Abstract
Despite recent tissue-engineering advances, there is no effective way of replacing all the functions of the larynx in those requiring laryngectomy. A recent clinical transplant was a success. Using quantitative immunofluorescence targeted at immunologically relevant molecules, we have studied the early (48 h and 1 week) immunological responses within larynxes transplantated between seven pairs of National Institutes of Health (NIH) minipigs fully homozygous at the major histocompatibility complex (MHC) locus. There were only small changes in expression of some molecules (relative to interindividual variation) and these were clearest in samples from the subglottic region, where the areas of co-expression of CD25+CD45RC-CD8- and of CD163+CD172+MHC-II- increased at 1 week after transplant. In one case, infiltration by recipient T cells was analysed by T cell receptor (TCR) Vβ spectratype analysis; this suggested that changes in the T cell repertoire occur in the donor subglottis mucosal tissues from day 0 to day 7, but that the donor and recipient mucosal Vβ repertoires remain distinct. The observed lack of strong immunological responses to the trauma of surgery and ischaemia provides encouraging evidence to support clinical trials of laryngeal transplantation, and a basis on which to interpret future studies involving mismatches.
Keywords: macrophages/monocytes, T cells, transplantation
Introduction
There were an estimated 1691 new cases of laryngeal cancer in England and Wales in 2006–07 [1] (DAHNO, 2008), and approximately one-third of these are likely to require laryngectomy for treatment of primary or recurrent disease at some point. Despite our recent success in clinical airway tissue engineering [2], there is no practical way of substituting the complex functions of the larynx using conventional surgery or existing regenerative medicine techniques. Hence, laryngeal transplantation remains one of the possible answers to the considerable quality-of-life impairment faced by laryngectomees [3,4], and has recently been used successfully clinically by surgeons in California and our group.
An orthotopic model of laryngeal transplantation in the National Institutes of Health (NIH) minipig has been developed [5,6] by our group. There are many immunological reagents available for swine [7], and genetically defined strains of swine exist with a choice of soluble liver antigen (SLA) expression ideal for transplant studies [8]. We have described previously the normal mucosal immune architecture of the pig larynx [9] and its neuromuscular anatomy [10,11]. Our primary aim was to measure the mucosal immune response of porcine larynxes to transplantation, initially in the absence of major histocompatibility complex (MHC)-directed rejection. Measurements were applied to several compartments: anatomical (supraglottis, subglottis, trachea), cellular (monocytes, lymphocytes) and T cell receptor repertoire (spectratyping).
Here we report mucosal immunological changes during the first week after revascularization of donor larynges in MHC-matched recipient pigs, including comparisons between donor and recipient, before and up to 1 week after transplantation of the larynx.
Materials and methods
Animals
NIH minipigs, 34 cc (median weight 27 kg, range 16–60 kg; median age 3·75 months, range 3–7·5 months; IAH, Compton, Berks, UK) were used. Animals were kept under conditions determined by local and national ethical guidelines. Following transportation animals were kept, in pairs, for 2 weeks before intervention to normalize stress responses.
Transplant experiments and sampling
Sixteen fully MHC-matched, non-immmunosuppressed transplants were performed using published techniques [5,6,12]. Transplants were female into female or female into male. Full details of the surgical technique, functional outcomes and peri-operative care are reported in separate papers [12,13].
Basic metabolic requirements were calculated as 2·621 × weight (kg)0·63 MJ/day [14] and two to four times this amount was fed as milk replacer (Parnutts Foods Ltd, Sleaford, UK) to compensate for the increased metabolic rate associated with recovery.
Mucosal biopsies (of supraglottis, subglottis and trachea) were taken from the donor larynx at induction (T0) after perfusion with ice-cold University of Wisconsin solution (Dupont Pharma, Newcastle, UK) (Tcold), on reperfusion (after transplantation) (Treperf), 48 h (T48) and 7 days (T7d) after transplant; and from the larynx of the recipient at the time of removal. In one case biopsies were taken 4 days after transplant rather than after 48 h (because of the availability of an anaesthetist). Mucosal biopsies were also taken from the tracheal stump of the recipient at T7d. Tonsillar biopsies were taken from the recipient at T7d and from the donor at T0; tonsillar biopsies were not taken from the recipient at T0 to reduce morbidity.
Biopsies were mounted, on randomly numbered cork discs, in optimal cutting temperature medium (OCT) (RALamb, Eastbourne, UK) and snap-frozen in liquid nitrogen. Biopsies from one transplant were halved and one half mounted and frozen as above and the other placed in RNAlater (Ambion, Slough, UK) and stored overnight at 4°C. All samples were stored at −80°C.
Quantitative multiple colour immunofluorescence histochemistry
OCT-embedded frozen biopsies of supraglottis, subglottis and trachea were prepared as described previously [9,15]. Briefly, 5 µm frozen tissue sections were mounted and subjected to two-step multiple-colour immunofluorescence. Optimal dilutions of primary antibody and isotype-specific secondary fluorochrome conjugates were determined by titration. Sections were stained with the antibodies and in the five combinations shown in Table 1, giving 40 possible staining patterns. Multiple fields of view, at ×20 and/or ×40 magnification, were captured by digitizing 16-bit greyscale images (one per colour channel) using a Hamamatsu Orca-ER camera, and Q-fluoro software, attached to a Leica DMR-A epifluorescence microscope (Leica, Milton Keynes, UK). Digital images were analysed using ImageJ software (Image-J; NIH, USA: http://rsb.web.nih.gov/ij/) and macros developed by our group [15]. Percentage areas of positive pixel staining were calculated for each fluorochrome and fluorochrome combination.
Table 1.
Antibodies, isotypes and combinations used in multiple colour immunofluorescence studies of the laryngeal mucosa. Five combinations, each of three antibodies, were used
| Primary antibody† to | Clone | Isotype | Secondary antibody‡ | False colour | Combination |
|---|---|---|---|---|---|
| Myeloid cells | |||||
| CD172 | 742215 | IgG2B | IgG2b-FITCa | Green | 1 |
| CD163 | 2A/10/11 | IgG1 | IgG1-TRITCa | Red | 1 |
| MHC-II | MSA3 EPICS | IgG2A | IgG2a-AF633b | Blue | 1 and 2 |
| CD14 | MIL2 | IgG2B | IgG2b-FITCa | Green | 2 |
| CD16c | G7 | IgG1 | IgG1-TRITCa | Red | 2 |
| Lymphoid cells | |||||
| CD45RC | MIL15 | IgM | IgM-FITCa | Green | 3 and 4 |
| CD25 | K231.3B2 | IgG1 | IgG1-TRITCa | Red | 3 and 4 |
| CD4 | MIL17 H-M | IgG2B | IgG2b-AF633b | Blue | 3 |
| CD8 | MIL12 | IgG2A | IgG2a-AF633b | Blue | 4 and 5 |
| γδ | PPT 27 | IgG2B | IgG2b-FITCa | Green | 5 |
| CD4 | STH293 | IgG1 | IgG1-TRITCa | Red | 5 |
Mouse anti-pig;
goat anti-mouse.
Southern Biotech, Birmingham, Alabama 35260, USA.
Molecular Probes, Oregon, USA.
AbD Serotec, Oxford, UK. All other antibodies were the generous gift of Dr K. Haverson, University of Bristol. Ig, immunoglobulin; FITC, fluorescein isothiocyanate; TRITC, tetramethyl rhodamine isothiocyanate.
Spectratyping
Our technique is based on published spectratyping assays [16] used to analyse variation in T cell receptor (TCR)-β complementarity determining region-3 (CDR3) lengths, and previously described porcine TCR-β sequences [17]–[19]. Biopsies preserved in RNA later were homogenized using a TissueLyser (Qiagen, Crawley, UK) and total RNA was extracted using the SV total RNA isolation system (Promega UK, Southampton, UK). Sample RNA concentration and quality were determined using the Experion automated electrophoresis system (Bio-Rad, Hemel Hempstead, UK). Reverse transcription (RT) was performed using ImProm II Reverse Transcription System (Promega) and random hexamers. To verify that RT was successful and to check for genomic contamination, a porcine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) real-time polymerase chain reaction (PCR) assay was performed for each sample using CDNA as template and a no-RT control. For each CDNA sample, labelled PCR products for TCR-β spectratype analysis were generated using one of 21 different porcine Vβ group-specific/subgroup-specific sense primers and a common Cβ-region antisense primer labelled with 5′ FAM, HEX or TET flourophore. Vβ PCR products were purified using the NucleoSpin 96 Extract II kit (ABgene, Epsom, UK) and a Sigma 4–15C centrifuge with a deep-well plate rotor 2 × 96 (Qiagen). Purifed Vβ PCR products were analysed by the University of Bristol Transcriptomics Facility using a MegaBACE 1000 DNA analysis system and MegaBACE 550-R ET size standards (GE Healthcare, Chalfont St Giles, UK). Electropherograms were analysed using MegaBACE Genetic Profiler version 1·5 (GE Healthcare). For each Vβ spectratype, the peak size [base pairs (bp)] and peak height (fluorescent intensity) was compiled and the peak height data normalized.
Statistical methods
Immunofluorescence data
Changes in the percentage areas of positively stained pixels were analysed using univariate and multivariate analysis of variance, repeated-measures analysis of variance or paired t-tests using spss version 14·0 for Windows (SPSS, Inc., Chicago, IL, USA). All analyses used log10 (n+2)-transformed data.
Spectratyping data
Hierarchical cluster analysis of the peak size and normalized peak height data for each Vβ generated from each sample was performed in spss using Ward's method.
Ethical guidelines
This project was approved by the University of Bristol Ethical Review Group, and was awarded a UK Home Office Project Licence (PPL 30/2374).
Results
Samples
Seven transplant recipients survived with healthy grafts until killed at 7 days. Samples from five of these were available for analysis. For anatomical reasons, it can be difficult to collect tracheal samples from live pigs without compromising intubation and causing unnecessary stress to the animal [20]. Therefore, only two samples of trachea were collected at induction (T0) and no attempt was made to collect tracheal samples at 48 h. Unsuccessful transplants were due either to problems with the airway (three cases) or failure of the graft to reperfuse; incremental improvements in the surgical technique resolved these problems [12].
Quantitative multiple colour immunofluorescence histochemistry
There was interpig variation in expression of all molecules measured (Tables 2–5 and Figs 1–3). Differences between epithelial and lamina propria compartments in the levels of expression of molecules on leucocytes were consistent with our previous observations ([6]; Table 2).
Table 2.
Comparison between supraglottic samples from donor (T0 and Tcold combined) and recipient animals at the start of the surgical procedure. Values are proportion (%) of total number of pixels (equivalent to area of positive staining)
| 95% Confidence interval | |||||
|---|---|---|---|---|---|
| Antigen expression | Type | Tissue | Mean | Lower | Upper |
| CD163+CD172-MHC-II- | Donor | Epithelium | 0·93 | 0·42 | 1·56 |
| Lamina propria | 6·63 | 5·19 | 8·35 | ||
| Recipient | Epithelium | 0·63 | 0·06 | 1·36 | |
| Lamina propria | 4·07 | 2·75 | 5·76 | ||
| CD163-CD172+MHC-II- | Donor | Epithelium | 2·58 | 1·53 | 3·94 |
| Lamina propria | 1·20 | 0·51 | 2·09 | ||
| Recipient | Epithelium | 0·77 | 0·00 | 1·85 | |
| Lamina propria | 0·31 | 0·00 | 1·21 | ||
| CD163+CD172+MHC-II- | Donor | Epithelium | 0·72 | 0·23 | 1·33 |
| Lamina propria | 1·68 | 1·05 | 2·44 | ||
| Recipient | Epithelium | 0·18 | 0·00 | 0·80 | |
| Lamina propria | 0·62 | 0·04 | 1·37 | ||
Table 5.
Myeloid antigens in tracheal samples from donor pigs at the start of cold ischaemia (Tcold) and 1 week after transplant (T7d), and from recipient pigs 1 week after transplant (T7d)
| CD163+CD172-MHC-II- | CD163+CD172+MHC-II- | ||||||
|---|---|---|---|---|---|---|---|
| 95% Confidence limits | 95% Confidence limits | ||||||
| Time | Source | Mean | Lower | Upper | Mean | Lower | Upper |
| Tcold | Donor | 2·16 | 0·68 | 4·45 | 0·84 | 0·10 | 1·84 |
| T7d | Donor | 8·69 | 4·07 | 16·83 | 3·90 | 1·44 | 8·14 |
| Recipient | 2·36 | 0·22 | 6·56 | 0·98 | 0·02 | 2·40 | |
Fig. 1.
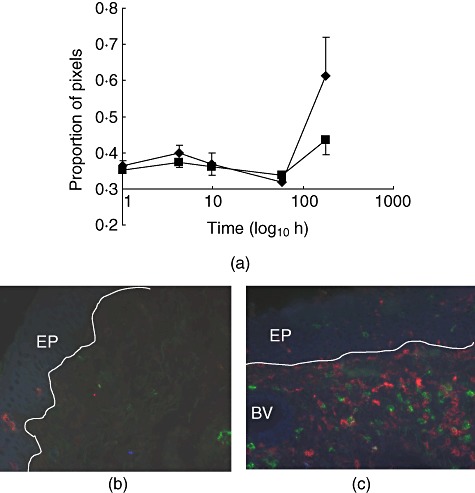
CD25 expression in the porcine subglottis. (a) Normalized log(proportion of pixels) CD25+CD45RC-CD4-◆ and CD25+CD45RC-CD8- ; values are means of five pigs with standard error. (b,c) Three-colour immunofluorescence images from biopsies taken at induction (b) and 7 days after transplantion (c). Red: CD25; green: CD45RC; blue: CD4; EP: epithelium; BV: blood vessel; line represents basement membrane.
; values are means of five pigs with standard error. (b,c) Three-colour immunofluorescence images from biopsies taken at induction (b) and 7 days after transplantion (c). Red: CD25; green: CD45RC; blue: CD4; EP: epithelium; BV: blood vessel; line represents basement membrane.
Fig. 3.
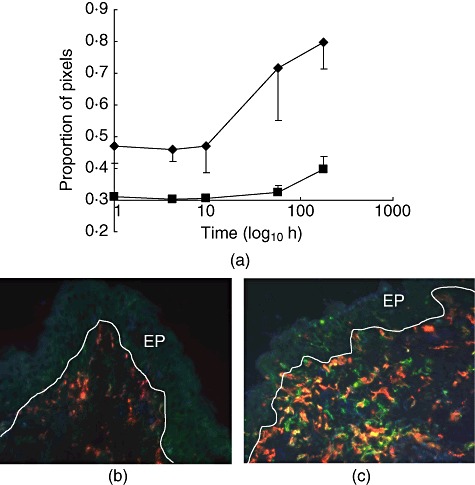
Macrophages in the porcine subglottis. (a) Normalized log(proportion of pixels) CD163+CD172+MHC-II-◆ and CD163+CD172+MHC-II+ values are means of five pigs with standard error. (b,c) Three-colour immunofluorescence images from biopsies taken at induction (b) and 7 days after transplantion (c). Red: CD163; green: CD172; blue: MHC-II; EP: epithelium; line represents basement membrane.
values are means of five pigs with standard error. (b,c) Three-colour immunofluorescence images from biopsies taken at induction (b) and 7 days after transplantion (c). Red: CD163; green: CD172; blue: MHC-II; EP: epithelium; line represents basement membrane.
Comparisons of donor and recipient groups prior to transplantation (donor T0versus recipient at time of removal)
We compared samples taken from the donors at induction (T0) with those taken from the recipients at the time the larynx was removed. We have only two values for the donor tracheas at T0; see above. Therefore, results from samples taken at T0 and Tcold were combined (seven samples in total for trachea, and 10 for each of supraglottis and subglottis) and compared with the five samples taken from the recipients at time of removal of the larynx.
No statistically significant differences between donors and recipients in expression of the molecules measured were seen in the trachea or the subglottis. However, in the supraglottis the areas expressing the combination of CD163-CD172+ MHC-II- (P = 0·008), of CD163+CD172-MHC-II- (P = 0·039) and of CD163+CD172+MHC-II- (P = 0·016) were all slightly greater in the donors than in the recipients (Table 2). The same result was seen in the more limited comparison of donor samples taken at T0 with those from the recipients.
Initial reaction to surgical procedures (T0versus Tcold and Treperfuse)
There were few statistically significant (P < 0·05) differences between the percentages of positively stained pixels recorded from samples taken from the donor larynx at T0 and those taken at Tcold, as indicated by paired t-test. In the supraglottis, the areas expressing the combination of CD16+CD14+MHC-II- (P = 0·012) (Table 3) and of CD16+CD14-MHC-II- (2·74% versus 4·58%, P = 0·023) were slightly smaller at Tcold than T0.
Table 3.
Myeloid-associated antigens in supraglottic samples from donor (transplanted) larynx. Values are proportion (%) of total pixels (equivalent to area of positive staining
| CD163+CD172-MHC-II- | CD163+CD172+MHC-II- | CD16+CD14+mhcMHC-II- | CD16-CD14+MHC-II- | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% Confidence limits | 95% Confidence limits | 95% Confidence limits | 95% Confidence limits | |||||||||
| Time | Mean | Lower | Upper | Mean | Lower | Upper | Mean | Lower | Upper | Mean | Lower | Upper |
| T0 | 3·12 | 1·56 | 5·36 | 1·64 | 0·86 | 2·64 | 1·83 | 1·22 | 2·54 | 0·68 | 0·43 | 0·95 |
| Tcold | 3·29 | 1·15 | 6·86 | 0·74 | 0·34 | 1·20 | 0·82 | 0·33 | 1·41 | 0·34 | 0·03 | 0·68 |
| Treperf | 2·39 | 0·91 | 4·62 | 1·09 | 0·48 | 1·85 | 1·71 | 1·05 | 2·52 | 0·90 | 0·21 | 1·80 |
| T48 | 2·13 | 0·89 | 3·89 | 2·81 | 1·01 | 5·68 | 0·90 | 0·21 | 1·80 | 1·88 | 1·03 | 2·96 |
| T7d | 4·36 | 1·76 | 8·77 | 2·95 | 1·04 | 6·06 | 3·99 | 1·83 | 7·35 | 0·61 | 0·26 | 1·02 |
At the end of cold ischaemia (Treperf) there were few statistically significant (P < 0·05) changes compared to T0 and Tcold. This was true whether the data were analysed using time–series analysis or paired t-test. In the subglottis the areas expressing the combination of CD163+CD172- and MHC-II- was slightly greater at Treperfuse than at T0 (2·52% versus 1·13%, P = 0·038), while in the supraglottis the areas of co-expression of CD45RC+CD4-CD25- were greater at Treperfuse (0·06% versus 0·34%, P = 0·009).
Changes within the graft after transplantation (T0versus T48 and T7d)
Forty-eight h after transplantation (T48) a small decrease in the area of positive staining was recorded for most molecules on lymphoid cells in samples from both the supraglottis and subglottis, exemplified by CD25 (Table 4 and Fig. 1).
Table 4.
CD25 in the supraglottis of donor larynges; values are proportion (%) of total number of pixels (equivalent to area of positive staining)
| CD25+CD45RC-CD4+ | CD25+CD45RC-CD8+ | |||||
|---|---|---|---|---|---|---|
| 95% Confidence limits | 95% Confidence limits | |||||
| Time | Mean | Lower | Upper | Mean | Lower | Upper |
| T0 | 0·84 | 0·11 | 1·82 | 0·59 | 0·00 | 1·39 |
| Tcold | 0·11 | 0·00 | 0·23 | 0·19 | 0·00 | 0·40 |
| Treperf | 0·46 | 0·00 | 1·44 | 0·34 | 0·00 | 0·83 |
| T48h | 0·30 | 0·10 | 0·53 | 0·14 | 0·02 | 0·28 |
| T7d | 0·48 | 0·00 | 1·33 | 0·87 | 0·00 | 3·07 |
There was a small increase in the areas of positive staining representing myeloid-associated molecules at T48, particularly those expressing the combination of CD163+CD172-MHC-II-, of CD163+CD172+MHC-II-, of CD16+CD14+MHC-II- and of CD16-CD14+MHC-II- in the subglottis (Figs 2 and 3). In the supraglottis changes were less consistent (Table 3).
Fig. 2.
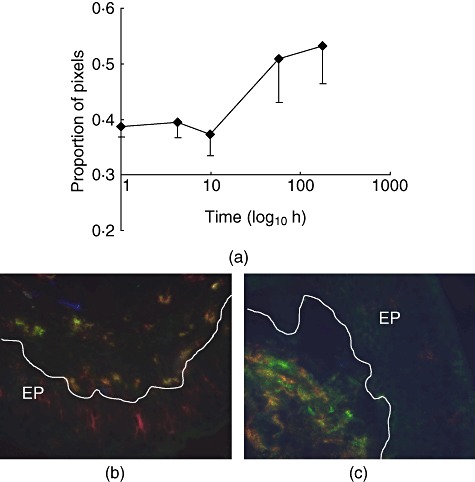
CD14 expression in the porcine subglottis. (a) Normalized log(proportion of pixels) CD14+CD16-MHC-II-◆ values are means of five pigs with standard error. (b,c) Three-colour immunofluorescence images from biopsies taken at induction (b) and 48 h after transplantion (c). Red: CD16; green: CD14; blue: MHC-II; EP epithelium; line represents basement membrane.
There was no evidence of a strong CD4+ or CD8+ T cell-mediated reaction at 1 week. However, subglottic CD25 staining increased significantly (CD25+CD45RC-CD4-: P = 0·048; CD25+CD45RC-CD8-: P = 0·044; Fig. 1). Similar, although not statistically significant, increases were seen in the supraglottis (Table 4). In the trachea there was an increase in the area expressing the combination of CD25+CD45RC-CD4- from 0·09% at Tcold to 0·41% at T7d (P = 0·035) and a smaller, not statistically significant, increase in the area expressing the combination of CD25+CD45RC-CD8- from 0·21% to 0·51% (P > 0·05). This increase was not in the CD4 or CD8 compartments, as shown by multiple-labelling.
The areas expressing molecules associated with myeloid cells tended to rise at 1 week; significant increases occurred in the subglottis in the area expressing the combination of CD16-CD14+MHC-II- (P = 0·05; Fig. 2) and of CD163+CD172+MHC-II- (indicative of macrophages; P = 0·01; Fig. 3). An increase in the area of co-expression of CD163+CD172+MHC-II+ molecules (indicative of ‘dendritic cells’) failed to reach statistical significance (P = 0·08, time–series and t-test; Fig. 3). Similarly, in the supraglottis increases were seen in the areas expressing the combination of CD163+CD172-MHC-II-, of CD163+CD172+MHC-II- and of CD16+CD14+MHC-II- (Table 3), although these were not statistically significant. In the trachea there was an increase in area expressing the combination CD163+, CD172-MHC-II- (F = 11·358, P = 0·008) and CD163+CD172+MHC-II- (F = 6·599, P = 0·030) (Table 5).
Comparisons of donor and recipient tissue 1 week after transplantation
We have tissue from the recipient tracheal stump to compare with tracheal tissue from the graft. No statistically significant differences (P < 0·05) were observed in the levels of antigen expression in tracheal samples from the recipient at start of surgery compared with those from the recipient after 1 week.
There were few differences between donor and recipient trachea 1 week after surgery, although the areas expressing the combination of CD163+CD172-MHC-II- and CD163+CD172+MHC-II- was greater in donor than in recipient (Table 5) (F = 9·882, P = 0·008 and F = 5·768, P = 0·033, respectively). There were also small but statistically non-significant (P < 0·05) increases in the area expressing the combination of CD16+CD14-MHC-II-, CD16+CD14+MHC-II- and CD16-CD14-MHC-II-.
Comparison between tonsillar samples from the donor at the start with samples from the recipient after 1 week also found no significant differences.
Spectratyping
TCR Vβ spectratypes were generated from samples collected during one transplant experiment using laryngeal subglottal biopsies taken from the recipient larynx at day 0 (biopsy mass 13·1 mg), and the donor larynx at days 0 (2·4 mg), 4 (4·4 mg) and 7 (13·4 mg). A sample of recipient spleen (10·5 mg) was also analysed. Hierarchical cluster analysis using data from all samples (Fig. 4) showed that the donor larynx Vβ repertoire was variable, but over time it became more similar to that of the recipient larynx. Clustering of the samples from the donor larynx at day 7 with the recipient larynx and spleen was demonstrated: however, the Vβ repertoires of the recipient larynx and spleen were still more similar to each other than to that of the donor larynx (Fig. 4).
Fig. 4.
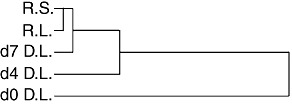
Hierarchical cluster analysis dendogram of normalized peak height data generated in Vβ spectratyping assays from samples of recipient larynx and spleen, and donor larynx collected at days 0, 4 and 7 post-transplantation. R, recipient; D, donor; L, larynx; S, spleen; d, day post-transplantation.
Discussion
Quantitative immunofluorescence immunohistochemistry
In our previous studies examining the early effects of ischaemia–reperfusion and surgical injury in a non-recovery transplant model, significant effects were observed at 8 h after reperfusion, but the magnitude of the effects was less than interpig variation at baseline [6]. Here we report the first detailed analysis of the acute and delayed mucosal immunological response to orthotopic laryngeal transplantation in a recovery model over the first week. The animals were fully matched at the MHC locus; minor histocompatability mismatches were unlikely to cause acute rejection. Donor and recipient had been housed together prior to the surgery; therefore we expected few differences between animals prior to transplantation. However, donor larynges expressed marginally more macrophage-related molecules than those they were replacing (Table 3). We hypothesize that this was due to the challenge, to the recipient, of a previous anaesthetic/laryngeal intubation performed 2–3 days previously [21].
Prior to the onset of cold ischaemia, there was a slight drop in expression of molecules associated with myeloid cells in the supraglottis and lymphocytic antigens in the supraglottis, but no change in the trachea. These small differences might be attributed to intra-operative blood loss. At the time of reperfusion there was no clear pattern to the minor changes observed, suggesting no hyperacute effect of this challenge on laryngeal immunological architecture. The length of cold ischaemia in this study (340 min) is between that in the only two reported human laryngeal transplants: 10 h [22] and 4 h (Birchall; personal communication).
At 48 h, we observed a small decrease in expression of MHC-II and lymphocyte-associated molecules, while there was a small increase in other myeloid-associated molecules. This is consistent with the observations of Friedman et al. [23] using heterotopic grafts in rats, where separate labelling of donor and recipient cells suggested depletion of donor leucocytes from the graft before the appearance of recipient cells.
An increase in some, but not all, myeloid-associated molecules was observed in the subglottis, supraglottis and trachea at 1 week after transplantation. In the subglottis there was a highly significant increase in areas expressing the combination of CD163+CD172+MHC-II- and a small increase in cells co-expressing CD163+CD172+MHC-II+ molecules. Previous studies in pigs have suggested that CD163+CD172+ cells in blood up-regulate surface MHC-II and differentiate effectively into dendritic cells [24] and the MHC-II- and MHC-II+ subsets may represent monocytes/macrophages and immature dendritic cells, respectively [25]. The similarity to the results of Friedman et al. [23] is striking. In both allograft and fully matched rat models, these authors found a significant increase in OX62+ cells, both with and without MHC-II co-expression, between days 5–7: again the strongest effect was seen in the subglottis. The proportion of OX62+ cells expressing MHC-II was low (25%), suggesting that the majority were either immature dendritic cells or other cells expressing the integrin receptor, such as macrophages [26]. Friedman et al.'s observations extended to 9 days, by which time expression of all antigens was falling towards baseline. The increases in myeloid-associated molecules we observed in our orthotopic pig model may also be transient. Similarly, we observed a rise in expression of CD14 molecules [in pigs, the lipopolysaccharide (LPS) receptor]; we saw an increase in number of CD14+CD16-MHC-II- cells and CD14+CD16+MHC-II- cells, but not in cells expressing MHC-II. We interpret this as further evidence that the population we are observing is primarily macrophages. Inspection of the images confirmed that the increase in CD14 staining was not localized to epithelium, endothelium or fibroblasts, all of which can also express this molecule [25], confirming that these were leucocyte changes.
In the absence of any obvious damage to the grafts at functional, macroscopic, microscopic and vascular levels, we hypothesize that the increase in myeloid cells in the subglottis at 1 week may be a response to a (possibly transient) change in mucosal flora. In support of this, colonization experiments in pigs have shown similar changes in bowel [27] and laryngeal [28,29] mucosa in response to bacterial challenge.
The observation that expression of CD25 in the absence of either CD4 or CD8 or CD45RC increased, but expression co-localized with CD4 or CD8 did not increase, indicates that the increase in CD25 was associated with a CD4-CD8-CD45RC- cell. Although CD25+CD4-CD8- cells occur in pigs, particularly in young animals as used here (Haverson, July 2008, personal communication), these are unlikely to be conventional T cells in which CD45RC indicates a particular state of differentiation. We hypothesize that unconventional cells expressing CD25 may be responsible for the subsequent recruitment of the myeloid populations seen in laryngeal transplants at 1 week in pig and rat. Alternatively, the CD25+ increase at 1 week could be associated with increased expression of the antigen on monocytes or natural killer (NK) cells.
The study of recipient trachea (proximal to the transplant) at the time of implantation and at death found no significant differences. The few differences that were observed between trachea from donors prior to implantation and recipient trachea at death may have been biased by the fact that the donor animals had slightly higher levels of expression of macrophage-associated molecules before surgery. This suggests that laryngeal transplantation surgery does not alter the immunological architecture of the host airway beyond the confines of the graft, at least in the first week after surgery. The apparent absence of changes in a smaller number of tonsillar biopsies supports this assertion.
Spectratyping
TCR-β spectratyping [30] is a technique that uses the variation in TCR-β CDR3 length as a surrogate measure of diversity of the T cell repertoire, and has been used to provide proxy measures of changes in the T cell repertoire occurring during transplantation, autoimmunity and inflammation. We have recently developed pig TCR Vβ spectratyping assays based upon published porcine Vβ sequences [17]–[19]. In this study we applied these assays to tissue biopsies from one transplantion experiment to establish if spectratype analysis is a feasible technique for use in our transplant model, and to determine if changes in T cell repertoires within laryngeal mucosal biopsies can be detected. Our results show that spectratype analysis can be performed successfully on laryngeal mucosal biopsies and has the potential to provide a better understanding of changes in the mucosal immunology of the grafted tissue. The donor and recipient laryngeal biopsy Vβ repertoires were initially distinct, consistent with interindividual variation [31]. Interestingly, post-transplantation, the Vβ repertoire of the donor laryngeal samples became more similar to that of the recipient larynx. As only a single transplant pair was analysed, firm conclusions cannot be drawn and further studies are required to determine if this observation is a consistent phenomenon in other laryngeal transplants. It is also possible that the differences in the T cell repertoires in the donor larynx may represent variation in biopsy sampling. Broadly, however, the results would support the hypothesis that between 0 and 7 days post-transplantation the donor larynx is gradually repopulated with recipient T cells, whose repertoire is representative of that normally associated with laryngeal mucosa in the recipient. These are likely to represent a pre-exisiting, laryngeal-homing T cell population, indicating normal recirculation of T cells through the graft. We hypothesize further that rejection will be associated with infiltration by T cells with characteristic repertoires. Spectratyping studies of grafts between mismatched animals would help to explore this question.
Conclusion
The results of this study reveal that in the first week following laryngeal transplantation a complex pattern of changes in expression of leucocyte-associated molecules occur in the subglottis and supraglottis mucosa, whereas expression of the same molecules in the tracheal mucosa remains more stable. This observation could lend support for the idea of using tissue engineering to replace damaged trachea [2]. However, in the case of the larynx, it may be that these intricate and often subtle immunological responses can only be replicated by another, transplanted larynx. Future work aiming to elucidate the functional significance of laryngeal mucosal responses during transplantation will be critical in resolving these issues and guiding advances in laryngeal transplantation and replacement. Together, the observed lack of strong responses to the trauma of surgery and ischaemia provides encouraging evidence to support clinical trials of laryngeal transplantation, and a basis on which to interpret future studies involving mismatches and immunosuppression.
Here, we present evidence that for at least at 1 week following laryngeal transplantation in an established orthotopic model in minipigs there is no major mucosal immune response to the twin challenges of ischaemia and reperfusion. In the future, the results of this study will provide baseline reference data to permit interpretation of immunological changes arising in the laryngeal and tracheal mucosa following grafting with varying but controlled levels of MHC mismatching, the clinical scenario for the two clinical laryngeal transplants reported to date.
Acknowledgments
This study was funded by the Wellcome Trust Clinical Research Leave Fellowship to Martin Birchall (GR061125). Ross Harley and Mick Bailey were supported by a Defra/Hefce award (VT0104). Some equipment was kindly provided by Karl Storz UK, Ltd. Numerous research fellows, ENT senior house officers and registrars, PhD students and veterinary nurses assisted with this project. They all gave of their time freely, including spending many hours performing high dependency care of pigs. Every one of them has played a part in the development of this complex, but ultimately successful, model and project. Kind advice was received from many parts of the School of Clinical Veterinary Science, University of Bristol, where Professor A. Waterman-Pearson, Dr K. Haverson, Dr C.F. Inman and Mr C. Chambers were particularly supportive.
Disclosure
The authors have no conflict of interest.
References
- 1.Data for Head and Neck Oncology (DAHNO) National Head and Neck Cancer Audit: key findings for England and Wales for the audit period October 2006–November 2007. Leeds: NHS information Centre Clinical Audit Support Program; 2008. [Google Scholar]
- 2.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 3.Meyer TK, Kuhn JC, Campbell BH, et al. Speech intelligibility and quality of life in head and neck cancer survivors. Laryngoscope. 2004;114:1977–81. doi: 10.1097/01.mlg.0000147932.36885.9e. [DOI] [PubMed] [Google Scholar]
- 4.Fung K, Lyden TH, Lee J, et al. Voice and swallowing outcomes of an organ-preservation trial for advanced laryngeal cancer. Int J Radiat Oncol Biol Phys. 2005;63:1395–9. doi: 10.1016/j.ijrobp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Birchall MA, Bailey M, Barker EV, et al. Model for experimental revascularized laryngeal allotransplantation. Br J Surg. 2002;89:1470–5. doi: 10.1046/j.1365-2168.2002.02234.x. [DOI] [PubMed] [Google Scholar]
- 6.Barker E, Murison P, Macchiarini P, et al. Early immunological changes associated with laryngeal transplantation in a major histocompatibility complex-matched pig model. Clin Exp Immunol. 2006;146:503–8. doi: 10.1111/j.1365-2249.2006.03232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haverson K. Overview of the third international workshop on swine leukocyte differentiation antigens. Vet Immunol Immunopath. 2001;80:5–23. doi: 10.1016/s0165-2427(01)00290-2. [DOI] [PubMed] [Google Scholar]
- 8.Sachs DH, Leight G, Cone J, et al. Transplantation in minature swine. Transplantation. 1976;22:559–67. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Barker E, Haverson K, Stokes CR, et al. The larynx as an immunological organ: immunological architecture in pig as a large animal model. Clin Exp Immunol. 2005;143:6–14. doi: 10.1111/j.1365-2249.2005.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight MJ, McDonald SE, Birchall MA. Intrinsic muscles and distribution of the recurrent laryngeal nerve in the pig larynx. Eur Arch Otorhinolaryngol. 2005;262:281–5. doi: 10.1007/s00405-004-0803-3. [DOI] [PubMed] [Google Scholar]
- 11.Kingham PJ, Birchall MA, Burt R, et al. Reinnervation of laryngeal muscles: a study of changes in myosin heavy chain expression. Muscle Nerve. 2005;32:761–6. doi: 10.1002/mus.20409. [DOI] [PubMed] [Google Scholar]
- 12.Birchall MA, Kingham PJ, Macchiarini P, et al. Laryngeal transplantation in minipigs: vascular, myologic and functional outcomes. Eur Arch Otorhinolaryngol. 2011;268:405–14. doi: 10.1007/s00405-010-1355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murison PJ, Jones A, Mitchard L, et al. Development of peri-operative care for pigs undergoing laryngeal transplantation. Lab Anim. 2009;43:338–43. doi: 10.1258/la.2009.008101. [DOI] [PubMed] [Google Scholar]
- 14.Agricultural Research Council. The Nutrient Requirements of Pigs/technical review by an Agricultural Research Council Working Party. Farnham Royal: Commonwealth Agricultural Bureaux; 1981. [Google Scholar]
- 15.Inman CF, Rees LEN, Barker EV, et al. Validation of computer-assisted pixed based analysis of multiple-colour immunofluorescence histology. J Immunol Methods. 2005;302:156–67. doi: 10.1016/j.jim.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Edwards AG, Weale AR, Denny AJ, et al. Antigen receptor V-segment usage in mucosal T cells. Immunology. 2008;123:181–6. doi: 10.1111/j.1365-2567.2007.02685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baron C, Sachs DH, LeGuern C. A particular TCR β variable region used by T cells infiltrating kidney transplants. J Immunol. 2001;166:2589–96. doi: 10.4049/jimmunol.166.4.2589. [DOI] [PubMed] [Google Scholar]
- 18.Butler JE, Wertz N, Sun J, et al. Comparison of expressed porcine Vbeta and Jbeta repertoire of thymocytes and peripheral T cells. Immunology. 2005;114:184–93. doi: 10.1111/j.1365-2567.2004.02072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe M, Iwasaki Y, Mita Y, et al. Porcine T-cell receptor β-chain: a genomic sequence covering Dβ1.1 to Cβ2 gene segments and the diversity of cDNA expressed in piglets including novel alternative splicing products. Mol Immunol. 2007;44:2332–43. doi: 10.1016/j.molimm.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Harrison DFN. The anatomy and physiology of the mammalian larynx. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- 21.Hughes OR, Ayling SM, Birchall MA. Immune response of the larynx to short term intubation. Clin Otolaryngol. 2008;33:304. [Google Scholar]
- 22.Strome M, Stein J, Esclamado R, et al. Laryngeal transplantation and 40-month follow-up. N Engl J Med. 2001;344:1676–9. doi: 10.1056/NEJM200105313442204. [DOI] [PubMed] [Google Scholar]
- 23.Friedman AD, Dan O, Drazba JA, et al. Postallograft donor and recipient dendritic cell trafficking in the rat larynx. Laryngoscope. 2007;117:1615–21. doi: 10.1097/MLG.0b013e3180959e1e. [DOI] [PubMed] [Google Scholar]
- 24.Ezquerra A, Revilla C, Alvarez B, et al. Porcine myelomonocytic markers and cell populations. Dev Comp Immunol. 2009;33:284–98. doi: 10.1016/j.dci.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Chen-Woan M, Delaney CP, Fournier V, et al. In vitro characterization of rat bone marrow-derived dendritic cells and their precursors. J Leukoc Biol. 1996;59:196–207. doi: 10.1002/jlb.59.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Summerfield A, Haverson K, Thacker E, et al. Differentiation of porcine myeloid bone marrow haematopoietic cell populations. Vet Immunol Immunopathol. 2001;80:121–9. doi: 10.1016/s0165-2427(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 27.Haverson K, Rehakova Z, Sinkora J, et al. Immune development in jejunal mucosa after colonization with selected commensal gut bacteria: a study in germ-free pigs. Vet Immunol Immunopathol. 2007;119:243–53. doi: 10.1016/j.vetimm.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Birchall M, Inman C, Laycock G, et al. The development of upper airway mucosal immune architecture depends on peri-natal bacterial colonisation. Clin Otolaryngol. 2008;33:299–300. [Google Scholar]
- 29.Thibeault SL, Rees L, Pazmany L, et al. At the crossroads: mucosal immunology of the larynx. Mucosal Immunol. 2009;2:122–8. doi: 10.1038/mi.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pannetier C, Even J, Kourilsky P. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today. 1995;16:176–81. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 31.Friedman TM, Statton D, Jones SC, et al. Vβ spectratype analysis reveals heterogeneity of CD4+ T-cell responses to minor histocompatibility antigens involved in graft-versus-host disease: correlations with epithelial tissue infiltrate. Biol Blood Marrow Transplant. 2001;7:2–13. doi: 10.1053/bbmt.2001.v7.pm11215694. [DOI] [PubMed] [Google Scholar]


