Abstract
Interferon (IFN)-γ is a cytokine with immunomodulatory properties, which has been shown previously to enhance the generation of tolerogenic dendritic cells (DC) when administered early ex vivo in 7-day monocyte-derived DC culture. To generate tolerogenic DC rapidly within 48 h, human monocytes were cultured for 24 h with interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) in the presence (IFN-γ-DC) or absence of IFN-γ (500 U/ml) (UT-DC). DC were matured for 24 h with TNF-α and prostaglandin E2 (PGE2). DC phenotype, signal transducer and activator of transcription-6 (STAT-6) phosphorylation and promotion of CD4+CD25+CD127neg/lowforkhead box P3 (FoxP3)hi T cells were analysed by flow cytometry. DC nuclear factor (NF)-κB transcription factor reticuloendotheliosis viral oncogene homologue B (RELB) and IL-12p70 protein expression were also determined. Phenotypically, IFN-γ-DC displayed reduced DC maturation marker CD83 by 62% and co-stimulation molecules CD80 (26%) and CD86 (8%). IFN-γ treatment of monocytes inhibited intracellular STAT6, RELB nuclear translocation and IL-12p70 production. IFN-γ-DC increased the proportion of CD4+CD25+CD127neg/lowfoxp3hi T cells compared to UT-DC from 12 to 23%. IFN-γ-DC primed T cells inhibited antigen-specific, autologous naive T cell proliferation by 70% at a 1:1 naive T cells to IFN-γ-DC primed T cell ratio in suppression assays. In addition, we examined the reported paradoxical proinflammatory effects of IFN-γ and confirmed in this system that late IFN-γ exposure does not inhibit DC maturation marker expression. Early IFN-γ exposure is critical in promoting the generation of regulatory DC. Early IFN-γ modulated DC generated in 48 h are maturation arrested and promote the generation of antigen-specific regulatory T cells, which may be clinically applicable as a novel cellular therapy for allograft rejection.
Keywords: dendritic cells, interferon-γ, NF-κB, STAT-6, transplantation
Introduction
Dendritic cells (DC) play a key role as sentinels of the immune system for the detection of pathogens and disturbance of the immunological milieu [1]. All transplanted organs possess a cellular component of passenger leucocytes, which migrate from the allograft to initiate rejection via the direct pathway of allorecognition [2]–[5]. Donor-derived DC are an important constituent of the passenger leucocyte population that initiate allograft rejection via direct allorecognition [6]–[8]. Recipient DC migrate into the allograft and sustain the rejection process via indirect allorecognition of donor-derived peptides to recipient T cells.
Under certain conditions, DC may be manipulated to promote potentially tolerogenic T cell responses. In particular, immature DC (iDC) have been shown to inhibit allogeneic T cell proliferation [9]–[11], while the injection of antigen-pulsed iDC block T cell responses in humans [12]. Unmodified iDC may undergo maturation in vivo and promote allorecognition, thereby limiting their tolerogenic potential. The modification of DC to stably inhibit maturation has been studied extensively in recent years, and a variety of pharmacological and immunological approaches including interleukin (IL)-10, vitamin D3, dexamethasone, aspirin and most recently curcumin have been shown to arrest DC in an immature state and promote tolerogenic responses in vitro and in vivo[13]–[18]. DC differentiation and maturation involve a myriad of intracellular signalling pathways. In particular, IL-4-activated signal transducer and activator of transcription-6 (STAT-6) signalling is involved specifically in DC development, as well as the activation of nuclear factor (NF)-κB, which also promotes DC maturation and are therefore important targets to promote tolerogenic DC [19]–[21].
Interferon (IFN)-γ is a potent proinflammatory cytokine secreted by CD4+ T helper type 1 (Th1) lymphocytes, CD8+ cytotoxic lymphocytes and natural killer (NK) cells that play key roles in allograft destruction [22,23]. IFN-γ has been used as a component of DC maturation cocktail when it is applied typically to monocyte-derived DC precursors after 5–7 days culture with granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4. However, IFN-γ may also play an important in allograft acceptance. IFN-γ knock-out (KO) models of skin and cardiac allograft transplantation have demonstrated that IFN-γ is crucial in negatively regulating alloimmune responses to promote allograft tolerance by co-stimulatory blockade [24]–[26]. IFN-γ is also required for protection against disease onset and severity in KO mouse models of experimental autoimmune encephalomyelitis and collagen-induced arthritis [27]–[30]. IFN-γ was shown to be necessary to regulate DC migration and T cell priming negatively in a KO mouse model [31]. We have also shown that early exposure of human monocytes to IFN-γ regulates negatively the stimulatory capacity of DC [32].
The use of tolerogenic DC to modify the recipient immune system to promote allograft acceptance is a potential alternative therapeutic approach, which has the advantage of not requiring conventional immunosuppressive therapy. However, their use in the clinic has faced significant hurdles – current protocols to generate monocyte-derived DC take 7–10 days [33], while rejection begins within hours of transplantation and is well established within 7 days [34]. Clinically useful DC for transplantation must therefore be generated in a short period of time. In 2003, Dauer and colleagues published a ‘FAST-DC’ protocol to generate potent immunostimulatory mature monocyte-derived DC for the purpose of cancer immunotherapy in 48 h [35].
In the present study, a complementary approach to rapidly generate stable immature DC, using early exposure of monocytes to IFN-γ, was developed to produce regulatory DC in a clinically applicable time-frame of 48 h. We demonstrate that the effect of early exposure of IFN-γ is mediated by the inhibition of STAT-6 phosphorylation and NF-κB activation resulting in DC maturational arrest and the development of a tolerogenic DC phenotype, unlike the late treatment of IFN-γ, which failed to inhibit the expression of DC maturation marker CD83. Rapidly generated regulatory DC only, via early IFN-γ exposure, support the generation of antigen-specific T regulatory cells in vitro providing a novel cellular therapeutic approach for transplant immunomodulation.
Methods and materials
Antibodies
The phenotypic profile of DC was defined using the following directly conjugated monoclonal antibodies (mAb): anti-CD83-fluorescein isothiocyanate (FITC) (HB15e), anti-CD86-FITC (FUN1), anti-CD80-FITC (L307·4), anti-DC-SIGN-FITC (DCN46), anti-human leucocyte antigen D-related (HLA-DR)-phycoerythrin-cyanin-5 (PE-Cy5) (G46-6) (BD Bioscience, San Jose, CA, USA) and rat anti-human immunoglobulin (Ig)-like transcript 4 (ILT4) (42D1) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as a primary mAb and FITC-conjugated anti-rat IgG was used for detection. STAT-6 phosphorylation was detected using anti-pY641-Alexa488 (clone: 18; BD Bioscience) and CD14-PE (M5E2). T cell phenotypes were determined using anti-CD25-PE-Cy7 (M-A251) (BD Bioscience), anti-CD4-peridinin chlorophyll (PerCP) 5.5 (OKT4) (eBiosciences, San Diego, USA) and anti-human FoxP3 PE-conjugated mAb (259D/C7 – BD Bioscience, San Jose, CA, USA). IL-4 receptor expression was detected using anti-human CD124 PE-conjugated (mouse IgG1, κ) (BD Bioscience). Anti-human reticuloendotheliosis viral oncogene homologue B (RELB) polyclonal antibody (Santa Cruz Biotechnology) was used as the primary antibody to detect localization of RELB by immunohistology.
Generation of ‘FAST’ human monocyte-derived dendritic cells
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coat of healthy human blood donors (Australian Red Cross Blood Service, Adelaide, South Australia) by Ficoll Paque (GE Healthcare, Little Chalfont, UK) density gradient centrifugation. Adherent monocytes were obtained from PBMC by incubating 6 × 107 PBMC in 75-cm2 flasks in 1% fetal calf serum (FCS) (Invitrogen, Mulgrave, Vic, Australia) for 1 h. Monocytes were cultured in RPMI-1640 containing 10% FCS, 1000 U/ml (1·2 × 107 U/mg) of granulocyte–macrophage colony-stimulating factor (GM-CSF)-Leucomax™ (Sandoz Australia, North Ryde, NSW, Australia) and 500 U/ml (1 × 107 U/mg) of IL-4 (eBiosciences) in the absence (UT-DC) or presence of 500 U/ml of IFN-γ (eBiosciences) (IFN-γ-DC) for 24 h. Cells were then treated with 10 ng/ml tumour necrosis factor (TNF)-α (R&D Systems, Minneapolis, MN, USA) and 1 µM PGE2 (Sigma, St Louis, MO, USA) for a further 24 h. All cell cultures were incubated under 5% CO2 at 37°C.
Fluorescence activated cell sorting (FACS) analysis
DC surface staining
DC were harvested and stained with monoclonal antibodies for 25 min at 4°C after blocking with heat-inactivated rabbit serum for 10 min. FACS lysing solution (4% paraformaldehyde solution) (BD Bioscience) was used as fixative.
Intracellular STAT-6 phosphorylation staining
PBMC were isolated from peripheral blood and treated with IL-4 (500 U/ml; eBioscience) and GM-CSF (1000 U/ml; Sandoz) in the presence or absence of 500 U/ml of IFN-γ (eBiosciences) for 10 min at 37°C. Cells were fixed immediately by adding an equal volume of prewarmed cytofix buffer (BD Bioscience). All samples were incubated with cytofix buffer for 10 min at 37°C. Cells were stained with anti-CD14 for 25 min 4°C. Cells were permeabilized with prechilled BD permeabilization buffer (BD Bioscience) for 30 min at 4°C; mAb targeting phosphorylated STAT-6 (pY641) samples were analysed within 1 h of staining.
Regulatory T cell (Treg) phenotype
For the enumeration of forkhead box P3 (FoxP3+) cells, T cells from the primary mixed leucocyte reaction (MLR) were harvested and stained with directly conjugated mAb targeting CD4, CD25 and CD127 for 25 min at room temperature. Cells were stained for the intracellular expression of FoxP3 (BD Bioscience), as per the manufacturer's instructions. Briefly, cells were fixed and then permeabilized before staining with anti-human FoxP3 (259D/C7) for 30 min at room temperature. Treg phenotype was assessed by flow cytometry (FACS Canto II; BD Bioscience). Data analysis was performed using FCS express (De Novo Software, Los Angeles, CA, USA). The FoxP3hi population of Tregs was determined by gating CD4+ T cells with a fluorescence intensity of greater than 102. Negative/positive gates were set according to fluorescence −1 controls, as described by Maecker and Trotter, for multi-colour flow cytometric analysis [36].
Cytokine cytometric bead array (CBA)
A human Th1/Th2 CBA cytokine kit (BD Bioscience) was used to determine the concentration of IFN-γ, IL-2, IL-10 and IL-4 in supernatants from DC-T cell co-cultures. DC were co-cultured with purified T cells (1:10 stimulator to responder ratio) for 5 days in complete RPMI-1640 medium (10% FCS and 1% glutamine) in 96-well round-bottomed plates (under 5% CO2 at 37°C). Supernatant was harvested and assayed according to the manufacturer's instructions. The lower detection limits for the tested cytokines were: IL-2: 2·6 pg/ml, IFN-γ: 7·1 pg/ml, IL-4: 2·6 pg/ml and IL-10: 3·0 pg/ml.
Allogeneic mixed lymphocyte reaction assay
Primary MLR
DC were washed with phosphate buffered saline (PBS) three times, irradiated (30 Gy) and used as stimulators in the MLR. Allogeneic T cells were purified from PBMC using nylon wool-packed columns, as described previously [10]. DC were co-cultured with T cell responders in 96-well round-bottomed plates (TPP, Trasadingen, Switzerland), at stimulator to responder ratios of 1:10, 1:100 and 1:1000. After 4 days cells were pulsed with 1 µCi [3H]-thymidine (Amersham Biosciences Ltd, Bucks, UK) for 18 h and harvested onto glass-fibre filters and counted in β-scintillation fluid using a Wallac Microbeta Counter (Turku, Finland). Proliferation was expressed as counts per minute (cpm) and expressed as the mean of five replicates ± standard deviation (s.d.).
Suppression assay
Primary MLR were performed using naive CD4+ T cells purified from PBMC using a human CD4+ T cell enrichment kit (Stem Cell Technologies, Vancouver, Canada). Naive CD4+ T cells and IFN-γ-DC were co-cultured at a ratio of 10:1. After 5 days, primed CD3+ T cells were harvested using a CD3+ selection kit (Stem Cell Technologies). These IFN-γ-DC primed T cells were co-cultured with a fixed number of autologous naive CD4+ T cells (105 cells per well) at varying ratios of 1:1, 1:2, 1:4 or 1:8. In addition, at the 1:1 ratio an irrelevant non-related third-party DC was used to determine the antigen specificity of Treg cells. Cells were cultured for 5 days and thymidine incorporation was used to measure proliferation following an 18 h pulse with 1 µCi of [3H]-thymidine.
Gene expression analysis
RNA was extracted from DC using RNAspin mini kit (GE Healthcare) and cDNA was synthesized using 1 µg total RNA by reverse transcription using an oligo-dT primer (Amersham Biosciences). Quantitative real-time polymerase chain reaction (PCR) based on a standard curve of copy numbers for each specific gene generated was used to analyse the expression of RELB (forward primer: 5′-TTT TAA CAA CCT GGG CAT CC, reverse primer: 5′-CGC AGC TCT GAT GTG TTT GT, cycling condition: 25 s at 95°C; 25 s at 55°C; 25 s at 72°C for 50 cycles), IL-12 (forward primer: 5′-TTT GGA GAT GCT GGG CAG TAC A, reverse primer: 5′-GAT GAT GTC CCT GAT GAA GAA GC, cycling condition: 25 s at 95°C; 25 s at 60°C; 25 s at 72°C for 50 cycles), interferon regulatory factor 4 (IRF4) (forward primer: 5′-AGT CCT GAG CGA AAA CAG GA, reverse primer: 5′-AAA GCC AAG AGG TGC GAG TA, cycling condition: 25 s at 95°C; 25 s at 55°C; 25 s at 72°C for 35 cycles). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward primer: 5′-ATC ACT GCC ACC CAG AAG ACT, reverse primer: 5′-CAT GCC AGT GAG CTT CCC GTT, cycling condition: 25 s at 95°C; 25 s at 55°C; 25 s at 72°C for 35 cycles). PCR was conducted according to optimized conditions. GAPDH was used as the housekeeping gene in order to verify RNA integrity and cDNA synthesis.
Immunohistology
DC (2 × 105) were spun onto slides at 20 g for 5 min using a Shandon Cytospin II (Thermo Scientific, Barrington, IL, USA). Slides were fixed with cold acetone for 5 min and air-dried. Anti-RELB polyclonal antibody (Santa Cruz Biotechnology) was used as the primary antibody, after blocking with 3% goat serum. Goat anti-rabbit IgG-FITC (Santa Cruz Biotechnology) was used to detect the protein localization of NF-κB transcription factor V-rel reticuloendotheliosis viral oncogene homologue B (RELB).
IL-12 enzyme-linked immunosorbent assay (ELISA)
DC (1 × 106 cells/ml) were stimulated with CD40L (500 ng/ml) and IFN-γ (1000 U/ml) (eBiosciences) for 48 h, as described previously [35]. Supernatants were harvested and assayed for the biologically active human IL-12p70, using the Ready-SET-go® ELISA kit, according to the manufacturer's instructions, sensitivity 4 pg/ml–500 pg/ml (eBiosciences).
Microarray analysis
FAST-DC were generated as described above. DC were cultured either in the absence of IFN-γ (no), treated with IFN-γ at time 0 h (early) or were exposed to IFN-γ after 24 h of culture with GM-CSF and IL-4 (late). All DC were matured with TNF-α and PGE2 after 24 h of culture with GM-CSF and IL-4. After 24 h of maturation DC were harvested and RNA was extracted. In brief, RNA was extracted by dissolving pellets in 500 µl TRIzol (Invitrogen, Carlsbad, CA, USA), then 100 µl of chloroform was added. The mixture was vortexed and left on ice for 15 min. Samples were then centrifuged (6500 g) for 30 min at 4°C. The upper aqueous phase was retained and mixed with an equal volume of 70% ethanol [in diethylpyrocarbonate (DEPC) H2O] prior to further purification using a Qiagen RNAeasy kit, according to the manufacturer's instructions. Samples were sent to the Adelaide Microarray analysis centre (Adelaide, South Australia), where samples were analysed using an Affymetrix Human Genearray chip (Affymetrix Inc., High Wycombe, UK). Data were analysed on Partek Genomics Suite (Partek, Santa Clara, CA, USA).
Statistical analysis
Analysis of variance (anova) statistical tests and t-tests were conducted using Prism Statistical Software where appropriate, with statistical significance at P < 0·05.
Results
IFN-γ in combination with the FAST-DC protocol generates phenotypically tolerogenic dendritic cells
The phenotype of monocyte-derived DC generated via the FAST-DC protocol following pretreatment with IFN-γ (IFNγ-DC) was examined using flow cytometry (Fig. 1). Monocyte-derived DCs that did not receive IFN-γ pretreatment (UT DC) were used as a control. After 24 h of differentiation with IL-4 and GM-CSF DC were either matured with TNF-α and PGE2 (Fig. 1a) or were left unmatured as immature DC controls (Fig. 1b). All groups showed marked up-regulation of the DC-specific marker CD209 (DC-SIGN), CD11c and HLA-DR molecules while showing down-regulation of CD14, consistent with a myeloid DC phenotype, as shown by previous studies [35]. Immature DC that did not undergo maturation with TNF-α and PGE2 (Fig. 1b) did not up-regulate DC maturation marker CD83 and had reduced expression of co-stimulatory molecules CD80 and CD86, consistent with an iDC phenotype. Pretreatment with IFN-γ reduced the number of cells expressing the DC maturation marker CD83 to 14% (compared to 76% in UT-DC), despite maturation with TNF-α and PGE2, without affecting CD209 expression. IFN-γ-DC showed decreased expression of positive co-stimulatory molecules CD80 and CD86 by 16 and 8%, respectively, compared to UT-DC. However, in comparison to immature DC, IFN-γ-DC had considerable expression of CD80 and CD86. The negative co-stimulatory molecule ILT4 was up-regulated from 17% of UT-DC expressing ILT4 to 30% of IFN-γ-DC, thus IFN-γ treatment increased ILT4 by 1·8-fold. Similarly, this was also observed in immature DC group ± IFN-γ.
Fig. 1.
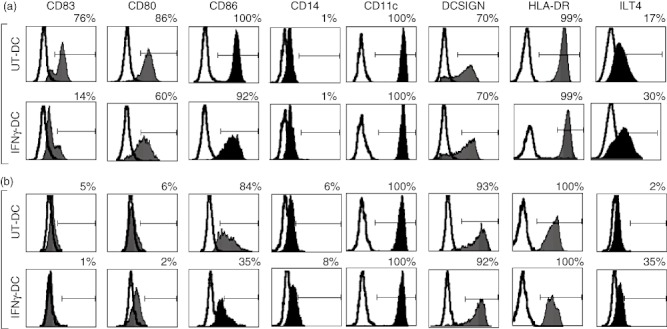
Interferon (IFN)-γ in combination with the FAST-dendritic cell (DC) protocol generates phenotypically tolerogenic DC. Monocytes were isolated from peripheral blood and cultured without (UT-DC) or with IFN-γ (IFN-γ-DC) in the presence of interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) for 24 h. DC were then either matured with tumour necrosis factor (TNF)-α (10 ng/ml) and prostaglandin E2 (PGE2) (1 µM) for another 24 h (a: plus TNF-α and PGE2, representative of six independent experiments) or were cultured for another 24 h in the absence (b: minus TNF-α and PGE2, representative of three independent experiments. Fluorescence activated cell sorter (FACS) analysis was used to determine phenotypic markers. The following co-stimulatory and linage markers were examined: CD83, CD80, CD86, immunoglobulin (Ig)-like transcript 4 (ILT4), CD11c, DC-SIGN, CD14, human leucocyte antigen D-related (HLA-DR). Line indicates the isotype control and solid histogram shows the tested monoclonal antibody. Negative gate was set to <0·5%.
At the transcriptional level, quantitative real-time PCR demonstrated that IFN-γ-DC produced 60% fewer transcripts of NF-κB transcription factor RELB (Fig. 2a), which was confirmed semiquantitatively at the protein level (Fig. 2b). Immunohistology also showed that UT-DC had distinct co-localization of RELB in the nucleus, indicating typical DC maturation in response to TNF-α and PGE2. However, IFN-γ-DC had reduced expression of RELB in the cytoplasm, with little or no translocation into the cell nucleus (Fig. 2b). IL-12p40 gene expression was reduced markedly in IFN-γ-DC both at the level of messenger RNA (decreased by 86% compared to UT-DC) and protein (IFN-γ-DC produced 62% less biologically active IL-12p70 compared to UT-DC) (Fig. 2c). Thus, pretreatment of human monocytes with IFN-γ prior to treatment with IL-4 and GM-CSF produces DC with a tolerogenic phenotype as assayed by cell surface molecule expression, transcriptional profile and cytokine production.
Fig. 2.
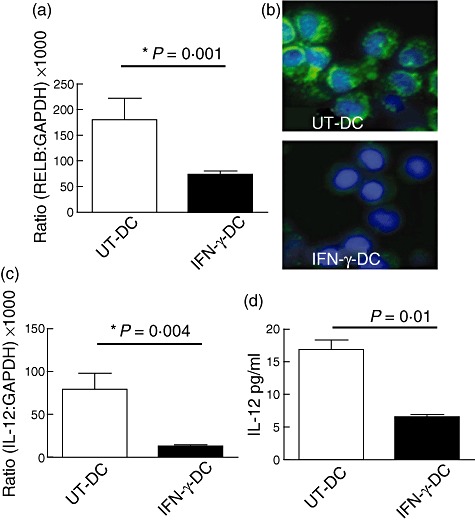
Interferon (IFN)-γ-treated dendritic cells (DC) have reduced expression and production of reticuloendotheliosis viral oncogene homologue B (RELB) and proinflammatory cytokine interleukin (IL)-12. Monocytes were treated with IFN-γ (IFN-γ-DC) or without (UT-DC) in the presence of IL-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) for 24 h and matured for another 24-h period with TNF-α and prostaglandin E2 (PGE2). Quantitative real-time polymerase chain reaction (PCR) was used to determine the expression of RELB (a) and IL-12 (c). Fluorescence microscopy was used to visualize the protein co-localization of RELB (b), blue depicts 4′,6-diamidino-2-phenylindole (DAPI) nucleus staining and green shows RELB expression. Enzyme-linked immunosorbent assay (ELISA) was used for the detection of biologically active IL-12p70 (d). Mean ± standard deviation. *P < 0·01. Representative of four independent experiments.
IFN-γ inhibits IL-4-driven STAT-6 in monocytes
To investigate the mechanism of IFN-γ-mediated effects on monocyte-derived DC, human PBMC were treated with or without IFN-γ in the presence of IL-4 and GM-CSF to induce the activation-mediated phosphorylation of STAT-6. Flow cytometric analysis showed that IFN-γ pretreatment inhibited the phosphorylation of STAT-6 in monocytes by 65% compared to IL-4 and GM-CSF alone (Fig. 3a). STAT-6 is known to be involved in the transcription of IRF4 molecule, which is up-regulated during DC maturation [37]. Accordingly, the downstream effects of STAT-6 inhibition on the transcription of IRF4 gene were investigated. PCR demonstrated that the inhibition of STAT-6 in IFN-γ-DC reduced the expression of IRF4 by 78–95% compared to UT-DC (Fig. 3b). The IL-4α chain of the IL-4 receptor is essential in the recruitment and phosphorylation of STAT-6, which in turn regulates its own IL-4α chain expression [38]. The cell surface expression of IL-4α receptor subunit was examined, and it was found that IFN-γ down-regulated the protein expression of IL-4α chain subunit in treated monocytes to 29% compared 42% in IL-4 and GM-CSF alone. Monocytes alone had a baseline expression of 38% (Fig. 3c).
Fig. 3.
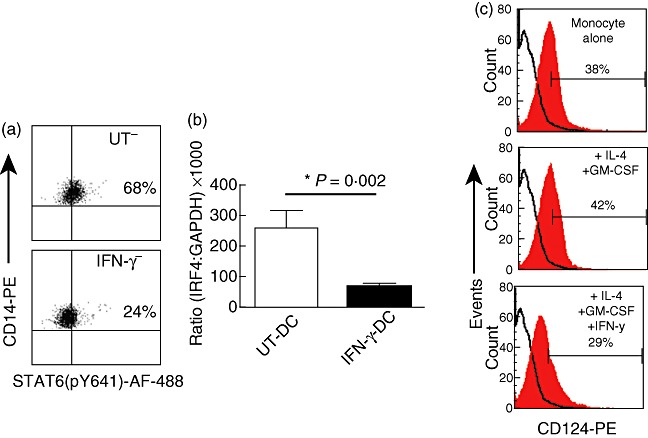
Interferon (IFN)-γ treatment of monocytes inhibits the phosphorylation of signal transducer and activator of transcription-6 (STAT-6), affecting the downstream expression of interferon regulatory factor 4 (IRF4). Isolated peripheral blood mononuclear cells (PBMC) treated with or without IFN-γ in the presence of interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) for 10 min were stained intracellularly with anti-STAT-6 (pY641)-AF-488 and surface-stained with CD14-phycoerythrin (PE) (a). IRF4 mRNA gene expression was determined by quantitative real-time polymerase chain reaction (PCR) in dendritic cells (DC) post-maturation with TNF-α and prostaglandin E2 (PGE2) (b). Mean ± standard deviation of quadruplicates. Representative of six independent experiments. IL-4 receptor expression was determined by fluorescence activated cell sorter (FACS) analysis using anti-CD124 24 h post-treatment with + or – IFN-γ (500 U) in the presence of IL-4 and GM-CSF. Solid black line indicates the isotype control and solid histogram shows the tested monoclonal antibody (c), representative of three independent experiments.
IFN-γ-DC induce T cell hyporesponsiveness associated with a reduction of IL-2 and IFN-γ
The stimulatory capacity of DC to induce T cell proliferation was measured in primary MLRs. IFN-γ-DC inhibited significantly the proliferation of allogeneic T cells by 71% at 1:10, 89% at 1:100 and 95% at 1:1000 DC to T cell ratios (Fig. 4). Analysis of supernatants demonstrated that T cell co-cultures with UT-DC contained significantly greater quantities of IFN-γ (UT-DC: 2300 pg/ml compared to IFN-γ-DC: 240 pg/ml, P = 0·003) and IL-2 (UT-DC: 40 pg/ml versus IFN-γ-DC: 20 pg/ml, P = 0·02), compared to IFN-γ-DC (Fig. 5).
Fig. 4.
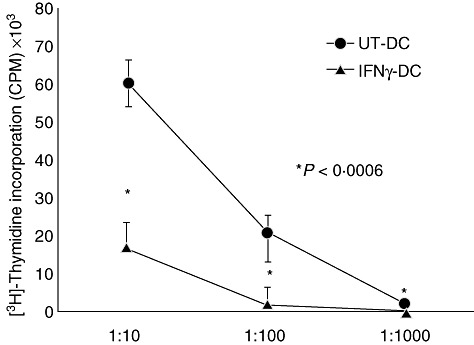
Interferon (IFN)-γ-dendritic cells (DC) cause T cell hyporesponsiveness. Monocytes were treated with 500 U of IFN-γ (IFN-γ-DC) or cultured in the absence of IFN-γ (UT-DC) for 24 h in the presence of interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF), then matured with tumour necrosis factor (TNF)-α and prostaglandin E2 (PGE2) for another 24 h. T cells (1 × 105) were incubated with DC, at a DC to T cell ratio of 1:10, 1:100 and 1:1000 for 5 days. Proliferation was determined by [3H]-thymidine incorporation (counts per minute). Mean ± standard deviation of quintuplicate within experiment. *P < 0·0006. Representative of 10 independent experiments.
Fig. 5.
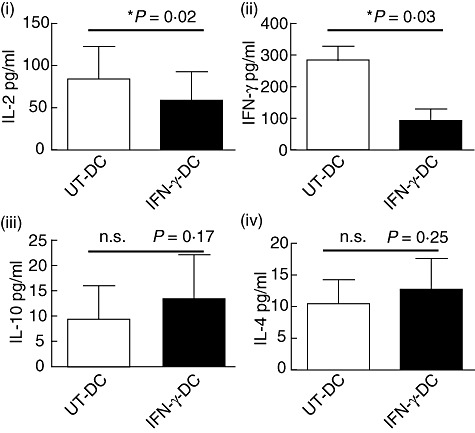
Interferon (IFN)-γ modulated dendritic cells (DC) inhibit the production of interleukin (IL)-2 and IFN-γ. DC were co-cultured with purified T cells at a 1:10 stimulator to responder ratio for 5 days. Supernatants from co-cultures were harvested and analysed by cytometric bead array assay for the production of IL-2 (i), IFN-γ (ii), IL-10 (iii) and IL-4 (iv). Mean ± standard deviation. *P < 0·02; n.s., not significant. Representative of four independent experiments.
IFN-γ-DC promote the generation of antigen-specific Tregs
It has been demonstrated previously that under conditions of human T cell activation FoxP3 protein can be expressed non-specifically in non-regulatory T cells, where the Foxp3hi population correlates with highly suppressive Tregs[39]. Based on this research, differences in the CD4+Foxp3hi T cell population from primary MLRs were assessed (for gating strategy please refer to Materials and methods) (Fig. 6). CD4+ T cells co-cultured with IFN-γ-DC had an increased frequency of CD4+Foxp3hi T cells of 23% compared to 12% that of T cells co-cultured with UT-DC (Fig. 6a). Cells within the Foxp3hi gate were also CD127neg/low and CD25+ (Fig. 6b)
Fig. 6.
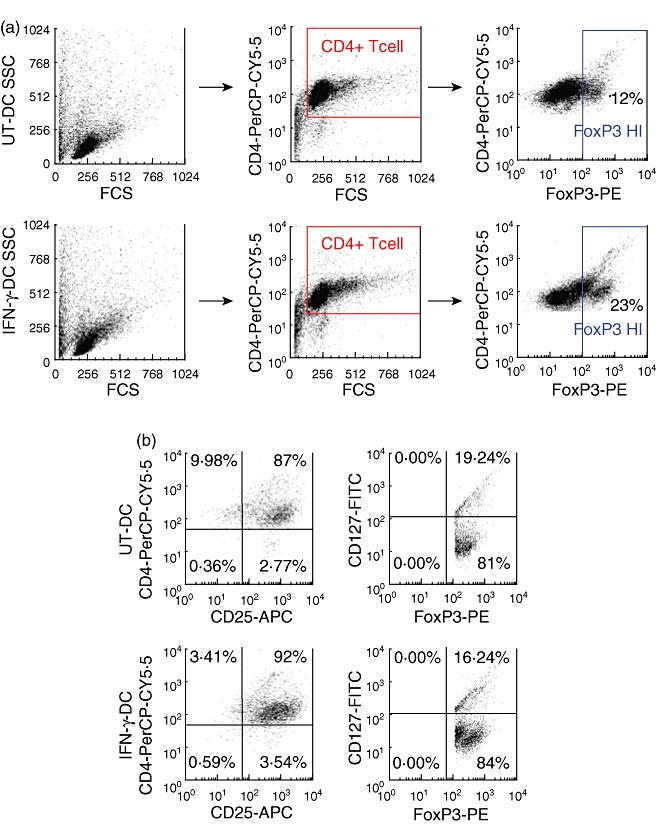
Interferon (IFN)-γ-dendritic cells (DC) promote the generation of CD4+CD25+CD127low/negforkhead box P3 (FoxP3+) T cells. DC were co-cultured for 5 days at a 1:10 DC to T cell ratio and then stained for surface expression of CD4, CD25, CD127 and intracellular expression of FoxP3. Samples were analysed using flow cytometry, which were gated for CD4+FoxP3hi T cell population; for gating strategy refer to Materials and methods. (a). Expression of CD25 and CD127 was also examined on CD4+FoxP3hi gated population (b). Fluorescence −1 (FMO) controls were used to determine negative and positive gates. This is a representative figure of four independent experiments.
Accordingly, the antigen-specific suppressive capacity of IFN-γ-DC primed T cells was assessed. IFN-γ-DC-primed T cells were co-cultured with a fixed number of autologous naive T cells at varying ratios (Fig. 7). T cell proliferation was suppressed by IFN-γ-DC primed T cells at 1:1 and 1:2 ratios by 70 and 49%, respectively. Moreover, at a 1:1 ratio, when stimulated instead with an irrelevant third-party donor DC, a significant increase in proliferation was observed compared to naive T cells alone (1:0 ratio) by 30% (P = 0·0004), indicating that IFN-γ-DC-generated Tregs are antigen-specific in their suppressive function.
Fig. 7.
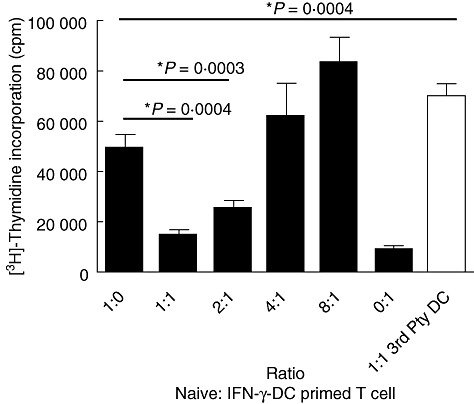
Interferon (IFN)-γ-dendritic cell (DC) primed T cells are antigen-specific in their suppressive function. IFN-γ-DC-primed T cells were isolated from primary mixed leucocyte reaction (MLR) and co-cultured with a fixed number of autologous naive CD4+ T cells (105) at varying ratios of 1 naive to 1 primed (1:1), 2:1, 4:1 or 8:1 and stimulated with UT-DC at a DC to naive T cell ratio of 1:10. At 1:1 naive to primed T cell co-cultures, an irrelevant third-party DC was used to determine the antigen specificity of regulatory T cells. Cells were cultured for 5 days and proliferation was determined by [3H]-thymidine incorporation (counts per minute). Mean ± standard deviation *P < 0·0004. Figure representative of four independent experiments.
Gene profiling of either early or late IFN-γ of rapidly generated DC by microarray analysis did not demonstrate major changes in gene signature
Our previous studies and studies by others have demonstrated that exposure of IFN-γ post-DC differentiation can act as a proinflammatory cytokine promoting DC to produce Th1 driving cytokines such as IL-12, thus involved in DC maturation. We also tested these opposing effects using the FAST-DC protocol and compared early versus late IFN-γ exposure. As shown in Fig. 8a, late IFN-γ exposure had minimal effects on the protein expression of DC maturation marker CD83 when compared to untreated DC, suggesting that DC maturation is not affected significantly when IFN-γ is added late after the first 24 h of differentiation. The differences in the effect of IFN-γ in gene expression signatures between the two treatments were analysed by microgene array. Post-data filtering and normalization, hierarchical clustering was performed to generate a heat map for data visualization, as shown in Fig. 8b, which demonstrates visually that IFN-γ exposure either early or late follow similar patterns of gene induction and reduction. Similarly, when the top seven genes that displayed increased gene expression above 20-fold, compared to no IFN-γ treatment (UT-DC) were graphed (Fig. 8c), both early and late IFN-γ exposure had similar induction of genes.
Fig. 8.
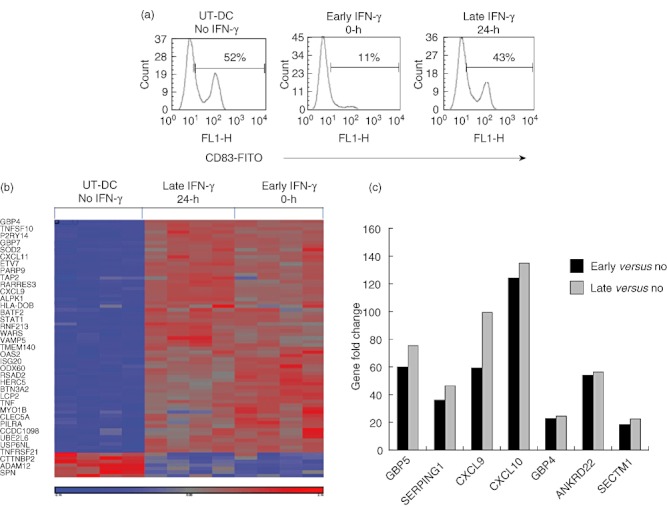
Early interferon (IFN)-γ exposure of FAST-dendritic cells (DC) do not have a significantly different gene expression profile compared to late IFN-γ exposure. DC were either treated with IFN-γ at time 0 h (early) or exposed to IFN-γ after 24 h of differentiation (late), or were left untreated (no). All DC were generated in the presence of interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF). DC were first stained for CD83, to confirm differences in IFN-γ exposure. (a) Representative of three independent experiments. Total RNA from DC samples were extracted and analysed by a human Affymetrix gene array chip for differences in gene signature, hierarchical clustering was used to generate a heat map for data visualization (b). Analysis based on four independent experiments. Genes of greater than 20-fold increase in comparison to UT-DC were graphed, based on step-up statistical analysis (c).
Discussion
The toxicities inherent in modern immunosuppressive therapy have prompted efforts to develop novel drug-free ways to manipulate or condition the immune system to promote allograft acceptance or tolerance. DCs are a prime cell type for manipulation through their unique ability to dampen immune responses and their known role in maintenance of self-tolerance at both central and peripheral sites.
IFN-γ is a pleiotropic cytokine generally implicated in inflammatory responses and well known for playing crucial roles in promoting DC maturation and production of Th1 cytokines such as IL-12. It is paradoxical to imply that IFN-γ may also act as a tolerogenic cytokine for in-vitro-generated DC, and at present it is unclear if these mechanisms exist in vivo, although a body of evidence already exists that IFN-γ has important tolerogenic function. Recent studies identified a key role in vivo for IFN-γ secreted from T cell receptor (TCR)-αβ+CD3+CD4-CD8-NKRP1- cells in the spleens of animals that received autologous tolerogenic DC [40]. Previous studies from Kathryn Wood's group have also shown that CD4+ Treg function is modulated through IFN-γ[41]. We and others have investigated previously the immunomodulatory role of IFN-γ using a standard 7-day protocol to generate monocyte-derived DC, and have shown a reduced capacity of these DC to induce T cell proliferation. IFN-γ-modulated DC are maturation-arrested with increased expression of inhibitory molecules [32,42]. IFN-γ released in an allogeneic MLR has also been shown to play a key role in promoting the generation of tolerogenic dendritic cells (Tol-DC) [43]. Thus, IFN-γ may also have immunoregulatory properties in addition to its well-known immunostimulatory role in immunity.
In this study, the mechanism of action of IFN-γ to promote tolerogenic DC phenotype was investigated and a protocol to promote the generation of maturation-arrested Tol-DC in 48 h was generated. Rapidly generated IFN-γ-DC express DC-specific marker c-type lectin, CD209 (DC-SIGN), CD11c and HLA-DR, but have reduced CD83 and B7 molecule expression (Fig. 1). In 1995 CD83 was characterized as a maturation marker for DC [44]. Early studies demonstrated that herpes simplex virus type 1-infected iDC failed to induce the expression of CD83 during maturation without affecting the expression of CD80 and CD86, resulting in a poor capacity to stimulate T cells. This was the first evidence to suggest that CD83 is essential in enhancing T cell activation [45]. More recently, the function of CD83 was investigated further with the aid of siRNA knock-down of CD83 showing that CD83 functions as an enhancer of T cell activation [46]. The inhibition of CD83 in IFN-γ-DC is therefore an important factor contributing to their poor stimulatory capacity of these antigen-presenting cells.
Mechanistically, IFN-γ reduced the expression NF-κB transcription factor RELB, which failed to co-localize into the nucleus of monocytes (Fig. 2b); this correlated with reduced RELB gene expression (Fig. 2a). The nuclear translocation of RELB is a known hallmark of DC maturation [20]. The importance of the NF-κB pathway in DC development was shown further in KO mice lacking components of NF-κB, where these mice failed to generate mature DC [21]. Translocation of NF-κB transcription factors to the nucleus are known to interact with κB sites in the regulatory region of target genes that control the expression of major histocompatibility complex (MHC) class II, CD80, CD86 and CD40 [47,48]. In addition, NF-κB regulates the expression of CD83 [49]. Thus, the down-regulation of positive co-stimulatory molecules, including CD83 seen in IFN-γ-DC, may be a downstream effect of the inhibition of RELB expression and nuclear translocation. Moreover, studies using maturation-resistant donor-derived RELB silenced DC functionally induce antigen-specific tolerance in vivo, thus prolonging murine heart allotransplantation [50]. Others have also reported the blocking of NF-κB using pharmacological agents, such as proteasome inhibitor PSI, LF 15–0195, aspirin, N-acetyl-cysteine, cyclosporin and tacrolimus, which subsequently inhibit DC maturation [16,51]–[54]. DC maturation arrest evident in IFN-γ-DC was accompanied with low production of biologically active IL-12p70 secretion and IL-12p40 gene expression (Fig. 2d,c).
The antagonist effects of IFN-γ on IL-4 were also observed by this study. IFN-γ-DC had reduced STAT-6 phosphorylation significantly compared to UT-DC (Fig. 3a). This is consistent with previous observations, showing that IFN-γ treatment of monocytes reduced IL-4 activation of STAT-6 by suppressing the expression of the IL-4 receptor [55]. We confirmed that rapidly generated IFN-γ-DC in the presence of IL-4 and GM-CSF also resulted in the downstream inhibition of the IL-4α receptor (Fig. 3c). Transcription factor IRF4, a molecule also implicated in DC development and maturation, was also down-regulated by the inhibition of STAT-6 phosphorylation (Fig. 3b). The IRF4 promoter region has a STAT-6-dependent element that specifically up-regulates IRF4 in response to IL-4 [37]. The relatively low expression of IRF4 may contribute to the lack of responsiveness of IFN-γ-DC to mature. IRF4 also binds to transcription factor PU.1 to negatively regulate the expression of ILT4. Reduced expression of IRF4 may therefore contribute to sustained expression of ILT4 (Fig. 1) [56]. The up-regulation of ILT3 and ILT4 via inhibition of NF-κB is also known to render human monocytes and DC tolerogenic [57].
T cell hyporesponsiveness induced by IFN-γ-DC (Fig. 4) correlates with reduced expression of positive co-stimulatory molecules and proinflammatory cytokines, making IFN-γ-DC poor T cell stimulators, a characteristic associated with promotion of a tolerogenic T cell response [58]. The reduction of IL-2 and IFN-γ in IFN-γ-DC co-cultures (Fig. 5) also potentially contributes to T cell hyporesponsiveness. T cell phenotypes from primary MLR were also examined by FACS (Fig. 6). Miyara et al. showed previously in that activated human T cells can express FoxP3 non-specifically in non-regulatory effector T cells defined by the FoxP3lo subpopulation of CD4+ T cells; the FoxP3hi subpopulation, however, is highly suppressive in its capacity to regulate T cell immune responses. In our previous study with 7-day cultures [32], Treg cells were only investigated using intracellular flow cytometry on CD4+ gating, which revealed a reduced number of Tregs promoted by day 0 IFN-γ-treated DC compared to UT-DC. Functional analysis of these cells was not performed. As the function of the cells may be more important than the absolute numbers of the cells, we performed a more sophisticated analysis of the potential Treg population in this study incorporating CD4, CD25 and CD127 fluorochromes (and fluorescence −1 controls) in addition to classical suppression assays. The Foxp3hi subpopulation of CD4+ T cells was examined in this study, which demonstrated that IFN-γ-DC promoted a higher frequency of CD4+Foxp3hi T cells which were also CD25+CD127neg/low when compared to UT-DC co-cultures. Moreover, our study demonstrated that IFN-γ-DC primed T cells are antigen-specific in their ability to functionally suppress the proliferation of naive T cells (Fig. 7).
To address the paradoxical effect of IFN-γ as a proinflammatory cytokine known to promote maturation, IFN-γ was added to monocytes 24 h post-treatment to IL-4 and GM-CSF (DC differentiation period) and added it in combination with maturation agents TNF-α and PGE2. At the protein level we observed that late IFN-γ exposure had minimal effects on the expression of DC maturation marker CD83 when compared to UT-DC, while early IFN-γ (at time 0 in the presence of IL-4 and GM-CSF) had a significant decrease. This difference in CD83 expression suggested that previously reported paradoxical effects of IFN-γ were also present in this study. To investigate these differences further we employed the use of microRNA gene analysis. Interestingly, we found that addition of IFN-γ either early or late induced similar gene expression patterns (Fig. 8b,c), despite the difference seen at the protein level, suggesting that the mechanistic difference in IFN-γ functions as proinflammatory or anti-inflammatory, probably occurring at the protein level rather than at the gene level. The diverse gene effects of IFN-γ also suggest that the mechanism of tolerance induction by IFNγ-DC may also be multi-factorial.
A recent study by Hill et al. demonstrated that IFN-γ-induced tolerance in an allogeneic rat heart transplant model was dependent on the expression of Epstein–Bar virus-induced gene-3 (EBI-3), expressed by autologous tolerogenic DC. EBI-3 is an IL-12 member that heterodimerizes to form immunoregulatory molecules IL-27 and IL-35 [40]. Accordingly, the expression of EBI-3 by tolerogenic DC may play an important role in in-vivo tolerance induction. The role and expression EBI-3 by IFN-γ-DC was not established in this study; however, in light of these new data it may also be an important pathway to investigate further in the context of IFN-γ-DC tolerance induction.
In conclusion, early exposure of CD14+ monocytes to IFN-γ inhibits efficient phosphorylation of IL-4-activated STAT-6 reducing downstream expression of IRF4. This, accompanied by the inhibition of NF-κB transcription factor RELB, a crucial molecule in DC maturation, contributes to the down-regulation of DC-positive co-stimulatory molecules including CD83, thus producing maturation-arrested ‘FAST’ DC that promote antigen-specific suppressive T cells. To our knowledge, this paper is the first of its kind to describe a rapid DC protocol to produce Tol-DC. The ability to generate Tol-DC in a short time-frame is fundamental in the ability for novel human DC cellular therapy to be transferred into the clinic for the potential treatment of allogeneic transplant rejection by adoptive cellular therapy.
Acknowledgments
The authors would like to acknowledge the support of the Queen Elizabeth Hospital Research Foundation, the Juvenile Diabetes Research Foundation (PTC) and National Health and Medical Research Council of Australia (PTC). Darling Rojas-Canales is a recipient of The Queen Elizabeth Hospital Research Foundation Postgraduate Pam and Ian Wall Scholarship and the University of Adelaide Faculty of Health Science Divisional Scholarship. Claire F. Jessup receiced a C.J. Martin Fellowship from NHMRC.
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Hart DN, Newton MR, Reece-Smith H, Fabre JW, Morris PJ. Major histocompatibility complex antigens in the rat pancreas, isolated pancreatic islets, thyroid, and adrenal. Transplantation. 1983;36:431–5. doi: 10.1097/00007890-198310000-00015. [DOI] [PubMed] [Google Scholar]
- 3.McKenzie JL, Beard ME, Hart DN. The effect of donor pretreatment on interstitial dendritic cell content and rat cardiac allograft survival. Transplantation. 1984;38:371–6. doi: 10.1097/00007890-198410000-00011. [DOI] [PubMed] [Google Scholar]
- 4.McKenzie JL, Beard ME, Hart DN. Depletion of donor kidney dendritic cells prolongs graft survival. Transplant Proc. 1984;16:948–51. [PubMed] [Google Scholar]
- 5.Coates PT, Thomson AW. Dendritic cells, tolerance induction and transplant outcome. Am J Transplant. 2002;2:299–307. doi: 10.1034/j.1600-6143.2002.20403.x. [DOI] [PubMed] [Google Scholar]
- 6.Talmage DW, Dart G, Radovich J, Lafferty KJ. Activation of transplant immunity: effect of donor leukocytes on thyroid allograft rejection. Science. 1976;191:385–8. doi: 10.1126/science.1082167. [DOI] [PubMed] [Google Scholar]
- 7.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–14. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lafferty KJ, Prowse SJ, Simeonovic CJ, Warren HS. Immunobiology of tissue transplantation: a return to the passenger leukocyte concept. Annu Rev Immunol. 1983;1:143–73. doi: 10.1146/annurev.iy.01.040183.001043. [DOI] [PubMed] [Google Scholar]
- 9.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newland A, Russ G, Krishnan R. Natural killer cells prime the responsiveness of autologous CD4+ T cells to CTLA4-Ig and interleukin-10 mediated inhibition in an allogeneic dendritic cell-mixed lymphocyte reaction. Immunology. 2006;118:216–23. doi: 10.1111/j.1365-2567.2006.02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–66. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 12.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Interleukin-10 prevents the generation of dendritic cells from human peripheral blood mononuclear cells cultured with interleukin-4 and granulocyte/macrophage-colony-stimulating factor. Eur J Immunol. 1997;27:756–62. doi: 10.1002/eji.1830270326. [DOI] [PubMed] [Google Scholar]
- 14.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 15.Xia CQ, Peng R, Beato F, Clare-Salzler MJ. Dexamethasone induces IL-10-producing monocyte-derived dendritic cells with durable immaturity. Scand J Immunol. 2005;62:45–54. doi: 10.1111/j.1365-3083.2005.01640.x. [DOI] [PubMed] [Google Scholar]
- 16.Hackstein H, Morelli AE, Larregina AT, et al. Aspirin inhibits in vitro maturation and in vivo immunostimulatory function of murine myeloid dendritic cells. J Immunol. 2001;166:7053–62. doi: 10.4049/jimmunol.166.12.7053. [DOI] [PubMed] [Google Scholar]
- 17.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 18.Rogers NM, Kireta S, Coates PT. Curcumin induces maturation-arrested dendritic cells that expand regulatory T cells in vitro and in vivo. Clin Exp Immunol. 2010;162:460–73. doi: 10.1111/j.1365-2249.2010.04232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lutz MB, Schnare M, Menges M, et al. Differential functions of IL-4 receptor types I and II for dendritic cell maturation and IL-12 production and their dependency on GM-CSF. J Immunol. 2002;169:3574–80. doi: 10.4049/jimmunol.169.7.3574. [DOI] [PubMed] [Google Scholar]
- 20.Neumann M, Fries H, Scheicher C, et al. Differential expression of Rel/NF-kappaB and octamer factors is a hallmark of the generation and maturation of dendritic cells. Blood. 2000;95:277–85. [PubMed] [Google Scholar]
- 21.Ouaaz F, Arron J, Zheng Y, Choi Y, Beg AA. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity. 2002;16:257–70. doi: 10.1016/s1074-7613(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 22.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 23.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–12. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 24.Konieczny BT, Dai Z, Elwood ET, et al. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998;160:2059–64. [PubMed] [Google Scholar]
- 25.Markees TG, Phillips NE, Gordon EJ, et al. Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4(+) T cells, interferon-gamma, and CTLA4. J Clin Invest. 1998;101:2446–55. doi: 10.1172/JCI2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saleem S, Konieczny BT, Lowry RP, Baddoura FK, Lakkis FG. Acute rejection of vascularized heart allografts in the absence of IFNgamma. Transplantation. 1996;62:1908–11. doi: 10.1097/00007890-199612270-00039. [DOI] [PubMed] [Google Scholar]
- 27.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–8. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 29.Manoury-Schwartz B, Chiocchia G, Bessis N, et al. High susceptibility to collagen-induced arthritis in mice lacking IFN-gamma receptors. J Immunol. 1997;158:5501–6. [PubMed] [Google Scholar]
- 30.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J Immunol. 1997;158:5507–13. [PubMed] [Google Scholar]
- 31.Wu X, Hou W, Sun S, et al. Novel function of IFN-gamma: negative regulation of dendritic cell migration and T cell priming. J Immunol. 2006;177:934–43. doi: 10.4049/jimmunol.177.2.934. [DOI] [PubMed] [Google Scholar]
- 32.Rojas D, Krishnan R. IFN-gamma generates maturation-arrested dendritic cells that induce T cell hyporesponsiveness independent of Foxp3(+) T-regulatory cell generation. Immunol Lett. 2010;132:31–7. doi: 10.1016/j.imlet.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–47. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 34.Einecke G, Mengel M, Hidalgo L, Allanach K, Famulski KS, Halloran PF. The early course of kidney allograft rejection: defining the time when rejection begins. Am J Transplant. 2009;9:483–93. doi: 10.1111/j.1600-6143.2008.02546.x. [DOI] [PubMed] [Google Scholar]
- 35.Dauer M, Obermaier B, Herten J, et al. Mature dendritic cells derived from human monocytes within 48 h: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170:4069–76. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 36.Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry. 2006;69:1037–42. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- 37.Lehtonen A, Veckman V, Nikula T, et al. Differential expression of IFN regulatory factor 4 gene in human monocyte-derived dendritic cells and macrophages. J Immunol. 2005;175:6570–9. doi: 10.4049/jimmunol.175.10.6570. [DOI] [PubMed] [Google Scholar]
- 38.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 39.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Hill M, Thebault P, Segovia M, et al. Cell therapy with autologous tolerogenic dendritic cells induces allograft tolerance through interferon-gamma and Epstein–Barr virus-induced Gene 3. Am J Transplant. 2011;11:2036–45. doi: 10.1111/j.1600-6143.2011.03651.x. [DOI] [PubMed] [Google Scholar]
- 41.Sawitzki B, Kingsley CJ, Oliveira V, Karim M, Herber M, Wood KJ. IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201:1925–35. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rongcun Y, Maes H, Corsi M, Dellner F, Wen T, Kiessling R. Interferon gamma impairs the ability of monocyte-derived dendritic cells to present tumour-specific and allo-specific antigens and reduces their expression of CD1A, CD80 AND CD4. Cytokine. 1998;10:747–55. doi: 10.1006/cyto.1998.0357. [DOI] [PubMed] [Google Scholar]
- 43.Eljaafari A, Li YP, Miossec P. IFN-gamma, as secreted during an alloresponse, induces differentiation of monocytes into tolerogenic dendritic cells, resulting in FoxP3+ regulatory T cell promotion. J Immunol. 2009;183:2932–45. doi: 10.4049/jimmunol.0804352. [DOI] [PubMed] [Google Scholar]
- 44.Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–35. [PubMed] [Google Scholar]
- 45.Kruse M, Rosorius O, Kratzer F, et al. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J Virol. 2000;74:7127–36. doi: 10.1128/jvi.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prechtel AT, Turza NM, Theodoridis AA, Steinkasserer A. CD83 knockdown in monocyte-derived dendritic cells by small interfering RNA leads to a diminished T cell stimulation. J Immunol. 2007;178:5454–64. doi: 10.4049/jimmunol.178.9.5454. [DOI] [PubMed] [Google Scholar]
- 47.Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–46. [PubMed] [Google Scholar]
- 48.Denk A, Wirth T, Baumann B. NF-kappaB transcription factors: critical regulators of hematopoiesis and neuronal survival. Cytokine Growth Factor Rev. 2000;11:303–20. doi: 10.1016/s1359-6101(00)00009-5. [DOI] [PubMed] [Google Scholar]
- 49.McKinsey TA, Chu Z, Tedder TF, Ballard DW. Transcription factor NF-kappaB regulates inducible CD83 gene expression in activated T lymphocytes. Mol Immunol. 2000;37:783–8. doi: 10.1016/s0161-5890(00)00099-7. [DOI] [PubMed] [Google Scholar]
- 50.Li M, Zhang X, Zheng X, et al. Immune modulation and tolerance induction by RelB-silenced dendritic cells through RNA interference. J Immunol. 2007;178:5480–7. doi: 10.4049/jimmunol.178.9.5480. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimura S, Bondeson J, Brennan FM, Foxwell BM, Feldmann M. Role of NFkappaB in antigen presentation and development of regulatory T cells elucidated by treatment of dendritic cells with the proteasome inhibitor PSI. Eur J Immunol. 2001;31:1883–93. doi: 10.1002/1521-4141(200106)31:6<1883::aid-immu1883>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 52.Yang J, Bernier SM, Ichim TE, et al. LF15-0195 generates tolerogenic dendritic cells by suppression of NF-kappaB signaling through inhibition of IKK activity. J Leukoc Biol. 2003;74:438–47. doi: 10.1189/jlb.1102582. [DOI] [PubMed] [Google Scholar]
- 53.Verhasselt V, Vanden Berghe W, Vanderheyde N, Willems F, Haegeman G, Goldman M. N-acetyl-L-cysteine inhibits primary human T cell responses at the dendritic cell level: association with NF-kappaB inhibition. J Immunol. 1999;162:2569–74. [PubMed] [Google Scholar]
- 54.Lee JI, Ganster RW, Geller DA, Burckart GJ, Thomson AW, Lu L. Cyclosporine A inhibits the expression of costimulatory molecules on in vitro-generated dendritic cells: association with reduced nuclear translocation of nuclear factor kappa B. Transplantation. 1999;68:1255–63. doi: 10.1097/00007890-199911150-00007. [DOI] [PubMed] [Google Scholar]
- 55.Bonder CS, Davies KV, Hosszu EK, Finlay-Jones JJ, Hart PH. IFN-gamma downregulates interleukin-4 functional activity on monocytes by multiple mechanisms. J Interferon Cytokine Res. 2002;22:287–93. doi: 10.1089/107999002753675703. [DOI] [PubMed] [Google Scholar]
- 56.Nakajima H, Asai A, Okada A, et al. Transcriptional regulation of ILT family receptors. J Immunol. 2003;171:6611–20. doi: 10.4049/jimmunol.171.12.6611. [DOI] [PubMed] [Google Scholar]
- 57.Chang CC, Ciubotariu R, Manavalan JS, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 58.Thomson AW, Turnquist HR, Zahorchak AF, Raimondi G. Tolerogenic dendritic cell-regulatory T-cell interaction and the promotion of transplant tolerance. Transplantation. 2009;87:S86–90. doi: 10.1097/TP.0b013e3181a2dcec. [DOI] [PMC free article] [PubMed] [Google Scholar]


