Abstract
Objective:
Having demonstrated short-term weight loss with liraglutide in this group of obese adults, we now evaluate safety/tolerability (primary outcome) and long-term efficacy for sustaining weight loss (secondary outcome) over 2 years.
Design:
A randomized, double-blind, placebo-controlled 20-week study with 2-year extension (sponsor unblinded at 20 weeks, participants/investigators at 1 year) in 19 European clinical research centers.
Subjects:
A total of 564 adults (n=90–98 per group; body mass index 30–40 kg m−2) enrolled, 398 entered the extension and 268 completed the 2-year trial. Participants received diet (500 kcal deficit per day) and exercise counseling during 2-week run-in, before being randomly assigned (with a telephone or web-based system) to once-daily subcutaneous liraglutide (1.2, 1.8, 2.4 or 3.0 mg, n=90–95), placebo (n=98) or open-label orlistat (120 mg × 3, n=95). After 1 year, liraglutide/placebo recipients switched to liraglutide 2.4 mg, then 3.0 mg (based on 20-week and 1-year results, respectively). The trial ran from January 2007–April 2009 and is registered with Clinicaltrials.gov, number NCT00480909.
Results:
From randomization to year 1, liraglutide 3.0 mg recipients lost 5.8 kg (95% confidence interval 3.7–8.0) more weight than those on placebo and 3.8 kg (1.6–6.0) more than those on orlistat (P⩽0.0001; intention-to-treat, last-observation-carried-forward). At year 2, participants on liraglutide 2.4/3.0 mg for the full 2 years (pooled group, n=184) lost 3.0 kg (1.3–4.7) more weight than those on orlistat (n=95; P<0.001). Completers on liraglutide 2.4/3.0 mg (n=92) maintained a 2-year weight loss of 7.8 kg from screening. With liraglutide 3.0 mg, 20-week body fat decreased by 15.4% and lean tissue by 2.0%. The most frequent drug-related side effects were mild to moderate, transient nausea and vomiting. With liraglutide 2.4/3.0 mg, the 2-year prevalence of prediabetes and metabolic syndrome decreased by 52 and 59%, with improvements in blood pressure and lipids.
Conclusion:
Liraglutide is well tolerated, sustains weight loss over 2 years and improves cardiovascular risk factors.
Keywords: liraglutide, GLP-1 analog, weight loss
Introduction
Obesity increases risk of developing type 2 diabetes mellitus1 and cardiovascular disease, the leading cause of death.2 Reduction of 5–10% body weight improves obesity-related cardiovascular and metabolic abnormalities,3, 4, 5 but achieving this target through diet and exercise is challenging.6, 7, 8 Pharmacological treatment is recommended in addition to diet and exercise when lifestyle intervention fails.4, 7, 8, 9
Liraglutide, an analog of the incretin hormone glucagon-like peptide-1 (GLP-1), is currently licensed for treatment of type 2 diabetes at doses up to 1.8 mg, after demonstrating improvements in glycemic control and weight loss in more than 4000 individuals of the Liraglutide Effect and Action in Diabetes (LEAD) program, with satisfactory tolerability.10, 11 In obese non-diabetic adults, liraglutide at doses up to 3.0 mg was more effective than orlistat, or diet and exercise alone, at reducing weight (primary outcome) over 20 weeks.12 Liraglutide also improved obesity-related risk factors, reducing waist circumference, blood pressure (BP) and the prevalence of prediabetes.
We now report 2-year results from the extension of the 20-week trial,12 as well as data after 1 year, the required duration for demonstrating weight loss/maintenance in confirmatory phase 3 trials.7 The primary outcome at 1 year was weight loss. In the extension at 2 years, we aimed to evaluate the long-term safety/tolerability of liraglutide (primary outcome) and efficacy for sustaining weight loss (secondary) of liraglutide, with dietary therapy and exercise, and also to examine the effects on cardiovascular risk factors.
Materials and methods
Participants
We performed a randomized, double-blind, placebo-controlled 20-week trial (RCT) with a 2-year extension. The data from the 20-week RCT has already been published.12 We recruited men and women aged 18–65 years, of stable weight, with body mass index 30–40 kg m−2 and fasting plasma glucose (FPG) <7 mmol l−1 (126 mg dl−1) at run-in, from 19 research sites in 8 European countries. Participants were recruited by local advertisement or were existing patients at an obesity clinic or research center. Key exclusion criteria were type 1 or 2 diabetes mellitus, drug-induced obesity, use of weight-lowering pharmacotherapy or participation in a weight-control study within the preceding 3 months, surgical obesity treatment and major medical conditions. There was no exclusion criterion based on psychiatric illness. Participants gave written informed consent. The protocol was approved by local ethics committees and the trial performed according to the Declaration of Helsinki13 and Good Clinical Practice Guidelines.
Study design and treatments
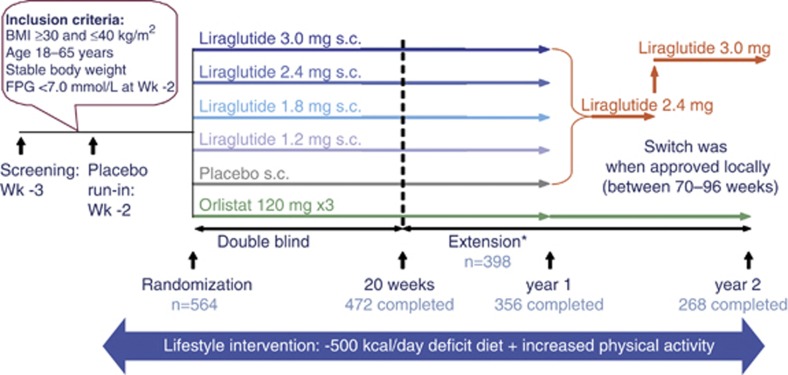
Study design is shown in Figure 1. The 20-week RCT12 consisted of a 2-week placebo run-in period one week after screening, then randomization to a 4-week dose-escalation period, and a 16-week constant-dose period. We randomly assigned individuals to once-daily liraglutide 1.2, 1.8, 2.4 or 3.0 mg (n=95, 90, 93 and 93) or placebo (n=98) by evening subcutaneous injection, starting at 0.6 mg per day and increasing weekly (dose escalation). The open-label comparator group (n=95) was randomized to receive orlistat capsules (3 × 120 mg) with each main meal for the full 2-year period. After 20 weeks, participants could consent to enroll in the extension, continuing on randomized treatment for 1 year, after which liraglutide or placebo-treated individuals switched to liraglutide 2.4 mg, considered the most favorable dose based on 20-week data analysis. However, when 1-year results became available, the 3.0 mg dose was deemed more favorable and participants switched to this dose between weeks 70–96, as sites obtained ethics committee approval.
Figure 1.
Study design. *From 20–52 weeks, participants/investigators remained blinded to liraglutide/placebo treatment but the sponsor was unblinded; after 1 year, all were unblinded.
In the main 20-week trial, eligible participants, sponsor and all study personnel were blinded to the random injection treatment assignment, performed using a sponsor-generated central telephone or web-based system, generated by the sponsor and concealed from trial investigators. A balanced (1:1) treatment allocation was specified, stratified by gender. We instructed participants receiving liraglutide or placebo to administer daily injections (liraglutide 6.0 mg ml−1 or vehicle in identical 3 ml cartridges) in the abdomen or thigh each evening, using a pen injector. Four different placebo injection volumes corresponded to the different liraglutide doses, thereby masking treatment (active/placebo).
From 20 to 52 weeks, participants/investigators remained blinded to liraglutide/placebo treatment but the sponsor/statistician was unblinded; after 1 year, all were unblinded. Throughout run-in and treatment, participants were advised on diet (about 30% of energy from fat, 20% from protein and 50% from carbohydrates), providing an energy deficit of ∼500 kcal per day, and encouraged to maintain or increase physical activity. To encourage adherence, pedometers were distributed, and a 3-day food diary was dispensed for completion 4 times during the trial, and reviewed by a dietician.
The trial ran from January 2007 to April 2009 and is registered with Clinicaltrials.gov, number NCT00480909.
Clinical outcomes
Efficacy endpoints included weight change from randomization to years 1 and 2 in the intention-to-treat population, the proportion losing >5 or >10% of randomization weight, and changes in waist circumference, BP, prevalence of prediabetes14 and metabolic syndrome,15, 16 glycemic parameters, fasting lipids and cardiovascular biomarkers, and quality of life.17 Prediabetes was defined14 as either impaired FPG (5.6–6.9 mmol l−1) or impaired glucose tolerance (7.8–11.0 mmol l−1) after 2-hour oral glucose tolerance test (75 g glucose). The diagnosis of metabolic syndrome was made according to updated National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) criteria.15, 16 Body composition in a subgroup of participants was measured at randomization and week 20 only by dual-energy X-ray absorptiometry and single-slice abdominal computerized axial tomography. Fully blinded specialists at SYNARC in Portland, USA (dual-energy X-ray absorptiometry) and Paris, France (computerized axial tomography) analyzed the scans. We measured weight and BP from screening, so effects on total weight-loss maintenance could be determined post hoc. All other efficacy parameters were measured from randomization, so that effects of liraglutide treatment versus placebo and orlistat could be assessed.7
Safety assessments included adverse events, recorded at every visit, standard laboratory tests and serum liraglutide antibodies. Antibody-positive samples were further assessed for neutralizing effect (against liraglutide) and crossreactivity (to GLP-1) in vitro. We measured serum calcitonin as thyroid C-cell tumors have been detected in liraglutide-treated rodents.18 MDS Pharma Services (Hamburg, Germany) performed the standard laboratory analyses, and Ligand Binding Services, MDS Pharma Services (Fehraltorf, Switzerland) analyzed the liraglutide antibody concentration. A safety committee for data surveillance was established.
Clinic visits over the 2-year trial period were weekly during dose escalation, otherwise, every 4 weeks. Weight was measured at every visit from screening. Standardized waist circumference assessments were made at randomization, then every 4 weeks. BP, glycemic parameters and lipids were measured at randomization, every 4 weeks to week 24, then at weeks 32, 44, 52, 64, 76, 88, 100 and 104; BP was also measured at screening. Metabolic syndrome status, prediabetes status, cardiovascular biomarkers and glycated hemoglobin (HbA1c) and quality of life were assessed at randomization, week 20, week 52 and week 104. Participants underwent physical examination and electrocardiogram at screening, week 20, week 52 and week 104. Pulse rate and laboratory parameters were measured at screening, run-in (laboratory parameters only), randomization, every 4 weeks to week 24, then at weeks 32, 44, 52 and 104. Calcitonin concentration was categorized by one of four categories: <lower level of quantification (LLOQ); ⩾LLOQ and <upper normal range (UNR); ⩾UNR and <2 × UNR; and ⩾2 × UNR.
Statistical analysis
Sample size estimation for the 20-week trial was based on the primary outcome weight. It was assumed that the s.d. for weight change at week 20 would be 5.6 kg;19 547 randomized individuals (91 per group) provided ⩾85% confidence to detect a clinically relevant 3 kg difference (P=0.05, two-sided) in mean weight between people dosed with liraglutide and placebo, based on Dunnett's test.20 A drop-out rate of 30% after 20 weeks was assumed. The objective of the extension was to evaluate safety and weight-loss durability. Data were analyzed according to a pre-established analysis plan. All analyses were performed on a modified intention-to-treat population, comprising all randomized individuals who received at least one treatment dose and had at least one post-randomization assessment of body weight, with the last-observation-carried-forward for efficacy endpoints (unless stated).7 Analysis of completers (those in the intention-to-treat population who completed the trial period) from screening was also performed post hoc. All analyses were two-sided, with 5% significance. The numbers of values that were imputed at year 1 and year 2 for the parameters weight, waist circumference, BP, fasting lipids, prediabetes and metabolic syndrome are shown in Supplementary Table 1.
We analyzed 1-year weight, waist, BP and 20-week body composition (also glycemic parameters, fasting lipids, cardiovascular biomarkers and pulse post hoc) by analysis of covariance (ANCOVA), investigating the superiority of each of the four doses of liraglutide over placebo (primary objective) and orlistat (secondary objective). At year 2, the superiority of liraglutide (in a pooled group of participants who were on 2.4/3.0 mg for 2 years) versus orlistat was assessed. Treatment, country and sex were fixed effects; the value of the respective parameter at randomization was a covariate. The primary null hypothesis was that there was no difference between treatments. We adjusted for multiplicity using Dunnett's method.20
In support of the ANCOVA analyses for weight, waist and BP, repeated measures analyses were performed using the longitudinal measurements available for the modified intention-to-treat population. These analyses were planned at year 1 and were performed post hoc at year 2. The repeated measures model included the same effects as the ANCOVA model described above. Interactions between treatment and visit, country and visit, and sex and visit were also included, with the restriction that, for visits in the dose-escalation period (weeks 1–4), means for treatment groups having received the same treatment up to the given visit were assumed to be equal. Evaluations of superiority of each liraglutide dose to placebo (primary objective) and to orlistat (secondary objective) were carried out using the Bonferroni adjustment for multiple testing.
The proportions of individuals losing >5 and >10% weight were analyzed by logistic regression, using the same model parameters (without country), with multiplicity adjustment using Bonferroni correction. As ‘country' showed no significant effect it was removed from the model, to allow inclusion of a greater number of variables and to increase the accuracy of the estimates. Prediabetes and calcitonin were also analyzed by logistic regression, as were metabolic syndrome status and nausea/vomiting incidence post hoc. The logistic regression analysis for calcitonin noted if a trial participant moved up one category from randomization to year 2. Randomization means for treatment groups in all the above analyses were assumed to be equal. We used the Statistical Analysis System software package (version 9.1; SAS Institute, Cary, NC, USA) for all analyses.
Results
Trial population
Of 616 individuals entering the 2-week run-in, 135 men and 429 women (n=564) were randomized to treatment, 398 entered the extension and 268/398 (67%) completed 2 years (see Supplementary Figure 1). Included in the trial were 21 individuals (<4% overall) classified as having a randomization glucose concentration in the range of type 2 diabetes mellitus; this had developed since screening. Three liraglutide-treated participants were excluded from the intention-to-treat population owing to missing post-randomization weight data. Major protocol deviations are described in the Supplementary Information.
Participant characteristics were comparable across groups at randomization (Supplementary Table 2)12 and entering the extension (not shown).
Weight and waist circumference from randomization
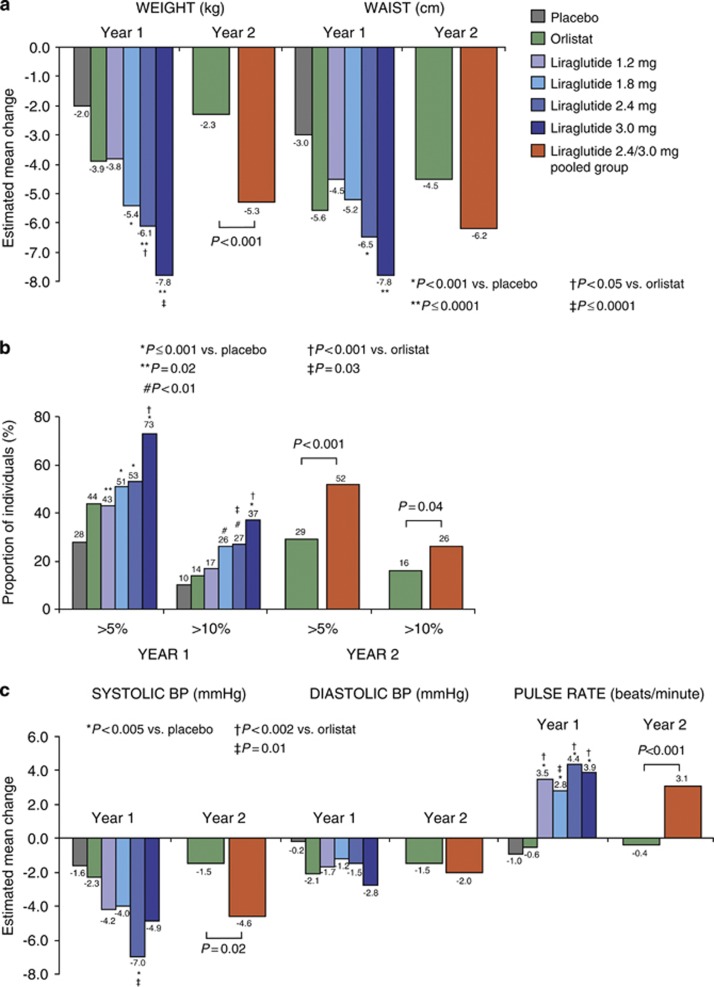
For the intention-to-treat population (with last-observation-carried-forward), estimated mean weight loss from randomization to year 1 was significantly greater with liraglutide 1.8–3.0 mg compared with placebo, and was dose-dependent (Figure 2a, ANCOVA). Placebo-subtracted mean weight loss was 5.8 kg (95% confidence interval 3.7–8.0) with liraglutide 3.0 mg. Mean change in waist circumference was significantly greater with liraglutide 2.4–3.0 mg versus placebo. Weight loss for those on liraglutide 2.4/3.0 mg for 2 years was significantly greater than with orlistat. No gender differences in weight loss were observed (data not shown). Body composition results in subgroup participants showed that 20-week weight loss with liraglutide was primarily from fat tissue (Table 1).
Figure 2.
(a) Mean changes in body weight and waist circumference from randomization to years 1 and 2. (b) Participants with >5 and >10% randomization weight loss at years 1 and 2. (c) Mean changes in BP and pulse rate from randomization to years 1 and 2. Estimated mean changes in weight, waist, BP and pulse rate (by ANCOVA), and in weight-loss responders (by logistic regression) are shown for the intention-to-treat population with the last observation carried forward.
Table 1. Body composition assessed by dual-energy X-ray absorptiometry and computerized axial tomography in a subgroup of participants at 20 weeks.
| Placebo n=14 |
Liraglutide |
Orlistat n=12 | ||||
|---|---|---|---|---|---|---|
| 1.2 mg n=15 | 1.8 mg n=13 | 2.4 mg n=15 | 3.0 mg n=15 | |||
| Dual-energy X-ray absorptiometry measurements: body composition at randomization (kg)a | ||||||
| Fat tissue | 45.8 (10.5) | 43.5 (7.6) | 45.0 (8.8) | 42.6 (6.1) | 43.9 (8.4) | 41.3 (6.7) |
| Lean tissue | 51.0 (11.0) | 55.0 (8.9) | 51.7 (11.3) | 50.6 (11.9) | 53.1 (10.3) | 47.4 (6.4) |
| Relative change at week 20 (%) | ||||||
| Fat tissueb | −11.9 (2.5) | −13.9 (2.7) | −13.0 (2.6) | −16.5 (2.5) | −15.4 (2.6) | −13.3 (2.9) |
| Change vs placeboc | — | −2.0 (−8.9 to 4.9); P=0.57 | −1.1 (−8.0 to 5.9); P=0.76 | −4.6 (−11.2 to 2.1); P=0.18 | −3.5 (−10.3 to 3.4); P=0.32 | — |
| Lean tissueb | −1.3 (1.0) | −0.9 (1.1) | −2.9 (1.1) | −2.6 (1.0) | −2.0 (1.1) | 0.9 (1.2) |
| Change vs placeboc | — | 0.4 (−2.4 to 3.3); P=0.77 | −1.6 (−4.4 to 1.3); P=0.28 | −1.3 (−4.1 to 1.4); P=0.33 | −0.7 (−3.6 to 2.1); P=0.61 | — |
| Computerized axial tomography measurements: body composition at randomization (cm2)a | ||||||
| Visceral fat | 136 (38) | 172 (77) | 121 (39) | 149 (76) | 145 (69) | 101 (40) |
| Subcutaneous fat | 474 (107) | 453 (68) | 476 (71) | 426 (75) | 434 (116) | 459 (113) |
| Relative change at week 20 (%) | ||||||
| Visceral fatb | −13.8 (5.7) | −19.0 (6.3) | −19.4 (6.0) | −23.0 (5.7) | −20.3 (6.0) | −20.2 (6.7) |
| Change vs placeboc | — | −5.1 (−21.2 to 11.0); P=0.53 | −5.6 (−21.8 to 10.6); P=0.49 | −9.2 (−24.7 to 6.4); P=0.25 | −6.4 (−22.1 to 9.2); P=0.42 | — |
| Subcutaneous fatb | −12.1 (3.0) | −15.6 (3.3) | −15.9 (3.6) | −19.3 (3.0) | −15.3 (3.3) | −17.9 (3.6) |
| Change vs placeboc | — | −3.5 (−11.8 to 4.9); P=0.41 | −3.8 (−12.6 to 5.1); P=0.40 | −7.1 (−15.2 to 1.0); P=0.09 | −3.1 (−11.5 to 5.2); P=0.45 | — |
Mean (s.d.).
Estimated mean (s.e.).
Estimated mean (95% CI); P-value.
Values are for participants who completed the substudy according to the protocol (PP completers).
Significantly more individuals on liraglutide 1.8–3.0 mg achieved weight losses >5 and >10% of randomization weight versus placebo at year 1 (Figure 2b). At year 2, significantly more on liraglutide 2.4/3.0 mg than on orlistat lost >5 and >10% weight. Of the 64% who achieved >5% weight loss with liraglutide 2.4/3.0 mg at year 1, >85% maintained this at year 2.
Blood pressure and pulse from randomization
Mean systolic and diastolic BP decreased in all groups over 1 year, and systolic BP remained significantly lower with liraglutide 2.4/3.0 mg than orlistat at 2 years, while pulse rate was 3.5 beats min−1 greater (Figure 2c).
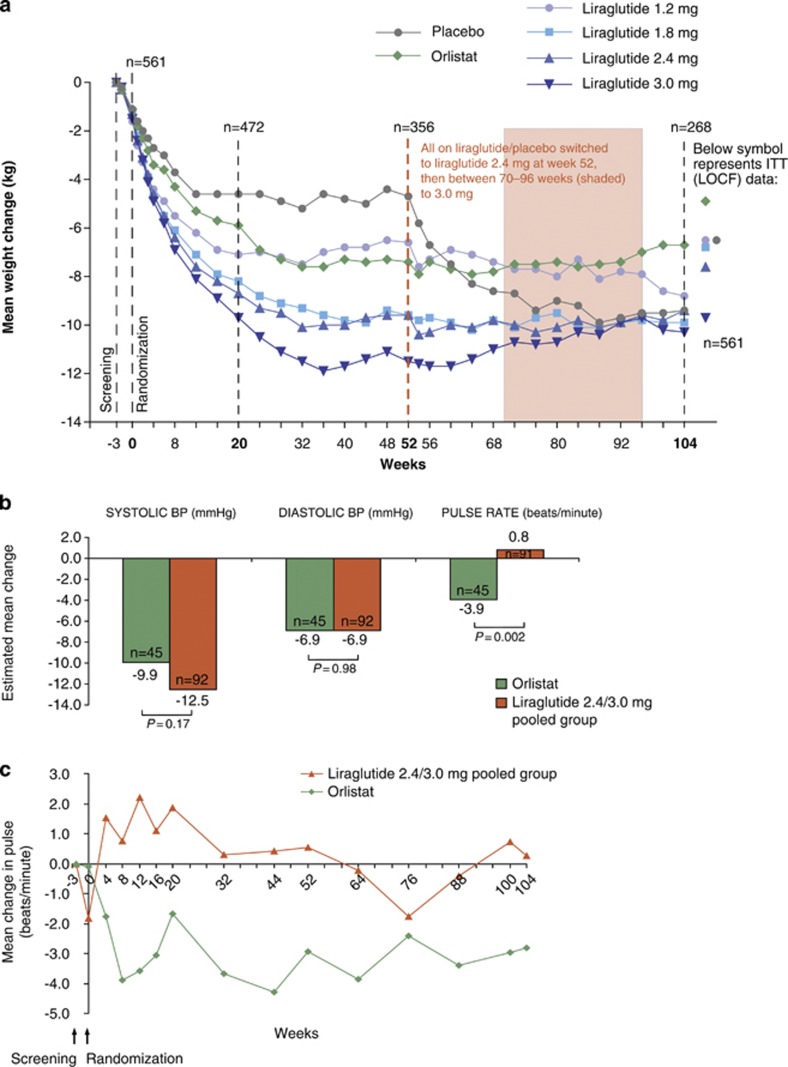
Maintenance from screening: weight loss, BP reduction and pulse
Mean weight reduction between screening and randomization was 1.3±1.4 kg across groups. Figure 3a shows that participants randomized to liraglutide 3.0 mg for 1 year (and then maintained on 2.4/3.0 mg for the second year) maintained a mean weight loss of 10.3±7.1 kg from screening over 2 years. Weight loss for the pooled group on liraglutide 2.4/3.0 mg for 2 years was estimated to be 7.8 kg by adjusted ANCOVA (Supplementary Figure 2a). Almost 70% of liraglutide 2.4/3.0 mg recipients maintained weight loss >5% of screening weight at year 2, 43% maintained >10% loss and 25% maintained >15% loss (Supplementary Figure 2b).
Figure 3.
(a) Change in body weight from screening over 2 years, presented as observed data for individuals completing each scheduled visit. (b) Estimated (ANCOVA) changes in BP and pulse rate from screening to year 2 for the completer population. (c) Mean change in pulse rate over 2 years, presented as observed data for the intention-to-treat population (with no imputation).
Between screening and randomization, across all groups, mean systolic BP decreased by 5.7±11.0 mm Hg, diastolic BP by 3.7±8.1 mm Hg and pulse rate fell by 0.9±10.1 beats min−1. For completers on liraglutide 2.4/3.0 mg, mean systolic BP had decreased from screening levels by 12.5 mm Hg at year 2 (Figure 3b). With liraglutide, mean pulse rate rose slightly from randomization in the first 30 weeks of the trial (Figure 3c), but subsequently fell, approximately to screening levels (Figures 3b and c).
Repeated measures analyses of weight, waist and BP
At years 1 and 2, the repeated measures analyses, performed in support of the primary ANCOVA analyses, in general gave slightly greater estimates for weight and waist change, slightly lower estimates for systolic BP and similar estimates for diastolic BP (Supplementary Tables 3 and 4). In terms of superiority, results of the repeated measures analyses were in general comparable to those of the ANCOVA analyses.
Safety and tolerability and quality of life
Over the 2-year period, 222 (39%) individuals withdrew from the trial: 34–43% of those randomized to liraglutide, and 40% of those randomized to placebo or orlistat. Adverse events include data previously reported for the 20-week trial.12 Summary data for year 1 are shown in Table 2, and in Supplementary Table 5 for year 2. The most frequent liraglutide-associated side effects were gastrointestinal (Table 2; Supplementary Table 6). In year 1, more participants reported nausea and/or vomiting with liraglutide 3.0 mg (49/93; 53%) than with lower doses, placebo (8/98; 8%) (P<0.0001) or orlistat (7/95; 7%)(P<0.0001). Most nausea/vomiting episodes started in weeks 1–6, were transient and >90% were of mild or moderate intensity. Few episodes were serious (2 vomiting). Nausea incidence over 2 years was similar for males and females (P=0.49).
Table 2. Summary of safety data, gastrointestinal disorders with an incidence of ⩾5% in any group and all psychiatric disorders in year 1.
| Placebo n=98 |
Liraglutide |
Orlistat n=95 | ||||
|---|---|---|---|---|---|---|
| 1.2 mg n=95 | 1.8 mg n=90 | 2.4 mg n=93 | 3.0 mg n=93 | |||
| N (%) E | N (%) E | N (%) E | N (%) E | N (%) E | N (%) E | |
| Summary of safety data | ||||||
| Overall withdrawal ratea | 24 (25) | 17 (18) | 20 (22) | 27 (29) | 18 (19) | 28 (30) |
| Participants with AEs | 87 (88.8) 374 | 88 (92.6) 362 | 84 (93.3) 430 | 88 (94.6) 485 | 89 (95.7) 492 | 89 (93.7) 372 |
| Participants with any SAE | 3 (3.1) 3 | 2 (2.1) 2 | 7 (7.8) 7 | 4 (4.3) 5 | 7 (7.5) 10 | 2 (2.1) 2 |
| Withdrawals due to AEs | 3 (3.1) 7 | 5 (5.3) 12 | 6 (6.7) 10 | 12 (12.9) 20 | 7 (7.5) 12 | 3 (3.2) 3 |
| Gastrointestinal disorders | 37 (37.8) 62 | 55 (57.9) 101 | 58 (64.4) 121 | 66 (71.0) 157 | 72 (77.4) 167 | 60 (63.2) 110 |
| Abdominal pain | 4 (4.1) 4 | 2 (2.1) 2 | 3 (3.3) 3 | 1 (1.1) 1 | 5 (5.4) 5 | 4 (4.2) 5 |
| Abdominal pain upper | 1 (1.0) 1 | 5 (5.3) 6 | 2 (2.2) 3 | 5 (5.4) 5 | 5 (5.4) 7 | 7 (7.4) 8 |
| Constipation | 12 (12.2) 14 | 15 (15.8) 17 | 11 (12.2) 12 | 21 (22.6) 24 | 17 (18.3) 18 | 7 (7.4) 8 |
| Diarrhea | 10 (10.2) 11 | 8 (8.4) 13 | 9 (10.0) 12 | 12 (12.9) 13 | 14 (15.1) 15 | 28 (29.5) 40 |
| Dyspepsia | 3 (3.1) 3 | 6 (6.3) 7 | 7 (7.8) 7 | 9 (9.7) 13 | 8 (8.6) 8 | 3 (3.2) 4 |
| Flatulence | 1 (1.0) 1 | — | 4 (4.4) 4 | 4 (4.3) 4 | 3 (3.2) 3 | 10 (10.5) 10 |
| Nausea | 7 (7.1) 8 | 23 (24.2) 27 | 29 (32.2) 33 | 35 (37.6) 48 | 45 (48.4) 68 | 7 (7.4) 7 |
| Steatorrhea | — | — | — | — | — | 5 (5.3) 5 |
| Toothache | 1 (1.0) 1 | 1 (1.1) 1 | 5 (5.6) 7 | 1 (1.1) 1 | — | 1 (1.1) 1 |
| Vomiting | 2 (2.0) 2 | 5 (5.3) 6 | 9 (10.0) 18 | 14 (15.1) 17 | 12 (12.9) 16 | 2 (2.1) 4 |
| Psychiatric disorders | 5 (5.1) 5 | 3 (3.2) 3 | 4 (4.4) 4 | 11 (11.8) 14 | 12 (12.9) 14 | 5 (5.3) 8 |
| Acute stress disorder | — | — | — | 1 (1.1) 1 | — | — |
| Affect lability | — | — | — | — | — | 1 (1.1) 1 |
| Alcohol abuse | — | — | — | — | — | 1 (1.1) 1 |
| Anxiety | 1 (1.0) 1 | — | — | 2 (2.2) 2 | 2 (2.2) 2 | — |
| Burnout syndrome | — | — | — | — | 1 (1.1) 1 | — |
| Depressed mood | — | 1 (1.1) 1 | — | 3 (3.2) 3 | 1 (1.1) 1 | — |
| Depression | — | — | 2 (2.2) 2 | 2 (2.2) 2 | 1 (1.1) 1 | 2 (2.1) 3 |
| Eating disorder | — | — | — | 1 (1.1) 1 | — | — |
| Food aversion | — | 1 (1.1) 1 | — | — | — | — |
| Insomnia | 2 (2.0) 2 | — | — | 2 (2.2) 2 | 5 (5.4) 6 | 2 (2.1) 3 |
| Mood altered | — | — | — | 1 (1.1) 1 | 1 (1.1) 1 | — |
| Nervousness | — | — | — | 2 (2.2) 2 | — | — |
| Restlessness | — | — | 1 (1.1) 1 | — | — | — |
| Stress | 2 (2.0) 2 | 1 (1.1) 1 | 1 (1.1) 1 | — | 2 (2.2) 2 | — |
Abbreviations: AE, adverse event; E, number of adverse event; N (%), number and proportion of participants with an adverse event; SAE, serious adverse event.
Does not include individuals who chose not to enroll in the extension period.
Mean 1-year weight loss from randomization with liraglutide 3.0 mg was 10.0 kg for those with nausea and/or vomiting (n=49) and 7.1 kg for those without (n=43)(difference 2.9 kg (95% confidence interval 0.5–5.3); P=0.02). Weight loss without nausea and/or vomiting was still 4.2 kg greater than placebo (P=0.0001) and 2.3 kg greater than orlistat (P=0.04).
No participant discontinued treatment owing to aversion to injections or injection-site disorders during run-in (Supplementary Figure 1). A total of 51 individuals (9%) withdrew from the trial over 2 years owing to adverse events. These were mostly gastrointestinal. Over 2 years, 15/371 (4%) liraglutide-treated individuals discontinued owing to nausea and/or vomiting; none on placebo (in year 1) or orlistat did so. Four on liraglutide 2.4 mg discontinued because of injection-site disorders (pain/extravasation; hematoma; irritation; and discomfort). Four individuals, all female and randomized to liraglutide treatment, were withdrawn owing to serious adverse events. One withdrew owing to a serious event of cholelithiasis, occurring simultaneously with acute pancreatitis, after 299 days on liraglutide 3.0 mg; the individual recovered without sequelae. Breast cancer occurred in an individual randomized to liraglutide 1.8 mg and treated with 2.4 mg at the time of the event, which was reported after 465 days of treatment. The individual subsequently withdrew from the trial. A serious intestinal adenocarcinoma was reported by a female participant after 410 days on liraglutide 2.4 mg, after a screening program for lung cancer during the trial revealed metastases in the liver. The individual withdrew from the trial and was not expected to recover. A serious anaphylactic reaction was reported by one individual after 692 days of treatment with liraglutide 3.0 mg. The event was due, according to the hospital to which the individual was admitted, to administration of diclofenac/misoprostol on the day of the reaction. The individual recovered but later withdrew from the trial. A further three serious adverse events of special interest and in the liraglutide group were events of atrial fibrillation, uterine leiomyoma and prostate cancer. A serious cardiovascular episode (atrial fibrillation) was reported in a male individual randomized to placebo but on liraglutide 3.0 mg at the time of the event, which occurred after 707 days. Treatment was temporarily discontinued; the participant recovered and completed the trial without further events. The uterine leiomyoma occurred in a participant on 2.4 mg liraglutide for 219 days, who was diagnosed with single fungal fibroid, underwent hysterectomy, but later recovered. Prostate cancer was detected in an individual on liraglutide 1.8 mg after 94 days of treatment. Trial drug continued unchanged throughout the event, the individual completed the trial and was reported as recovering.
A psychiatric medical history was present in 96/564 (17%) participants. In year 1, the most frequently reported disorder coded as ‘psychiatric' was insomnia (Table 2). Other frequently reported disorders included stress, depression, depressed mood and anxiety. All were non-serious and of mild or moderate severity. Overall, there were more psychiatric disorders in general reported by participants on 2.4 and 3.0 mg liraglutide than those on placebo, but there did not seem to be any pattern to the disorders reported, with specific events (other than insomnia) being reported by ⩽3 participants in any group. Two participants withdrew because of anxiety (placebo) and food aversion (liraglutide 1.2 mg).
Over 2 years, 13 self-reported events of symptomatic hypoglycemia (unconfirmed by blood glucose measurement, non-serious) were reported by 9 individuals: 1 event in placebo and 12 in liraglutide-treated participants. No changes in calcitonin concentration were noted at 2 years. Most calcitonin assessments were below the upper normal limit during the 2-year trial period, and no differences in mean concentrations were observed between liraglutide 2.4/3.0 mg and orlistat by logistic regression (estimated odds ratio for liraglutide 2.4/3.0 mg versus orlistat was 0.5 (95% confidence interval 0.17–1.6); P=0.26).
Seven individuals developed antibodies to liraglutide over the 2-year trial period (6 on liraglutide and 1 on orlistat). One subject randomized to liraglutide 1.2 mg had antibodies that crossreacted to GLP-1 in vitro at the end of the trial (the subject was exposed to both liraglutide 2.4 and 3.0 mg during the extension period).
Quality of life improved in all groups at year 1 (Supplementary Table 7) and year 2 (not shown).
Other secondary endpoints from randomization
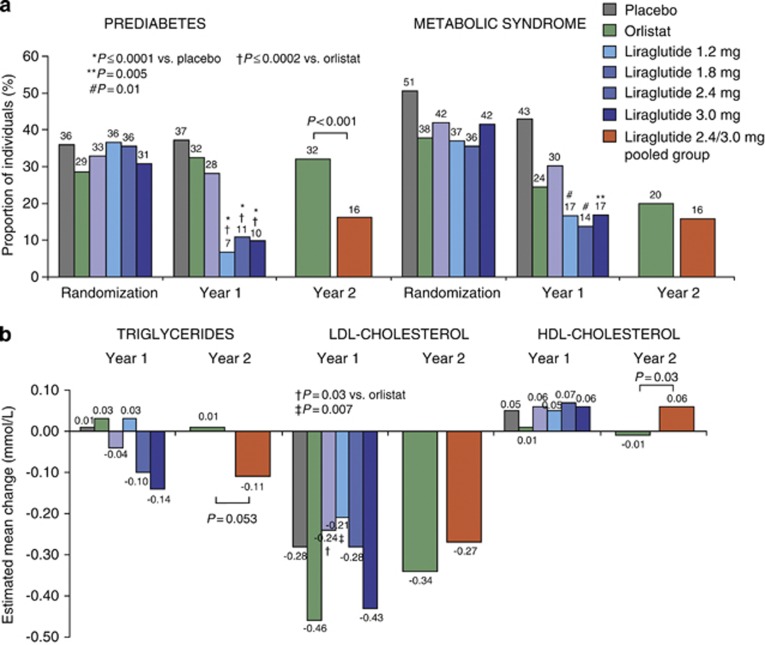
At randomization, 176/564 (31%) individuals had prediabetes, and 229/564 (41%) met criteria for metabolic syndrome. Prediabetes prevalence was significantly reduced with liraglutide 1.8–3.0 mg versus both placebo and orlistat at year 1, and with liraglutide 2.4/3.0 mg versus orlistat at year 2 (Figure 4a). Between 52–62% of liraglutide-treated individuals with prediabetes at randomization achieved normal glucose tolerance at year 2, compared with 26% of those on orlistat. At year 1, the prevalence of metabolic syndrome decreased significantly with liraglutide 1.8–3.0 mg compared with placebo, and was reduced with both liraglutide 2.4/3.0 mg and orlistat at year 2.
Figure 4.
(a) The prevalence of prediabetes and the metabolic syndrome at randomization and after 1 and 2 years of treatment. Metabolic syndrome is defined by updated NCEP-ATP III criteria.16 (b) Mean changes in lipids from randomization to years 1 and 2. Estimated (ANCOVA) changes are shown for the intention-to-treat population with the last observation carried forward.
Fasting lipids, glycemic parameters and cardiovascular risk factors were first measured at randomization, so changes associated with weight loss between screening and randomization are not accounted for. No effects of liraglutide versus placebo on fasting lipids were apparent after 1 year (Figure 4b). At year 2, high-density lipoprotein cholesterol significantly increased with liraglutide 2.4/3.0 mg versus orlistat (treatment difference 0.07 mmol l−1, P=0.03), and both low-density lipoprotein cholesterol and triglycerides decreased from randomization with liraglutide 2.4/3.0 mg (treatment difference −0.12 mmol l−1 for triglycerides, P=0.053 versus orlistat). Mean FPG and glycated hemoglobin (HbA1c) decreased significantly with all liraglutide doses versus placebo at year 1 and with liraglutide 2.4/3.0 mg versus orlistat at year 2 (Supplementary Figure 3).
Plasminogen activator inhibitor-1 significantly decreased with liraglutide 3.0 mg versus placebo (Supplementary Table 8); otherwise, no significant liraglutide effects on cardiovascular biomarkers were observed at year 1. Fibrinogen concentrations decreased across groups, while adiponectin levels increased.
Discussion
Over 2 years, liraglutide with a diet and exercise program was well tolerated, produced sustained weight loss and reduced important cardiovascular risk factors in obese non-diabetic adults. Estimated weight loss of 7.8 kg and systolic BP decrease of 12.5 mm Hg was sustained with liraglutide 2.4/3.0 mg in completers from screening. In obesity trials, the intervention includes weight loss achieved during run-in, before drug exposure, during which lifestyle changes are initiated, and, from a patient perspective, represents the total effect on weight loss. Furthermore, biochemical and other parameters change during this weight-loss period: for example, the initial weight loss of 1.3 kg seen in the current trial was associated with an immediate reduction in systolic BP of 5.7 mm Hg (in completers), so where screening data exist we have additionally reported these. From randomization, reductions in FPG, HbA1c, and in the prevalence of prediabetes and metabolic syndrome, were observed during the trial.
At year 1, superior weight loss with liraglutide (7.8 kg) over both placebo (2.0 kg) and orlistat (3.9 kg) was demonstrated. Mean placebo-subtracted weight loss of 5.8 kg from randomization with liraglutide 3.0 mg was 1.5 kg more than with sibutramine in similar 1-year trials.21 Weight loss stabilized by about 36 weeks, similar to trials with other weight-loss agents,22, 23 was maintained over 2 years and was significantly greater with liraglutide 2.4/3.0 mg than with orlistat. Importantly, almost 70% of participants on liraglutide 2.4/3.0 mg over 2 years maintained a >5% weight loss from screening, associated with improvements in several cardiovascular risk factors and metabolic abnormalities.4, 5
Liraglutide-associated weight loss, in this population not selected for hypertension, was accompanied by decreased systolic BP and unchanged pulse rate at 2 years from screening. Compared with orlistat, BP was lower and pulse rate higher. The clinical significance of the initial pulse increase with liraglutide, as has been reported previously,10 in the context of decreased systolic BP, remains unknown.
At year 2, the prevalence of prediabetes was reduced with liraglutide 2.4/3.0 mg by over 50%. Mean FPG and HbA1c concentrations were also reduced, as in previous studies with liraglutide in type 2 diabetes.10, 11 A significant increase in high-density lipoprotein cholesterol, and marked (nonsignificant) decrease in triglycerides, was noted with liraglutide 2.4/3.0 mg versus orlistat. Changes in low-density lipoprotein cholesterol were comparable to those observed with orlistat, whose mode of action includes reduced dietary fat absorption.
Obesity is associated with altered expression of adipocytokines and risk factors for cardiovascular disease.24, 25 In agreement with previous studies,25 weight loss in all groups increased concentrations of adiponectin and reduced fibrinogen levels.26 Concentrations of plasminogen activator inhibitor-1, which inhibits endogenous fibrinolysis, are reduced by diet and exercise,25 as observed here for all groups except orlistat; liraglutide 3.0 mg produced significantly greater reduction than diet and exercise alone at year 1. These data concur with effects demonstrated with liraglutide in patients with type 2 diabetes.27 Reductions in highly sensitive C-reactive protein were also observed with liraglutide and orlistat, as noted previously.25 In the light of the current focus on cardiovascular safety of weight-loss drugs,28, 29 the favorable effect on cardiovascular risk factors in the current trial seems promising.
Liraglutide was generally well tolerated and improved quality of life. Adverse events were mostly mild or moderate. Gastrointestinal events (particularly nausea and vomiting), consistent with the known physiological effects of GLP-1, were more frequent than with placebo. At year 1, nausea and/or vomiting was associated with greater weight loss with liraglutide 3.0 mg, but even those who did not experience these events lost more weight than those on placebo or orlistat. The injection regimen did not impair adherence or cause significant withdrawal during treatment or run-in.
The main study limitations are the complex study design and the open-label nature of the orlistat treatment arm, although this represents existing best pharmacological practice and provides a comparison for the long-term safety data of liraglutide over the 2-year period. The lack of the placebo comparison at 2 years is also a limitation, but it did not seem feasible in terms of participant retention to maintain the placebo arm. The presence of a diet and exercise run-in period in the study design complicates analyses, and limits the capacity of the trial to anticipate the total maintained effects of treatment. Most secondary endpoints were not measured at screening, therefore several biochemical changes brought about by weight loss during run-in cannot be evaluated. Lipids are likely to have improved; a 6% decrease in low-density lipoprotein and total cholesterol concentrations was previously observed during 2-week run-in in a trial of orlistat versus placebo treatment.30 Analyses from randomization should be interpreted in this light. The initial choice of 2.4 mg as a long-term maintenance dose after 1 year was based on 20-week data. However, when the 12-month data were analyzed, and the decision taken to move up to 3.0 mg, the non-uniform time for dose-switch from liraglutide 2.4 to 3.0 mg during year 2 (as a result of differences in local ethical committee efficiency) is also a limitation. No participants had the benefit of the 3.0 mg dose for the full 2 years, and some for as little as 8 weeks.
In conclusion, results of this study indicate the ability of liraglutide, with diet and exercise, to provide sustained weight loss over 2 years, greater efficacy compared to orlistat and improvements in many of the important obesity-associated metabolic and cardiovascular risk factors. There were no major safety issues, confirming data from the LEAD trials in people with type 2 diabetes at liraglutide doses up to 1.8 mg.10, 11 However, it will be necessary to confirm these results in a larger phase 3 program in obese adults, both in terms of efficacy and (particularly) safety and tolerability.
Acknowledgments
We thank the study participants and acknowledge the members of the NN8022-1807 study group, their staff and clinical trial personnel, without whom this trial would not have been possible. We also thank Helle Hartvig (Novo Nordisk), who performed the statistical analyses.
Appendix
NN8022-1807 Investigators
Belgium: Luc Van Gaal. Czech Republic: Stepan Svacina, Marie Kunesova. Denmark: Arne Astrup, Bjørn Richelsen, Kjeld Hermansen, Steen Madsbad. Finland: Aila Rissanen, Leo Niskanen, Markku Savolainen. Netherlands: Mazin Al Hakim. Spain: Guillem Cuatrecasas Cambra, Belén Sádaba, Raffaele Carraro, Basilio Moreno. Sweden: Stephan Rössner, Martin Ridderstråle. United Kingdom: Michael Lean, Nick Finer, Mike Sampson.
Liraglutide is a Novo Nordisk proprietary compound under development for the treatment of obesity. AA has done commercially sponsored research for Novo Nordisk, is an advisory board member for Novo Nordisk, 7TM, Neuro-Search, Amylin/Takeda, Pathway Genomics, Jenny Craig, and has been a consultant to the following companies within the last 3 years: Orexigen, Vivus, Arena, GSK, Novo Nordisk, 7TM, Pharma, Neurosearch, and Johnson and Johnson. SR has done paid lecturing and commercially sponsored research for Novo Nordisk. LVG was an advisory board member for Novo Nordisk and Eli Lilly, and has done commercially sponsored research for Novo Nordisk. AR has done commercially sponsored research and/or been an advisory board member for Novo Nordisk, Novartis, Johnson and Johnson, Boeringer Ingelheim and Eli Lilly. LN has done paid lecturing and commercially sponsored research for Eli Lily, Merck, Novo Nordisk, Boehringer Ingelheim, Astra Zeneca, Sanofi Aventis, Bristol-Myers Squibb and Pfizer, and is an advisory board member for Novo Nordisk and Astra Zeneca. NF was an advisory board member and has done paid lecturing and commercially sponsored research for Novo Nordisk, Abbott laboratories and Sanofi Aventis, and is an advisory board member for Pfizer, Allergan and Takeda. MEJL and MJS have done commercially sponsored research for Novo Nordisk, and MEJL has done paid lecturing for Novo Nordisk. RC has done paid lecturing and commercially sponsored research for Astra Zeneca, Novo Nordisk, Novartis and Sanofi Aventis. MK has done commercially sponsored research for Novo Nordisk and Boehringer Ingelheim. AH and MFR are employees of Novo Nordisk and own stock in Novo Nordisk. The work was funded by Novo Nordisk A/S, Denmark.
Footnotes
Supplementary Information accompanies the paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary Material
References
- Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes Res. 1998;6 (Suppl 2:51S–209S. [PubMed] [Google Scholar]
- Mertens IL, Van Gaal LF. Overweight, obesity, and blood pressure: the effects of modest weight reduction. Obes Res. 2000;8:270–278. doi: 10.1038/oby.2000.32. [DOI] [PubMed] [Google Scholar]
- Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement Health Technol Assess 20048iii–iv.1. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration FDA Guidance for Industry: developing products for weight management, 2007
- NHS, National Institute for Health and Clinical Excellence Obesity - guidance on the prevention, identification, assessment and management of overweight and obesity in adults and childrenNICE clinical guideline 43, 2006 [PubMed]
- EMEA, Committee for Medicinal Products for Human Use (CHMP) Guideline on clinical evaluation of medicinal products used in weight control, 15 November 2007.
- Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courrèges JP, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30:1608–1610. doi: 10.2337/dc06-2593. [DOI] [PubMed] [Google Scholar]
- Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- World Medical Association Declaration of Helsinki Ethical principles for medical research involving human subjects. JAMA. 2000;284:3043–3045. [PubMed] [Google Scholar]
- Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the national heart, lung, and blood institute/American heart association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9:102–111. doi: 10.1038/oby.2001.13. [DOI] [PubMed] [Google Scholar]
- Knudsen LB, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473–1486. doi: 10.1210/en.2009-1272. [DOI] [PubMed] [Google Scholar]
- Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142:532–546. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- Dunnett CW. A multiple comparisons procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev. 2004;3:CD004094. doi: 10.1002/14651858.CD004094.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak H, Ziegler O, Keller U, Hamann A, Godin C, Wittert G, et al. X-PERT: weight reduction with orlistat in obese subjects receiving a mildly or moderately reduced-energy diet. Early response to treatment predicts weight maintenance. Diabetes Obes Metab. 2005;7:699–708. doi: 10.1111/j.1463-1326.2005.00483.x. [DOI] [PubMed] [Google Scholar]
- McMahon FG, Fujioka K, Singh BN, Mendel CM, Rowe E, Rolston K, et al. Efficacy and safety of sibutramine in obese white and African American patients with hypertension - a 1-year, double-blind, placebo-controlled, multicenter trial. Arch Intern Med. 2000;160:2185–2191. doi: 10.1001/archinte.160.14.2185. [DOI] [PubMed] [Google Scholar]
- Nguyen XM, Lane J, Smith BR, Nguyen NT. Changes in inflammatory biomarkers across weight classes in a representative US population: a link between obesity and inflammation. J Gastrointest Surg. 2009;13:1205–1212. doi: 10.1007/s11605-009-0904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi-Sunyer FX. The relation of adipose tissue to cardiometabolic risk. Clin Cornerstone. 2006;8 (Suppl 4:S14–S23. doi: 10.1016/s1098-3597(06)80040-2. [DOI] [PubMed] [Google Scholar]
- Belza A, Toubro S, Stender S, Astrup A. Effect of diet-induced energy deficit and body fat reduction on high-sensitive CRP and other inflammatory markers in obese subjects. Int J Obes. 2009;33:456–464. doi: 10.1038/ijo.2009.27. [DOI] [PubMed] [Google Scholar]
- Courreges JP, Vilsbøll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabet Med. 2008;25:1125–1131. doi: 10.1111/j.1464-5491.2008.02484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup A. Is cardiometabolic risk improved by weight-loss drugs. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60999-3. [DOI] [PubMed] [Google Scholar]
- James WPT, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- Muls E, Kolanowski J, Scheen A, Van GL. The effects of orlistat on weight and on serum lipids in obese patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled, multicentre study. Int J Obes Relat Metab Disord. 2001;25:1713–1721. doi: 10.1038/sj.ijo.0801814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.