Abstract
Leishmaniasis is a group of important parasitic diseases affecting millions worldwide. To understand more clearly the quality of T helper type 1 (Th1) response stimulated after Leishmania infection, we applied a multiparametric flow cytometry protocol to evaluate multifunctional T cells induced by crude antigen extracts obtained from promastigotes of Leishmania braziliensis (LbAg) and Leishmania amazonensis (LaAg) in peripheral blood mononuclear cells from healed cutaneous leishmaniasis patients. Although no significant difference was detected in the percentage of total interferon (IFN)-γ-producing CD4+T cells induced by both antigens, multiparametric flow cytometry analysis revealed clear differences in the quality of Th1 responses. LbAg induced an important proportion of multifunctional CD4+ T cells (28% of the total Th1 response evaluated), whereas LaAg induced predominantly single-positive cells (68%), and 57% of those were IFN-γ single-positives. Multifunctional CD4+T cells showed the highest mean fluorescence intensity (MFI) for the three Th1 cytokines assessed and MFIs for IFN-γ and interleukin-2 from those cells stimulated with LbAg were significantly higher than those obtained after LaAg stimulation. These major differences observed in the generation of multifunctional CD4+ T cells suggest that the quality of the Th1 response induced by L. amazonensis antigens can be involved in the mechanisms responsible for the high susceptibility observed in L. amazonensis-infected individuals. Ultimately, our results call attention to the importance of studying a Th1 response regarding its quality, not just its magnitude, and indicate that this kind of evaluation might help understanding of the complex and diverse immunopathogenesis of American tegumentary leishmaniasis.
Keywords: human American cutaneous leishmaniasis, Leishmania amazonensis, Leishmania braziliensis, multifunctional T cells
Introduction
Leishmaniasis is a group of sandfly-transmitted diseases caused by different species of protozoan parasites from the genus Leishmania, affecting 88 countries around the world [1]. The diverse clinical presentations depend upon which Leishmania species is involved and also upon host-related factors. American tegumentary leishmaniasis (ATL) is endemic in widespread areas of Latin America, and the main causative agents include species from the subgenus Viannia (Leishmania (Viannia) braziliensis, L. (V.) guyanensis, L. (V.) panamensis) and the subgenus Leishmania (Leishmania (Leishmania) amazonensis, L. (L.) mexicana) [1]. In addition to being a public health problem in the New World, ATL is a risk for those who travel to Latin America [2].
Human infection with Leishmania braziliensis leads to a broad spectrum of clinical, immunological and histopathological manifestations, ranging from self-healing cutaneous lesions to the severe and destructive clinical form named mucocutaneous leishmaniasis (ML) [3]–[5]. The localized cutaneous form (CL) usually manifests as one or a few ulcers with elevated borders and sharp crater that increase rapidly in size and heal slowly without treatment [6]. L. braziliensis can also cause disseminated leishmaniasis, in which up to hundreds of lesions erupt as a result of haematogenous spread of parasite [7,8]. L. amazonensis has also been isolated from patients with diverse clinical forms, including CL and diffuse cutaneous leishmaniasis (DCL) [9]. Patients with DCL are often resistant to chemotherapy, have negative leishmanin skin test and low or negative responses after Leishmania antigen-specific stimulation in vitro, but remain responsive for other unrelated antigens, such as tuberculin [3,10]. The mechanisms responsible for this specific cell-mediated immune response suppression remain unclear. A high degree of variability in cross-immunity between the New World Leishmania species in humans as well as in simian models has also been observed [11]–[14].
Currently, it is well established that the T helper type 1 (Th1) immune response is important for protection against intracellular parasites. Previous studies have demonstrated that CL caused by L. braziliensis is associated with an early establishment of efficient parasite-killing mechanisms with a balance between Th1 and Th2 responses, which is associated with the control of exacerbated inflammatory responses and lesion healing. In contrast, individuals who develop ML display an exacerbated Th1 response associated with lower levels of interleukin (IL)-10 and lower expression of IL-10 receptors, in comparison to CL patients [15]–[18]. Even though we have made great progress in understanding the immunopathology of human ATL, many questions still remain, especially regarding Leishmania-specific Th1 response induction, regulation and persistence.
After specific activation, naive CD4+T cells undergo a complex differentiation programme before developing into Th1 cells [19]. The amount and duration of antigenic stimulation [20], the type of antigen-presenting cell [21], the anatomic site of immunization and the cytokine milieu [22] all seem to determine the magnitude and quality of the Th1 response elicited. Differences in cytokine production can also have profound implications in this fine-tuned differentiation programme, as CD4+T cells that secrete only IFN-γ have a self-limited capacity to develop into memory T cells when compared to IL-2+- or IL-2+IFN-γ+-producing cells [23,24]. CD4+T cells obtained from human immunodeficiency virus (HIV)-infected long-term non-progressors (LTNP) individuals, or those treated with antiretroviral therapy, have increased frequencies of IL-2+ or IL-2+IFN-γ+ T cells, whereas progressors had predominantly IFN-γ single-producing CD4+T cells [25]–[28]. Similar results were found in chronic hepatitis C virus (HCV) [29] and Mycobacterium tuberculosis infections [30]. Using the multiparametric flow cytometry approach, and including tumour necrosis factor (TNF)-α production as another parameter of investigation, it clearly demonstrated a correlation between protective immunity and the induction of a high frequency of IFN-γ+TNF-α+IL-2+-producing CD4+T cells (termed multifunctional T cells) after vaccination with protein plus cytosine–phosphate–guanosine oligodeoxynucleotide (CpG ODN) in experimental L. major infection. Conversely, poor or non-protective vaccine strategies induced mainly T cells producing only one or two different cytokines [31]. The same pattern was observed in vaccine studies for tuberculosis [32,33], malaria [34] and Chlamydia infection [35].
To first evaluate the generation of multifunctional T cells in human leishmaniasis we performed a multiparametric flow cytometry analysis in peripheral blood mononuclear cells (PBMC) obtained from healed Brazilian CL patients after stimulation in vitro with total crude antigen extracts obtained from stationary phase promastigotes of L. amazonensis, the causative agent of DCL, and also from L. braziliensis, regarded as the most important cause of ATL in Brazil [36]. A better understanding in the induction of multifunctional T cells in human disease may help to clarify mechanisms associated with the diverse clinical manifestations of ATL and the immunopathological factors involved in cure and protection, which will certainly help in the development of vaccines and/or immunotherapeutical strategies against human leishmaniasis.
Materials and methods
Patients
A group of 18 ATL patients with clinical history of localized CL lesions (11 male and seven female, aged 40·3 ± 16 years) was recruited from Evandro Chagas Clinical Research Institute (IPEC), Oswaldo Cruz Foundation (FIOCRUZ) in Rio de Janeiro, Brazil. PBMC were obtained from the patients approximately 110 days after completing the antimonial therapy, when lesions were considered healed. They were diagnosed based on immunological and parasitological criteria, as described previously [37], and treated with meglumine antimoniate. Parasites were isolated from the lesions of 15 patients and L. braziliensis infection was confirmed by characterization with isoenzyme electrophoresis [38], using five enzymatic loci: 6-phosphogluconate dehydrogenase (6PGDH; EC.1·1.1·43); phosphoglucose isomerase (GPI; EC.5·3.1·9); nucleoside hydrolase (NH; two loci, EC.3·2.2·1); glucose-6-phosphate dehydrogenase (G6PDH; EC.1·1.1·49); and phosphoglucomutase (PGM; EC.1·4.1·9). Reference samples of L. (Viannia) braziliensis (MHOM/BR/75/M2903) were used in all the electrophoretic runs. A control group from non-endemic areas, comprised of 14 healthy subjects (six male and eight female, aged 28 ± 7·1 years), was also evaluated in parallel. This study was approved by the National Ethical Clearance Committee of Brazil (CONEP), as well as by the Ethical Committee for Human Research from IPEC/FIOCRUZ, all of which adhere to the principles laid out in the Declaration of Helsinki. Informed consent was obtained from all participants.
Antigens
Promastigotes of L. braziliensis (MCAN/BR/98/R69) and L. amazonensis (IFLA/BR/67/PH8) were cultured in Schneider's medium supplemented with antibiotics (200 IU penicillin and 200 µg streptomycin/ml) and 10% inactivated fetal calf serum (all from Sigma-Aldrich, St Louis, MO, USA). Stationary phase promastigotes were washed three times in phosphate-buffered saline (PBS), and disrupted by 10 freeze and thaw cycles, followed by ultrasonication (Ultra-tip Labsonic System; Laboratory-Line, Melrose Park, IL, USA), at 40 watts for 15 min in an ice bath, to generate the crude extracts of L. braziliensis (LbAg) and L. amazonensis (LaAg). All antigenic preparations were adjusted to 1 mg/ml protein nitrogen in PBS and stored at −70°C until use.
PBMC culture and IFN-γ enzyme-linked immunosorbent assay (ELISA)
PBMCs were isolated from heparinized venous blood by Ficoll–Hypaque gradient centrifugation (Sigma). After being washed three times in PBS, the PBMC were resuspended in RPMI-1640 medium (Sigma) supplemented with 10% human AB serum, 10 mM HEPES, 1·5 mM l-glutamine, 0·04 mM 2-mercaptoethanol and antibiotics (200 IU/ml penicillin and 200 mg/ml streptomycin) (all from Sigma). Cells were adjusted to 3 × 106 cells/ml, added to 24-well plates and kept unstimulated or were stimulated with 50 µg/ml of each Leishmania crude antigen or 20 µg/ml of concanavalin A (ConA; Sigma) for 5 days at 37°C, in a 5% CO2 incubator. After this time, the supernatants were collected and stored frozen at −70°C until analysed for IFN-γ production by a commercial ELISA kit (BD Pharmingen, San Diego, CA, USA). The procedures were performed according to the manufacturer's instructions. Samples were tested in duplicate and concentration was analysed using the SOFTmax®PRO version 4·0 program (Life Sciences Edition; Molecular Devices Corporation, Sunnyvale, CA, USA). Results were expressed as picograms per millilitre. The minimum IFN-γ level detected was 7·8 pg/ml.
Multiparametric flow cytometry
A total of 3 × 106 PBMCs of each individual were kept at rest, unstimulated, or were stimulated with 50 µg/ml of either Leishmania crude antigens in the presence of 2 µg/ml antibody to CD28 (e-Bioscience, San Diego, CA, USA) for 2 h at 37°C, in a 5% CO2 incubator. ConA was also used as a positive control (20 µg/ml; Sigma). Brefeldin A (BFA; Sigma) was added to all cultures at a final concentration of 10 µg/ml and cells were incubated for an additional 12 h before staining. Cells were then harvested and stained for surface markers using allophycocyanin-cyanin-7 (APC-Cy7)-labelled anti-CD4, phycoerythrin (PE)-Texas red-labelled anti-CD8 (Invitrogen, Carlsbad, CA, USA) and PE-Cy5-labelled anti-CD25 monoclonal antibodies by incubation for 30 min in PBS containing 0·1% bovine serum albumin (BSA) and 0·05% sodium azide (all from Sigma-Aldrich), followed by washes and fixation using 4% formaldehyde solution (Sigma-Aldrich). These cells were permeabilized with 0·5% saponin solution in PBS/BSA (SAP buffer). After 1 h permeabilization at 4°C cells were incubated, for additional 30 min, with the cytokine antibodies PE-Cy7-labelled anti-IFN-γ, fluorescein isothiocyanate (FITC)-labelled anti-TNF-α, APC-labelled anti-IL-2 and PE-labelled anti-IL-10, washed with SAP buffer and resuspended in PBS/BSA. All antibodies were purchased from e-Bioscience except when noted. A minimum of 50 000 events per sample were acquired inside the lymphocytes gate, based on size and granularity properties, in a CyAn ADP flow cytometer device (Beckman-Coulter/Dako, Brea, CA, USA) and analysed using FlowJo software (Tree Star Inc., Ashland, OR, USA).
Statistical analysis
Statistical comparisons were performed by a two-tailed Wilcoxon matched-pairs signed-ranks, Mann–Whitney U-test (in the comparison between patients and control groups) and Spearman's correlation tests, using GraphPad Prism version 5·0 software (GraphPad Software, La Jolla, CA, USA). All cytokine frequencies, mean fluorescence intensity (MFI) and iMFI values reported are after background subtraction of the frequency, MFI or integrated MFI (iMFI) of the identically gated population of cells from the same sample cultured without antigen. Statistical significance was assigned to P ≤ 0·05.
Results
Single-parameter evaluation of cytokine producing CD4+ T cells: analysis via iMFI of cytokine-expressing cells can make a difference
The majority of studies that evaluate immune responses in human leishmaniasis usually estimate the frequency of antigen-specific IFN-γ and other Th1-related cytokine-producing cells, as a key immune correlate of a protective response. In a former report, Darrah et al. [31] developed a metric approach in order to evaluate the total response of a given population of cytokine-producing cells that combine the magnitude and quality of T cell responses multiplying the frequency of cytokine-expressing cells by the cytokine MFI, termed iMFI. After applying this novel metric approach to our data we were able to detect more pronounced differences between healed CL patients and control groups for both Leishmania crude antigen preparations than when using only the frequencies of cytokine-positive cells (Fig. 1a and b). More significantly, we found that LbAg-stimulated CD4+T cells have considerably higher iMFIs for IFN-γ, TNF-α and IL-2 in comparison to LaAg (Fig. 1b) in the healed CL group, while only the frequencies of IL2+CD4+ T cells differ between both antigens in the same group (Fig. 1a). These findings indicate that LbAg induces higher cytokine production by CD4+T cells than LaAg, rather than a higher percentage of cytokine-producing cells. In fact, the levels of IFN-γ protein detected in supernatants of LbAg-stimulated PBMC cultures (3871·74 ± 887 pg/ml) were higher than after LaAg stimulation (2849·42 ± 712·9 pg/ml) in the healed CL patient group (P = 0·001 Wilcoxon's matched-pairs signed-ranks test), although we were not able to find a positive correlation between the amount of protein and the iMFIs.
Fig. 1.
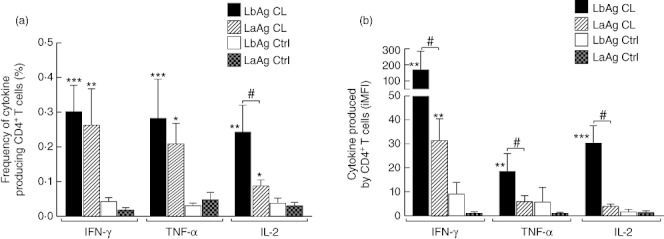
Response of cytokine-producing CD4+T cells analysed by the frequency of cytokine positive cells (a) or by the integrated mean fluorescence intensity (iMFI) of interferon (IFN)-γ, tumour necrosis factor (TNF)-α and interleukin (IL)-2 (b) after stimulation with Leishmaniasis braziliensis (LbAg) and L. amazonensis (LaAg) total antigen extracts of peripheral blood mononuclear cells (PBMCs) obtained from healed cutaneous leishmanaisis (CL) patients and uninfected controls (Ctrl). #Different from LaAg CL Wilcoxon matched-pairs signed-ranks test, P < 0·05; *different from Ctrl group; Mann–Whitney U-test; *P < 0·05; **P < 0·005; ***P < 0·0005.
Distinct quality of CD4 Th1 cells is induced after stimulation with L braziliensis and L. amazonensis crude antigen extracts
It has been suggested that multifunctional CD4+T cells, able to produce simultaneously IFN-γ, IL-2 and TNF-α, are associated with protective immunity or a beneficial outcome in chronic infectious diseases, such as HIV [25]–[28] and HCV [29]. We therefore evaluated the quality of Th1 responses induced by LbAg and LaAg in healed CL patients, based on their ability to secrete these three major Th1-related cytokines at the single-cell level. Using multiparametric flow cytometry, seven distinct populations of cytokine-producing cells can be delineated based on any of the possible combinations of IFN-γ+, IL-2+ and TNF-α+ producers, and the relative frequency of these distinct populations defines the quality of the Th1 response. The percentages of cytokine-producing cells were shown to be higher in the healed CL patient group than in healthy controls, and we were able to observe statistically significant differences between those groups for triple-positive (3+) multifunctional T cells (with both LbAg and LaAg), IFN-γ single-positive cells after LaAg stimulation and for IFN-γ+IL-2+ cells stimulated with LbAg (Fig. 2a). When comparing the quality of the Th1 response elicited by each Leishmania antigen evaluated we could observe that LbAg induces significantly higher percentages of multifunctional CD4+T cells and IFN-γ+IL-2+ cells than LaAg stimulation in the healed CL patient group (Fig. 2a). The quality of the Th1 response was also evaluated by analysing the contribution of each phenotype in the total Th1 response, and is represented pictorially by pie charts (Fig. 2b). This kind of representation demonstrates clearly that LbAg induced a major proportion of multifunctional CD4+T cells (in red – 28% of the total Th1 response evaluated) and double-positive CD4+T cells (in blue – comprising 44% of the total Th1 response), while LaAg induced predominantly single-positive cells (68%). More than half of the single-positive cells induced by LaAg were IFN-γ single-positive. In the control group, the majority of responsive cells were single-positives (>60%), and no major differences were observed concerning LbAg and LaAg stimulation.
Fig. 2.
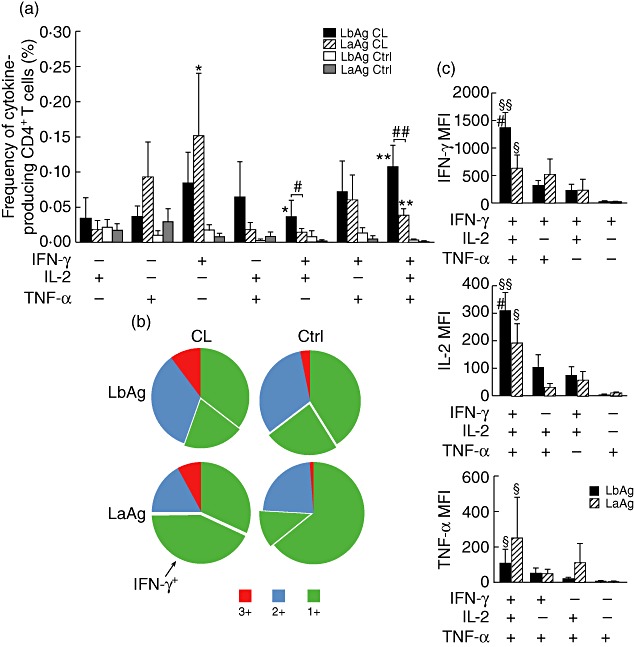
Distinct quality of CD4 T helper type 1 (Th1) cells induced by LbAg and LaAg. Multiparametric flow cytometry was used to determine (a) the frequency of cells expressing each of the seven possible combinations of interferon (IFN)-γ, interleukin (IL)-2 and tumour necrosis factor (TNF)-α and (b) the fraction of the total response comprising cells expressing all three cytokines (3+), any two cytokines (2+) or any one cytokine (1+). Detached from the pie charts are the fraction that consists of IFN-γ single-positive cells. (c) The IFN-γ, IL-2 and TNF-α median fluorescence intensities (MFIs) of antigen-specific 3+, 2+, and 1+ cells for Leishmaniasis braziliensis (LbAg) and L. amazonensis (LaAg). Shown is the mean ± standard deviation of the mean. *Different from the Ctrl group. Mann–Whitney U-test; *P < 0·05; **P < 0·005. #Different from LaAg Wilcoxon matched-pairs signed-ranks test; #P < 0·05;##P < 0·005. §Mann–Whitney test, P < 0·05; §different from single-positive cells; §§different from all other phenotypes.
Having shown that LbAg induced higher cytokine production by CD4+T cells than LaAg in healed CL patients (Fig. 1b), we also investigated the relative cytokine concentrations produced by all distinct Th1 phenotypes induced by LbAg and LaAg, measured as the geometric MFIs. The highest MFI values for all three cytokines were found among triple-positive multifunctional CD4+T cells (both after LbAg and LaAg stimulation) (Fig. 2c) and a progressive decrease in the MFIs for all cytokines was observed as the degree of functionality decreased (3+ to single-positives). MFIs for IFN-γ and IL-2 from multifunctional T cells stimulated with LbAg were significantly higher than those obtained after LaAg stimulus (Fig. 2c). Although LbAg induces significantly higher percentages of triple-positive (3+) cells, which also produces more IFN-γ than LaAg, we could not find any correlation between the percentage of 3+ cells and the amount of IFN-γ detected in LbAg- or LaAg-stimulated culture supernatants.
Discussion
Localized CL is the most frequent clinical form of ATL [18,36,39]. It can be caused by all pathogenic Leishmania species with dermal tropism, including L. braziliensis and L. amazonensis[18]. Clinical and histopathological differences have been described between human infections with these two species: L. braziliensis causes mucosal leishmaniasis, a clinical form associated with the up-regulation of Th1-type responses [15]–[18], whereas L. amazonensis is the aetiological agent of anergic diffuse cutaneous leishmaniasis, a condition associated with specific impairment of the cell-mediated immune response [3,10,18,37,40]. Furthermore, a respectable amount of data in the murine model indicates impairment in multiple immune functions after L. amazonensis infection [41]–[47]. Taken together, these observations suggest major differences in cell-mediated immunity against these Leishmania species, and that the mechanisms responsible for susceptibility to L. amazonensis are complex and deserve more thorough investigation.
In the present study, we were able to show that crude promastigotes extracts obtained from L. braziliensis and L. amazonensis induce a different magnitude and quality of the Th1 response in PBMCs from healed CL patients. To our knowledge, this is the first time that multifunctional CD4+T cells have been evaluated in human leishmaniasis. Corroborating previous data [48], in this study we confirmed that LbAg induces higher levels of IFN-γ than LaAg, and are now able to demonstrate that this fact was related not to a higher percentage of cytokine-producing cells, but to a higher amount of protein produced by individual CD4+T cells (Fig. 1a and b). Furthermore, using multiparametric flow cytometry approach, we were also able to indicate that it might be associated to differences in the quality of Th1 CD4+T cells induced by both antigen extracts (Fig. 2).
Because the same results regarding IFN-γ levels induced by LbAg and LaAg were observed in PBMCs obtained from ATL patients before therapy [48], and that parasites isolated from patients of the former and current studies were characterized as L braziliensis, it could be expected that their T cells would respond more strongly to antigens from the homologous species with which they have been infected than to antigens from species belonging to a different subgenus. Conversely, it has been demonstrated that LbAg is a more potent stimulator of T cell response than LaAg in individuals infected with L. amazonensis, as well as in individuals infected with parasites from the Viannia subgenus, before and after therapy [49]. It has also been shown that LaAg or live L. amazonensis promastigotes are able to induce high levels of IL-10 in PBMCs obtained from LaAg-vaccinated volunteers [50] or from healthy subjects [51], suggesting that the differences observed between LbAg and LaAg stimulation can be related to suppressive factors that are triggered by L. amazonensis parasites. We could not detect CD4+ that were able to produce IL-10 and IFN-γ simultaneously and did not observe any differences in the frequency of IL-10+CD4+T cells, or in CD4+CD25highIL10+ regulatory T cells between LbAg and LaAg stimulation (data not shown). There are indications that L. amazonensis infection induces IL-10 production by macrophages [51]–[53] and regulatory B cells [54], which were not evaluated in the present work. These possibilities are currently being investigated, as we are now also looking for IL-10 production by other cell types.
As shown in Fig. 2a and b, LbAg induced significantly higher proportions of multifunctional triple-positive (3+) CD4+T cells than LaAg, corresponding to 28% of the total Th1 response observed. Forty-four per cent (44%) of the LbAg responding cells were double-positives and 21% were single-positives for IFN-γ. Conversely, 68% of the Th1 responses induced by LaAg were composed of single-positive cells and more than half of those were IFN-γ single-positives (covering 32% of the total Th1 response). Only 10% of the Th1 cells induced by LaAg were capable of producing all three cytokines simultaneously (Fig. 2b). As it has been well demonstrated that IFN-γ single-positive cells are short-lived [24,25], and fail to induce protection in murine L. major vaccine-studies [32], it is possible that one of the mechanisms involved in the poor parasite-specific Th1 response observed in DCL patients is the induction of a great number of short-lived IFN-γ single-positive cells. L. amazonensis could induce a state of functional exhaustion of CD4 Th1 cells, as was shown recently for CD8+T cells in L. mexicana-infected DCL patients [55]. In our system we were able to detect low percentages of Leishmania-specific cytokine-producing CD8+T cells. All of them were IFN-γ single-positives, but no difference could be observed between LaAg and LbAg stimulation (data not shown). L. amazonensis can also cause localized cutaneous leishmaniasis, and DCL patients may display temporary remission of lesions after therapy, when eventually they can produce low levels of IFN-γ after in vitro Leishmania antigen stimulation [18]. It would be most interesting to study the quality of parasite-specific CD4+T cells generated after LaAg and LbAg stimulation in L. amazonensis-infected patients to evaluate a possible correlation between the induction of multifunctional T cells or IFN-γ single-positive T cells, and the development of CL or DCL in L. amazonensis-infected individuals.
We also investigated the relative cytokine concentrations produced by all seven Th1 phenotypes induced by LbAg and LaAg by comparing the geometric MFIs. Multifunctional CD4+ T cells stimulated with LbAg and LaAg presented the highest MFIs for the three Th1 cytokines assessed when compared with double-positive and single-positive cells. Interestingly, MFIs for IFN-γ and IL-2 from multifunctional T cells stimulated with LbAg were significantly higher than those obtained after LaAg stimulation (Fig. 2c). Although this last result corroborates the ELISA data for IFN-γ protein detection in Leishmania antigen-stimulated PBMCs, we could not find any correlation between protein levels in the culture supernatants and the IFN-γ MFIs of multifunctional triple-positive CD4+T cells after LaAg or LbAg stimulation. The same lack of correlation was observed when we compared the ELISA data with the total percentages of multifunctional T cells or the iMFIs of total IFN-γ-producing CD4+T cells. Because in the ELISA technique supernatants are analysed after 5 days of antigen stimulation, with the participation of other cell types besides CD4+ or CD8+T lymphocytes that can also produce IFN-γ, this lack of correlation could be expected.
In experimental leishmaniasis it has been shown that subcutaneous injections with LaAg alone can significantly increase the susceptibility of Rhesus monkeys to experimental infection with L. amazonensis, despite the enhanced IFN-γ production and increased delayed-type cutaneous hypersensitivity [56]. Similarly, intramuscular LaAg was found to increase the susceptibility of BALB/c mice to cutaneous leishmaniasis, in a manner associated with up-regulated transforming growth factor (TGF)-β overcoming the increased IFN-γ[57]. In humans, L. amazonensis whole-cell extract has also been tested for both immunoprophylaxis and immunotherapy. As observed in experimental models, although capable of eliciting T cell-mediated responses in immunized volunteers and the production of expressive amounts of IFN-γ[58]–[60], a vaccine candidate composed of killed L. amazonensis promastigotes from the same strain utilized in the present work failed to induce protection in a Phase III clinical trial [61]. Conversely, the same preparation was shown to be extremely suitable for immunotherapeutic practice, especially in individuals who are resistant to the usual antimonial therapy and those with counterindications such as cardiopathy and nephropathy [62,63]. As IFN-γ single-positive CD4+T cells are short-lived [22,23], our results can offer a possible explanation for the diverse results observed in the prophylaxis and immunotherapy studies with L. amazonensis whole-cell extracts. LaAg stimulation induces a substantial amount of IFN-γ single-positive CD4+T cells, which may not be sufficient to induce long-term and good-quality protection against reinfection, but could be effective when a rapid and transient Th1 response is needed, as in the case of immunotherapeutic interventions. It has also been shown that in-vitro stimulation with LaAg in PBMCs obtained from LaAg vaccinated volunteers induce a higher number of IL-10 producers (measured by ELISA) than IFN-γ producers, while the opposite effect was observed in PBMCs from LTA patients before therapy [51]. The capacity of LaAg to induce IL-10 secretion in PBMCs obtained from ATL patients, together with the generation of short-lived IFN-γ-producing CD4+T cells, could result in equilibrium between inflammatory and anti-inflammatory responses, allowing parasite clearance and lesion resolution, as observed in the immunotherapeutic protocols tested so far.
Currently we are performing multiparametric flow cytometry studies with PBMCs obtained from CL, ML and disseminated CL patients infected with L. braziliensis before and after therapy, in an attempt to find better immune parameters that could correlate with the clinical manifestation and effective healing of lesions. It is to be expected that understanding the induction of Leishmania-specific multifunctional T cells in the diverse clinical manifestations of ATL will help understanding of the complex immunopathogenesis of this neglected tropical disease, and bring new and important parameters that can help in the selection of antigens or adjuvants that will have better chances of working in prophylactic or therapeutic interventions against human leishmaniasis.
Based on our data, we are very tempted to suggest that the quality of the Th1 response induced by L. amazonensis antigens, involving a poor generation of multifunctional CD4+T cells and a high proportion of IFN-γ single-positive CD4+T cells, in association with its well-known capacity of inducing IL-10 production [45]–[47,51,53,54], can be involved in the mechanisms responsible for the susceptibility to L. amazonensis observed in ATL patients and in experimental models. In this sense we have shown, for the first time, that multiparametric flow cytometry can bring new important aspects to the studies of ATL immunopathogenesis, and reinforce the importance of evaluating not just the magnitude, but the quality of a pathogen-specific Th1 immune response by multiple parameters at a single-cell level, to find better and more effective biomarkers of disease and protection.
Acknowledgments
We thank the following funding agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq – PAPES V), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ-APQ1) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for fellowship. We are also grateful to Dr Joseli de Oliveira Ferreira for critical reading of the manuscript.
Disclosure
None.
References
- 1.World Health Organization (WHO) Leishmaniasis. Available at: http://www.who.int/leishmaniasis/en/ (accessed 27 June 2011)
- 2.Schwartz E, Hatz C, Blum J. New world cutaneous leishmaniasis in travellers. Lancet Infect Dis. 2006;6:342–9. doi: 10.1016/S1473-3099(06)70492-3. [DOI] [PubMed] [Google Scholar]
- 3.Grimaldi G, Jr, Tesh RB. Leishmaniases of the New World: current concepts and implications for future research. Clin Microbiol Rev. 1993;6:230–50. doi: 10.1128/cmr.6.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Oliveira-Neto MP, Mattos MS, Perez MA, et al. American tegumentary leishmaniasis (ATL) in Rio de Janeiro State, Brazil: main clinical and epidemiologic characteristics. Int J Dermatol. 2000;39:506–14. doi: 10.1046/j.1365-4362.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 5.Guerra JA, Prestes SR, Silveira H, et al. Mucosal leishmaniasis caused by Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis in the Brazilian Amazon. PLoS Negl Trop Dis. 2011;5:e980. doi: 10.1371/journal.pntd.0000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bittencourt A, Silva N, Straatmann A, et al. Post-kala-azar dermal leishmaniasis associated with AIDS. Braz J Infect Dis. 2003;7:229–33. doi: 10.1590/s1413-86702003000300009. [DOI] [PubMed] [Google Scholar]
- 7.Costa JM, Marsden PD, Llanos-Cuentas EA, et al. Disseminated cutaneous leishmaniasis in a field clinic in Bahia, Brazil: a report of eight cases. J Trop Med Hyg. 1986;89:319–23. [PubMed] [Google Scholar]
- 8.Turetz ML, Machado PR, Ko AI, et al. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. Braz J Infect Dis. 2002;186:1829–34. doi: 10.1086/345772. [DOI] [PubMed] [Google Scholar]
- 9.Almeida RP, Barral-Netto M, De Jesus AM, et al. Biological behavior of Leishmania amazonensis isolated from humans with cutaneous, mucosal, or visceral leishmaniasis in BALB/C mice. Am J Trop Med Hyg. 1996;54:178–84. doi: 10.4269/ajtmh.1996.54.178. [DOI] [PubMed] [Google Scholar]
- 10.Petersen EA, Neva FA, Barral A, et al. Monocyte suppression of antigen-specific lymphocyte responses in diffuse cutaneous leishmaniasis patients from the Dominican Republic. J Immunol. 1984;132:2603–6. [PubMed] [Google Scholar]
- 11.Lainson R, Bray RS. Studies on the immunology and serology of leishmaniasis. II. Cross-immunity experiments among different forms of American cutaneous leishmaniasis in monkeys. Trans R Soc Trop Med Hyg. 1966;60:526–32. doi: 10.1016/0035-9203(66)90278-1. [DOI] [PubMed] [Google Scholar]
- 12.Lainson R, Shaw JJ. Studies on the immunology and serology of leishmaniasis. 3. On the cross-immunity between Panamanian cutaneous leishmaniasis and Leishmania mexicana infection in man. Trans R Soc Trop Med Hyg. 1966;60:533–5. doi: 10.1016/0035-9203(66)90279-3. [DOI] [PubMed] [Google Scholar]
- 13.Lainson R, Shaw JJ. Leishmaniasis in Brazil: XII. Observations on cross-immunity in monkeys and man infected with Leishmania mexicana mexicana, L. m. amazonensis, L. braziliensis braziliensis, L. b. guyanensis and L. b. panamensis. J Trop Med Hyg. 1977;80:29–35. [PubMed] [Google Scholar]
- 14.Porrozzi R, Teva A, Amaral VF, et al. Cross-immunity experiments between different species or strains of Leishmania in rhesus macaques (Macaca mulatta. Am J Trop Med Hyg. 2000;71:297–305. [PubMed] [Google Scholar]
- 15.Bacellar O, Lessa H, Schriefer A, et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–40. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faria DR, Gollob KJ, Barbosa J, Jr, et al. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005;73:7853–9. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaze ST, Dutra WO, Lessa M, et al. Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scand J Immunol. 2006;63:70–8. doi: 10.1111/j.1365-3083.2005.01707.x. [DOI] [PubMed] [Google Scholar]
- 18.Silveira FT, Lainson R, De Castro Gomes CM, et al. Immunopathogenic competences of LeishmaniaVbraziliensis and LLamazonensis in American cutaneous leishmaniasis. Parasite Immunol. 2009;31:423–31. doi: 10.1111/j.1365-3024.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- 19.Glimcher LH, Murphy KM. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 2000;14:1693–711. [PubMed] [Google Scholar]
- 20.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 21.Maldonado-Lopez R, Moser M. Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin Immunol. 2001;13:275–82. doi: 10.1006/smim.2001.0323. [DOI] [PubMed] [Google Scholar]
- 22.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 23.Wu CY, Kirman JR, Rotte MJ, et al. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002;3:852–8. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 24.Foulds KE, Rotte MJ, Paley MA, et al. IFN-gamma mediates the death of Th1 cells in a paracrine manner. J Immunol. 2008;180:842–9. doi: 10.4049/jimmunol.180.2.842. [DOI] [PubMed] [Google Scholar]
- 25.Younes SA, Yassine-Diab B, Dumont AR, et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med. 2003;198:1909–22. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harari A, Petitpierre S, Vallelian F, et al. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood. 2004;103:966–72. doi: 10.1182/blood-2003-04-1203. [DOI] [PubMed] [Google Scholar]
- 27.Boaz MJ, Waters A, Murad S, et al. Presence of HIV-1 Gag-specific IFN-γ+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J Immunol. 2002;169:6376–85. doi: 10.4049/jimmunol.169.11.6376. [DOI] [PubMed] [Google Scholar]
- 28.Duvall MG, Jaye A, Dong T, et al. Maintenance of HIV-specific CD4+ T cell help distinguishes HIV-2 from HIV-1 infection. J Immunol. 2006;176:6973–81. doi: 10.4049/jimmunol.176.11.6973. [DOI] [PubMed] [Google Scholar]
- 29.Semmo N, Day CL, Ward SM, et al. Preferential loss of IL-2-secreting CD4+ T helper cells in chronic HCV infection. Hepatology. 2005;41:1019–28. doi: 10.1002/hep.20669. [DOI] [PubMed] [Google Scholar]
- 30.Millington KA, Innes JA, Hackforth S, et al. Dynamic relationship between IFN-γ and IL-2 profile of Mycobacterium tuberculosis specific T cells and antigen load. J Immunol. 2007;178:5217–26. doi: 10.4049/jimmunol.178.8.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 32.Lindenstrøm T, Agger EM, Korsholm KS, et al. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol. 2009;182:8047–55. doi: 10.4049/jimmunol.0801592. [DOI] [PubMed] [Google Scholar]
- 33.Derrick SC, Yabe IM, Yang A, et al. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine. 2011;29:2902–9. doi: 10.1016/j.vaccine.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Draper SJ, Biswas S, Spencer AJ, et al. Enhancing blood-stage malaria subunit vaccine immunogenicity in rhesus macaques by combining adenovirus, poxvirus, and protein-in-adjuvant vaccines. J Immunol. 2010;185:7583–95. doi: 10.4049/jimmunol.1001760. [DOI] [PubMed] [Google Scholar]
- 35.Yu H, Karunakaran KP, Kelly I, et al. Immunization with live and dead Chlamydia muridarum induces different levels of protective immunity in a murine genital tract model: correlation with MHC class II peptide presentation and multifunctional Th1 cells. J Immunol. 2011;186:3615–21. doi: 10.4049/jimmunol.1002952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silveira FT, Lainson R, Corbett CE. Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: a review. Mem Inst Oswaldo Cruz. 2004;99:239–51. doi: 10.1590/s0074-02762004000300001. [DOI] [PubMed] [Google Scholar]
- 37.Mendonça SC, Coutinho SG, Amendoeira RR, et al. Human American cutaneous leishmaniasis (Leishmania b. braziliensis) in Brazil: lymphoproliferative responses and influence of therapy. Clin Exp Immunol. 1986;64:269–76. [PMC free article] [PubMed] [Google Scholar]
- 38.Cupolillo E, Grimaldi G, Jr, Momen H. A general classification of new world leishmania using numerical zymotaxonomy. Am J Trop Med Hyg. 1994;50:296–311. doi: 10.4269/ajtmh.1994.50.296. [DOI] [PubMed] [Google Scholar]
- 39.Reithinger R, Dujardin JC, Louzir H, et al. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–96. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 40.Pinheiro RO, Pinto EF, Benedito AB, et al. The T-cell anergy induced by Leishmania amazonensis antigens is related with defective antigen presentation and apoptosis. An Acad Bras Cienc. 2004;76:519–27. doi: 10.1590/s0001-37652004000300006. [DOI] [PubMed] [Google Scholar]
- 41.Afonso LC, Scott P. Immune responses associated with susceptibilityof C57BL/10 mice to Leishmania amazonensis. Infect Immun. 1993;61:2952–59. doi: 10.1128/iai.61.7.2952-2959.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soong L, Chang C, Sun J, et al. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J Immunol. 1997;158:5374–83. [PubMed] [Google Scholar]
- 43.Jones DE, Buxbaum LU, Scott P. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. J Immunol. 2000;165:364–72. doi: 10.4049/jimmunol.165.1.364. [DOI] [PubMed] [Google Scholar]
- 44.Lemos de Souza V, Ascenção JS, Correia TMS, et al. Different Leishmania species determine distinct profiles of immune and histopathological responses in CBA mice. Microbes Infect. 2000;2:1807–15. doi: 10.1016/s1286-4579(00)01340-x. [DOI] [PubMed] [Google Scholar]
- 45.Ji J, Sun J, Hai J, et al. Analysis of T helper cell responses during infection with Leishmania amazonensis. Am J Trop Med Hyg. 2002;66:338–45. doi: 10.4269/ajtmh.2002.66.338. [DOI] [PubMed] [Google Scholar]
- 46.Jones DE, Ackermann MR, Wille U, et al. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect Immun. 2002;70:2151–8. doi: 10.1128/IAI.70.4.2151-2158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji J, Sun J, Soong L. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect Immun. 2003;71:4278–88. doi: 10.1128/IAI.71.8.4278-4288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Telino E, De Luca PM, Matos DCS, et al. In vitro responses of human peripheral blood mononuclear cells to whole-cell, particulate and soluble extracts of Leishmania promastigotes. Clin Exp Immunol. 2005;143:338–44. doi: 10.1111/j.1365-2249.2006.02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silveira FT, Blackwell JM, Ishikawa EA, et al. T cell responses to crude and defined leishmanial antigens in patients from the Lower Amazon region of Brazil infected with different species of Leishmania of the subgenera Leishmania and Viannia. Parasite Immunol. 1998;20:19–26. doi: 10.1046/j.1365-3024.1998.t01-1-00126.x. [DOI] [PubMed] [Google Scholar]
- 50.Azeredo-Coutinho RB, Matos DC, Armôa GG, et al. Contrasting human cytokine responses to promastigote whole-cell extract and the Leishmania analogue receptor for activated C kinase antigen of L. amazonensis in natural infection versus immunization. Clin Exp Immunol. 2008;153:369–75. doi: 10.1111/j.1365-2249.2008.03705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coêlho ZC, Teixeira MJ, Mota EF, et al. In vitro initial immune response against Leishmania amazonensis infection is characterized by an increased production of IL-10 and IL-13. Braz J Infect Dis. 2010;14:476–82. [PubMed] [Google Scholar]
- 52.Pereira RM, Teixeira KL, Barreto-de-Souza V, et al. Novel role for the double-stranded RNA-activated protein kinase PKR: modulation of macrophage infection by the protozoan parasite Leishmania. FASEB J. 2010;24:617–26. doi: 10.1096/fj.09-140053. [DOI] [PubMed] [Google Scholar]
- 53.Yang Z, Mosser DM, Zhang X. Activation of the MAPK, ERK, following Leishmania amazonensis infection of macrophages. J Immunol. 2007;178:1077–85. doi: 10.4049/jimmunol.178.2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veras PS, Welby-Borges M, de Santana CD, et al. Leishmania amazonensis: participation of regulatory T and B cells in the in vitro priming (PIV) of CBA/J spleen cells susceptible response. Exp Parasitol. 2006;113:201–5. doi: 10.1016/j.exppara.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Hernández-Ruiz J, Salaiza-Suazo N, Carrada G, et al. CD8 cells of patients with diffuse cutaneous leishmaniasis display functional exhaustion: the latter is reversed, in vitro, by TLR2 agonists. PLoS Negl Trop Dis. 2010;4:e871. doi: 10.1371/journal.pntd.0000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kenney RT, Sacks DL, Sypek JP, et al. Protective immunity using recombinant human IL-12 and alum as adjuvants in a primate model of cutaneous leishmaniasis. J Immunol. 1999;163:4481–8. [PubMed] [Google Scholar]
- 57.Pinheiro RO, Pinto EF, Lopes JRB, et al. TGF-β-associated enhanced susceptibility to leishmaniasis following intramuscular vaccination of mice with Leishmania amazonensis antigens. Microbiol Infect. 2005;7:1317–23. doi: 10.1016/j.micinf.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 58.Marzochi KB, Marzochi MA, Silva AF, et al. Phase 1 study of an inactivated vaccine against American tegumentary leishmaniasis in normal volunteers in Brazil. Mem Inst Oswaldo Cruz. 1998;93:205–12. doi: 10.1590/s0074-02761998000200014. [DOI] [PubMed] [Google Scholar]
- 59.Velez ID, Agudelo S, Arbelaez P, et al. Safety and immunogenicity of a killed LeishmaniaLamazonensis vaccine against cutaneous leishmaniasis in Colombia: a randomized controlled trial. Trans R Soc Trop Med Hyg. 2000;94:698–703. doi: 10.1016/s0035-9203(00)90239-6. [DOI] [PubMed] [Google Scholar]
- 60.De Luca PM, Mayrink W, Pinto JA, et al. A randomized double blind placebo-controlled trial to evaluate the immunogenicity of a candidate vaccine against American tegumentary leishmaniasis. Acta Trop. 2001;80:251–60. doi: 10.1016/s0001-706x(01)00181-4. [DOI] [PubMed] [Google Scholar]
- 61.Velez ID, Gilchrist K, Arbelaez MP, et al. Failure of a killed Leishmania amazonensis vaccine against American cutaneous leishmaniasis in Colombia. Trans R Soc Trop Med Hyg. 2005;99:593–8. doi: 10.1016/j.trstmh.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Toledo VPCP, Mayrink W, Gollob KJ, et al. Immunochemotherapy in American cutaneous leishmaniasis: immunological aspects before and after treatment. Mem Inst Oswaldo Cruz. 2001;96:89–98. doi: 10.1590/s0074-02762001000100010. [DOI] [PubMed] [Google Scholar]
- 63.Convit J, Ulrich M, Zerpa O, et al. Immunotherapy of American cutaneous leishmaniasis in Venezuela during the period 1990–99. Trans R Soc Trop Med Hyg. 2003;97:469–72. doi: 10.1016/s0035-9203(03)90093-9. [DOI] [PubMed] [Google Scholar]


