Abstract
Mantle cell lymphoma is a mature B cell neoplasm constituting 5–7% of all non-Hodgkin lymphoma. Overall prognosis with current therapeutics remains poor, thus numerous novel agents are currently under investigation. In this review we focus on early phase trials that have demonstrated promise in mantle cell. Constitutive activation of signaling components downstream of the B cell receptor play an important role in the pathobiology of mantle cell lymphoma. Targeting of this signaling pathway has become a focus with specific agents under development including inhibitors of spleen tyrosine kinase, phosphoinositide-3-kinase and Bruton’s tyrosine kinase. Promsing data also supports further development of BH-3 mimetics, a crucial component of anti-apoptotic signaling. Histone deacetylase inhibitors have an established role in cutaneous T cell lymphoma and are now under investigation in mantle cell lymphoma as well. With further understanding of cellular signaling, the armamentarium of treatment options will be enhanced, with the hope of improving the prognosis of this disease.
Keywords: Mantle Cell Lymphoma, Novel Agents, B cell receptor
Introduction
Mantle Cell Lymphoma (MCL) is a mature B cell neoplasm first described in 1994 that constitutes 5–7% of all non-Hodgkin Lymphoma. This disease is characterized by the overexpression of cyclin D1 as a result of the translocation of t(11:14)(q13;q32). (1) MCL can be viewed as an incurable disease. Historically, survival at the time of diagnosis was estimated to be 3–5 years, however recent data suggest that patients can live greater than seven years.
The standard of care has previously focused on aggressive upfront systemic chemotherapy with the option of autologous transplant, but recent literature has raised the question of an initial conservative approach with observation. Despite improved survival data in the younger population, therapy for the elderly or refractory/relapsed patient remains limited, and the prognosis quite poor. The role of proteasome inhibition and immunomodulatory therapy are well established, here we focus on novel agents recently identified as having a role in the treatment of mantle cell lymphoma.
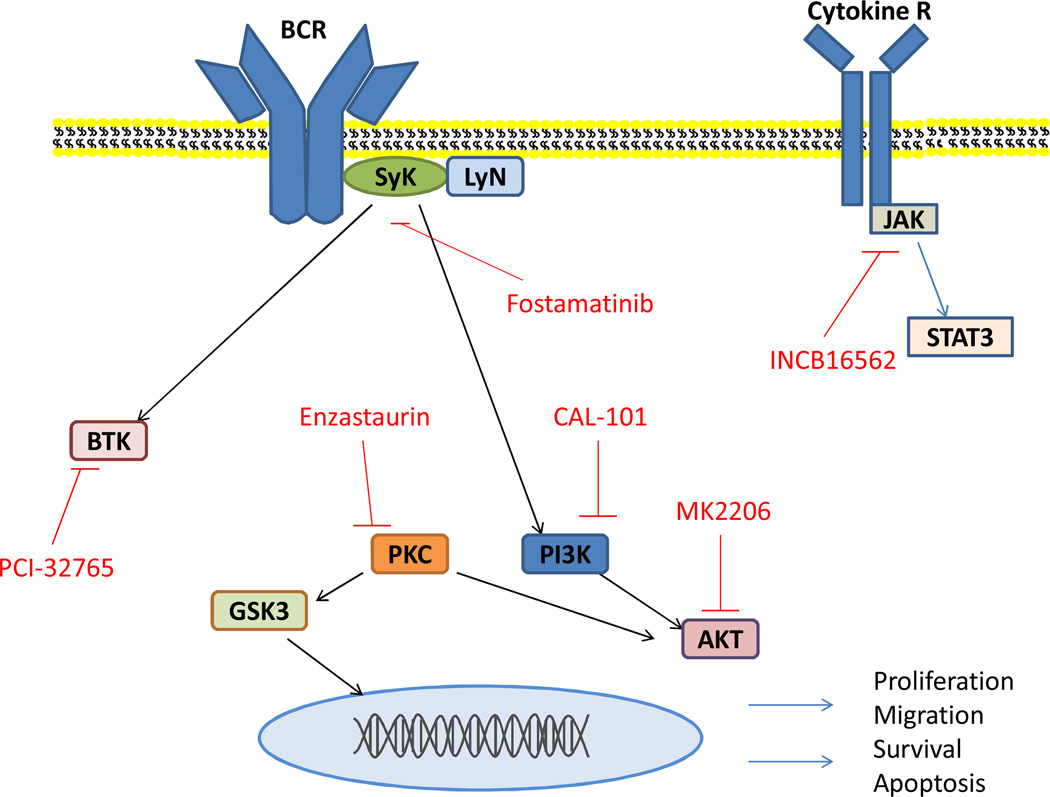
At the crux of newer agents in MCL lie alterations of the B cell receptor (BCR). In normal B cell activation, initial antigen binding activates the SRC-family kinase LYN as depicted in figure 1. Spleen tyrosine kinase (SYK) is then activated via phosphorylation and autophosphorylation. (2) Once activated through association with B cell linker protein (BLNK), SYK facilitates downstream activation of Bruton tyrosine kinase (BTK) and subsequently phospholipase Cγ2 (PLC-γ2), in addition to activating phosphoinositide 3-kinase (PI3K). Downstream from these signaling molecules, second messenger activation generates release of intracellular calcium and activated protein kinase C, ultimately activating various transcription factors including NF-κB. (2) Other key downstream targets include mammalian target of rapamycin (mTOR) and AKT.
Figure 1.
Key signaling pathways in relation to the B-cell receptor. Novel agents labeled in red are depicted at their particular focus of inhibition.
Current research in MCL indicates that BCR signal components are constitutively activated contributing to tumor proliferation and survival. (3) Modulation of this pathway has shown great promise in mantle cell, particularly in the otherwise refractory patient. A number of agents have been developed as logical targets for induction of apoptosis and will be addressed below, also highlighted in tables 1 and 2.
Table 1.
Novel Agents in various stages of clinical development within Mantle Cell Lymphoma.
| TARGET | DRUGS | PHASE OF CLINICAL DEVELOPMENT |
|---|---|---|
| Spleen Tyrosine Kinase | Fostamatinib | Phase II |
| Bruton's tyrosine Kinase | PCI-32765, AVL-292 | Phase II |
| PI3K | CAL-101, SAR245409 | Phase II |
| HDAC | Vorinostat, Randepsin, Panobinostat | Phase II |
| Cyclin D1 | Flavopiridol | Phase II |
| Janus-Associated Kinase | INCB16562 | Phase I |
| Protein Kinase C | Enzastaurin | Phase II |
| PARP | ABT-888, CEP-9722, BMN 673 | Phase I |
| AKT | MK 2206 | Phase II |
| Aurora Kinase | MLN 8237, AT9283 | Phase I |
| Bcl-2 family | GX15-070, ABT-737, ABT 263 | Phase I |
Table 2.
Monoclonal Antibodies in various stages of clinical development within Mantle Cell Lymphoma.
| Antibody Target | Drug | Phase of Clinical Development |
|---|---|---|
| CD 20 | Ofatumumab, Veltizumab | Phase II |
| CD 27 | CDY-1127 | Phase II |
| CD 40 | Dacetuzumab | Phase I |
| CD 74 | Milatuzumab | Phase I |
Spleen Tyrosine Kinase
Syk is a crucial component of the BCR pathway. It was first described in B lymphocytes, but is expressed in many cells including epithelial, fibroblasts, hepatocytes, and vascular smooth muscle cells. (4) Once recruited to the BCR complex, Syk is phosphorylated at which time it can activate several downstream targets including BLNK and PLCγ. Work in knockout mice reveal those that are Syk deficient have impaired lymphocyte development at the pre-pro B cell stage.
Experiments in four established mantle cell lines demonstrated elevated phosphorylated Syk protein levels. These cell lines were treated with a Syk inhibitor, piceatannol, and one cell line was found to have 80% cell growth arrest at 12.5 umol/liter.(5) Of note, cell growth was less impaired in those mantle cell lines that had lower levels of Syk expression.
Fostamatinib disodium (R788) is a prodrug of R406, a potent and selective inhibitor of spleen tyrosine kinase that is available in oral formulation. A phase 1 and 2 clinical trial of fostamatinib in non-Hodgkin lymphoma and chronic lymphocytic leukemia enrolled patients with relapsed or refractory disease over a one year period beginning in February 2007. (6) The phase 1 portion consisted of two cohorts who were sequentially assigned to receive doses of either 200 mg PO twice daily or 250 mg PO twice daily. Thirteen patients in total were enrolled in the cohorts of phase 1, (3 of which were mantle cell lymphoma) with a median age of 74.
In phase 2, patients were assigned to groups based on histology and each received fostamatinib disodium twice daily. A total of 63 patients were enrolled in the phase 2 portion, 9 with mantle cell lymphoma at a dose of 200 mg twice daily. Grade 3/4 neutropenia and anemia were the most common adverse events seen in 18% and 7% of patients respectively. Dose limiting toxicity was thought to be anemia, neutropenia, and thrombocytopenia.
Of the 9 mantle cell patients in the phase 2 portion, 1 achieved a partial response and 4 demonstrated stable disease. The average progression free survival was 3.8 months and the median time to response was 57 days. This initial trial evaluating a Syk inhibitor was the first to demonstrate the promise of BCR signaling inhibition in B cell malignancies including patients with heavily pretreated mantle cell lymphoma.
Bruton’s Tyrosine Kinase
BTK, a member of the Tec family, has an established role in BCR signaling. BTK is activated by kinases Blk, Lyn, and Fyn and then activates PLCγ which subsequently leads to Ca2+ activation of NF-κB and MAP kinase pathways. NF-κB and MAP kinase are both vital to cell survival, thus a logical target of promoting cell death. (7)
Previously recognized to play a role in B lymphocyte development, mutations of BTK in humans leads to an inherited disorder, X-linked agammaglobulinemia. This particular disorder is defined by severe B cell-specific defects that manifest via signifiantly decreased levels of immunoglobulin production and the lack of peripheral B cells. BTK has been indentified as an essential kinase in activated B cell subtype of diffuse large B-cell lymphoma where a BTK inhibitor promoted apoptosis in vitro. (8) Agents designed to inhibit this kinase are currently being tested.
PCI-32765 is a Cys-481 selective and potent orally available BTK inhibitor. PCI-32765 inhibits phosphorylation of Phospholipase-Cγ in addition to downstream phosphorylation of Erk. A study using PCI-32765 in canine lymphoma models was developed to test the inhibition of BCR-signaling in lymphoma. (7) PCI-32765 was dosed orally, and inhibition of BTK was monitored in vivo. In five dogs, a single administration of PCI-32765 was sufficient to fully occupy BTK in both peripheral blood and in malignant tumor for 24 hours. Eight dogs in total were treated, a partial response was observed in three and three others demonstrated stable disease.
In humans subjects, Phase I trials evaluating PCI-32765 are underway. Fowler et al conducted a phase I study of PCI-32765 in patients with relapsed/refractory B-cell malignancies. (9) Patients were treated with escalating doses over 6 cohorts starting with 1.25 mg/kg/day then escalating to 2.5, 5.0, 8.3, 8.3 and 12.5 mg/kg/day. Of the 47 patients enrolled on study 20 (43%) achieved an objective response (OR) including 3 complete remissions (CR) and 17 partial remissions (PR). Fourteen of 47 patients have been on study for > 6 months with 8/14 achieving a (PR) and 6/14 with stable disease.
With regard to mantle cell patients, 3/4 demonstrated an objective response, 2 with a complete response and 1 with a partial response. The responses were independent of pre-treatment variables including performance status, lactate dehydrogenase and overall disease burden. Grade 3 toxicities were observed in 9/47 (19%) patients. Five patients had to discontinue treatment for reasons including grade 3 neutropenia, grade 3 hypersensitivity, and grade 3 small bowel obstruction. There have been no treatment related deaths reported and there is no evidence of cumulative hematologic toxicity.
A phase II trial is currently underway investigating PCI-32765 using 560 mg daily in patients with relapsed or refractory mantle cell lymphoma with two separate arms; one including patients previously treated with bortezomib and the other in bortezomib naïve patients.
Other BTK inhibitors have been developed and are currently being investigated in B cell malignancies. AVL-292 is a more selective inhibitor of BTK compared to PCI-32765, by preventing BTK autophosphorylation and phosphorylation of the substrate without inhibiting upstream kinases Lyn of Syk. Similarities between the two agents include their daily oral administration. A phase 1B trial with AVL-292 is enrolling in relapsed refractory non-Hodgkin lymphoma.
Phosphoinositide 3-kinase
Many human cancers contain mutations in the cellular signaling pathway leading to over activation of PI3K. The PI3K signaling pathway has been proven to play a significant role in the development of human malignancies particularly those of B cell origin and specifically mantle cell lymphoma. PI3K is an enzyme that acts on the plasma membranes phospholipids and has been identified as a critical mediator in B-cell activation and differentiation. (10)
In hematologic malignancies, activation of this pathway results in constitutive B cell receptor activation; pathway activation appears to be mediated by Class 1 isoforms (α, β, δ, γ). The P110 δ isoform is expressed in cells of hematologic origin, predominately in leukocytes. (11) Pharmacologic inhibition of the P110 δ isoform has demonstrated its role in signaling through the normal B cell receptor in addition to demonstrating early signs of significant efficacy in B cell malignanies. (11)
CAL-101 is a specific potent oral inhibitor of the P110 δ isoform that decreases cellular viability by inhibiting PI3K. In-vitro studies in CLL reveal that in addition to causing cell death through disruption of intracellular signaling, CAL-101 impairs crosstalk between leukemic cells and the microenvironment. The resulting impairment in chemokine receptor function leads to diminished leukemia cell chemotaxis. (12)
In vitro, primary leukemic cells from 134 patients and 5 controls were tested for response to CAL-101. (11) Increased sensitivity to CAL-101 was seen in > than 20% of B cell acute lymphoblastic leukemia and only 3% of myeloid samples. The control samples showed no sensitivity to CAL-101. With regards to PIK3 signaling, 5 out of 5 mantle cell lines expressed constitutive levels of pAKT that were reduced by administration CAL-101. By inhibiting the PIK3 pathway vital signals for cell survival are lost thereby resulting in apoptosis. In taking this model forward, CAL-101 is being investigated at the phase 1 and II levels.
Kahl and colleagues conducted a large phase 1 trial of CAL 101 in individuals with relapsed or refractory hematologic malignancies. (13) Patients were enrolled at progressively higher doses with CAL-101 administered orally once or twice daily for 28 days for up to 12 cycles. Doses ranged from 50 mg twice daily to 350 mg BID. Tumor response was measured using standard criteria without the addition of PET/CT. Of the 55 patients enrolled, 18 had mantle cell lymphoma, 72 % previously received bortezomib. Objective responses were observed in 73% (8/11) and 40% (2/5) of MCL patients with relapsed and refractory disease respectively. However, the median duration of response was a modest 3 months. Correlative studies demonstrated plasma drug concentrations of chemokines CCL 22 and CCL 17 were elevated at baseline and decreased following administration of CAL-101.
An ongoing phase 1 trial is investigating the safety of CAL-101 combined with rituximab and/or bendamustine in patients with relapsed or refractory B-cell lymphoma. (14) CAL-101 is being administered at 100 mg BID in 28 days cycles with rituximab 375 mg/m2 weekly for 8 weeks or bendamustine 90 mg/2 on the first two days of each cycle for 6 cycles. Although mantle cell patients are not specified, preliminary results demonstrate that of the three patients with non-Hodgkin lymphoma two had a partial response (1 each in the CAL-101 + rituximab group and CAL -101 + bendamustine group) and 1 a complete response from the CAL-101+ bendamustine group.
Other PI3K inhibitors are currently being developed in B cell malignancies, some of which inhibit additional PI3K isoforms in addition to delta. Give the objective response rates with CAL-101, combination studies are being planned in MCL
AKT
Downstream from PI3K, activated AKT targets important regulator signals of the cell cycle leading to apoptosis and protein translation. (15) As an antiapototic factor, inhibition of AKT is a logical intracellular target for therapy. Small oral potent AKT inhibitors are being developed including MK-2206. This drug has been tested in heavily pretreated patients with solid tumor and hematologic malignancies.(16) Based on this safety data and the importance of signaling through the PI3K-mTOR-AKT pathway in lymphoma early phase trials are underway in patients with relapsed or refractory disease, including those with mantle cell histology.
Protein Kinase C
Downstream from Syk is Protein Kinase C β (PKCβ) the major isoform of PKC involved in BCR signaling. PKCβ is a modulator of angiogenic activity of vascular endothelial growth factor (VEGF). (17) Elevated levels of VEGF receptor are associated with a poor prognosis in mantle cell lymphoma.
Enzastaurin is a selective inhibitor of PKCβ, and in preclinical models has induced apoptosis in diffuse large B cell lines. To test its efficacy in mantle cell lymphoma, a phase II trial of enzastaurin was initiated enrolling 60 patients. (18) The drug was given orally once daily for 28 days per cycle up to six cycles. 22 patients were free from progression for 3 cycles and 6 of the 22 were free from progression for 6 months, however no objective responses were reported. The most common toxicities were fatigue and diarrhea; there were no grade 4 toxicities.
Janus-Associated Kinase
The Janus Kinase-2 pathway plays a vital role in the propagation of the malignant hematologic cell. (17) Western blotting of MCL lines have shown that phosphorylated STAT3 (ser 737) is constitutively activated (19) contributing to malignant cell proliferation via deregulated protein expression. (20) Furthermore, STAT 3 induces tumor angiogenesis, thus a potential target for inhibition. STAT proteins are often phosphorylated by Janus-associated kinase (JAK) providing rationale for the investigation of JAK inhibitors.
INCB16562 is a small molecule JAK1/JAK2 inhibitor recently tested on a panel of lymphoma cell lines (n=17) which included mantle cell lymphoma. (21) Cells were incubated with INCB1656 2 for up to 72 hours with subsequent evaluation of cell viability. IC50 values ranged from 1–9 uM with DLBCL lines being the most sensitive. The bcl-2 family proteins Bcl-xl and MCL-1 were both downregulated in the most sensitive cell lines after treatment with INCB16562. Furthermore, INCB16562 was found to be synergistic with bcl-2 inhibition, suggesting promise for future combination studies.
BCL-2 Family
The inhibition of programmed cell death is essential in the pathogenesis of the malignant cell. In MCL, bcl-2 is constitutively overexpressed leading to the inhibition of apoptosis. (22) The bcl-2 family of proteins (of which includes BH-3) are key regulators of apoptosis, including those which potentiate cell survival and proteins that promote cell death. When activated, the BH-3 proteins lead to a cascade of signals and eventual cell death. (23) Inhibitors of this pathway have been developed including the molecule Obataclax (GX15-070), a BH-3 mimetic. GX15-070 was tested in 5 mantle cell lines and produced cell death at various rates. GX15-070 was also tested in conjunction with bortezomib and synergistic apoptosis was observed. (23)
A second BH-3 mimetic, ABT-737 selectively binds to bcl-2 and bcl-xl displacing BH-3 from bcl-2 or bcl-xl leading to the activation of downstream caspases. The compound was tested in vitro with MCL lines. (24) Cell death was observed in 4 of 10 cell lines with ABT-737. Genetic testing revealed that amplification of chromosome 18q21, causing overexpression of bcl-2, was present in the four sensitive cell lines.
Another bcl-2 family inhibitor, ABT-263, is a potent orally bioavailable BH-3 mimetic that has also been evaluated in the pre-clinical setting. The in vivo properties of ABT-263 were tested in aggressive xenograft models of mantle cell lymphoma in combination with rituximab. Dosed at 100 mg/kg PO for 17 days, treatment with ABT-263 resulted in 44% tumor growth inhibition. (25) ABT -263 was also tested alone and in combination with R-CHOP in a GRANTA-519 xenograft MCL model. Single agent treatment with ABT-263 for 21 days resulted in 40% growth inhibition compared to R-CHOP alone which invoked 68% tumor growth inhibition with 20% complete responses. When ABT-263 was combined with R-CHOP, complete tumor response was seen in all animals without evidence of re-growth in 4 of 9 tumors. (25) Toxicity includes a rapid but reversible thrombocytopenia. Based on these results further studies in patients with relapsed/refractory lymphoma are in progress.
Novel antibodies
The role of rituximab has well been established in mantle cell lymphoma. Given this success, new antibodies targeting CD 20, CD 22, CD 40 and CD 74 have been developed and are in various stages of clinical development. These are highlighted in table 2.
Ofatumumab is a new human antibody that binds to the epitope of CD20 with a greater avidity than rituximab. (26) Preclincal work in CLL demonstrated that ofatumumab produced in vitro cell death more effectively that rituximab. (27) A phase I/II trial evaluating ofatumumab in 40 patients with relapsed / refractory follicular lymphoma at doses of 300 mg, 500 mg, 700 mg, and 1000 mg revealed responses in 63%, 33%, 20%, and 50% respectively. Furthermore, in 14 patients who had been previously treated with rituximab, the response rate was 64%. (28) Given these results, as well as previous results targeting CD 20 in MCL, four phase I/II trials investigating ofatummab in combination are now recruiting MCL patients.
Another antibody currently being investigated is dacetuzumab, a humanized anti CD-40 monoclonal antibody. CD 40 is a type 1 transmembrane protein that is expressed on dendritic cells, activated B lymphocytes and activated monocytes. (29) CD-40 is also expressed on many malignancies of B-cell origin including non-Hodgkin lymphoma, multiple myeloma and chronic lymphocytic leukemia. In a phase 1 dose escalation study, dacetuzumab was administered in several cohorts to patients with lymphoma. Of the 10 mantle cell patients 1 achieved a partial response. (29)
Other antibodies undergoing investigation in MCL include milatuzumab. This compound is a fully humanized anti CD 74 antibody, found to exert its effect as a signaling molecule and survival receptors in the maturation of B cells through activation of the PI3K/Akt and NF-κβ pathways. Milatuzumab has been studied in combination with rituximab in mantle cell lines resulting in improved cell survival when compared to controls. (30)
Histone Deacetylase
The epigenetic modulation of gene expression is a crucial component of cellular biology. In the normal cell, DNA is packaged into an organized nucleosome that is composed of a strand of DNA wound around eight histone proteins. (31) The amino acid residues on the histone tails are modified by post –translational acetylation, methylation, and phosphorylation. As a result, the accessibility of transcription factors to gene promoters is increased. On the contrary, deacetylation, demethylation and dephosphorylation of histone decreases the accessibility of transcriptions factors to the promoter region. Histone deacetylases (HDAC) control the removal of lysine residues leading to hypoacetylation of histone gene promoters and subsequent inhibition of DNA transcription. At least 18 human HDACs have been identified varying in function and substrate. Knockout mice that are deficient in class 1 HDAC die in the perinatal setting. HDACs also play a role in the balance of pro and antiapoptotic proteins. Malignant cells have a high level of expression of HDAC resulting in hypoacetylation of histone. Inhibition of HDACs thus leads to cell cycle arrest followed by apoptosis. Furthermore, inhibition of HDAC leads to decreased expression of vascular endothelial growth factor. (31)
Given the above, development of HDAC inhibitors has become a promising area of advancement within the realm of oncology. These agents achieve their desired effect by binding to a critical Zn2+ ion required for catalytic function of the HDAC enzyme. (31) Each inhibitor has varying action with regard to potency and affinity for substrate. They are divided into 4 groups and more than 10 have been tested in clinical trials. (32) Vorinostat was the first HDAC inhibitor to reach the market, approved for cutaneous T-cell lymphoma in 2006.
In a phase II clinical trial, Kirschbaum et al investigated the efficacy of vorinostat in patients with refractory lymphoma. Nine MCL patients were included, and although none achieved a measurable response, one patient demonstrated stable disease for 26 months. (33) The most common grade 3 and 4 toxicities were myelosuppression and fatigue.
Yazbeck et al investigated the combination of a mTOR inhibitor in combination with vorinostat in MCL lines. (34) Results from their study suggest that temsirolimus works via autophagy, (the degradation of long-lived proteins) and vorinostat via apoptosis. In combination with temsirolimus, small concentrations of vorinostat activated the caspase pathway leading to apoptosis. (34) Inhibition of ERK phosphorylation was seen without reducing levels of bcl-2. Observed in vitro synergy may warrant further clinical investigation of this combination.
Panobinostat (LBH589), a pan-histone deacetylase inhibitor, was combined with everolimus in a phase I/II trial of patients with relapsed non-Hodgkin lymphoma. A total of 12 patients were enrolled, 2 of which had MCL. At 20 mg, grade 3 and 4 toxicities included thrombocytopenia (45%) and fatigue (18%) with one death on study possibly secondary to a pulmonary embolus. 10 of 11 patients that were evaluated had documented tumor reduction ranging from 31–63% with changes of cytokines levels including VEGF, IL-10 and IL-13. Based on these results, this dose level was recommended for phase II evaluation.
Aurora Kinase
The aurora kinases are a family of mitotic serine/threonine protein kinase that play a role in regulating the mitotic phase of the cell cycle. Three aurora homologues have been identified. Aurora A is involved in centrosome maturation and separation while Aurora B & C are essential for chromosomal separation. (35) Inhibition of Aurora A in tumor cells leads to a delay in mitotic entry leading to G2/M cell cycle arrest. Tissue microarray analysis has demonstrated that Aurora kinase A & B are over expressed in mantle cell patients. Furthermore, over-expression is associated with a poor prognosis. There are at least twelve Aurora inhibitors that are currently being developed including those specific to the A & B isoform and pan Aurora inhibitors.
Alisertib (MLN8237) is an ATP-competitive oral inhibitor of Aurora A phosphorylation and histone-H3 phosphorylation leading to apoptosis. In mantle cell lines, MLN8237 decreased protein levels of Aurora A to a greater extent that Aurora B. (35) When studied in combination with docetaxel (microtubule targeting agent) in a SCID mouse xenograft model of MCL, significant tumor growth inhibition was seen compared to control.
In a phase II study of MLN8237 in 48 NHL patients including 13 with mantle cell lymphoma, 1 CR, 2 PRs and 6 patients with stable disease were observed. (36) The most common adverse events included neutropenia (73%) fatigue (60%) and diarrhea (56%). Further combinations are being investigated in B cell NHL, including mantle cell lymphoma.
Cyclin Dependent Kinase
Mantle cell lymphoma is characterized by increased expression of cyclin D1 secondary to the translocation between the IgH and BCL-1 genes. Cyclin D1, in conjunction with the cyclin dependent kinases CDK 4 and CDK 6, regulates the transition of the cell from G1 to S. The constitutive expression of cyclin D1 is thought to accelerate the progression of lymphoma through the G1/S checkpoint of the cell cycle. (37)
Flavopiridol is a synthetic pan-CDK 1 inhibitor that decreases both cyclin D1 and cyclin D3 leading to cell arrest of the G1 phase of the cell cycle. When tested in mantle cell lines, flavopiridol induced apoptosis via down regulation of cyclin D1 and inhibition of CDK 4.
At the phase I level, flavopirodol was tested in combination with rituximab and fludarabine in patient with B cell malignancies. (38) 10 of 38 patients had MCL, 7 of which achieved a complete response and 1 a partial response. The median progression free survival in mantle cell patients was 21.9 months. Substantial myelosuppression was noted with the combination.
Poly (ADP-ribose) polymerases
Poly (ADP-ribose) polymerases (PARPs) are a family of enzymes involved in DNA repair, transcriptional regulation, protein degradation, spindle maintenance and apoptosis. (39) PARPs bind to site of DNA breaks and facilitate DNA repair via base excision repair and non-homologous end joining. A number of PARP inhibitors have been developed and are currently under investigation in phase 1 trials. Veliparib (ABT-888) is a potent, novel PARP inhibitor. In a phase 0 study, patients with advanced malignancies (including non-Hodgkin lymphoma) were treated with ABT-888 and found to have decreased PARP levels after a single dose. (39) In vivo studies of MCL mouse models treated with olaparib, a PARP inhibitor, resulted in a significant reduction of tumor in the bone marrow and a trend towards spleen size reduction in 8 mice. (40) Consequently, combinations incorporating PARP inhibitors are being investigated in mantle cell lymphoma.
Conclusion
Progress in the treatment of MCL may depend on the development of small molecules. It is clear from pre-clinical and early phase trials that clinical efficacy can be achieved with targeted inhibition of constitutively activated pathways. Future efforts should be focused on novel agent development, and combinations of targeted therapies. With this in mind, it is reasonable to believe that based on the more favorable side effect profile, Bendamustine may be a better platform for combination with novel agents when compared to CHOP or Hyper-CVAD, a strategy being tested in the cooperative group setting. Having demonstrated feasibility with bendamustine, the incorporation of agents targeting BCR signaling will likely be a focus of future clinical trials. Furthermore, ongoing laboratory work suggests that combining multiple agents targeting relevant signaling pathways in MCL may lead to clinically significant combinations that do not include chemotherapy. As we obtain an enhanced understanding of these pathways and the complicated cellular interactions, drug develop will continue to become increasingly targeted, ultimately improving the prognosis of mantle cell lymphoma.
Acknowledgement
This work was supported in part by the University of Rochester SPORE in lymphoma P50 CA13080503 and the James P. Wilmot Foundation. Dr. Friedberg is a Scholar in Clinical Research of the Leukemia and Lymphoma Society. Dr. Barr is a Lymphoma Research Foundation Clinical Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest disclosure
The authors report no relevant conflict of interest.
References
- 1.Martin P, Chadburn A, Christos P, Furman R, Ruan J, Joyce MA, et al. Intensive treatment strategies may not provide superior outcomes in mantle cell lymphoma: overall survival exceeding 7 years with standard therapies. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO [Research Support, Non-U.S. Gov't] 2008 Jul;19(7):1327–1330. doi: 10.1093/annonc/mdn045. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson FK, Krysov S, Davies AJ, Steele AJ, Packham G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011 Oct 20;118(16):4313–4320. doi: 10.1182/blood-2011-06-338855. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. [Research Support N.I.H., Intramural Review] 2011 Jan 6;117(1):26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pighi C, Gu TL, Dalai I, Barbi S, Parolini C, Bertolaso A, et al. Phospho-proteomic analysis of mantle cell lymphoma cells suggests a pro-survival role of B-cell receptor signaling. Cell Oncol (Dordr). [Research Support, Non-U.S. Gov't] 2011 Apr;34(2):141–153. doi: 10.1007/s13402-011-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinaldi A, Kwee I, Taborelli M, Largo C, Uccella S, Martin V, et al. Genomic and expression profiling identifies the B-cell associated tyrosine kinase Syk as a possible therapeutic target in mantle cell lymphoma. British journal of haematology. [Research Support, Non-U.S. Gov't] 2006 Feb;132(3):303–316. doi: 10.1111/j.1365-2141.2005.05883.x. [DOI] [PubMed] [Google Scholar]
- 6.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. [Clinical Trial, Phase I Clinical Trial, Phase II Research Support N.I.H. Extramural Research Support, Non-U.S. Gov't] 2010 Apr 1;115(13):2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences of the United States of America. 2010 Jul 20;107(29):13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. [Research Support N.I.H. Extramural Research Support, Non-U.S. Gov't] 2011 Jun 9;117(23):6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler N, Sharman JP, Smith SM, Boyd T, Grant B, Kolibaba KS, et al. The Btk Inhibitor, PCI-32765, Induces Durable Responses with Minimal Toxicity In Patients with Relapsed/Refractory B-Cell Malignancies: Results From a Phase I Study. ASH Annual Meeting Abstracts; 2010 November 19; 2010. p. 964. [Google Scholar]
- 10.Chantry D, Vojtek A, Kashishian A, Holtzman DA, Wood C, Gray PW, et al. p110delta, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. The Journal of biological chemistry. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't P.H.S.] 1997 Aug 1;272(31):19236–19241. doi: 10.1074/jbc.272.31.19236. [DOI] [PubMed] [Google Scholar]
- 11.Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011 Jan 13;117(2):591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3'-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. [Research Support, Non-U.S. Gov't] 2011 Sep 29;118(13):3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahl B, Byrd JC, Flinn IW, Wagner-Johnston N, Spurgeon S, Benson DM, Jr, et al. Clinical Safety and Activity In a Phase 1 Study of CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110{delta}, In Patients with Relapsed or Refractory Non-Hodgkin Lymphoma. ASH Annual Meeting Abstracts; 2010 November 19; 2010. p. 1777. [Google Scholar]
- 14.Flinn IW, Schreeder MT, Wagner-Johnston N, Boccia RV, Leonard JP, Coutre SE, et al. A Phase 1 Study of CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110{delta}, In Combination with Rituximab and/or Bendamustine In Patients with Relapsed or Refractory B-Cell Malignancies. ASH Annual Meeting Abstracts; 2010 November 19; 2010. p. 2832. [Google Scholar]
- 15.Rudelius M, Pittaluga S, Nishizuka S, Pham TH, Fend F, Jaffe ES, et al. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood. [Research Support. N.I.H. Intramural] 2006 Sep 1;108(5):1668–1676. doi: 10.1182/blood-2006-04-015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Molecular cancer therapeutics. [Research Support, Non-U.S. Gov't] 2010 Jul;9(7):1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 17.Reeder CB, Ansell SM. Novel therapeutic agents for B-cell lymphoma: developing rational combinations. Blood. [Review] 2011 Feb 3;117(5):1453–1462. doi: 10.1182/blood-2010-06-255067. [DOI] [PubMed] [Google Scholar]
- 18.Morschhauser F, Seymour JF, Kluin-Nelemans HC, Grigg A, Wolf M, Pfreundschuh M, et al. A phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO [Clinical Trial, Phase II Research Support, Non-U.S. Gov't] 2008 Feb;19(2):247–253. doi: 10.1093/annonc/mdm463. [DOI] [PubMed] [Google Scholar]
- 19.Pham LV, Tamayo AT, Li C, Bornmann W, Priebe W, Ford RJ. Degrasyn potentiates the antitumor effects of bortezomib in mantle cell lymphoma cells in vitro and in vivo: therapeutic implications. Molecular cancer therapeutics. [Research Support. N.I.H. Extramural Research Support, Non-U.S. Gov't] 2010 Jul;9(7):2026–2036. doi: 10.1158/1535-7163.MCT-10-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baran-Marszak F, Boukhiar M, Harel S, Laguillier C, Roger C, Gressin R, et al. Constitutive and B-cell receptor-induced activation of STAT3 are important signaling pathways targeted by bortezomib in leukemic mantle cell lymphoma. Haematologica. [Clinical Trial Multicenter Study Research Support, Non-U.S. Gov't] 2010 Nov;95(11):1865–1872. doi: 10.3324/haematol.2009.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derenzini E, Lemoine M, Brighenti E, Davis RE, Zinzani PL, Younes A. The Small Molecule JAK1/JAK2 Inhibitor INCB16562 Shows Single Agent Activity and Strongly Synergizes with Bcl-2 Inhibitors in Lymphoma. ASH Annual Meeting Abstracts; 2011 November 18; 2011. p. 2731. [Google Scholar]
- 22.Touzeau C, Dousset C, Bodet L, Gomez-Bougie P, Bonnaud S, Moreau A, et al. ABT-737 induces apoptosis in mantle cell lymphoma cells with a Bcl-2high/Mcl-1low profile and synergizes with other antineoplastic agents. Clinical cancer research : an official journal of the American Association for Cancer Research. [Research Support, Non-U.S. Gov't] 2011 Sep 15;17(18):5973–5981. doi: 10.1158/1078-0432.CCR-11-0955. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. [Evaluation Studies Research Support, Non-U.S. Gov't] 2007 May 15;109(10):4441–4449. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- 24.Beltran E, Fresquet V, Martinez-Useros J, Richter-Larrea JA, Sagardoy A, Sesma I, et al. A cyclin-D1 interaction with BAX underlies its oncogenic role and potential as a therapeutic target in mantle cell lymphoma. Proceedings of the National Academy of Sciences of the United States of America. [Research Support N.I.H. Extramural Research Support, Non-U.S. Gov't] 2011 Jul 26;108(30):12461–12466. doi: 10.1073/pnas.1018941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer research. 2008 May 1;68(9):3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD. Ofatumumab, a novel anti-CD20 monoclonal antibody for the treatment of B-cell malignancies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. [Review] 2010 Jul 20;28(21):3525–3530. doi: 10.1200/JCO.2010.27.9836. [DOI] [PubMed] [Google Scholar]
- 27.Nightingale G. Ofatumumab: a novel anti-CD20 monoclonal antibody for treatment of refractory chronic lymphocytic leukemia. The Annals of pharmacotherapy. 2011 Oct;45(10):1248–1255. doi: 10.1345/aph.1P780. [DOI] [PubMed] [Google Scholar]
- 28.Hagenbeek A, Gadeberg O, Johnson P, Pedersen LM, Walewski J, Hellmann A, et al. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase 1/2 trial. Blood. [Clinical Trial, Phase I Clinical Trial, Phase II Multicenter Study Research Support, Non-U.S. Gov't] 2008 Jun 15;111(12):5486–5495. doi: 10.1182/blood-2007-10-117671. [DOI] [PubMed] [Google Scholar]
- 29.Advani R, Forero-Torres A, Furman RR, Rosenblatt JD, Younes A, Ren H, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin's lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. [Clinical Trial, Phase I Multicenter Study] 2009 Sep 10;27(26):4371–4377. doi: 10.1200/JCO.2008.21.3017. [DOI] [PubMed] [Google Scholar]
- 30.Alinari L, Yu B, Christian BA, Yan F, Shin J, Lapalombella R, et al. Combination anti-CD74 (milatuzumab) and anti-CD20 (rituximab) monoclonal antibody therapy has in vitro and in vivo activity in mantle cell lymphoma. Blood. [In Vitro Research Support N.I.H. Extramural Research Support, Non-U.S. Gov't] 2011 Apr 28;117(17):4530–4541. doi: 10.1182/blood-2010-08-303354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. [Review] 2009 Nov 10;27(32):5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 32.Xargay-Torrent S, Lopez-Guerra M, Saborit-Villarroya I, Rosich L, Campo E, Roue G, et al. Vorinostat-induced apoptosis in mantle cell lymphoma is mediated by acetylation of proapoptotic BH3-only gene promoters. Clinical cancer research : an official journal of the American Association for Cancer Research. [Research Support, Non-U.S. Gov't] 2011 Jun 15;17(12):3956–3968. doi: 10.1158/1078-0432.CCR-10-3412. [DOI] [PubMed] [Google Scholar]
- 33.Kirschbaum M, Frankel P, Popplewell L, Zain J, Delioukina M, Pullarkat V, et al. Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin's lymphoma and mantle cell lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. [Clinical Trial, Phase II Research Support N.I.H. Extramural] 2011 Mar 20;29(9):1198–1203. doi: 10.1200/JCO.2010.32.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yazbeck VY, Buglio D, Georgakis GV, Li Y, Iwado E, Romaguera JE, et al. Temsirolimus downregulates p21 without altering cyclin D1 expression and induces autophagy and synergizes with vorinostat in mantle cell lymphoma. Experimental hematology. 2008 Apr;36(4):443–450. doi: 10.1016/j.exphem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Qi W, Cooke LS, Liu X, Rimsza L, Roe DJ, Manziolli A, et al. Aurora inhibitor MLN8237 in combination with docetaxel enhances apoptosis and anti-tumor activity in mantle cell lymphoma. Biochemical pharmacology. [Research Support N.I.H. Extramural] 2011 Apr 1;81(7):881–890. doi: 10.1016/j.bcp.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Session 14: signalling pathways in lymphoma. Annals of Oncology. 2011 Jun 1;22(suppl 4):iv135–iv137. 2011. [Google Scholar]
- 37.Venkataraman G, Maududi T, Ozpuyan F, Bahar HI, Izban KF, Qin JZ, et al. Induction of apoptosis and down regulation of cell cycle proteins in mantle cell lymphoma by flavopiridol treatment. Leukemia research. 2006 Nov;30(11):1377–1384. doi: 10.1016/j.leukres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Lin TS, Blum KA, Fischer DB, Mitchell SM, Ruppert AS, Porcu P, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. [Clinical Trial, Phase I Research Support. N.I.H. Extramural Research Support, Non-U.S. Gov't] 2010 Jan 20;28(3):418–423. doi: 10.1200/JCO.2009.24.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kummar S, Kinders R, Gutierrez ME, Rubinstein L, Parchment RE, Phillips LR, et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. [Clinical Trial Research Support N.I. H. Extramural] 2009 Jun 1;27(16):2705–2711. doi: 10.1200/JCO.2008.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weston VJ, Oldreive CE, Skowronska A, Oscier DG, Pratt G, Dyer MJ, et al. The PARP inhibitor olaparib induces significant killing of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood. [Research Support, Non-U.S. Gov't] 2010 Nov 25;116(22):4578–4587. doi: 10.1182/blood-2010-01-265769. [DOI] [PubMed] [Google Scholar]