Abstract
The segregation and random assortment of characters observed by Mendel have their basis in the behavior of chromosomes in meiosis. But showing this actually to be the case requires a correct understanding of the meiotic behavior of chromosomes. This was achieved only gradually, over several decades, with much dispute and confusion along the way. One crucial step in the understanding of meiosis was provided in 1909 by Frans Alfons Janssens who published in La Cellule an article entitled “La théorie de la Chiasmatypie. Nouvelle interprétation des cinèses de maturation,” which contains the first description of the chiasma structure. He observed that, of the four chromatids present at the connection sites (chiasmata sites) at diplotene or anaphase of the first meiotic division, two crossed each other and two did not. He therefore postulated that the maternal and paternal chromatids that crossed penetrated the other until they broke and rejoined in maternal and paternal segments new ways; the other two chromatids remained free and thus intact. This allowed him also to propose that the chromatids distributed in the four nuclei issued from the second meiotic division had various combinations of maternal and paternal segments of each chromosome. And conversely, permitted the appreciation that the laws of Mendelian segregation required breakage and joining (crossing over) between homologous non-sister chromatids. Although Janssens’s article found a broad appreciative audience and had a large influence on the chromosomal theory at that time, his theory was resisted by both geneticists and cytologists for several decades. This Perspectives aims to highlight the novelty of Janssens’s chiasmatype theory by examining the historical background and our actual understanding of meiotic recombination.
FRANS A. Janssens (1863–1924) was a Belgian cytologist. Cytology itself was a new field, having emerged during the 19th century concomitantly with the development and improvement of microscope techniques, notably in Belgium (Figure 1). Janssens was first the student of, and then the successor to, J. B. Carnoy, at the University of Louvain. Carnoy had been the founder of La Cellule, the first journal dedicated to cytology. Two other well-known cytologists, Theodore Schwann (1810–1882) and Edouard Van Beneden (1846–1910) (who had shown that both parental gametes contributed an equal number of chromosomes during fertilization), were also teaching in Louvain before moving to the neighboring University of Liège, and their work was familiar to Janssens. He was also aware of the hypotheses favoring a role for chromosomes in Mendel’s laws (Bateson 1902; Sutton 1903; Boveri 1889, 1904). Hugo De Vries himself, who contributed to the rediscovery of Mendel, wrote in Die Mutationstheorie (“The Mutation Theory”) (De Vries 1901): “We may assume that these units [Mendel’s determinants] are represented in the hereditary substance of the cell-nucleus by definite bodies too small to be seen, but constituting together the chromosomes.”
Figure 1.

Original painting, (Katholieke Universiteit Leuven \x{2013} Catholic University of Leuven). François Alphonse Ignace Marie (Frans) Janssens was born on July 23, 1863, in Sint-Niklaas-Waas near Anvers (Antwerpen), Belgium, into a wealthy family. His father was a senator for >35 years and held several high-powered positions for which he was knighted under the name of Janssens de Varebeke. Frans and three of his brothers became Catholic priests. While studying theology, Frans defended a zoology thesis in 1890 at the Louvain (Leuven) Catholic University UCL) under the supervision of Jean-Baptiste Carnoy. Carnoy, also a priest, contributed to the emergence of cytology both locally in his university and also worldwide. In 1884, Frans Janssens founded La Cellule, the first journal entirely dedicated to this new field of biology. Janssens received some post-doctoral training in various European laboratories, visiting marine biology centers in France and Italy. He worked also with E. C. Hansen, a renowned yeast expert from the Carlsberg Laboratory in Copenhagen. Under his influence, Janssens started to address the debated existence of a nucleus in the fungi phylum Torula sp. Moving back to Belgium in 1896, Frans Janssens then joined the UCL faculty of sciences where he took over some of Carnoy’s (deceased prematurely in 1899) courses. His research focused mostly on the chromosome organization found in nuclei of batrachians and insects. During the First World War Janssens found refuge in his Wichelen estate, where he established a private laboratory and made most of the observations described in his late and final work, the voluminous “Chiasmatype in insects” published in 1924 in La Cellule. Sick and almost blind, Janssens resigned from all university appointments in 1924 and died on October 8 of the same year. Ten days later, the editor of La Cellule received a manuscript from T. H. Morgan. In this article, Morgan wrote: “The evidence for crossing-over in Amphibia and in Orthoptera that Janssens has brought forward is of great importance both for genetics and for cytology” (Morgan 1925).
In 1909, Janssens published in La Cellule an article entitled “La théorie de la Chiasmatypie. Nouvelle interprétation des cinèses de maturation,” which contains the first description of the chiasma structure (Janssens 1909).1 Interestingly, in an unusual but significant gesture, Janssens deposited in 1908 a sealed document at the Royal Academy of Belgium, of which he was not a member, 1 year before submitting his complete article.2 This document reports part of his observations and gives most of the conclusions developed in the 1909 article. Rereading this article today reveals how substantial and innovative the chiasmatype theory was at the time, relying on a remarkable intuition and a careful analysis of diplotene and anaphase I configurations of Batracoseps attenuatus (California salamander) and meiosis in several triton species.
Meiosis, as It Is Now Understood
Regular meiosis in diploids begins with chromosomal replication followed by two nuclear divisions with no intervening replication. Following replication, maternal and paternal chromosomes (homologs), each now comprising a pair of sister chromatids (each chromatid being a DNA duplex), condense into visible structures at a stage called “leptotene” (thin threads) and begin to pair side-by-side, initiating the stage designated “zygotene” (paired threads), which is followed, when pairing is completed, by the “pachytene” stage (thick threads). Pachytene is said to end when homologs begin to separate, initiating diplotene (two threads). From leptotene through pachytene, non-sister chromatids cross over (recombine) via breakage-and-joining at certain local sites. The positions of these crossover events become visible only later, especially in mid-diplotene, where they comprise discrete local connections between homologs called chiasmata. Each crossover event gives rise to a reciprocally related pair of chromatids, each with a segment of maternal origin joined to a segment of paternal origin. After diplotene, homologous paternal and maternal chromosomes move to opposite poles (anaphase I), thus completing the first division. The second division of meiosis thus begins with each chromosome comprising two sister chromatids, which then segregate as they do during mitosis, the two cellular products of the first meiosis thus giving rise, in total, to four haploid nuclei.
In both meiotic divisions, as during mitosis, chromosome segregation is governed by spindle tension, which senses the presence of a physical connection between the segregating entities (e.g., Paliulis and Nicklas 2005). During mitosis, segregating sister chromatids are initially connected to one another by a complex of proteins called cohesins (review in Nasmyth 2001), which are released when centromeres/kinetochores are attached to the spindle microtubules, thus allowing chromatids to segregate to opposite poles. During the first meiotic division, homologous chromosomes segregate to poles. In this case, homolog-chromosome connectedness is provided by chiasmata, which result from the combined effects of the reciprocal exchange provided by crossovers (above) and the connection of the sister chromatids (by cohesins) all along their arms. Correspondingly, when either crossing over or sister cohesion is defective, homologous chromosomes segregate randomly to the poles, giving rise to aneuploidy and sterile gametes (review in Kleckner et al. 2012). Thus, in most organisms, aside from promoting genetic diversity, crossing over plays a critical direct mechanical role in ensuring regular meiotic chromosome segregation.
Meiosis as Known in 1909, When Janssens Published His Article
At the time of Janssens’s article, Mendel’s laws were generally accepted, as was, at least by some authors, the notion that the corresponding heritable entities were present on the chromosomes (the “chromatic part of the nucleus,” i.e., that which, in fixed preparations, stains colorfully with certain dyes). However, the latter notion, that chromosomes were the bearers of heritable entities, was still not universally accepted, as illustrated by Morgan’s comments of 1910: “Since the number of chromosomes is relatively small and the characters of the individual are very numerous, it follows on the theory that many characters must be contained in the same chromosome. Consequently, many characters must “Mendelize” together. Do the facts conform to this requisite of the hypothesis? It seems to me that they do not.” Later in the text, Morgan adds: “Our general conclusion is, therefore, that the essential process of the two kinds of gametes of hybrids is a reaction or response of the cell, and is not due to a material segregation of the two kinds of materials contributed by the germ cells of the two parents” (Morgan 1910).
Cytological studies had established certain basic chromosomal events of meiosis. The zygotene and pachytene stages of prophase (“amphitene” and “pachytene,” respectively, the latter also called “conjugation”) were understood to comprise the coming together of maternal and paternal homologous chromosomes (“twin chromosomes”) into a single morphological unit (e.g., Weismann 1885, Strasburger 1888; Montgomery 1901; Grégoire 1904). The dyad chromosome configuration that emerges between pachytene and the first meiotic division, at diplotene/diakinesis, was understood to comprise a set of maternal and paternal chromosomes (e.g., Weismann 1885; Wilson 1900; Grégoire 1904). In most organisms studied at that time, the two chromosomes at this stage are seen wound around one another, i.e., are “relationally coiled.” This stage was thus called the “strepsitene coiling stage.” The existence of metaphase I, of segregation of chromosomes at anaphase I via microtubules (“fibers”) attached at centromere/kinetochore regions (“fiber attachment points”), and of the occurrence of a second round of segregation rapidly succeeding the first round had also been described. Moreover, both Boveri (1889) and Sutton (1902, 1903) had shown that meiosis is a process of halving the chromosome number to compensate for the addition in fertilization and that the separation of paternal and maternal chromosomes during the first meiotic division paralleled Mendel’s separation of characters. Moreover, Sutton showed that the independence of orientation of the homologs leads to a large number of combinations of the two sets of chromosomes in the gametes, again a parallel with Mendel’s observation of independent inheritance of different characters (see details in Hegreness and Meselson 2007).
Also, remarkably, Boveri wrote in 1904 concerning his experiment on sea urchins: “This will lead to the conclusion that the characters located in one chromosome distribute themselves over both of the daughter cells during the reduction division, which indicates that an exchange of parts of homologous chromosomes takes place” (Boveri 1904). In fact, Boveri was not the first to make this suggestion. Rückert (1892) had already suggested that chromosomes exchanged material at the points where they came together at diplotene. Thus, the idea of an exchange of chromosome parts related to genetic characters was already in the literature several years before Janssens’s theory. Those preceding experiments and conclusions do not diminish the remarkable nature of Janssens’s insights, but they were also per se important contributions.
The question, then, was how to relate chromosome morphologies seen through meiotic stages to the requirements of Mendel’s laws. The prevailing theory prior to Janssen’s article was that of Weismann (1885). He proposed that chromosome segregation at meiosis I cleanly segregated maternally and paternally derived chromosomes of each dyad to opposite poles. This process would fully “reduce” the genetic complement at all locations throughout the genome. Meiosis I was thus named the “reductional” division (“cinese”). This process would satisfy Mendel’s law asserting random segregation of the maternal and paternal versions of a given hereditary unit. By the end of the meiosis I division, each homolog is seen to have become split along its length (“longitudinal cleavage”) by separation of what we now call sister chromatids. By Weismann’s hypothesis, the two separated units would be genetically identical to one another (and to the parental chromosome from which they originated). At meiosis II, the two split entities (sisters) would segregate to opposite poles as in mitosis. Meiosis II was thus called the “equational” division (“cinese”). Weismann’s ideas were called the “heterotypie-homeotypie” theory because the first division segregated genetically different units while the second division segregated identical units.
However, as stated in Janssens’s article, some authors still argued that the second division was reductional and the first division equational or, like Bonnevie (1907) and Vejdovsky (1907), that both divisions were equational. Even Sutton thought that separation of parental chromosomes took place during the second division; and even as late as 1931, some authors like Carothers thought that reduction could occur in either meiotic division (see discussion in Hegreness and Meselson 2007).
Why Janssens Was Not Satisfied by the “Heterotypie-Homeotypie” Theory
Janssens questioned whether the heterotypie-homeotypie theory, while attractive, was really complete. As Janssens describes in the first part of his article, Weismann’s theory provided no explanation for several important conundrums.
If segregation of maternal and paternal chromosomes is complete at the heterotypic division (first division), why is there a second division and thus four meiotic products (“tetraspores”) rather than two?
What is the meaning of the unique and very long duration of the pachytene stage of meiosis?
As addressed extensively in Janssens’s study, how can Weismann’s theory explain the diverse configurations seen after pachytene at the diplotene/diakinesis stages (the “strepsitene coiling stage”), which are both characteristic of meiosis and commonly observed in the studied organisms and which also persist through onset of anaphase of the first division.
Mendel’s second law dictates independent assortment of the hereditary units (genes) for different traits. In Weismann’s theory, all units on the same chromosome would always segregate together. However, Janssens was aware of the fact that the known number of independently segregating genetic units was greater than the number of chromosomes.
How Janssens Resolved These Conundrums
Janssens tried to resolve these conundrums through careful studies of fixed chromosome configurations from meiocytes of the Californian salamander B. attenuatus and of different triton species at the two “maturation divisions,” i.e., the post-pachytene stages through meiosis I and meiosis II. Analyses were done on dyads at diplotene, and especially importantly, at anaphase I onset. Images were drawn through a camera lucida, which allows a simultaneous optical superimposition of the figure being viewed in the microscope and the drawing surface (through a silvered mirror tilted at 45° and a negative lens that creates a virtual image). Because at that time photomicroscopy either was not possible or was expensive, the camera lucida was a standard tool of cytologists. Furthermore, most observations were made on sections of embedded material, and the camera lucida, which gives a 3D stereo-like image, allowed clearer visualizations of the structures that cytologists wished to document than those provided by micrographs (which give a flat and thus less-clear image of sections). However, Janssens was aware of the limits of cytological and morphological analyses and explained that some figures are “delicate to analyze” or emphasized that certain conclusions were “only [addressable] through […] ideal fixations of appropriate samples.”
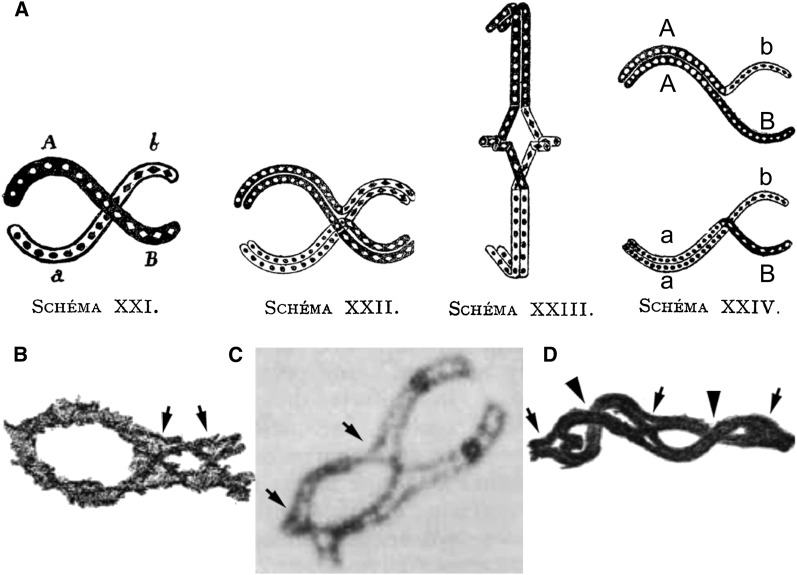
Janssens studied in special detail the onset of anaphase I. This choice allowed him to actually see what we now call chiasmata as connections between homologs that remain in the equatorial region while the two centromere-proximal regions are already well-separated by pole-ward movement (Figure 2). Had Janssens observed only diplotene figures, as others had done before him [he cites Schreiner and Schreiner (1905), Foot and Strobell (1905), and Bonnevie (1908) from whom he reproduces the drawings], the tight association of sister chromatids all along their lengths at this stage would have made it difficult, and likely also for him, to understand the underlying structure of diplotene dyads. He analyzed several types of figures with different dispositions, ring chromosomes, and E- and D-shaped dyads and carefully analyzed the relationship of the four chromatids (“filaments”) at the sites of connections between chromosomes, including examples of multiple chiasmata involving more than two chromatids (Figure 2 and Figure 3, A–D). Janssens also describes the complications due to the fact that fibers (chromatids) twist at the chiasma sites, leading to asymmetrical arrangements, especially when the spindle fiber attachment is near the end of the chromosome.
Figure 2.
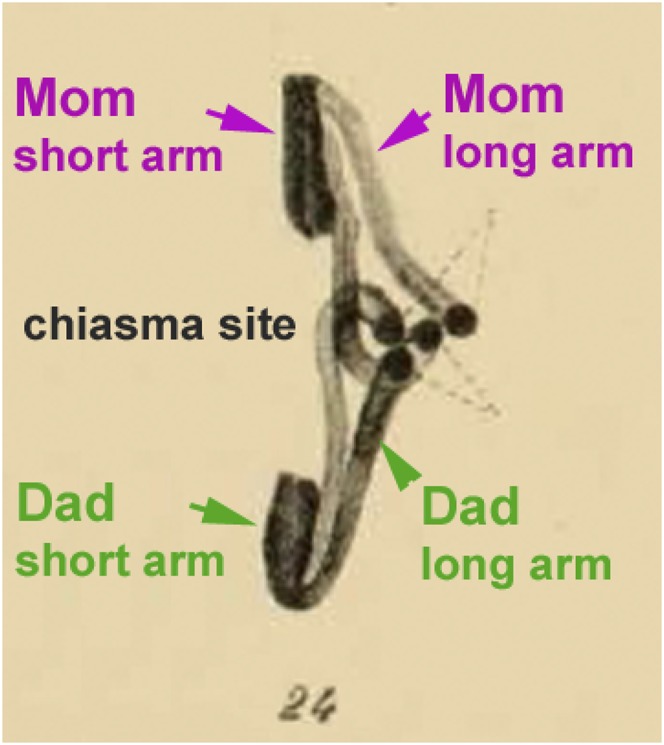
Anaphase I from Janssens’s original article: segregation of a pair of homologous chromosomes exhibiting one chiasma (figure 24 in Janssens’s 1909 article). Green and pink arrows as well as explanations were added by the authors on the original picture.
Figure 3.
Drawings from Janssens’s 1909 article illustrating the exchange of chromosomal segments in chiasmata. (A) Schema XXI: drawing of a single chiasma between homologous chromosomes A, B (black) and a, b (white). Schema XXII represents the four chromatids (of chromosomes AB and ab) and the site where, according to Janssens, the break and reciprocal exchange occurred between two homologous chromatids of this diplotene “dyad.” Schema XXIII: anaphase I. Schema XXIV illustrates the consequence of the exchange between two of the four chromatids. Two of the resulting chromatids will remain parental, and thus AB and ab, and two will have recombined as Ab and aB. (B) Drawing from Janssen’s article illustrating the presence of two chiasmata (arrows) in a diplotene dyad. (C) Micrograph of a diplotene bivalent from the salamander Oedipina poelzi. In this case, the separation of the four chromatids allows a clear picture of the two chiasmata sites (arrows). The dark balls correspond to the centromeres. This picture was taken by the late James Kezer and kindly provided by Geoffrey K. Rickards. (D) Dyad with multiple chiasmata drawn by Janssens (figure 15 of his article) and used by Morgan in his book The Theory of the Gene (Morgan 1926, figure 27, p. 42) as a plausible, but not conclusive, cytological illustration of genetic crossing over. Note that three of the five “connections” are clearly chiasmata as indicated by the opening where two of the four chromatids exchange partners (arrows) while the two other connections (arrowheads) likely correspond to a superposition of the two homologs without exchange. It is this latter type of configuration (common in some organisms) that was used by several cytologists to criticize Janssens’s theory on the ground that there was no evidence for breakage and rejoining of chromatids at diplotene. Arrows in B, C, and D were added by the authors.
Janssens’s Arguments for the Chiasmatype Theory
Janssens’s hypothesis is based primarily on his own cytological studies of figures seen at diplotene and anaphase I, supplemented by previously published images of chromosome figures in the existing literature (see plates of original article1 and Figure 3, B and D). As Janssens reports, analysis of anaphase I figures allowed him to ascertain three main facts:
That the longitudinal cleavage (i.e., sister separation) did not occur at anaphase but much earlier, at metaphase I at the latest, and thus that diplotene-to-anaphase dyads were formed of four filaments (chromatids).
The most pertinent observation, which clearly helped him to understand what a chiasma is, was the fact that, in most dyads seen at early–mid anaphase I, two of the four chromosome arms had moved to the pole while the two others were still “connected at the equatorial plane” (Figure 2). Janssens concluded that, if Weismann’s theory were correct, “it is unlikely that one of the two elements [arms] resulting from a longitudinal cleavage should remain united [with its homolog partner element] longer than the other element (arm) [e.g., the long and short arm, respectively, in Figure 2]. They [both arms] should separate [from their partner] simultaneously.” He reports that similar observations had bothered other “distinguished authors” (Foot and Strobell 1905; Bonnevie 1907), preventing them from accepting the “hetero-homeotype theory” but without explaining them.
Janssens observed that, of the four filaments (chromatids) present at the connection sites (chiasmata sites) at diplotene or anaphase I, two crossed each other and two did not. He therefore postulated that the maternal and paternal chromatids that crossed penetrated the other until they broke (he shows examples) and rejoined maternal and paternal segments in new ways. The other two chromatids (also one maternal and one paternal) remained free and thus intact (Figure 3A).
Janssens’s brilliant idea of concentrating on early–mid anaphase I was also appreciated much later by Darlington (1937) who stresses that “The observation of structures of anaphase chromosomes is of great importance. It shows, first, that what might have been regarded at metaphase as ‘points of contact’ between the chromosomes really were chiasmata. It shows, second, the relationship of the exchange undergone at one chiasma to that undergone at the next.” (His book is dedicated to Janssens, William Charles Frank Newton, and Karl Belar).
The conclusions reported in Janssens’s 1909 article are the following:
“It is unlikely that dyads result from a simple coiling of two anatomically independent elements.”
“At these sites (of connection), the chromosomes more or less co-penetrate one another.”
“There is inter-penetration of two chromosomes and secondary fusion of the filaments at this site.” And finally,
“Interactions between chromosomes in dyads are far from being as simple as believed until now. When chromosomes are in contact with each other[’s] at chiasmata sites, which according to us, is the rule, we do not think that they remain independent. Their filaments are involved in contacts that can modify their organization from one segment to the next. This will generate new segmental combinations, which will be different for the two filaments of a same chromosome, or which can affect the whole chromosomal segment.”
Janssens gave very clear illustrations to show how fusion of filaments would generate new combinations of chromosome segments (e.g., Figure 3A). This allowed him also to propose that the chromatids distributed in the four nuclei issued from the second division had various combinations of maternal and paternal segments of each chromosome. This could also provide the raison d’être of the two meiotic divisions.
The Chiasmatype Theory
Janssens’s hypothesis synthetically unites observations from cytology and genetics into a single economical “chiasmatype hypothesis” wherein physical connections between homologous chromosomes (the chiasmata) correspond to the positions of genetic crossing over between non-sister chromatids of homologs at homologous positions. It must have been a thrilling moment for Janssens when he realized that he had understood something new, original, and deeply meaningful about the fundamental processes that underlie sexual reproduction. He actually said it in his 1909 article in a nice way: “We were able to find among our samples and in figures from the literature indications that convinced us that dyads had very often not been sufficiently scrutinized and that they are still holding some secrets. We even believe that we have discovered some of the secrets. Are we being presumptuous? Time will tell.”
Janssens ends his article with the following list of features explained by his chiasmatype theory.
“This theory especially provides a clear explanation for the figures seen during anaphases of the first maturation division, which are so embarrassing for the ‘heterotype theory.’ ”
“Only this theory can explain and account for the otherwise completely inexplicable interweavings observed between the filaments (chromatids) which segregate to one of the pole[s] during the heterotype anaphases, whereas the filaments pulled towards the other pole remain completely parallel.”
“The ‘heterotype’ division now appears as an ordinary division (i.e., mitotic-like) as far as longitudinal cleavage of chromosomes is concerned.”
“The theory explains the most ‘striking’ figures observed during the ‘homeotype’ prophases and anaphases. It provides a very simple interpretation of the strepsinema (diplotene) stage, which otherwise remains an enigma.”
“It outlines the meaning of chromosome conjugation (synapsis), which, likely already during the pachytene stage, brings pieces of their segments into connection in preparation for strepsinema.”
“It explains the ‘raison d’être’ of the two maturation divisions, both of them being potentially reductional.”
“The theory allows us to understand the existence of the tetraspore [four nuclei]”.
“It opens the way to a broader cytological application of Mendel’s theory.”
And conversely, permitted the appreciation that the laws of Mendelian segregation required breakage and joining (crossing over) between homologous non-sister chromatids.
Janssens’s Article in the History of Meiosis
Although his 1909 article found a broad appreciative audience and had a large influence on the chromosomal theory at that time, as one would expect for any truly imaginative contribution, Janssens’s theory was actively resisted by both geneticists and cytologists. As described above, although Boveri and Sutton’s remarkable discoveries (1889 and 1902 and 1903, respectively) that the separation of paternal and maternal chromosomes during the first meiotic division paralleled Mendel’s separation of characters, Morgan still questioned the chromosomal basis of heredity in 1910. By the following year, however, Morgan (1911), citing Janssens (1909), had changed his view, concluding that the degree of linkage between Mendelian factors depended on “the linear difference apart of the chromosomal materials that represent the factors.” Nevertheless, it was only later that the chromosome heredity of Mendelian laws was clearly established. This was accomplished first by Sturtevant (1913) who showed that the proportion of crossovers between factors on the Drosophila X chromosome could be represented by a linear map and, later, by Bridges in 1914 when he showed that certain abnormal segregations were due to the presence of two linked (attached) X chromosomes and a Y chromosome in Drosophila females (Bridges 1914). Muller (1916) questioned the timing of crossing over specified in Janssens’s theory because of the apparent discrepancy between the precision needed for crossing over to occur and Janssens’s supposition that it occurs in thick diplotene chromosomes. Nevertheless, Muller invokes Janssens’s idea as an explanation for crossing-over interference because “On Janssens’s theory that crossing over takes place in the strepsinema stage, when the chromosomes are twisted in loose loops, crossing-over would seldom take place at two points very near together, for this would require a tight twisting of the chromosomes.” In retrospect, Muller’s objection was unfounded because crossing over at the DNA/genetic level occurs “invisibly,” before diplotene (see below for details).
Although he specifically discusses breakage and fusion at the sites of chiasmata in his article, Janssens had no proof that chiasmata result from actual breakage and rejoining of chromatids. In 1909, the evidence for exchange of maternal and paternal chromatid segments could come only from genetic approaches. Evidence that crossing over occurred only between two of the four chromatids—and thus after chromosome duplication (and not before)—was given by Bridges (1916a,b) in his analysis of XXY strains of Drosophila and by Bridges and Anderson (1925) in studies of triploid Drosophila with sex-linked characters. Also, in 1920, in a article written with Morgan (Wilson and Morgan 1920), Wilson built models in clay that could be viewed obliquely and that clearly showed “at each node (chiasma) two threads that are connected by a chiasma and two that are not thus connected; but if the model be rotated through an angle of 90° the appearance is reversed, the ‘chiasma’ now appearing between the two threads that previously seemed unconnected, and vice versa.” In fact, Wilson tried all kinds of models to reconcile the cytological observations with Janssens’s theory. He suggested: “To admit this (the chiasmatype theory), diagrams should be modified as to indicate exchanges which have earlier taken place between the synaptic mates.” And Wilson concluded, “We are in no position to predict when the plodding progress of cytology may be able to close the gap: nevertheless we have every reason to hope that the physical mechanism of the recombination-phenomena may in the end prove to be accessible to decisive cytological demonstration.” This hope was fulfilled only half a century later with the isotope-labeling studies of Taylor (1965), Peacock (1970), and Jones (1971).
Although Morgan became convinced that chromosomes were the bearers of heredity, after some battles concerning the representation and meaning of chiasmata in the “looping of a pair of chromosomes,” he concluded that “If we compare this latest scheme of Janssens with the figures that we have recently published [“Physical basis of heredity,” 1919] in which, following some of Janssens’s earlier diagrams only two of the strands cross over at each node, it is perfectly clear that these later schemes of ours give the same number of cross-overs per complete twist as do Janssens’s present diagram” (Wilson and Morgan 1920). Definite demonstration that crossovers should occur only between two of the four chromatids was established in 1933 by Lindegren’s (1933) tetrad analyses of linked characters in the fungus Neurospora crassa.
Criticisms came also from cytologists, who remained skeptical longer than the geneticists. One criticism was that Janssens could not tell exactly when splitting/division of the chromosomes into two chromatids occurred, and therefore the possibility remained that chiasmata could result from a change of pairing partner without breakage and joining. This latter hypothesis was strongly defended by McClung who wrote in 1927: “the ‘chiasms’ [chiasmata] are optical effects and not structural conditions” and blamed Janssens for being too unfamiliar with his subject to analyze them correctly McClung (1927). The same criticisms also came later from Carothers (1931) and Sax (1932). Only Belling (1928) proposed that breakage and joining could not be seen at diplotene because his observations of different plants suggested that it occurred much earlier—at the preceding pachytene stage (as already proposed by Muller in 1916).
The definitive demonstration of “cytological crossing over” came only in 1931 from analyses of Creighton and McClintock (1931) of maize and Stern (1931) of Drosophila. Chromosome 9 of maize possesses a heterochromatic knob at the end of its short arm and thus its distribution through crossing is similar to that of a gene. In a cross between a plant with no knob and a plant with a knob, “in the resulting F1 individuals only one member of the homologous pair possesses a knob. When such an individual is back-crossed to one having no knob on either chromosome, half of the offspring are heterozygous for the knob and half possess no knob at all.” However, although the data were clear, and the article of Stern on Drosophila translocations of chromosomes X and Y (with broken X’s also allowing cytological analyses) yielded the same conclusions, skepticism persisted (e.g., Brink and Cooper 1935, working with maize translocations). Therefore, Creighton and McClintock made a new set of experiments in 1935 because “We feel forced to add more data merely to counteract any suspicion that the evidence previously presented constituted insufficient proof.” They examined 260 more individuals cytologically for the presence or absence of the knob contributed by the F1 parent in parallel with genetic analysis for crossovers in five regions of chromosome 9 (Creighton and McClintock 1935). And their conclusion was the following: “These data, therefore, supplement those given in our previous publication and indicate the soundness of the conclusions drawn.” Next Haldane (1931), using Maeda’s data on chiasma frequencies in the plant Vicia faba (Maeda 1930), found that chiasmata showed interference as crossovers did (shown by Muller 1916 and Sturtevant 1913), another indication that chiasmata corresponded to crossovers. Even so, there was no proof that cytological crossing over resulted from actual breakage and joining, as various template-switching schemes (“copy choice”) could not be entirely ruled out.
The first direct evidence for the chiasmatype theory came only 56–62 years later when isotope labeling by Taylor (1965), Peacock (1970), and Jones (1971) proved beyond any doubt that crossing over does indeed involve breakage and rejoining of homologous non-sister chromatids. In parallel, molecular studies demonstrated that DNA recombination in bacteriophage λ involved the breaking and joining of two DNA duplexes (Meselson and Weigle 1961). Since then, and especially in recent years, combined genetics and molecular biological and cytological approaches in diverse organisms have definitively shown that Janssens’s explanation of a chiasma was correct.
It is now clear that halving of the number of chromosomes in meiosis is accomplished by a single round of DNA replication followed by two rounds of chromosome segregation and that each chromosome entering meiosis comprises two sister chromatids. These latter, being tightly linked by a series of proteins called cohesins (reviewed in Nasmyth 2001), are not visibly distinguishable before diplotene in most organisms, thus explaining some of the criticisms made by the cytologists (e.g., C. E. McClung, E. E. Carothers, and K. Sax; see above). It is also clear now that, in most organisms, the connections between homologs (Janssens’s chiasmata), are established by programmed formation of DNA double-strand breaks (DSBs) at leptotene, which, after identification of the homologous region on a non-sister chromatid and the ensuing biochemical changes in the two involved DNA duplexes, are finally resolved into reciprocal exchanges between homologous chromatids (crossover) (e.g., Hunter and Kleckner 2001; Keeney 2007; Lichten and De Massy 2011). In addition, in many organisms, interaction of a DSB with its partner duplex also mediates pairing of homologs, prior and prerequisite to formation of crossovers (review in Bishop and Zickler 2004). The series of biochemical events involved in these processes have been elucidated primarily from physical analysis of DNA events in synchronous budding yeasts (Padmore et al. 1991; Hunter and Kleckner 2001). The program of events, however, is likely to be universal (Guillon et al. 2005; review in Kleckner et al. 2012).
Crossover formation involves a unique branched structure, the double Holliday junction, which matures specifically to crossover products via breakage and resealing of appropriate pairs of strands, as predicted by Janssens (Schwacha and Kleckner 1995; review in Hunter 2006). Resolution of chiasma-mediated interhomolog connections is achieved not by resolution of recombination intermediates but by loss of sister cohesion via cohesin cleavage at the onset of anaphase I (e.g., Kudo et al. 2006). This timing can now be followed cytologically through antibodies against recombination complexes or by fluorescent tags, thus fulfilling Wilson’s wish of 1920 (above) (e.g., De Boer and Heyting (2006). Moreover, the complexes that mediate recombination are physically associated with chromosome axes as revealed from EM studies identifying crossover-correlated “nodules” and also as seen by immunostaining of recombination proteins (review in Zickler and Kleckner 1999; De Boer and Heyting 2006; Storlazzi et al. 2010). As recombination is completed, crossover “nodules” remain visible through late pachytene and, occasionally, into diplotene at chiasma sites (e.g., Moens et al. 2007), providing another proof of Janssens’s theory. Finally, as shown by Muller (1916) and Haldane (1931) (above), the positions of crossovers (and chiasmata) are not randomly distributed but, instead, show specific spatial patterning, including a prominent tendency for even spacing due to “interference,” i.e., the fact that if crossover designation occurs at one position, there is a reduced probability that another crossover designation will occur nearby (review in Jones and Franklin 2006).
However, >100 years after Janssens’s contribution to our understanding of meiosis, several questions remain unanswered. Mechanisms that determine the positioning of DSBs, the spatial patterning of crossovers and thus chiasmata, the fact that meiotic recombination occurs preferentially between homologous non-sister chromatids rather than between sisters all remain to be defined. In addition, several pieces important to the understanding of meiotic recombination and its relationship with chromosome axes and higher-order chromatin structure are still missing. Also, the critical question posed by Janssens—why are there two divisions?—remains unanswered, leaving open at least several more years of investigation. Finally, we can conclude with Janssens that chiasmata “are still holding some secrets.”
Supplementary Material
Acknowledgments
We gratefully acknowledge Nancy Kleckner for her help in the preparation of the manuscript and for stimulating discussions. We also recommend to the reader the detailed and stimulating documentation of the subject provided by chapters 5–9 of H. L. K. Whitehouse’s book Towards an Understanding of the Mechanism of Heredity, published in 1973 by Edward Arnold Publishers Ltd. We extend special gratitude to Dominique Lambert, Françoise Thomas, Jacques Pasteels, and René Thomas from the Royal Academy of Belgium who gave us access to the archives containing F. A. Janssens’s deposit, including the 1908 sealed document. This latter was originally requested by M. Meselson nearly 50 years ago in a letter to R. Thomas asking that he attempt to obtain it from the Academy. The Academy responded that the letter could not be found. The “secret letter” was finally kindly provided by R. Thomas after his election to the Academy. This allowed and stimulated the translation into English of both Janssens’s 1909 article and the sealed document. We thank Geoff Rickards for the bivalent chromosome picture made by the late James Kezer and for comments on the manuscript. R.K. was supported by the 7th Framework Program for Research FP7/2007-2013 under grant agreement no. 231093.
Footnotes
Communicating editor: R. A. Isaacson
Literature Cited
- Bateson W., 1902. Mendel’s Principles of Heredity: A Defense, Cambridge University Press, Cambridge, UK [Google Scholar]
- Belling J., 1928. Nodes and chiasmas in the bivalents of Lilium with regard to segmental interchange. Biol. Bull. 54: 465–470 [Google Scholar]
- Bishop D. K., Zickler D., 2004. Meiotic crossover interference prior to stable strand exchange and synapsis. Cell 117: 9–15 [DOI] [PubMed] [Google Scholar]
- Bonnevie K., 1907. “Heterotypical” mitosis in Nereis limbata (Ehlers). Biol. Bull. 13: 57 [Google Scholar]
- Bonnevie K., 1908. Chromosome studies II, Vol. II. Arch Zellforschung, Bd; II, 201–278 (in German) [Google Scholar]
- Boveri T., 1889. Ein geschlechtlich erzeugter Organismus ohne mütterliche Eigenschaften. Gesel. für Morph. und Physiol. München 5: 73–83 (translated by T. H. Morgan 1893, as “An organism produced sexually without characteristics of the mother”). Am. Nat. 27: 222–232 [Google Scholar]
- Boveri T., 1904. Results Concerning the Chromosome Substance of the Cell Nucleus, p. 65 Fischer, Jena, Germany (in German) [Google Scholar]
- Bridges C. B., 1914. Direct proof through non-disjunction that the sex-linked genes of Drosophila are borne by the X-chromosome. Science 40: 107–109 [DOI] [PubMed] [Google Scholar]
- Bridges C. B., 1916a Non-disjunction as proof of the chromosome theory of heredity. Genetics 1: 1–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges C. B., 1916b Non-disjunction as proof of the chromosome theory of heredity (concluded). Genetics 1: 107–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges C. B., Anderson E. G., 1925. Crossing over in the X chromosomes of triploid females of Drosophila melanogaster. Genetics 10: 418–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink R. A., Cooper D. C., 1935. A proof that crossing over involves an exchange of segments between homologous chromosomes. Genetics 20(1): 22–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carothers E. E., 1931. The maturation divisions and segregation of heteromorphic homologous chromosomes in Acrididae (Othoptera). Biol. Bull. 61: 324–349 [Google Scholar]
- Creighton H., McClintock B., 1931. A correlation of cytological and genetical crossing-over in Zea Mays. Proc. Natl. Acad. Sci. USA 17: 492–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton H., McClintock B., 1935. The correlation of cytological and genetical crossing-over in Zea Mays: a corroboration. Proc. Natl. Acad. Sci. USA 21: 148–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington C. D., 1937. Recent Advances in Cytology, Ed. 2nd Blakiston’s Son & Co., Philadelphia [Google Scholar]
- De Boer E., Heyting C., 2006. The diverse roles of transverse filaments of synaptonemal complexes in meiosis. Chromosoma 115: 220–234 [DOI] [PubMed] [Google Scholar]
- De Vries H, 1901. Die Mutationstheorie VI. (English translation entitled “The Mutation Theory,” 2 vols. London, Kegan Paul, Trench, Trübner & Co., 1910 and 1911, 582 and 683 pp [Google Scholar]
- Foot K., Strobell E., 1905. Prophase and metaphase of the first maturation spindle of Allolobophora foetida. Am. J. Anat. 4: 193–243 [Google Scholar]
- Grégoire V., 1904. Reduction of the chromosome number and maturation divisions (La réduction numérique des chromosomes et les cinèses de maturation). Cellule 21: 297–314 (in French) [Google Scholar]
- Guillon H., Baudat F., Grey C., Liskay R. M., de Massy B., 2005. Crossover and noncrossover pathways in mouse meiosis. Mol. Cell 20: 563–573 [DOI] [PubMed] [Google Scholar]
- Haldane J. B. S., 1931. The cytological basis of genetical interference. Cytologia (Tokyo) 3: 54–65 [Google Scholar]
- Hegreness M., Meselson M., 2007. What did Sutton see? Thirty years of confusion over the chromosomal basis of Mendelian genetics. Genetics 176: 1939–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N., 2006. Meiotic recombination, pp. 381–442 in Molecular Genetics of Recombination, edited by Aguileira A., Rothstein R. Springer, Berlin/Heidelberg, Germany [Google Scholar]
- Hunter N., Kleckner N., 2001. The single-end invasion: an asymmetric intermediate at the double-strand break to double-Holliday junction transition of meiotic recombination. Cell 106: 59–70 [DOI] [PubMed] [Google Scholar]
- Janssens F. A, 1909. The chiasmatype theory. A new interpretation of the maturation divisions. Cellule 25: 389–411. (Translated from the French; reprinted in Genetics 191: 319–346.)
- Jones G. H., 1971. The analysis of exchanges in tritium-labeled meiotic chromosomes. II. Stethophyma grossum. Chromosoma 34: 367–382 [Google Scholar]
- Jones G. H., Franklin F. C., 2006. Meiotic crossing-over: obligation and interference. Cell 126: 246–248 [DOI] [PubMed] [Google Scholar]
- Keeney S., 2007. Spo11 and the formation of DNA double-strand breaks in meiosis, pp. 81–123 Recombination and Meiosis, edited by Lakenau D. H. Springer-Verlag, Heidelberg, Germany: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Zhang L., Weiner B., Zickler D., 2012. Meiotic chromosome dynamics, pp. 487–533 Genome Organization and Function in the Cell Nucleus, chap 19., edited by Rippe K. John Wiley-VCH Verlag GmbH & Co, Weinheim, Germany [Google Scholar]
- Kudo N. R., Wassmann K., Anger M., Schuh M., Wirth K. G., et al. , 2006. Resolution of chiasmata in oocytes requires separase-mediated proteolysis. Cell 126: 135–146 [DOI] [PubMed] [Google Scholar]
- Lichten M., de Massy B., 2011. The impressionistic landscape of meiotic recombination. Cell 147: 267–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegren C. C., 1933. The genetics of Neurospora III. Pure bred stocks and crossing-over in N. crassa. Bull. Torrey Bot. Club 60: 133–154 [Google Scholar]
- Maeda T., 1930. The meiotic divisions in pollen mother cells of the sweet-pea (Lathyrus odoratus) with special reference to the cytological basis of crossing-over. Mem. Coll. Sci. Kyoto Imp. Univ. 5: 125–137 [Google Scholar]
- McClung C. E., 1927. The chiasmatype theory of Janssens. Q. Rev. Biol. 2: 344–366 [Google Scholar]
- Meselson M. S., Weigle J. J., 1961. Chromosome breakage accompanying genetic recombination in bacteriophage. Proc. Natl. Acad. Sci. USA 47: 857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B., Marcon E., Shore J. S., Kochakpour N., Spyropoulos B., 2007. Initiation and resolution of interhomolog connections: crossover and non-crossover sites along mouse synaptonemal complexes. J. Cell Sci. 120: 1017–1027 [DOI] [PubMed] [Google Scholar]
- Montgomery T. H., 1901. A study of the chromosomes of the germ cells of metazoa. Trans. Am. Phil. Soc. 20: 154–230 [Google Scholar]
- Morgan T. H., 1910. Chromosomes and heredity. Am. Nat. 44: 449–496 [Google Scholar]
- Morgan T. H., 1911. Random segregation vs. coupling in Mendelian inheritance. Science 34: 384. [DOI] [PubMed] [Google Scholar]
- Morgan T. H., 1925. The bearing of genetics on the cytological evidence for crossing-over. Cellule 36: 113–123 [Google Scholar]
- Morgan T. H., 1926. ••• The Theory of the Gene, Yale University Press, New Haven [Google Scholar]
- Muller H. J., 1916. The mechanism of crossing-over. Am. Nat. 50: 193–221 [Google Scholar]
- Nasmyth K., 2001. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet. 35: 673–745 [DOI] [PubMed] [Google Scholar]
- Padmore R., Cao L., Kleckner N., 1991. Temporal comparison of recombination and synaptonemal complex formation during meiosis in S. cerevisiae. Cell 66: 1239–1256 [DOI] [PubMed] [Google Scholar]
- Paliulis L. V., Nicklas R. B., 2005. Kinetochore rearrangement in meiosis II requires attachment to the spindle. Chromosoma 113: 440–446 [DOI] [PubMed] [Google Scholar]
- Peacock W. J., 1970. Replication, recombination and chiasmata in Goniaea australasiae (Orthoptara: Acrididae). Genetics 65: 593–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückert J., 1892. Development of ovary in Selachii (Zur Entwicklungsgeschichte des Ovarialeies bei Selachiern). Anat. Anz. 7: 107–158 (in German) [Google Scholar]
- Sax K., 1932. Meiosis and chiasma formation in Paeonia suffruticosa. J. Arnold Arbor. 13: 375–384 [Google Scholar]
- Schreiner K. E., Schreiner A., 1905. New studies on chromatin maturation in germinal cells, vol. I (Neue Studien über die Chromatinreifung der Geschlechtszellen I). Arch. Biol. 22. [Google Scholar]
- Schwacha A., Kleckner N., 1995. Identification of double Holliday junctions as intermediates in meiotic recombination. Cell 83: 783–791 [DOI] [PubMed] [Google Scholar]
- Stern C., 1931. Cytological-genetic studies as evidence for Morgan's theory of factor exchanges (Zytologisch-genetische Untersuchungen als Beweis für die Morgansche Theorie des Faktorenaustausches). Biol. Zentralbl. 51: 547–587 (in German) [Google Scholar]
- Storlazzi A., Gargano S., Ruprich-Robert G., Falque M., David M., et al. , 2010. Recombination proteins mediate meiotic spatial chromosome organization and pairing. Cell 141(1): 94–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasburger E., 1888. Nuclear and cell division in the plant kingdom, in addition to an annex on fertilization. Histological contributions (Uber Kern-undZelltheilung in Planzenreiche, nebst einem Anhang über Befruchtung. Histologische Beiträge), p. 258, Vol. I Gustav Fischer, Jena, Germany (in German) [Google Scholar]
- Sturtevant A. H., 1913. The linear arrangement of six sex-linked factors in Drosophila, as shown by their mode of association. J. Exp. Zool. 14: 43–59 [Google Scholar]
- Sutton W. S., 1902. On the morphology of the chromosome group in Brachystola magna. Biol. Bull. 4: 24–39 [Google Scholar]
- Sutton W. S., 1903. The chromosomes in heredity. Bio. Bull. 4: 231–251 [Google Scholar]
- Taylor J. H., 1965. Distribution of tritium-labeled DNA among chromosomes during meiosis I spermatogenesis in the grasshopper. J. Cell Biol. 25: 57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejdovsky F., 1907. New studies on the maturation and fertilization (Neue Untersuchungen über die Reifung und Befrüchtung), Königl. Böhm. Ges. d Wiss. in Prag (in German) [Google Scholar]
- Weismann A, 1885. The continuity of the germ-plasm as the foundation of a theory of heredity (Die Kontinuität des Keimplasmas: eine Theorie der Vererbung) pp. 161–248 Essays upon Heredity and Kindred Biological Problems. Clarendon Press, Oxford (in German) [Google Scholar]
- Wilson E. B., 1900. The Cell in Development and Inheritance, Ed. 2nd Macmillan, New York [Google Scholar]
- Wilson E. B., Morgan T. H., 1920. Chiasmatype and crossing over. Am. Nat. 54(632): 193–219 [Google Scholar]
- Zickler D., Kleckner N., 1999. Meiotic chromosomes: integrating structure and function. Annu. Rev. Genet. 33: 603–754 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.