Abstract
In clinical practice it is possible to find patients with clinical signs suggestive of anti-phospholipid syndrome (APS) who are persistently negative for the routinely used anti-phospholipid antibodies (aPL). Therefore, the term proposed for these cases was seronegative APS (SN-APS). We investigated the clinical usefulness of thin-layer chromatography (TLC) immunostaining in detecting serum aPL in patients presenting clinical features of SN-APS. Sera from 36 patients with SN-APS, 19 patients with APS, 18 patients with systemic lupus erythematosus (SLE), 20 anti-hepatitis C virus (HCV)-positive subjects and 32 healthy controls were examined for aPL using TLC immunostaining. Anti-β2-glycoprotein-I, anti-annexin II, anti-annexin V and anti-prothrombin antibodies were tested by enzyme-linked immunosorbent assays (ELISA). Eahy926, a human-derived endothelial cell line, was incubated with immunoglobulin (Ig)G fraction from SN-APS patients and analysis of phospho-interleukin (IL)-1 receptor-associated kinase (IRAK) and phospho-nuclear factor (NF)-κB was performed by Western blot, vascular cell adhesion molecule 1 (VCAM-1) expression by cytofluorimetric analysis and supernatants tissue factor (TF) levels by ELISA. TLC immunostaining showed aPL in 58·3% of SN-APS patients: anti-cardiolipin in 47·2%, anti-lyso(bis)phosphatidic acid in 41·7% and anti-phosphatidylethanolamine in 30·5%. Six of 36 patients showed anti-annexin II. Incubation of Eahy926 cells with IgG from SN-APS induced IRAK phosphorylation, NF-κB activation, VCAM-1 surface expression and TF cell release. TLC immunostaining could identify the presence of aPL in patients with SN-APS. Moreover, the results suggest the proinflammatory and procoagulant effects in vitro of these antibodies.
Keywords: anti-phospholipid antibodies, anti-phospholipid syndrome, thin layer chromatography immunostaining
Introduction
Anti-phospholipid syndrome (APS) is a disease characterized by arterial and venous thrombosis, recurrent miscarriages or fetal loss associated with circulating anti-phospholipid antibodies (aPL). Anti-cardiolipin (aCL) and anti-β2-glycoprotein-I (aβ2-GPI) antibodies detected by enzyme linked immunosorbent assay (ELISA) and the lupus anti-coagulant (LA), detected by clotting assays, are the recommended tests for the detection of aPL [1]. Classification of APS requires the combination of at least one clinical and one laboratory criterion. Nevertheless, in daily clinical practice it is possible to find patients with clinical signs suggestive of APS who are persistently negative for the routinely used aCL, aβ2-GPI and LA. Therefore, for these cases the term ‘seronegative APS’ (SN-APS) was proposed [2].
Although aPL are largely directed against β2-GPI and/or prothrombin, new antigenic targets for aPL in the APS syndrome have been investigated recently. In particular, it has been shown that antibodies directed to the lyso(bis)phosphatidic acid (aLBPA) may represent a marker of APS showing similar sensitivity and specificity compared to aβ2-GPI [3]. In addition, aLBPA are associated strongly with the presence of LA [3,4]. Moreover, anti-prothrombin antibodies (aPT) have been reported as the sole antibodies detected in a few patients with systemic lupus erythematosus (SLE) and a history of thrombosis but persistently negative for aCL or LA [5]. Anti-phosphatidylethanolamine antibodies (aPE) were detected in 15% of a cohort of thrombotic patients and found mainly in the absence of the other laboratory criteria of APS, but the retrospective design of the study did not permit evaluation of the persistence of aPE positivity [6]. Recently, using a proteomic approach, we identified vimentin/cardiolipin as a ‘new’ target of the APS, also detectable in SN-APS patients [7].
We demonstrated the possibility of detecting aPL by immunostaining on thin layer chromatography (TLC) plates [8]. This non-quantitative technique identifies the reactivity of serum aPL with purified phospholipid molecules with a different exposure compared to ELISA methods.
The aim of this study, proposed at the sixth meeting of the European Forum on anti-phospholipid antibodies [9], was to investigate the potential clinical usefulness of TLC immunostaining in detecting serum aPL in patients with so-called SN-APS and to evaluate their biological activity.
Materials and methods
Patients
This study included 36 consecutive patients, 27 attending the Lupus Clinic at Saint Thomas' Hospital in London (UK) and nine attending the Rheumatology Division of the Sapienza University of Rome. All the patients presented clinical features consistent with a diagnosis of APS but tested persistently negative (at least two times 12 weeks apart) for conventional aCL, aβ2-GPI and LA tests. Clinical manifestations included venous and/or arterial thrombosis and pregnancy morbidity, as stated in the classification criteria for definite APS [1]. Sera were collected at several times and stored at −20°C until use. Moreover, all patients showed normal screening for other causes of thrombophilia, such as anti-thrombin III, protein C and protein S deficiency, hyperhomocysteinaemia, Factor V Leiden and prothrombin mutations. For each patient two serum samples were studied far apart for at least 12 weeks.
Thirty-seven consecutive out-patients, attending the Rheumatology Division of Sapienza University of Rome, were also studied. Nineteen patients had APS, diagnosed according to the Sapporo criteria [1], primary (n = 8) or associated to SLE (n = 11); 18 patients had SLE fulfilling the ACR revised criteria for the classification of SLE [10]. Finally, 20 patients with chronic hepatitis C virus (HCV) infection and 32 healthy subjects (normal blood donors) matched for age and sex were studied as controls.
This study was approved by the local ethic committees and participants gave written informed consent.
Detection of aPL by TLC immunostaining
Cardiolipin (CL) (bovine heart) was obtained from Sigma Chemical Co. (St Louis, MO, USA). Lyso(bis)phosphatidic acid (LBPA), phosphatidylethanolamine (PE), phosphatidylinositol (PI) and phosphatidylcholine (PC) were obtained from Avanti Polar Lipids (Alabaster, AL, USA). TLC immunostaining was performed as described previously, with slight modification [8,11,12]. Briefly, this assay was performed using 2 µg of each phospholipid. Notably, all TLC immunostaining assays were performed on all the phospholipids. Phospholipids were run on aluminium-backed silica gel 60 (20 × 20) high-performance thin-layer chromatography (HPTLC) plates (Merck Co, Inc., Darmastdt, Germany) preincubated with 1% potassium oxalate in methanol/water (2:3, v/v) for 1 h at room temperature, dried and then activated at 100°C for 5 min. Chromatography was performed in chloroform : acetone : methanol : acetic acid : water (40:15:13:12:8) (v/v/v/v/v). The dried chromatograms were soaked for 90 s in a 0·5% (w/v) solution of poly(isobutyl methacrylate) beads (Polysciences, Inc., Eppelheim, Germany) dissolved in hexane. After air-drying, the chromatograms were incubated at room temperature for 1 h with 1% [bovine serum albumin (BSA)] in phosphate-buffered saline (PBS) to eliminate non-specific binding. The blocking solution was removed and replaced by a washing buffer (PBS). The chromatograms were then incubated for 1 h at room temperature with sera, diluted 1:100 in the blocking solution. Sera were removed and chromatograms were washed three times for 10 min with PBS. Bound antibodies were visualized with horseradish peroxidase (HRP)-conjugated goat anti-human immunoglobulin (Ig)G diluted 1:1000 in 1% BSA in PBS, incubated at room temperature for 1 h, and immunoreactivity was assessed by chemiluminescence reaction using the enhanced chemiluminescence (ECL) Western blotting system (Amersham Pharmacia Biotech, Buckinghamshire, UK).
ELISA for aPL and anti-phospholipid-binding proteins
aCL and aβ2-GPI ELISA kits were obtained from Diamedix (Miami, FL, USA). ELISA for aLBPA, anti-annexin II, anti-annexin V and anti-prothrombin were performed as described previously [3,11]–[14].
In vitro exposure of endothelial cells to IgG fraction from SN-APS patients
IgG were isolated from sera of three SN-APS patients (Supplementary Table S1, patients 32, 34 and 35), from three APS patients and from three healthy donors by precipitation with 33% ammonium sulphate [15]. For in vitro studies, Eahy926, a human-derived endothelial cell line, was maintained in Dulbecco's modified Eagle's medium (high glucose), containing 10% fetal calf serum (FCS), hypoxanthine/aminopterin/thymidine (HAT supplement), 2 mM l-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin and 250 pg/ml Fungizone (Gibco, Grand Island, NY, USA) at 37°C in a humified 5% CO2 atmosphere. Experiments were performed in cells grown to 60–70% confluence. Eahy926 were incubated with IgG fraction from SN-APS patients (SN-APS IgG; 200 µg/ml), with IgG fraction from normal human serum (NHS-IgG; 200 µg/ml), IgG fraction from APS patients (APS IgG; 200 µg/ml), lipopolysaccharide (LPS) (100 ng/ml) or tumour necrosis factor (TNF)-α (20 ng/ml) as positive controls or with IgG fraction from SN-APS patients (SN-APS IgG; 200 µg/ml), preadsorbed with CL or LBPA, for different incubation times at 37°C [16]–[18]. All in vitro experiments were performed using purified IgG from three patients and three controls. We preliminarily determined the optimal IgG concentration and incubation time on the basis of a time–IgG concentration curve, but all the experiments were shown at the best concentration and incubation time.
In order to investigate the specificity of the assay, adsorption tests of purified IgG with both CL and LBPA were performed according to the technique described elsewhere [3]. All the materials contained less the 0·00025 ng endotoxin/mg protein, as detected by the Limulus amebocyte lysate (LAL) test, performed at Associates of Cape Cod (Falmouth, MA, USA).
Western blot analysis of phospho-interleukin (IL)-1 receptor-associated kinase (IRAK) and phospho-nuclear factor (NF)-κB
Equal amounts of whole or nuclear extracts proteins [19] (from unstimulated or stimulated Eahy926 with SN-APS IgG fraction, NHS-IgG fraction, LPS, APS IgG fraction or SN-APS IgG fraction preadsorbed with CL or LBPA for 45 min at 37°C, 5% CO2) were separated in 7·5 sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred electrophoretically to nitrocellulose membrane (Bio-Rad Laboratories, Richmond, CA, USA) and then, after blocking with PBS, containing 1% albumin, probed with polyclonal rabbit anti-phospho-IRAK (Cell Signaling, Inc., Danvers, MA, USA) or polyclonal rabbit anti-phospho-NF-κB p65 (Cell Signaling, Inc.), as reported previously [18].
Detection of surface vascular cell adhesion molecule 1 (VCAM-1) expression by flow cytometry
Indirect immunofluorescence was performed to analyse VCAM-1 expression on the cell plasma membrane of Eahy926 cells. One × 106 cells (from unstimulated or stimulated Eahy926 with SN-IgG fraction, NHS-IgG fraction, TNF-α, APS IgG fraction or SN-APS IgG fraction, preadsorbed with CL or LBPA, 8 h at 37°C, 5% CO2) placed on ice, washed once in PBS and scraped in PBS, were fixed in 4% formaldehyde/PBS for 20 min at 4°C. After washing three times with PBS, cells were incubated with monoclonal anti-human VCAM-1 (GeneTex, Inc., Irvine, CA, USA) for 1 h at 4°C. Fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (Sigma Chemical Co.) was then added and incubated at 4°C for 30 min. After washing with PBS, fluorescence intensity was analysed with a Becton Dickinson cytometer.
Tissue factor (TF) assay
Eahy926 cells were incubated with SN-APS IgG fraction, NHS-IgG fraction, LPS, APS IgG fraction and SN-APS IgG fraction preadsorbed with CL or LBPA, for 4 h at 37°C in 5% CO2, after treatment supernatants were removed and tested for TF levels, using commercially available ELISA kits (American Diagnostica, Stamford, CT, USA), according to the manufacturer's instructions.
Statistical analysis
Differences between numerical variables were tested with the Wilcoxon test. Correlation was tested with Spearman's rank-order or Pearson's correlation coefficient. For comparison of categorical variables or percentages we used Fisher's exact and χ2 tests when appropriate. P-values less than 0·05 were considered significant.
Results
Demographic and clinical characteristics of patients
All SN-APS patients included in this study were Caucasian women with a mean age of 46·4 years (range 23–82) and a mean disease duration of 16·2 years (range 0·4–57). The clinical characteristics of SN-APS patients are reported in Supplementary Table S1. APS patients (two male and 17 female) showed a mean age of 43·4 years (range 27–71), and a mean disease duration of 9·2 years (range 0·1–34). SLE patients (18 female) showed a mean age of 38·8 years (range 18–59) and a mean disease duration of 13·4 years (range 0·8–36). Clinical characteristics of the three patient groups are summarized in Table 1. None of the healthy subjects or chronic HCV infection experienced arterial or venous thrombosis or recurrent fetal loss.
Table 1.
Clinical characteristics of patients studied
| Characteristics n (%) | SN-APS (n = 36) | APS (n = 19) | SLE (n = 18) |
|---|---|---|---|
| Other autoimmune disease | 31 (86·1) | 11 (57·8) | 6 (33·3) |
| SLE | 13 (20·6) | 11 (57·8) | – |
| Sjögren syndrome secondary to SLE | 7 (19·4) | 4 (21) | 5 (27·7) |
| Lupus-like disease | 8 (22·2) | 0 | 0 |
| Primary Sjögren syndrome | 2 (5·5) | 0 | 0 |
| Urticarial vasculitis | 2 (5·5) | 0 | 0 |
| Rhupus | 1 (2·7) | 0 | 0 |
| Mixed connective tissue disease | 1 (2·7) | 0 | 0 |
| Undifferentiated connective tissue disease | 1 (2·7) | 0 | 0 |
| Adult onset Still disease | 1 (2·7) | 0 | 0 |
| Vitiligo | 1 (2·7) | 0 | 0 |
| Pernicious anaemia | 1 (2·7) | 0 | 0 |
| Myasthenia gravis | 0 | 1 (5·2) | 1 (5·5) |
| None | 5 (13·9) | 8 (42·1) | – |
| Vascular thrombosis | 21 (58·3) | 19 (100) | 4 (22·2) |
| Venous thrombosis | 14 (38·9) | 16 (84·2) | 1 (5·5) |
| Arterial thrombosis | 8 (22·2) | 7 (36·8) | 3 (16·6) |
| Recurrent thrombosis | 6 (20·7) | 9 (47·3) | 0 |
| Pregnancy morbidity | 21 (58·3) | 8 (42·1) | 1 (5·5) |
| Normal fetus deaths | 9 (25·0) | 1 (5·2) | 0 |
| Premature births | 5 (13·9) | 0 | 0 |
| Spontaneous abortions | 12 (33·3) | 8 (42·1) | 1 (5·5) |
| Vascular thrombosis and pregnancy morbidity | 6 (20·7) | 8 (42·1) | 1 (5·5) |
| Non-criteria APS features | |||
| Livedo reticularis | 22 (61.l) | 4 (21) | 1 (5·5) |
| Thrombocytopenia | 1 (2·8) | 3 (15·7) | 2 (11·1) |
| Cognitive dysfunctions | 15 (41·7) | 6 (31·5) | 7 (38·8) |
| Migraine | 11 (30·6) | 5 (26·3) | 5 (27·7) |
| Seizures | 2 (5·5) | 4 (21) | 2 (11·1) |
| Brain MRI scan abnormalities | 10/23 (43·5) | 5/9 (55·5) | 3/13 (23) |
| Thrombotic risk factors | |||
| Hypercholesterolemia | 12 (33·3) | 9 (47·3) | 2 (11·1) |
| Smoking | 9 (25·0) | 4 (21) | 1 (5·5) |
| Hypertension | 7 (19·4) | 6 (31·5) | 2 (11·1) |
| OC/HRT | 3 (8·3) | 1 (5·2) | 1 (5·5) |
| Diabetes mellitus | 1 (2·8) | 0 | 1 (5·5) |
MRI, magnetic resonance imaging; OC/HRT, oral contraceptive or hormone replacement therapy use; SLE, systemic lupus erythematosus; SN-APS, seronegative anti-phospholipid syndrome.
A statistically significant correlation was found between vascular thrombosis (arterial and/or venous) and pregnancy morbidity in SN-APS (P < 0·0001).
Detection of aPL by TLC immunostaining
In SN-APS patients the results obtained by TLC immunostaining with the first sample showed the presence of aPL in 21 of 36 SN-APS patients (58·3%): antibodies against CL were detected in 17 (47·2%), against LBPA in 15 (41·7%) and PE in 11 (30·5%). Figure 1 shows a representative TLC immunostaining with two positive and one negative samples. A statistically significant correlation was found among aCL, aLBPA and aPE positivity (P < 0·02). No reactivity was observed against the other phospholipids tested (PI and PC). TLC immunostaining performed with a second sample obtained at least 12 weeks from the previous immunostaining confirmed the same result except in five sera; in the case of three patients the positive result was not confirmed with the second sample. Conversely, two patients who had resulted negative with the first serum displayed aPL reactivity by TLC immunostaining with the second.
Fig. 1.
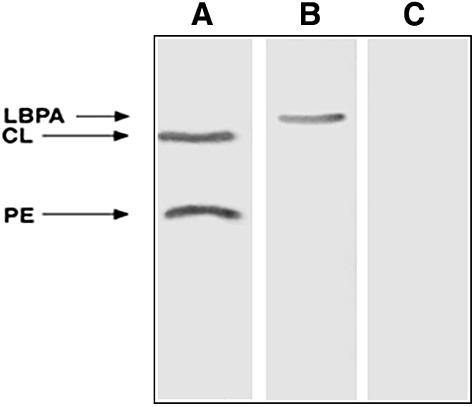
Representative thin-layer chromatography (TLC) immunostaining. TLC immunostaining was performed on all the phospholipids simultaneously, as reported previously [7]. Lane A, reactivity of the serum from a patient positive for both cardiolipin (CL) and phosphatidylethanolamine (PE); lane B, reactivity of the serum from a patient positive for lyso(bis)phosphatidic acid antibodies (LBPA); lane C, no background staining was observed with the serum from a healthy subject.
In APS patients TLC immunostaining showed the presence of antibodies against CL in 13 of 19 (68·4%), against LBPA in 12 of 19 (63·1%) and PE in 8 of 19 (42·1%) patients. In SLE patients TLC immunostaining showed the presence of antibodies against CL in 11 of 18 (61·1%), against LBPA in 11 of 18 (61·1%) and PE in 6 of 18 (33·3%) patients. Considering the two patient populations (APS and SLE) as a single group, a statistically significant correlation was found among aCL, aLBPA and aPE positivity (P < 0·03). Finally, none of the healthy subjects or patients with chronic HCV infection showed aPL reactivity by TLC immunostaining.
Detection of aPL and anti-phospholipid-binding proteins by ELISA
Six of 36 SN-APS patients (16·7%) showed serum antibodies (IgG class) against annexin II; none resulted positive for antibodies against CL, β2-GPI, LBPA, annexin V and prothrombin. Again, all sera but one showing reactivity against annexin II were also positive for aPL by TLC [P = not significant (n.s.)]. The results with the second sample were the same as the first.
Anti-CL reactivity (IgG and/or IgM) was observed in 19 of 19 (100%) APS and 14 of 18 (77·7%) SLE patients. Anti-β2-GPI reactivity (IgG and/or IgM) was observed in 14 (73·6%) APS and seven (38·8%) SLE patients. Finally, none of the 32 healthy subjects displayed positivity for the autoantibodies tested.
Associations between autoantibodies and clinical features
Table 2 shows the prevalence of autoantibodies in SN-APS patients with different clinical manifestations. The prevalence of the clinical features in SN-APS patients positive for aPL (by TLC immunostaining and anti-annexin II ELISA) was not statistically different from that observed in SN-APS patients negative for aPL by these assays.
Table 2.
Autoantibody prevalence in seronegative anti-phospholipid syndrome (SN-APS) patients (n = 36) according to the clinical manifestations
| Autoantibodies (assay) | Total thrombosis n = 21 | Arterial thrombosis n = 8 | Venous thrombosis n = 14 | Recurrent thrombosis n = 6 | Pregnancy morbidity n = 21 |
|---|---|---|---|---|---|
| aCL (TLC) n (%) | 10 (47·6) | 4 (50·0) | 7 (50·0) | 2 (33·3) | 9 (42·8) |
| aLBPA (TLC) n (%) | 9 (42·8) | 4 (50·0) | 6 (42·8) | 3 (50·0) | 9 (42·8) |
| aPE (TLC) n (%) | 6 (28·6) | 3 (37·5) | 4 (28·6) | 2 (33·3) | 6 (28·6) |
| Anti-annexin II (ELISA) n (%) | 3 (14·3) | 0 | 3 (14·3) | 0 | 3 (14·3) |
| No autoantibodies n (%) | 6 (28·6) | 2 (25·0) | 4 (28·6) | 2 (33·3) | 7 (33·3) |
aCL, anti-cardiolipin antibodies; aLBPA, anti-lyso(bis)phosphatidic acid antibodies; aPE, anti-phosphatidylethanolamine antibodies; ELISA, enzyme-linked immunosorbent assay; TLC, thin-layer chromatography.
IgG from SN-APS induce IRAK phosphorylation and NF-κB activation
Western blot analysis of cell lysates showed that IgG fractions from SN-APS, as well as LPS and IgG fractions from APS, induced IRAK phosphorylation, as revealed by anti-phospho-IRAK antibodies reactivity (Fig. 2a, Supplementary Fig. S1a). Conversely, cells stimulated with control human IgG did not show anti-phospho-IRAK reactivity. Because IRAK phosphorylation leads to NF-κB activation, we investigated the effects of IgG fractions on p65 NF-κB [20].
Fig. 2.
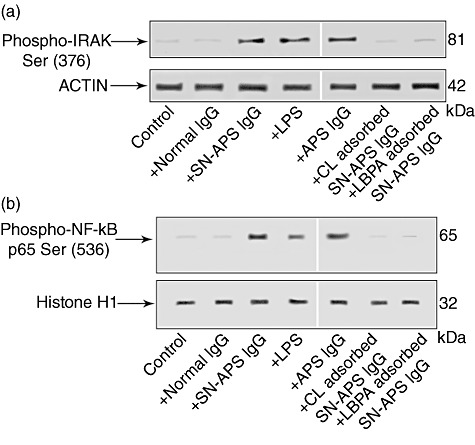
Interleukin (IL)-1 receptor-associated kinase (IRAK) phosphorylation assay and nuclear factor (NF)-κB activation by seronegative anti-phospholipid syndrome (SN-APS) immunoglobulin (Ig)G fraction. Eahy926 cells were incubated with normal human serum IgG (NHS-IgG) (200 µg/ml), SN-APS IgG (200 µg/ml), lipopolysaccharide (LPS) (100 ng/ml) APS IgG (200 µg/ml), cardiolipin (CL)-adsorbed SN-APS IgG or lyso(bis)phosphatidic acid antibodies (LBPA)-adsorbed SN-APS IgG, for 45 min at 37°C, and thereafter whole and nuclear extracts were probed with polyclonal rabbit anti-phospho-IRAK or polyclonal rabbit anti-phospho-NF-κB p65, respectively. Bound antibodies were visualized with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG and immunoreactivity was assessed by enhanced chemiluminescence (ECL). (a) SN-APS IgG, LPS or APS IgG (200 µg/ml) induced IRAK phosphorylation; conversely, cells stimulated with control human IgG, as well as unstimulated cells (control), did not show anti-phospho-IRAK reactivity. Anti-phospho-IRAK reactivity was inhibited significantly by pre-adsorption of SN-APS IgG with CL or LBPA. As a control for loading, IRAK blots were stripped and reprobed with polyclonal anti-actin antibody. (b) SN-APS IgG, LPS (100 ng/ml) or APS IgG (200 µg/ml) induced NF-κB phosphorylation; conversely, cells stimulated with control human IgG did not show anti-phospho-NF-κB p65 reactivity. NF-κB phosphorylation was inhibited significantly by pre-adsorption of SN-IgG with CL or LBPA. As a control for loading and purity of preparation, phospho-NF-κB p65 blots were stripped and reprobed with polyclonal anti-histone H1.
Western blot analysis of nuclear extracts revealed that IgG fractions from SN-APS, as well as LPS and IgG fractions from APS, induced NF-κB phosphorylation, as revealed by anti-phospho-NF-κB p65 antibody reactivity (Fig. 2b, Supplementary Fig. S1b). Conversely, cells stimulated with control human IgG did not shown anti-phospho-NF-κB p65 reactivity.
Interestingly, both anti-phospho-IRAK reactivity (Fig. 2a) and NF-κB activation (Fig. 2b) were inhibited significantly by preadsorption of SN-APS IgG with CL or LBPA.
IgG from SN-APS induce VCAM-1 surface expression and tissue factor release by endothelial cells
Flow cytometric analysis of VCAM-1 expression on endothelial cell plasma membrane, after incubation with IgG fractions from SN-APS, as well as with TNF-α or APS-IgG (not shown), revealed a shift of mean fluorescence intensity compared to unstimulated cells or cells stimulated with human control IgG (Fig. 3).
Fig. 3.
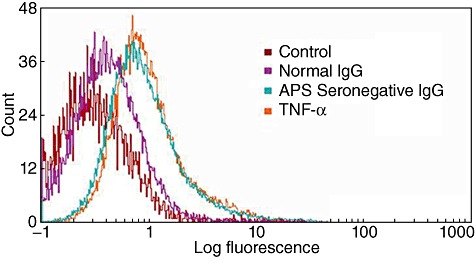
Vascular cell adhesion molecule 1 (VCAM-1) expression by seronegative anti-phospholipid syndrome (SN-APS) immunoglobulin (Ig)G fraction. Cytofluorimetric analysis of VCAM-1 expression on the surface of Eahy926 cells. The cells were stimulated with IgG fraction from SN-APS patients, with IgG from normal human serum (NHS) for 8 h or with tumour necrosis factor (TNF)-α as positive control. All the experiments were performed using purified IgG from each patient and control. We showed only one patient (Table S1, patient 35) and control representative of the other samples. Thus, the figure is representative of all sera tested. Thereafter, the cells were incubated with monoclonal anti-VCAM-1 for 1 h at 4°C. Fluorescence intensity was analysed after incubation with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG with a Becton Dickinson cytometer. Histograms represent log fluorescence versus cell number, gated on cell population of a side-scatter/forward-scatter (SS/FS) histogram. Cell number is indicated on the y-axis and fluorescence intensity is represented in three logarithmic units on the x-axis. Fluorescence intensity was analysed by a flow cytometer. VCAM-1 expression on endothelial cell plasma membrane, after incubation with IgG fractions from SN-APS, as well as with TNF-α, revealed a shift of the mean fluorescence intensity, compared to unstimulated cells (control) or cells stimulated with IgG from NHS (P < 0·001).
Moreover, we analysed TF release following cell incubation with IgG fractions from SN-APS (Fig. 4, Supplementary Fig. S2). TF release by cells stimulated with IgG fractions from SN-APS, LPS or IgG fractions from APS was increased significantly compared to untreated endothelial cells, as well as cells stimulated with human control IgG. TF release was inhibited significantly by preadsorption of SN-APS IgG with CL or LBPA (Fig. 4).
Fig. 4.
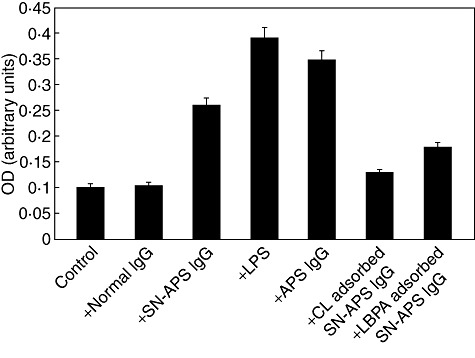
Tissue factor (TF) release by seronegative anti-phospholipid syndrome (SN-APS) immunoglobulin (Ig)G fraction. Cells were stimulated with normal human serum IgG (NHS-IgG) (200 µg/ml), SN-APS IgG (200 µg/ml), lipopolysaccharide (LPS) (100 ng/ml), APS IgG (200 µg/ml), cardiolipin (CL)-adsorbed SN-APS IgG or lyso(bis)phosphatidic acid antibodies (LBPA)-adsorbed SN-APS IgG, for 4 h at 37°C. After treatment, the supernatants were collected and analysed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit. Results are expressed as mean ± standard deviation from three different experiments. SN-APS versus control (unstimulated cells) P < 0·05, SN-APS versus normal IgG (P < 0·05).
Discussion
To our knowledge, this is the first study showing aPL detected by TLC immunostaining associated with clinical features of APS in patients repeatedly negative for the laboratory criteria of APS, i.e. aCL, aβ2-GPI and LA. Moreover, the results suggest that the biological activity of these antibodies is able to trigger a signal transduction pathway(s) in endothelial cells with consequent proinflammatory and procoagulant effects.
Current laboratory criteria for the classification of APS include aCL and aβ2-GPI measured by standardized ELISA and LA, detected by clotting assays [1,21]. However, the term SN-APS has been suggested recently for patients with a clinical profile suggestive of APS who are persistently negative for the routinely used assays [2,22,23]. We studied here a cohort of patients affected mainly by autoimmune systemic diseases presenting a clinical picture suggestive of APS, i.e. vascular thrombosis and/or pregnancy morbidity associated with several non-criteria APS features, persistently negative for the routinely used aPL. Interestingly, a statistically significant correlation was observed between thrombosis and pregnancy morbidity in these patients. In the absence of positive blood tests, more clinical features would make a diagnosis of SN-APS more convincing. We identified the presence of aPL in about 60% of such patients using a method (TLC immunostaining) in which the antigen was run on aluminium-backed silica gel plates; in this way it may mimic phospholipid exposure after protein binding [8,12]. Interestingly, a strong correlation was observed between these aPL specificities demonstrated by TLC immunostaining.
The prevalence of aPL detected by TLC test in SLE patients without APS was similar to that showed by ELISA (61% and 78%, respectively). Although these aPL antibodies are probably of low affinity and are not associated with any clinical manifestation, long-term prospective studies could clarify their clinical relevance.
With regard to the so-called SN-APS patients, the discrepancies between ELISA and immunostaining on TLC plates in detecting antibodies against CL, LBPA and PE may be due to the different antigenic presentation of phospholipids on chromatograms compared to the surface of microtitre wells. In addition, six (16·7%) of the SN-APS patients showed serum IgG antibodies against annexin II detected by ELISA. Anti-annexin II have been associated recently with thrombosis in patients with APS, even though they can be detected in serum of patients with rheumatoid arthritis and other autoimmune systemic disorders [14,24]. It is noteworthy that all but one of the SN-APS patients presenting anti-annexin II were also positive for aPL as detected by TLC immunostaining. Thus, we do not exclude that, in SN-APS patients, phospholipid-binding proteins may also be involved in anti-phospholipid reactivity, as TLC immunostaining does not exclude this possibility. However, at present the involvement of phospholipid-binding proteins other than annexin II remains unclear. Because, in recent years, our research has focused on the identification of endothelial autoantigens involved in different autoimmune diseases, studies based on the screening of endothelial cDNA expression libraries also identified vimentin as a new phospholipid-binding protein autoantigen in SN-APS [7].
Interestingly, in almost all the patients the positive result obtained by TLC assay was confirmed with the second result after at least 12 weeks; conversely, two patients negative with the first sample displayed aPL reactivity with the second sample. Of note, one of the last such cases was a 26-year-old female with SLE and proteinuria; histological evaluation of the kidney biopsy showed diffuse global lupus nephritis (class IV-G) associated with thrombotic microangiopathy suggestive of APS.
Recently, it was demonstrated that aPL may exert their pathogenic role by triggering a signal transduction pathway involving IRAK phosphorylation, NF-κB activation and translocation with consequent release of proinflammatory and procoagulant factors by endothelial and/or monocytic cells [18,20,25]. In order to verify the possible pathogenic role of the autoantibodies we demonstrate that purified IgG from sera of SN-APS patients induce IRAK serine phosphorylation with consequent NF-κB activation. Interestingly, we demonstrated that aCL as well as aLBPA were involved in this signalling pathway triggering, as these autoantibodies failed to induce IRAK phosphorylation if they were previously adsorbed with highly purified aCL or LBPA.
Previous studies demonstrated that aPL induce monocyte and endothelial cell TF expression through the simultaneous activation of NF-κB-related proteins as well as aPL induce VCAM-1 on endothelial cells surface and that these effects are correlated with increased adhesion of leucocytes to endothelium [18,25,26]. According to these findings we demonstrate that IgG from SN-APS patients triggering resulted in the expression of VCAM-1, as well as release of TF from endothelial cells, which may contribute to the pathogenesis of thrombosis in patients with APS.
Deep vein thrombosis, myocardial infarction and stroke are the major causes of morbidity and death among APS patients due to the high risk of recurrence; therefore, it is mandatory to identify among patients with suspected APS repeatedly negative for conventional aPL tests, those with a true APS to offer them long-term anti-coagulation, as widely recommended for secondary thromboprophylaxis in this disease [27,28]. Similarly, as APS is now recognized as the most common treatable cause of recurrent miscarriage, for women with a history of recurrent early abortions or fetal loss a diagnosis of APS leads them towards treatments which significantly improve the rate of live births [28].
The most relevant finding of this study is that TLC immunostaining could potentially identify the presence of aPL in patients with clinical features suggestive of APS not ascertained by traditional tests for aPL, and such identification could have a major impact on the prognosis and therapeutic approach. Moreover, our results suggest the biological activity of these antibodies that are able to trigger a signal transduction pathway(s) in endothelial cells with consequent proinflammatory and procoagulant effects in vitro. However, currently testing for TLC immunostaining is not suitable for screening purposes, and larger prospective studies are needed to assess its clinical relevance as a rescue test for patients with suspected APS but persistently negative for conventional aPL.
Acknowledgments
This work was supported by grants from Fondazione Umberto di Mario ONLUS, MIUR-PRIN 2007.
Disclosure
A patent relating to the content of the manuscript is applying.
Supporting information
Additional supporting information may be found in the online version of this article.
Fig. S1. Interleukin (IL)-1 receptor-associatedkinase (IRAK) phosphorylation assay and nuclear factor(NF)-κB activation by seronegative anti-phospholipid syndrome(SN-APS) immunoglobulin (Ig)G fraction from three differentpatients. Eahy926 cells were incubated with SN-APS IgG (200μg/ml) from three different patients (Table S1, patients 32, 34and 35, respectively) for 45 min at 37°C and thereafter wholeand nuclear extracts were probed with polyclonal rabbitanti-phospho-IRAK (a) or polyclonal rabbit anti-phospho-NF-κBp65 (b), respectively. Bound antibodies were visualized withhorseradish peroxidase (HRP)-conjugated anti-rabbit IgG andimmunoreactivity was assessed by enhanced chemiluminescence (ECL).As a control for loading, IRAK blots were stripped and reprobedwith polyclonal anti-actin antibody (a), phospho-NF-κB p65blots were stripped and reprobed with polyclonal anti-histone H1(b).
Fig. S2. Tissue factor (TF) release byseronegative anti-phospholipid syndrome (SN-APS) IgG fraction fromthree different patients. Cells were stimulated with SN-APSimmunoglobulin (Ig)G (200 μg/ml) from three different patients(Table S1, patients 32, 34 and 35, respectively) for 4 h at37°C. After treatment, the supernatants were collected andanalysed using a commercially available enzyme-linked immunosorbentassay (ELISA) kit. Results are expressed as mean ± standard deviation from three different experiments.
Table S1. Clinical and serological profile of seronegative anti-phospholipid syndrome (SN-APS) patients.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Hughes GRV, Khamashta MA. Seronegative antiphospholipid syndrome. Ann Rheum Dis. 2003;62:1127. doi: 10.1136/ard.2003.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessandri C, Bombardieri M, Di Prospero L, et al. Anti-lysobisphosphatidic acid antibodies in patients with anti-phospholipid syndrome and systemic lupus erythematosus. Clin Exp Immunol. 2005;140:173–80. doi: 10.1111/j.1365-2249.2005.02727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorice M, Ferro D, Misasi R, et al. Evidence for anticoagulant activity and beta2-GPI accumulation in late endosomes of endothelial cells induced by anti-LBPA antibodies. Thromb Haemost. 2002;87:735–41. [PubMed] [Google Scholar]
- 5.Bertolaccini ML, Gomez S, Pareja JF, et al. Antiphospholipid antibody tests: spreading the net. Ann Rheum Dis. 2005;64:1639–43. doi: 10.1136/ard.2005.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanmarco M, Gayet S, Alessi MC, et al. Antiphosphatidylethanolamine antibodies are associated with an increased odds ratio for thrombosis. A multicenter study with the participation of the European Forum on antiphospholipid antibodies. Thromb Haemost. 2007;97:949–54. [PubMed] [Google Scholar]
- 7.Ortona E, Capozzi A, Colasanti T, et al. Vimentin/cardiolipin complex as a new antigenic target of the antiphospholipid syndrome. Blood. 2010;116:2960–7. doi: 10.1182/blood-2010-04-279208. [DOI] [PubMed] [Google Scholar]
- 8.Sorice M, Griggi T, Circella A, et al. Detection of antiphospholipid antibodies by immunostaining on thin layer chromatography plates. J Immunol Methods. 1994;173:49–54. doi: 10.1016/0022-1759(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 9.Rotar Z, Rozman B, de Groot PG, et al. Sixth meeting of the European Forum on antiphospholipid antibodies. How to improve the understanding of the antiphospholipid syndrome? Lupus. 2009;18:53–60. doi: 10.1177/0961203308097569. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 11.Alessandri C, Sorice M, Bombardieri M, et al. Antiphospholipid reactivity against cardiolipin metabolites occurring during endothelial cell apoptosis. Arthritis Res Ther. 2006;8:R180. doi: 10.1186/ar2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorice M, Pittoni V, Griggi T, et al. Specificity of anti-phospholipid antibodies in infectious mononucleosis: a role for anti-co-factor protein antibodies. Clin Exp Immunol. 2000;120:301–6. doi: 10.1046/j.1365-2249.2000.01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaburaki J, Kuwana M, Yamamoto M, Kawai S, Ikeda Y. Clinical significance of anti-annexin V antibodies in patients with systemic lupus erythematosus. Am J Hematol. 1997;54:209–13. doi: 10.1002/(sici)1096-8652(199703)54:3<209::aid-ajh6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Cesarman-Maus G, Ríos-Luna NP, Deora AB, et al. Autoantibodies against the fibrinolytic receptor, annexin 2, in antiphospholipid syndrome. Blood. 2006;107:4375–82. doi: 10.1182/blood-2005-07-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mc Neil HP, Krillis SA, Chesterman CN. Purification of anti-phospholipid antibodies using a new affinity method. Thromb Res. 1988;52:641–8. doi: 10.1016/0049-3848(88)90136-3. [DOI] [PubMed] [Google Scholar]
- 16.Margutti P, Sorice M, Conti F, et al. Screening of an endothelial cDNA library identifies the C-terminal region of Nedd5 as a novel autoantigen in systemic lupus erythematosus with psychiatric manifestations. Arthritis Res Ther. 2005;7:R896–903. doi: 10.1186/ar1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delunardo F, Conti F, Margutti P, et al. Identification and characterization of the carboxy-terminal region of Sip-1, a novel autoantigen in Behçet's disease. Arthritis Res Ther. 2006;8:R71. doi: 10.1186/ar1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorice M, Longo A, Capozzi A, et al. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum. 2007;56:2687–97. doi: 10.1002/art.22802. [DOI] [PubMed] [Google Scholar]
- 19.Nourbakhsh M, Kalble S, Dorrie A, Hauser H, Resch K. The NF-kappa B repressing factor is involved in basal repression and interleukin (IL)-1-induced activation of IL-8 transcription by binding to a conserved NF-kappa B-flanking sequence element. J Biol Chem. 2001;276:4501–8. doi: 10.1074/jbc.M007532200. [DOI] [PubMed] [Google Scholar]
- 20.Raschi E, Testoni C, Bosisio D, et al. Role of the MyD88 transduction signaling pathway in endothelial activation by antiphospholipid antibodies. Blood. 2003;101:3495–500. doi: 10.1182/blood-2002-08-2349. [DOI] [PubMed] [Google Scholar]
- 21.Edwards CJ, Hughes GR. Hughes syndrome (the antiphospholipid syndrome): 25 years old. Mod Rheumatol. 2008;18:119–24. doi: 10.1007/s10165-008-0042-3. [DOI] [PubMed] [Google Scholar]
- 22.Bertolaccini ML, Khamashta MA. Laboratory diagnosis and management challenges in the antiphospholipid syndrome. Lupus. 2006;15:172–8. doi: 10.1191/0961203306lu2293rr. [DOI] [PubMed] [Google Scholar]
- 23.Bertolaccini ML, Khamashta MA, Hughes GR. Diagnosis of antiphospholipid syndrome. Nat Clin Pract Rheumatol. 2005;1:40–6. doi: 10.1038/ncprheum0017. [DOI] [PubMed] [Google Scholar]
- 24.Salle V, Mazière JC, Smail A, et al. Anti-annexin II antibodies in systemic autoimmune diseases and antiphospholipid syndrome. J Clin Immunol. 2008;28:291–7. doi: 10.1007/s10875-008-9188-1. [DOI] [PubMed] [Google Scholar]
- 25.Meroni PL, Raschi E, Testoni C, Borghi MO. Endothelial cell activation by antiphospholipid antibodies. Clin Immunol. 2004;112:169–74. doi: 10.1016/j.clim.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Meroni PL, Raschi E, Testoni C, Parisio A, Borghi MO. Innate immunity in the antiphospholipid syndrome: role of the Toll-like receptors in endothelial cell activation by antiphospholipid antibodies. Autoimmun Rev. 2004;3:510–5. doi: 10.1016/j.autrev.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Cervera R, Khamashta MA, Shoenfeld Y, et al. Morbidity and mortality in the antiphospholipid syndrome during a 5-year period: a multicenter prospective study of 1000 patients. Ann Rheum Dis. 2009;68:1428–32. doi: 10.1136/ard.2008.093179. [DOI] [PubMed] [Google Scholar]
- 28.Tuthill JI, Khamashta MA. Management of antiphospholipid syndrome. J Autoimmun. 2009;33:92–8. doi: 10.1016/j.jaut.2009.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


