Abstract
Marine cyanobacteria are noted for their ability to excrete metabolites with biotic properties. This paper focuses on such exometabolites obtained from the culture of the marine filamentous cyanobacterium Geitlerinema sp. strain, their purification and subsequent analyses. By this means the recoveries of the active compounds, a prerequisite for properly determining their concentration, are quantified here for the first time. We demonstrate a new procedure using Amberlite XAD-1180 resin in combination with the eluent isopropanol for extraction of the culture media and gas chromatography as simplified chemical analysis. This procedure reduced necessary bacteria cultivation time (from 150 to 21 days) at low volumes of culture media (300 mL) required for identification of two selected bioactive compounds: 4,4′-dihydroxybiphenyl and harmane.
Keywords: 4,4′-Dihydroxybiphenyl; Harmane; Analytical bioseparation; Cyanobacterial culture media; GC method; Recovery
Introduction
Marine microorganisms are proving to be promising new sources of a huge number of biologically active products of commercial interest in the pharmaceutical, agricultural and food industries (Tan 2007; Tan et al. 2010; Le Gal and Muller-Feuga 1998; Debbab et al. 2010). Apart from original products, they produce a wide variety of secondary metabolites that cannot be produced by terrestrial microorganisms (Liu et al. 2008). These metabolites may possess pharmaceutically attractive properties (e.g. antitumor, antiviral, antifungal, antibiotic), but they may also be toxic (e.g. Patterson et al. 1994; Thiel and Imhoff 2003; Kelecom 2002).
Recently, two surveys of antibiotic compounds produced by cyanobacteria (blue-green algae) demonstrated the antifouling potential of this phylum of bacteria (Dahms et al. 2006; Gademann 2007). Although cyanobacterial metabolites are accumulated mostly in the cyanobacterial biomass, some of them are excreted into the environment (Kreitlow et al. 1999; Tan et al. 2010). The compounds with biotic properties are typically excreted into the growth media as it was reported for the phenolic compound 4,4′-dihydroxybiphenyl and the indole alkaloid norharmane (9H-pyrido(3,4-b)indole) from the filamentous cyanobacteria Nostoc insulare and Nodularia harveyana, respectively (Volk and Furkert 2006), both belonging to the order Nostocales. Another indole alkaloid containing a β-carboline skeleton —harmane (1-methyl-9H-pyrido(3,4-b)indole)—was isolated from the algicidal bacterium Pseudomonas sp. K44-1 by Kodani et al. (2002) and the tunicate-associated bacterium Enterococcus faecium from a marine bacterium (Aassila et al. 2003), but not from cyanobacteria (Okunade et al. 2007; Tan et al. 2010). The structures of these compounds are presented in Fig. 1. The concentrations of these metabolites, for example norharmane, detected in the cyanobacterial culture media, depend not only on the species but are also related to the age of the culture (Volk 2007).
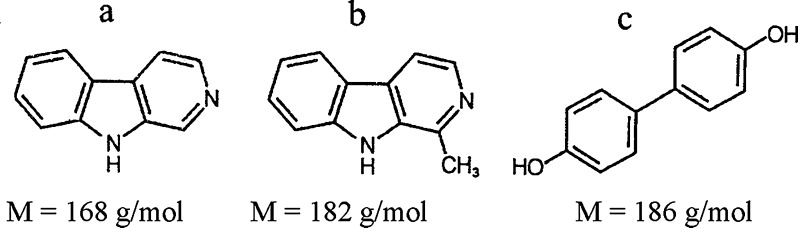
Fig. 1.
The chemical structure of a norharmane, b harmane, c 4,4′-dihydroxybiphenyl
The above-mentioned metabolites elicit various pharmaceutical effects, e.g. they exhibit antimicrobial, antifouling and anticyanobacterial activities (Volk and Furkert 2006; Volk 2007; Reza and Abbas 2007). Moreover, harmane and norharmane are characterized by a co-mutagenic activity (Totsuka et al. 1999, 2004), so these substances should be analysed because of their toxicological properties too.
One of the techniques most often used as a first step in downstream processing to separate these compounds from the growth media of cyanobacteria is the use of polymeric resins: Amberlite XAD-1180 or Amberlite XAD-16 (Volk and Furkert 2006; Volk 2007; Armstrong et al. 2000). The choice of the proper resin is crucial because it can govern parameters such as selectivity, affinity and adsorption capacity (Abdullah et al. 2008). The elution of the adsorbed analytes depends on solute–polymer interactions. These analytes are commonly desorbed from the resin using methanol. However, in some cases other methods of weakening these interactions need to be applied, such as increasing the temperature or changing the solvent. The methanolic solution is evaporated to dryness and the residue is dissolved in ethanol, filtered, evaporated to dryness again, dissolved in demineralized water and freeze-dried to obtain the crude culture medium extract. In the next steps, the crude culture medium extract is subjected to qualitative and quantitative analyses using one of the following techniques: TLC, HPLC and/or HPLC-MS (Volk 2005). GC techniques have not been reported for this type of problem.
Literature data on natural products produced by the marine filamentous cyanobacterium Geitlerinema sp. belonging to the order Oscillatoriales are very limited (Tan 2007). So far, no exometabolites excreted into the culture media have been described (Tan 2007). Andrianasolo et al. (2005, 2007) isolated two cytotoxic natural products from the cells of the marine cyanobacterium Geitlerinema sp. strain: a linear peptide (IC50 460 nM to NCI-H460 human lung tumour cells) and Swinholide A (previously identified in the heterotrophic bacteria of sponges).
Recently we reported that culture medium extracts, which were obtained by supernatant's extraction with Amberlite XAD-1180 from cultures of a cyanobacterial strain Geitlerinema sp, were biologically active upon fungi and bacteria (Caicedo et al. 2011).
Here we present analytical bioseparation procedures for the detection of bioactive compounds from crude culture medium of Geitlerinema sp for the purpose of identification and quantitative analyses of β-carbolines and phenolic compounds by means of TLC and Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) in comparison with Gas Chromatography–Flame Ionization Detection (GC-FID) and Gas Chromatography–Mass Spectrometry (GC-MS) methods developed for this purpose.
Materials and Methods
Cultivation of Geitlerinema Strain So-11, Preparation of the Crude Culture Medium Extract and Test for Antimicrobial Activity
The marine filamentous cyanobacterium Geitlerinema strain So-11 was obtained from a culture collection of the Department of Marine Microbiology at the University of Bremen. This strain was cultivated separately in three cylindrical glass columns containing each 300 mL of artificial sea water medium ASN III/2 plus 8.8 mM NaNO3 (pH 8.5; Rippka et al. 1979) at a constant temperature of 25°C with continuous aeration and under continuous illumination (10 μEm−2 s−1 photon flux). The cultivation time was fixed to 3 weeks as it is referred by Caicedo et al. (2011) (the stock cultures of So-11(b) and So-11(c); Table 1), and simultaneously one column, containing a stock culture, was kept under the same conditions during 150 days (the stock culture of So-11(a)). The supernatant of each culture was obtained after centrifugation (15 min at 4000 × g) and separation from the biomass. The bioactive compounds from the supernatant were separated on Amberlite XAD-1180 resin according to Volk's procedure (Volk and Furkert 2006; Volk 2007). The crude culture medium extracts thus obtained (Table 1) were tested for their antimicrobial activity towards Bacillus subtilis according to Caicedo et al. (2011) and subjected to a qualitative alkaloid test (Mayer's and Wagner's reagents) in the conditions presented by Scholz and Liebezeit (2006).
Table 1.
Crude media extracts prepared from the cultivation of Geitlerinema sp strain So-11
| Sample | Volume supernatant (mL) | Cultivation time (days) | Solvent for elution | Mass of crude extract (g) | % inhibition upon B subtilis | Qualitative analysis for alkaloid |
|---|---|---|---|---|---|---|
| So-11(a) | 50 | 150 | Methanol | 0.0440 | 100 | Positive |
| So-11(b) | 300 | 21 | Methanol | 0.1154 | 100 | Negative |
| So-11(c) | 300 | 21 | Isopropanol | 0.2935 | 99.5 | Positive |
Detection of Target Compounds in the Crude Culture Medium Extract
Target Compounds
Standard solutions of harmane (Sigma-Aldrich, Steinheim, Germany), norharmane (Acros Organics BVBA, Geel, Belgium) and 4,4′-dihydroxybiphenyl (Sigma-Aldrich, Steinheim, Germany) were prepared in methanol (Sigma-Aldrich, Steinheim, Germany).
LC-MS/MS
The MS/MS analysis of the methanolic solution of the crude culture medium extract So-11(a) (30 mg in 0.2 mL), having been passed through a 0.45-μm syringe filter, was performed on a Thermo Finnigan LCQ Deca LC-MS/MS system equipped with an electrospray ionization (ESI) source and syringe pump. The following MS measurement parameters were applied: sheath gas 50 arb; aux gas 5 arb; spray voltage 6.00 kV; capillary temperature 250°C; capillary voltage 35.00 V; tube lens offset −10.00 V; flow rate 8 μL/min. All MS analyses were performed in positive ion mode. The following m/z values for identification of the target were applied: norharmane ([M + 1 (H)]+ = 169; [M + 23 (Na)]+ = 191; [M + 39 (K)]+ = 207); harmane [M + 1 (H)]+ = 183; [M + 23 (Na)]+ = 205; [M + 39 (K)]+ = 221); 4,4′-dihydroxybiphenyl [M + 1 (H)]+ = 187; [M + 23 (Na)]+ = 209; [M + 39 (K)]+ = 225). Additionally, the MS full scan and MS/MS spectra of the standard solution of harmane in methanol (0.1 mg mL−1) and distilled methanol were recorded. Because of the poor results of the performed LC-MS/MS analysis of extract So-11(a) (150 days cultivation of a stock culture medium), the methanolic solutions of the crude culture medium extracts So-11(b) and So-11(c) have been not analysed using this method.
TLC
TLC analysis was carried out on a prefabricated plate (20 × 20 cm; Merck, Germany) coated with silica gel 60 F254 50 μL of the solution containing 20 mg of the crude culture medium extract So-11(b) in 150 μL 50% methanol and 10 μL of each of the reference solutions of harmane, norharmane and 4,4′-dihydroxybiphenyl in 250 μL 50% methanol (0.16 mg mL−1) were pipetted onto the plate. The mobile phase was ethyl acetate/methanol/demineralized water (100:16.5:13.5, v/v/v). Harmane and norharmane were detected at 254 and 366 nm, but 4,4′-dihydroxybiphenyl only at 366 nm. The same procedure was applied for TLC analysis of the solution of the crude culture medium extract So-11(a) (10 mg in 150 μL 50% methanol).
GC-FID and GC-MS Measurements
The GC-FID analysis of the methanolic solution of the crude culture medium extract So-11(a) (30 mg in 0.2 mL), having been passed through a 0.45-μm syringe filter, was performed on a Clarus 500 gas chromatograph (Perkin Elmer) equipped with a fused-silica capillary column BGB-5 (30 m × 0.25 mm I.D., 0.25 μm film thickness, BGB Analytik AG) with the following temperature programme: 100°C to 320°C at 4°C, 15 min isothermally at 320°C. Injections (5 μL) were carried out in split mode (ratio 1:5). The temperature of the injector was 320°C, that of the detector 320°C. The carrier gas was argon with a flow rate of 2.0 mL/min. GC-MS measurements were performed on an AutoSpec mass spectrometer (EI 70 eV) coupled with a Hewlett-Packard 5890 gas chromatograph using the same column and chromatographic conditions, except that helium and not argon was the carrier gas. GC peaks were identified by their retention times and their characteristic MS spectra in GC-MS analysis. GC-FID measurement was used for quantitative analysis and GC-MS for the qualitative analysis of this extract.
Development of a GC-FID Method for Determination of Target Compounds in Crude Culture Media Extracts
Internal Standard
4,4′-Dipyridyl (Sigma-Aldrich, Steinheim, Germany) was used as the internal standard (IS).
GC-FID Analysis of Target Compounds in the Native Forms
The stock solutions (250 μg mL−1) of each target compound (harmane, norharmane and 4,4′-dihydroxybiphenyl) and IS (4,4′-dipyridyl) were prepared in methanol and analysed directly by GC-FID under the chromatographic conditions described above.
Derivatization Procedure
Derivatization was carried out using a working solution (100 μg mL−1) of each target compound and IS. Fifty microliters of this solution was transferred to a 1.5-mL GC vial and evaporated to dryness in a stream of nitrogen. After evaporation of the working solution, 100 μL of the mixture of 99% N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) and 1% trimethylchlorosilane (TMCS) (Sigma-Aldrich, Steinheim, Germany) were added. The sample was kept at 90°C for 60 min and analysed (2 μL) directly using GC-FID and GC-MS. GC-FID measurements were performed using the same column and chromatographic conditions, except that the following temperature programme—100°C to 320°C at 6°C, 15 min isothermally at 320°C—was applied.
Validation of the GC-FID Method
Ten different concentrations of each target compound in the working solutions (125, 100, 80, 40, 20, 10, 5, 1, 0.5 and 0.25 μg mL−1) were prepared in methanol. Each solution (100 μL) was transferred to a separate 1.5-mL vial and derivatized using the BSTFA/TMCS mixture under the conditions described above. Subsequently, all the samples (2 μL) were analysed by GC-FID under the same chromatographic conditions as presented above with three replicate injections. The GC peak areas were integrated manually. A number of validation parameters were established: linearity, sensitivity, precision and accuracy of the method, as well as the limits of detection (LODinst) and quantification (LOQinst) of the instrument. The linearity of the method was determined by a minimum of five different concentrations with three replicates. The LOQinst was selected as the lowest amount of analyte that could be quantified by the method with good accuracy and precision, i.e. the lowest concentration for which the value of the relative standard deviation (RSD, %) was less than 10%. The LOD of the instrument was calculated as LODinst = LOQinst/3. The intra-day precision of the method was determined by calculating the RSD for the replicated measurements (n = 3). The accuracy of the method was determined by assessing the agreement between the measured and known concentrations of the analysed samples.
Determination of the RRFs of Target Compounds
The relative response factor (RRF) was calculated using the following equation:
where A IS—internal standard peak area (4,4′-dipyridyl), C S—target analyte concentration (in micrograms per milliliters), A S—target analyte peak area, C IS—internal standard concentration (4,4′-dipyridyl) (in micrograms per milliliters) and was given as a mean value. The RRF was determined on the basis of working solutions with concentrations 100 μg mL−1 of IS and 100, 80 and 40 μg mL−1 of each target compound. These samples were derivatized and analysed with three replicates.
Estimation of the Recovery of Target Compounds from Growth Culture Medium
Growth culture medium was prepared using the operations above. Then the samples were shaken for 3 h. Subsequently, 1 mL of each of the obtained liquors was transferred to 1.5 mL GC vials, evaporated to dryness under a stream of nitrogen, derivatized, and analysed by GC-FID and GC-MS using the same column and chromatographic conditions as described in Section “Derivatization procedure”. GC peaks were identified by their retention times and characteristic MS spectra. The GC peak areas were integrated manually.
Standard Addition to Growth Culture Medium and Subsequent Preparation Using Volk's Procedure
One hundred fifty milliliters of the growth medium was spiked with the solution of the target compounds and IS (commercial compounds as specified above) to a concentration of 1,000 μg L−1 of each compound in the medium. The sample was extracted according to Volk's procedure (Volk and Furkert 2006; Volk 2007). The crude culture medium extract obtained was dissolved in 3 mL of methanol, and 10 mg of anhydrous Na2SO4 was added.
Standard Addition to Growth Culture Medium and Subsequent Preparation Using a Simplified Volk's Procedure
The sample was prepared exactly as described above and then extracted on Amberlite XAD-1180 resin. The methanolic solution obtained was evaporated to dryness, but the further steps in Volk's procedure (dissolution in ethanol, filtration, a second evaporation to dryness, dissolution in demineralized water and lyophilization) were omitted. The residue was dissolved in 3 mL methanol, and 10 mg of anhydrous Na2SO4 was added.
Standard Addition to the Growth Culture Medium and Subsequent Preparation Using an Alternative Procedure
The sample was prepared exactly as described above but without the IS and extracted on Amberlite XAD-1180 resin using isopropanol as eluent; the lyophilization step was omitted. The extract obtained (5.5 mg) was dissolved in 2.7 mL of methanol, and 300 μL of the IS stock solution (500 μg mL−1) was added.
Extraction of the Exometabolites from Culture Medium Using the Alternative Procedure
The bioactive compounds from 300 mL of the culture medium of the cyanobacterium Geitlerinema strain So-11 obtained after 3-week cultivation time was separated on Amberlite XAD-1180 resin using the alternative procedure described above. The obtained extract So-11(c) (Table 1) was dissolved in 2.7 mL methanol and spiked with 300 μL of the stock solution of IS (250 μg mL−1). Next, 1 mL of methanolic solutions of this extract was subjected to derivatization step and GC-FID and GC-MS analyses using the same chromatographic conditions as was mentioned in the Section “Derivatization procedure”. For comparison, the GC-FID and GC-MS analyses of the extract So-11(b) spiked with known amount of IS were also performed.
Results
Antimicrobial Activity of the Crude Extract from the Culture Medium of Geitlerinema Strain So-11
All extract samples obtained from the respective supernatant volumes showed growth inhibition of the bacterium B. subtilis (Table 1) and two of them gave a positive response to the qualitative alkaloid test (So-11(a) and So-11(c)).
Analysis of the Native Crude Culture Medium Extract So-11
In general the following target compounds could be identified: harmane, norharmane and 4,4′-dihydroxybiphenyl. However, with the LC techniques used several problems arose. The mass spectrum of harmane recorded in MS full scan mode contained a high intensity signal at m/z 183 [M + 1 (H)]+, which was used as the precursor ion in the full scan MS/MS mode measurement. The fragmentation amplitude and isolation width (CE = 40%, with ±1.5, respectively) were optimized manually to increase the sensitivity and selectivity of the method. The mass spectrum obtained from the signal at m/z 183 in MS/MS mode showed ions at m/z 108 (7%), 142 (44%), 155 (100%), 156 (63%) and 182 (3%). During analysis of the crude culture medium extract So-11(a), two problems cropped up: (a) the capillary in the ESI interface of the LC-MS/MS system was still blocked by the residues of salts present in the extract, and (b) the intensities of the analysed ions in the MS full scan mode were very weak. Analysis of distilled methanol enabled the intensity of the background ions to be determined. Analysis of the crude culture medium extract So-11(a) methanol extract showed that the intensities of only two ions, at m/z 221 and 225, respectively, increased slightly.
TLC analysis of the standards of harmane, norharmane and 4,4′-dihydroxybiphenyl was performed in order to establish the R f parameters for each compound. Although the TLC chromatogram obtained for the reference substances showed well-separated bands for each compound, non-separated band was observed for the sample So-11(a), therefore calculation of Rf parameters and confirmation of the presence of the target compounds in the extract was impossible. On the other hand, no bands were observed during the analysis of the crude extract from the culture medium of Geitlerinema strain So-11(b).
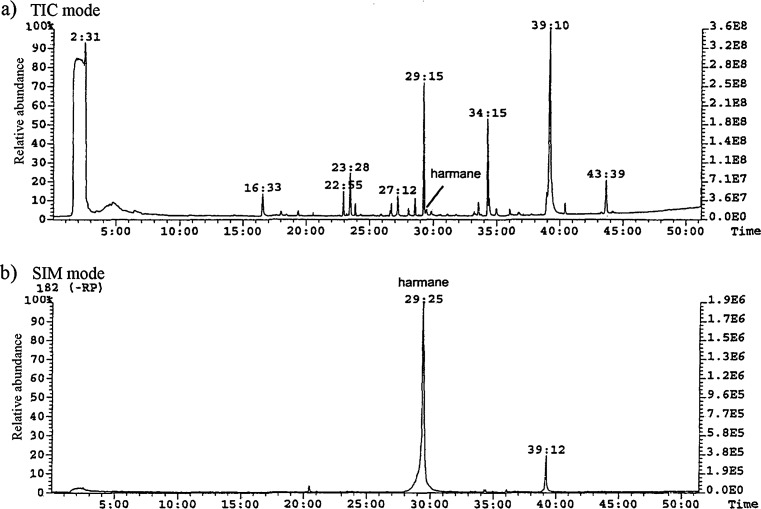
In order to get analytical methods that are more robust with respect to matrix effects, we developed GC-FID and GC-MS methods as alternative techniques for analysing these types of samples. Our analyses of the crude extract from the culture medium of Geitlerinema strain So-11(a) now revealed the presence of harmane in the culture medium. GC-MS analyses of the culture medium were performed in both total ion chromatogram mode (Fig. 2a) and single ion monitoring mode (m/z = 182) (Fig. 2b). Additionally, the identification of harmane was confirmed by co-injection with the standard in GC-FID and by analysis of the GC-MS mass spectra.
Fig. 2.
Chromatogram of the crude extract of the culture medium of Geitlerinema strain So-11(a) obtained during GC-MS analyses performed a in TIC (total ion chromatogram) mode, b in SIM (single ion monitoring) mode at m/z = 182
Development of the GC-FID Method
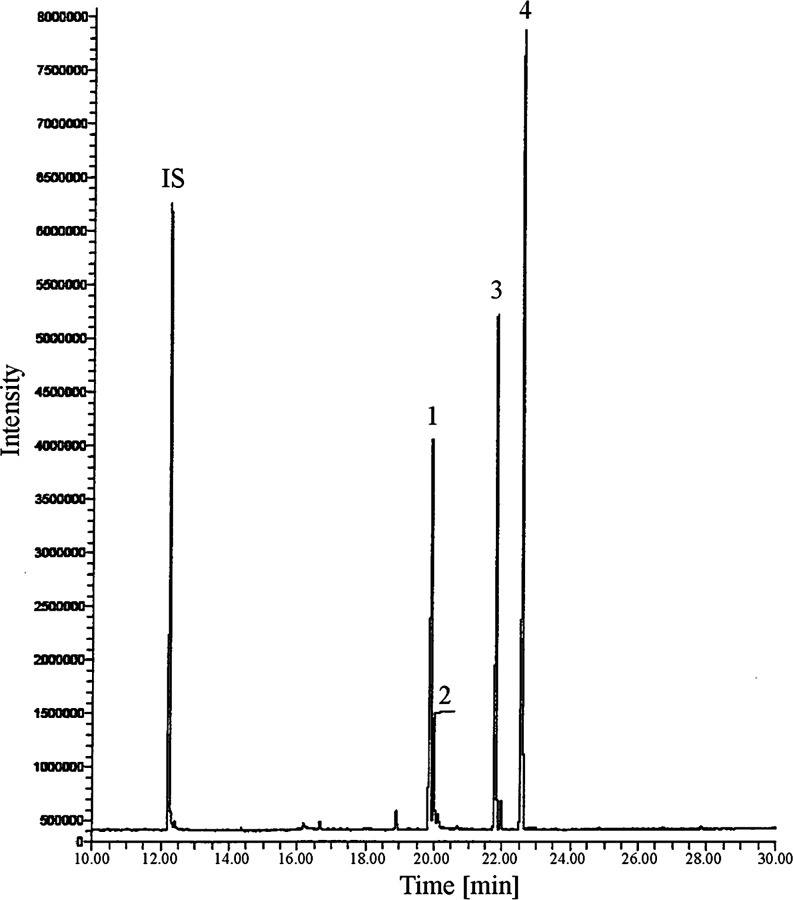
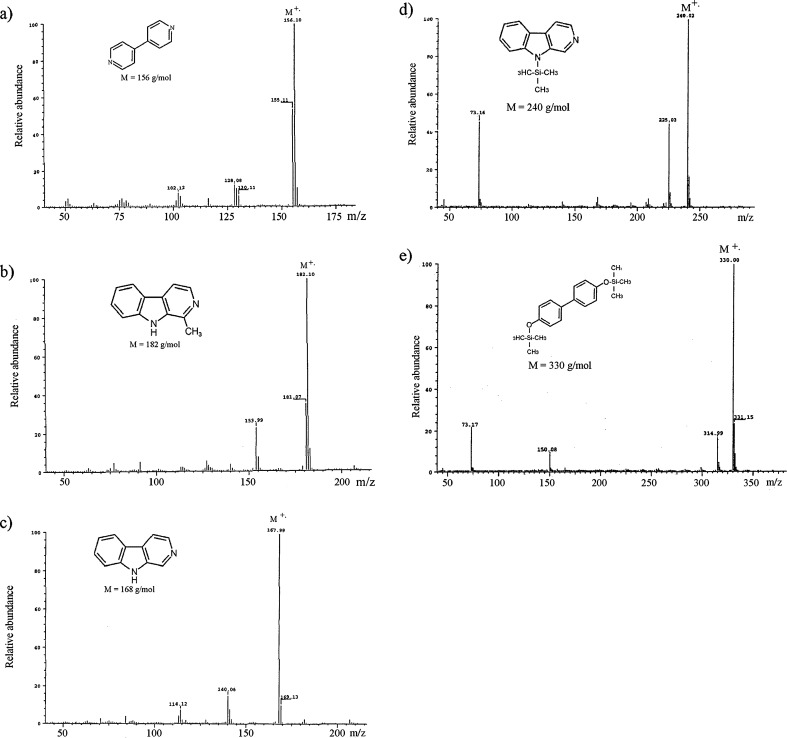
GC-FID analyses of the stock solutions of 4,4′-dipyridyl (an internal standard), harmane, norharmane and 4,4′-dihydroxybiphenyl in the native form showed (a) a well-separated chromatographic peak of 4,4′-dipyridyl, (b) a higher intensity peak of harmane in comparison to that recorded for the same amount of norharmane, and (c) no chromatographic signal for 4,4′-dihydroxybiphenyl. In the next step, before GC-FID and GC-MS measurements, derivatization using a mixture of 99% BSTFA and 1% TMCS was applied. The gas chromatogram of a solution containing each of the target compounds and IS (100 μg mL−1 of each compound) and derivatized by GC-FID is presented in Fig. 3 and the GC-MS mass spectra in Fig. 4. The RRFs for these compounds are as follows: RRFharmane = 1.2607, RRF4,4′-dihydroxybiphenyl = 0.6169 and RRFnorharmane = 0.9866.
Fig. 3.
GC-FID analysis of the target compounds and IS-preceded silylation procedure (stock solution 100 μg mL−1). IS 4,4′-dipyridyl, 1 harmane, 2 native norharmane, 3 silylated norharmane, 4 silylated 4,4′-dihydroxybiphenyl
Fig. 4.
EI mass spectra of a 4,4′-dipyridyl, b harmane, c native norharmane, d silylated norharmane, e silylated 4,4′-dihydroxybiphenyl
Recovery
GC-FID and GC-MS analyses of the extract obtained from the growth culture medium spiked with each target compound and IS to a concentration of 1,000 μg L−1 in the medium using Volk's procedure yielded no chromatographic signals for any of the target compounds. Elimination of the lyophilization step from Volk's procedure meant that only a very weak chromatographic signal of silylated 4,4′-dihydroxybiphenyl was observed and a trace concentration of harmane was detected. IS and norharmane were still not found in the extract. Modifying Volk's procedure by using isopropanol as eluent (instead of methanol) and omitting the lyophilization step provided an extract containing both IS and the expected target analytes. The first time recoveries of analytes from the culture medium are now be calculated yielding 6.4% for harmane and norharmane, and 3.6% for 4,4′-dihydroxybiphenyl. The numbers are substantially high for determining concentrations. However, they also demonstrate the potential for an improvement of the extraction procedure as described below.
Identification of Harmane and 4,4-Dihydroxybiphenyl in the Crude Culture Medium Extract of the Cyanobacterium Geitlerinema Strain So-11
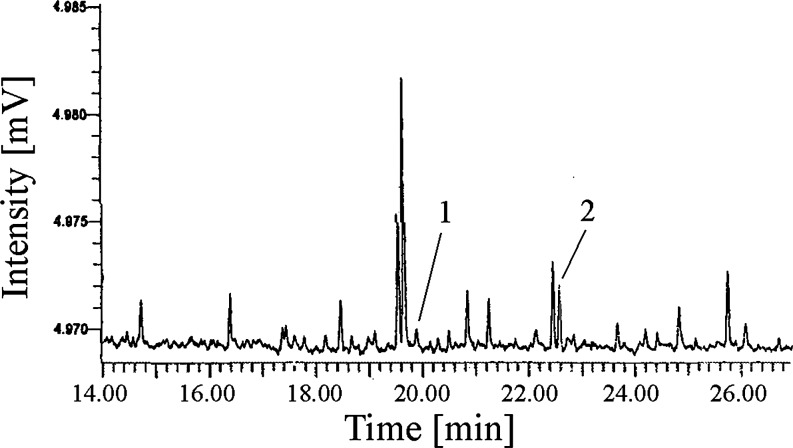
Figure 5 shows the gas chromatogram of the GC-FID analysis of the crude culture medium extract So-11(c) obtained using a modified version of Volk's procedure with isopropanol as eluent, without lyophilization, and with subsequent derivatization. The identification of harmane and 4,4'-dihydroxybiphenyl in the extract was confirmed by co-injection with standards in GC-FID measurement and by analysis of the mass spectra obtained from GC-MS analysis.
Fig. 5.
GC-FID analysis of the silylated extract of the culture medium of the cyanobacterium Geitlerinema strain So-11(c) (Table 1). 1 harmane, 2 4,4′-dihydroxybiphenyl
Discussion
The qualitative and quantitative analysis of crude culture medium extract using established LC-(ESI)-MS/MS and TLC showed to be of limited usefulness. Although the ESI-MS/MS conditions using a methanolic solution of harmane were optimized without any problems and the ESI mass spectrum of harmane obtained was similar to the one presented by Teichert et al. (2008), the analysis of the crude culture medium extract So-11(a) by LC-MS/MS was much more complicated. The fused silica and heated capillary in the ESI interface were blocked by the residues of salts present in the extract (for example, the concentrations of NaCl, KCl and MgCl2·6 H2O in the medium were 12,500 mg L−1, 250 mg L−1 and 1,000 mg L−1, respectively), and the intensities of the analysed ions in the MS full scan mode were very weak. Only two ions—at m/z 221 and 225—increased slightly during the analysis of the crude culture medium extract So-11(a) in MS/MS mode. It was thought that the ion at m/z 221 could indicate the presence of harmane in the extract ([M + 39 (K)]+ = 221), whereas that at m/z 225 the presence of 4,4′-dihydroxybiphenyl ([M + 39 (K)]+ = 225). Although the main ion in the full scan mass spectrum of standard harmane was [M + 1 (H)]+ at m/z 183, the [M + 39 (K)]+ rather than the [M + 1 (H)]+ adducts are probably more stable during ESI ionization of harmane and 4,4′-dihydroxybiphenyl in the presence of salts. Good quality mass spectra of these ions in the MS/MS mode were not obtained, so harmane and 4,4′-dihydroxybiphenyl in the crude culture medium extract So-11(a) could not be definitively identified.
Poor results were obtained from the TLC analyses of the crude culture medium extracts So-11(a) and So-11(b) (performed according to Volk and Furkert 2006). This was probably due to the presence of large amount of salts in the samples (low resolution of the bands in the sample So-11(a)) and low concentration of these bioactive compounds in the extracts (especially in the crude culture medium extract So-11(b)). The analysed crude extracts in this work were obtained from a smaller culture volume and shorter cultivation times (Table 1) compared to a similar sample preparation for other cyanobacterium (250 L and 150 days) reported by Volk (2007).
Due to these problems GC methods were adopted from food analysis methods. Direct GC-FID and GC-MS analyses of the crude extract from the Geitlerinema strain So-11(a) culture medium showed that this strain excretes harmane into the culture medium, but no chromatographic signal was observed for 4,4′-dihydroxybiphenyl (Fig. 3). Since LC-MS/MS analysis of the crude extract So-11(a) indicated the presence of 4,4′-dihydroxybiphenyl, a suitable GC method for identifying also this compound was developed. 4,4′-Dipyridyl (similar structure, formulation of a well-separated chromatographic peak) was used as the internal standard in the quantitative analysis of the target compounds.
GC-FID analyses of the stock solutions (250 μg mL−1) showed to be more sensitive to the presence of harmane than norharmane (for the same concentration of analytes a higher intensity chromatographic peak for harmane was obtained). Further no chromatographic signal for 4,4′-dihydroxybiphenyl was found. From this we could conclude that a derivatization step before GC measurement is required.
For such a derivatization, β-carbolines can be reacted with N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide, but tert-butyldimethylsilyl derivatives are obtained only for norharmane (Casal et al. 2004). In our investigation, we thus decided to use the mixture of 99% BSTFA and 1% TMCS. As shown in Fig. 3, the following chromatographic signals were obtained for the stock solution containing each of the target compounds and IS: at 12.23 min for native IS, at 19.89 min for native harmane, at 19.98 min for native norharmane, at 21.80 min for silylated norharmane and at 22.57 min for silylated 4,4′-dihydroxybiphenyl. The intensity of the GC signal of silylated norharmane increased noticeably in comparison to the native form of this compound recorded for the stock solution of norharmane containing the same amount of this analyte.
Some validation parameters of the determination of harmane, norharmane (based on the silylated norharmane signal) and 4,4′-dihydroxybiphenyl by GC-FID were established (Table 2). As shown, the linearity, sensitivity, precision and accuracy of the technique and the LODinst and LOQinst indicated that this method could be successful in the quantitative analysis of these compounds in crude culture media extracts, as well as other matrices. Determination of the RRFs for harmane, norharmane and 4,4′-dihydroxybiphenyl was crucial before the quantitative analyses of these compounds could be performed in the extracts based on 4,4′-dipyridyl as internal standard.
Table 2.
Validation parameters of the GC-FID method of determining harmane, norharmane and 4,4′-dihydroxybiphenyl based on silylation
| Compounds | Linearity range (μg mL−1) | Reggresion equation | Correlation coefficient | Accuracy (%) | Intra-day precision RSD (%) | LOQinst (ng) | LODinst (ng) |
|---|---|---|---|---|---|---|---|
| Harmane | 5–80 | 125427c–281899 | 0.992 | 86.7–112.4 | <3.76 | 6 | 2 |
| Norharmane | 5–125 | 194739c–657395 | 0.997 | 89.15–109.12 | <2.49 | 6 | 2 |
| 4,4′-Dihydroxybiphenyl | 0.25–125 | 267004c–16993 | 0.996 | 86.84–113.97 | <2.27 | 0.5 | 0.17 |
Estimation of the concentrations of the target compounds in the culture medium of the cyanobacterium Geitlerinema strain So-11 has required data about the recoveries of these analytes from the culture medium during their extraction. Reports so far indicate that yields of norharmane isolated from culture media of N. harveyana have been very low (Volk and Furkert 2006), but no data about recoveries of harmane and 4,4′-dihydroxybiphenyl are available.
We obtained, when using Volk's extraction procedure, GC-FID and GC-MS chromatograms without chromatographic signals of any of the target compounds, indicating very poor analyte recoveries from the culture medium. In order to improve these recoveries the lyophilization step was omitted from Volk's procedure (the high volatility of e.g. β-carbolines allows to analyse them directly by GC). This was not sufficient, as GC-FID and GC-MS measurements still did not indicate the presence of IS and norharmane in the extract. Only harmane and a larger amount of 4,4′-dihydroxybiphenyl was found. These results showed clearly that the lyophilization step in the extraction procedure is not the main reason for the poor recoveries of the target compounds from the culture media.
Isolation of exometabolites from culture media in Volk's procedure is based on the interaction between analytes and the polymeric resin Amberlite XAD-1180. These interactions depend strongly on the available surface area, polarity, contact time, pH and the hydrophobicity of the adsorbent and adsorbate (Abdullah et al. 2008) and govern a subsequent elution. Up to this point, the eluent was methanol. In the next step, however, the less hydrophilic compound isopropanol was used as eluent, again without lyophilization. Now the gas chromatographic signals were well separated from the baseline peaks. The GC-FID analysis of the So-11(c) extract is illustrated in Fig. 5.
The presence of harmane and 4,4′-dihydroxybiphenyl in the extract was confirmed by co-injection with standards in the GC-FID measurement and by analysis of the GC-MS spectra. This is the first report of the indole alkaloid harmane being detected in a cyanobacterial culture medium. In the crude extract So-11(b) only 4,4′-dihydroxybiphenyl was found. This result explains the negative response to the qualitative alkaloid test obtained for this sample (Table 1). Here methanol was used during purification.
However, the recoveries of the analytes from the culture medium were still relatively small: 6.39% for harmane and norharmane, and 3.58% for 4,4′-dihydroxybiphenyl. This suggests to test a series of eluents with increasing hydrophobicity or, if this is not successful in the aqueous test system, to test adsorbents with weaker binding strength for the analytes.
We could demonstrate that more sensitive analytical methods are necessary for a successful small-scale screening of culture media from marine cyanobacteria. Modification of the extraction procedure and the application of GC technique allow reducing both time (cultivation of the bacteria from 150 to 21 days; shorter time of the extraction procedure) and volume of the cyanobacterial culture media (from 250 L to 300 mL).
Acknowledgements
The authors express their gratitude for the financial support provided by the Polish Ministry of Research and Higher Education under grant DS/8200-4-0085-1 and the German Academic Exchange Service (DAAD).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Nelson H. Caicedo, uacocjunior@googlemail.com
Jolanta Kumirska, Phone: +48-58-5235470, FAX: +48-58-5235454, Email: kumirska@chem.univ.gda.pl.
Jennifer Neumann, Email: j.neumann@uni-bremen.de.
Stefan Stolte, Email: stefan.stolte@uni-bremen.de.
Jorg Thöming, Email: thoeming@uni-bremen.de.
References
- Aassila H, Bourguet-Kondracki ML, Rifa S, Fassouane A, Guyot M. Identification of harman as the antibiotic compound produced by a tunicate-associated bacterium. Mar Biotechnol. 2003;5:163–166. doi: 10.1007/s10126-002-0060-7. [DOI] [PubMed] [Google Scholar]
- Abdullah M, Chiang L, Nadeem M. Comparative evaluation of adsorption kinetics and isotherms of a natural product removal by Amberlite polymeric adsorbents. Chem Eng J. 2008;146:370–376. doi: 10.1016/j.cej.2008.06.018. [DOI] [Google Scholar]
- Andrianasolo EH, Gross H, Goeger D, Musafija-Girt M, McPhail K, Leal RM, Mooberry SL, Serwick WH. Isolation of swinholide A and related glycosylated derivatives from two field collections of marine cyanobacteria. Org Letter. 2005;7:1375–1378. doi: 10.1021/ol050188x. [DOI] [PubMed] [Google Scholar]
- Andrianasolo EH, Goeger D, Gerwick WH. Mitsoamide: a cytotoxic linear lipopeptide from the Madagascar marine cyanobacterium Geitlerinema sp. Pure Appl Chem. 2007;79(4):593–602. doi: 10.1351/pac200779040593. [DOI] [Google Scholar]
- Armstrong E, Boyd KG, Pisacane A, Peppiatt CJ, Burgess JG. Marine microbial natural products in antifouling coating. Biofouling. 2000;16:221–232. doi: 10.1080/08927010009378446. [DOI] [Google Scholar]
- Caicedo NH, Heyduck-Söller B, Fischer U, Thöming J. Bioproduction of antimicrobial-compounds by using marine-filamentous cyanobacterium cultivation. J Appl Phycol. 2011;23:811–818. doi: 10.1007/s10811-010-9580-0. [DOI] [Google Scholar]
- Casal S, Mendes E, Fernandes JO, Oliveira MBPP, Ferreira MA. Analysis of heterocyclic aromatic amines in foods by gas chromatography–mass spectrometry as their tert-butyldimethylsilyl derivatives. J Chromatogr A. 2004;1040:105–114. doi: 10.1016/j.chroma.2004.03.054. [DOI] [PubMed] [Google Scholar]
- Dahms HU, Ying X, Pfeiffer C. Antifouling potential of cyanobacteria: a mini-review. Biofouling. 2006;22:317–327. doi: 10.1080/08927010600967261. [DOI] [PubMed] [Google Scholar]
- Debbab A, Aly AH, Lin WH, Proksch P. Bioactive compounds from marine bacteria and fungi. Microbial Biotechnol. 2010;3:544–563. doi: 10.1111/j.1751-7915.2010.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gademann K. Cyanobacterial natural products for the inhibition of biofilm formation and biofouling. Chimia. 2007;61:373–377. doi: 10.2533/chimia.2007.373. [DOI] [Google Scholar]
- Kelecom A. Secondary metabolites from marine microorganisms. Annals Brazilian Acad Sci. 2002;74:151–170. doi: 10.1590/S0001-37652002000100012. [DOI] [PubMed] [Google Scholar]
- Kodani S, Imoto A, Mitsutani A, Murakami M. Isolation and identification of the antialgal compound, harmane (1-methyl-β-carboline), produced by the algicidal bacterium, Pseudomonas sp. K44-1. J Appl Phycol. 2002;14:109–114. doi: 10.1023/A:1019533414018. [DOI] [Google Scholar]
- Kreitlow S, Mundt S, Lindequist U. Cyanobacteria—a potential source of new biologically active substances. J Biotech. 1999;70:61–63. doi: 10.1016/S0168-1656(99)00058-9. [DOI] [PubMed] [Google Scholar]
- Le Gal Y, Muller-Feuga A. Marine microorganisms for industry. Versailles: Ifremer; 1998. [Google Scholar]
- Liu X, Xu F, Shao C, She Z, Linand Y, Chan WL. Bioactive metabolites from marine microorganisms. Studies Nat Prod Chem. 2008;35:197–310. doi: 10.1016/S1572-5995(08)80007-5. [DOI] [Google Scholar]
- Okunade AL, Elvin-Lewis MPF, Lewis WH. Natural antimycobacterial metabolites: current status. Phytochem. 2007;65:1017–1032. doi: 10.1016/j.phytochem.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Patterson GML, Larsen LK, Moore RE. Bioactive natural products from blue-green algae. J Appl Phycol. 1994;6:151–157. doi: 10.1007/BF02186069. [DOI] [Google Scholar]
- Reza VRM, Abbas H. Cytotoxicity and antimicrobial activity of harman alkaloids. J Pharmacol Toxicol. 2007;2:677–680. doi: 10.3923/jpt.2007.677.680. [DOI] [Google Scholar]
- Rippka R, Deuelles J, Waterbury JB, Herdman M, Stainer RY. Generic assignment strain histories and properties of pure cultures of cynobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- Scholz B, Liebezeit G. Chemical screening for bioactive substances in culture media of microalgae and cyanobacteria from marine and brackish water habitats. Pharm Biol. 2006;44:544–549. doi: 10.1080/13880200600883114. [DOI] [Google Scholar]
- Tan LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochem. 2007;68:954–979. doi: 10.1016/j.phytochem.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Tan LT, Goh BP, Tripathi A, Lim MG, Dickinson GH, Lee SS, Teo SL. Natural antifoulants from the marine cyanobacterium Lyngbya majuscula. Biofouling. 2010;26:685–695. doi: 10.1080/08927014.2010.508343. [DOI] [PubMed] [Google Scholar]
- Teichert A, Lübken T, Schmidt J, Kuhnt C, Huth M, Porzel A, Wessjohann L, Arnold N. Determination of β-carboline alkaloids in fruiting bodies of Hygrophorus spp. by liquid chromatography/electrospray ionisation tandem mass spectrometry. Phytochem Anal. 2008;19:335–341. doi: 10.1002/pca.1057. [DOI] [PubMed] [Google Scholar]
- Thiel V, Imhoff JF. Phylogenetic identification of bacteria with antimicrobial activities isolated from different Mediterranean sponges. J Biomolec Engin. 2003;20:421–423. doi: 10.1016/S1389-0344(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Totsuka Y, Ushiyama H, Ishihara J, Sinha R, Goto S, Sugimura T, Wakabayashi K. Quantification of the co-mutagenic beta-carbolines, norharman and harman, in cigarette smoke condensates and cooked foods. Cancer Lett. 1999;143:139–143. doi: 10.1016/S0304-3835(99)00143-3. [DOI] [PubMed] [Google Scholar]
- Totsuka Y, Takamura-Enya T, Nishigaki R, Sugimura T, Wakabayashi K. Mutagens formed from beta-carbolines with aromatic amines. J Chromatogr B. 2004;802:135–141. doi: 10.1016/j.jchromb.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Volk RB. Studies on culture age versus exometabolite production in batch cultures of the cyanobacterium Nostoc insulare. J Appl Phycol. 2007;19:491–495. doi: 10.1007/s10811-007-9161-z. [DOI] [Google Scholar]
- Volk RB (2005) Screening of microbial culture media for the presence of algicidal compounds and isolation and identification of two bioactive metabolites, excreted by the cyanobacteria Nostoc insulare and Nodularia harveyana J Appl Phycol 17:339–347
- Volk RB, Furkert F. Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiol Res. 2006;161:180–186. doi: 10.1016/j.micres.2005.08.005. [DOI] [PubMed] [Google Scholar]