Abstract
Bone marrow-derived mesenchymal stromal cells (BM-MSCs) have immunosuppressive properties and have been used to treat steroid-refractory acute graft-versus-host disease (GVHD) in stem cell transplant patients. Cells with similar capacities can also be found in term placental tissue. We have isolated stromal cells from term fetal membrane (FMSCs), umbilical cords (UCSCs) and placental villi (PVSCs) as well as from bone marrow and compared their immunoregulatory capacity in allogeneic settings. We found that FMSCs and UCSCs suppressed proliferation significantly in mixed lymphocyte reactions (MLRs), whereas PVSCs showed inconsistent suppressive effects. When added to MLR cultures, FMSCs suppressed the production of interferon (IFN)-γ and interleukin (IL)-17, whereas UCSCs and PVSCs promoted the production of IL-17 instead. Secretion of IL-10 was increased after addition of FMSCs and UCSCs. In this setting, BM-MSCs had no significant effect on secretion of IFN-γ, IL-17 or IL-10 in MLR cultures. When analysing the expression of adhesion markers, we noted that FMSCs expressed the highest levels of CD29 (β1), CD49d (α4) and CD54 (ICAM-1) compared to the other types of stromal cells. Thus, our data indicate that stromal cells isolated from term fetal membrane have great immunosuppressive capacity in terms of proliferation and production of proinflammatory cytokines from alloreactive T cells, and also promote anti-inflammatory IL-10. They express high levels of integrins that may be of importance in homing to inflamed tissues. Fetal membrane may provide a valuable source of cells with immunosuppressive properties and could possibly be used for treatment of acute GVHD and other inflammatory disorders.
Keywords: adhesion molecules, allotransplantation, immune regulation, immunotherapy, stem cell transplantation
Introduction
Haematopoietic stem cell transplantation (SCT) is an established therapy for treatment of patients with malignancies and inborn immune disorders [1,2]. New treatment modalities have improved the outcome for SCT patients, but there are still problems in handling transplant-related disorders. One of the most serious conditions in this setting is steroid-refractory acute graft-versus-host disease (GVHD), which develops when donor-derived T cells recognize and become activated by alloreactive recipient tissue.
Bone marrow-derived mesenchymal stromal cells (BM-MSCs) have immunoregulatory functions, and have been shown to suppress alloantigen-induced T cell effector functions in vitro[3]. The mechanisms of suppression by BM-MSCs are still largely unknown, but human BM-MSCs appear to mediate their immunomodulatory capacity mainly through soluble factors [4,5], although recent studies have indicated that contact-dependent mechanisms may also play a role [6]. Another essential feature of BM-MSCs is their low immunogenicity, which – together with their immunomodulatory effects in the allogeneic setting – has provided a rationale for their clinical use in GvHD or tissue toxicity. Infusion of third-party BM-MSCs has been used to treat steroid-refractory acute GVHD [7,8] and haemorrhagic cystitis [9], but there is still a need for further development of this kind of cell therapy.
Fetomaternal tolerance is of great importance for pregnancy success, as the maternal immune system recognizes the developing fetus and placenta as semi-allografts [10]. Many systemic and local mechanisms have been proposed to explain this transient maternal tolerance to fetal histoincompatibility [11]. As the fetus is never in direct contact with the maternal immune system, the tolerance mechanisms may occur at the placental–maternal interface. Placental tissue may therefore be a valuable source of cells with intrinsic immunosuppressive properties, which might be suited for transplant settings.
Stromal cells from term placental and umbilical cord tissue share many features with BM-MSCs in terms of differentiation potential and surface marker expression [12]–[17]. Placenta-derived stromal cells are accessible with little or no need for ethical considerations and without any invasive procedures, as the placenta is normally discarded after delivery. Interestingly, fetal membranes from term placentas have been used for almost a century to treat burn injuries [18]. Most studies on stromal cells derived from placenta and umbilical cord have focused on possible uses in regenerative medicine, but there are indications that term placental tissues may also provide a valuable source of suppressive cells for adoptive therapy in inflammatory disorders. Indeed, a few studies have indicated that placental stromal cells are better at suppressing alloreactive T cells than are MSCs from adults [19,20].
The fetal part of the placenta is composed of the fetal membranes, the umbilical cord and placental villi. The fetal membranes consist of the amnion and the chorion. The amnion is a thin membrane consisting of a mesoderm layer and an epithelial layer, which encloses the amniotic cavity and the developing fetus [13]. The chorion is the outer fetal membrane and consists of mesodermal and trophoblastic regions. Fibroblast-like stromal cells have been isolated from all these placental compartments, but until now their effects on alloantigen-specific immunity and expression of adhesion molecules have not been compared directly. The main aim of this study was to determine which placental stromal cells that would be best suited for clinical protocols in the setting in which immune suppression and capacity to home to target tissue are the most important properties.
Materials and methods
Isolation of stromal cells from fetal membrane, villous placenta and umbilical cord
Term placentas were obtained from healthy mothers, with informed consent after caesarean section. Ethical approval was obtained from the Institutional Ethical Review Board. The placenta was washed in Hanks's balanced salt solution (Life Technologies, Gaithersburg, MD, USA) several times to remove contaminating blood. For isolation of membrane stromal cells, fetal membrane (both the amnion and the chorion) was dissected mechanically by carefully cutting the membrane free of the placenta 3–4 cm from the placental structure, and was thereafter cut into smaller pieces (n = 4). Villous placental cells were expanded from pieces of chorionic villi that were harvested in the spongy tissue from the area close to the attachment of the umbilical cord and minced (n = 4) according to a previously published method [21]. For generation of umbilical cord stromal cells (n = 4), the amniotic membrane was removed from the cord and small pieces of Wharton's jelly were cut from the cord and minced. To isolate stromal cells from the amnion and chorion, the membranes were separated mechanically from each other. The pieces of different tissues were plated under a cover glass in six-well plates. All tissues were therefore processed without enzymatic digestion, to avoid unnecessary damage to the cells and to limit the number of reagents that would have to be considered regarding good manufacturing practice (GMP) if adopted for clinical use. In experiments examining amniotic stromal cells (ASC) and amniotic epithelial cells (AEC), the amniotic membrane was digested in trypsin-ethylenediamine tetraacetic acid (EDTA) (Gibco BRL, Grand Island, NY, USA) according to a previously published paper [22] to isolate AEC before plating the amniotic membrane. The stromal cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Life Technologies) with 10% fetal calf serum (PAA Laboratories GmbH, Pasching, Austria) and penicillin–streptomycin (complete DMEM). When colonies of fibroblast-like cells appeared after 2–7 days, the pieces of tissue were removed and culture was continued until the wells became 80% confluent. The cells were harvested with trypsin-EDTA and transferred to a T75 or T175 flask for further expansion. Stromal cells were expanded up to passage three and then used for experiments.
Preparation of bone marrow-derived mesenchymal stromal cells
BM-MSCs were isolated and expanded from bone marrow aspirated from the iliac crest of adult volunteers, following approval by the ethics Committee of Huddinge University Hospital, Sweden, and were cultured as reported previously [3]. Briefly, mononuclear cells were isolated from Percoll-separated bone marrow and resuspended in complete DMEM. Cultures were maintained for two to four passages and harvested by treatment with trypsin. The cells were classified as BM-MSCs based on their ability to differentiate into bone, fat and cartilage [3] and by flow cytometric analysis (positive for CD44, CD29, CD73 and CD105 but negative for CD14, CD34 and CD45).
Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats from blood donors by density gradient centrifugation over Lymphoprep (Nycombed, Oslo, Norway).
Proliferation assay
PBMCs were plated in triplicate at 2 × 105 per well in 96-well plates in RPMI-1640 medium with 5% human AB serum and were stimulated with irradiated allogeneic PBMCs (2 × 105 per well) in mixed lymphocyte reactions (MLRs), the absence or presence of 2 × 104 irradiated placental stromal cells or BM-MSCs per well (stromal cell to effector cell ratio 1:10). After 5 days, PBMCs were pulsed with 1 µCi [3H]-thymidine (Amersham Biosciences, Little Chalfont, UK) per well for 18 h. To study the proliferative response of purified T cells, CD3+ cells were first separated from normal donor PBMCs using the Pan T cell isolation kit (Miltenyi Biotech, Bergisch Gladbach, Germany), according to the manufacturer's directions. CD3+ T cells were stimulated with anti-CD3- and anti-CD28-coated beads (Miltenyi Biotech) for 4 days in the presence or absence of FMSCs. [3H]-Thymidine incorporation was measured with a MicroBeta TriLux (Perkin-Elmer, Weiterstadt, Germany). The n-numbers in the figure legends refer to the number of MLR experiments performed with different responder PBMC, expressed as the mean of triplicates.
ELISA
Levels of interferon (IFN)-γ, interleukin (IL)-10, IL-17 and IL-6 in supernatants from MLR cultures and purified CD3+ T cells after stimulation for 5 days and 3 days, respectively, were analysed by enzyme-linked immunosorbent assay (ELISA) with paired capture and biotinylated detection antibodies according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). The detection limits for the different cytokines were 37 pg/ml for IFN-γ, 12 pg/ml for IL-10, 6 pg/ml for IL-17 and 6 pg/ml for IL-6. When no cytokine was detected the lowest detectable value was used.
Flow cytometry
For all flow cytometric analyses, a fluorescence activated cell sorter (FACS) Sort flow cytometer (BD Biosciences, San José, CA, USA) was used to acquire data and Summit version 4·1 software (Dako, Fort Collins, CO, USA) was used to analyse the results. The different kinds of stromal cells were stained with the following antibodies: CD14, CD45, CD34, CD90, CD73, CD29, CD49d, CD54, CD31, stage-specific embryonic antigen (SSEA)-3, SSEA-4 (BD Biosciences), CD105 (Ancell Corporation, Bayport, MN), CD44 (Immunotech, Marseille, France), human leucocyte antigen (HLA) class II, HLA class I (Dako), CXCR4, PD-L1 (BioLegend, San Diego, CA, USA) and CD13 (Harlan Sera-Lab, Loughborough, UK).
To analyse the effect of an inflammatory environment on the expression of markers on FMSCs and BM-MSCs, stromal cells were preincubated in the presence or absence of 100 U IFN-γ per ml for 48 h before analysis.
Expression of various markers on stromal cells is either presented as percentage of positive cells after setting the gates using fluorochrome-labelled isotype control antibodies or as mean fluorescence intensity (MFI) in cases where the majority of the cells were positive for the molecule examined.
Osteogenic and adipogenic differentiation potential
The capacity of placental stromal cells to differentiate along osteogenic and adipogenic lineages was analysed as described previously [3]. Briefly, for osteogenic differentiation the cells were grown in complete DMEM supplemented with dexamethasone (0·1 µM), ascorbic acid (0·05 mM) and glycerophosphate (10 mM). The cells were stained for calcified structures using Alizarin red stain. For adipogenic differentiation, the cells were grown in complete DMEM supplemented with 1-methyl-3-isobutylxanthine (0·5 mM), dexamethasone (1 µM), insulin (10 µg/ml) and indomethacin (0·2 µM). Adipogenesis was measured by the accumulation of neutral lipids in fat vacuoles, stained with oil red O.
Statistical analysis
Wilcoxon signed-rank test (GraphPad Prism Software, San Diego, CA, USA) was used to compare the proliferation of or cytokine production from MLR cultures with or without stromal cells. Mann–Whitney U-test was used to compare the expression of surface markers on the different stromal cells.
Results
Culture and morphology of placental tissue-derived stromal cells
We have developed protocols for isolation of stromal cells from fetal membrane, umbilical cord and placental villi, in which dissected parts of term placental tissues were cultured without enzymatic digestion. After 2–7 days, cells began to migrate from the tissue and were left to adhere to and proliferate on tissue culture plates. For the fetal membranes, the primary cell culture was a heterogeneous population of epithelial cells and fibroblast-like stromal cells (Fig. 1a), but after 1–2 passages the cultures were dominated by cells that morphologically resembled mesenchymal stromal cells (Fig. 1b). For the umbilical cord (Fig. 1c) and placental villus-derived cultures (Fig. 1e), the majority of cells protruding from the tissues had a fibroblast-like morphology. These retained their morphological structure when expanded (Fig. 1d,f). The stromal cells were cultured up to passage three and used for subsequent experiments. In an attempt to generate a large quantity of cells from one of the FMSC cultures, we managed to expand up to 600 million cells in passage two, which would be an adequate number for treating at least four to five GVHD or toxicity patients with the same cell dose that is used for BM-MSC infusion (1–2 × 106 cells per kg).
Fig. 1.
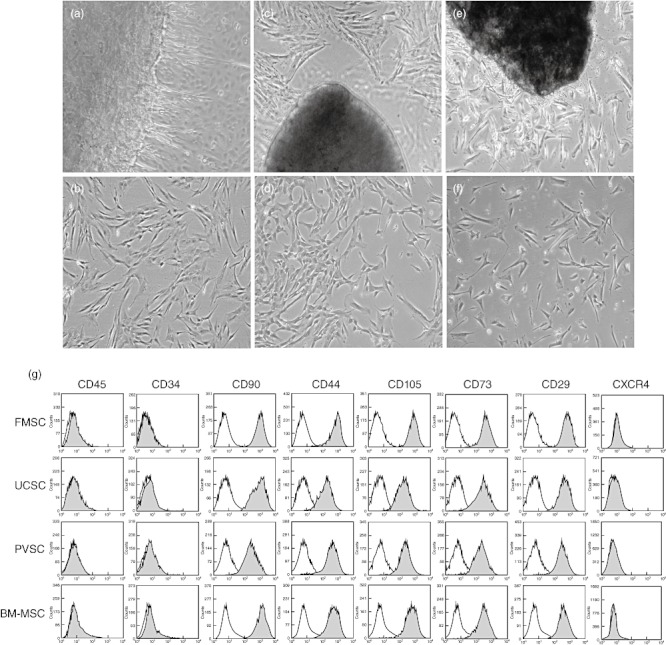
Photomicrographs and phenotypic characterization of stromal cells from placental tissues. Panels (a), (c) and (e) illustrate primary cultures of fetal membrane, umbilical cord and placental villi, respectively, with cells protruding from the tissues. Original magnification: ×4. Panels (b), (d) and (f) show stromal cells from fetal membrane, umbilical cord and placental villi, respectively, at passage 3. Original magnification: ×10. (g) Phenotypic analysis of stromal cells isolated from fetal membrane (FMSC), umbilical cord (UCSC), placental villi (PVSC) and bone marrow (BM-MSC) in passage 3. Grey and white histograms depict the corresponding markers and isotype controls, respectively.
The cells were negative for haematopoietic cell markers CD45 and CD34, but were positive for the typical MSC markers CD29, CD44, CD105, CD90 and CD73 (Fig. 1g). All kinds of stromal cells showed surface expression of MHC class I, but were negative for CXCR4 (Fig. 1g), MHC class II, SSEA-3, CD31 and CD14 and negative or weakly positive for SSEA-4 (data not shown).
Differentiation capacity of placenta-derived stromal cells
Stromal cells from different placental tissues (n = 3 from each subset, passage three) were grown in osteogenic and adipogenic induction media for 3 weeks. BM-MSCs showed great differentiation capacities into these lineages, but all placenta-derived stromal cells failed to differentiate to fat and bone under these conditions (data not shown). Therefore, due to their poor ability to differentiate into mesodermal lineages, the placenta-derived cells are not referred to as mesenchymal cells.
Stromal cells from the fetal membrane have strong immunosuppressive capacity
To examine the suppressive ability of stromal cells derived from different parts of placental tissue, we stimulated PBMCs with irradiated allogeneic PBMCs in the absence or presence of irradiated FMSCs, placental villi (PVSCs) or UCSCs (10 responder cells per stromal cell) and measured proliferative responses. We found that stromal cells isolated from fetal membranes and from umbilical cords inhibited alloantigen-induced proliferation, whereas cells from placental villi generally showed poor suppressive capacity (Fig. 2a). Overall, when comparing all the data with control MLRs, FMSCs showed the highest median suppressive capacity (47%, P < 0·0001) compared to UCSCs (29%, P = 0·015) and bone marrow-derived MSCs (41% suppression, P = 0·014) (Fig. 2b). As shown in Fig. 2c, FMSCs from all four donors tested showed similar suppression. Thus, stromal cells isolated from the fetal membrane appeared to have the most consistent and greatest inhibition of alloantigen-induced proliferation compared to stromal cells isolated from other placental tissues, and it was at least as good as that shown by BM-MSCs. Addition of conditioned medium from FMSC cultures to the MLRs had no suppressive effect on proliferation (n = 6, data not shown).
Fig. 2.
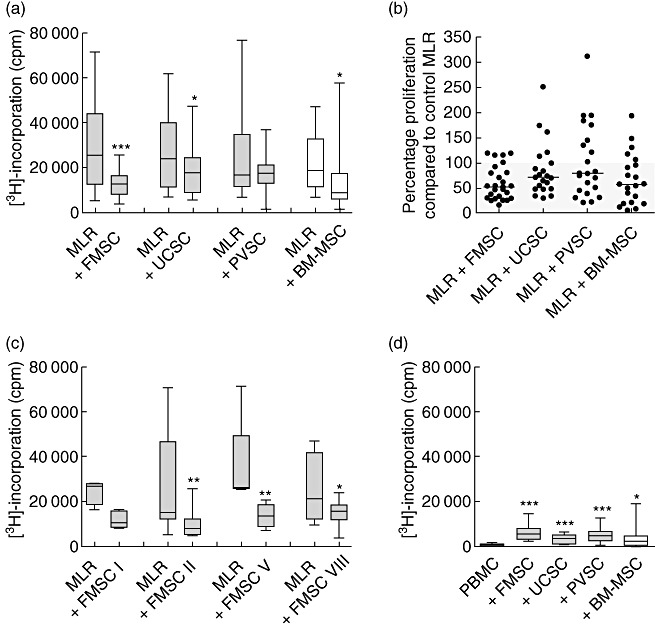
Fetal membrane stromal cells show strong immunosuppressive capacity. (a) Effect of proliferation after addition of stromal cells from fetal membrane (FMSCs) [n = 27 mixed lymphocyte reactions (MLRs) with different responder peripheral blood mononuclear cells (PBMCs)], umbilical cord (UCSCs) (n = 21 MLRs), placental villi (PVSCs) (n = 21 MLRs) and bone marrow-derived mesenchymal stem cells (BM-MSCs) (n = 21 MLRs) to MLR cultures (responder PBMC to stromal cell ratio 10:1). Proliferation was measured on day 6. (b) Percentage proliferation in MLRs after addition of the indicated type of stromal cells compared to control MLRs without stromal cells. (c) Alloantigen-induced proliferation with or without FMSCs from four different donors (from left to right: n = 4, 5, 8 and 8 responder PBMCs, respectively). (d) Stromal cell-induced proliferation of PBMCs (n = 15). The data are presented as box-and-whiskers with the maximum, minimum, median, 25th and 75th quartiles, and were generated by using FMSC, UCSC, PVSC and BM-MSC from four, three, three and three donors, respectively. *P < 0·05; **P < 0·01; ***P < 0·001 compared to the MLRs (panels a and c) or PBMCs (panel d) in the absence of stromal cells, as analysed by Wilcoxon's matched-pairs test.
BM-MSCs have been found previously to have low immunostimulatory effects on allogeneic T cells [4,23]. We therefore examined the immunogenicity of third-party stromal cells on unstimulated PBMCs. These experiments showed that all kinds of placental stromal cells, as well as BM-MSCs, induced proliferation of PBMCs to some extent (Fig. 2d). FMSCs, UCSCs, PVSCs, and BM-MSCs induced median values of 6·2-, 4·1-, 6·7- and 3·1-fold higher proliferative response than background proliferation of unstimulated PBMCs, respectively.
Stromal cells isolated from different placental tissues promote different cytokine patterns
Stromal cells may influence cytokine production from T cells in an alloresponse. In order to examine how alloantigen-stimulated T cells are affected by stromal cells from different placental tissues, we next measured IFN-γ, IL-10 and IL-17 levels in the supernatants of MLR cultures stimulated in the presence or absence of placental stromal cells. As shown in Fig. 3a, we found that the production of IFN-γ was reduced significantly when either FMSCs or UCSCs were added to the MLR (P = 0·0013 and P = 0·0067, respectively), whereas there was a trend of a reduction in IFN-γ secretion in the presence of PVSCs (P = 0·08). Only a low and non-significant effect on alloantigen-induced IFN-γ was observed for BM-MSCs (P = 0·27). None of the stromal cells tested showed an IFN-γ-inducing effect from unstimulated PBMCs (Fig. 3a).
Fig. 3.
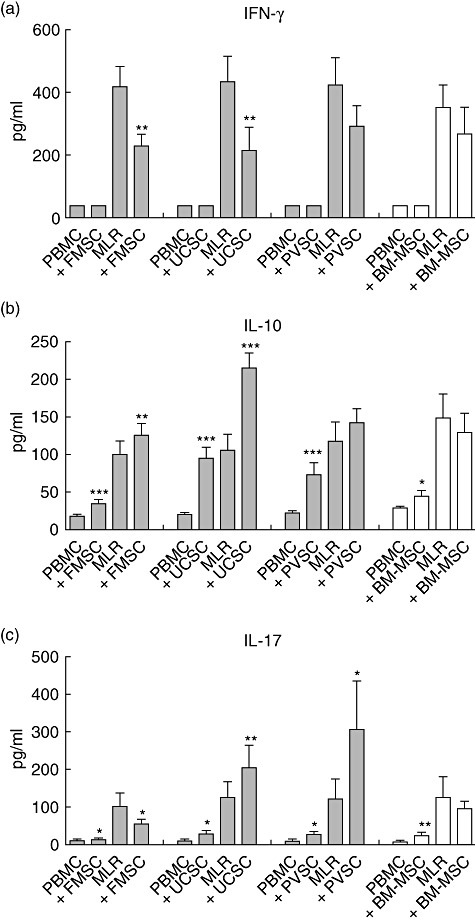
Fetal membrane stromal cells suppress interferon (IFN)-γ and interleukin (IL)-17 production, but induce IL-10 production. Peripheral blood mononuclear cells (PBMCs) were stimulated with irradiated allogeneic PBMCs [mixed lymphocyte reactions (MLRs)] or were left unstimulated (PBMCs) in the presence or absence of stromal cells from fetal membrane (FMSCs) (n = 30 MLRs with different responder PBMCs), umbilical cord (UCSCs) (n = 22 MLRs), placental villi (PVSCs) (n = 22 MLRs) and bone marrow-derived mesenchymal stem cells (BM-MSCs) (n = 20 MLRs). Supernatants were harvested on day 5 and levels of (a) IFN-γ, (b) IL-17 and (c) IFN-γ were analysed by enzyme-linked immunosorbent assay. The data are presented as the mean cytokine production ± standard error of the mean, and were generated by using FMSC, UCSC, PVSC and BM-MSC from four, three, three and three donors, respectively. *P < 0·05; **P < 0·01; ***P < 0·001 compared to the MLRs or PBMCs in the absence of stromal cells, as analysed by Wilcoxon's matched-pairs test.
Levels of IL-10 were significantly higher in PBMC and MLR cultures when FMSCs or UCSCs were added (P = 0·0071 and P = 0·0001, respectively), whereas PVSCs and BM-MSCs induced higher levels of IL-10 when cultured with unstimulated PBMCs, but not in alloantigen-stimulated cultures (Fig. 3b).
Previous studies have shown that BM-MSCs may promote production of IL-17 [24], which is a cytokine that may have harmful effects in patients with inflammatory disorders. We found that the level of IL-17 in supernatants from MLRs cultured with UCSCs and PVSCs was significantly higher than in those from control MLRs, indicating that stromal cells isolated from placental villi and umbilical cord induced production of IL-17 (Fig. 3c). In contrast, FMSCs showed the opposite effect with significantly reduced levels of IL-17 when added to the MLR cultures. For the BM-MSCs, there was no significant difference between the MLRs with or without MSCs. All kinds of stromal cells promoted low levels of IL-17 from unstimulated PBMCs, but FMSCs only induced an increase in IL-17 levels by a factor of mean 1·3-fold compared to resting PBMCs, whereas the corresponding mean figures for UCSCs, PVSCs and BM-MSCs were 3·9-, 4·1- and 3·1-fold, respectively.
There was no production of IFN-γ, IL-10 or IL-17 from stromal cells cultured alone in the absence of PBMC (data not shown).
IL-6 is a pleiotropic cytokine that is produced by stromal cells, and this cytokine has been shown to be implicated in the development of IL-17-producing T helper type 17 (Th17) cells [25]. We therefore measured the levels of IL-6 in our cultures and found that addition of any of the stromal cells tested dramatically increased the secretion of IL-6, both under unstimulated and alloantigen-stimulated conditions (Fig. 4a). However, the amount of IL-6 produced was higher in cultures to which UCSCs, PVSCs and BM-MSCs were added, compared to those that received FMSCs. In line with these observations, when measuring the level of IL-6 secreted from stromal cells in the absence of PBMCs, we found that the UCSCs, PVSCs and BM-MSCs produced significantly higher levels of this cytokine than FMSCs (Fig. 4b). We also measured IL-23 levels, but there were no detectable amounts of this cytokine in these cultures (data not shown).
Fig. 4.
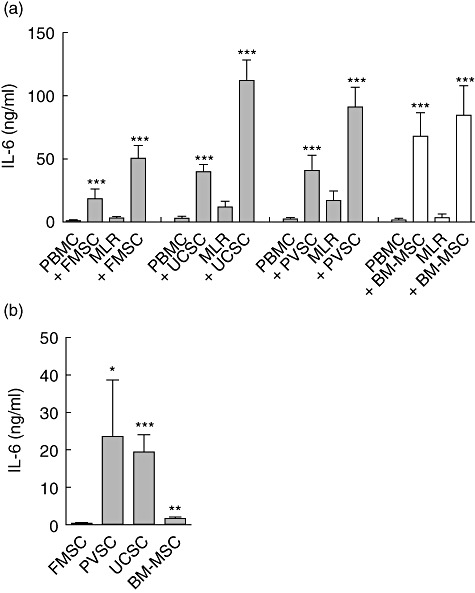
Fetal membrane stromal cells produce low levels of interleukin (IL)-6. (a) Peripheral blood mononuclear cells (PBMCs) were stimulated with irradiated allogeneic PBMCs [mixed lymphocyte reactions (MLRs)] or left unstimulated (PBMCs) in the presence or absence of stromal cells from fetal membrane (FMSCs) (n = 30 MLRs with different responder PBMCs), umbilical cord (UCSCs) (n = 22 MLRs), placental villi (PVSCs) (n = 22 MLRs) and bone marrow-derived mesenchymal stem cells (BM-MSCs) (n = 20 MLRs). Supernatants were harvested on day 5 and levels of IL-6 were determined by enzyme-linked immunosorbent assay. (b) Levels of IL-6 were determined in supernatants from the type of stromal cell indicated after 5 days of culture. The data are presented as the mean cytokine production ± standard error of the mean and were generated by using FMSC, UCSC, PVSC and BM-MSC from four, three, three and three donors, respectively. *P < 0·05; **P < 0·01; ***P < 0·001 compared to the MLRs or PBMCs in the absence of stromal cells, as analysed by Wilcoxon's matched-pairs test (a). The Mann–Whitney U-test was used to compare the levels of IL-6 produced from different types of stromal cells relative to the level produced by FMSCs (b).
Fetal membrane stromal cells express high levels of adhesion markers
We then examined adhesion markers that are important for migratory properties and for cell–cell interactions. CD49d (α4-integrin) is of importance for the ability of cells to migrate over endothelial barriers to sites of inflammation. As shown in Fig. 5a, FMSCs were found to express higher levels of this marker than stromal cells isolated from other placental tissues. BM-MSCs showed the lowest expression of CD49d with a median proportion of 3·6% of cells as opposed to 41·8% for FMSCs (P = 0·03). Similarly, the intensity of expression of CD29 (β1-integrin), which together with CD49d forms VLA-4 that binds to VCAM-1 on endothelial cells, was highest on FMSCs (Fig. 5b). CD54 intercellular adhesion molecule-1 (ICAM-1) is a cell–cell adhesion molecule, and almost all FMSCs and UCSCs were positive for this marker (median 92·6% and 99·6%, respectively), whereas the median proportion of PVSCs and BM-MSCs expressing CD54 was 63·8% and 33·6%, respectively (Fig. 5c). CD44, another adhesion molecule, tended to be expressed at higher intensity on FMSCs than on other placental tissue stromal cells and BM-MSCs (Fig. 5d), but this difference did not reach statistical significance.
Fig. 5.
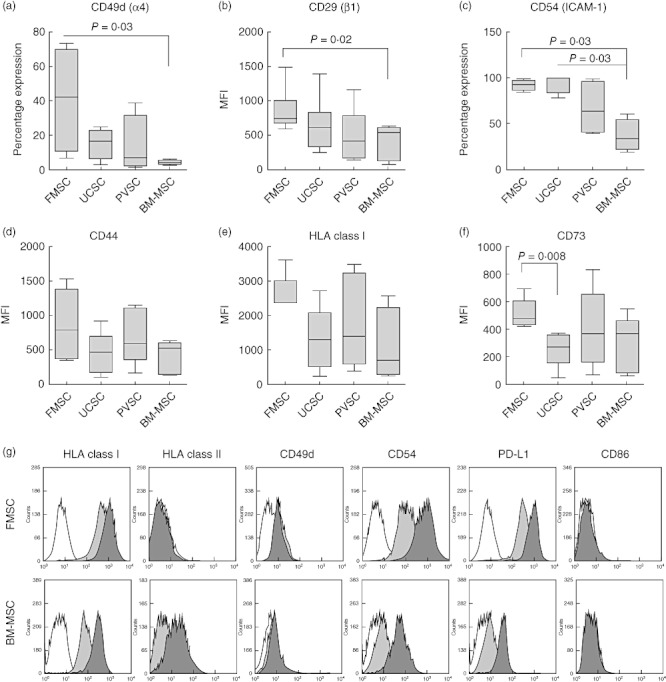
Fetal membrane stromal cells express high levels of adhesion molecules. (a–f) Expression of different markers on stromal cells from fetal membrane (FMSCs), umbilical cord (UCSCs), placental villi (PVSCs) and bone marrow-derived mesenchymal stromal cells (BM-MSCs) were analysed by flow cytometry (n = 4 for each type of stromal cell). The Mann–Whitney U-test was used to compare the mean fluorescence intensity (MFI) or the percentage expression of cell surface markers on different types of stromal cells. The data are presented as box-and-whiskers with the maximum, minimum, median, 25th and 75th quartiles. (g) Representative histograms of expression of different markers on unstimulated (light grey histograms) or interferon (IFN)-γ-stimulated (dark grey histograms) FMSCs (n = 6) and BM-MSCs (n = 3). White histograms depict isotype controls.
FMSCs showed the highest intensity of expression of HLA class I antigen compared to UCSCs, PVSCs and BM-MSCs (median MFI of 2390, as opposed to 1315, 1412 and 700, respectively), but this observation was not statistically significant (Fig. 5e). CD73, a 5'-ectonucleotidase that produces extracellular adenosine, which has been implicated in regulatory T cell-mediated suppression [26], was expressed in significantly higher amounts on FMSCs than on UCSCs (Fig. 5f), but it also tended to be expressed at greater intensity on FMSCs than on BM-MSCs (P = 0·09). There was no difference in the expression of other markers, including CD13, CD90, CD105 and SSEA-4, when we compared the different stromal cells (data not shown).
In order to study how an inflammatory environment affects the expression of MHC molecules and other markers, we next stimulated FMSCs and BM-MSC with IFN-γ before analysis. We found that IFN-γ increased the expression of HLA class I molecules on FMSCs and BM-MSCs, but had little effect on the expression of HLA class II on FMSC, whereas BM-MSC readily up-regulated the expression of this marker (Fig. 5g). The expression of CD49d was not affected by an inflammatory environment on either cell type, whereas IFN-γ induced an up-regulation of CD54. IFN-γ further strongly induced the expression the negative co-stimulatory marker PD-L1 on FMSCs and BM-MSCs, but the intensity of the expression of this marker was significantly higher on FMSCs than on BM-MSCs both in unconditioned and in IFN-γ-stimulated stromal cells (P = 0·02). Neither FMSCs nor BM-MSC expressed the co-stimulatory marker CD86 after IFN-γ treatment.
Stromal cells generated from intact fetal membrane provide at least as good suppression as cells from separated chorion and amnion
When performing MLRs with chorion stromal cells (CSCs) and amnion stromal cells (ASCs) isolated separately, and with amnion epithelial cells (AECs), we found that FMSCs were at least as good, or even better, at depriving proliferation compared to cells isolated from separated amniotic or chorionic membranes (Fig. 6a). This indicates that contaminating AECs are unlikely to have affected the suppressive capacity of FMSCs and that crude preparations of unseparated fetal membrane generates stromal cells that have great suppressive effects.
Fig. 6.
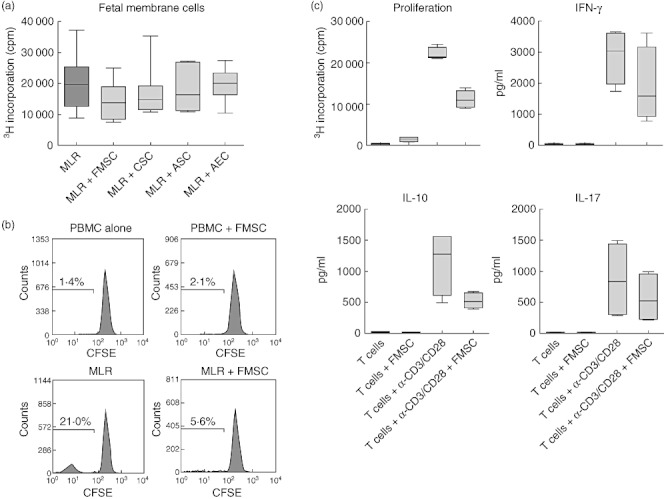
Fetal membrane stromal cells mediate their suppressive effect on T cells. (a) Effect on proliferation in mixed lymphocyte reactions (MLRs) [n = 7 different responder peripheral blood mononuclear cells (PBMCs)] after addition of stromal cells generated from intact fetal membrane (FMSC), separated chorion (CSC) or amnion (ASC) or amnion epithelial cells (AEC) (n = 2 for each type of stromal cell). (b) Responder PBMCs were labelled with carboxyfluorescein succinimidyl ester (CFSE) before stimulation with allogeneic PBMC (MLR) and the effect of FMSCs were analysed on T cells by gating on CD3+ cells. (c) Effect of FMSCs on proliferation and cytokine production from purified CD3+ T cells after stimulation with anti-CD3- and CD28-coated beads (n = 4). The data are presented as box-and-whiskers with the maximum, minimum, median, 25th and 75th quartiles.
T cells are targets for fetal membrane stromal cell-mediated suppression
We next tried to identify the target cell type upon which FMSCs have its effect. By labelling responder PBMCs with CFSE before stimulation with alloantigen, we found that proliferation of CD3+ cells were inhibited in the MLR when FMSC were added to the culture (Fig. 6b). To confirm further that T cells are targeted by FMSC-mediated suppression, we examined how purified CD3+ T cells were affected in a system devoid of antigen-presenting cells. We found that CD3+ T cells were suppressed strongly when stimulated with anti-CD3/anti-CD28-coated beads in the presence of FMSCs (Fig. 6c). In this system, production of IFN-γ, IL-17 and IL-10 tended to be suppressed when FMSC were added to the purified T cell cultures (Fig. 6c), which contrasts with the results with MLRs, where the production of IL-10 was increased. It should also be noted that FMSCs did not induce production of either IL-10 or IL-17 from unstimulated T cells.
Discussion
Bone marrow-derived MSCs have been used therapeutically in several indications, including acute GVHD, Crohn's disease and multiple sclerosis [7,8,27,28]. Very little is known about the qualities of these cells, which are important for their tissue-repairing and immunosuppressive effects. However, an ability to inhibit alloantigen- or autoantigen-induced proliferation and promotion of an anti-inflammatory milieu may be of particular importance. The capacity to migrate to inflamed tissue after infusion may also be a significant feature of the cells. Although BM-MSCs have shown promising results for treatment of acute GVHD [7,8], there is still a need for improvement of this kind of immunotherapy. In this study, we have isolated cells from different placental compartments and examined immunological properties that are of importance in allogeneic transplantation settings.
In accordance with the results from other studies, we have shown that stromal cells from fetal membrane [29]–[31] and umbilical cord [32,33] inhibit proliferative responses in allogeneic settings. We were also able to show that these cells suppress IFN-γ secretion and promote IL-10 production when added to MLR cultures. In our hands, stromal cells derived from placental villi had no significant suppressive effect, but there have been other studies showing that cells isolated from this part of the placenta have an inhibitory effect [19,34]. We noted a suppressive effect in the responder cells from more than half the donors, whereas in other experiments the cells promoted proliferation instead (Fig. 2b). A stimulating effect in MLR, especially when using low numbers of stromal cells, has been reported previously for BM-MSCs [3]. Overall, none of the PVSCs isolated from any of the four donors showed consistent suppression. Differences in isolation techniques might account for the discrepancies in suppressive effect between this and the above studies, as the other authors prepared their cells by enzymatic digestion of the tissues.
When examining the immunogenicity of third-party stromal cells on unstimulated PBMCs, we found that all kinds of placental stromal cells, as well as BM-MSCs, induced proliferation of PBMCs to some extent. This may be due either to an expansion of CD4+CD25+forkfead box protein 3 (FoxP3+) regulatory T cells, which has been reported previously for BM-MSC [35], or to the fact that the cells express MHC class I which promotes an alloreactive response.
There have been studies showing that BM-MSCs may promote IL-17 production when CD4+ T cells are stimulated with phytohaemagglutinin (PHA) [24], and that they can aggravate collagen-induced arthritis in an animal model by increased production of IL-17 and IL-6 [36]. This contrasts with two other studies showing that murine BM-MSCs can suppress IL-17 secretion in vitro[37] and in vivo in an experimental autoimmune encephalomyelitis model [38]. However, how different populations of stromal cells from placental tissue affect IL-17 secretion has not been described. We found that FMSCs suppressed the secretion of IL-17 in MLR cultures, whereas UCSCs and PVSCs promoted production of IL-17 instead. BM-MSCs had no significant effect on IL-17 production when added to the cultures. The reduced production of IFN-γ and IL-17 in the MLR culture in the presence of FMSCs might reflect the repressed proliferative responses. However, UCSC inhibited proliferation but still induced production of IL-17, indicating that factors produced from these cells promoted IL-17 secretion. In line with these observations, UCSCs and PVSCs produced significantly higher levels of IL-6 than FMSCs, and IL-6 has been suggested to be important for induction of IL-17 production from T cells [25]. However, purified T cells did not produce IL-17 when FMSCs were added to the cultures, indicating that other cells in PBMCs provide factors that are needed for IL-17 production, such as IL-23 and IL-1β, which can be produced from antigen-presenting cells [39].
The pattern of IL-10 secretion was also different in purified T cell cultures compared to MLR cultures, as this cytokine was not induced when FMSCs were added to unstimulated or anti-CD3/CD28 stimulated T cells. This indicates that other cells than T cells, such as monocytes or B cells, are responsible for the production of IL-10 or needed for induction of IL-10 from T cells.
We further examined potential trafficking molecules on placental stromal cells. α4β1, which is also known as VLA-4, is composed of CD49d and CD29. We have shown that FMSCs express significantly higher levels of both these markers than BM-MSCs; they also tended to be expressed at higher intensity compared to stromal cells isolated from other parts of the placenta. Although BM-MSCs expressed CD29 at high intensity, CD49d was virtually absent on the surface of these cells. Expression of the ligand for α4β1, vascular cell adhesion molecule-1 (VCAM-1), is up-regulated on endothelium of inflamed tissue, and the capacity of cells to enter the site of inflammation in an injury setting is dependent on the interaction between these molecules [40]. Thus, our data indicate that FMSCs may have enhanced properties for homing to damaged tissue relative to BM-MSCs, but it is not known whether expression of CD49d also may affect the suppressive effects of the cells. Interestingly, Brooke et al. have shown that stromal cells isolated from term villous placenta show functional binding to VCAM-1, whereas BM-MSCs fail to show such an interaction [41].
Another adhesion molecule, ICAM-1, is important for homing in tissue repair and for cell–cell contacts, in particular for T cell interactions with antigen-presenting cells. Ren et al. used ICAM-1-deficient MSCs from ICAM-1−/− mice to demonstrate that ICAM-1 is essential for the immunosuppressive effects of murine BM-MSCs, both in vitro and in vivo, as cell–cell proximity was important for the efficient delivery of soluble suppressive factors [6]. In contrast, Najar et al. were unable to detect any involvement of ICAM-1 in BM-MSC-mediated suppression when they blocked this molecule with antibodies [42]. Whether or not ICAM-1 is of importance for the suppressive effect, it may nevertheless be crucial for homing to damaged tissue, and our data indicate that FMSCs express higher levels of this adhesion marker than the other stromal cells tested.
It has been reported that amniotic epithelial cells (AECs) may become stromal-like with expansion [43,44]. As the fetal membrane cultures were contaminated previously by AECs in the first passage it is possible that the FMSCs may contain AECs that have undergone epithelial–mesenchymal transition and that this may have affected the results. AEC can be isolated by digesting the amnion with trypsin, which yields a practically pure preparation of epithelial cells, but there will be a considerable number of AECs still attached to the membrane which may contaminate the amnion stromal cells. We have therefore not been able to start with pure stromal cell cultures that are completely devoid of AEC. However, in our hands, purified AECs survived for one only passage and thereafter stopped growing without becoming stromal-like, indicating that our culture conditions did not induce epithelial–mesenchymal transition. This was repeated three times with the same results (data not shown). Furthermore, chorionic stromal cells, which were isolated from chorion that were separated mechanically from amnion and hence devoid of AEC before culturing, should contain a pure population of stromal cells. These cells showed a better suppressive effect than AEC, indicating that contaminating AECs are unlikely to have affected the suppressive capacity of FMSCs.
The placenta-derived stromal cells described in this study showed a poor capacity to differentiate into fat and bone. Others have previously shown that stromal cells from term fetal membranes, umbilical cord and placental villi have osteogenic, adipogenic and chondrogenic differentiation potential [12,45]–[48], but there are indications that placenta-derived stromal cells have lower osteogenic [49] or adipogenic [12,34] differentiation capacities than bone marrow-derived MSCs. However, the capacity of stromal cells to differentiate into different mesodermal lineages may not be of relevance for their immunosuppressive and tissue repairing effect in vivo.
The mechanisms by which FMSC have a stronger suppressive effect compared to stromal cells isolated from other placental compartments is still not known. It could be due potentially to differences in expression of HLA-G on the different placental cell types or inductive capacity for regulatory T cells, but these assumptions remain to be determined.
Understanding the fetal escape from an attack by the maternal immunological system is a central question in maternal–fetal physiology. It is likely that a number of classical pregnancy complications, such as preterm labour and pre-eclampsia, might be associated with a disturbed maternal tolerance. Thus, it will be important to further elucidate the role of FMSC in this context.
In conclusion, we have shown that stromal cells isolated from term fetal membranes have strong immunosuppressive capacity and express adhesion molecules that are important for homing to damaged tissue. Apart from these immunological features, another advantage of placental tissue-derived cells is that they are easily isolated by non-invasive procedures, with little or no ethical considerations. Fetal membrane stromal cells may have great therapeutic capability, as this placental tissue has been shown previously to have wound-healing effects [18].
Acknowledgments
We thank Boel Niklasson and the other midwives at the Department of Obstetrics and Gynecology, Karolinska University Hospital in Huddinge for their invaluable help in recruiting parents and in collecting placentas. This study was supported by grants from the Swedish Cancer Society, the Children's Cancer Foundation, the Swedish Research Council, the Cancer Society in Stockholm, the Swedish Society of Medicine, the Axel och Signe Lagerman Foundation and Tissue and Motion, Karolinska Institutet.
Disclosure
The authors declare that there are no competing financial or commercial interests.
References
- 1.Thomas ED, Buckner CD, Banaji M, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49:511–33. [PubMed] [Google Scholar]
- 2.Hoogerbrugge PM, Brouwer OF, Bordigoni P, et al. Allogeneic bone marrow transplantation for lysosomal storage diseases. The European Group for Bone Marrow Transplantation. Lancet. 1995;345:1398–402. doi: 10.1016/s0140-6736(95)92597-x. [DOI] [PubMed] [Google Scholar]
- 3.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 4.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 5.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–50. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Ren G, Zhao X, Zhang L, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–8. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 8.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–7. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 9.Ringden O, Uzunel M, Sundberg B, et al. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia. 2007;21:2271–6. doi: 10.1038/sj.leu.2404833. [DOI] [PubMed] [Google Scholar]
- 10.Mincheva-Nilsson L. Immune cells and molecules in pregnancy: friends or foes to the fetus? Expert Rev Clin Immunol. 2006;2:457–70. doi: 10.1586/1744666X.2.3.457. [DOI] [PubMed] [Google Scholar]
- 11.Trowsdale J, Betz AG. Mother's little helpers: mechanisms of maternal–fetal tolerance. Nat Immunol. 2006;7:241–6. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 12.Portmann-Lanz CB, Schoeberlein A, Huber A, et al. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol. 2006;194:664–73. doi: 10.1016/j.ajog.2006.01.101. [DOI] [PubMed] [Google Scholar]
- 13.Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first International Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300–11. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 14.Alviano F, Fossati V, Marchionni C, et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11. doi: 10.1186/1471-213X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakuragawa N, Kakinuma K, Kikuchi A, et al. Human amnion mesenchyme cells express phenotypes of neuroglial progenitor cells. J Neurosci Res. 2004;78:208–14. doi: 10.1002/jnr.20257. [DOI] [PubMed] [Google Scholar]
- 16.Karahuseyinoglu S, Cinar O, Kilic E, et al. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319–31. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- 17.Ilancheran S, Moodley Y, Manuelpillai U. Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta. 2009;30:2–10. doi: 10.1016/j.placenta.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Kesting MR, Wolff KD, Hohlweg-Majert B, Steinstraesser L. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res. 2008;29:907–16. doi: 10.1097/BCR.0b013e31818b9e40. [DOI] [PubMed] [Google Scholar]
- 19.Chang CJ, Yen ML, Chen YC, et al. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466–77. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- 20.Roelen DL, van der Mast BJ, in't Anker PS, et al. Differential immunomodulatory effects of fetal versus maternal multipotent stromal cells. Hum Immunol. 2009;70:16–23. doi: 10.1016/j.humimm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Huang HI. Isolation of human placenta-derived multipotent cells and in vitro differentiation into hepatocyte-like cells. Curr Protoc Stem Cell Biol. 2007;Chapter 1 doi: 10.1002/9780470151808.sc01e01s1. Unit 1E. [DOI] [PubMed] [Google Scholar]
- 22.Miki T, Marongiu F, Ellis E, Strom CS. Isolation of amniotic epithelial stem cells. Curr Protoc Stem Cell Biol. 2007;Chapter 1:Unit 1E 3. doi: 10.1002/9780470151808.sc01e03s3. [DOI] [PubMed] [Google Scholar]
- 23.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–6. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 24.Guo Z, Zheng C, Chen Z, et al. M-derived mesenchymal stem cells promote the expansion of human Th17 cells, but inhibit the production of Th1 cells. Eur J Immunol. 2009;39:2840–9. doi: 10.1002/eji.200839070. [DOI] [PubMed] [Google Scholar]
- 25.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–25. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 28.Duijvestein M, Vos AC, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a Phase I study. Gut. 2010;59:1662–9. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 29.Wolbank S, Peterbauer A, Fahrner M, et al. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007;13:1173–83. doi: 10.1089/ten.2006.0313. [DOI] [PubMed] [Google Scholar]
- 30.Bailo M, Soncini M, Vertua E, et al. Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation. 2004;78:1439–48. doi: 10.1097/01.tp.0000144606.84234.49. [DOI] [PubMed] [Google Scholar]
- 31.Magatti M, De Munari S, Vertua E, Gibelli L, Wengler GS, Parolini O. Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells. 2008;26:182–92. doi: 10.1634/stemcells.2007-0491. [DOI] [PubMed] [Google Scholar]
- 32.Cutler AJ, Limbani V, Girdlestone J, Navarrete CV. Umbilical cord-derived mesenchymal stromal cells modulate monocyte function to suppress T cell proliferation. J Immunol. 2010;185:6617–23. doi: 10.4049/jimmunol.1002239. [DOI] [PubMed] [Google Scholar]
- 33.Chen K, Wang D, Du WT, et al. Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol. 2010;135:448–58. doi: 10.1016/j.clim.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Jones BJ, Brooke G, Atkinson K, McTaggart SJ. Immunosuppression by placental indoleamine 2,3-dioxygenase: a role for mesenchymal stem cells. Placenta. 2007;28:1174–81. doi: 10.1016/j.placenta.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 35.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(high) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–60. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen B, Hu J, Liao L, et al. Flk-1+ mesenchymal stem cells aggravate collagen-induced arthritis by up-regulating interleukin-6. Clin Exp Immunol. 2010;159:292–302. doi: 10.1111/j.1365-2249.2009.04069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatara R, Ozaki K, Kikuchi Y, et al. Mesenchymal stromal cells inhibit Th17 but not regulatory T-cell differentiation. Cytotherapy. 2011;13:686–94. doi: 10.3109/14653249.2010.542456. [DOI] [PubMed] [Google Scholar]
- 38.Rafei M, Campeau PM, Aguilar-Mahecha A, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 39.Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev. 2008;226:112–31. doi: 10.1111/j.1600-065X.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemler ME, Elices MJ, Parker C, Takada Y. Structure of the integrin VLA-4 and its cell–cell and cell–matrix adhesion functions. Immunol Rev. 1990;114:45–65. doi: 10.1111/j.1600-065x.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 41.Brooke G, Tong H, Levesque JP, Atkinson K. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 2008;17:929–40. doi: 10.1089/scd.2007.0156. [DOI] [PubMed] [Google Scholar]
- 42.Najar M, Raicevic G, Id Boufker H, et al. Modulated expression of adhesion molecules and galectin-1: role during mesenchymal stromal cell immunoregulatory functions. Exp Hematol. 2010;38:922–32. doi: 10.1016/j.exphem.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Bilic G, Zeisberger SM, Mallik AS, Zimmermann R, Zisch AH. Comparative characterization of cultured human term amnion epithelial and mesenchymal stromal cells for application in cell therapy. Cell Transplant. 2008;17:955–68. doi: 10.3727/096368908786576507. [DOI] [PubMed] [Google Scholar]
- 44.Stadler G, Hennerbichler S, Lindenmair A, et al. Phenotypic shift of human amniotic epithelial cells in culture is associated with reduced osteogenic differentiation in vitro. Cytotherapy. 2008;10:743–52. doi: 10.1080/14653240802345804. [DOI] [PubMed] [Google Scholar]
- 45.Soncini M, Vertua E, Gibelli L, et al. Isolation and characterization of mesenchymal cells from human fetal membranes. J Tissue Eng Regen Med. 2007;1:296–305. doi: 10.1002/term.40. [DOI] [PubMed] [Google Scholar]
- 46.In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–45. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 47.Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–7. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 48.Barlow S, Brooke G, Chatterjee K, et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17:1095–107. doi: 10.1089/scd.2007.0154. [DOI] [PubMed] [Google Scholar]
- 49.Pilz GA, Ulrich C, Ruh M, et al. Human term placenta-derived mesenchymal stromal cells are less prone to osteogenic differentiation than bone marrow-derived mesenchymal stromal cells. Stem Cells Dev. 2011;20:635–46. doi: 10.1089/scd.2010.0308. [DOI] [PubMed] [Google Scholar]


