Abstract
The aim of this study was to investigate the effects of beta-alanine supplementation on exercise capacity and the muscle carnosine content in elderly subjects. Eighteen healthy elderly subjects (60–80 years, 10 female and 4 male) were randomly assigned to receive either beta-alanine (BA, n = 12) or placebo (PL, n = 6) for 12 weeks. The BA group received 3.2 g of beta-alanine per day (2 × 800 mg sustained-release Carnosyn™ tablets, given 2 times per day). The PL group received 2 × (2 × 800 mg) of a matched placebo. At baseline (PRE) and after 12 weeks (POST-12) of supplementation, assessments were made of the muscle carnosine content, anaerobic exercise capacity, muscle function, quality of life, physical activity and food intake. A significant increase in the muscle carnosine content of the gastrocnemius muscle was shown in the BA group (+85.4%) when compared with the PL group (+7.2%) (p = 0.004; ES: 1.21). The time-to-exhaustion in the constant-load submaximal test (i.e., TLIM) was significantly improved (p = 0.05; ES: 1.71) in the BA group (+36.5%) versus the PL group (+8.6%). Similarly, time-to-exhaustion in the incremental test was also significantly increased (p = 0.04; ES 1.03) following beta-alanine supplementation (+12.2%) when compared with placebo (+0.1%). Significant positive correlations were also shown between the relative change in the muscle carnosine content and the relative change in the time-to-exhaustion in the TLIM test (r = 0.62; p = 0.01) and in the incremental test (r = 0.48; p = 0.02). In summary, the current data indicate for the first time, that beta-alanine supplementation is effective in increasing the muscle carnosine content in healthy elderly subjects, with subsequent improvement in their exercise capacity.
Keywords: Acidosis, Buffering capacity, Ergogenic aid, Elderly people
Introduction
Ageing is associated with both a loss of skeletal muscle mass and skeletal muscle function leading to varying degrees of sarcopenia as defined by the European Working Group on Sarcopenia in Older People (EWGSOP) (Cruz-Jentoft et al. 2010). Changes include a decrease in the cross sectional area of type II muscle fibres (Verdijk et al. 2010) as well as a loss of both type I and II muscle fibres (Doherty 2003), and a replacement of fibres with fat. Ageing is also associated with a significant reduction in skeletal muscle carnosine (Stuerenburg and Kunze 1999; Tallon et al. 2007) resulting in a decline in the buffering capacity of the muscle. As a result elderly subjects may experience a decrease in their capacity to undertake anaerobic activity where this is limited by intramuscular cell pH decrease (Stout et al. 2008). Combined with the loss in muscle mass, progressive changes in muscle function will contribute to an increased sense of frailty in elderly men and women with impairments in balance, gait speed, and an increased risk of falls (Madureira et al. 2010). With the increase in longevity of industrialized populations, sarcopenia is emerging as a major health and financial concern at the national level. Regular exercise, incorporating some form of resistance training, is considered one of the most effective measures to slow, and even reverse the progression of sarcopenia (Snijders et al. 2009; Verdijk et al. 2009a, b).
Carnosine is a dipeptide synthesized in muscle and other tissues involved in intracellular buffering and is composed of the two amino acids histidine and beta-alanine (Artioli et al. 2010). The availability of the is limiting to the in vivo synthesis of carnosine under normal physiological conditions (Harris et al. 2006). It has been previously shown that supplementation with beta-alanine has the potential to increase muscle carnosine content by 60–80% in healthy young adults, and that this is accompanied by an improvement in the ability to perform high-intensity exercise (Harris et al. 2006; Hill et al. 2007; Kendrick et al. 2008, 2009).
Improving the buffering capacity of muscle could be important for muscle function and daily-life activities in the elderly. A previous study (Stout et al. 2008) demonstrated a 28.6% increase in the physical working capacity at the neuromuscular fatigue threshold after 90 days of beta-alanine supplementation (3 × 800 mg/day) in men and women aged 55–92 years. The authors suggested that the increase in working capacity could have importance in the prevention of falls, and the maintenance of health and independent living of elderly men and women. However, the authors did not assess muscle carnosine content. In fact, to the best of our knowledge, no study has directly investigated the effect of beta-alanine supplementation on the muscle carnosine content in elderly individuals. It remains to be seen if (a) muscle increases in carnosine can be achieved to a similar extent as has been reported in younger subjects, and (b) if any such increases can be correlated with changes in exercise capacity.
Therefore, the aim of this study was to investigate the effects of beta-alanine supplementation on exercise performance capacity and the muscle carnosine content in elderly subjects. It was hypothesized that beta-alanine supplementation would increase the muscle carnosine content in the elderly, which would be paralleled by an increase in exercise capacity.
Materials and methods
Subjects
Eighteen subjects (60–80 years) who had not engaged in any exercise programme for at least 1 year were recruited to the study. Exclusion criteria were assessed by a physician and were as follows: joint disease or other causes of limited mobility that would prevent the subject undertaking the exercise tests, cardiovascular diseases which had not been treated, the use of nutritional supplements within the past 6 months (e.g., protein, amino acids and creatine), and the use of any drug that could induce myopathy (e.g., simvastatin, glucocorticoid). Volunteers were instructed to refrain from any exercise-training programme during the course of the study.
The Local Ethical Committee approved the study and all subjects gave their consent in writing after the purpose of the study and the risks involved had been explained.
Experimental protocol
A double-blind, placebo-controlled study was conducted between October 2010 and May 2011 in Sao Paulo (Brazil).
Each participant was randomly assigned to either the beta-alanine (BA, n = 12) or placebo (PL, n = 6) group using a computer-generated randomization code. An unbalanced design was adopted a priori to reduce the cost of the trial and to gain more experience in using beta-alanine supplementation in elderly subjects. The BA group received 3.2 g of beta-alanine per day (2 × 800 mg sustained-release Carnosyn™ tablets, given 2 times per day after lunch and dinner). The PL group received 2 × (2 × 800 mg) placebo made up of maltodextrin, which was identical in appearance. The supplements were obtained from Natural Alternatives International, San Marcos, USA. Carnosyn™ and placebo tablets were from the same batch as used by Sale et al. (2011) and were tested by HFL Sports Science (Newmarket, UK) before use to ensure no contamination with steroids or stimulants according to ISO 17025-accredited tests. A researcher called the subjects on daily basis to verify the compliance to supplementation intake.
Muscle carnosine, anaerobic exercise capacity, muscle function and quality of life were assessed at baseline (PRE) and after 12 weeks (POST-12) of supplementation. All of the subjects underwent one familiarization session prior to the muscle function tests. Food intake was assessed at baseline and after the intervention and physical activity levels were assessed only at baseline. The closer monitoring of physical activity patterns and diet was not possible and it is a limitation of this study.
All the subjects were physically inactive (as assessed by the international physical activity questionnaire—IPAQ) (Voorrips et al. 1991) and well nourished (as assessed by the Mini Nutritional Assessment (MNA) (Guigoz et al. 1994) at baseline and after the intervention. To characterize the sample, measurements of body composition were also assessed at PRE and POST-12 by Dual-energy X-ray absorptiometry (DXA, Hologic QDR 4500, Discovery model Bedford, MA, USA). Subjects’ demographic characteristics are given in Table 1.
Table 1.
Subjects’ demographic characteristics at baseline
| Beta-alanine (n = 12) | Placebo (n = 6) | |
|---|---|---|
| Gender (M/F) | 6/6 | 2/4 |
| Age (years) | 65 ± 4 | 64 ± 7 |
| Height (cm) | 162 ± 11 | 162 ± 5 |
| Body mass (kg) | 78.2 ± 11.8 | 74.7 ± 12.4 |
| BMI (kg/cm2) | 29.6 ± 2.7 | 28.4 ± 4.3 |
| Body fat (kg) | 25.9 ± 4.1 | 26.1 ± 6.8 |
| Body fat (%) | 33.4 ± 5.2 | 34.8 ± 8.2 |
| LBM (kg) | 50.1 ± 9.7 | 47.1 ± 10.1 |
| BMD (g/cm2) | 1.1 ± 0.1 | 1.0 ± 0.1 |
| BMC (kg) | 2.1 ± 0.4 | 2.0 ± 0.2 |
Data are expressed as mean ± SD. There were no significant differences between groups at baseline (p > 0.05)
M males, F females, BMI body mass index, LBM lean body mass, BMD body mineral density, BMC body mineral content
Muscle carnosine content
Muscle carnosine content was assessed in vivo by 1H-MRS using a whole body 3.0-T MRI scanner (Achieva Intera, Philips Best, the Netherlands) and an eight channel knee coil. In brief, the calf muscle of the left leg was centred within the knee coil fixed with pads to avoid leg motion during acquisition. Conventional anatomical T1-weighted magnetic resonance images were obtained in three orthogonal planes to select the voxel position in the gastrocnemius muscle for MRS measurements. Voxel size was 40 mm (I–S) × 30 mm (A–P) × 12 mm (L–R). Single-voxel point-resolved spectroscopy sequence (PRESS) with the following parameters was used: repetition time (TR) = 2,000 ms; echo time (TE) = 31 ms; number of repetitions = 256; 2,048 data points; spectral bandwidth of 2,000 Hz. Total acquisition time of the spectrum was 9 min. Spectra raw data were analysed with Java Magnetic Resonance User Interface software (Naressi et al. 2001). For quantification purposes, only the carnosine H2 peak at 8.0 ppm was taken into account. Carnosine values were normalized by the internal water content in the voxel, measured from the water unsuppressed reference acquisitions. Water and carnosine signals were quantified using a Hankel–Lanczos singular value decomposition (HLSVD) algorithm (Pijnappel et al. 1992). No corrections for the effect of relaxation times were applied. The coefficient of variation (CV) for muscle carnosine content was 2.5%. Carnosine data were expressed relatively to the water signal. The lack of a carnosine phantom (i.e., external reference) in the current study precluded us to quantify absolute carnosine concentrations.
Physical capacity tests
The participants were required to visit the laboratory on two occasions. At the first visit, subjects performed an incremental test on a motorized treadmill (Centurion 200, Micromed, Brazil). The starting speed was set at 1.5 mph with increments of 0.5 mph every min up to 3.5 mph; then slope increments (2% increase every min) were made until exhaustion. The ventilatory anaerobic threshold (VAT) and VO2 peak were determined according to previously described criteria (Howley et al. 1995). In the next session, the subjects performed a constant-load protocol on the treadmill which consisted of a single repetition square-wave transition from rest to an exercise intensity corresponding to 75% of the difference between VAT and VO2 peak. This intensity was maintained to the limit of tolerance (TLIM). In both tests, the time-to-exhaustion was assessed as a measure of exercise tolerance. The subjects received strong verbal encouragement to continue as long as possible.
Muscle function tests
Assessment of muscle function was through “timed-stands” and “timed-up-and-go” tests. The “timed-stands” test (Newcomer et al. 1993) evaluates the maximum number of stand-ups that a subject can perform from a standard height (i.e., 45 cm) armless chair within 30 s and the “timed-up-and-go” test evaluates the time that subjects required to rise from a standard arm chair, walk to a line on the floor 3 m away, turn, return, and sit down again, which has been previously validated to measure improvements in daily-life activities (Podsiadlo and Richardson 1991).
Quality of life assessment
The Brazilian version of the Short-Form Health Survey (SF-36) was used to assess quality of life (Ciconelli et al. 1999). The SF-36 consists of eight subscales: physical functioning, role limitations due to physical health problems (so-called physical role function), bodily pain, general health perceptions, vitality, social role functioning, role limitations due to emotional health problems (so-called emotional role functioning) and mental health. Items were answered according to standardized response choices. Raw scores are transformed to scale scores ranging from 0 to 100, with higher scores indicating better levels of functioning.
Food intake assessment
Food intake was assessed at baseline and after 12 weeks of supplementation by means of three 24 h dietary recalls undertaken on separate days (2 weekdays and 1 weekend day) using a visual aid photo album of real foods. The 24 h dietary recall consists of the listing of foods and beverages consumed during 24 h prior to the recall. Energy and macronutrient intakes were analysed by the Brazilian software DietPro 5.1 (Minas Gerais, Brazil).
Blood and urinary parameters
Blood and urine samples for clinical biochemistry (i.e., liver, muscle, kidney function tests) and haematology were taken at PRE and POST-12 from all subjects.
Statistical analysis
Intention-to-treat analysis was used for each comparison, irrespective of the compliance with the intervention. Shapiro–Wilk test revealed normal distribution of the data. Unpaired t tests were used to assess relative changes between groups for muscle carnosine and physical capacity parameters. Quality of life data were tested by Wilcoxon test. The remaining variables were tested by a Mixed Model procedure. Pearson correlations were performed between relative changes in muscle carnosine content and performance parameters. Effect sizes (ES) for muscle carnosine and physical capacity parameters were estimated for the posttest assessments using the pooled standard deviation of the two independent samples at POST-12 to determine the practical significance of the present findings. The significance level was previously set at p < 0.05. Data are expressed as mean ± SD.
Results
According to the subjects’ self-reported compliance on tablet intake, full adherence to the supplementation protocol was achieved. Three patients (two male and one female from the BA group) presented unreliable MRS scans at either PRE or POST-12 and were excluded from all analyses involving muscle carnosine determination, and all correlations. After exclusion of these subjects, baseline values for all measurements remained non-significantly different between the groups. Body mass and body composition (data not shown), and food intake (Table 2) did not significantly differ within (between Pre and Post) or between groups.
Table 2.
Food intake at baseline and after 12 weeks following either beta-alanine or placebo supplementation
| Beta-alanine (n = 12) | Placebo (n = 6) | |||
|---|---|---|---|---|
| PRE | POST-12 | PRE | POST-12 | |
| Energy (kcal/d) | 2263 ± 332 | 2068 ± 513 | 2184 ± 409 | 2083 ± 352 |
| Carbohydrate | ||||
| % of energy | 45 ± 7 | 44 ± 5 | 54 ± 5 | 46 ± 1 |
| g/day | 257 ± 55 | 228 ± 51 | 298 ± 23 | 238 ± 35 |
| Fat | ||||
| % of energy | 38 ± 5 | 36 ± 4 | 31 ± 5 | 36 ± 1 |
| g/day | 96 ± 17 | 83 ± 20 | 76 ± 26 | 84 ± 15 |
| Protein | ||||
| % of energy | 16 ± 3 | 20 ± 3 | 14 ± 1 | 18 ± 1 |
| g/day | 93 ± 25 | 103 ± 44 | 77 ± 20 | 94 ± 19 |
Data are expressed as mean ± SD. There were no significant differences within or between groups
PRE baseline, POST-12 after 12 weeks of beta-alanine or placebo supplementation
Muscle carnosine content
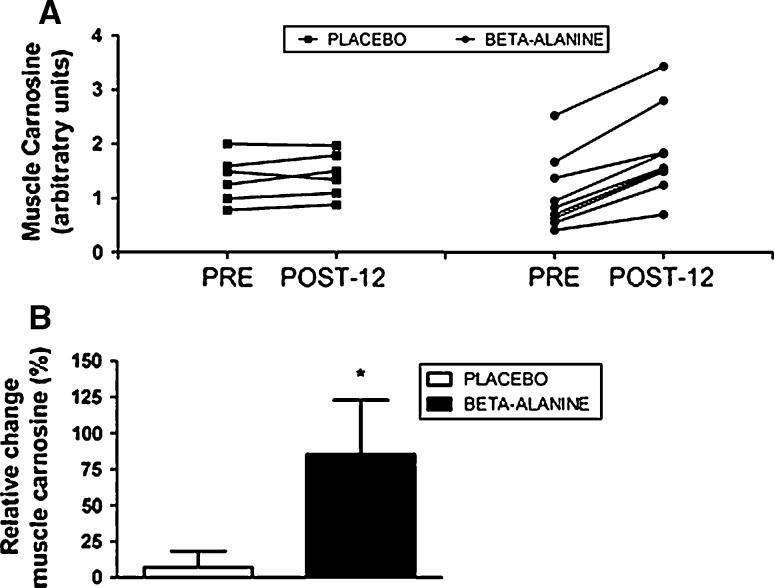
A significant increase in muscle carnosine content was shown in the BA group (+85.4%) when compared with the PL group (+7.2%) (p = 0.004; ES: 1.21) (Fig. 1a, b).
Fig. 1.
a Individual data for muscle carnosine content (arbitrary units) at baseline (PRE) and after 12 weeks of beta-alanine supplementation (POST-12). b Relative change (%) in muscle carnosine content (from PRE to POST-12). Asterisk indicates p < 0.05 when compared with the placebo group
Physical capacity
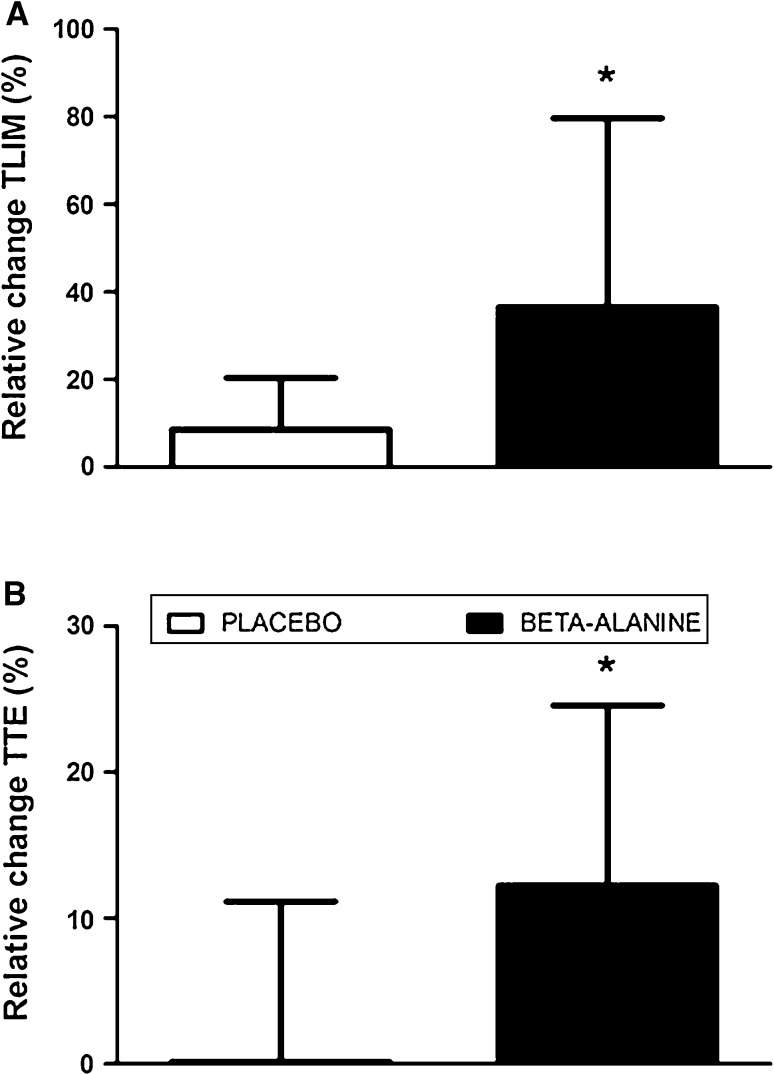
The time-to-exhaustion in the TLIM test was significantly improved (p = 0.05; ES: 1.71) in the BA group (PRE: 5.2 ± 1.9, POST-12: 6.6 ± 1.7 min; 36.5%) versus the PL group (PRE: 4.4 ± 1.1; POST-12: 4.7 ± 1.2 min; 8.6%) (Fig. 2a). Similarly, the time-to-exhaustion in the incremental test was significantly increased (p = 0.04; ES: 1.03) following beta-alanine supplementation (PRE: 11.0 ± 2.1; POST-12: 12.1 ± 2.1 min; 12.2%) when compared with placebo (PRE: 12.6 ± 1.7; POST-12: 12.5 ± 1.5 min; 0.1%) (Fig. 2b).
Fig. 2.
a Relative change (%) in total time of exercise during the TLIM test (TLIM) from baseline to 12 weeks of beta-alanine supplementation. b Relative change (%) in time to exhaustion during the incremental test (TTE). Asterisk indicates p < 0.05 when compared with the placebo group
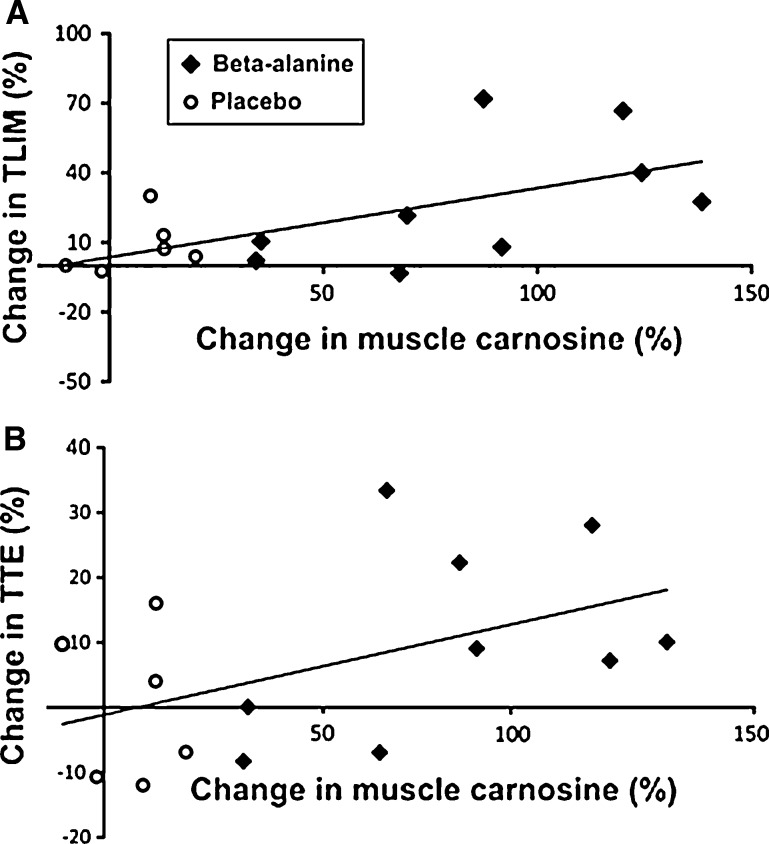
A significant and positive correlation was observed between the relative change in the muscle carnosine content and the relative change in the time-to-exhaustion in the TLIM test (r = 0.62; p = 0.01) and in the incremental test (r = 0.48; p = 0.02). These correlations are illustrated in Fig. 3.
Fig. 3.
a Correlation between the relative change (%) in the time-to-exhaustion in the TLIM test and the relative change (%) in the muscle carnosine content. b Correlation between the relative change (%) in the time-to-exhaustion in the incremental test and the relative change (%) in the muscle carnosine content
Muscle function
No significant within- or between-group changes were observed in the timed-stands test after beta-alanine supplementation (PRE: 16 ± 2; POST-12: 17 ± 2 repetitions) when compared with the PL group (PRE: 15 ± 3; POST-12: 16 ± 3 repetitions). Similarly, no significant changes were observed in the timed-up-and-go test after beta-alanine supplementation (PRE: 6.3 ± 0.8; POST-12: 6.2 ± 1.1 s) when compared with PL group (PRE: 6.3 ± 1.2; POST-12: 6.2 ± 1.1 s).
Quality of life
No significant changes were observed between groups for quality of life parameters (Table 3).
Table 3.
Short Form Health Survey (SF36) data at baseline and after 12 weeks following either beta-alanine or placebo supplementation
| Domain | Beta-alanine (n = 12) | Placebo (n = 6) | ||
|---|---|---|---|---|
| PRE | POST-12 | PRE | POST-12 | |
| Physical functioning | 74 ± 9 | 70 ± 13 | 61 ± 16 | 74 ± 7 |
| Physical role functioning | 16 ± 8 | 18 ± 6 | 11 ± 9 | 16 ± 5 |
| Bodily pain | 63 ± 18 | 71 ± 20 | 59 ± 13 | 61 ± 20 |
| General health perceptions | 77 ± 14 | 82 ± 11 | 70 ± 13 | 72 ± 12 |
| Vitality | 73 ± 11 | 78 ± 12 | 59 ± 18 | 63 ± 17 |
| Social role functioning | 89 ± 16 | 91 ± 15 | 69 ± 19 | 73 ± 23 |
| Emotional role functioning | 21 ± 7 | 22 ± 2 | 15 ± 7 | 14 ± 11 |
| Mental health | 79 ± 15 | 83 ± 9 | 75 ± 14 | 81 ± 16 |
Data are expressed as mean ± SD. There were no significant differences within or between groups
PRE baseline, POST-12 after 12 weeks of beta-alanine or placebo supplementation
Blood and urinary parameters and side effects
Laboratory parameters were unchanged after the intervention (Table 4). Additionally, there were no self-reported side effects throughout the course of the study.
Table 4.
Biochemical parameters and haematology at baseline and after 12 weeks following either beta-alanine or placebo supplementation
| Beta-alanine (n = 12) | Placebo (n = 6) | |||
|---|---|---|---|---|
| PRE | POST-12 | PRE | POST-12 | |
| Aldolase (U/L) | 3.4 ± 0.9 | 3.0 ± 1.2 | 4.0 ± 0.3 | 4.1 ± 0.7 |
| CK (U/L) | 141.7 ± 82.6 | 123.9 ± 65.2 | 117.0 ± 87.7 | 148. ± 107.0 |
| ALT (U/L) | 28.2 ± 15.0 | 26.7 ± 7.5 | 23.2 ± 6.6 | 23.0 ± 10.9 |
| AST (U/L) | 24.3 ± 7.0 | 22.6 ± 4.7 | 23.3 ± 2.1 | 21.8 ± 4.0 |
| GGT (U/L) | 29.00 ± 8.71 | 28.09 ± 7.54 | 17.83 ± 5.91 | 15.50 ± 5.82 |
| Total bilirubin (mg/dL) | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.2 |
| Glycemia (mg/dL) | 109.7 ± 15.7 | 114.4 ± 17.9 | 89.0 ± 9.0 | 95.0 ± 8.4 |
| LDH (U/L) | 334.4 ± 34.4 | 323.2 ± 28.9 | 385.0 ± 35.7 | 401.7 ± 52.3 |
| Urea (mg/dL) | 37.58 ± 8.13 | 38.45 ± 7.09 | 35.67 ± 6.77 | 36.67 ± 9.46 |
| Erythrocytes (1 million/mm3) | 4.94 ± 0.34 | 4.94 ± 0.31 | 4.82 ± 0.45 | 4.80 ± 0.53 |
| Haemoglobin (g/dL) | 15.05 ± 1.31 | 14.85 ± 1.09 | 15.05 ± 0.92 | 14.72 ± 1.09 |
| Haematocrit (%) | 44.33 ± 3.20 | 44.63 ± 2.50 | 44.30 ± 3.12 | 44.37 ± 3.17 |
| Leukocytes (1,000/mm3) | 7.4 ± 1.6 | 7.5 ± 2.7 | 6.1 ± 1.0 | 6.6 ± 1.5 |
| Neutrophils (1,000/mm3) | 3.9 ± 1.2 | 3.7 ± 1.5 | 3.0 ± 0.6 | 3.7 ± 1.5 |
| Eosinophils (1,000/mm3) | 0.2 ± 0.1 | 0.5 ± 1.0 | 0.3 ± 0.2 | 0.2 ± 0.2 |
| Basophils (1,000/mm3) | 0.01 ± 0.03 | 0.02 ± 0.04 | 0.02 ± 0.04 | 0.02 ± 0.04 |
| Lymphocytes (1,000/mm3) | 2.6 ± 0.5 | 2.6 ± 0.8 | 2.2 ± 0.6 | 2.1 ± 0.8 |
| Monocytes (1,000/mm3) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| Platelets (1,000/mm3) | 266.5 ± 59.8 | 261.0 ± 61.3 | 244.7 ± 35.8 | 253.0 ± 67.2 |
| Urinary creatinine (g/L) | 1.3 ± 0.6 | 1.2 ± 0.4 | 1.0 ± 0.8 | 0.9 ± 0.3 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.83 ± 0.2 |
| Microalbuminuria (mg/L) | 6.3 ± 3.2 | 6.7 ± 3.4 | 6.8 ± 6.5 | 4.5 ± 3.6 |
| Proteinuria (g/L) | 0.07 ± 0.05 | 0.08 ± 0.04 | 0.07 ± 0.05 | 0.07 ± 0.05 |
Data are expressed as mean ± SD. There were no significant differences within or between groups
PRE baseline, POST-12 after 12 weeks of beta-alanine or placebo supplementation, AST aspartate amino transferase, ALT alanine aminotransferase, LDH lactate dehydrogenase, CK creatine kinase, GGT glutamyltransferase
Discussion
The main and novel finding of the present study is that beta-alanine supplementation is able to increase the muscle carnosine concentration in elderly (60–80 yrs) subjects. Importantly, the study also showed compelling evidence indicating that the increase in muscle carnosine was paralleled by an improvement in exercise tolerance with no evidence of any adverse effect. The present data is in accordance with a growing body of evidence obtained in younger subjects suggesting that beta-alanine supplementation results in increased carnosine synthesis in muscle (Harris et al. 2006; Hill et al. 2007). The present data further demonstrate that within the age group measured, ageing does not impair intramuscular beta-alanine uptake or intramuscular carnosine synthesis.
Although one study (Kim 2009) demonstrated a normal muscle carnosine content in older individuals with glucose intolerance, Tallon et al. (2007) reported a 53% reduction in carnosine in type II, but not in type I muscle fibres, in older subjects with osteoarthritis when compared with younger healthy subjects. Similarly Stuerenburg and Kunze (1999) reported a significant age-related reduction in skeletal muscle carnosine. These dissonant findings may be explained, at least in part by dietary differences, as the Korean population studied by Kim (2009) is described as eating a typical Korean diet including chicken, pork and beef meat, as well as fish. As noted by Kim (2009) Korean cooking is typically done “in the pot” or by barbeque with the result that loss of dipeptides is minimal. Changing dietary patterns in the elderly due to loss of appetite will reduce beta-alanine intake from the ingestion of histidine containing dipeptides (carnosine, anserine and balenine). Even where dietary intake of protein may be adequate, dietary levels of beta-alanine may fall. Declining levels of carnosine in muscle may also occur with preferential loss of type II muscle fibres with age, or with a reduction in cross sectional area of type II muscle fibres, since in humans these have up to two times the level of carnosine compared to type I (Harris et al. 1998; Hill et al. 2007). In such circumstances beta-alanine supplementation could be beneficial in maintaining or even elevating muscle carnosine levels with possible improvements in physical exercise capacity and life quality.
In the present study the mean increase of 85.4% in muscle carnosine content after 12 weeks beta-alanine supplementation was almost identical to the increase observed after 10 weeks by Hill et al. (2007), albeit using a dosing level twice that used in this study. Hill et al. (2007) administered beta-alanine in a hard gelatin capsule, which disintegrates rapidly on ingestion and gave rise to a peak in the plasma concentration after 30 to 45 min. In the present study a proprietary sustained-release tablet formulation was used, which for the same dose results in a lower and later peak plasma concentration and reduced urinary loss, but generates the same area-under-the-plasma-concentration curve (Stellingwerff et al. 2011). By slowing the rate of beta-alanine release, less is excreted in urine with a greater percentage retained for carnosine synthesis in muscle.
Importantly in the present study changes in TLIM and TTE were positively correlated to the changes in muscle carnosine. Similar correlations have been shown in younger subjects with improved performances in cycling (Hill et al. 2007) and rowing (Baguet et al. 2010) tracking the increase in muscle carnosine content with beta-alanine supplementation. Although Stout et al. (2008) have earlier shown an improvement in the time to neuromuscular fatigue in the elderly with beta-alanine supplementation, the absence of carnosine measurements did not enable comparison to any increase in the muscle carnosine content. The emerging correlations in the current study, between carnosine increase and exercise performance, are consistent with the role of carnosine as an intramuscular H+ buffer. In the context of the elderly, those still active in such activities as cycling, skating, mountain climbing, even gardening, as examples of strenuous leisure activities involving bouts of intense isometric and dynamic exercise, are likely to gain both immediate benefits from an increase in muscle carnosine, as well as long-term benefits if the current high level of activity can be extended into more senior years. Whether much less active older people are able to derive a similar level of benefit remains to be shown but many simple tasks, for instance stair climbing, which in earlier years might have been accomplished by aerobic exercise, invoke increasing levels of anaerobic effort as muscle mass progressively declines.
In the present study there were no improvements in the “timed-stands” and “timed-up-and-go” tests. Although these have previously been validated to measure improvements in daily-life activities (Podsiadlo and Richardson 1991, Newcomer et al. 1993) this is based on the study of populations far less active than investigated here. The lack of changes in the quality of life may be due to the fact that subjects were healthy and functional at baseline. A future goal, therefore, should be to study the effects of beta-alanine supplementation in patients who are less active and demonstrate a greater degree of sarcopenia and frailty, and poor quality of life.
In any decade, whether it is 60+, 70+ or even 90+, there will be a broad spectrum of activity levels set against a background of declining musculo-skeletal function. Moderating the rate of decline would help maintain active participation in leisure activities and independence. Regular exercise involving some form of resistance training is considered the most effective treatment in slowing the progression of sarcopenia (Snijders et al. 2009; Verdijk et al. 2009a, b). We suggest that beta-alanine supplementation by increasing the capacity for resistance exercise and lessening the fatigue burden associated with this, whether performed as part of a prescriptive training programme, in the course of leisure, or simply as part of normal everyday activities, will encourage prolongation of an active life style in elderly people. This assumption needs to be tested in future studies.
In conclusion the present study indicates that beta-alanine supplementation is effective in increasing the muscle carnosine content in the elderly (60–80 years) with improvements in physical exercise capacity. Benefits in maintaining a high muscle carnosine content may be both immediate and long-term, if this encourages subjects to maintain a more active life. Beta-alanine supplementation possibly represents one of the few evidenced-based dietary interventions which may help delay the decline in muscle function with ageing.
Acknowledgments
The authors would like to thank Natural Alternatives International (NAI) which partially sponsored this study and supplied both beta-alanine (Carnosyn™) and placebo tablets. This study was also partially supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (Grant 2010/11221-0).
Conflict of interest
Dr John Wise is a former employee of NAI and today is a consultant to NAI on scientific affairs. Whilst at the Animal Health Trust, Newmarket, the Royal Veterinary College, London, and the University of Chichester, Chichester, all in the UK, Professor Roger Harris held research grants for beta-alanine research from UK national funding bodies, and from NAI jointly with colleagues at the Korea National Sport University. Today, Professor Harris is a named inventor, along with other colleagues from these early studies, on patents owned by NAI describing methods to increase carnosine levels in muscle using beta-alanine supplements. Professor Harris today acts as an unpaid consultant to NAI. The other authors have no conflict of interests.
References
- Artioli GG, Gualano B, Smith A, Stout J, Lancha AH., Jr Role of beta-alanine supplementation on muscle carnosine and exercise performance. Med Sci Sports Exerc. 2010;42(6):1162–1173. doi: 10.1249/MSS.0b013e3181c74e38. [DOI] [PubMed] [Google Scholar]
- Baguet A, Bourgois J, Vanhee L, Achten E, Derave W. Important role of muscle carnosine in rowing performance. J Appl Physiol. 2010;109(4):1096–1101. doi: 10.1152/japplphysiol.00141.2010. [DOI] [PubMed] [Google Scholar]
- Ciconelli R, Ferraz M, Santos W, Meinão I, Quaresma M. Tradução para a língua portuguesa e validação do questionário genérico de avaliação de qualidade de vida SF-36 (Brasil SF-36) Rev Bras Reumatol. 1999;39:143–150. [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel J-P, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis, report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Guigoz Y, Vellas B, Garry P. Mini nutritional assessment: a practical assessment tool for grading the nutritional state of elderly patients. Facts Res Gerontol. 1994;4(Suppl 2):15–59. [Google Scholar]
- Harris RC, Dunnett M, Greenhaff PL. Carnosine and taurine contents in individual fibres in human vastus lateralis muscle. J Sports Science. 1998;16:639–643. doi: 10.1080/026404198366443. [DOI] [Google Scholar]
- Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids. 2006;30(3):279–289. doi: 10.1007/s00726-006-0299-9. [DOI] [PubMed] [Google Scholar]
- Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA. Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids. 2007;32(2):225–233. doi: 10.1007/s00726-006-0364-4. [DOI] [PubMed] [Google Scholar]
- Howley ET, Bassett DR, Jr, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27(9):1292–1301. [PubMed] [Google Scholar]
- Kendrick IP, Harris RC, Kim HJ, Kim CK, Dang VH, Lam TQ, Bui TT, Smith M, Wise JA. The effects of 10 weeks of resistance training combined with beta-alanine supplementation on whole body strength, force production, muscular endurance and body composition. Amino Acids. 2008;34(4):547–554. doi: 10.1007/s00726-007-0008-3. [DOI] [PubMed] [Google Scholar]
- Kendrick IP, Kim HJ, Harris RC, Kim CK, Dang VH, Lam TQ, Bui TT, Wise JA. The effect of 4 weeks beta-alanine supplementation and isokinetic training on carnosine concentrations in type I and II human skeletal muscle fibres. Eur J Appl Physiol. 2009;106(1):131–138. doi: 10.1007/s00421-009-0998-5. [DOI] [PubMed] [Google Scholar]
- Kim HJ. Comparison of the carnosine and taurine contents of vastus lateralis of elderly Korean males, with impaired glucose tolerance, and young elite Korean swimmers. Amino Acids. 2009;36(2):359–363. doi: 10.1007/s00726-008-0092-z. [DOI] [PubMed] [Google Scholar]
- Madureira MM, Bonfá E, Takayama L, Pereira RM. A 12-month randomized controlled trial of balance training in elderly women with osteoporosis: improvement of quality of life. Maturitas. 2010;66(2):206–211. doi: 10.1016/j.maturitas.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12(2–3):141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- Newcomer KL, Krug HE, Mahowald ML. Validity and reliability of the timed-stands test for patients with rheumatoid arthritis and other chronic diseases. J Rheumatol. 1993;20(1):21–27. [PubMed] [Google Scholar]
- Pijnappel W, Boogaart A, Beer R, Ormondt D. SVD-based quantification of magnetic resonance signals. J Magn Reson. 1992;97:122–134. doi: 10.1016/0022-2364(92)90241-X. [DOI] [Google Scholar]
- Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Sale C, Saunders B, Hudson S, Wise JA, Harris RC, Sunderland CD (2011) Effect of beta-alanine plus sodium bicarbonate on high-intensity cycling capacity. Med Sci Sports Exerc doi:10.1249/MSS.0b013e3182188501 [DOI] [PubMed]
- Snijders T, Verdijk LB, Loon LJ. The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing Res Rev. 2009;8(4):328–338. doi: 10.1016/j.arr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Stellingwerff T, Decombaz J, Harris RC and Boesch C (2011) Optimizing in vivo dosing and delivery of beta-alanine supplements. Amino Acids (This issue) [DOI] [PubMed]
- Stout JR, Graves BS, Smith AE, Hartman MJ, Cramer JT, Beck TW, Harris RC. The effect of beta-alanine supplementation on neuromuscular fatigue in elderly (55–92 years): a double-blind randomized study. J Int Soc Sports Nutr. 2008;5:21. doi: 10.1186/1550-2783-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuerenburg HJ, Kunze K. Concentrations of free carnosine (a putative membrane-protective antioxidant) in human muscle biopsies and rat muscles. Arch Gerontol Geriatr. 1999;29(2):107–113. doi: 10.1016/S0167-4943(99)00020-5. [DOI] [PubMed] [Google Scholar]
- Tallon MJ, Harris RC, Maffulli N, Tarnopolsky MA. Carnosine, taurine and enzyme activities of human skeletal muscle fibres from elderly subjects with osteoarthritis and young moderately active subjects. Biogerontology. 2007;8(2):129–137. doi: 10.1007/s10522-006-9038-6. [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64(3):332–339. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, Loon LJ. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009;89(2):608–616. doi: 10.3945/ajcn.2008.26626. [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Snijders T, Beelen M, Savelberg HH, Meijer K, Kuipers H, Loon LJ. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc. 2010;58(11):2069–2075. doi: 10.1111/j.1532-5415.2010.03150.x. [DOI] [PubMed] [Google Scholar]
- Voorrips LE, Ravelli AC, Dongelmans PC, Deurenberg P, Staveren WA. A physical activity questionnaire for the elderly. Med Sci Sports Exerc. 1991;23(8):974–979. [PubMed] [Google Scholar]