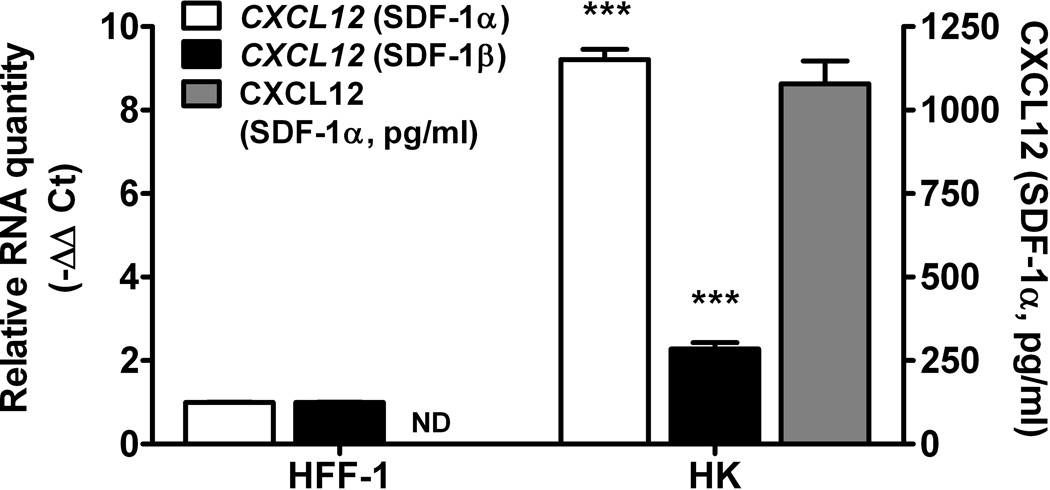
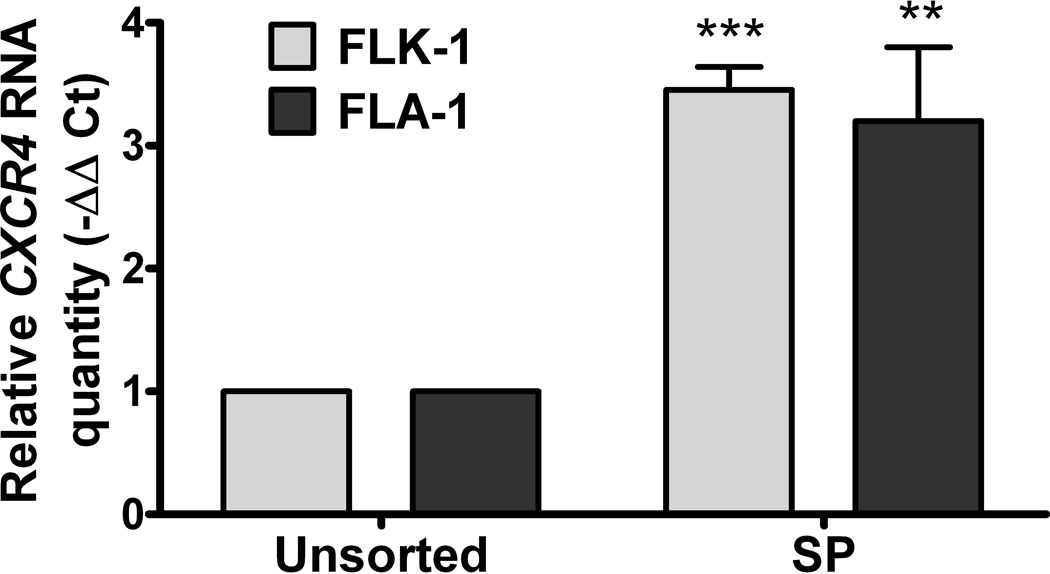
We next investigated the potential role of CXCL12/CXCR4 signalling in the interaction between FL-derived SP cells and stromal HK cells. First, we measured CXCL12 secretion by HK cells and CXCR4 expression by FLK-1 and FLA-1 derived SP cells. Quantitative real-time polymerase chain reaction (PCR) showed CXCL12 was expressed over 9-fold higher in HK cells than control fibroblasts (HFF-1, Fig 3A). CXCL12 protein levels produced by HK cells were confirmed by enzyme-linked immunosorbent assay (ELISA) (Fig 3A, grey bar). CXCR4 mRNA expression was more than 3-fold upregulated in SP versus parental cells in both FLK-1 and FLA-1 cells (Fig 3B). These results suggest that HK cells produce CXCL12 whereas the SP fractions of FLK-1 and FLA-1 cells express CXCR4.
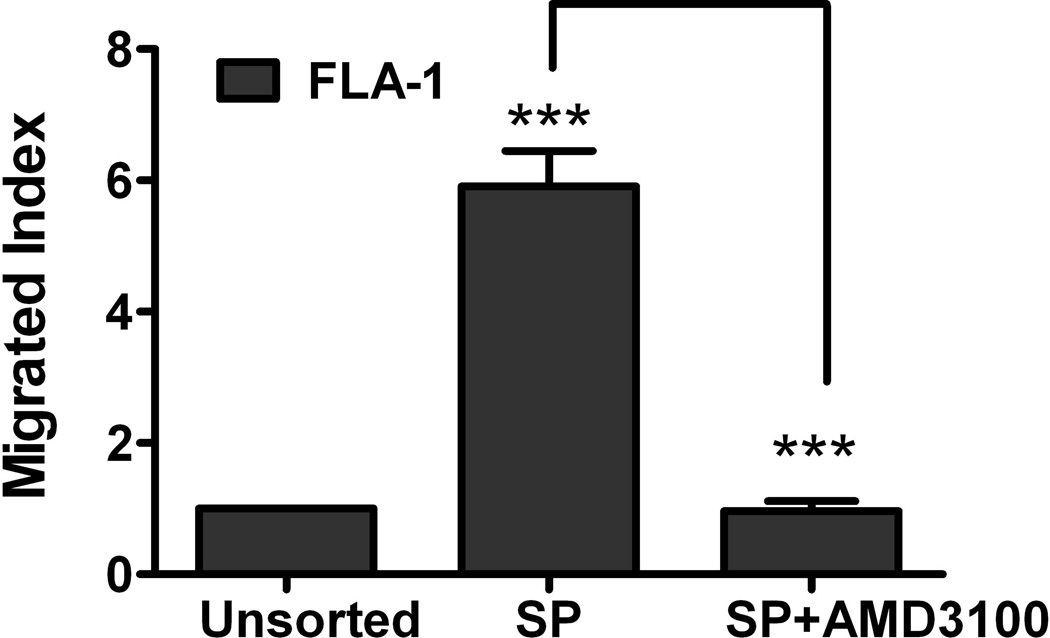
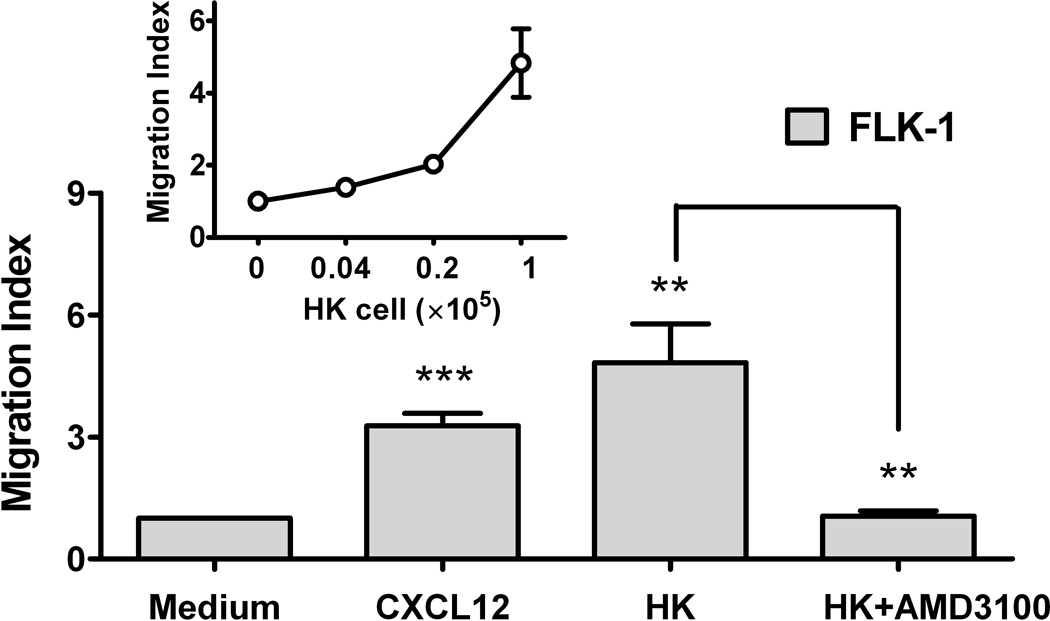
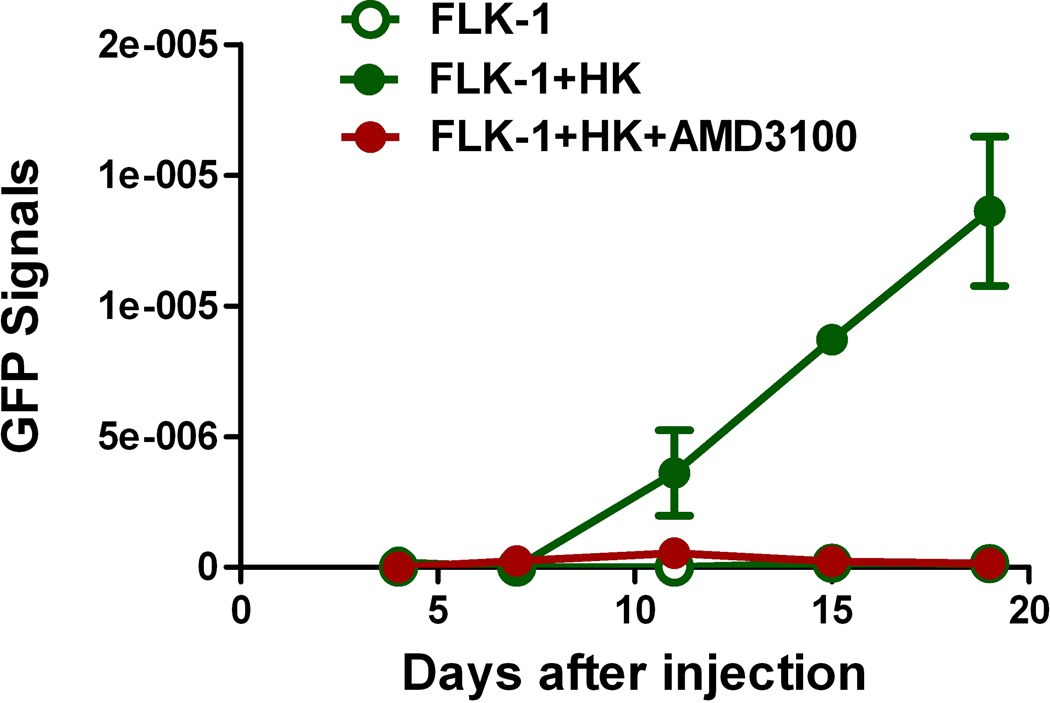
Fig. 3. CXCL12 is a microenvironmental factor for FL-SC.
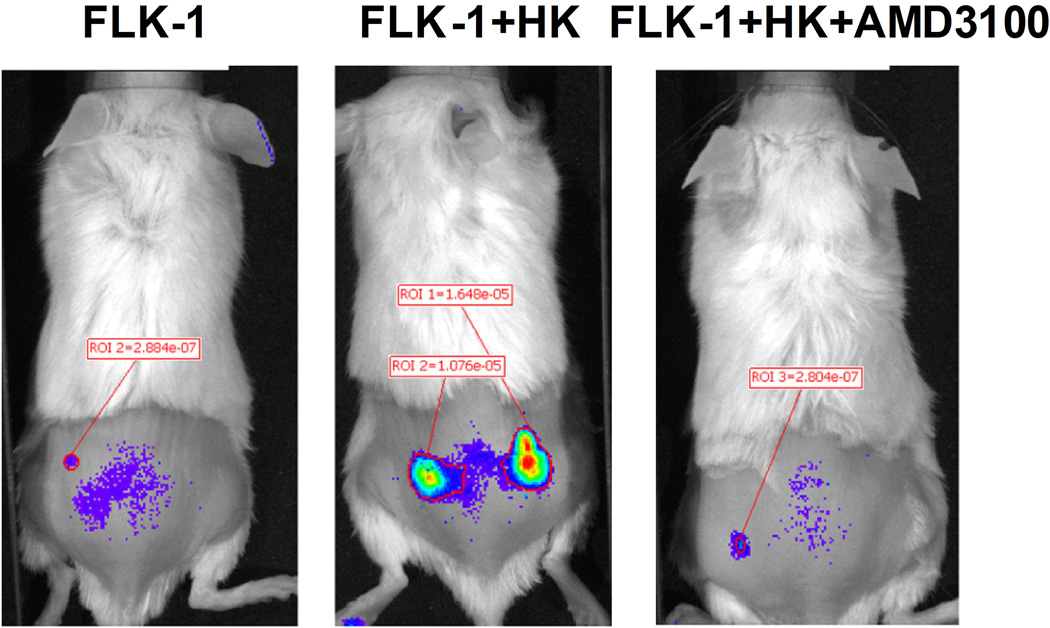
(A) Quantitative real-time polymerase chain reaction (PCR) results show CXCL12 (SDF-1α, clear bars, and SDF-1β, solid bars) expression in HK cells. Fibroblasts (HFF-1) are used as control. Cumulative levels of CXCL12 (SDF-1α) in HK cells (0.2 million cells seeded in 24-well plates in 1 ml culture media and cultured for 72 h) were detected by ELISA (grey bar). ND: not tested. (B) Quantitative real-time PCR results show FLK-1 and FLA-1 cell SP cells express higher levels of CXCR4. (C) FLA-1 SP cells migrate toward CXCL12 with a significantly higher index compared with unsorted cells, and migration is inhibited by the presence of AMD3100. (D) FLK-1 cells migrate toward CXCL12 or HK cells (insert), and pre-treatment of lymphoma cells with small molecule inhibitor (AMD3100) prevented this migration. Migration index of lymphoma cells are shown. (E and F) GFP-tagged FLK-1 cell tumour formation assisted by HK cells is inhibited by pretreatment of lymphoma cells with AMD3100. Tumour growth curves (E) and images (F) of GFP-FLK-1 cell tumours detected with IVIS Lumina Imaging System are shown. ROI, region of interest.
Second, we performed the migration study to evaluate the functionality of the chemokine receptor CXCR4 expressed on the FLK-1 and FLA-1 SP cells. When CXCL12 was added to the media in the lower compartments of transwells, we found that the FLA-1 SP cells migrated towards CXCL12 at a significantly higher rate (more than 5-fold) compared with parental cells (Fig 3C), and their migration was inhibited by the presence of AMD3100, a specific small molecule inhibitor of CXCL12/CXCR4 signalling. We also found similar results with FLK-1 cells (3-fold, Fig 3D). These data suggest that the chemokine receptor CXCR4 is active on these cells, and cells migrated in response to chemokine CXCL12.
Third, we evaluated the role of HK cells in the maintenance of FLK-1 derived SP cells. Because HK cells were adherent, whereas FLK-1 cells were in suspension in culture, FLK-1 cells were easily separated from HK cells to perform the experiments. Two populations of FLK-1 cells emerged when they were co-cultured with HK cells, one represented FLK-1 cells remaining suspended in the medium and the other represented FLK-1 cells adhering to HK cells. Evaluation of the SP fraction identified a 55-fold enrichment of the SP fraction in FLK-1 cells that were adherent to HK cells when compared to the parental cells (Control, Table 1). It is not likely that co-culture with HK cells induced non-SP cells to express SP phenotype, because FLK-1 cells need HK cells in order to survive in vitro and in vivo. These results suggest the enrichment of FLK-1 derived SP cells in FLK-1 cells that interact with HK cells.
Fourth, in a transwell migration assay, we further evaluated the migration of FLK-1 cells towards HK cells with or without treatment of AMD3100. FLK-1 cells migrated toward HK cells in a dose-dependent manner (Fig 3D, insert). The migratory FLK-1 fraction was 60-fold enriched in SP cells compared with parental cells (Control, Table 1). AMD3100 treatment completely blocked FLK-1 cell migration towards HK cells (Fig 3D). Thus, these results suggest that FLK-1 derived SP cells migration towards HK cells is dependent on CXCL12/CXCR4 signalling.
Taken together, the results suggest that the stromal cells, represented by HK cells, interact with the CSC fraction of FLK-1, the SP cells, in a CXCL12/CXCR4-dependent manner to enhance maintenance, growth, and migration of the SP cells in vitro.
FL-SC interact with FDC in a CXCL12/CXCR4 dependent manner in vivo
To investigate the in vivo interaction between FLK-1 SP cells and FDCs, GFP-tagged FLK-1 cells were mixed with HK cells and injected subcutaneously to NOD/SCID mice for in vivo monitoring of tumour growth. In some of the tumour formation experiments, AMD3100-treated GFP-tagged FLK-1 cells were used. We first confirmed the rapid tumour growth of the FLK-1 cells when co-injected with HK cells. Next, we found that the AMD3100 pre-treated groups showed no tumour growth (Fig 3E and 3F). These data suggest that FL-SC may be dependent on FDC via CXCL12 and CXCR4 paracrine signalling in tumour formation in vivo.
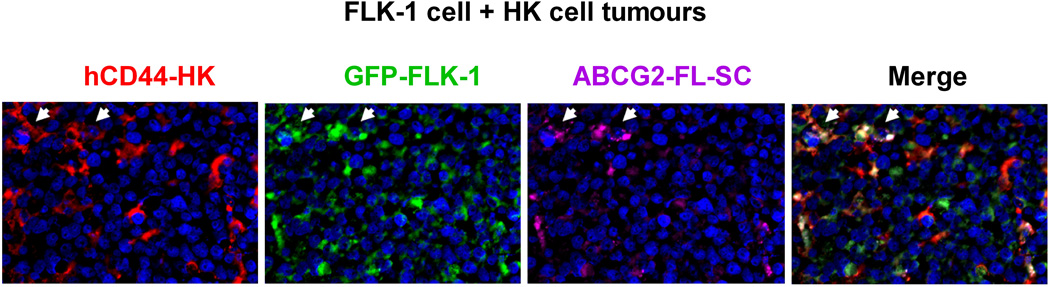
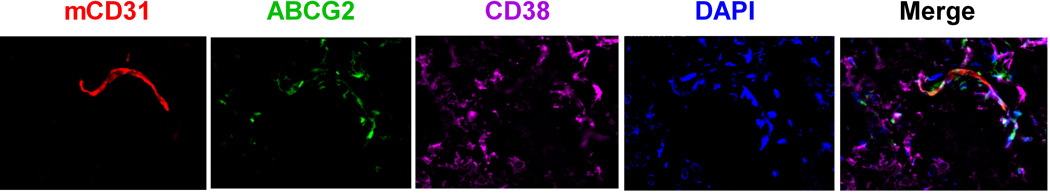
Next, we took advantage of the GFP-tagged FLK-1 cells to perform a co-localization study of SP cells with HK cells. In this study, ABCG2 expression in the GFP-tagged FLK-1 cells was considered representative of SP cells. Established tumours were removed for immunohistochemistry staining. HK cells were detected by CD44 expression (Li, et al 2004). In xenografts, it is known that the host inflammatory cells are attracted to the tumour site, including CD31+ endothelial cells which are essential for neovascularization (Gilbertson and Rich 2007). As an indicator of CSC niche formation, we also studied the presence of mouse CD31+ endothelial cells.
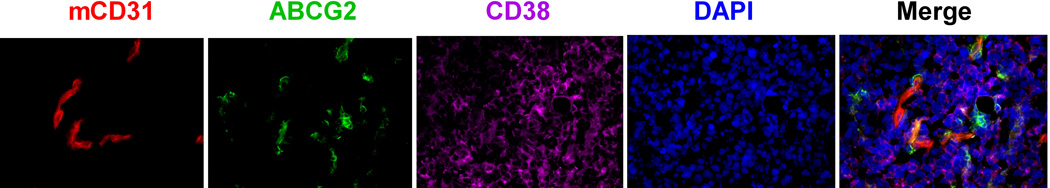
We first confirmed ABCG2+ expression in the GFP+ FLK-1 cells (Fig 4A). Then, we observed that the ABCG2+GFP+ FLK-1 cells were in close contact with CD44+ HK cells within 2 weeks after lymphoma cell injection (Fig 4A). Most importantly, similar to what was observed with CSC and the vascular niche in glioblastoma (Gilbertson and Rich 2007), ABCG2+CD38+ lymphoma cells were in close contact with CD31+ mouse endothelial cells 4 weeks after lymphoma cell injection (Fig 4B). These data suggest that FLK-1 SP cells and the stromal HK cells stay in close contact, and FLK-1 SP cells may also interact with the host vasculature cells. Taken together, we conclude that the FLA-1 SP cells interact with HK cells in a CXCL12/CXCR4-dependent manner to promote in vivo tumour growth of the SP cells.
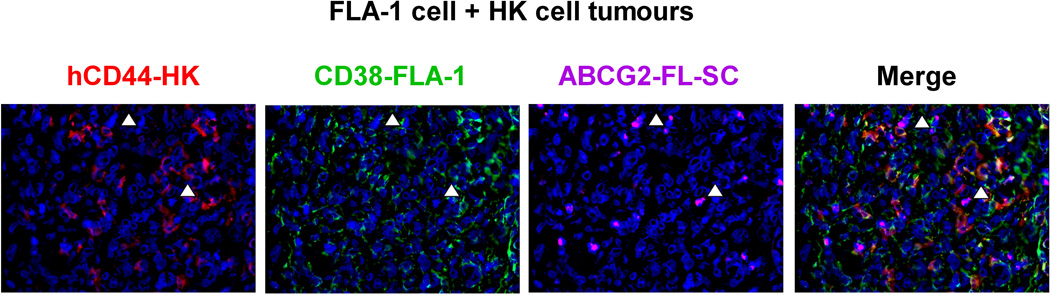
Fig. 4. A stem cell niche for FL-SC.
(A and C) Tumour tissue sections from 7 days post-injection of GFP-tagged FLK-1 cells (A) or FLA-1 cells (C, CD38, green) and HK cells were stained with anti-hCD44 (red) for HK cells and anti-ABCG2 (purple) for FL-SC. (B and D) Tumour tissue sections from 4 weeks post-injection of FLK-1 (B) or FLA-1 (D) and HK cells were stained with CD31 (red) for mouse endothelial cells, ABCG2 (green) for FL-SC, and CD38 (purple) for lymphoma cells. DAPI (blue) was used as a nuclear counter-stain. Arrowheads show ABCG2+ lymphoma cells. The images were captured by a deconvoluting microscope using SlideBook software. Original magnification x400.
FL patient LN specimens contain ABCG2+ and Oct-3/4+ cells
The presence of an FL-SC population found in our FLK-1 cell line experiments was then confirmed with clinical specimens from FL patients. First, we identified that FL-patient LN cell suspensions contained 0.37% SP cells, and FL-patient lymphoma cells isolated from ascitic fluid (FLA-1 cells) contained 0.62% SP cells. Both SP cell fractions were sensitive to verapamil treatment (Table 1).
Similar to our observation with FLK-1 cells, primary FL tumour-derived FLA-1 cells also demonstrated the close interaction between ABCG2+ lymphoma cells and HK cells in the early stage (Fig 4C) or CD31+ host vasculatures in the late stage of tumour formation (Fig 4D). These data further suggest that the interaction between FL-SP cells and stromal cells may not be restricted to cell line alone, but might represent a general phenomenon of the FL-SP cells’ ability to interact with host stromal cells in vivo to maintain tumourigenicity. Hence, to investigate the possibility that the ABCG2-expressing FL tumour cells may be present in FL tumours, we utilized an immunohistochemical-based clinical study.
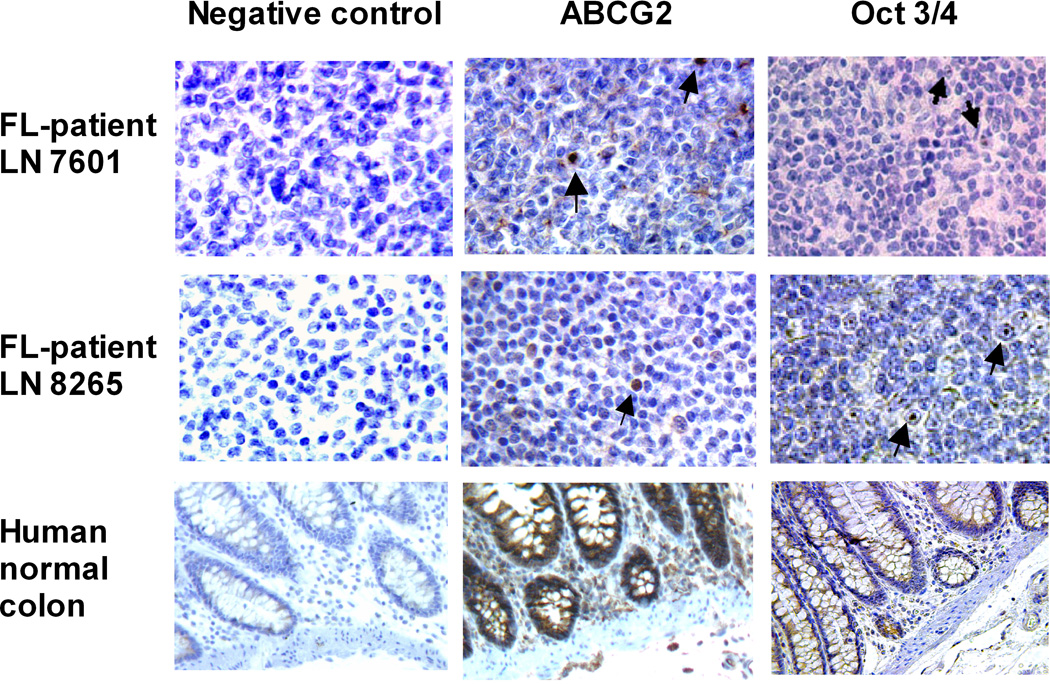
We obtained paraffin-embedded tissue samples from FL patients and observed ABCG2 expressing cells. Positive staining was scored by counting all cells in 10 high power fields (magnification ×400) to calculate the percentage of positive cells (Table 3). Normal human colon specimens were used as positive control tissues for ABCG2 and Oct-3/4 staining (Fig 5A). We found 0.6−2.4% ABCG2+ cells in FL patient LN specimens (N=6). Positive staining was specific because all tissues used were negative when isotype control Ab (negative control) was used in place of stem cell-specific Abs (Fig 5A). These data in clinical FL samples further suggest the presence of ABCG2 expressing cells in the FL samples.
Table 3.
Cancer stem cells detected in biopsies from follicular lymphoma patients
| Patient samples | LN 7601 | LN 8265 | LN 12608 | LN 21727 | LN 21728 | LN 25138 |
|---|---|---|---|---|---|---|
| Age (years) | 69 | 57 | 59 | 41 | 68 | 65 |
| Gender | Male | Female | Female | Female | Female | Male |
| Diagnosis | FL | FL | FL | FL | FL | FL |
| Grade | 2/3 | 1/3 | 1/3 | 2/3 | 3/3 | 2/3 |
|
Bone marrow involvement |
Yes | Yes with metastases |
ND | ND | ND | No |
| Oct 3/4+ | 6.8% (402/5906) | 8.7% (620/7099) | 7.2% (298/4114) | 4.4% (95/2144) | 6.4% (116/1808) | 4.1% (164/4039) |
| ABCG2+ | 0.6% (14/2235) | 1.7% (52/3051) | 1.5% (51/3487) | 2.4% (55/2305) | 2.3% (37/1613) | 1.1% (33/2981) |
Fig. 5. Stem cell markers detected by immunohistochemistry in FL lymph node tissues.
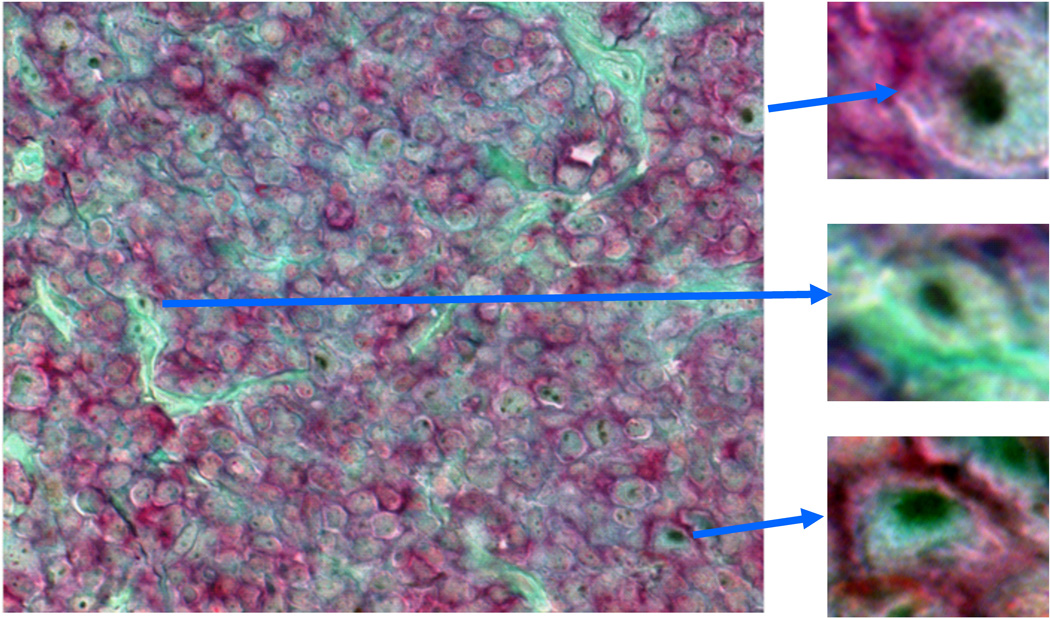
(A) Paraffin-embedded normal colon, FL patient 8265 and 7601 lymph node (LN) tissue sections were stained with mAb against ABCG2 (clone BXP-21) followed by biotin-conjugated goat anti-mouse IgG Ab or stained with biotin-conjugated goat Ab against Oct 3/4. Slides were then stained with horseradish-conjugated streptavidin, and DAB substrate to develop the brown colour deposits (arrows). (B). Paraffin embedded FL-patient LN 12608 tissue sample was double stained with anti-CD20 (red) and Oct 3/4 (brown) Abs. Light green was used as counter stain. Inserts show enlarged individual cells. Original magnification ×100 (normal colon) and ×400 (LN). Percentages of Oct 3/4+ and ABCG2+ cells in LN tissue section of 6 FL patients are summarized in Table 3.
Next, we evaluated the expression of Oct 3/4, which is increasingly found to be expressed in CSC of diverse tumours (Bentivegna, et al 2010, Koh, et al 2011). We found 4.1−8.7% Oct-3/4+ cells in these same FL patient LN specimens. We further confirmed that these lymphoma cells expressed Oct 3/4 by double staining with mAb CD20 and Oct 3/4. Oct 3/4+ (brown, nuclear staining pattern) lymphoma cells were also CD20+ (Fig 5B; red, membrane staining pattern, upper and lower inserts). Taken together, we confirmed the existence of ABCG2 and Oct 3/4 expressing cells in clinical FL samples. These data are consistent with our observations in the FLK-1 cells and primary FLA-1 cells, that there is a rare cell population with stem-cell characteristics, including expression of ABCG2 and Oct 3/4.
DISCUSSION
FL is often indolent and responsive to initial chemotherapy. However, nearly all patients relapse (Horning 2000, Tan and Horning 2008), suggesting the existence of a small, chemotherapy-resistant population responsible for regeneration of the tumours. Here, we found that a rare ABCG2 expressing SP cell fraction demonstrated CSC characteristics in FL. We identified an SP fraction both within the established FL cell line FLK-1 as well as from lymphoma cells from patients with FL. We convincingly demonstrated that this SP fraction exhibits CSC function, including increased tumourigenicity in NOD/SCID mice, higher expression of stem cell associated genes, and resistance to chemotherapy agents and radiation. CSC were initially described in acute myeloid leukaemia (Bonnet and Dick 1997), and have now been characterized in various solid and haematological malignancies, including recent reports in murine mantle cell lymphoma, multiple myeloma, and human Hodgkin lymphomas (Huff and Matsui 2008, Jones, et al 2009, Vega, et al 2010). Our work is the first report of the presence of CSC-like cells in FL.
The CSC models suggest that niche cell signalling may play an important role in CSC-mediated tumourigenesis and evasion of chemotherapy. Here, in vitro and in vivo, we demonstrated that drug-resistant FL-SC interact with stromal cell FDC by CXCL12/CXCR4 signalling to maintain tumourigenicity. Previous reports suggest that FDC is present in the GC of lymphoid follicles maintain a pro-tumourigenic microenvironment by producing the appropriate cytokines (Li and Choi 2002, Torlakovic, et al 2002). In this study, our findings suggest that the CXCL12 is one of the candidate cytokines that FDC might secret to modulate the tumourigenicity of FL-SC.
One of the limitations of the study of FL-SC and its interactions with the stromal cells is the lack of representative in vitro and in vivo models of FL. In our experiments, we have used the FLK-1 cell line, established by co-culturing patient-derived FL cells with an FDC cell line HK cells (Kagami, et al 2001). FLK-1 cells possess several features characteristic of FL, including cytogenetics with translocation t(14;18)(q32;q21), common to FL, as well as the requirement of FDC for tumour formation in NOD/SCID mice (Supplementary Fig 2A). Thus, we used the FLK-1 cell line as a reasonable exploratory pre-clinical model to study the biology of FL-SC interaction with the FDC, with confirmation of results with FL clinical specimens.
This observed interaction between FL-SC and FDC provides critical insights into the mechanism of FL relapse and drug resistance. Drug resistance and transformation to more aggressive forms of lymphoma, such as large B cell lymphoma (De Jong and de Boer 2009), are the major causes of mortality from FL. The molecular mechanism of such transformation is not yet known, however, it may be associated with embryonic stem cell-like gene expression signatures (Gentles, et al 2009). We hypothesize that the FL-SC and stromal cell interaction through CXCL12/CXCR4 signalling may allow the FL-SC to evade chemotherapy and transform to a more aggressive phenotype. This is supported by our findings reported in this work, as well as previous findings that CXCL12 expression in B cell lymphoma was associated with poor prognosis (Lenz, et al 2008).
CXCL12 and CXCR4 expression may also serve as prognostic markers for risk of disease transformation. Recently, distinct stromal gene signatures predictive of large B cell lymphoma patient survival were identified (Lenz, et al 2008), and CXCL12 was among the unfavorable markers. A deeper understanding of the essential signals produced by FDC to promote survival in B cell lymphoma could suggest new therapeutic targets. Recent reports showed that migrating populations enrich CSC in neuroblastoma SP cells (Das, et al 2008), and migrating CD133+CXCR4+ CSC are essential for pancreatic adenocarcinoma metastasis (Hermann, et al 2007). In a transwell experiment, we similarly found that those FLK-1 cells that migrated towards HK cells contained a 60-fold enriched SP. In addition, HK cells were in close contact with ABCG2+ lymphoma cells during early stages of tumour formation. This is similar to clinical FL where FDCs are associated with the lymphoma mass. It has been hypothesized that FDCs attract FL-SC by producing survival factors, such as CXCL12 (Burger and Kipps 2006), which is consistent with our findings, that SP cells express higher levels of CXCR4, and that inhibition of CXCL12/CXCR4 interaction abolished FLK-1 cell tumour formation in NOD/SCID mice. Therefore, targeting the stem cell niche interaction is an attractive approach to target CSCs. In this regard, our findings suggest that the CXCL12/CXCR4 signalling may be an attractive target to eliminate FL-SC.
The FLK-1 and HK interaction in vivo model described here could serve as a humanized microenvironment model to identify and analyse key interactions and signalling molecules in FL and evaluate response to targeted agents. In this interaction, we found that both chemotherapy and radiation treatment increased the number of SP cells in the FLK-1. Thus, the lymphoma cells with SP phenotype and the environment that protect them could be the therapeutic targets to eradicate relapses. It is reported that AMD3100 inhibited infiltration of lymphomatous cells into liver and lung tissues by inhibiting CXCL12/CXCR4 signalling. Additionally, antibody mediated inhibition of CXCL12/CXCR4 signalling completely abrogated the CXCL12-mediated cell migration of lymphocytic leukaemias and lymphomas, as well as the migration of lymphoma cells towards lymph node stromal cells (Kawaguchi, et al 2009, O'Callaghan, et al 2012, Zhang, et al 2006). AMD3100 has been used clinically in the setting of mobilization of normal haematopoietic stem cells prior to autologous stem cell transplantation. Clinical trials are ongoing, investigating the safety and efficacy of AMD3100 in combination with chemotherapy in a variety of haematological malignancies, including lymphomas (ClinicalTrials.gov Identifier: NCT00694590 and NCT00512252). It is hypothesized that AMD3100 disrupts the CSC niche and makes the CSC more susceptible to chemotherapy. Therefore, our findings, that inhibition of the CXCL12/CXCR4 signalling reduced the migration of lymphoma cells towards stromal cells and inhibited tumour formation, is consistent with previous reports and highlight that the one mechanism of action of AMD3100 in this disease may be the elimination of FL-SC.
The in vivo interaction between FL-SC and the FDC may also promote angiogenesis, and therefore, targeting angiogenesis may further reduce the SP cell fraction in vivo. In our lymphomagenesis model, the signals produced by FDC and lymphoma cells promote host angiogenesis in the tumour site that sustains tumour formation after the initial survival stage. After tumour formation, ABCG2+ lymphoma cells were found in close contact with CD31+ mouse endothelial cells. The microenvironment, first supported by HK cells followed by host vessels, may form a niche to sustain FL-SC in xenoplants. These niches may involve a complex interplay of short- and long-range signals between FL-SC, their differentiated daughter cells, and neighbouring stromal cells (Watt and Hogan 2000). Thus, the FL-SC niche may also represent a novel target to prevent disease recurrence.
In summary, we identified a novel CSC population in FL and its signalling mechanism of interaction with the niche cells. Current treatments for FL are not curative for the majority of patients and were designed and deemed successful based on the response of the bulk population of tumour cells (Reya, et al 2001). Effects on a rare CSC population or on cells in the supportive microenvironment are unknown but presumed to be inadequate given the rates of relapse in FL. Understanding the essential signals for tumour chemoresistance and survival produced by FDC in FL may help develop novel therapeutic strategies (Burger, et al 2009). FL-SC biomarkers may also serve as prognostic markers to classify patients at higher risk of transformation or suggest alternative treatments. The overall limitation of the current study is the considerable reliance on cell line-based studies with confirmation of the results using patient specimens. Given the limited availability of clinical specimens and the rare CSC population, it is a reasonable strategy to use a cell line model for exploratory experiments but requiring confirmation in clinical specimens. In future studies of FL, a much larger effort would be required to perform lymph node biopsies rather than fine needle aspirations in FL patients in order to collect a larger number of cancer cells to perform FL-SC studies. We believe that our studies, which identify and characterize the FL-SC and its interactions with the tumour microenvironment, are an important step in the development of biologically-based, curative treatments for FL.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Mr. Allen Tucker for technical assistance in side population FACS sorting, Drs. Yong Sung Choi, Herman Yeger, and Isodore Lossos for scientific discussion, Dr. SunOK Yoon for manuscript proof reading, Dr. Jeong-Ran Kim for establishing GFP-FLK-1 cell lines, Ms. Rosemary Velasquez for pathology expertise, Ms. Melissa Aycock and Ms. Rachel Regn for excellent technical assistance. This work was partially supported by NIH/NCI grant R01 CA089057 (LL) and Ladies Leukemia League, Metairie, Louisiana (LL).
Footnotes
AUTHOR CONTRIBUTIONS
LL designed the research study, performed research, analysed data and wrote the paper. CGL and BD performed the research, analysed the data, and wrote the paper. CG, XZ, TL and LY performed the research. TLL and JC contributed essential reagents, and wrote the paper. LH contributed essential reagents.
REFERENCES
- Arai J, Yasukawa M, Yakushijin Y, Miyazaki T, Fujita S. Stromal cells in lymph nodes attract B-lymphoma cells via production of stromal cell-derived factor-1. Eur J Haematol. 2000;64:323–332. doi: 10.1034/j.1600-0609.2000.90147.x. [DOI] [PubMed] [Google Scholar]
- Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- Bentivegna A, Conconi D, Panzeri E, Sala E, Bovo G, Vigano P, Brunelli S, Bossi M, Tredici G, Strada G, Dalpra L. Biological heterogeneity of putative bladder cancer stem-like cell populations from human bladder transitional cell carcinoma samples. Cancer Sci. 2010;101:416–424. doi: 10.1111/j.1349-7006.2009.01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–3375. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia Enhances Tumor Stemness by Increasing the Invasive and Tumorigenic Side-Population Fraction. Stem Cells. 2008;26:1818–1830. doi: 10.1634/stemcells.2007-0724. [DOI] [PubMed] [Google Scholar]
- De Jong D, de Boer JP. Predicting transformation in follicular lymphoma. Leuk Lymphoma. 2009:1–6. doi: 10.1080/10428190903093815. [DOI] [PubMed] [Google Scholar]
- Gentles AJ, Alizadeh AA, Lee SI, Myklebust JH, Shachaf CM, Shahbaba B, Levy R, Koller D, Plevritis SK. A pluripotency signature predicts histologic transformation and influences survival in follicular lymphoma patients. Blood. 2009;114:3158–3166. doi: 10.1182/blood-2009-02-202465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- Horning SJ. Follicular lymphoma: have we made any progress? Ann Oncol. 2000;11(Suppl 1):23–27. [PubMed] [Google Scholar]
- Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Huff CA, Matsui W. Multiple myeloma cancer stem cells. J Clin Oncol. 2008;26:2895–2900. doi: 10.1200/JCO.2007.15.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubikova J, Adamia S, Kost-Alimova M, Klippel S, Cervi D, Daley JF, Cholujova D, Kong SY, Leiba M, Blotta S, Ooi M, Delmore J, Laubach J, Richardson PG, Sedlak J, Anderson KC, Mitsiades CS. Lenalidomide targets clonogenic side population in multiple myeloma: pathophysiologic and clinical implications. Blood. 2011;117:4409–4419. doi: 10.1182/blood-2010-02-267344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RJ, Gocke CD, Kasamon YL, Miller CB, Perkins B, Barber JP, Vala MS, Gerber JM, Gellert LL, Siedner M, Lemas MV, Brennan S, Ambinder RF, Matsui W. Circulating clonotypic B cells in classic Hodgkin lymphoma. Blood. 2009;113:5920–5926. doi: 10.1182/blood-2008-11-189688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami Y, Jung J, Choi YS, Osumi K, Nakamura S, Morishima Y, Seto M. Establishment of a follicular lymphoma cell line (FLK-1) dependent on follicular dendritic cell-like cell line HK. Leukemia. 2001;15:148–156. doi: 10.1038/sj.leu.2402002. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A, Orba Y, Kimura T, Iha H, Ogata M, Tsuji T, Ainai A, Sata T, Okamoto T, Hall WW, Sawa H, Hasegawa H. Inhibition of the SDF-1alpha-CXCR4 axis by the CXCR4 antagonist AMD3100 suppresses the migration of cultured cells from ATL patients and murine lymphoblastoid cells from HTLV-I Tax transgenic mice. Blood. 2009;114:2961–2968. doi: 10.1182/blood-2008-11-189308. [DOI] [PubMed] [Google Scholar]
- Kim HS, Zhang X, Klyushnenkova E, Choi YS. Stimulation of germinal center B lymphocyte proliferation by an FDC-like cell line, HK. J Immunol. 1995;155:1101–1109. [PubMed] [Google Scholar]
- Klein U, Goossens T, Fischer M, Kanzler H, Braeuninger A, Rajewsky K, Kuppers R. Somatic hypermutation in normal and transformed human B cells. Immunol Rev. 1998;162:261–280. doi: 10.1111/j.1600-065x.1998.tb01447.x. [DOI] [PubMed] [Google Scholar]
- Koh MY, Lemos R, Jr., Liu X, Powis G. The hypoxia-associated factor switches cells from HIF-1alpha- to HIF-2alpha-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res. 2011;71:4015–4027. doi: 10.1158/0008-5472.CAN-10-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger JA, Kaplan CD, Luo Y, Zhou H, Markowitz D, Xiang R, Reisfeld RA. Characterization of stem cell like cancer cells in immune competent mice. Blood. 2006;108:3906–3912. doi: 10.1182/blood-2006-05-024687. [DOI] [PubMed] [Google Scholar]
- Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, Xu W, Tan B, Goldschmidt N, Iqbal J, Vose J, Bast M, Fu K, Weisenburger DD, Greiner TC, Armitage JO, Kyle A, May L, Gascoyne RD, Connors JM, Troen G, Holte H, Kvaloy S, Dierickx D, Verhoef G, Delabie J, Smeland EB, Jares P, Martinez A, Lopez-Guillermo A, Montserrat E, Campo E, Braziel RM, Miller TP, Rimsza LM, Cook JR, Pohlman B, Sweetenham J, Tubbs RR, Fisher RI, Hartmann E, Rosenwald A, Ott G, Muller-Hermelink HK, Wrench D, Lister TA, Jaffe ES, Wilson WH, Chan WC, Staudt LM. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Choi YS. Follicular dendritic cell-signaling molecules required for proliferation and differentiation of GC-B cells. Semin Immunol. 2002;14:259–266. doi: 10.1016/s1044-5323(02)00058-1. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang X, Kovacic S, Long AJ, Bourque K, Wood CR, Choi YS. Identification of a human follicular dendritic cell molecule that stimulates germinal center B cell growth. J Exp Med. 2000;191:1077–1084. doi: 10.1084/jem.191.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yoon SO, Fu DD, Zhang X, Choi YS. Novel follicular dendritic cell molecule, 8D6, collaborates with CD44 in supporting lymphomagenesis by a Burkitt lymphoma cell line, L3055. Blood. 2004;104:815–821. doi: 10.1182/blood-2004-01-0292. [DOI] [PubMed] [Google Scholar]
- Margolin DA, Silinsky J, Grimes C, Spencer N, Aycock M, Green H, Cordova J, Davis NK, Driscoll T, Li L. Lymph Node Stromal Cells Enhance Drug-Resistant Colon Cancer Cell Tumor Formation through SDF-1alpha/CXCR4 Paracrine Signaling. Neoplasia. 2011;13:874–886. doi: 10.1593/neo.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan K, Lee L, Nguyen N, Hsieh MY, Kaneider NC, Klein AK, Sprague K, Van Etten RA, Kuliopulos A, Covic L. Targeting CXCR4 with cell-penetrating pepducins in lymphoma and lymphocytic leukemia. Blood. 2012;119:1717–1725. doi: 10.1182/blood-2011-04-347518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Seigel GM, Campbell LM, Narayan M, Gonzalez-Fernandez F. Cancer stem cell characteristics in retinoblastoma. Mol Vis. 2005;11:729–737. [PubMed] [Google Scholar]
- Singh RR, Kunkalla K, Qu C, Schlette E, Neelapu SS, Samaniego F, Vega F. ABCG2 is a direct transcriptional target of hedgehog signaling and involved in stroma-induced drug tolerance in diffuse large B-cell lymphoma. Oncogene. 2011;30:4874–4886. doi: 10.1038/onc.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D, Horning SJ. Follicular lymphoma: clinical features and treatment. Hematol Oncol Clin North Am. 2008;22:863–882. doi: 10.1016/j.hoc.2008.07.013. viii. [DOI] [PubMed] [Google Scholar]
- Torlakovic E, Torlakovic G, Brunning RD. Follicular pattern of bone marrow involvement by follicular lymphoma. Am J Clin Pathol. 2002;118:780–786. doi: 10.1309/EG2M-YHB9-WEFW-7H1R. [DOI] [PubMed] [Google Scholar]
- van Oers MH, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD, Jack A, Van't Veer M, Vranovsky A, Holte H, van Glabbeke M, Teodorovic I, Rozewicz C, Hagenbeek A. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295–3301. doi: 10.1182/blood-2006-05-021113. [DOI] [PubMed] [Google Scholar]
- Vega F, Davuluri Y, Cho-Vega JH, Singh RR, Ma S, Wang RY, Multani AS, Drakos E, Pham LV, Lee YC, Shen L, Ambrus J, Jr., Medeiros LJ, Ford RJ. Side population of a murine mantle cell lymphoma model contains tumour-initiating cells responsible for lymphoma maintenance and dissemination. J Cell Mol Med. 2010;14:1532–1545. doi: 10.1111/j.1582-4934.2009.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67:3716–3724. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268:1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Zhang C, Cui GH, Liu F, Wu QL, Chen Y. Inhibitory effect of triptolide on lymph node metastasis in patients with non-Hodgkin lymphoma by regulating SDF-1/CXCR4 axis in vitro. Acta Pharmacol Sin. 2006;27:1438–1446. doi: 10.1111/j.1745-7254.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhang Y, Mao L, Zhang Z, Chen W. Side population in oral squamous cell carcinoma possesses tumor stem cell phenotypes. Cancer Lett. 2009;277:227–234. doi: 10.1016/j.canlet.2008.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.