Abstract
Secretory vesicles are used during spermatogenesis to deliver proteins to the cell surface. In Caenorhabditis elegans, secretory membranous organelles (MO) fuse with the plasma membrane to transform spermatids into fertilization-competent spermatozoa. We show that, like the acrosomal vesicle of mammalian sperm, MOs undergo acidification during development. Treatment of spermatids with the V-ATPase inhibitor bafilomycin blocks both MO acidification and formation of functional spermatozoa. There are several spermatogenesis-defective mutants that cause defects in MO morphogenesis, including spe-5. We determined that spe-5, which is on chromosome I, encodes one of two V-ATPase B paralogous subunits. The spe-5 null mutant is viable but sterile because it forms arrested, multi-nucleate spermatocytes. Immunofluorescence with a SPE-5-specific monoclonal antibody shows that SPE-5 expression begins in spermatocytes and is found in all subsequent stages of spermatogenesis. Most SPE-5 is discarded into the residual body during spermatid budding, but a small amount remains in budded spermatids where it localizes to MOs as a discrete dot. The other V-ATPase B subunit is encoded by vha-12, which is located on the X chromosome. Usually, spe-5 mutants are self-sterile in a wild-type vha-12 background. However, an extrachromosomal transgene containing wild-type vha-12 driven by its own promoter allows spe-5 mutant hermaphrodites to produce progeny, indicating that VHA-12 can at least partially substitute for SPE-5. Others have shown that the X chromosome is transcriptionally silent in the male germline, so expression of the autosomally located spe-5 gene ensures that a V-ATPase B subunit is present during spermatogenesis.
Keywords: V-ATPase, lysosomes, SPE-5, spermatogenesis, Caenorhabditis elegans
VESICULAR organelles in eukaryotic cells frequently maintain an acidic pH (reviewed by Paroutis et al. 2004) that is created by the vacuolar H+-ATPase (V-ATPase). The V-ATPase is a large (∼910-kDa) molecular machine that couples ATP hydrolysis to the movement of protons across biological membranes. The V-ATPase has a V0-sector that creates the pore-for-proton translocation through the lipid bilayer and a V1-sector, located in the cytoplasm, that is the site of ATP hydrolysis. Each V-ATPase holoenzyme is composed of 14 different subunits, some of which are present in multiple copies (reviewed by Toei et al. 2010). In yeast, there is one gene for each V-ATPase subunit, except for the “a” subunit, which is encoded by two genes (reviewed by Kane 2006). The physiological properties of the V-ATPase are in part determined by which of these two “a” subunits it contains (Kawasaki-Nishi et al. 2001). In humans and other animals, the “a” and other V-ATPase subunits are encoded by more than one gene (reviewed by Toei et al. 2010). Subunit diversity presumably allows the V-ATPase to be either customized for a specific function or utilized in a tissue-specific fashion.
In this article, we use pH-sensitive vital dyes and specific inhibitors to show that sperm-specific MOs use the V-ATPase to acidify their interior as spermatids mature. Pseudopodial extension during spermatid maturation into spermatozoa requires V-ATPase activity, suggesting a mechanistic connection between this process and MO acidification. We examined the microarray data for the 21 V-ATPase subunits encoded by Caenorhabditis elegans and found that there are two B-subunit paralogs with strikingly different patterns of expression (Reinke et al. 2000; Jiang et al. 2001). While the X chromosome-located vha-12 gene showed somatic expression and the accompanying article in this issue by Ernstrom et al. (2012) shows the null phenotype of this gene to be lethal, the second gene encoding a B subunit showed upregulated expression during spermatogenesis. We used positional cloning techniques to show that the previously described spe-5 gene, which is on chromosome I (Machaca and L’Hernault 1997), encodes this sperm-expressed B subunit. A null mutation in spe-5 severely affects spermatogenesis, but it is not lethal, so we could examine the role of the V-ATPase during this process. spe-5 mutants have defective MOs (Machaca and L’Hernault 1997) that do not get acidified during development, and immunofluorescence with specific antibodies revealed that SPE-5 is located partly on MOs. Finally, placing vha-12, driven by its own promoter, in an extrachromosomal array allows this gene to partly rescue the self-sterility associated with spe-5 null mutants, presumably due to VHA-12 misexpression during spermatogenesis. This suggests that SPE-5 and VHA-12 are functionally similar and that spe-5 may have arisen to allow V-ATPase function during spermatogenesis when the X chromosome is transcriptionally quiescent (Kelly et al. 2002).
Materials and Methods
Strains, culture, and nomenclature
C. elegans var. Bristol (N2) (Brenner 1974) was the wild-type strain used for most experiments. Culturing, manipulation, and genetic analyses were performed as described (Brenner 1974) except as noted, and standard C. elegans nomenclature was employed (Horvitz et al. 1979). The following genetic markers were used: dpy-5(e61)I (Brenner 1974), unc-38(x20)I (Lewis et al. 1980), mat-1(ax212)I (Golden et al. 2000), fer-1(b232 (L’Hernault et al. 1988), fem-3(q23gf)IV (Barton et al. 1987), fem-3(e1996)IV, spe-17(hcDf1)IV (L’Hernault et al. 1993), him-8(e1489)IV, him-5(e1490)V (Hodgkin et al. 1979), let-44(mg41)X, and lon-2(e678)X (Brenner 1974). spe-5 is located on chromosome I, and six alleles—eb29, eb30, hc93, hc110, oz3, and oz46—have been described previously (L’Hernault et al. 1988; Machaca and L’Hernault 1997). The spe-5(ok1054) deletion allele was provided by the C. elegans Knockout Consortium. Both balancer chromosomes sDp2 (Howell et al. 1987) and JK2739 lin-6(e1466) dpy-5(e61) I/hT2[bli-4(e937) let-?(q782) qIs48](I;III)hT2[qIs48]/+ I; hT2/+ III cover the left arm of chromosome I and include the genes unc-38, spe-5, and dpy-5. The balancer mnDp31 (X;f) (Herman et al. 1979) covers both let-44 and lon-2.
Genetic mapping and cloning of spe-5
Prior work placed spe-5 between unc-38 and dpy-5 (Machaca and L’Hernault 1997). We mapped spe-5 left of mat-1 (see http://www.WormBase.org), which is located at −0.4 (Shakes et al. 2003) and relative to single nucleotide polymorphisms (SNPs) (Jakubowski and Kornfeld 1999). Primers (Supporting Information, Table S1) were used to PCR-amplify intervals between unc-38 and mat-1 from both the N2 and CB4856 Hawaiian (HI) strains (Hodgkin and Doniach 1997). These PCR products were sequenced and aligned to the complete N2 genomic sequence (Abbott et al. 1998) to identify SNPs (see Table S2) useful for mapping (Jakubowski and Kornfeld 1999). As summarized in Table S3, 20 recombinant worm strains were isolated from unc-38(x20) spe-5(hc10) dpy-5(e61)/+ (HI) F1 and used for SNP mapping.
Sperm cytological analyses
Individual hermaphrodites were picked to separate plates and transferred daily, and progeny and oocytes were counted until the worms stopped laying (L’Hernault et al. 1988). Males that either were or were not spe-5(ok1054)I and either him-5(e1490)V, dpy-5(e61)I or him-8(e1489)IV were analyzed; these him and dpy mutations did not obviously affect sperm cytology, consistent with earlier observations (e.g., L’Hernault et al. 1988). Individual males were dissected in 5–10 μl of 1× sperm medium (SM) [50 mM HEPES (pH 7.8), 25 mM KCl, 45 mM NaCl, 1 mM MgSO4, 5 mM CaCl2] supplemented with 10 mM dextrose [SM (pH 7.8)/dextrose] Machaca et al. 1996). Spermatids were activated to become spermatozoa in vitro with Pronase or triethanolamine (TEA) Ward et al. 1983; L’Hernault and Roberts 1995). The vital dyes LysoSensor Yellow/Blue DND-160 or LysoSensor Blue DND-192 were used to stain MOs. These dyes were provided at 1 mM (in dimethyl sulfoxide) from the manufacturer (Molecular Probes, Eugene, OR), and they were diluted to 1–10 μM into SM pH 7.8/dextrose just before use. The fungal toxin bafilomycin A1 (LC Laboratories, Woburn, MA) was prepared as a 25 mM stock in dimethyl sulfoxide and diluted to 25 μM in SM (pH 7.8)/dextrose. Males were dissected in SM (pH 7.8)/dextrose containing a LysoSensor vital dye, either with or without bafilomycin and/or the TEA activator, and incubated in a moist chamber for 15 min, after which slides were sealed with silicone grease and viewed.
An Olympus BX60 equipped with either a Plan-Apo 1.4 numerical aperture (N.A.) ×60 or a 1.35 N.A. ×100 oil-immersion objective lenses or a Zeiss Axiophot compound microscope equipped with either a Plan-Apo 1.4 N.A. ×63 or a 1.4 N.A. ×100 oil-immersion objective lens was used to view sperm. Images in Figures 2 and 7 were captured with either a DAGE 300T CCD (DAGE Instruments, Michigan City, IN) or a SensiCam digital camera (Cooke, Auburn Hills, MI) using either National Institutes of Health Image or Image-Pro PLUS (Media Cybernetics, Silver Spring, MD) together with VolumeScan 3.1 (VayTek, Fairfield, IA). Twelve-bit images from the SensiCam were converted to 8 bits in Image-Pro PLUS. In the case of Figures 3 and 8, Z-axis stacks of 18–23, 12-bit images were collected every 0.3 μm using SlideBook software (Intelligent Imaging Innovations, Denver, CO). Resulting images were subject to a nearest-neighbor deconvolution algorithm and exported as 16-bit tif images. Images were compiled using Canvas 9 (ACD Systems, Miami, FL) or PhotoShop CS3 (Adobe Systems, San Jose, CA). The deconvolved images in Figure 8 are shown as individual slices for the red, green, and red/green/blue triple overlays and also as complete Z-axis stacks for the triple overlay.
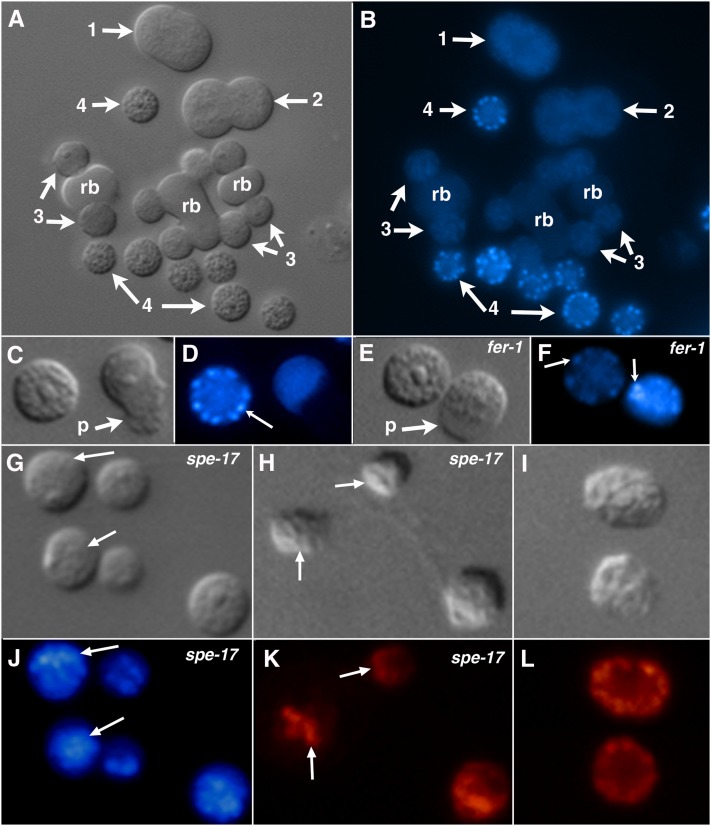
Figure 2 .
The position and staining of MO secretory vesicles during C. elegans spermatogenesis. (A, C, E, G, H, and I) Nomarski DIC. (B, D, F, and J) LysoSensor Y/B fluorescent vital staining of MO secretory vesicles. (K and L) Indirect immunofluorescence of fixed cells with the 1CB4 antibody to reveal MO secretory vesicles. (A and B) Various cellular stages of spermatogenesis. (1) Primary spermatocyte in anaphase of meiosis I. (2) Secondary spermatocytes forming from a dividing primary spermatocyte. (3) Spermatids budding from residual bodies (rb). (4) Spermatids that have completed budding from the residual body. (C and D) A wild-type spermatid (left) and spermatozoon (right). (E and F) A fer-1(b232) spermatid (left) and spermatozoon (right) spermatozoon. (G and J, H and K) Respective pairs of DIC and fluorescent images of spe-17(hcDf1) spermatids. (I and L) DIC and fluorescent images of wild-type spermatids. p, pseudopod of the spermatozoon. Arrows in D, F, J, and K point to MO secretory vesicles in fluorescent images or, in the case of G and H, their location in DIC images.
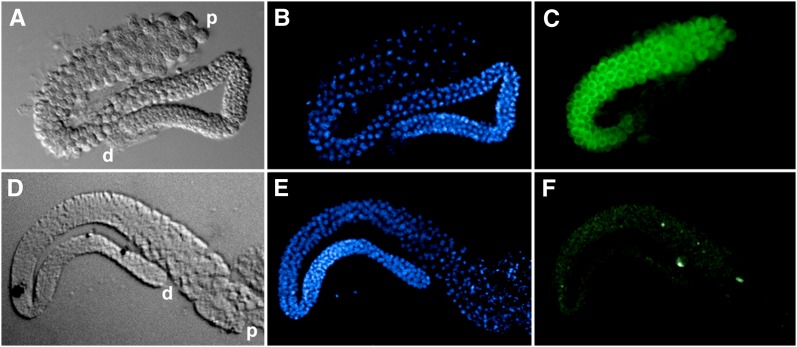
Figure 7 .
Testes localization of SPE-5 expression. Male-derived him-8 control (A–C) and spe-5(ok1054); him-8 (D–F) dissected testes were viewed by DIC (A and D), after DAPI staining to visualize DNA (blue in B and E) or after SPE-5 Mab staining that was enhanced by tyramine signal amplification (green in C and F).
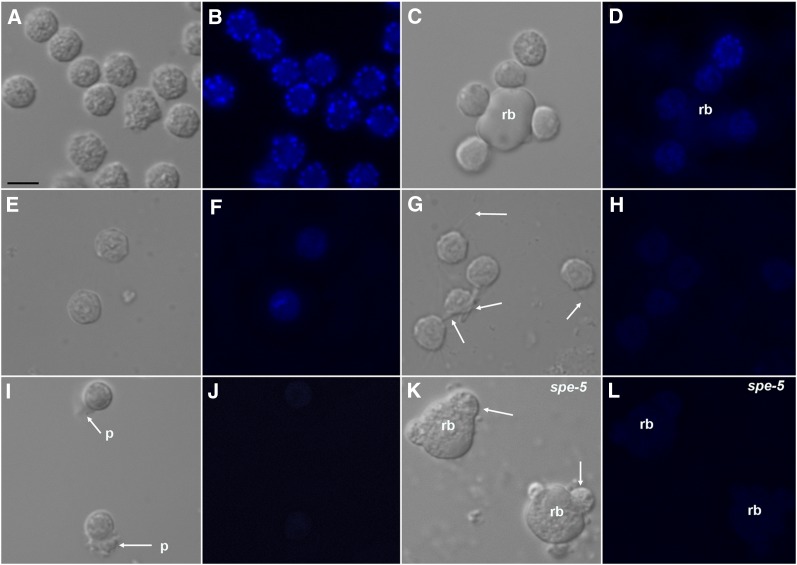
Figure 3 .
Bafilomycin A1 affects vital dye staining of MO secretory vesicles in C. elegans spermatids. (A, C, E, G, I, and K) Nomarski DIC. (B, D, F, H, J, and L) LysoSensor Blue DND-192 fluorescent staining of MO secretory vesicles. (A, B, C, and D) Wild-type spermatids and spermatids budding from residual body (rb) under control (no drug) conditions. (E, F, G, and H) Wild-type spermatids treated with 25 μM bafilomycin A1. (G, H, I, and J) Wild-type spermatids activated with 70 mM TEA (pH 7.8). Arrows in G point to fine processes extending from cells. (I) p, pseudopods on spermatozoa. (K and L) spe-5(ok1054) budding-arrested terminal spermatocytes.
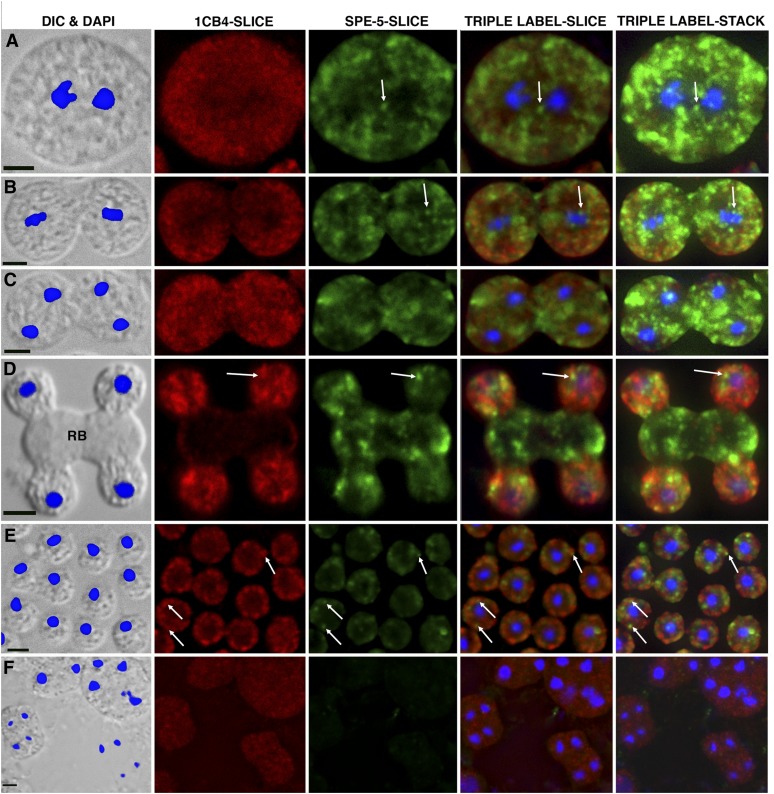
Figure 8 .
SPE-5 localization during spermatogenesis. Sperm cytology was visualized by DIC overlaid by DAPI staining of nuclei (blue on gray panels) or indirect immunofluorescence with either antibody 1CB4 that recognizes FB-MOs (Texas Red; red panels) or SPE-5 Mab enhanced by tyramine signal amplification (Alexa 488; green panels). For each field of cells, a z-series of fluorescent image slices in the DAPI, Texas Red, and Alexa 488 channels was collected, and each resulting wide-field image was deconvolved. For each slice, DAPI, red, and green channels were overlaid to create a triple-labeled slice. All triple-labeled slices for each field of cells were also stacked to create a through-field projection image stack (far right column of images). (A, B, C, and E) dpy-5 him-5 control (23 plane slices, slice #16 from the bottom shown). (D) him-8 control (21 plane slices, slice #16 from the bottom shown). (F) spe-5(ok1054) him-8 (18 plane slices, slice #10 shown). Differentiation stages: (A) primary spermatocytes, (B and C) secondary spermatocytes, (D) budding spermatids, (E) budded spermatids, and (F) spe-5 terminally arrested spermatocytes. Arrows indicate SPE-5 accumulation in dots. For D and E, arrows point to SPE-5 accumulation that is close to MOs. RB, residual body. Scale bar: 5 μm for each row of images.
Real-time PCR
Total RNA was isolated from him-5(e1490) males, him-5(e1490) hermaphrodites, spe-5(ok1054) him-8(e1489) males, spe-5(ok1054) him-8(e1489) hermaphrodites, fem-3(q23gf) hermaphrodites with a masculinized germline, and fem-3(e1996 lf) hermaphrodites with a feminized germline. For each genotype, 30 adult worms were picked into 10 μl of a 1:10 dilution of worm lysis buffer [50 mM KCl, 10 mM Tris-Cl (pH 8.3), 2.5 mM MgCl2, 0.45% NP-40, 0.45% Tween-20] and frozen in liquid N2. The frozen worms were treated with Trizol/chloroform, RNAse free glycogen was added as carrier, and RNA was precipitated with isopropanol (see http://www.pristionchus.org/wiki/index.php/RNA_preparation_from_few_worms). RNA was resuspended in RNase/DNase-free water. cDNA was synthesized using 200 ng of total RNA template with the iScript cDNA synthesis kit (Bio-Rad, Richmond, CA). Quantitative RT-PCR (qRT-PCR) was performed with 10 ng of total cDNA using the SsoFast EvaGreen Supermix (Bio-Rad) in a Bio-Rad CFX-96 real-time PCR system. The following primers were used to amplify cDNA levels: spe-5 (prMH26 and prMH27), vha-12 (prMH24 and prMH25), and rpa-1 (prMH30 and prMH31) (see Table S1). The RNA sequences of spe-5 and vha-12 are >75% identical throughout most of their sequences (not shown), so primers were designed to unique regions that showed no cross hybridization between these two paralogous sequences. A dilution series of him-5 hermaphrodite cDNA was used to calculate an arbitrary amount for each sample and primer set. The arbitrary amounts were normalized to the standard, which was the ubiquitously expressed mRNA (rpa-1) (Spieth et al. 1991), and the results were plotted as fold difference. RNA was isolated three independent times for worms of each genotype. The three RNA samples prepared for each worm genotype were subjected to qRT-PCR in triplicate (nine PCRs), and error bars representing standard error of the mean are shown.
Synthesis of cDNA and expression constructs
Total RNA was prepared from fem-3(q23gf) masculinized hermaphrodites (Barton et al. 1987) and used to create cDNA libraries according to published procedures (Gleason et al. 2006). The 5′ end of the spe-5 gene was amplified from the cDNA library by a nested PCR reaction using the Clontech SMART II primer and spe-5 gene-specific reverse primers PDHLa, PDHLb, and PDHLa2 (Table S1). The 3′ end of the spe-5 gene was amplified by a nested PCR reaction using the Clontech CDS primer and two spe-5 gene-specific forward primers, PDHRa and PDHRb (Table S1).
Three C. elegans full-length cDNA constructs were used to create bacterial expression constructs for spe-5, vha-12 (F20B6.2, a spe-5 paralog), and W05B10.1 (histone 3.3) by previously described techniques (Gleason et al. 2006).
Construction of a vha-12 transgene
The plasmid pRW05 was created by cutting the recombinant cosmid C04E10 insert with ApaLI and subcloning the 6592-bp ApaLI fragment that included the vha-12-coding sequence (F20B6.2) into the Litmus 28 plasmid vector (New England Biolabs, Ipswich, MA). pRW05 has 3029 bp 5′ to the vha-12 start codon and 1933 bp 3′ to the stop codon (1520 bp 3′ from the end of the predicted transcript for vha-12). pRW05 (2 μg/ml), pPD118.20 (10 μg/ml; 1997 Fire Lab Vector Kit), a plasmid that contains the myo-3 body-wall muscle promoter driving GFP, and carrier pBluescript II KS+ (76 μg/ml; Stratagene) were microinjected into let-44(mg41) lon-2(e678)X; mnDp31(X;f) hermaphrodites by standard techniques (Mello and Fire 1995). Lon animals that were GFP+ were selected to recover the oxEx551 extrachromosomal transgenic array; let-44(mg41) proved to be an allele of vha-12 (R. Weimer and E. Jorgensen, personal communication).
DNA sequencing
DNA sequencing was performed at either Iowa State University or the University of Michigan using standard ABI (Perkin-Elmer, Foster City, CA) automated fluorescent sequencing methods for PCR products. Genomic DNA was prepared from picked spe-5 homozygous mutants (Barstead et al. 1991) and used to PCR-amplify the chromosome I Y110A7A.12 gene (Table S1). PCR products were pooled, purified using either GeneClean II (Bio 101, Carlsbad, CA) or the Qiaquick PCR purification kit (Qiagen, Valencia, CA), and sequenced to identify the mutant lesions.
Antisera preparation
We screened preimmune sera by immunoblot and immunofluorescence and identified rabbits that did not react with C. elegans proteins. Peptides CTNHRTALIQNYSTKPKLT and CGRNNETVN, respectively, corresponded to residues 23–40 and 211–218 of the SPE-5 polypeptide sequence (Figure 4), except for the underlined N-terminal cysteine. Peptide sequences were selected on the basis of antigenic probability (Hopp and Woods 1983) and low sequence identity to the C. elegans VHA-12 paralog. The N-terminal cysteine was used to conjugate these peptides to keyhole limpet hemocyanin carrier protein. Each conjugated peptide was used to immunize two rabbits using standard protocols (Zymed Laboratories, South San Francisco, CA), and only the polyclonal antiserum R1 (from a rabbit immunized with the 23-40 peptide) proved specific. Affinity purification of SPE-5 antiserum R1 was performed as described previously (Gleason et al. 2006), and the glycine-eluted fraction was used for all experiments.
Figure 4 .
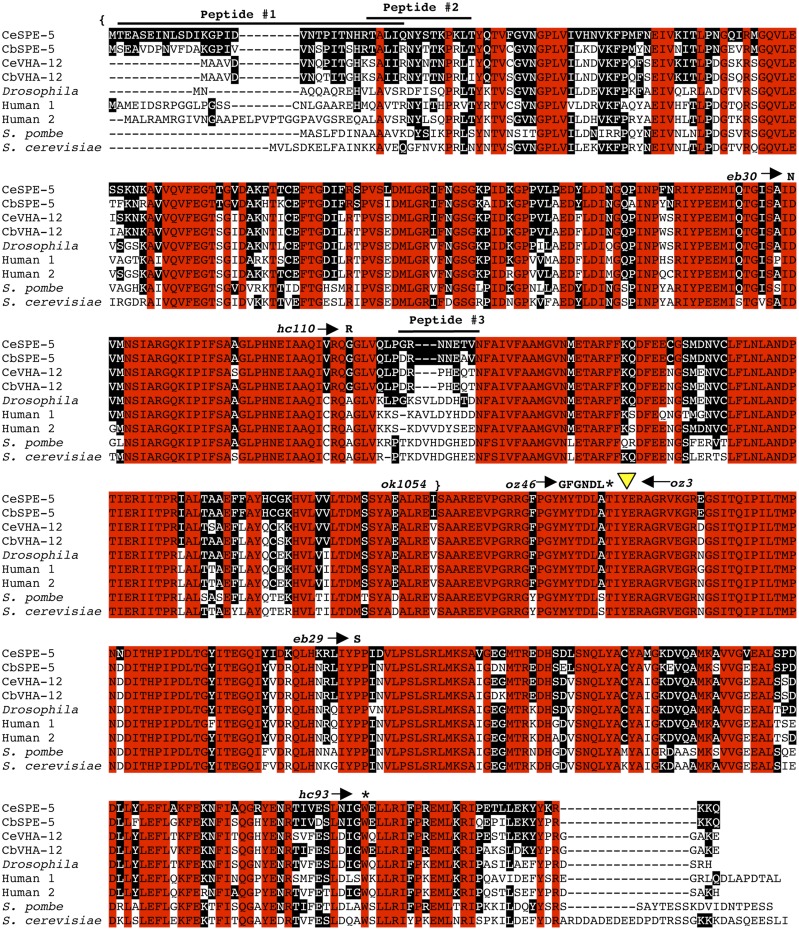
Alignment of V-ATPase B polypeptides from C. elegans, C. briggsae, Drosophila melanogaster, human, Schizosaccharomyces pombe, and Saccharomyces cerevisiae. Red boxes indicate identical residues among all shown B polypeptides. Black boxes show identity to the C. elegans sequence. Both the spe-5(hc93) point mutation and the spe-5(oz46) deletion mutation introduce premature nonsense mutations in C. elegans SPE-5, and they are indicated by asterisks (*). The amino acid change(s) in the missense spe-5(eb30), spe-5(hc110), and spe-5(eb29) mutants is indicated after an arrow. The position of a Tc1 insertion in the spe-5(oz3) mutant is indicated by a yellow triangle. Braces indicate the portion of the SPE-5 polypeptide deleted in the spe-5(ok1054) mutant; the deletion actually extends into the 5′ noncoding region of the spe-5 gene (not shown). Alignment was performed using the ClustalW2 program (Larkin et al. 2007). The Genbank accession number for C. elegans SPE-5 is NP_491518.1; for C. briggsae SPE-5, CAE65728.1; for C. elegans VHA-12, NP_508711.1; for C. briggsae VHA-12, CAE68535.1; for Drosophila, NP_476908.1; for human 1, NP_001683.2; for human 2, AAH30640.1; for S. pombe, NP_594623.1; and for S. cerevisiae, NP_009685.1.
For monoclonal antibody production, the multiple antigen peptide (MAP) TEASEINLSDIKPIDVNTPITNHRTALIQ (residues 2–31) was synthesized (Tam 1989) and used to immunize mice. Tail bleeds were screened for MAP reactivity, the spleen from a reactive mouse was excised, and individual splenocytes were fused to multiple myeloma cells by polyethylene glycol-mediated fusion. Resulting hybridomas were screened with the MAP by limit dilution screening, and one reactive cell clone was recovered. This clone was grown in Dulbecco’s modified Eagle’s medium with hypoxantine/amonopterin/thymine selection, and the culture supernatant, referred to below as the SPE-5 monoclonal antibody (Mab), was used in the experiments described in this article.
Immunoblots
The C. elegans SPE-5, VHA-12, and histone 3.3 (W05B10.1) fusion proteins were prepared from recombinant BL21 (DE3) Codon Plus RIPL Escherichia coli (Stratagene) that were induced with 1 mM IPTG at 25°. One milliliter of induced cultures was pelleted by centrifugation, resuspended in 100 μl 2× Laemelli sample buffer (Laemmli 1970), and boiled for 5 min, and 20-μl aliquots were loaded onto gels. Larger-scale SPE-5 fusion protein purification or sperm lysates and Western blotting were as described previously (Gleason et al. 2006). Western blots were probed with a monoclonal antibody to the c-MYC epitope [2 ng/ml; Clontech; used to confirm the authenticity of the SPE-5::Myc fusion protein (not shown)], SPE-5 R1 glycine affinity-purified primary antibody (5 or 50 μg/mL), or the SPE-5 Mab (1:1000) followed by a goat anti-rabbit (R1) or goat anti-mouse (c-MYC and SPE-5 Mab) IgG HRP-conjugated secondary antibody at 1:5000 (BioRad). ECL Plus (GE Healthcare) and BioMax MS Film (Kodak) were used for signal detection.
Immunocytochemistry
Sperm from male worms were prepared for immunohistochemistry by modifications to the freeze/crack methanol fixation method (Miller and Shakes 1995). Dissections of worms were performed on Colorfrost/Plus microscope slides (Fisher Scientific, Pittsburgh, PA) to facilitate adhesion. The SPE-5 Mab was incubated with samples overnight at a 1:1000 dilution for all immunofluorescent experiments described in this article. The secondary antibody was a goat anti-mouse IgG conjugated to horseradish peroxidase (Molecular Probes), and visualization was by 5 min of tyramine signal amplification (TSA) (Bobrow et al. 1989; Chao et al. 1996) with Alexa Fluor 488 conjugated to tyramine (Invitrogen). Mouse monoclonal antibody 1CB4 (Okamoto and Thomson 1985; Arduengo et al. 1998) was used after completion of the SPE-5 Mab incubation/TSA enhancement step at 1:2000 to detect the fibrous body-membranous organelle (FB-MO) complexes. 1CB4 was visualized using a Texas Red-conjugated, affinity-purified goat anti-mouse IgG secondary antibody (Fab1)2 fragment at a final concentration of 0.75 μg/ml (Jackson ImmunoResearch Laboratories, West Grove, PA). Control experiments indicated that the labeled secondary (Fab1)2 fragment used to visualize 1CB4 localization did not also generate a detectable level of fluorescence due to cross-reaction with the SPE-5 Mab (data not shown). Resulting slides were mounted in ProLong Antifade (Molecular Probes), dried overnight, and viewed through appropriate filters in an Olympus BX60 microscope, and images were analyzed as described above (see Sperm cytological analyses).
Results
Secretory vesicles become acidified during C. elegans spermatogenesis
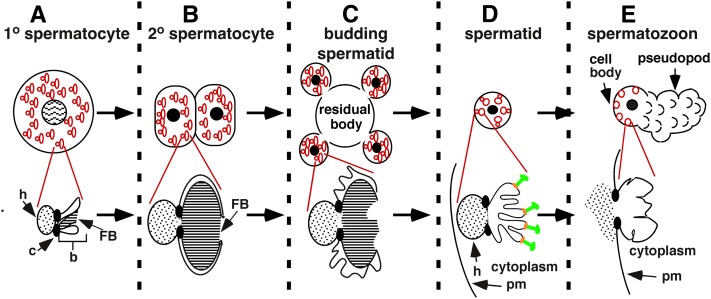
During C. elegans spermatogenesis, the unusual secretory vesicles FB-MOs play a major role in the accumulation and transport of macromolecules into developing spermatids (Figure 1; reviewed by L’Hernault 2009; Nishimura and L’Hernault 2010). The FB-MOs segregate into spermatids as they bud from the anucleate residual body (Figure 1C). As budding is completed, the membrane surrounding the FB retracts and the major sperm protein fibrous contents depolymerize into constituent dimers. The remaining portion of the FB-MO, now called the MO, localizes close to the plasma membrane (Figure 1D). Subsequently, the sessile spermatid activates to become a spermatozoon that crawls via its single pseudopod (Figure 1E), as is the case in many other nematodes (reviewed by Stewart and Roberts 2005). Successful participation of a spermatozoon in fertilization requires that MOs fuse with and insert new proteins into the plasma membrane. They also secrete their contents onto the cell surface, but the role of this process in sperm function is not yet clear (reviewed by L’Hernault 2009; Nishimura and L’Hernault 2010).
Figure 1 .
C. elegans spermatogenesis and its coordination with fibrous body-membranous organelle (FB-MO) morphogenesis. (A) Primary spermatocytes contain many nascent FB-MOs (in red) that each have a membranous organelle head (h) separated by a collar (c) from the body (b), which envelopes the growing fibrous body (FB). (B) Secondary spermatocytes frequently divide incompletely and contain full-sized FB-MOs. (C) Spermatids retain FB-MOs as they bud from the residual body and the FB disassembles. (D) Budded spermatids contain MOs that localize beneath the plasma membrane (pm) and have active V-ATPase (V0 sector in orange and V1 sector in green; not drawn to scale). (E) Maturation of spermatids into spermatozoa is associated with fusion of MOs with the plasma membrane (pm) and extension of a motile pseudopod. The presence of the V1 subunit in association with V0 in spermatozoa has not yet been conclusively shown. Red lines lead from one representative FB-MO (A–C) or MO (D and E) in a cell to an expanded view of this structure (modified from L’Hernault 2006).
The secretory role shown by MOs (Figure 1E) suggests that they could be related to lysosomes or the acrosome (found in flagellated sperm), both of which can fuse with the cell surface (reviewed by Moreno and Alvarado 2006; Luzio et al. 2007). Both lysosomes and the acrosome can be stained with acidotropic vital dyes (Lin et al. 2001, 2003; Sun-Wada et al. 2002; Codelia et al. 2005), and we tried this approach to determine if FB-MO secretory vesicles maintain an acidic interior. We screened a variety of pH-sensitive fluorescent vital dyes and discovered three useful ones. LysoTracker Blue DND-192, LysoTracker Green DND-26, and LysoSensor Yellow/Blue (Y/B). These dyes fluoresce maximally under low-pH conditions (Molecular Probes/Invitrogen, Carlsbad, CA), and all showed similar staining patterns.
Developing wild-type sperm show a remarkable staining pattern after treatment with LysoSensor Y/B (Figure 2, A and B). Primary spermatocytes (1 in Figure 2B), secondary spermatocytes (2 in Figure 2B), and budding spermatids (3 in Figure 2B) all show a blue staining pattern that is both uniform and dim. Spermatids (4 in Figure 2A) that have separated from the residual body (rb) have bright blue dots superimposed on dim background staining (4 in Figure 2B). The blue dots observed in spermatids (left cell in Figure 2, C and D) disappear after they mature into spermatozoa (right cell in Figure 2, C and D). The size and position of the blue dots suggest that they are MO secretory vesicles (reviewed by L’Hernault 2009; Nishimura and L’Hernault 2010).
Certain spermatogenesis-defective (spe or fer) mutants change the position and/or function of sperm secretory vesicles, and they were examined to help determine the identity of the above-described blue dots. The fer-1 mutant makes MO secretory vesicles, but they do not fuse with the cell surface when a pseudopod is extended (Ward et al. 1981). Spermatids from a fer-1 mutant (Figure 2, E and F, left cell) stain with LysoSensor Y/B in a dotted pattern (Figure 2F, left cell) that is similar to wild type (Figures 2D, left cell). However, unlike wild type (right cell in Figure 2D), fer-1 spermatozoa continue to show cortical blue dots (right cell in Figure 2F), presumably because secretory vesicles do not fuse with the plasma membrane. Staining spe-17 mutants with an antibody (1CB4) specific for MO secretory vesicles (Okamoto and Thomson 1985; Arduengo et al. 1998) reveals that they cluster opposite the nucleus in this mutant (Figure 2, H and K), rather than exhibiting the cortical location seen in wild-type spermatids (Figure 2, I and L). Like 1CB4 staining, the LysoSensor Y/B staining pattern in spe-17 mutant spermatids was clumped to one side (Figure 2, G and J, arrows). These observations confirm that LysoSensor Y/B stains MO secretory vesicles in C. elegans spermatids. Taken together, these data indicate that MO secretory vesicles are acidified during development and that H+ is released from these organelles during maturation of spermatids into spermatozoa.
Acidification of MO secretory vesicles and sperm activation require V-ATPase function
The above data indicate that secretory vesicles are acidified in a manner that is coordinated with spermatogenesis. We found that treating developing spermatids with bafilomycin A1, a specific inhibitor of the V-ATPase (Drose and Altendorf 1997), prevented LysoSensor Blue DND-192 staining of the MO secretory vesicles during C. elegans spermatogenesis (compare cortical dots in the Figure 3, B and D, control to their absence in Figure 3F, which is bafilomycin treated). We also attempted to activate spermatids into spermatozoa in the presence of bafilomycin. While control treatment with TEA (Ward et al. 1983) causes spermatids to extend a pseudopod and begin to move (Figure 3, I and J), bafilomycin treatment prevents completion of spermatid activation (Figure 3, G and H). Instead, bafilomycin-treated spermatids arrest at an intermediate stage (Shakes and Ward 1989) since they extend surface spikes that do not transition into a normal pseudopod (Figure 3G, arrows). As expected, no detectable MO staining was observed in TEA-treated spermatids that became spermatozoa in the absence of bafilomycin (Figure 3J) or were inhibited from maturing into spermatozoa by bafilomycin treatment (Figure 3H).
V-ATPase mutant that specifically affects spermatogenesis
The C. elegans genome encodes 21 V-ATPase subunits (see Figure 1A of the accompanying article in this issue by Ernstrom et al. 2012), and we examined their expression and RNA interference characteristics (see http://www.wormbase.org/, Jiang et al. 2001, and Reinke et al. 2000). Only the Y110A7A.12 gene, which encodes a 502-amino-acid B subunit, showed both elevated expression during spermatogenesis and a viable (not dead) RNA interference (RNAi) phenotype when assayed by feeding; these are characteristics of many sperm-expressed genes (Kamath et al. 2003). Y110A7A.12 is between unc-38 (−0.65) and mat-1 (−0.4) on chromosome I, and our mapping placed spe-5 within this interval (see Materials and Methods) between SNP 64 (left) and SNP 79 (right) (Table S2 and Figure S1; detailed data available on request). Y110A7A.12 was amplified from the seven spe-5 alleles, and each was found to have a unique mutation in this gene (Figure 4).
Three spe-5 EMS-induced mutants each contain a missense mutation. The spe-5(eb30) g-to-a transition mutation changes codon 172 from Asp to Asn. The spe-5(hc110) g-to-a transition mutation changes codon 204 from Gly to Arg. The spe-5(eb29) c-to-t transition mutation changes codon 382 from Pro to Ser. Two of these missense mutations (eb29 and eb30) caused nonconservative changes in residues that are identical in all cloned B subunits from yeast to mammals. hc110 changes an amino acid that, although conserved among nematode sequences, is not conserved in other eukaryotes (Figure 4).
The spe-5(oz3) mutation arose in a mut-3 genetic background (Collins et al. 1987), and this allele has a Tc1 insertion at position 1073 in exon 3 of the spe-5 genomic sequence. This mutant has been extensively outcrossed to Bristol N2, and the Tc1 insertion in spe-5(oz3) is stably inherited in this genetic background. However, PCR of spe-5(oz3) genomic DNA with primers that flank the mutation site always show two bands, one where the ∼1.6-kb Tc1 is inserted into spe-5 and a second, smaller band of approximately the wild-type size (not shown). This second, smaller band contains a TATG footprint at the point where Tc1 was inserted. Presumably, Tc1 is imprecisely excised in somatic tissue but maintained stably in the germline, including during spermatogenesis, as would be expected for the Bristol strain (Emmons and Yesner 1984). When imprecise excision occurs, the spe-5(oz3) gene would encode 328 amino acids of SPE-5 plus 1 amino acid encoded by Tc1 sequence before termination at a Tc1-derived stop codon. The spe-5(oz3) mutant, with an intact Tc1 insertion, is predicted to encode the first 327 amino acids of SPE-5 plus 18 amino acids encoded by Tc1 sequence before termination at a stop codon derived from Tc1 sequence. Therefore, spe-5(oz3) mutants are predicted to produce a truncated SPE-5 protein that is ∼65% the wild-type size, whether or not the Tc1 in spe-5 is intact or imprecisely excised (Figure 4).
Two EMS-induced spe-5 alleles (hc93 and oz46) cause nonsense mutations. The spe-5(hc93) g-to-a transition mutation is located near the 3′ end of the gene, where it changes codon 473 into an UGA opal stop codon. The spe-5(oz46) mutation is an 8-bp deletion that removes codons 319, 320, and the first two bases of codon 321. This results in 318 amino acids of the correct SPE-5 protein sequence followed by a frame-shift mutation that causes insertion of 6 novel amino acids before termination at a premature UGA opal stop codon; the missing 183-amino-acid region is almost identical in all sequenced V-ATPase B subunits (Figure 4).
The C. elegans Knockout Consortium recovered the spe-5(ok1054) deletion mutation that removes the spe-5 start codon, the first two exons and introns, and part of exon 3, so it is a candidate null allele. spe-5 is the third gene in an ∼4.6-bp operon designated CEOP1216, and the spe-5(ok1054) deletion also removes the 72-bp 5′ UTR, including the predicted acceptor for the SL2 spliced leader (that we empirically confirmed by RT-PCR) (Figure S2), plus another 100 bp of intergenic sequence. The second gene in this operon is chp-1 and GeneFinder predicts that 57 bp separate the spe-5(ok1054) deletion from the predicted 3′ end of the chp-1 transcript. Like all other spe-5 point and transposon mutants characterized previously (Machaca and L’Hernault 1997), spe-5(ok1054) causes a spermatogenesis-defective phenotype and no evident embryonic or somatic defects (data not shown). In contrast, RNAi knockdown of chp-1 expression causes an embryonic lethal phenotype (e.g., Fraser et al. 2000; Sonnichsen et al. 2005), which suggests that the ok1054 deletion affects only spe-5. These data indicate that the null phenotype of spe-5 is likely to be spermatogenesis-defective without any obvious somatic defects. We examined spe-5(ok1054) mutants with pH-sensitive dyes and found, as expected, that there is no detectable staining of MOs (Figure 3L: compare to Figure 3D control).
spe-5 expression
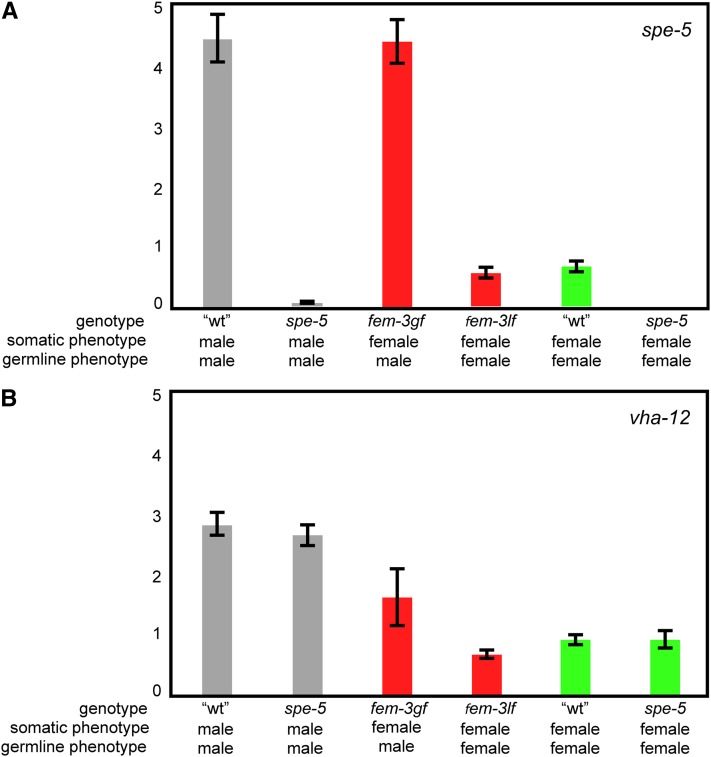
We used a qRT-PCR approach to explore the relationship between spermatogenesis and mRNA expression of both spe-5 (Figure 5A) and its paralog vha-12 (Figure 5B). Three matched pairs of mRNA samples were evaluated for spe-5 and vha-12 expression: males with and without spe-5 function (Figure 5, gray bars), somatic “females” with either a male or a female germline (Figure 5, red bars), and post-spermatogenesis adult hermaphrodites with and without spe-5 function (Figure 5, green bars). spe-5 mRNA is dramatically upregulated in wild type males as compared to spe-5 mutant males (Figure 5A, gray bars) and also in fem-3 gain-of-function worms (gf; female soma, male germline; Figure 5A, left red bar) as compared to fem-3 loss-of-function worms (lf; female soma, female germline; Figure 5A, right red bar). Adult wild-type hermaphrodites continue to show significant spe-5 expression after they have completed spermatogenesis (Figure 5A, green bar), as do fem-3 loss of function (lf; female soma, female germline) worms (Figure 5A, right red bar). These data indicate that, while spe-5 expression is upregulated during spermatogenesis, it is also expressed in one or more other tissues.
Figure 5 .
qRT-PCR on total RNA. (A) spe-5 mRNA levels. (B) vha-12 mRNA levels. Gray bars represent mRNA levels from him-5 male worms (“wt”) and spe-5(ok1054) him-8 males. Red bars indicate mRNA levels in fem-3(q23gf) masculinized hermaphrodites and fem-3(e1996lf) feminized hermaphrodites. Green bars show mRNA levels from him-5 hermaphrodites (“wt”) and spe-5(ok1054) him-8 hermaphrodites. Three independent RNA samples were isolated and, for each of these three samples, qRT-PCR was performed in triplicate; error bars are the standard error of the mean. Matched colored bars represent data that were normalized relative to rpa-1, which is a ubiquitously expressed housekeeping gene (Spieth et al. 1991).
Within each of the above-discussed matched pairs of mRNA samples, vha-12 showed none of the expression biases observed for spe-5. However, vha-12 expression was higher in worms with a somatic male phenotype (Figure 5B, gray bars) as compared to worms with a somatic female phenotype (Figure 5B, red and green bars), suggesting that one or more male somatic tissues contributed to this signal; males show significant anatomical differences from females in many tissues (Emmons 2005).
vha-12 can partially substitute for its spe-5 paralog
Perhaps the small amount (∼15%) of sequence differences between SPE-5 and its VHA-12 paralog help tailor the V-ATPase to play a sperm-specific role. To test this possibility, we examined if an extrachromosomal transgene containing vha-12-coding sequence and its 5′ and 3′ flanking regulatory sequences (oxEx551; see Materials and Methods) could affect the self-sterility of spe-5 strains. While spe-5(hc93) dpy-5 mutant hermaphrodites are nearly self-sterile (mean brood size = 2 ± 2, n = 12), oxEx551/spe-5(hc93) dpy-5 have elevated self-fertility (mean brood size = 53 ± 11, n = 8). This brood size is ∼36% of the dpy-5 control worms (mean brood size = 146 ± 24, n = 12), and it indicates that VHA-12 misexpression during spermatogenesis can partially replace the SPE-5 that is missing in spe-5 loss-of-function mutants. Like the germline expression of other transgenes (Kelly et al. 1997), this vha-12-containing transgene undergoes epigenetic silencing, and its effect on spe-5-associated self-sterility progressively diminished as the strain was propagated (data not shown). Nonetheless, this experiment shows that vha-12 can, at least partially, substitute for spe-5.
SPE-5 expression during spermatogenesis
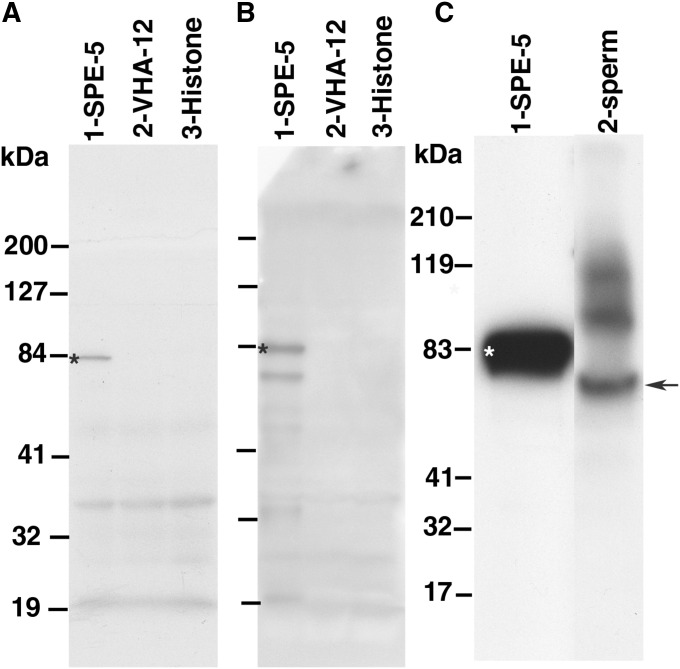
We tested our antibodies on bacterially expressed SPE-5, VHA-12, and a histone control to allow rigorous characterization of specificity. Immunoblots of gels containing these crude bacterial lysates reveal that both the mouse monoclonal (Figure 6A) and the R1 affinity-purified rabbit polyclonal antiserum (Figure 6B) were SPE-5-specific and showed no cross-reaction to either VHA-12 or the histone control.
Figure 6 .
Characterization of SPE-5-specific antibodies on Western blots. (A and B) Blots of gels that were each loaded with crude bacterial lysates prepared from cells induced to express SPE-5 (1), VHA-12 (2), or histone 3.3 (3) (W05B10.1) pDual fusion proteins. Blot in A was probed with a monoclonal antibody raised to a SPE-5-derived peptide. Blots in B and C were probed with an affinity-purified polyclonal antiserum raised to a SPE-5-derived peptide. (C) Blot of a gel that was loaded with affinity-purified SPE-5 fusion protein (1) and 1.6 × 107 purified sperm (2). For each blot, the position of the SPE-5 fusion protein is indicated by an asterisk. Short lines to the left of each panel mark the positions of protein standards. The molecular weight standards to the left of A (in kDa) apply to A and B, while C has different standards that are indicated to the left of that panel.
These antisera were then characterized on immunoblots of whole-worm lysates prepared from somatically female animals with germlines that produced only oocytes (fem-1lf) or only sperm (fem-3gf). SPE-5 expression apparently occurs at a very low level or is somehow partly masked, so long exposures (∼20 min) were required to visualize it in whole-worm lysates, where a band appears in fem-3gf worms that contain sperm but is missing in fem-1lf worms that never contain sperm (data not shown). The SPE-5 protein is more prominent and easily detected (exposure times of <1 min) in purified sperm (>90% spermatids) that were isolated from fem-3gf worms (Figure 6C, arrow). The deduced protein sequence of SPE-5 indicates that the molecular weight (MW) should be ∼56 kDa, and the epitope-tagged recombinant clone expressed in bacteria is expected to encode a protein that is ∼63 kDa. However, we consistently observed that both the bacterially expressed (with an apparent MW of ∼83 kDa) and the worm-encoded forms (with an apparent MW of ∼68 kDa) of SPE-5 show a significantly higher-than-expected molecular weight when subjected to SDS gel electrophoresis, perhaps due to anomalous detergent binding as has been observed for a wide variety of other proteins (e.g., Rath et al. 2009). Nonetheless, these data suggest that SPE-5 expression occurs during spermatogenesis.
SPE-5 localization in the testes and sperm
We next subjected male gonads from (wild-type-like) him-8 control and spe-5(ok1054) him-8 to immunofluorescence with the anti-SPE-5 antisera. The R1 affinity-purified rabbit polyclonal SPE-5 antiserum yielded only background staining that persisted in spe-5(ok1054) mutant gonads (not shown); it was judged not suitable for immunofluorescence. However, the mouse monoclonal antibody showed that SPE-5 expression was coincident with spermatocytes, where it was distributed throughout the cytoplasm and excluded from the nucleus (Figure 7C). A faint mottled pattern was observed in the cytoplasm, suggestive of punctate staining. spe-5(ok1054) him-8 gonads lack spermatocyte staining with the mouse monoclonal antibody beyond a faint nonspecific background (Figure 7F). Neither the control nor the spe-5(ok1054) male gonads had significant staining with the anti-SPE-5 monoclonal antibody in their respective distal gonad arms (d in Figure 7: compare A to C and D to F), which is where germ-cell proliferation occurs (reviewed by Kimble and Crittenden 2005). These data suggest that significant SPE-5 expression occurs during spermatogenesis in the male germline and that spe-5(ok1054) is a molecular null mutant, as would be expected for this deletion mutation.
Subcellular localization of SPE-5 was also performed on sperm released from dissected males. For these studies, the SPE-5 distribution was examined in cells co-stained with the 1CB4 antibody (Okamoto and Thomson 1985), which visualizes the MO secretory vesicles and their precursors during spermatogenesis (Arduengo et al. 1998). As spermatocytes bud from the proximal end of the rachis (as in Figure 7, A–C), they enter meiosis I (Figure 8A). During meiosis I, SPE-5 appears distributed throughout the cytoplasm, some of it in discrete dots (Figure 8, A and B, arrows). The second meiotic division occurs just before spermatid formation (Figure 8C), and the resulting haploid nuclei segregate into spermatids that bud from the residual body (RB in Figure 8D). MO secretory vesicles segregate with budding spermatids and are not observed within the residual body as judged by 1CB4 staining (red in Figure 8D) and as shown previously (Arduengo et al. 1998; reviewed by L’Hernault 2009; Nishimura and L’Hernault 2010). In contrast, SPE-5 staining (green in Figure 8D) is observed in both spermatids and the residual body (RB in Figure 8D), and much of the staining in both regions is in discrete dots. Budded spermatids have distinct MOs just below their plasma membrane (arrows at red spheres in Figure 8E). Most of the SPE-5 signal is also localized near the plasma membrane, much of it as discrete dots (green in Figure 8E) that are adjacent to MOs. In certain favorable regions (Figure 8E, arrows), it appears that there is one green dot per MO. These observations indicate that SPE-5 is located near the MO secretory vesicles of spermatids, consistent with mediating MO acidification. As expected, no SPE-5 signal above the low nonspecific background was detected in terminally arrested spe-5(ok1054) spermatocytes (Figure 8F).
Discussion
Much prior work has shown that C. elegans spermatogenesis requires the proper morphogenesis and function of its MO secretory vesicles. While many mutants that affect the ultrastructure and cellular position of MO secretory vesicles are known (reviewed by L’Hernault 2006, 2009), little is known about how the affected proteins alter cell physiology. In this article, we show that morphogenesis of MO secretory vesicles is associated with a physiological transition when this compartment becomes acidified. This acidification is apparently synchronous and occurs coincident with spermatid budding from the residual body, which is when each FB-MO matures into a MO secretory vesicle (Figure 1, D and E). This acidification requires the V-ATPase, which is composed of a V0 sector that forms a pore in the membrane and a V1 sector that hydrolyzes ATP to provide the electromotive force for proton transport through V0 (reviewed by, e.g., Toei et al. 2010). spe-5 mutants do not show this MO acidification. In addition, blockage of V-ATPase activity with bafliomycin A1 in wild-type spermatids prevents both this acidification and spermatid activation into spermatozoa.
Spermatid budding places all ribosomes into the residual body (reviewed by L’Hernault 2009), so the V-ATPase activity that we observe in MOs cannot be due to de novo protein synthesis in the spermatid. One common way to regulate the V-ATPase is to maintain V1 in a cytoplasmic pool and to regulate its association with V0, as was first shown in Maduca sexta (Sumner et al. 1995) and is now realized to be a widespread phenomenon (reviewed by Beyenbach and Wieczorek 2006). Much of the observed SPE-5 immunofluorescent signal during spermatogenesis prior to spermatid budding does not obviously localize with FB-MOs (Figure 8, A–C). In contrast, most SPE-5 is near the plasma membrane in spermatids, suggesting that any V1 not destined to associate with MOs is discarded in the residual body. One SPE-5 dot is observed next to certain MOs, so it is possible that there is either only one V-ATPase molecule per MO or, perhaps, multiple V-ATPase molecules clustered at one location. However, there are multiple ∼800-nm MOs in each 5- to 6-μm spermatid, so establishing that each MO has only one V-ATPase dot is beyond the resolution of our light microscopy equipment.
When spermatids become spermatozoa (Figure 1, D and E), many MO secretory vesicles fuses with the plasma membrane, and a single pseudopod is extended (Figure 1E) (reviewed by L’Hernault 2009; Nishimura and L’Hernault 2010). Prior work has shown that the bulk pH of the cytoplasm rises ∼0.5 pH unit during spermiogenesis induced by TEA treatment (Ward et al. 1983), and resulting spermatozoa are fertilization-competent during artificial insemination, indicating that TEA treatment does not adversely affect sperm physiology (LaMunyon and Ward 1994). We found that spermatids do not form a normal pseudopod after TEA treatment when they are simultaneously treated with the V-ATPase inhibitor bafilomycin A1. Instead, spermatid activation arrests at what is normally an intermediate stage, characterized by extension of fine spike-like processes (Shakes and Ward 1989). This suggests that the V-ATPase participates in the bulk cytoplasmic pH rise that occurs during spermiogenesis (Ward et al. 1983) and that this process is somehow mechanistically linked to pseudopodial extension.
Topologically, the V-ATPase V1 sector (green, Figure 1D) must be oriented in the spermatid cytoplasm so that protons move through V0 (orange, Figure 1D) in the MO membrane to acidify the MO interior. Our vital dye data indicate that fusion of the MO with the plasma membrane, when a spermatid (Figure 2D) matures into a spermatozoon (Figure 2E), releases protons within the MO interior to the extracellular space. The fused MOs are permanently opened to the extracellular space (Figure 1E), and the V-ATPase would likely be oriented so it could pump additional protons out of the cytoplasm, assuming that V1 (green, Figure 1D) remains associated with V0 (orange, Figure 1D) at this stage.
Before SPE-5 was known to be a V-ATPase subunit, electron microscopy revealed that FB-MO morphogenesis was severely disrupted in spe-5 mutants, starting in spermatocytes (for wild type, see Figure 1, A and B) (Machaca and L’Hernault 1997). spe-5 mutant spermatocytes do not usually divide to form spermatids, which might be a direct consequence of a nonfunctional or mispositioned V-ATPase in FB-MOs. However, mutants affecting either the cell cycle or other diverse biochemical processes during sperm morphogenesis all show defects in both MO position and spermatid formation, so this failure of spe-5 mutants to form spermatids is not an unusual defect seen only in this V-ATPase mutant (reviewed by L’Hernault 2009). Furthermore, our immunocytochemistry shows that most of the SPE-5 seen in spermatocytes is discarded into the residual body during spermatid budding. This discarded SPE-5 is over and above that needed in MOs for them to become acidified in budded spermatids. We currently do not know what, if anything, the SPE-5 not seen associated with MOs is doing in spermatocytes.
The null phenotype of spe-5 mutants is severe disruption of spermatogenesis, but an exclusive role for SPE-5 during spermatogenesis is not consistent with several observations. First, our qRT-PCR results (Figure 5) indicate that the spe-5-encoded mRNA is present in “female” animals that are not engaged in spermatogenesis. This indicates that spe-5 is expressed in somatic tissues and/or oocytes. Studies published by others show that mutations and/or RNAi of a variety of V-ATPase-encoding genes, including both spe-5 and vha-12, partially suppresses the necrotic cell death of touch neurons found in mec-4gf dominant mutants (Syntichaki et al. 2005). In addition, RNAi of spe-5 lengthened life span by 23% (Curran and Ruvkun 2007) and caused decreased survival following exposure of worms to hypertonic stress (Choe and Strange 2008). Collectively, these data indicate that spe-5 is expressed in a variety of somatic tissues and, under the right conditions, is required in those tissues.
Many prior studies indicate that the B subunit is absolutely required for V-ATPase function (e.g., reviewed by Marshansky and Futai 2008). We showed that a high-copy-number extrachromosomal array containing the vha-12 promoter and coding sequence allows (normally self-sterile) spe-5 mutants to produce small broods. This indicates that, under the right circumstances, VHA-12 can (at least partly) replace SPE-5 and leads one to question why there are two paralogs in C. elegans. The probable explanation is that the vha-12 gene normally resides on the X chromosome and transcription from the X chromosome is usually silenced during spermatogenesis (Kelly et al. 2002). Placing vha-12 on an extrachromosomal transgene allows it to escape X chromosome silencing and be expressed in the testes. The very weak self-fertility of all spe-5 mutants, including the spe-5(ok1054) null mutant, might be due to occasional incomplete vha-12 transcriptional repression. If true, vha-12 misexpression could allow phenotypic suppression of the spe-5 self-sterile phenotype, at least to a limited degree; we are currently exploring this possibility further.
Caenorhabditis briggsae also encodes two V-ATPase B subunits and, like C. elegans, the spe-5 ortholog is on chromosome I and the vha-12 ortholog is on the X chromosome (Stein et al. 2003; Hillier et al. 2007). For each nematode species, we used bootstrap methods to create a phylogenetic tree and derive an ancestral consensus sequence (Figure S3A). SPE-5 had more amino acid changes than did VHA-12 when compared to the ancestral sequence, and these results were true for both species (Figure S3, A and C). Like C. elegans, the C. briggsae vha-12 ortholog is not part of an operon. The C. briggsae spe-5 ortholog is the third gene in an operon that shows the same syntenic relationship to pas-3 and chp-1 as exists in the equivalent C. elegans operon (CEOP1216; see above). Since organizing nematode genes into operons is a derived condition not seen in most animal groups (Blumenthal 2005), this suggests that gene duplication and localization of spe-5 within an operon on chromosome I preceded the reproductive isolation of C. elegans from C. briggsae 80–110 million years ago (Hillier et al. 2007). The relatively more rapid evolution of SPE-5 as compared to VHA-12 might indicate that this V-ATPase B subunit is becoming specialized for some as-yet-unknown role.
Supplementary Material
Acknowledgments
We thank Erik Jorgensen for providing unpublished data and the vha-12 transgene, Shozo Yokoyama for help in deciphering the evolutionary relationship between spe-5 and vha-12, Richard Meagher for selecting the peptide sequence used to generate the SPE-5 monoclonal antibody, Andy Golden for mat-1 strains, and Vince LaTerza for useful discussions and facilitating S.W.L.’s collaboration with Abeome Inc. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources. P.D.H., K.L.H.-H., and M.H. were supported, respectively, by the Emory University SURE summer program (funded by Howard Hughes Medical Institute Award 52003071 to the Emory University Center for Science Education), NIH Training Grant T32GM008490, and NIH FIRST grant 2K12GM000680. This work was supported by NIH grants GM040697 and GM082932 to S.W.L. and R43 GM071317 to Abeome Inc.
Note added in proof: See Ernstrom et al. 2012 (pp. 461–475) in this issue for a related work.
Footnotes
Communicating editor: O. Hobert
Literature Cited
- Abbott A., Abu-Threideh J., Ainscough R., Ahrens C., Alexander E., et al. , 1998. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282: 2012–2018 [DOI] [PubMed] [Google Scholar]
- Arduengo P. M., Appleberry O. K., Chuang P., L’Hernault S. W., 1998. The presenilin protein family member SPE-4 localizes to an ER/Golgi derived organelle and is required for proper cytoplasmic partitioning during Caenorhabditis elegans spermatogenesis. J. Cell Sci. 111: 3645–3654 [DOI] [PubMed] [Google Scholar]
- Barstead R. J., Kleiman L., Waterston R. H., 1991. Cloning, sequencing, and mapping of an alpha-actinin gene from the nematode Caenorhabditis elegans. Cell Motil. Cytoskeleton 20: 69–78 [DOI] [PubMed] [Google Scholar]
- Barton M. K., Schedl T. B., Kimble J., 1987. Gain-of-function mutations of fem-3, a sex-determination gene in Caenorhabditis elegans. Genetics 115: 107–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyenbach K. W., Wieczorek H., 2006. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J. Exp. Biol. 209: 577–589 [DOI] [PubMed] [Google Scholar]
- Blumenthal T., 2006. Trans-splicing and operons (June 25, 2005), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.5.1, http://www.wormbook.org
- Bobrow M. N., Harris T. D., Shaughnessy K. J., Litt G. J., 1989. Catalyzed reporter deposition, a novel method of signal amplification. Application to immunoassays. J. Immunol. Methods 125: 279–285 [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J., DeBiasio R., Zhu Z., Giuliano K. A., Schmidt B. F., 1996. Immunofluorescence signal amplification by the enzyme-catalyzed deposition of a fluorescent reporter substrate (CARD). Cytometry 23: 48–53 [DOI] [PubMed] [Google Scholar]
- Choe K. P., Strange K., 2008. Genome-wide RNAi screen and in vivo protein aggregation reporters identify degradation of damaged proteins as an essential hypertonic stress response. Am. J. Physiol. Cell Physiol. 295: C1488–C1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codelia V. A., Cortes C. J., Moreno R. D., 2005. Inhibition of the vacuolar H(+)-pump with bafilomycin A1 does not induce acrosome reaction or activate proacrosin in mouse spermatozoa. Biochem. Biophys. Res. Commun. 337: 1337–1344 [DOI] [PubMed] [Google Scholar]
- Collins J., Saari B., Anderson P., 1987. Activation of a transposable element in the germ line but not the soma of Caenorhabditis elegans. Nature 328: 726–728 [DOI] [PubMed] [Google Scholar]
- Curran S. P., Ruvkun G., 2007. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 3: e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drose S., Altendorf K., 1997. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200: 1–8 [DOI] [PubMed] [Google Scholar]
- Emmons S. W., 2005. Male development (November 10, 2005), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.33.1, http://www.wormbook.org [Google Scholar]
- Emmons S. W., Yesner L., 1984. High-frequency excision of transposable element Tc1 in the nematode Caenorhabditis elegans is limited to somatic cells. Cell 36: 599–605 [DOI] [PubMed] [Google Scholar]
- Ernstrom G. G., Weimer R., Pawar D. R. L., Watanabe S., Hobson R. J., et al. , 2012. V-ATPase V1 sector is required for corpse clearance and neurotransmission in Caenorhabditis elegans. Genetics 191: 461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A. G., Kamath R. S., Zipperlen P., Martinez-Campos M., Sohrmann M., et al. , 2000. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408: 325–330 [DOI] [PubMed] [Google Scholar]
- Gleason E. J., Lindsey W. C., Kroft T. L., Singson A. W., L’Hernault S. W., 2006. spe-10 encodes a DHHC-CRD zinc-finger membrane protein required for endoplasmic reticulum/Golgi membrane morphogenesis during Caenorhabditis elegans spermatogenesis. Genetics 172: 145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Sadler P. L., Wallenfang M. R., Schumacher J. M., Hamill D. R., et al. , 2000. Metaphase to anaphase (mat) transition-defective mutants in Caenorhabditis elegans. J. Cell Biol. 151: 1469–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. K., Madl J. E., Kari C. K., 1979. Duplications in Caenorhabditis elegans. Genetics 92: 419–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier L. W., Miller R. D., Baird S. E., Chinwalla A., Fulton L. A., et al. , 2007. Comparison of C. elegans and C. briggsae genome sequences reveals extensive conservation of chromosome organization and synteny. PLoS Biol. 5: e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J., Doniach T., 1997. Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics 146: 149–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J., Horvitz H. R., Brenner S., 1979. Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics 91: 67–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R., 1983. A computer program for predicting protein antigenic determinants. Mol. Immunol. 20: 483–489 [DOI] [PubMed] [Google Scholar]
- Horvitz H. R., Brenner S., Hodgkin J., Herman R. K., 1979. A uniform genetic nomenclature for the nematode Caenorhabditis elegans. Mol. Gen. Genet. 175: 129–133 [DOI] [PubMed] [Google Scholar]
- Howell A. M., Gilmar S. G., Mancebo R. A., Rose A. M., 1987. Genetic analysis of a large autosomal region in Caenorhabditis elegans by the use of a free duplication. Genet. Res. 49: 207–213 [Google Scholar]
- Jakubowski J., Kornfeld K., 1999. A local, high-density, single-nucleotide polymorphism map used to clone Caenorhabditis elegans cdf-1. Genetics 153: 743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Ryu J., Kiraly M., Duke K., Reinke V., et al. , 2001. Genome-wide analysis of developmental and sex-regulated gene expression profiles in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 98: 218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., et al. , 2003. Systemic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237 [DOI] [PubMed] [Google Scholar]
- Kane P. M., 2006. The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol. Mol. Biol. Rev. 70: 177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki-Nishi S., Nishi T., Forgac M., 2001. Yeast V-ATPase complexes containing different isoforms of the 100-kDa a-subunit differ in coupling efficiency and in vivo dissociation. J. Biol. Chem. 276: 17941–17948 [DOI] [PubMed] [Google Scholar]
- Kelly W. G., Xu S., Montgomery M. K., Fire A., 1997. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics 146: 227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly W. G., Schaner C. E., Dernburg A. F., Lee M. H., Kim S. K., et al. , 2002. X-chromosome silencing in the germline of C. elegans. Development 129: 479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J., Crittenden S. L., 2005. Germline proliferation and its control (August 15, 2005), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.13.1, http://www.wormbook.org
- Laemmli U. K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- LaMunyon C. W., Ward S., 1994. Assessing the viability of mutant and manipulated sperm by artificial insemination of Caenorhabditis elegans. Genetics 138: 689–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., et al. , 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948 [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Wu C. H., Berg H., Levine J. H., 1980. The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics 95: 905–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Hernault S. W., 2006. Spermatogenesis (February 20, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.85.1, http://www.wormbook.org
- L’Hernault S. W., 2009. The genetics and cell biology of spermatogenesis in the nematode C. elegans. Mol. Cell. Endocrinol. 306: 59–65 [DOI] [PubMed] [Google Scholar]
- L’Hernault S. W., Roberts T. M., 1995. Cell biology of nematode sperm, pp. 273–301 in Caenorhabditis elegans: Modern Biological Analysis of an Organism, edited by Epstein H. F., Shakes D. C. Academic Press, San Diego [Google Scholar]
- L’Hernault S. W., Shakes D. C., Ward S., 1988. Developmental genetics of chromosome I spermatogenesis-defective mutants in the nematode Caenorhabditis elegans. Genetics 120: 435–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Hernault S. W., Benian G. M., Emmons R. B., 1993. Genetic and molecular characterization of the Caenorhabditis elegans spermatogenesis-defective gene spe-17. Genetics 134: 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. J., Herman P., Kang J. S., Lakowicz J. R., 2001. Fluorescence lifetime characterization of novel low-pH probes. Anal. Biochem. 294: 118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. J., Herman P., Lakowicz J. R., 2003. Fluorescence lifetime-resolved pH imaging of living cells. Cytometry A 52: 77–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio J. P., Pryor P. R., Bright N. A., 2007. Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8: 622–632 [DOI] [PubMed] [Google Scholar]
- Machaca K., L’Hernault S. W., 1997. The Caenorhabditis elegans spe-5 gene is required for morphogenesis of a sperm-specific organelle and is associated with an inherent cold-sensitive phenotype. Genetics 146: 567–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaca K., DeFelice L. J., L’Hernault S. W., 1996. A novel chloride channel localizes to Caenorhabditis elegans spermatids and chloride channel blockers induce spermatid differentiation. Dev. Biol. 176: 1–16 [DOI] [PubMed] [Google Scholar]
- Marshansky V., Futai M., 2008. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr. Opin. Cell Biol. 20: 415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C., Fire A., 1995. DNA transformation, pp. 451–482 in Caenorhabditis elegans: Modern Biological Analysis of an Organism, edited by Epstein H. F., Shakes D. C. Academic Press, San Diego [Google Scholar]
- Miller D. M., Shakes D. C., 1995. Immunofluorescence microscopy, pp. 365–394 Caenorhabditis elegans: Modern Biological Analysis of an Organism, edited by Epstein H. F., Shakes D. C. Academic Press, San Diego [Google Scholar]
- Moreno R. D., Alvarado C. P., 2006. The mammalian acrosome as a secretory lysosome: new and old evidence. Mol. Reprod. Dev. 73: 1430–1434 [DOI] [PubMed] [Google Scholar]
- Nishimura H., L’Hernault S. W., 2010. Spermatogenesis-defective (spe) mutants of the nematode Caenorhabditis elegans provide clues to solve the puzzle of male germline functions during reproduction. Dev. Dyn. 239: 1502–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Thomson J. N., 1985. Monoclonal antibodies which distinguish certain classes of neuronal and supporting cells in the nervous tissue of the nematode Caenorhabditis elegans. J. Neurosci. 5: 643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroutis P., Touret N., Grinstein S., 2004. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology (Bethesda) 19: 207–215 [DOI] [PubMed] [Google Scholar]
- Rath A., Glibowicka M., Nadeau V. G., Chen G., Deber C. M., 2009. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc. Natl. Acad. Sci. USA 106: 1760–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke V., Smith H. E., Nance J., Wang J., Van Doren C., et al. , 2000. A global profile of germline gene expression in C. elegans. Mol. Cell 6: 605–616 [DOI] [PubMed] [Google Scholar]
- Shakes D. C., Ward S., 1989. Initiation of spermiogenesis in C. elegans: a pharmacological and genetic analysis. Dev. Biol. 134: 189–200 [DOI] [PubMed] [Google Scholar]
- Shakes D. C., Sadler P. L., Schumacher J. M., Abdolrasulnia M., Golden A., 2003. Developmental defects observed in hypomorphic anaphase-promoting complex mutants are linked to cell cycle abnormalities. Development 130: 1605–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen B., Koski L. B., Walsh A., Marschall P., Neumann B., et al. , 2005. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature 434: 462–469 [DOI] [PubMed] [Google Scholar]
- Spieth J., Shim Y. H., Lea K., Conrad R., Blumenthal T., 1991. elt-1, an embryonically expressed Caenorhabditis elegans gene homologous to the GATA transcription factor family. Mol. Cell. Biol. 11: 4651–4659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. D., Bao Z., Blasiar D., Blumenthal T., Brent M. R., et al. , 2003. The Genome sequence of Caenorhabditis briggsae: a platform for comparative genomics. PLoS Biol. 1: E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M., Roberts T. M., 2005. Cytoskeleton dynamics powers nematode sperm motility. Adv. Protein Chem. 71: 383–399 [DOI] [PubMed] [Google Scholar]
- Sumner J. P., Dow J. A., Earley F. G., Klein U., Jager D., et al. , 1995. Regulation of plasma membrane V-ATPase activity by dissociation of peripheral subunits. J. Biol. Chem. 270: 5649–5653 [DOI] [PubMed] [Google Scholar]
- Sun-Wada G. H., Imai-Senga Y., Yamamoto A., Murata Y., Hirata T., et al. , 2002. A proton pump ATPase with testis-specific E1-subunit isoform required for acrosome acidification. J. Biol. Chem. 277: 18098–18105 [DOI] [PubMed] [Google Scholar]
- Syntichaki P., Samara C., Tavernarakis N., 2005. The vacuolar H+ -ATPase mediates intracellular acidification required for neurodegeneration in C. elegans. Curr. Biol. 15: 1249–1254 [DOI] [PubMed] [Google Scholar]
- Tam J. P., 1989. High-density multiple antigen-peptide system for preparation of antipeptide antibodies. Methods Enzymol. 168: 7–15 [DOI] [PubMed] [Google Scholar]
- Toei M., Saum R., Forgac M., 2010. Regulation and isoform function of the V-ATPases. Biochemistry 49: 4715–4723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S., Argon Y., Nelson G. A., 1981. Sperm morphogenesis in wild-type and fertilization-defective mutants of Caenorhabditis elegans. J. Cell Biol. 91: 26–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S., Hogan E., Nelson G. A., 1983. The initiation of spermiogenesis in the nematode Caenorhabditis elegans. Dev. Biol. 98: 70–79 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.