The digestive and respiratory systems have different physiological functions (nutrition, food transit and evacuation for the first; breathing and oxygen supply to the blood for the second) and are generally considered and studied as two independent structures. Nevertheless, although at birth they are separated, they both derive from a common and transient developmental structure, the foregut, which is the anterior part of the gastrointestinal (GI) tract (1). The GI tract is a remarkably complex, three-dimensional, specialized and vital system that is derived from a simple tube-like structure. The vertebrate GI tract includes the luminal digestive system (i.e., esophagus, stomach, intestine and colon, which we will designate on the whole as “gut”) and the GI-tract derivatives (i.e., thyroid, lungs, liver and pancreas). The gut is composed of three germ layers: mesoderm (which forms the smooth muscle layer), endoderm (which forms the epithelial lining) and ectoderm (which includes the enteric nervous system). The gut develops from two invaginations at the anterior (anterior intestinal portal, AIP) and posterior (caudal intestinal portal, CIP) end of the embryo that elongate and fuse to form a straight tube. The primitive gut tube is initially patterned into three broad domains along its anterior-posterior (AP) axis: the fore-, mid- and hindgut. As they develop, each region of the gut is characterized by unique mesodermal and endodermal morphologies, which can easily be discerned by gross and microscopic examination. Specifically, these tissues show regional differentiation along the AP axis that specifies pharynx, esophagus and stomach (the foregut), small intestine (the midgut) and large intestine (hindgut). This regionalization is maintained throughout life and is essential and necessary for normal gut function. These patterning events are remarkably conserved across species (1) and patterning anomalies are likely to be responsible for many of the human gut malformation syndromes, such as tracheo-esophageal atresia (TE), infantile hypertrophic pyloric stenosis or anal atresia (1,2).
Foregut development
Lung and gut are two independent systems that originate from one common embryonic organ, the foregut. The development of the foregut is not well documented in comparison to that of other parts of the digestive system. The respiratory system originates from the formation of an endodermal diverticulum in the ventral wall of the foregut, whereas the esophagus forms from the foregut dorsal wall (1). Specifically, the foregut endoderm evaginates and pushes the surrounding mesenchyme to form the two presumptive lung buds. In avian embryos, these processes manage to form two independent and separate endodermal structures, dorsally the esophagus and ventrally the lung buds, in a very short time (around 10 hours). Later on, the lung buds grow caudally, leading to the formation of a temporary TE septum. The appearance of the TE septum is followed first by the expansion of the trachea and by the final separation of the two endodermally-derived systems.
Molecular pathways involved in foregut development
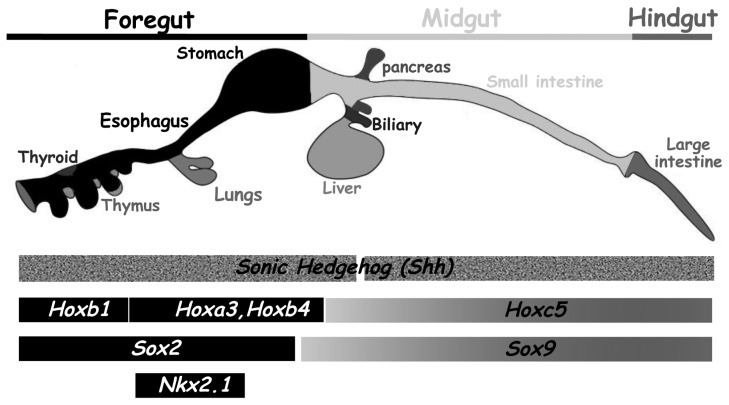
The cellular events that contribute to foregut specification, lung development and separation of trachea and esophagus are regulated by different and specific molecular pathways. The regionalization of the different parts of the gut is controlled by the localized expression of different Hox genes, which are homeobox-containing transcription factors (3). For example, Hoxa3 and Hoxb4 are specifically expressed in the foregut endoderm, whereas Hoxc5 and Hoxa13 are expressed more caudally, respectively in the midgut and hindgut endoderm (3,4). Sonic Hedgehog (Shh), a member of the Hedgehog (Hh) family of morphogens is expressed in the entire endodermal layer but not in the pancreas (3). Moreover, Ligtingting and colleagues reported a specific and dynamic expression pattern of shh during TE development with earlier expression and patterning of the ventral foregut by Shh and transient inhibition of shh expression in the tracheal endoderm (5). Others transcription factors also show specific expression profiles in the endoderm, such as the SRY-related High-Mobility Group (HMG) transcription factors Sox2 (foregut endoderm) and Sox9 (midgut/hindgut endoderm) (6,7). The homeodomain transcription factor Nkx2.1 (also called TTF1 or T/EBP) is specifically expressed in the anterior part of the foregut and in the endoderm of the developing trachea but not of the esophagus (8). All these genes are expressed in the foregut endoderm in a spatially regulated manner (Figure 1) and therefore their deregulation could be implicated in the genesis of TE malformations.
Figure 1.
Molecular pathways involved in the regionalization of the foregut endoderm. Expression boundaries of selected factors expressed in the endoderm along the antero-posterior axis. The embryonic GI tract is divided into fore-, mid- and hindgut. Sonic hedgehog (shh) is expressed in the entire endodermal layer sparing the pancreas. Hox genes which are homeobox-containing transcription factors harbor a specific localized expression into the GI endoderm. SRY-related High Mobility Group (HMG) transcription factors Sox2 and Sox9 present exclusive pattern in GI endoderm. The transcription factor Nkx2.1 is expressed into the foregut. Adapted from Zorn and Wells (2009) (15).
In order to investigate their function(s) and requirement during foregut development, these genes have been inactivated in different transgenic mouse lines. For instance, inactivation of Nkx2.1 is associated with the presence of a common TE lumen suggesting that NKX2.1 is essential for the development and separation of TE endoderm (9). Inactivation of shh in mice, like for Nkx2.1, leads to a common TE lumen, demonstrating an essential function of Shh during TE development. Shh is a ligand and activator of the Hh signaling pathway. During the development of the GI tract, shh is localized to the gut endoderm, but it acts on the gut mesenchyme to control endodermal-mesenchymal interactions (3). After processing by the Golgi apparatus, Shh is secreted by endodermal cells and then binds to its receptor PATCHED (or PTCH1), which is expressed in the surrounding mesenchymal cells. PATCHED activation is followed by cleavage and activation of the GLI1/2 transcription factors, which regulate various genetic networks and the expression of mesenchymal-specific genes, such as Ptch1, Gli1 and Bone Morphogenetic Protein 4 (Bmp4) (3,7).
Epithelial-mesenchymal interactions during the foregut development
Epithelial-mesenchymal interactions are essential for the development and differentiation of the GI tract (7). However, the contribution of the mesenchyme during the development of the foregut is not often commented. Different studies reported a significant mesenchymal condensation at the site of TE separation (10,11). Moreover, in rats, treatment with the teratogenic agent adriamycin reproducibly induces esophageal atresia/TE fistula. Such malformations are associated with lower cellularity and disorder of the surrounding mesenchymal cells already at early stages of foregut misdevelopment (11), supporting the notion of a major involvement of the foregut mesenchyme during foregut development.
As previously commented, the Hh signaling pathway regulates foregut development through epithelial-mesenchymal interactions and different members of this pathway, such as the transcription factor Gli2 (12), are expressed in the foregut mesenchyme. To analyze the interplay of the different components of the Hh pathway that are expressed in the foregut mesenchyme, Motoyama and colleagues inactivated Gli2 in mice and observed the presence of TE malformation associated with alterations of visceral smooth muscle cells (13). In addition, inactivation of both Gli2 and Gli3 caused a stronger phenotype with a common TE structure, demonstrating that inhibition of genes of the Hh pathway, which are normally expressed in the foregut mesenchyme, triggers similar phenotypes as inactivation of the endodermally expressed shh.
Recently, also the function of the BMP signaling pathway during TE development was investigated by the Hogan’s laboratory. This signaling pathway is activated in the gut mesenchyme following expression and activation of shh and Patch. While shh is localized to the ventral foregut endoderm, Bmp4 is expressed in the neighboring mesenchyme but not in the dorsal part of the foregut, which will give rise to the esophagus (14) and where Noggin, the inhibitor of the BMP pathway, is expressed. To analyze the function of the BMP pathway during foregut development, Que and colleagues inactivated Noggin and observed the presence of a common TE structure (14). In a rescue experiment, where Noggin−/− mice where crossed with BMP4+/− mice to decrease the activity of the BMP pathway, the progeny showed normal separation and formation of trachea and esophagus, supporting the idea that the level of BMP activity is crucial for the development of these two structures. These examples demonstrate that the foregut mesenchyme is essential for the correct development of the foregut and that the mesenchyme could actively participate in the development of these two structures.
Conclusion and perspectives
Candidate factors for foregut development include known pattern formation genes that were first identified in Drosophila, such as nuclear homeotic transcription factors (HOX, SOX and NKX factors) and secreted factors (BMP and Hh factors) (3,6,15,16). Genetic evidences from different animal models indicate that these molecules play multiple and crucial roles in the development and septation of trachea and esophagus. The identification of NMYC and chromodomain helicase DNA binding (CDH) CDH7 mutations in syndromic forms of TE bring us new factors that could be involved during the development of the trachea and esophagus (16). To better understand the molecular basis of human malformations and syndromes, such as TE atresia, it is essential to better dissect normal foregut development at the molecular level and to stimulate translational research between scientists and clinicians.
Acknowledgments
Supported by grants from Agence Nationale pour la Recherche (ANR-07-JCJC-0112), Association Française contre les Myopathies, Région Languedoc-Roussillon (Chercheur d’Avenir) and Ligue Contre le Cancer (Comité de l’Aude).
References
- 1.de Santa Barbara P, Van den Brink GR, Roberts DJ. Molecular etiology of gut malformations and diseases. Am J Med Genet. 2002;115:221–30. doi: 10.1002/ajmg.10978. [DOI] [PubMed] [Google Scholar]
- 2.Geneviève D, de Pontual L, Amiel J, et al. An overview of isolated and syndromic oesophagal atresia. Clin Genet. 2007;71:392–99. doi: 10.1111/j.1399-0004.2007.00798.x. [DOI] [PubMed] [Google Scholar]
- 3.Roberts DJ, Johnson RL, Burke AC, et al. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–74. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- 4.de Santa Barbara P, Roberts DJ. Tail gut endoderm and gut/genitourinary/tail development: a new tissue-specific role for Hoxa13. Development. 2002;129:551–61. doi: 10.1242/dev.129.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 6.Moniot B, Biau S, Faure S, et al. SOX9 specifies the pyloric sphincter epithelium through mesenchymal-epithelial signals. Development. 2004;131:3795–804. doi: 10.1242/dev.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Santa Barbara P, van den Brink GR, Roberts DJ. Development and differentiation of the intestinal epithelium. Cell Mol Life Sci. 2003;60:1322–32. doi: 10.1007/s00018-003-2289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- 9.Minoo P, Su G, Drum H, et al. Defects in tracheoesophageal and lung morphogenesis in Nkx2. 1(−/−) mouse embryos. Dev Biol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- 10.Kluth D, Fiegel H. The embryology of the foregut. Semin Pediatr Surg. 2003;12:3–9. doi: 10.1053/spsu.2003.50003. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki T, Kusafuka T, Okada A. Analysis of the development of normal foregut and tracheoesophageal fistula in an adriamycin rat model using three-dimensional image reconstruction. Surg Today. 2001;31:133–9. doi: 10.1007/s005950170197. [DOI] [PubMed] [Google Scholar]
- 12.Hui CC, Slusarski D, Platt KA, et al. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm-and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162:402–13. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- 13.Motoyama J, Liu J, Mo R, et al. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20:54–7. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- 14.Que J, Choi M, Ziel JW, et al. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–37. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 15.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–51. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunner HG, van Bokhoven H. Genetic players in esophageal atresia and tracheosephageal fistula. Curr Opin Genet Dev. 2005;15:341–7. doi: 10.1016/j.gde.2005.04.010. [DOI] [PubMed] [Google Scholar]